Application of flow chemistry in toluene nitrification
A technology of flow chemistry and nitration reaction, applied in the field of chemical materials, can solve the problems of difficult control of the reaction process, potential safety hazards, and overheating of the reaction, achieve high selectivity of the target product, reduce the possibility of accidents, and reduce the o/p value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The invention provides a technical solution: an application of flow chemistry in toluene nitration.
[0035] In this embodiment, preferably, toluene is used as a solvent, nitric acid is used as a nitrating agent, and sulfuric acid is used as a catalyst to carry out the nitration reaction in a microchannel reactor.
[0036] In the present embodiment, preferably, the specific steps of the nitration reaction are as follows:
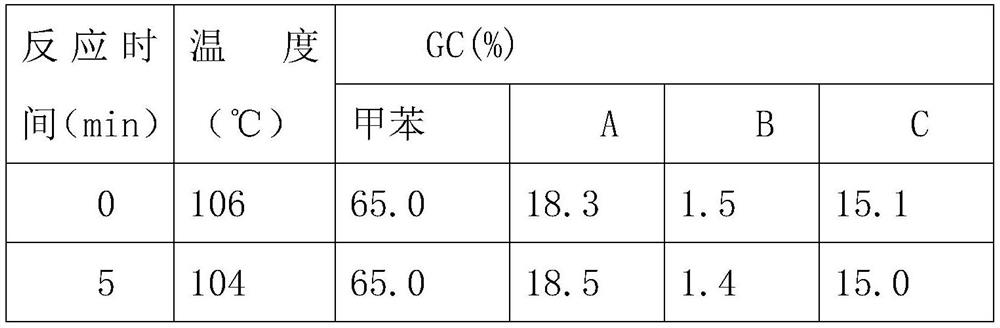
[0037] Step 1, mix the nitric acid of 1.0g, 15.2ml / mol and the sulfuric acid catalyst of 0.1g, obtain the mixed solution, then the mixed solution and the toluene of 10.0g, 21.7ml / mol are transported to 50ml micro Two inlets of the channel reactor;
[0038] Step 2, after the two liquids are mixed and contacted in the microchannel reactor and reacted, they flow out from the outlet of the microchannel reactor into the collection tank;
[0039] Step 3, the solution in the collection tank is left to stand for stratification, the upper layer is a mixture ...
Embodiment 2
[0060] The invention provides a technical solution: an application of flow chemistry in toluene nitration.
[0061]In this embodiment, preferably, toluene is used as a solvent, nitric acid is used as a nitrating agent, and sulfuric acid is used as a catalyst to carry out the nitration reaction in a microchannel reactor.
[0062] In the present embodiment, preferably, the specific steps of the nitration reaction are as follows:
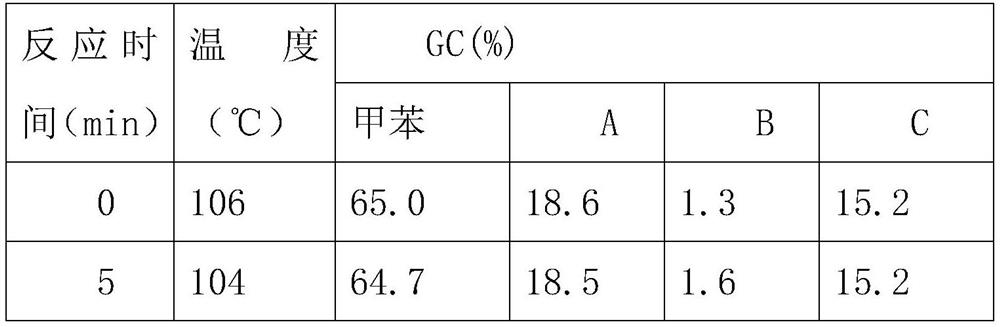
[0063] Step 1, mix the nitric acid of 2.0g, 30.4ml / mol and the sulfuric acid catalyst of 0.1g, obtain mixed solution, then the toluene of mixed solution and 10.0g, 21.7ml / mol is transported to 50ml micro Two inlets of the channel reactor;
[0064] Step 2, after the two liquids are mixed and contacted in the microchannel reactor and reacted, they flow out from the outlet of the microchannel reactor into the collection tank;
[0065] Step 3, the solution in the collection tank is left to stand for stratification, the upper layer is a mixture of sulfuri...
Embodiment 3
[0086] The invention provides a technical solution: an application of flow chemistry in toluene nitration.
[0087] In this embodiment, preferably, toluene is used as a solvent, nitric acid is used as a nitrating agent, and sulfuric acid is used as a catalyst to carry out the nitration reaction in a microchannel reactor.
[0088] In the present embodiment, preferably, the specific steps of the nitration reaction are as follows:
[0089] Step 1, mix the nitric acid of 2.0g, 45.7ml / mol and the sulfuric acid catalyst of 0.1g, obtain the mixed solution, then the mixed solution and the toluene of 10.0g, 21.7ml / mol are transported to 50ml micro Two inlets of the channel reactor;
[0090] Step 2, after the two liquids are mixed and contacted in the microchannel reactor and reacted, they flow out from the outlet of the microchannel reactor into the collection tank;
[0091] Step 3, the solution in the collection tank is left to stand for stratification, the upper layer is a mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com