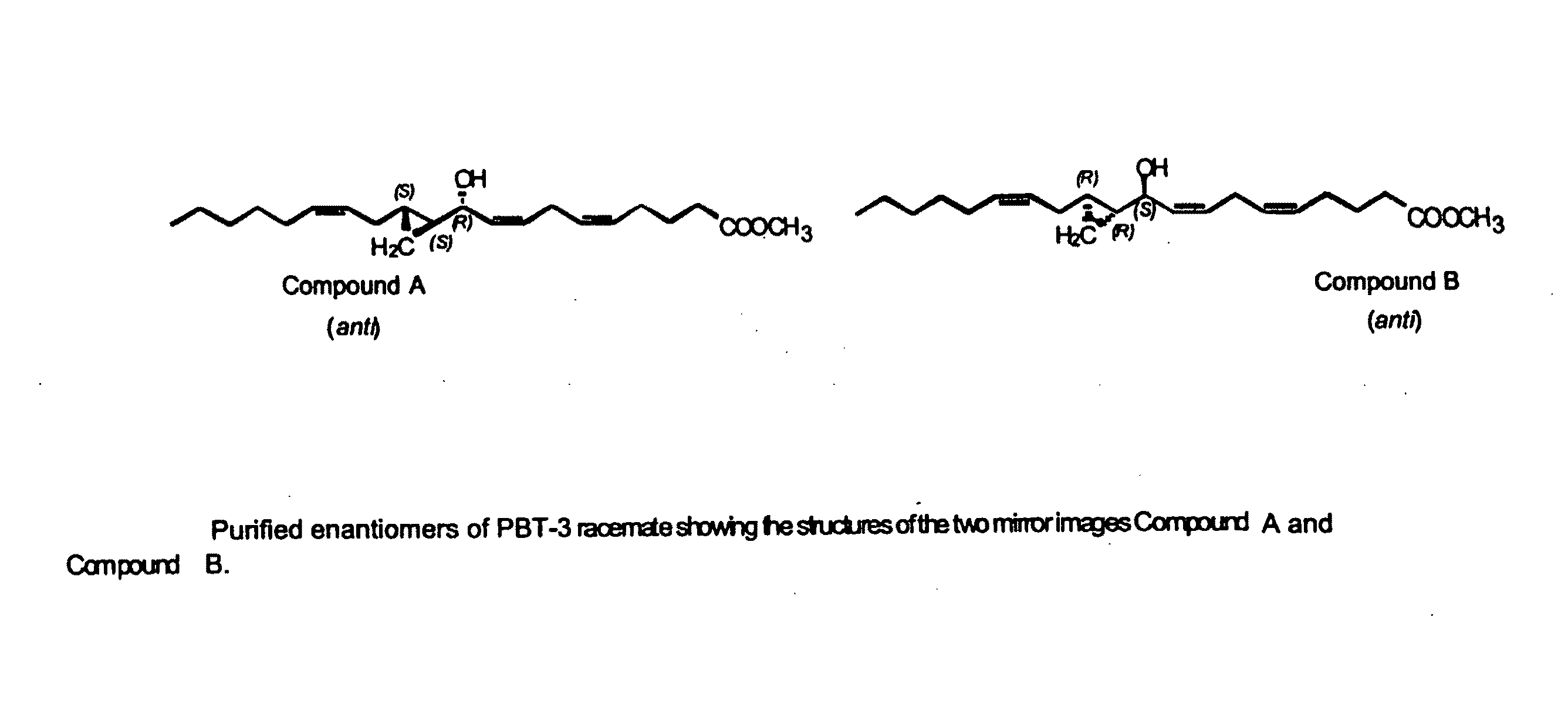
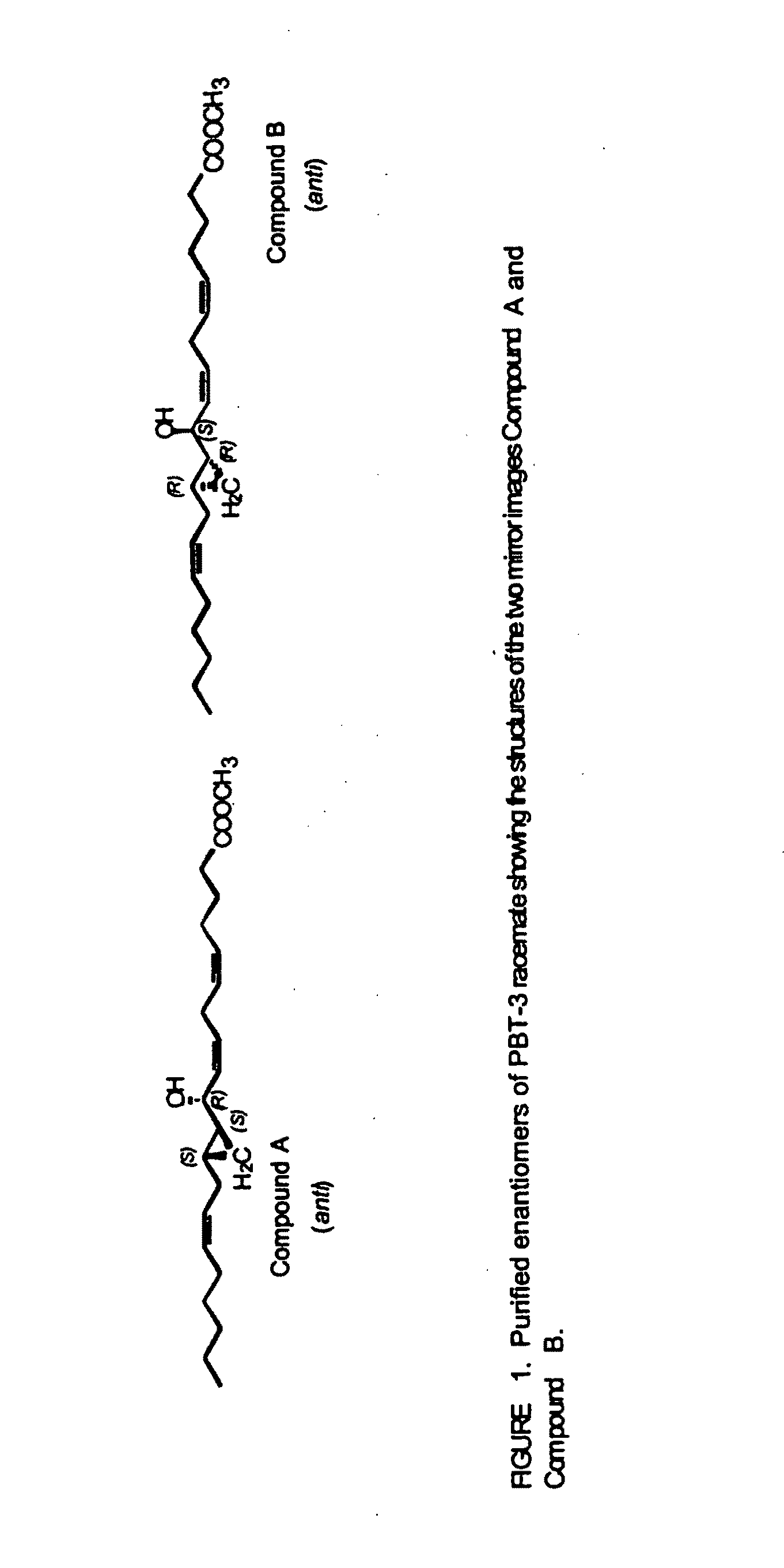
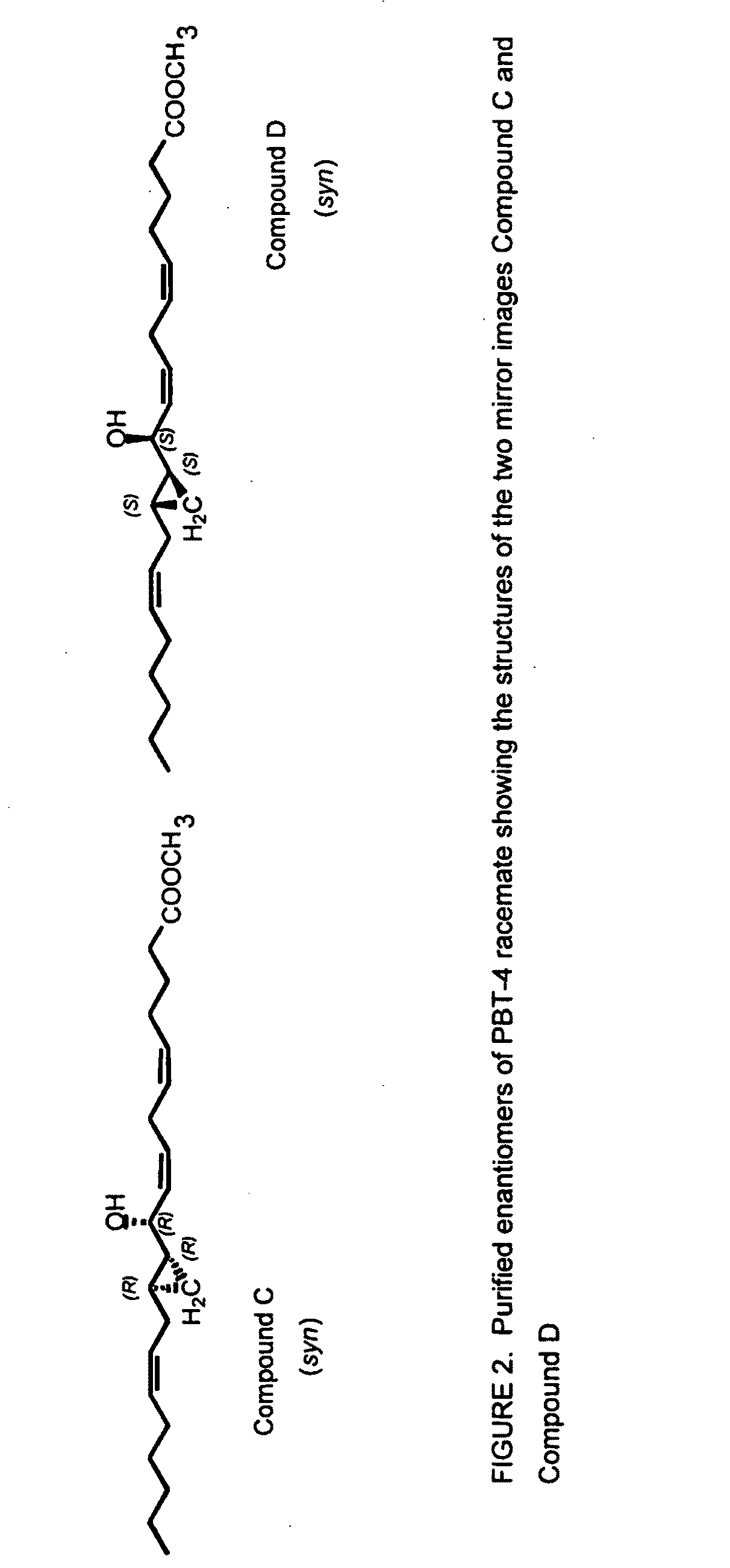
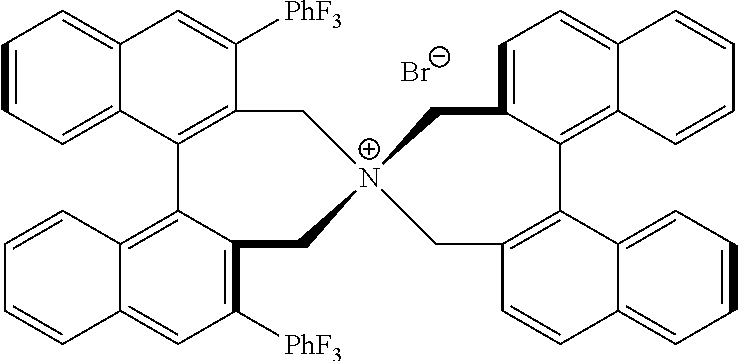
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Chiral phase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chiral phases. Chiral stationary phases are the most common first approach for enantiomer separation. To be able to separate racemic mixture of stereoisomers , the chiral phase has to form a diastereomeric complex with one of the isomers, or has to have any other type of stereospecific interactions.
Thermostable pigments, films and effect coatings, and mixtures for their production
InactiveUS6423246B1Reduce interactionDegrade solventLiquid crystal compositionsBoltsLiquid crystallineColor shift
A mixture of crosslinkable liquid-crystalline substances having a chiral phase (LC mixture), containing polymerizable groups, where at least 90% of the polymerizable groups are part of molecules containing at least two polymerizable groups (crosslinker molecules), wherein from 3.2 to 15 mmol of polymerizable groups are present per g of LC mixture. The crosslinked pigments show little color shift in the presence of solvents or upon application to substrate different temperatures.
Owner:SICPA HLDG SA
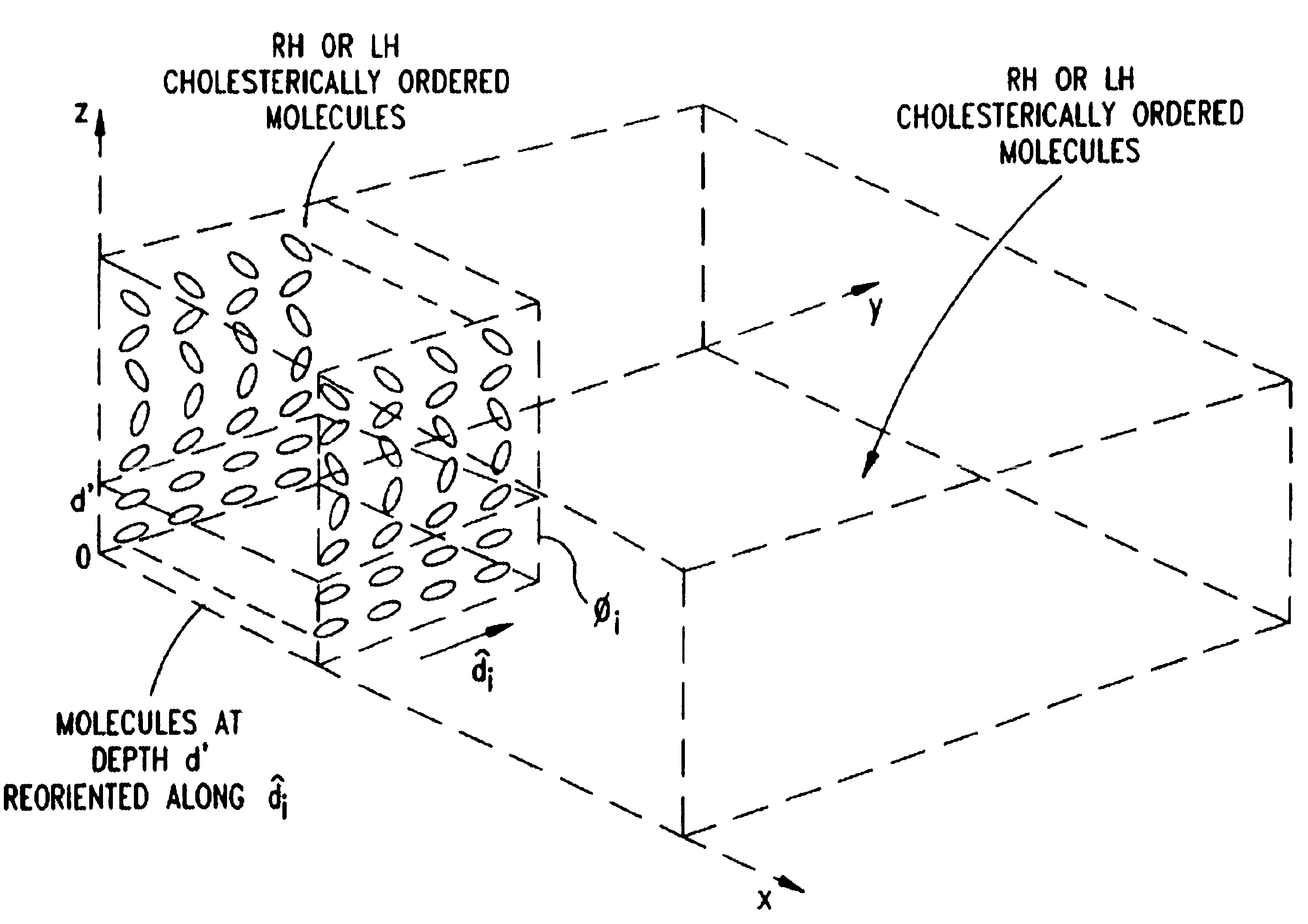
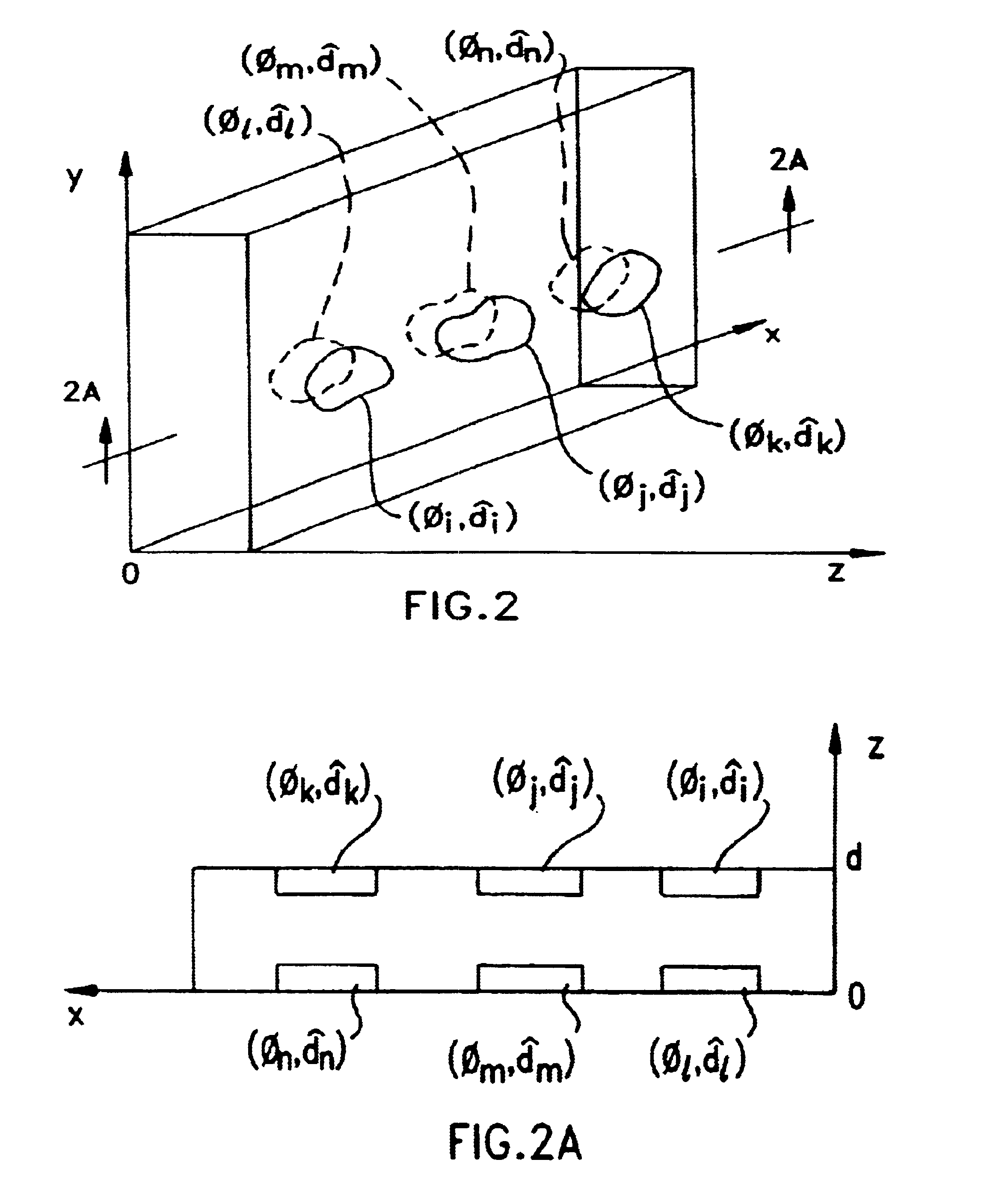
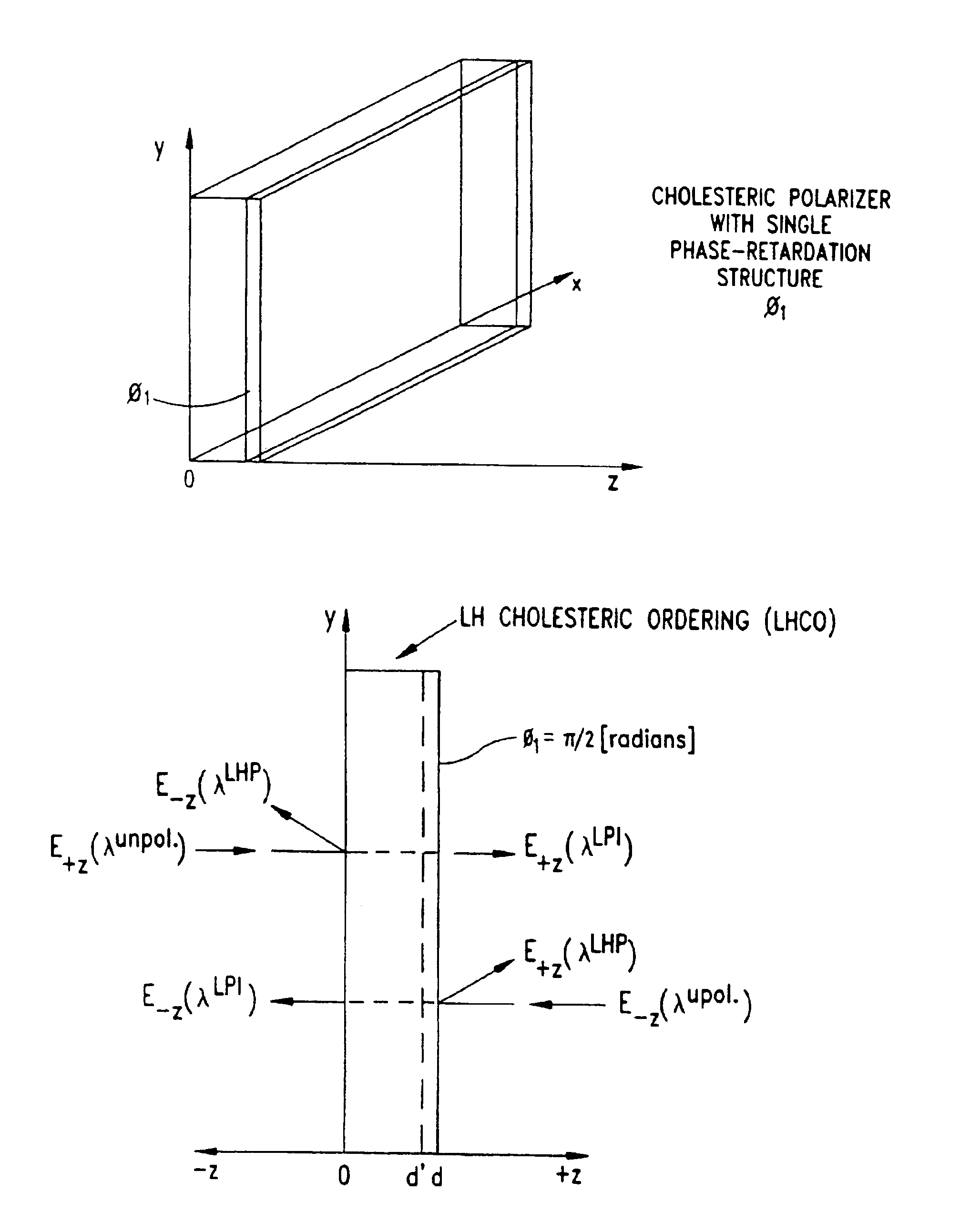
Liquid crystal film structures with phase-retardation surface regions formed therein and methods of fabricating the same
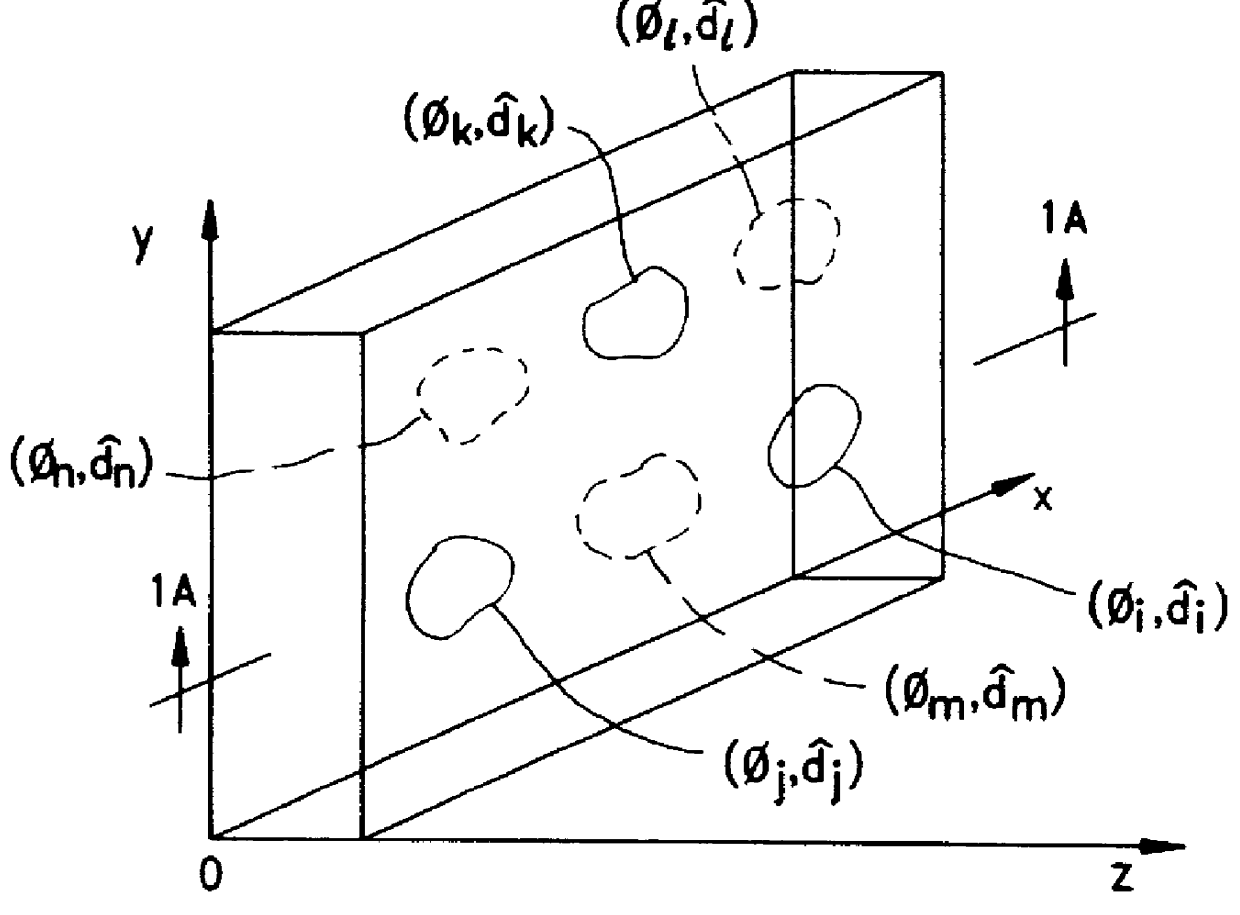
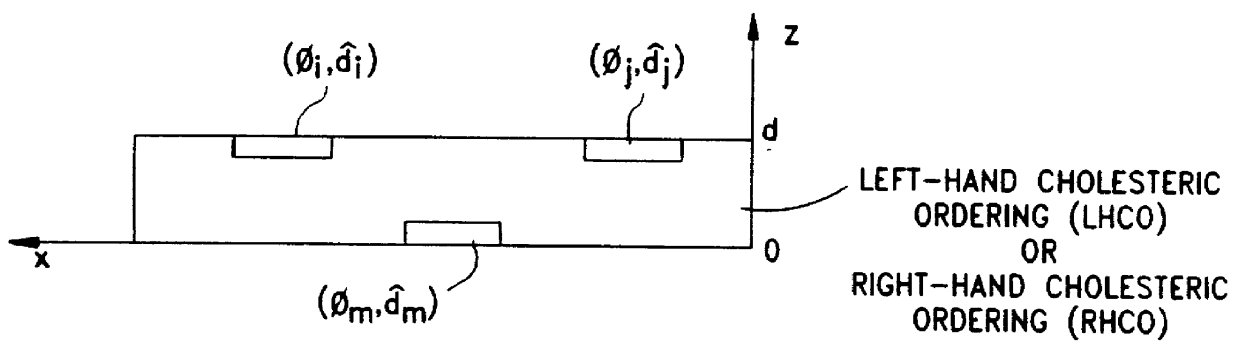
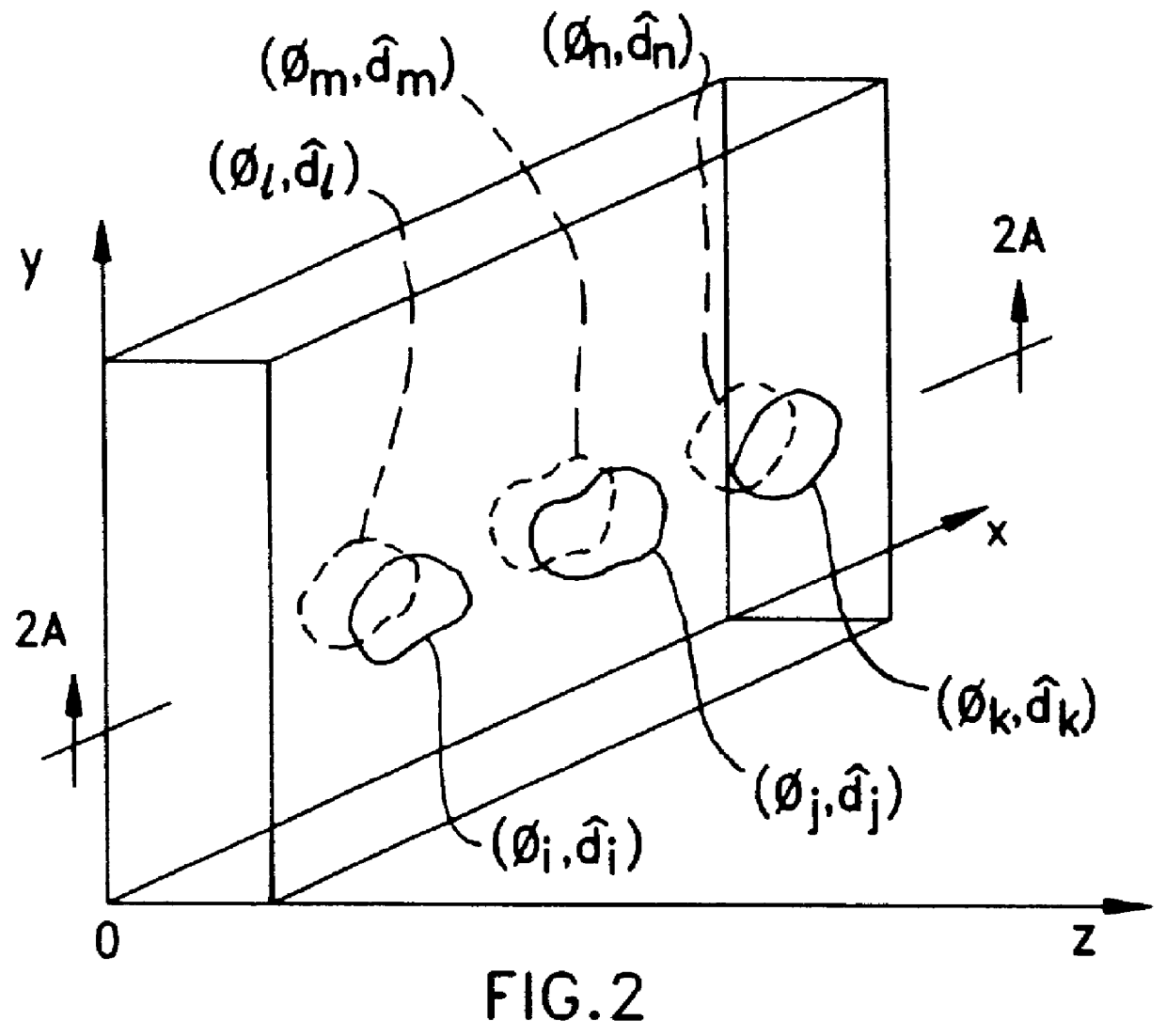
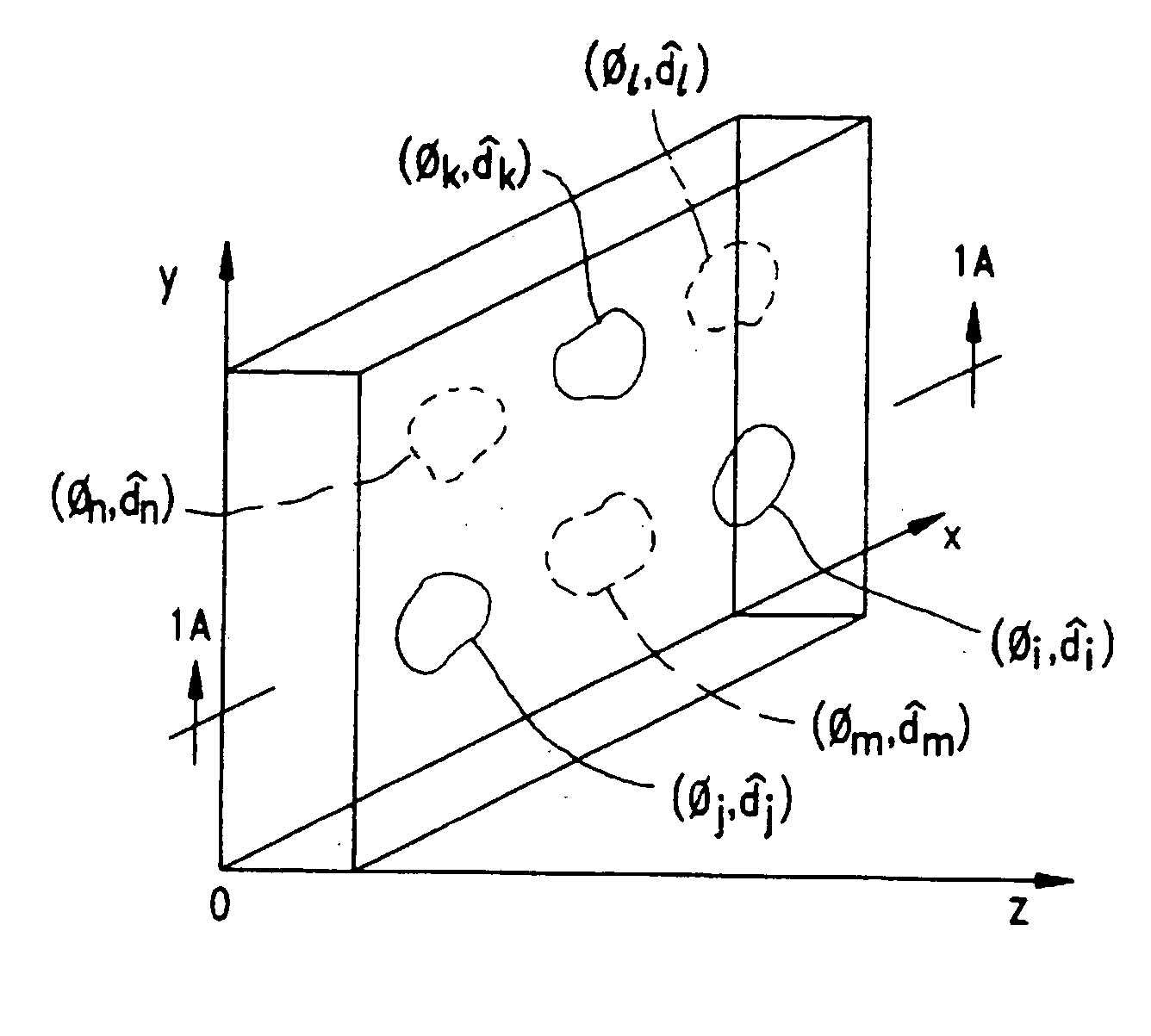
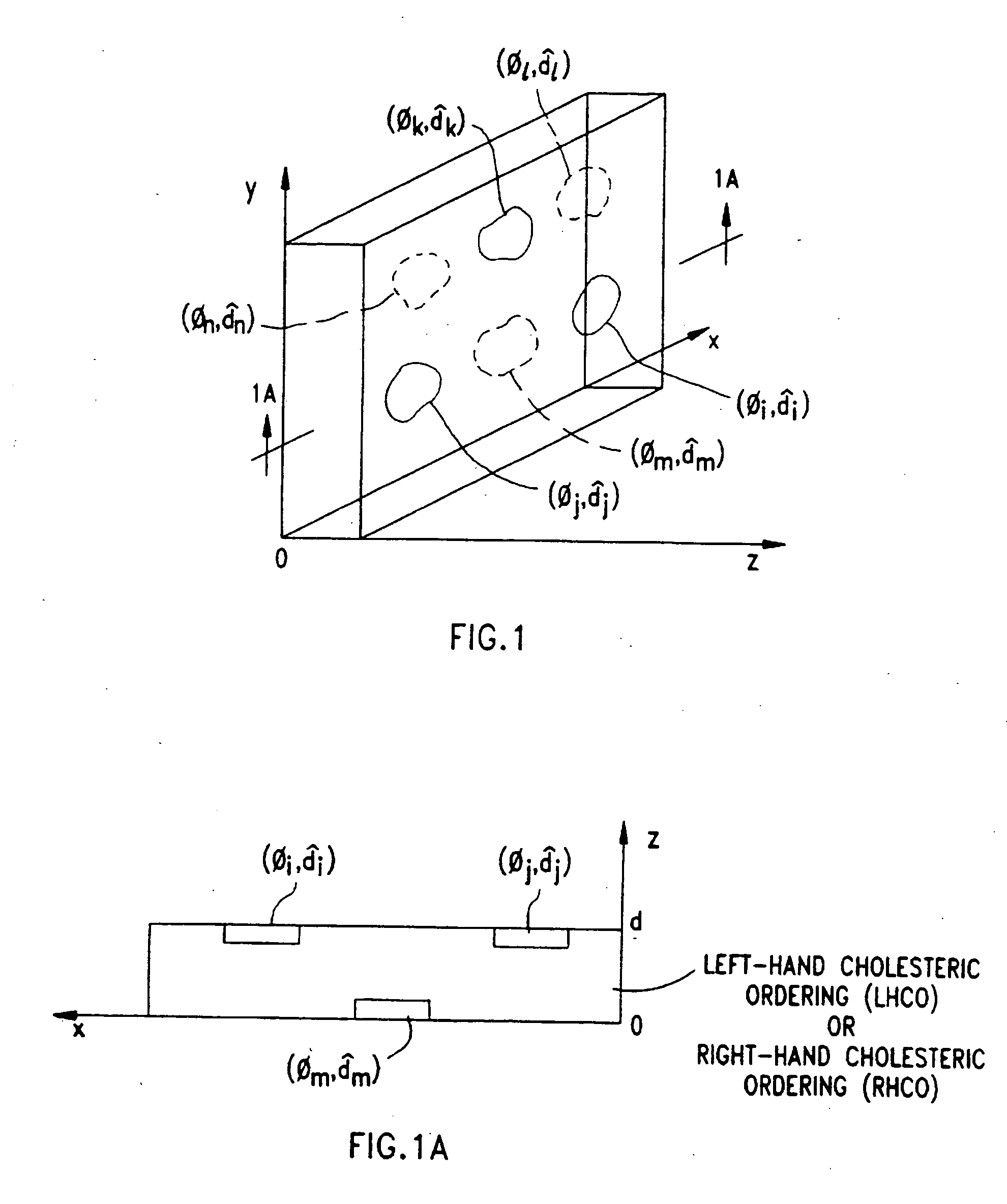
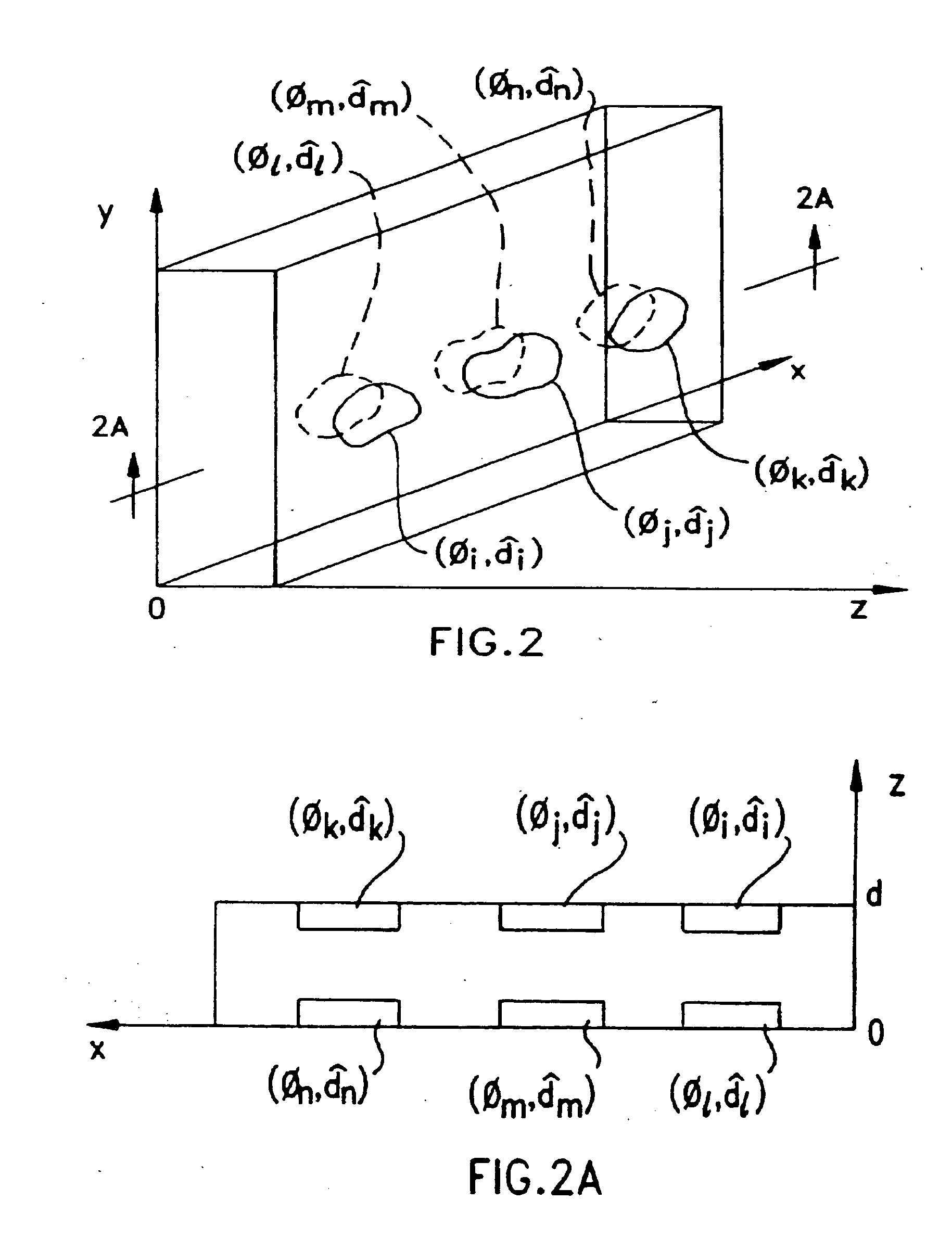
Disclosed are liquid crystal (LC) phase-retarders and linear polarizers and methods and apparatus for making the same. The liquid crystal phase-retarder is realized by a liquid crystal film structure having one or more phase retardation regions formed therein. Each phase retardation region has an optical axis specified by the direction and depth of orientation of liquid crystal molecules along the surface of the liquid crystal film structure. The liquid crystal linear polarizer is realized by a liquid crystal film structure having a chiral phase region within which liquid crystal molecules are cholesterically ordered. One or more nematic phase regions are formed along the surface of the liquid crystal film structure within which liquid crystal molecules are oriented along a direction and to a surface depth sufficient to realize one or more phase retardation regions therein having optical axes along the direction of liquid crystal molecule orientation.
Owner:REVEO
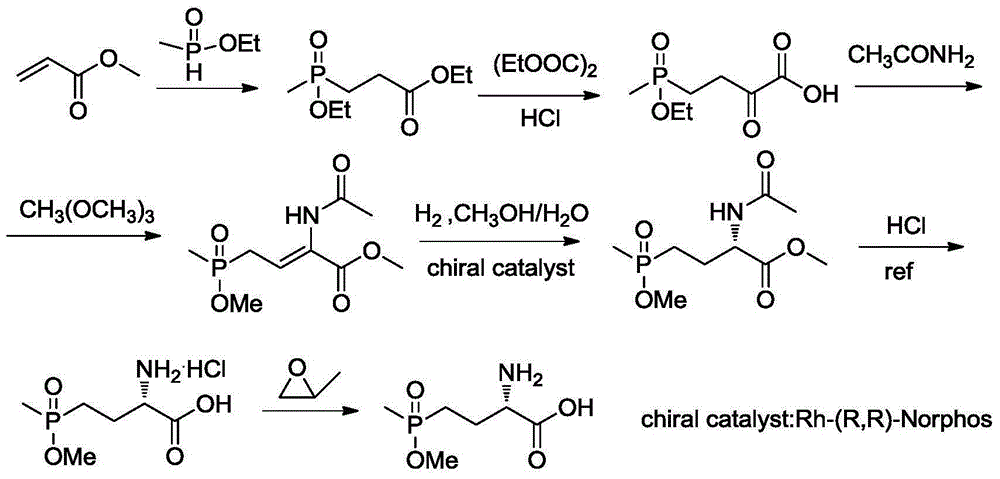
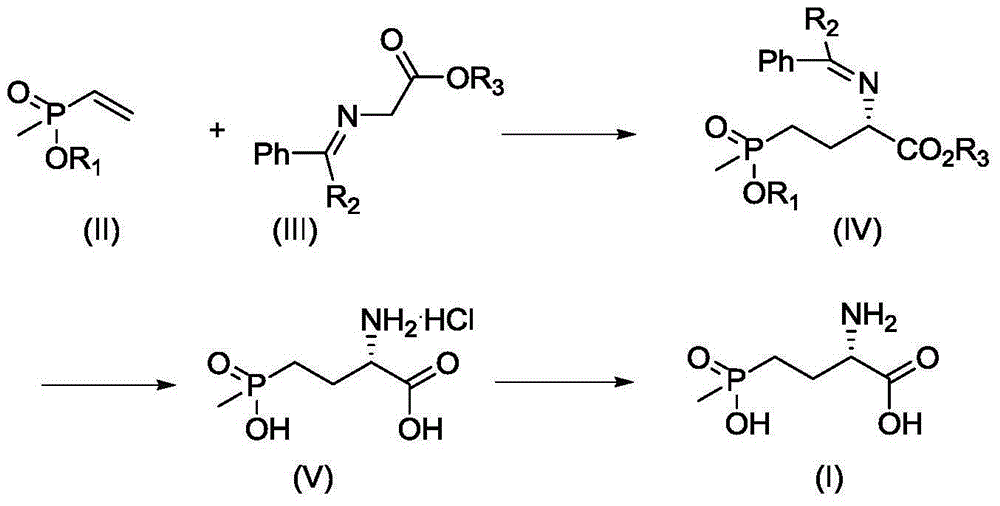
Synthetic method for L-phosphinothricin
InactiveCN105131032AEasy to synthesizeHigh yieldGroup 5/15 element organic compoundsHydrolysisHydrochloride
The invention discloses a synthetic method for L-phosphinothricin. The synthetic method has a reaction formula as shown in a formula described in the specification. The method comprises the following steps: with a methylvinylphosphonate compound (II) and a benzylideneglycinate compound (III) as raw materials, carrying out an addition reaction so as to obtain an intermediate, i.e., an S-benzylidenephosphonobutyrate compound (IV); then carrying out hydrolysis so as to obtain hydrochloride (V); and carrying out neutralization so as to obtain the L-phosphinothricin (I). The method provided by the invention uses chiral phase transfer catalysis (a cinchonidine chiral quaternary ammonium salt derivative) as a means to construct a chiral center of a phosphinothricin molecular structure, so a chiral herbicide, i.e., the L-phosphinothricin, is obtained.
Owner:XIAN MODERN CHEM RES INST
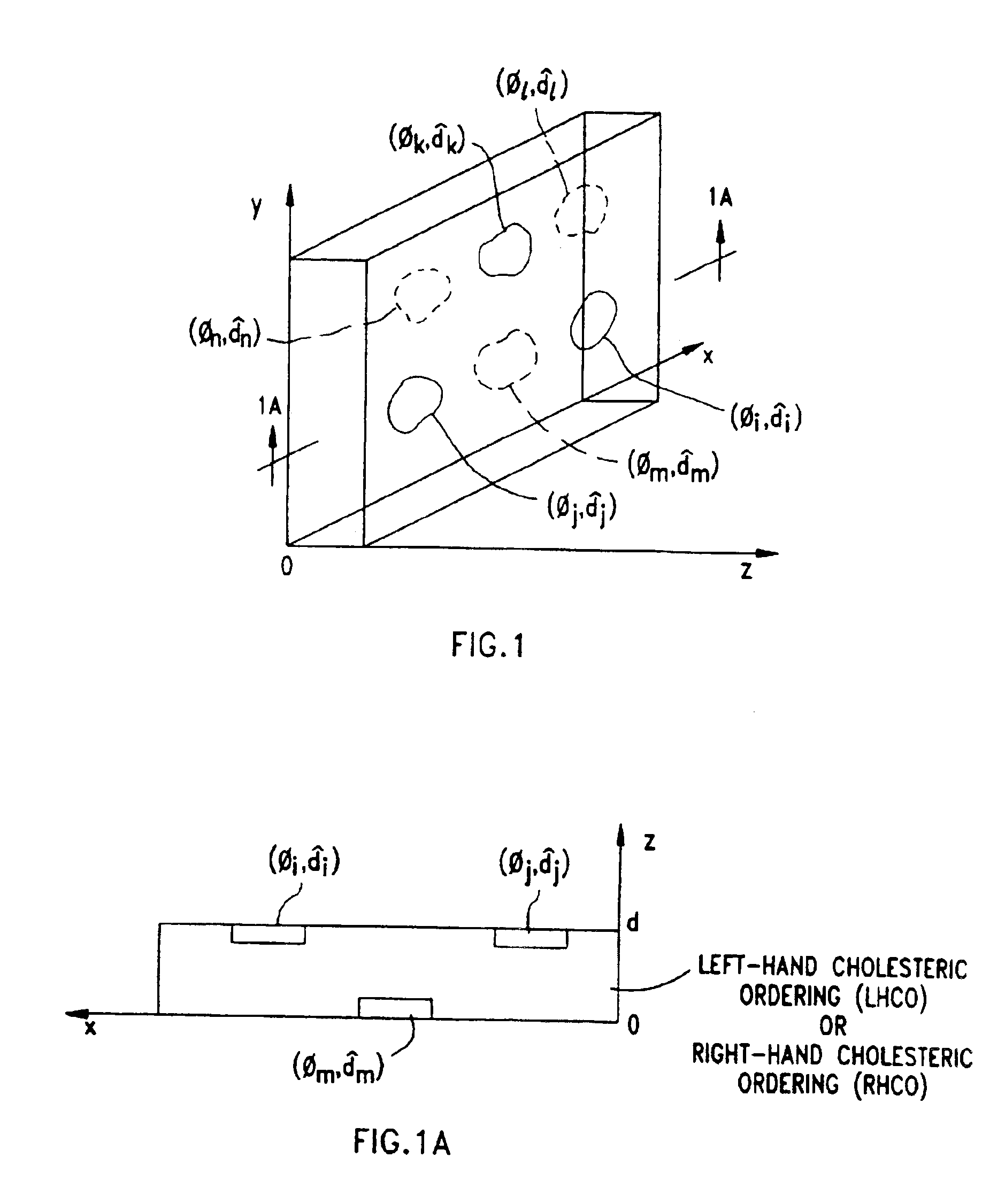
Liquid crystal film structures with phase-retardation surface regions formed therein
Disclosed are liquid crystal phase-retarders and linear polarizers and methods and apparatus for making the same. The liquid crystal phase-retarder is realized by a liquid crystal film structure having one or more phase retardation regions formed therein. Each phase retardation region has an optical axis specified by the direction and depth of orientation of liquid crystal molecules along the surface of the liquid crystal film structure. The liquid crystal linear polarizer is realized by a liquid crystal film structure having a chiral phase region within which liquid crystal molecules are cholesterically ordered (FIG. 3). One or more nematic phase regions are formed along the surface of the liquid crystal film structure within which liquid crystal molecules are oriented along a direction and to a surface depth sufficient to realize one or more phase retardation regions therein having optical axes along the direction of liquid crystal molecules orientation.
Owner:REVEO
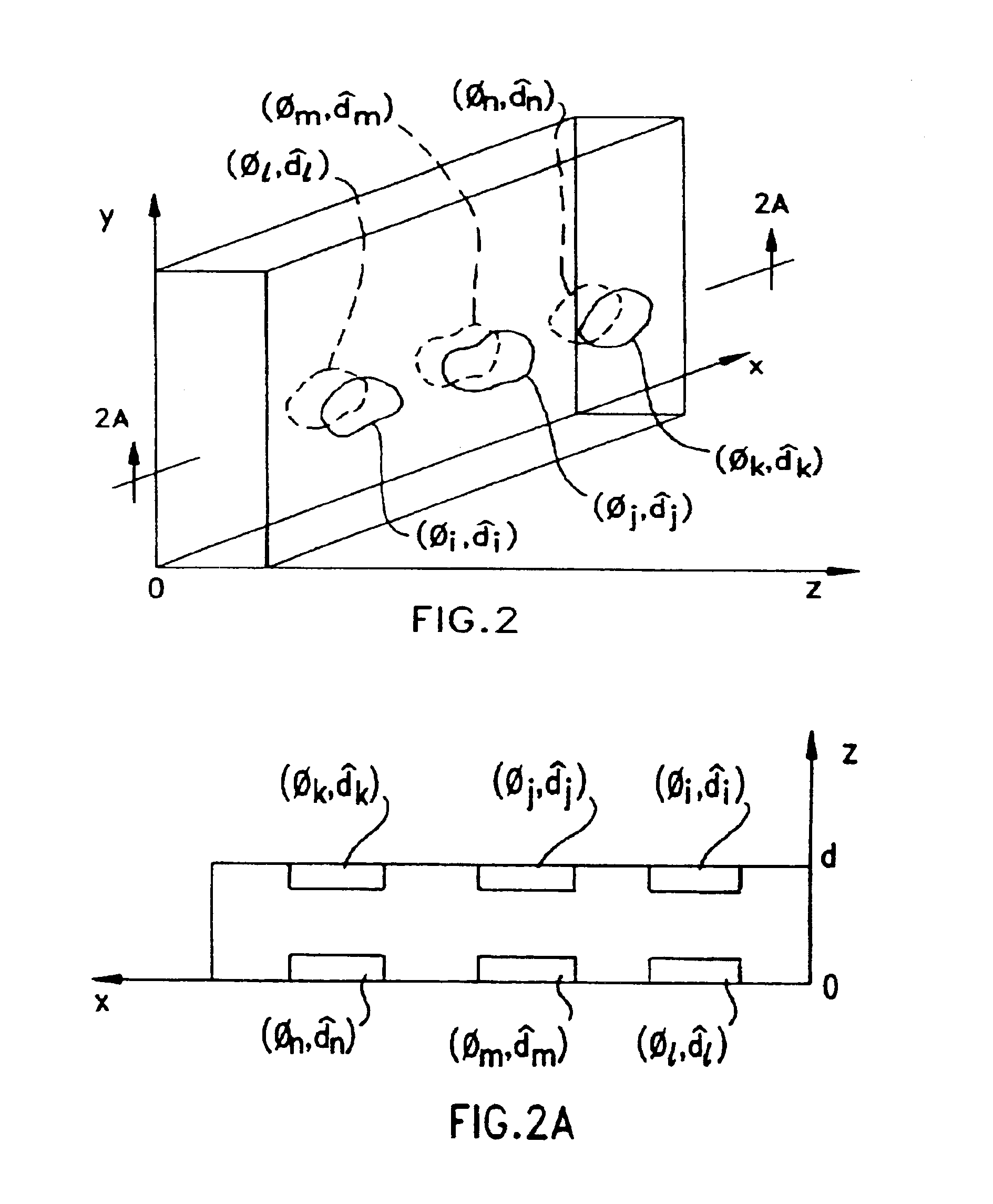
Liquid crystal film structures with phase-retardation surface regions formed therein
Disclosed are liquid crystal (LC) phase-retarders and linear polarizers and methods and apparatus for making the same. The liquid crystal phase-retarder is realized by a liquid crystal film structure having one or more phase retardation regions formed therein. Each phase retardation region has an optical axis specified by the direction and depth of orientation of liquid crystal molecules along the surface of the liquid crystal film structure. The liquid crystal linear polarizer is realized by a liquid crystal film structure having a chiral phase region within which liquid crystal molecules are cholesterically ordered. One or more nematic phase regions are formed along the surface of the liquid crystal film structure within which liquid crystal molecules are oriented along a direction and to a surface depth sufficient to realize one or more phase retardation regions therein having optical axes along the direction of liquid crystal molecule orientation.
Owner:REVEO
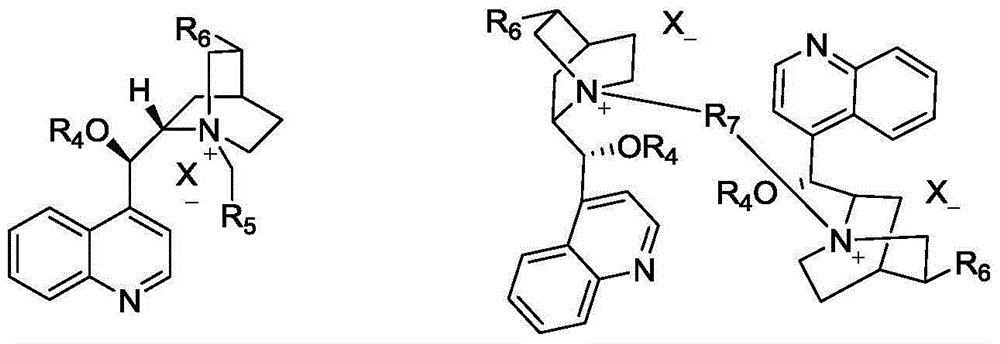
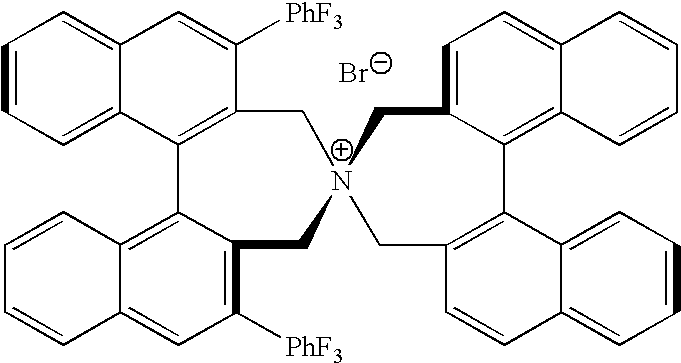
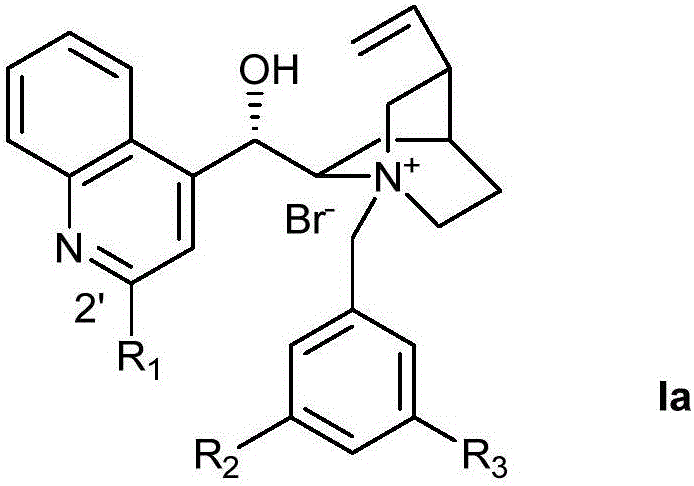
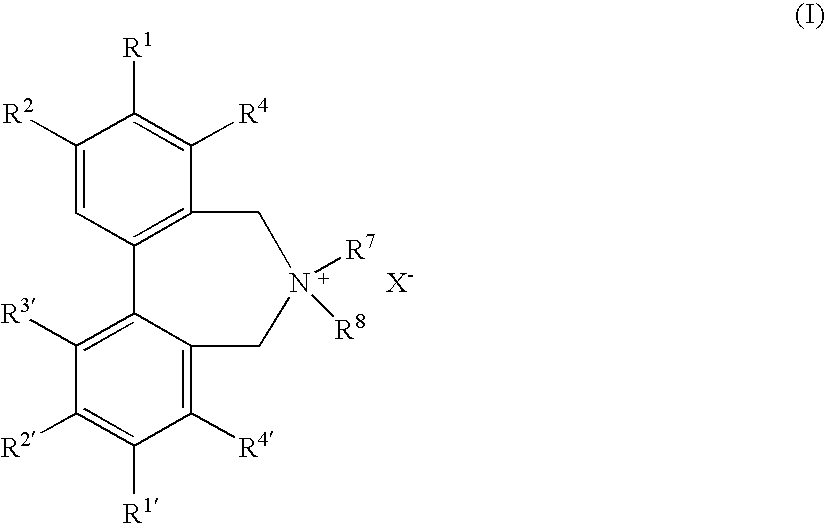
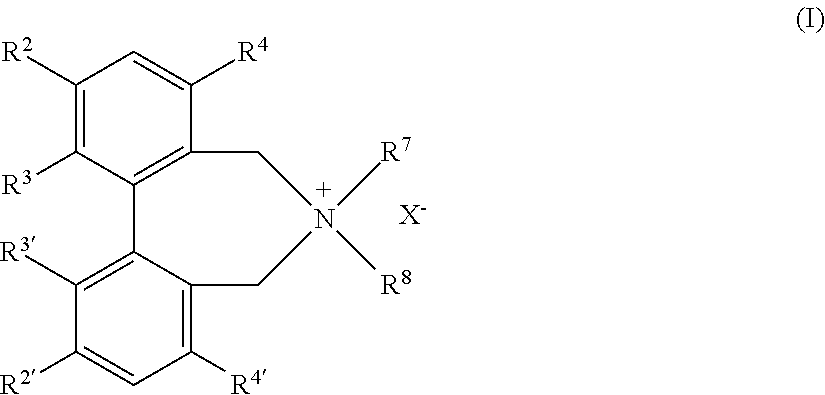
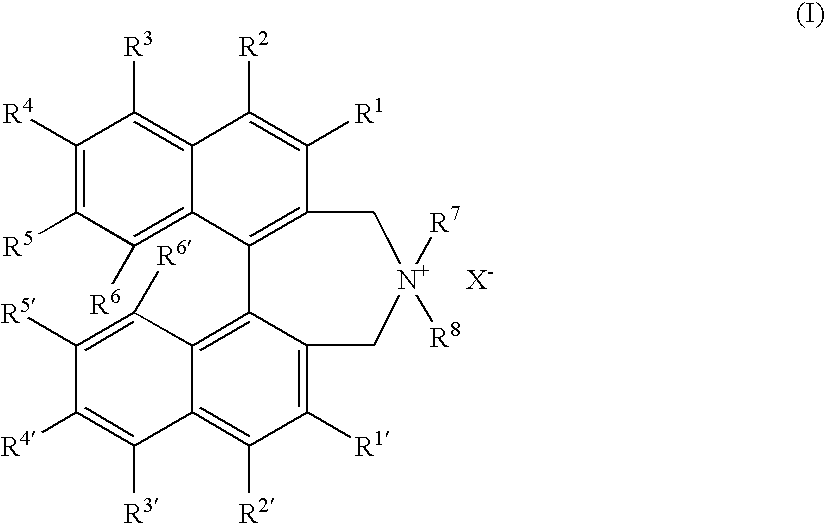
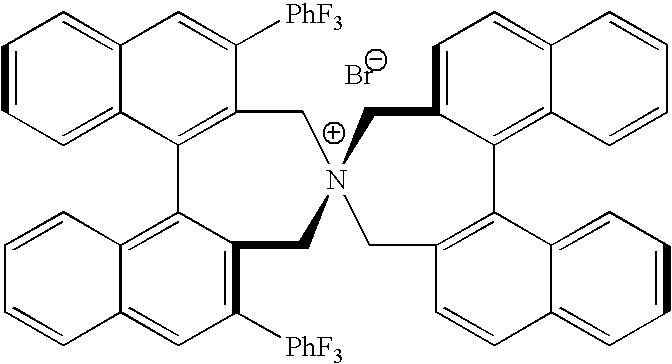
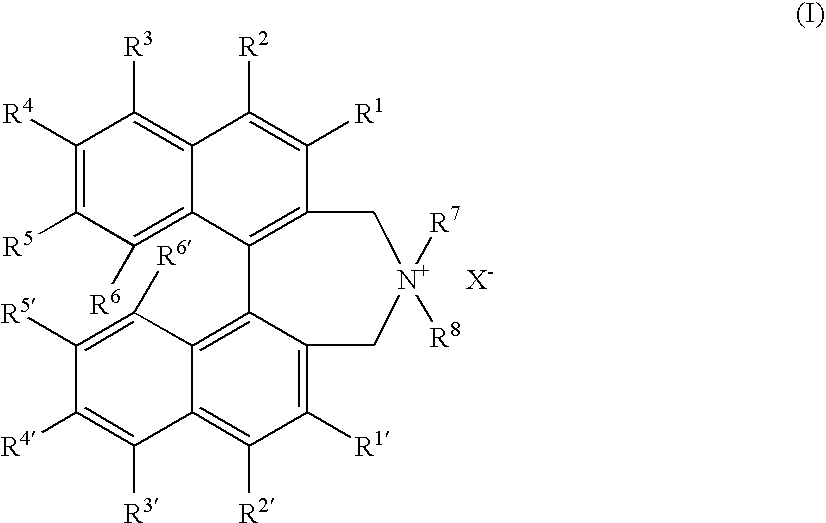
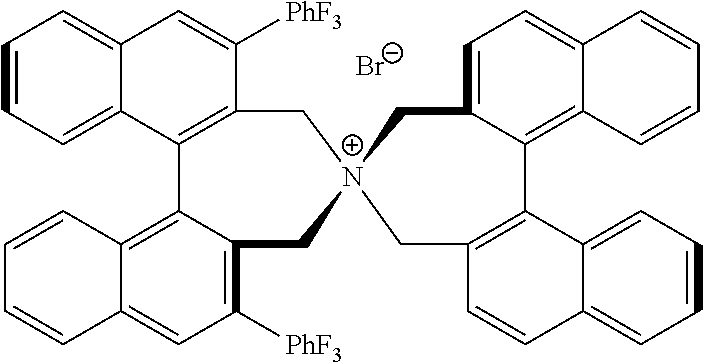
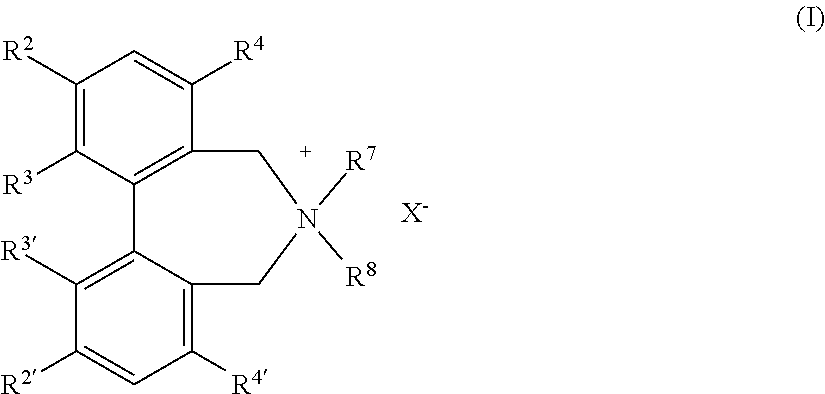
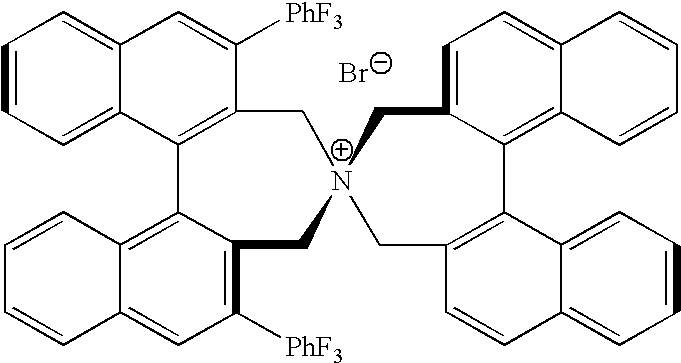
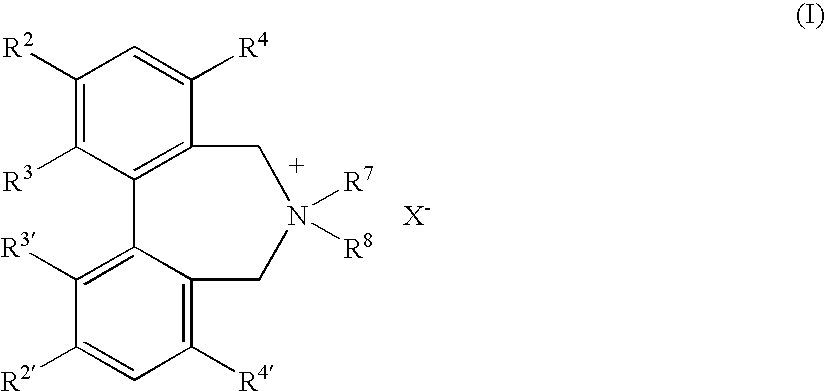
Optically active quaternary ammonium salt having axial asymmetry and process for producing alpha-amino acid and derivative thereof with the same
ActiveUS20070161624A1Simple structureEasy to provideBiocideOrganic compound preparationMedicinal chemistryAlpha amino acid
The present invention provides a compound of the following formula (I) below. This compound (I) can be produced by reacting a 2,2′-dimethylene bromide-1,1′-binaphthyl derivative, which can be produced by a relatively small number of processes, with an easily available secondary amine. This compound (I) is useful as a chiral phase-transfer catalyst.
Owner:KISHIDA CHEM
Spherical liquid-crystal laser
ActiveCN103201914AEasy to change wavelengthActive medium materialLiquid laser constructional detailsDopantSelective reflection
The patent refers to one or more droplets of chiral liquid crystals used as point source(s) of laser light. The source is shaped as a droplet of chiral liquid crystals (1) and an active medium preferably dispersed in the liquid crystals. The source is spherical and with a size of preferably between a few nanometres and 100 micrometres. A droplet consists of chiral liquid crystals (1) that have selective reflection in the range of the active medium's emission and can be cholesteric liquid crystals, a mixture of nematic liquid crystals and a chiral dopant or any other chiral liquid-crystal phase, preferably the blue phase, the ferroelectric phase, the antiferroelectric phase, any of the ferrielectric phases or another chiral phase of a soft substance, that need not be chiral by itself.
Owner:INSTITUT JOZEF STEFAN
Invisible, machine-detectable security marking, production of the security marking, and security system comprising this security marking
InactiveUS20030173539A1Improve the level ofLevel of complexityLiquid crystal compositionsOther printing matterEngineeringSecurity system
The invention relates to a security marking whose level of proof against forgery is greater than that of known security markings, comprising liquid-crystalline material with chiral phase, wherein the security marking is imperceptible to the eye and the properties of the liquid-crystalline material with chiral phase can be detected with the aid of detection systems.
Owner:SICPA HLDG SA
Optically active quaternary ammonium salt having axial asymmetry, and method for producing alpha-amino acid and derivative thereof by using the same
ActiveUS20100029935A1Simple structureReduce the numberOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsQuaternary ammonium cationPhase-transfer catalyst
The present invention discloses an optically active quarternary ammonium salt having axial asymmetry and a method for producing an α-amino acid and a derivative thereof using the same. The optically active quarternary ammonium salt having axial asymmetry of the present invention is a chiral phase-transfer catalyst that has a simple structure and that can be produced in a smaller number of process steps. The compound of the present invention is very useful as a phase-transfer catalyst in the synthesis of an α-alkyl-α-amino acid and a derivative thereof as well as an α,α-dialkyl-α-amino acid and a derivative thereof. Therefore, the compound of the present invention can be used in the development of novel foods and pharmaceuticals.
Owner:KISHIDA CHEM
OPTICALLY ACTIVE QUATERNARY AMMONIUM SALT HAVING AXIAL ASYMMETRY AND PROCESS FOR PRODUCING alpha-AMINO ACID AND DERIVATIVE THEREOF WITH THE SAME
ActiveUS20070135654A1Simple structureEasy to provideOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMedicinal chemistryAlpha amino acid
The present invention provides a compound of the following formula (I) below. This compound (I) can be produced by reacting a 2,2′-dimethylene bromide-1,1′-binaphthyl derivative, which can be produced by a relatively small number of processes, with an easily available secondary amine. This compound (I) is useful as a chiral phase-transfer catalyst.
Owner:KISHIDA CHEM
Novel method for asymmetric alpha-hydroxylation of photo-oxygenation beta-dicarbonyl compound based on C-2' phase transfer catalyst
InactiveCN105732387AHigh catalytic activityGood substrate applicabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhotosensitizerOrganic synthesis
The invention belongs to the technical fields of organic synthesis and provides a novel method for asymmetric alpha-hydroxylation of a photo-oxygenation beta-dicarbonyl compound based on a C-2' phase transfer catalyst.The beta-dicarbonyl compound, the cinchona alkaloid C-2' phase transfer catalyst and an organic photosensitizer are stirred in a solvent, alkali is added, strong stirring reaction is performed in the air under the condition of visible light, the reaction temperature is 50-70 DEG C, the reaction time is 1-4 hours, and a chiral alpha-hydroxyl-beta-dicarbonyl compound with the yield no lower than 70% and the enantiomeric excess selectivity no lower than 60% ee is obtained; derivatization is conducted on a cheap cinchona alkaloid C-2' potential easy to obtain a series of chiral phase transfer catalysts having higher catalytic activity, molecules are successively oxidized into an oxidant, and the asymmetric alpha-hydroxylation of the photo-oxygenation beta-dicarbonyl compound is achieved.The method has good substrate applicability and environmental friendliness.
Owner:DALIAN UNIV OF TECH
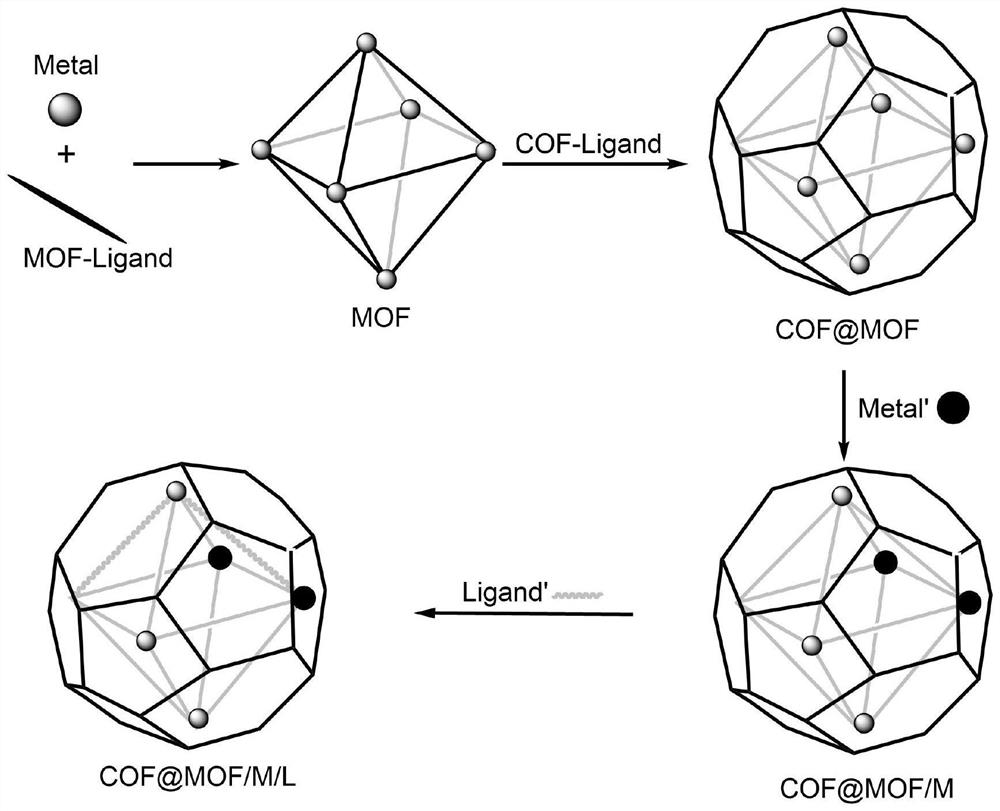
COF-coated MOF/M/L composite material and preparation method thereof
ActiveCN112604714AEfficient catalytic degradationRapid catalytic degradationGas treatmentOrganic-compounds/hydrides/coordination-complexes catalystsIon exchangeComposite material
The invention discloses a COF-coated MOF / M / L composite material, which comprises a COF-coated MOF material with a core-shell structure synthesized by a COF-coated MOF crystal material, the MOF of the COF-coated MOF material is firstly subjected to synthesis, then is subjected to metal ion exchange, and then is subjected to ligand exchange to form the COF-coated MOF / M / L composite material with metal ions and chiral ligands. Meanwhile, the invention discloses a preparation method of the COF-coatedMOF / M / L composite material. The COF-coated MOF / M / L composite material disclosed by the invention has the characteristics of a COF-coated MOF material with a core-shell structure; meanwhile, metal ions such as copper or iron with an efficient catalytic degradation function and chiral functional organic ligands such as Llactic acid or histidine are introduced, so that nodes of the MOF have high catalytic activity of monatomic catalysis, and chiral toxic pollutants such as cis-permethrin and cis-permethrin are effectively identified by utilizing chiral-chiral interaction; chiral pollutants can be selectively, quickly, efficiently and thoroughly catalytically degraded into non-toxic substances.
Owner:杭州阿德旺斯材料科技有限公司
Optically active quaternary ammonium salt having axial asymmetry and process for producing alpha-amino acid and derivative thereof with the same
InactiveUS20090270614A1Simple structureReduce the number of stepsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBiphenyl derivativesAlpha amino acid
The present invention provides a chiral phase-transfer catalyst of the following formula (I):The compound (I) can be produced by reacting a 2,2′-dimethylene bromide-1,1′-biphenyl derivative, which can be produced through comparatively small number of steps, with an easily available secondary amine.
Owner:KISHIDA CHEM
Invisible, machine-detectable security marking, production of the security marking, and security system comprising this security marking
InactiveUS7401817B2Improve the level ofLevel of complexityPolarising elementsPattern printingChiralitySecurity system
The invention relates to a security marking whose level of proof against forgery is greater than that of known security markings, comprising liquid-crystalline material with chiral phase, wherein the security marking is imperceptible to the eye and the properties of the liquid-crystalline material with chiral phase can be detected with the aid of detection systems.
Owner:SICPA HLDG SA
Process for synthesizing 6-18F-L-dopa
InactiveCN1408705AEasy to purifyHigh yieldOrganic compound preparationAmino-carboxyl compound preparationAlkyl transferHydrolysis
The synthesis process 6-18F-L-Dopa (18FDOPA) with 6-nitro piperonaldehyde as precursor includes four steps: nucleophilic fluorination, reducing bromination, chiral phase transferring catalytic alkylation and hydrolysis. The process has high production efficiency, no need of purifying precursor, easy to purify final product, and low cost. The product may be used in animal experiment and conventional clinical application.
Owner:NAN FANG HOSPITAL
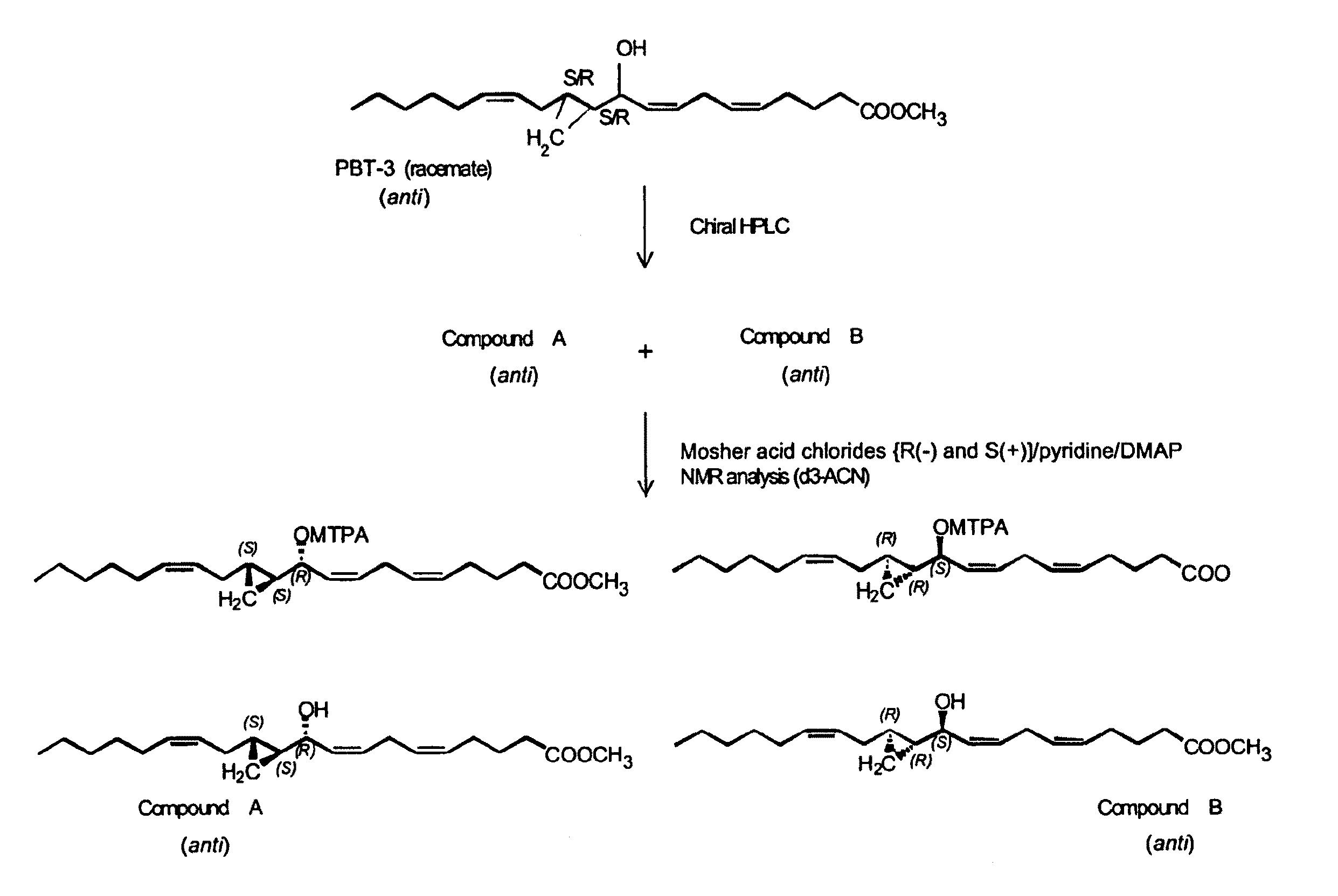
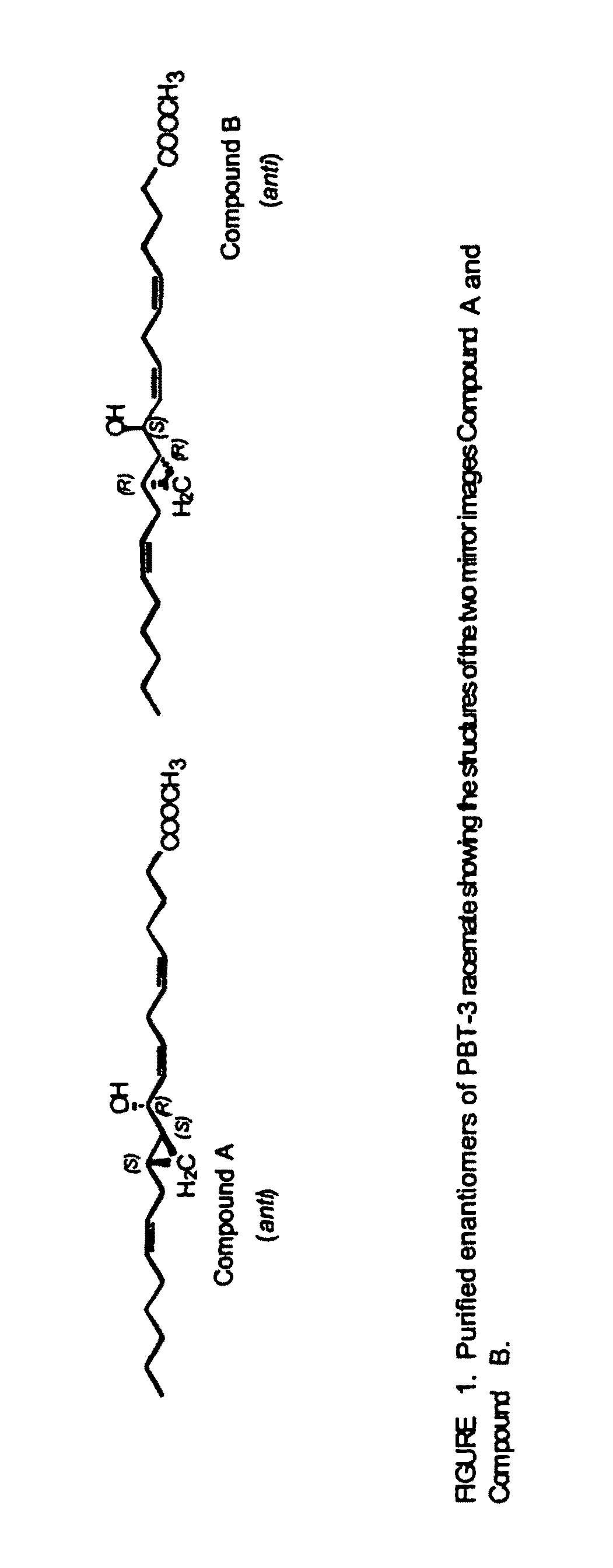
Hepodxilin analog enantiomers
The present invention relates to enantiomeric forms of hepoxilin analogs of Formula I-VIII, pharmaceutical compositions thereof, a method for the separation of said enantiomeric forms of hepoxilin analogs comprising applying said hepoxilin to a chiral phase HPLC column and eluting said hepoxilin with an alkane and alcohol solvent mixture. Said enantiomeric forms of hepoxilin analogs of Formula I-VIII were found to be useful in controlling the biological effects of PPAR mediated transcriptional control for the treatment of diseases such as cancer, thromboxane-mediated diseases and for modulating intracellular calcium concentration.
Owner:CECIL PACE ASCIAK +1
Hepoxilin analog enantiomers
ActiveUS20100210727A1Enhanced chemilluminescenceIncreased cleavageBiocideSenses disorderAlkaneDisease
The present invention relates to enantiomeric forms of hepoxilin analogs of Formula I-VIII, pharmaceutical compositions thereof, a method for the separation of said enantiomeric forms of hepoxilin analogs comprising applying said hepoxilin to a chiral phase HPLC column and eluting said hepoxilin with an alkane and alcohol solvent mixture. Said enantiomeric forms of hepoxilin analogs of Formula I-VIII were found to be useful in controlling the biological effects of PPAR mediated transcriptional control for the treatment of diseases such as cancer, thromboxane-mediated diseases and for modulating intracellular calcium concentration.
Owner:CECIL PACE ASCIAK +1
Optically active quaternary ammonium salt having axial asymmetry, and method for producing alpha-amino acid and derivative thereof by using the same
InactiveUS20120095218A1Simple structureReduce the numberOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsQuaternary ammonium cationPhase-transfer catalyst
The present invention discloses an optically active quarternary ammonium salt having axial asymmetry and a method for producing an α-amino acid and a derivative thereof using the same. The optically active quarternary ammonium salt having axial asymmetry of the present invention is a chiral phase-transfer catalyst that has a simple structure and that can be produced in a smaller number of process steps. The compound of the present invention is very useful as a phase-transfer catalyst in the synthesis of an α-alkyl-α-amino acid and a derivative thereof as well as an α,α-dialkyl-α-amino acid and a derivative thereof. Therefore, the compound of the present invention can be used in the development of novel foods and pharmaceuticals.
Owner:NAGASE & COMPANY
Optically active quaternary ammonium salt having axial asymmetry and process for producing α-amino acid and derivative thereof with the same
ActiveUS7928224B2Simple configurationImprove featuresOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsQuaternary ammonium cationChemical compound
The present invention provides a compound of the following formula (I) below. This compound (I) can be produced by reacting a 2,2′-dimethylene bromide-1,1′-binaphthyl derivative, which can be produced by a relatively small number of processes, with an easily available secondary amine. This compound (I) is useful as a chiral phase-transfer catalyst.
Owner:KISHIDA CHEM
Optically active ilaprazole and synthesis method thereof
The invention relates to optically active ilaprazole and a synthesis method thereof, belongs to the field of pharmaceutical technology, and discloses a method for synthesizing optically active ilaprazole. According to the method, corresponding thiol is used as a starting raw material, and reacts with acrylate or halopropionate to obtain a thioether compound, the thioether compound is oxidized to obtain racemic sulfoxide, and the racemic sulfoxide reacts with another fragment under the action of a chiral phase transfer catalyst to obtain the chiral ilaprazole. According to the present invention, the preparation of the chiral ilaprazole is firstly reported, the synthesis method of the chiral ilaprazole does not require the use of heavy metals and the use of dangerous peroxides, and the excessive oxidation by-product is generated at the penultimate chemical step so as to easily control; and the method has characteristics of novel process, few steps, mild reaction conditions and the like.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
Optically active quaternary ammonium salt having axial asymmetry, and method for producing alpha-amino acid and derivative thereof by using the same
ActiveUS20120095252A1Simple structureReduce the numberOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPhase-transfer catalystMedicinal chemistry
The present invention discloses an optically active quarternary ammonium salt having axial asymmetry and a method for producing an α-amino acid and a derivative thereof using the same. The optically active quarternary ammonium salt having axial asymmetry of the present invention is a chiral phase-transfer catalyst that has a simple structure and that can be produced in a smaller number of process steps. The compound of the present invention is very useful as a phase-transfer catalyst in the synthesis of an α-alkyl-α-amino acid and a derivative thereof as well as an α,α-dialkyl-α-amino acid and a derivative thereof. Therefore, the compound of the present invention can be used in the development of novel foods and pharmaceuticals.
Owner:KISHIDA CHEM
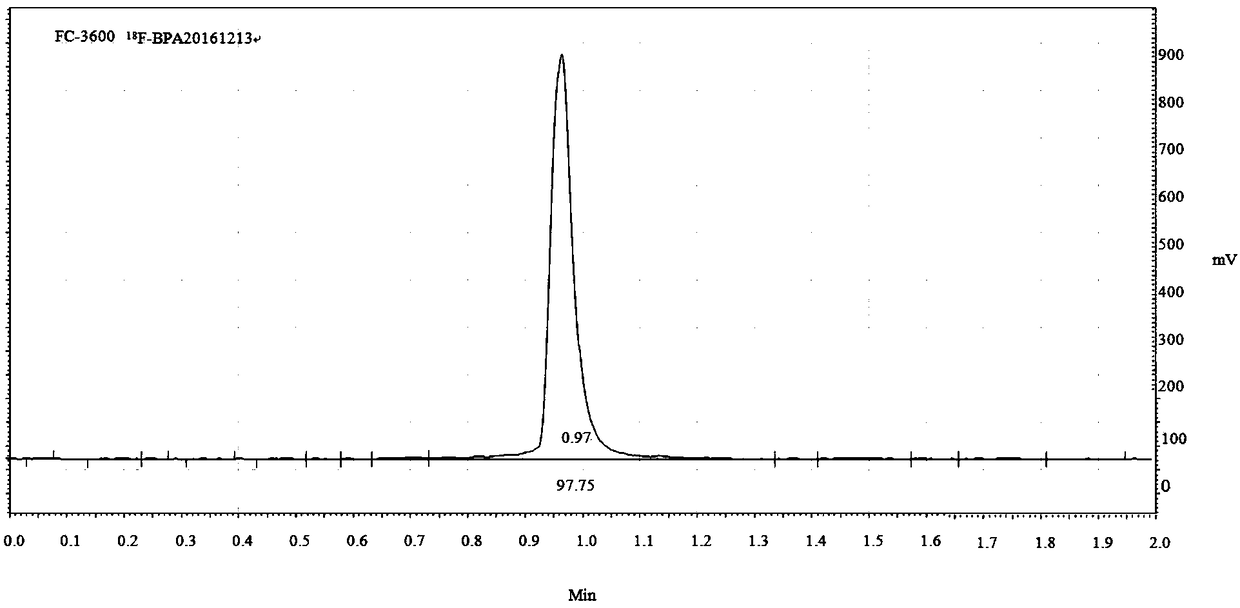
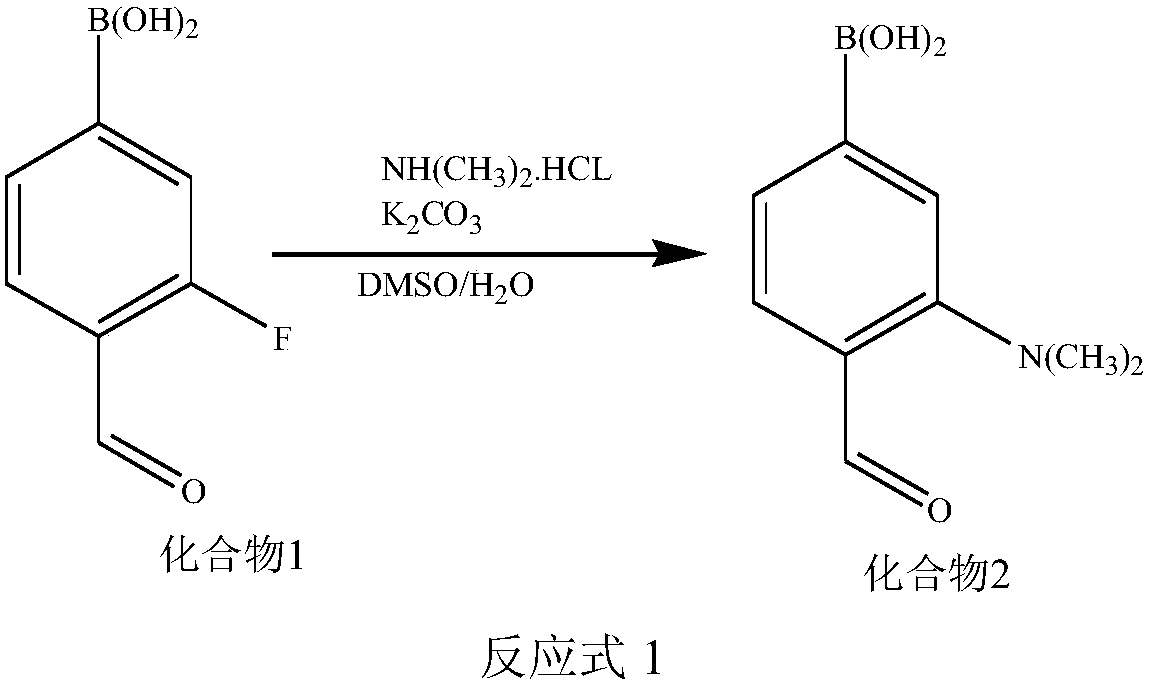
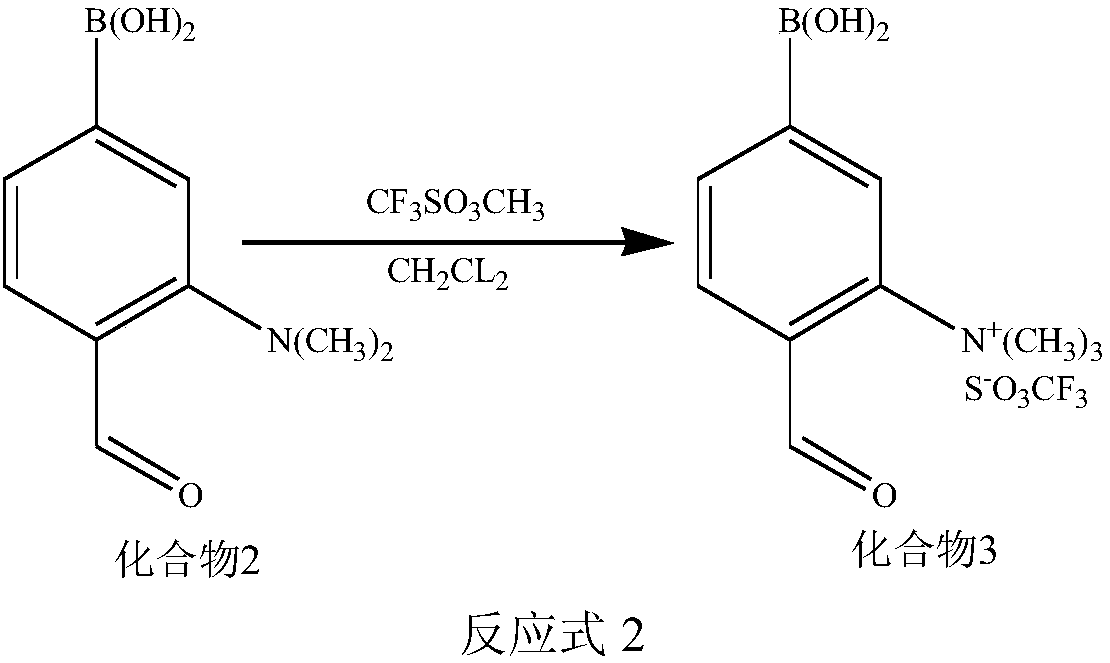
Synthetic methods of F-BPA and F-BPA intermediates, intermediates and application of intermediates
ActiveCN108299482AMeet the requirements of clinical applicationImprove image qualityGroup 3/13 element organic compoundsIsotope introduction to acyclic/carbocyclic compoundsAqueous solutionPhase-transfer catalyst
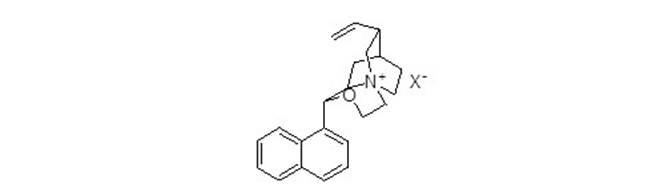
The invention provides a nucleophilic fluorination based synthetic method of F-BPA, a synthetic method of intermediates, the intermediates and an application of the intermediates. The nucleophilic fluorination based synthetic method of F-BPA comprises the following steps: 1, synthesizing a compound 2 from raw materials including a compound 1, dimethylamine hydrochloride and the like; 2, synthesizing a compound 3 from raw materials including the compound 2, methyl-trifluoromethanesulfonate and the like; 3, synthesizing a compound 4 from raw materials including the compound 3, K2.2.2 and the like; 4, synthesizing a compound 5 from raw materials including the compound 4, an NaBH4 aqueous solution, an HI aqueous solution and the like; 5, synthesizing the target product from raw materials including the compound 5, N-(diphenylmethyl)tert-butyl glycinate and the like under the catalytic action of an Maruoka chiral phase transfer catalyst.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
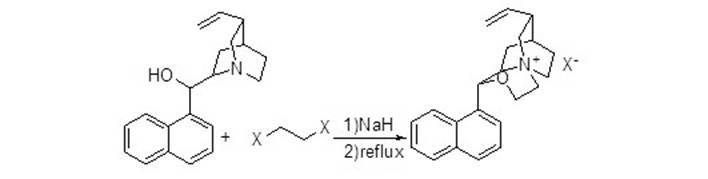
Chiral phase transfer catalyst and synthesizing method thereof
InactiveCN102626654ARich applicabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical synthesisOrganic layer
The invention discloses a chiral phase transfer catalyst and a synthesizing method thereof, which relate to chemical synthesis and in particular relates to the technical field of synthesis of the novel chiral phase transfer catalyst. The method comprises the following steps of dissolving quinine in solvent, reacting with sodium hydride, reacting by adding hydrocarbon dihalide, extracting to obtain an organic layer, drying, and re-separating for removing the solvent so as to obtain the chiral phase transfer catalyst. The chiral phase transfer catalyst is novel in structure, and central nitrogen-atoms of the chiral phase transfer catalyst has good hidden performance, and therefore, the chiral phase transfer catalyst has certain special catalyzing effects, and can be suitable for requirements of catalytic reactions with various types. The chiral phase transfer catalyst prepared by the method can be applied to tert-Butyl glycinate ramification asymmetrical alkylating reaction, achieves excellent results, and is an excellent chiral phase transfer catalyst proved by practices.
Owner:YANGZHOU TIANHE PHARM CO LTD
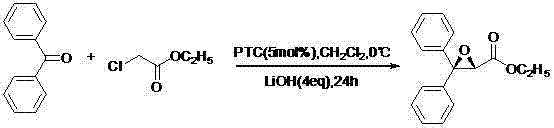
Stereoselectivity preparation method for Letairis
InactiveCN103709106AReduce usageReduce generationOrganic chemistryNucleophilic substitutionHydrolysis
The invention discloses a stereoselectivity preparation method for a compound shown in the formula (I). First, chiral phase-transfer catalysts of quaternary ammonium salts are employed to catalyze a stereoselective Darzen reaction of diphenyl ketone and alpha-halogenated acetic ester, and optically pure epoxides are obtained. Then ring opening, nucleophilic substitution and ester group hydrolysis reaction are carried out in order and optically pure Letairis is obtained. The method is aimed to achieve industrial preparation of optically pure Letairis. By utilization of the stereoselectivity preparation method, generation of another isomer is avoided, the yield of the target compound is increased, and the method accords with requirements of green synthesis.
Owner:SHIJIAZHUANG BOCE BIO TECH
New ultrahigh efficiency, superficially porous particle (SPP) chiral phases for liquid chromatography
ActiveUS20170197156A1Enhanced chiral separationEasy to separateOther chemical processesOrganic chemistry methodsStationary phaseTheoretical plate
The present invention relates to a novel stationary phase support for liquid chromatographic chiral separations. The specific combination of the special underlying support material and certain classes of known chiral selectors according to the invention produces far superior chiral (enantiomeric) separations than those obtained on any conventionally known supports. These chiral (enantiomeric) separations are enhanced in terms of significantly higher efficiencies (theoretical plate numbers), higher resolutions (Rs), shorter retention times and either equivalent or slightly higher selectivities than those obtained on conventional supports.
Owner:AZYP
Optically active quaternary ammonium salt having axial asymmetry, and method for producing alpha-amino acid and derivative thereof by using the same
ActiveUS8110680B2Organic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPhase-transfer catalystMedicinal chemistry
Owner:KISHIDA CHEM
Synthesis method of single optical isomer nitrendipine
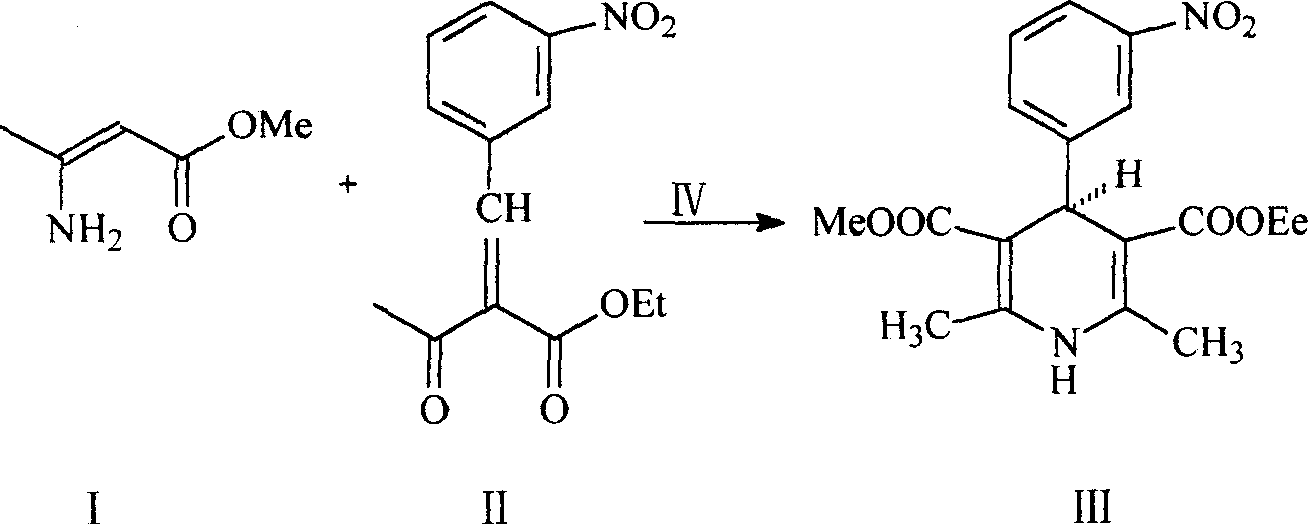
A process for synthesizing the simple optical isomer nitrendipine features that under existance of quinine-ammonium benzylbromide as chiral phase-transfer catalyst, 2-(3-nitrobenzilidene) ethyl acetylacetate and beta-methyl amino crotonate take part in stereoselective condensating reaction in absolute alcohol to obtain target product.
Owner:JIAXING UNIV
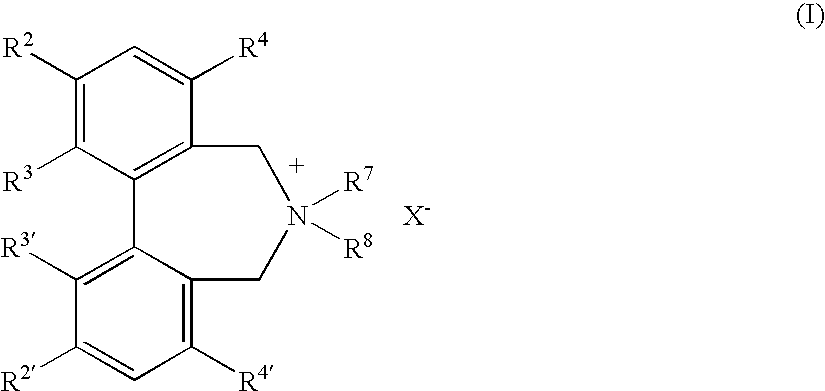
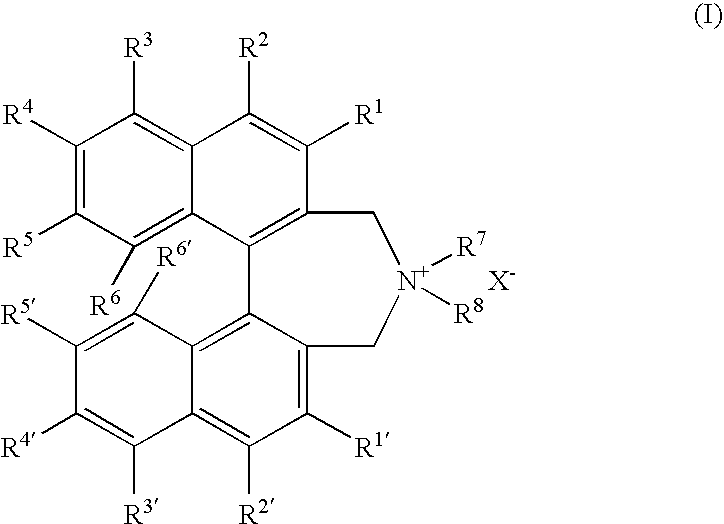
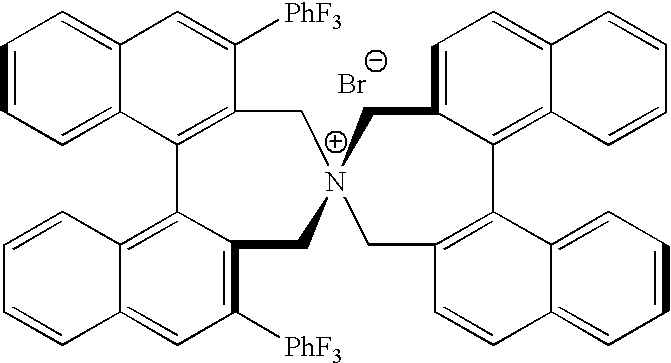
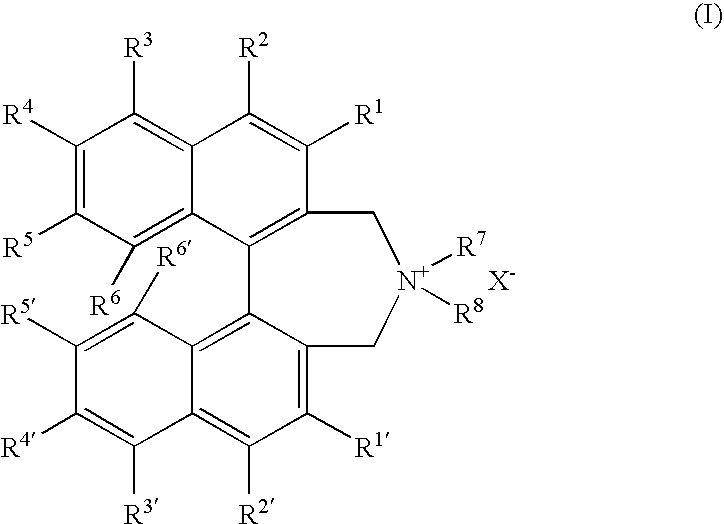
Optically active dibenzazepine derivatives
The invention provides novel optically active dibenzazepine derivatives having high utility value as chiral phase-transfer catalyst, more specifically, optically active 6,7-dihydro-5H-dibenz-[c,e]azepine derivatives represented by general formula (1') [wherein R is a divalent organic bridging group for joining the 1- and 11-positions; R1 and R2 may be the same or different and are each hydrogen, halogeno, or an organic group, or R1 and R2 may together represent an divalent organic group; R3' and R4' may be the same or different and are each a monovalent organic group, or R3' and R4' may together represent a cyclic organic group containing the onium nitrogen atom; Ar is a monovalent organic group; * represents optical activity, that is, an excess of one axially chiral isomer over the other axially chiral isomer, both isomers being due to the bond axis constituting the biphenyl structure of the derivative; and X- is a counter anion].
Owner:NIPPON SODA CO LTD
Liquid crystal film structures with phase-retardation surface regions formed therein
Disclosed are liquid crystal (LC) phase-retarders and linear polarizers and methods and apparatus for making the same. The liquid crystal phase-retarder is realized by a liquid crystal film structure having one or more phase retardation regions formed therein. Each phase retardation region has an optical axis specified by the direction and depth of orientation of liquid crystal molecules along the surface of the liquid crystal film structure. The liquid crystal linear polarizer is realized by a liquid crystal film structure having a chiral phase region within which liquid crystal molecules are cholesterically ordered. One or more nematic phase regions are formed along the surface of the liquid crystal film structure within which liquid crystal molecules are oriented along a direction and to a surface depth sufficient to realize one or more phase retardation regions therein having optical axes along the direction of liquid crystal molecule orientation.
Owner:REVEO
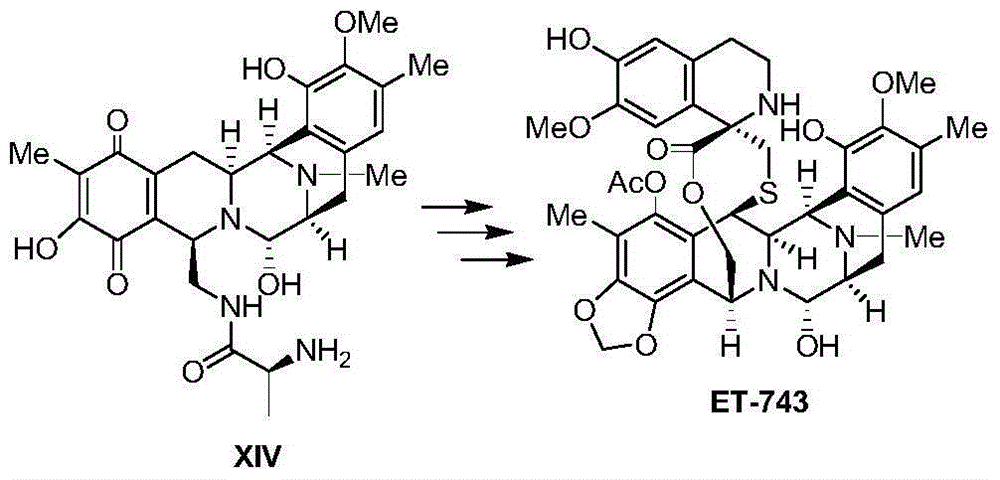
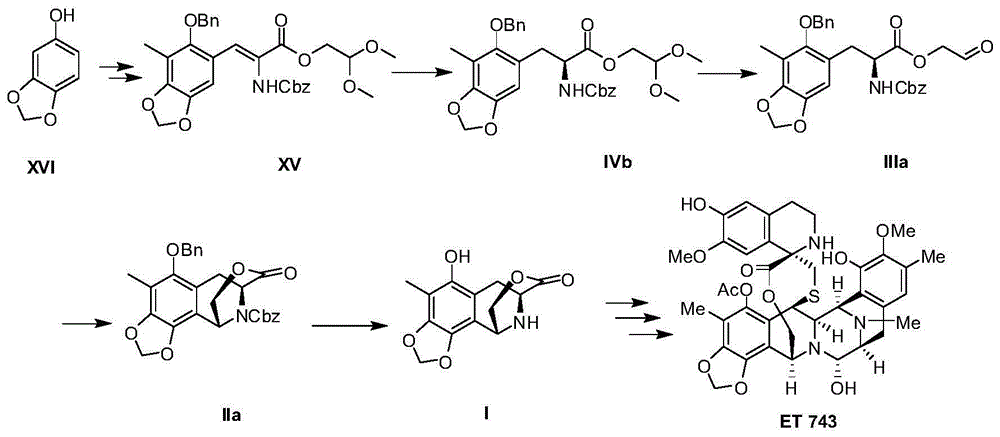
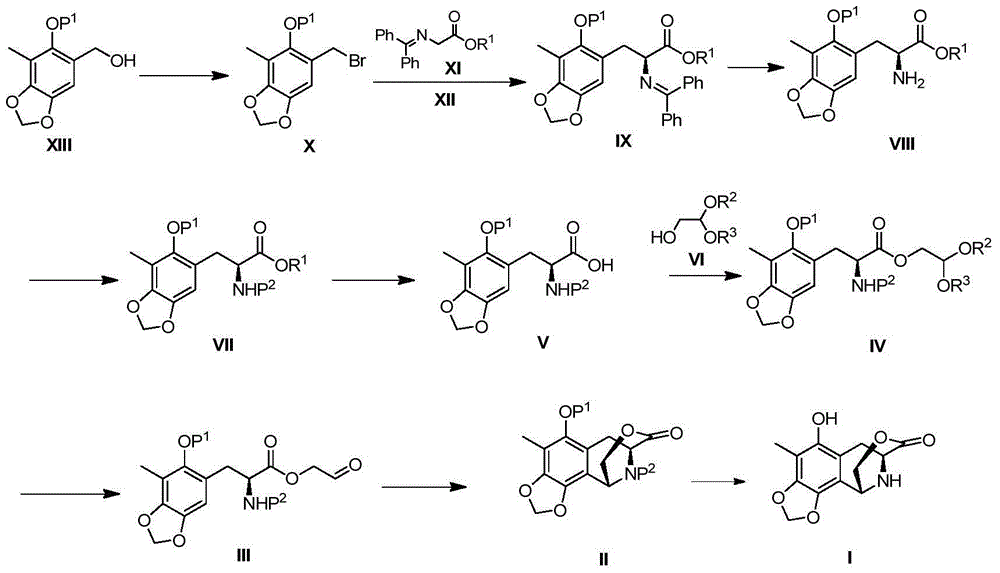
Method for preparing intermediate of ecteinascidin-743
The invention relates to a method for preparing an intermediate of ecteinascidin-743 and particularly relates to a method for preparing an intermediate which is used for synthesizing ecteinascidin-743 and is represented by the formula I as shown in the description. The method comprises the following steps of carrying out protecting group conversion on a compound represented by the formula X as shown in the description in the presence of a chiral phase transfer catalyst to obtain a chiral intermediate represented by the formula V as shown in the description, carrying out esterification reaction on the intermediate V to obtain an intermediate represented by the formula IV as shown in the description and then carrying out intramolecular cyclization reaction and removing the protecting groups to obtain the intermediate which is used for synthesizing ecteinascidin-743 and is represented by the formula I. The method has the advantages of mild reaction conditions, simplicity in operation, low synthesis cost and the like and is suitable for mass production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com