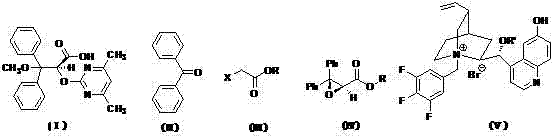
Stereoselectivity preparation method for Letairis
A basic and selected technology, which is applied in the field of stereoselective preparation of ambrisentan, can solve the problems of increased production cost, failure to meet the requirements of environmentally friendly green technology, low utilization rate of chiral drug raw materials, etc., and achieve cost reduction , the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Ambrisentan【(+)-(S)-2-[(4,6-dimethylpyrimidine)-2-oxyl]-3-methoxy-3,3-diphenylpropionic acid】
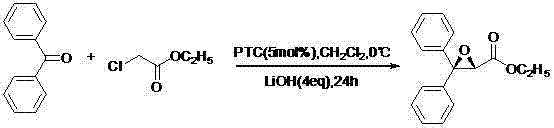
[0033] (1) Preparation of ethyl (2S)-3,3-diphenyl-2,3-epoxypropionate
[0034]
[0035] Add benzophenone (45.56g, 0.25mol), dichloromethane (75ml), PTC and lithium hydroxide to a 500ml three-necked reaction flask with stirring, and slowly add ethyl chloroacetate (46.12g, 0.42mol), after dropping, keep stirring for 24h. Add 125ml of water, separate, extract the aqueous layer with dichloromethane (50ml×2), combine the organic layers, wash with saturated brine until neutral, anhydrous Na 2 SO 4 After drying, filtering, and evaporating the solvent to dryness, 59.75 g of light yellow liquid (IV) was obtained, with a yield of 89.2%. It was directly used in the next reaction without further treatment.
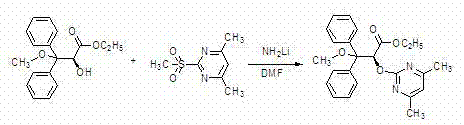
[0036] (2) Preparation of ethyl (2S)-2-hydroxy-3-methoxy-3,3-diphenylpropionate
[0037]
[0038] The oily compound (50.0 g, 0.186 mol) obtained above ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com