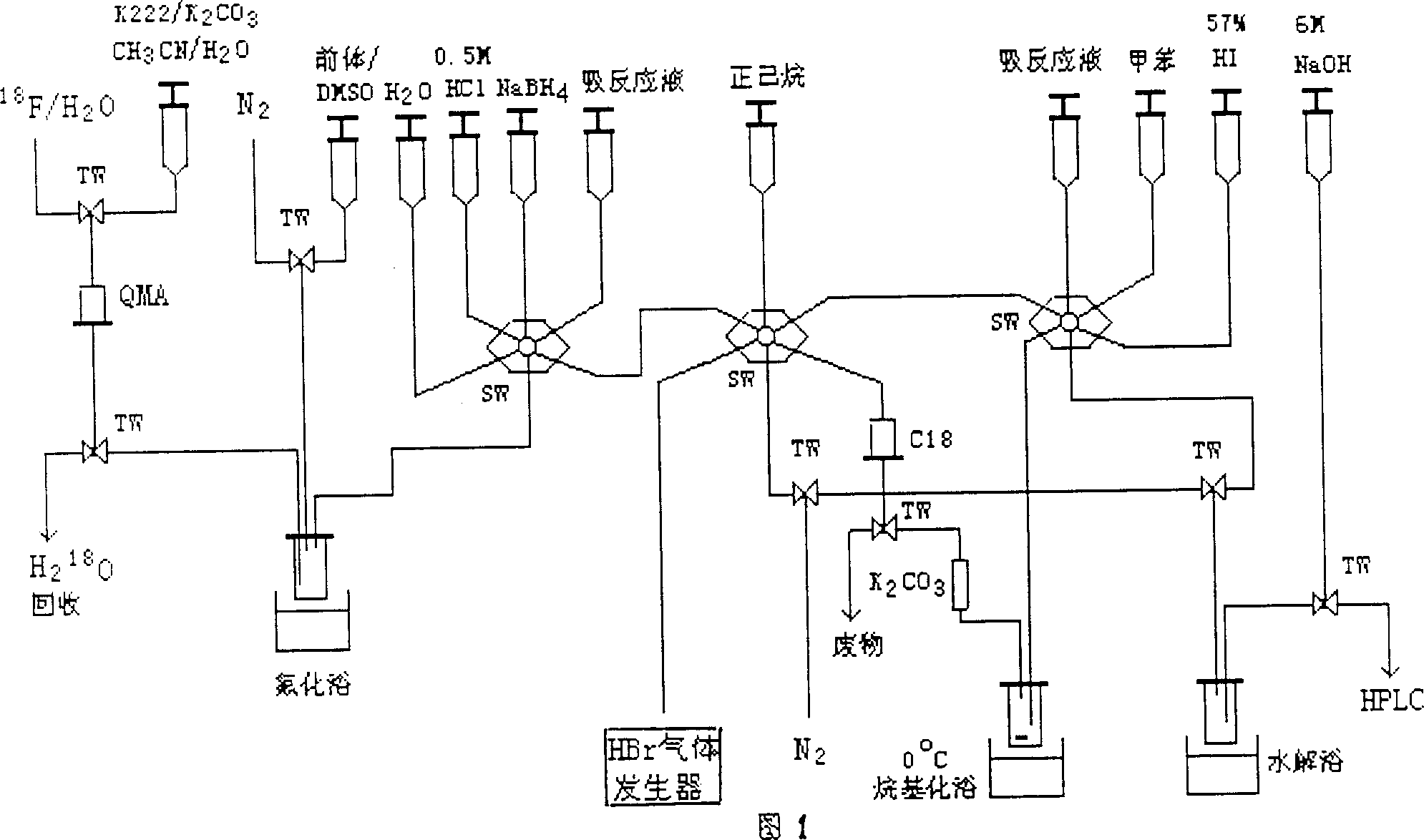
Process for synthesizing 6-18F-L-dopa
A synthesis process and preparation process technology, applied in the field of synthesis process of radioactive substance 6-18F-L-dopa, can solve the problem of shortening the total radiochemical synthesis time of precursors, etc. The effect of high rate and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (K / K / 222) +18 f - preparation of
[0017] Using a cyclotron through 18 O(p,n) 18 F nuclear reaction, continuous bombardment of 1.5mL H with 16.5MeV, 25μA proton beam 2 18 O target 30~60min, production 18 f - . contain 18 f - After the target water passes through the anion-exchange column QMA post produced by Waters, other anion-exchange columns can also be used to use the acetonitrile aqueous solution containing potassium carbonate, the phase transfer catalyst Kryptofix 222 between the crown ether liquid phases produced by Sigma or Merck Rinse, and other crown ethers can also be used to collect the eluate. Take part of the eluent, heat at 120°C, and blow dry with nitrogen to obtain a white solid residual active complex (K / K / 222) +18 f - .
[0018] The acetonitrile aqueous solution of potassium carbonate and crown ether is preferably acetonitrile water (96 / 4, v / v) solution containing potassium carbonate 4mg (0.029mmol) and 22mg (0.058mmol) Kryptofix 222. Th...
Embodiment 2
[0020] 6-[ 18 F] Preparation of fluoropiperonal (3)
[0021] To the above residue, add a DMSO (1 mL) solution containing 12-17 mg of 6-nitropiperonal (2) (Mr 195, 0.061-0.087 mmol), seal it, and heat at 135° C. for 20 min. Cool the reaction mixture, add 20 mL of water, pass through a C18 Sep-Pak column, wash with 5 mL of 0.5 mol / L hydrochloric acid and 10 mL of water respectively, discard the washing solution, and 6-[ 18 F] Fluoropiperonal (3) was retained on the C18Sep-Pak column. Rinse with a small amount of ether, and the radiochemical purity determined by HPLC and TLC is greater than 95%. HPLC analysis: C18 column, mobile phase is methanol / water (volume ratio is 65:35, pH=4, containing acetic acid 0.05mol / L), flow rate is 0.7mL / min; TLC analysis: chromatographic solution is 100% dichloro methane.
Embodiment 3
[0023] 2-[ 18 F] Preparation of fluoro-4,5-methylenedioxybenzyl bromide (4)
[0024] NaBH 4 15 mg dissolved in H 2 O 0.5mL, through the C18 Sep-Pak column, 6-[ 18 F] fluoropiperonal (3) is reduced to generate 6-[ 18 F] Fluoropiperol, add water 5mL and n-hexane 5mL rinse respectively, 6-[ 18 F] Fluoropiperol remains on the column. with NaBr and concentrated H 3 PO 4 The reaction prepares HBr gas, and the dried HBr gas passes through the C18 Sep-Pak column, 6-[ 18 F] fluoropiperol rapidly (within 30s) bromination reaction occurs with HBr on the column to generate 2-[ 18 F] Fluoro-4,5-methylenedioxybenzyl bromide (4) remained on the column. Rinse the C18Sep-Pak column with 1.5-2.5 mL of anhydrous toluene, and pass the eluent through anhydrous K 2 CO 3 Short column, directly used for the next step of alkylation reaction. The radiochemical purity was greater than 95% as determined by TLC. Conditions: The chromatographic solution is 100% dichloromethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com