Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Cyclooctanes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A group of compounds with an 8-carbon ring. They may be saturated or unsaturated.
8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists
Owner:THERAVANCE BIOPHARMA R&D IP LLC
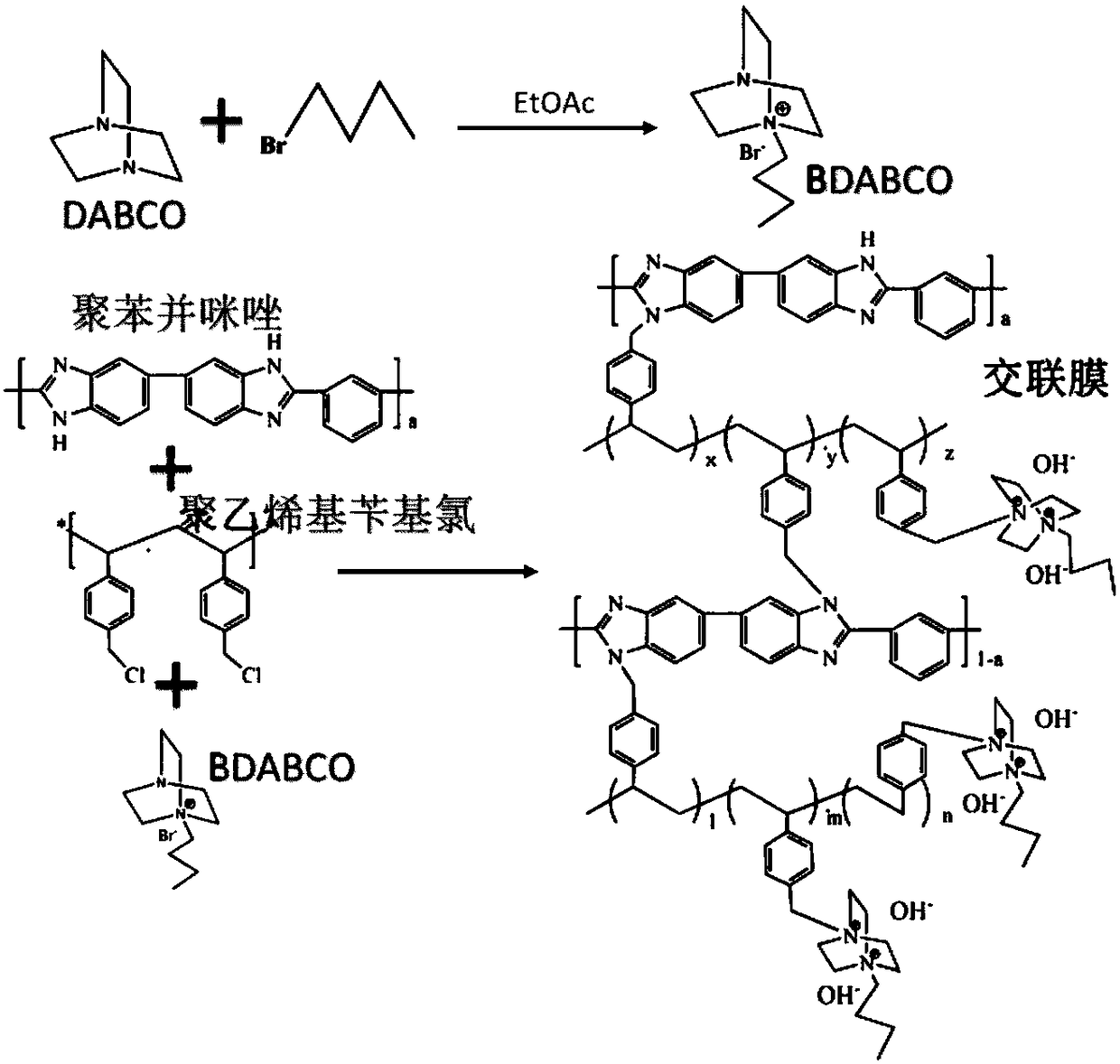
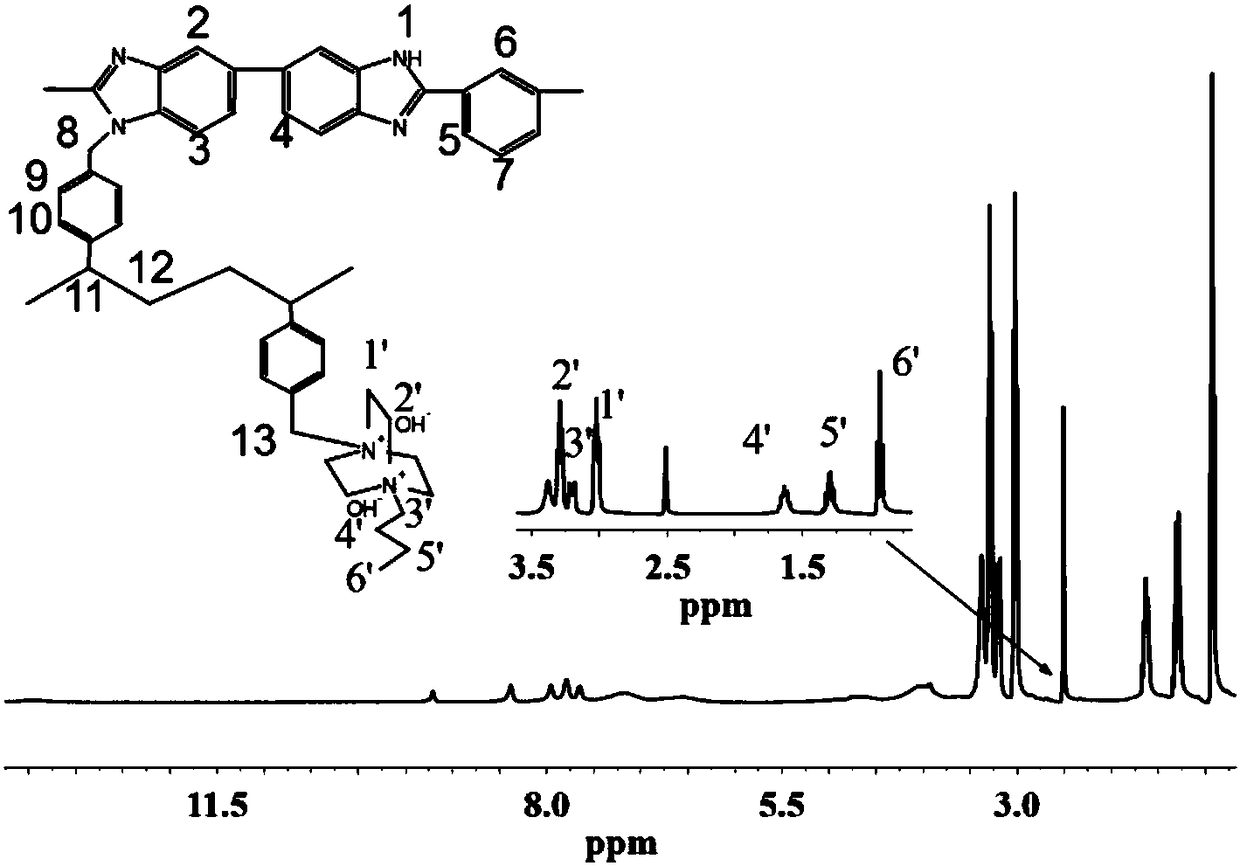
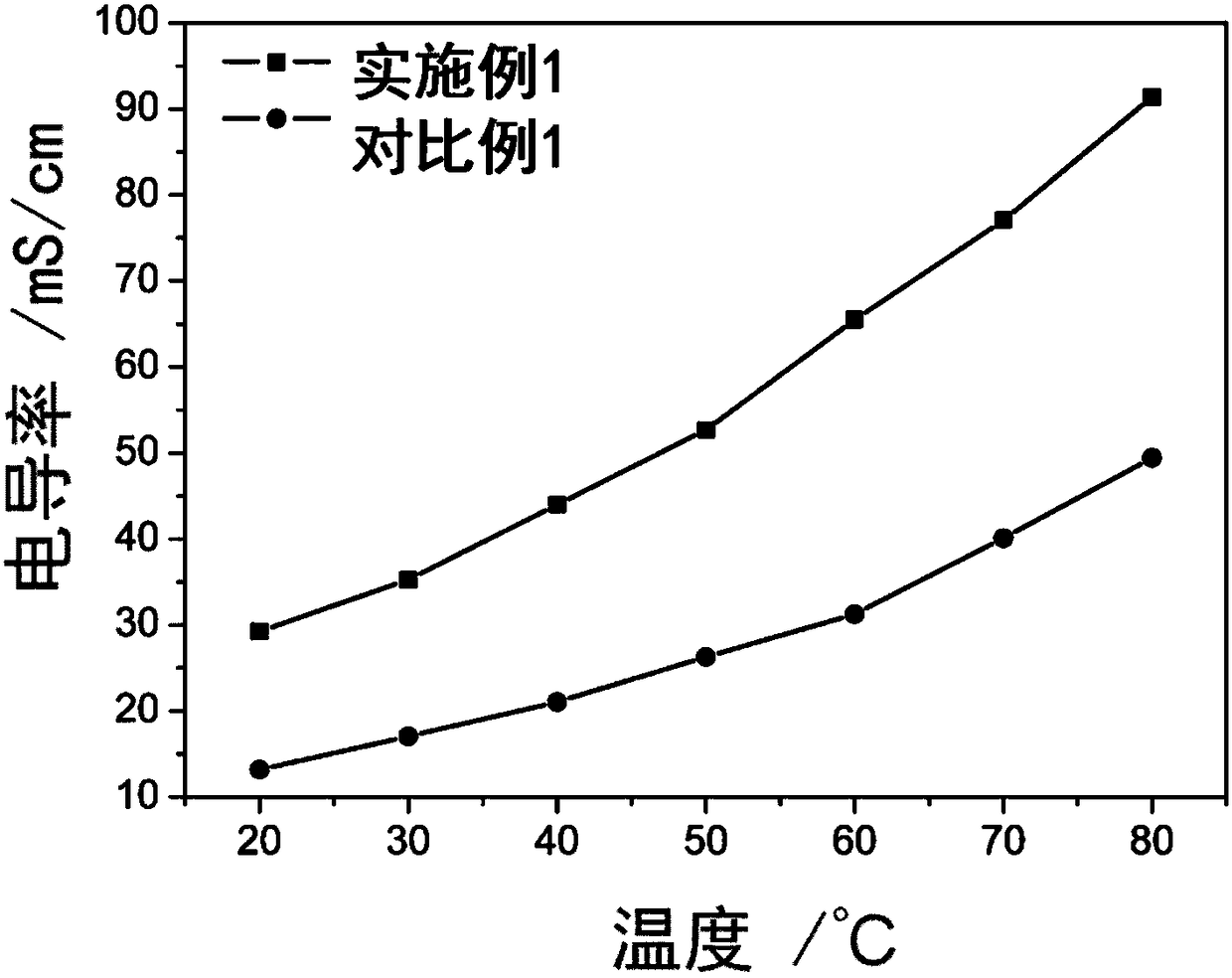
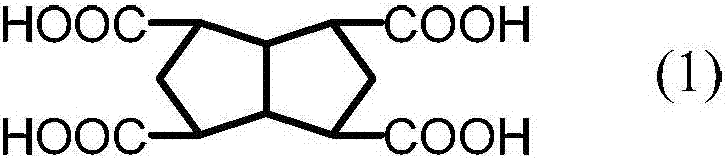
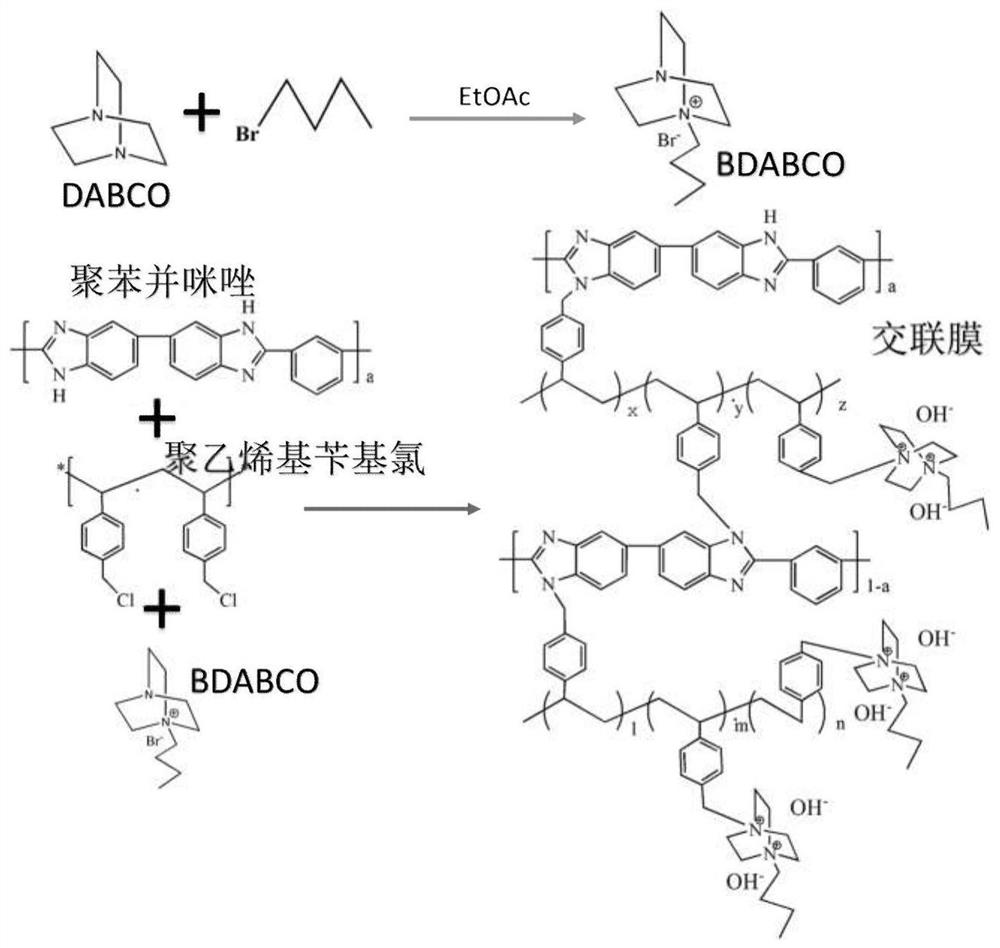
Cross-linked polybenzimidazole basic anion exchange membrane, and preparation and application thereof
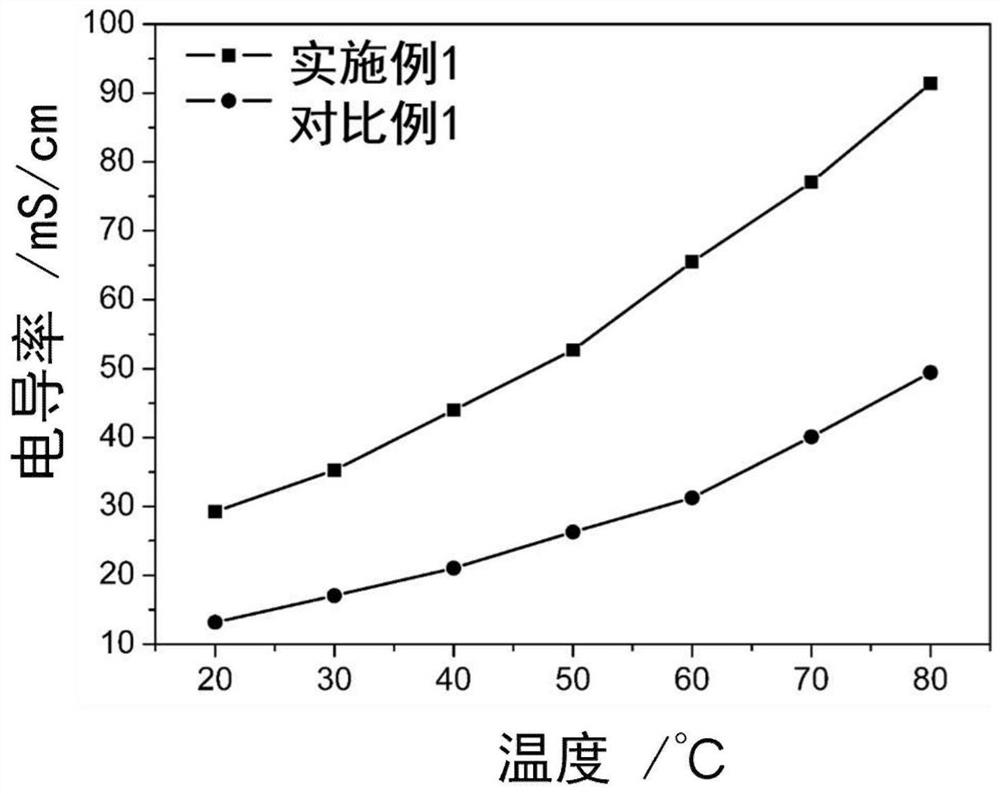
ActiveCN109390617AHigh mechanical strengthGood dimensional stabilityOrganic chemistryFuel cellsCross-linkAlkane
The invention specifically relates to a cross-linked polybenzimidazole basic anion exchange membrane, and preparation and application thereof, belonging to basic anion exchange membrane fuel cells. The method comprises the following steps: synthesis of N1-long-chain alkane substituted-1,4-diazabicyclo[2.2.2]octane; and preparation of the N1-long-chain alkane substituted-1,4-diazabicyclo[2.2.2]octane functionalized polybenzimidazole-polyvinylbenzyl chloride cross-linked basic anion exchange membrane. According to the invention, the cross-linked anion exchange membrane is prepared by using a homogeneous method, and cross-linking and quaternization are completed in one step, so operation is simple and efficient, and the mechanical strength and dimensional stability of the cross-linked membrane are improved; a homogeneous reaction adopted in the process of preparation effectively improves the efficiency of quaternization, and a microscopic phase separation structure in the membrane allowsthe cross-linked membrane to have higher conductivity; and the cross-linked polybenzimidazole basic anion exchange membrane has the advantages of excellent dimensional stability, chemical stability and the like, and has potential application prospects in basic anion exchange membrane fuel cells.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Liquid crystal aligning agent, liquid crystal alignment film, and liquid crystal display element
ActiveCN103797408AImprove friction resistanceExcellent voltage retention characteristicsNon-linear opticsHydrogenHydrogen atom
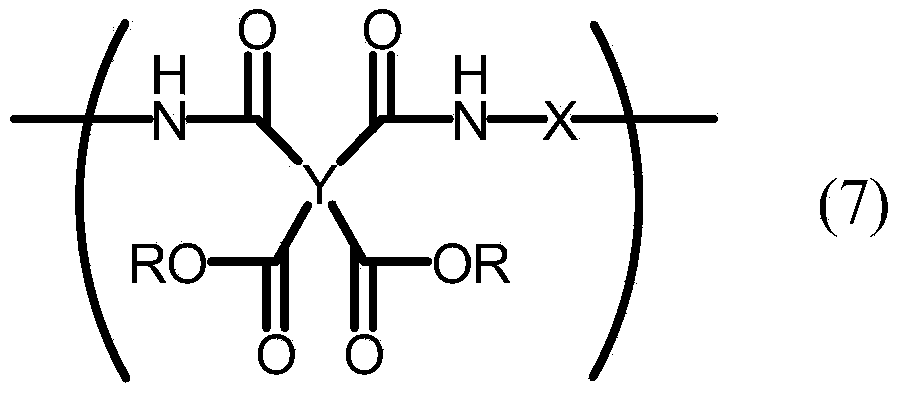
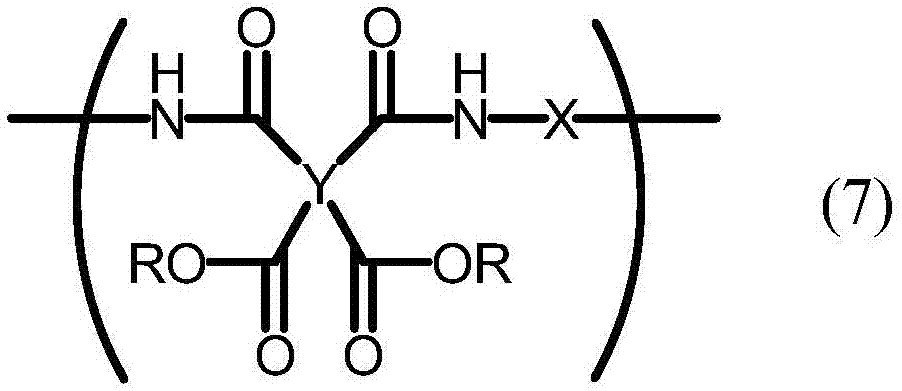
A liquid crystal aligning agent which contains a specific polymer (A) and a specific polymer (B) described below. Specific polymer (A): a polyamic acid which is obtained by reacting a tetracarboxylic acid dianhydride component that contains bicyclo[3,3,0]octane-2,4,6,8-tetracarboxylic acid dianhydride with a diamine component that contains at least one diamine compound as typified by 4,4'-diaminodiphenyl amine and 3,6-diaminocarbazole. Specific polymer (B): a polyimide precursor which has a structural unit represented by formula (7). (In formula (7), R represents a hydrogen atom or an alkyl group; Y represents a tetravalent organic group; and X represents a divalent organic group, with 10-100% by mole of X being a divalent organic group having one of the moieties represented by formulae (8)-(10) in the structure or a paraphenylene group.) (In formula (8), m1 represents an integer of 2-18.) (In formula (9), one or more arbitrary hydrogen atoms on the benzene rings may be substituted by monovalent organic groups other than primary amino groups; and m2 represents an integer of 1-8.) (In formula (10), one or more arbitrary hydrogen atoms on the benzene rings may be substituted by monovalent organic groups other than primary amino groups; and m3 represents an integer of 1-4.)
Owner:NISSAN CHEM IND LTD
Photoresist polymeric compound and photoresist resin composition
InactiveUS20050014087A1Well-balanced solubilityForming accuratelyRadiation applicationsPhotomechanical apparatusResistSolubility
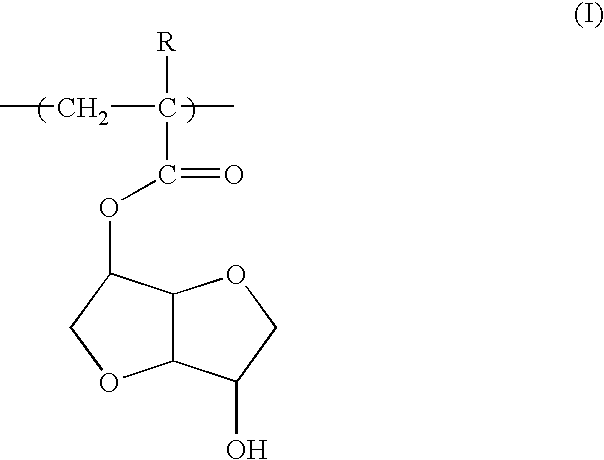
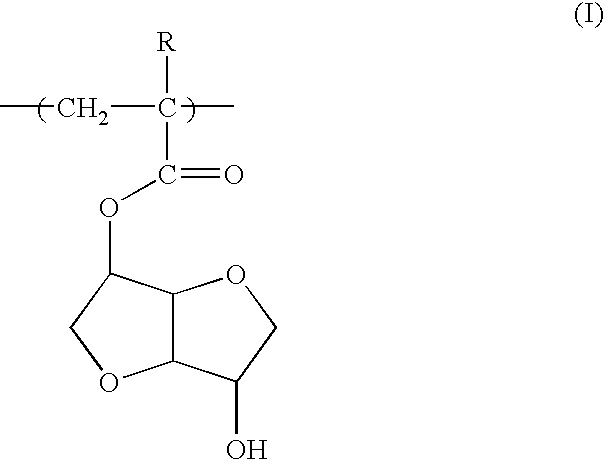
A polymeric compound for photoresist of the present invention includes a monomer unit having 2,6-dioxabicyclo[3.3.0]octane skeleton in the structure. The monomer unit having 2,6-dioxabicyclo[3.3.0]octane skeleton includes a monomer unit represented by the following Formula (I): wherein R is a hydrogen atom or a methyl group. The polymeric compound for photoresist may include a monomer unit having 2,6-dioxabicyclo[3.3.0]octane skeleton, a monomer unit having a group of adhesion to substrate, and a monomer unit having an acid-eliminating group. The polymeric compound for photoresist of the present invention exhibits not only adhesion to substrate, acid-eliminating property and resistance to dry-etching but also has well-balanced solubility in solvents for photoresist and alkali-soluble property.
Owner:DAICEL CHEM IND LTD
CHA type aluminum-silicon molecular sieve and preparation method and application of SCR catalyst
PendingCN111871455AReasonably acidicGood hydrothermal stabilityMolecular sieve catalystsInternal combustion piston enginesChemical synthesisMolecular sieve
The invention discloses a CHA type aluminum-silicon molecular sieve and a preparation method and application of an SCR catalyst, and belongs to the field of chemical synthesis technology and application thereof. The CHA type aluminosilicate zeolite molecular sieve is synthesized by adopting an N,N,N-trialkylbicyclo[2.2.2]octylammonium compound as an organic template agent, wherein the molar ratiorange of silicon dioxide to aluminum oxide in the product is 6-80, the average grain diameter is less than or equal to 500 nm, the total specific surface area is more than or equal to 400m2 / g, the total pore volume is more than or equal to 0.20 ml / g, the micropore volume is not less than 0.10 ml / g, and the grain diameter in the crystal face (-210) direction of the molecular sieve is 50-160 nm. After hydrothermal treatment of the molecular sieve at 600-800 DEG C, the tetra-coordinated aluminum accounts for more than or equal to 90% of the total aluminum amount, and the hexa-coordinated aluminumaccounts for less than or equal to 10% of the total aluminum amount. The molecular sieve provided by the invention has high hydrothermal stability without large crystal grains, shows high nitrogen oxide reduction characteristics, and especially shows a catalyst with high nitrogen oxide reduction characteristics in a temperature range of 200-550 DEG C.
Owner:CHINA CATALYST HLDG CO LTD
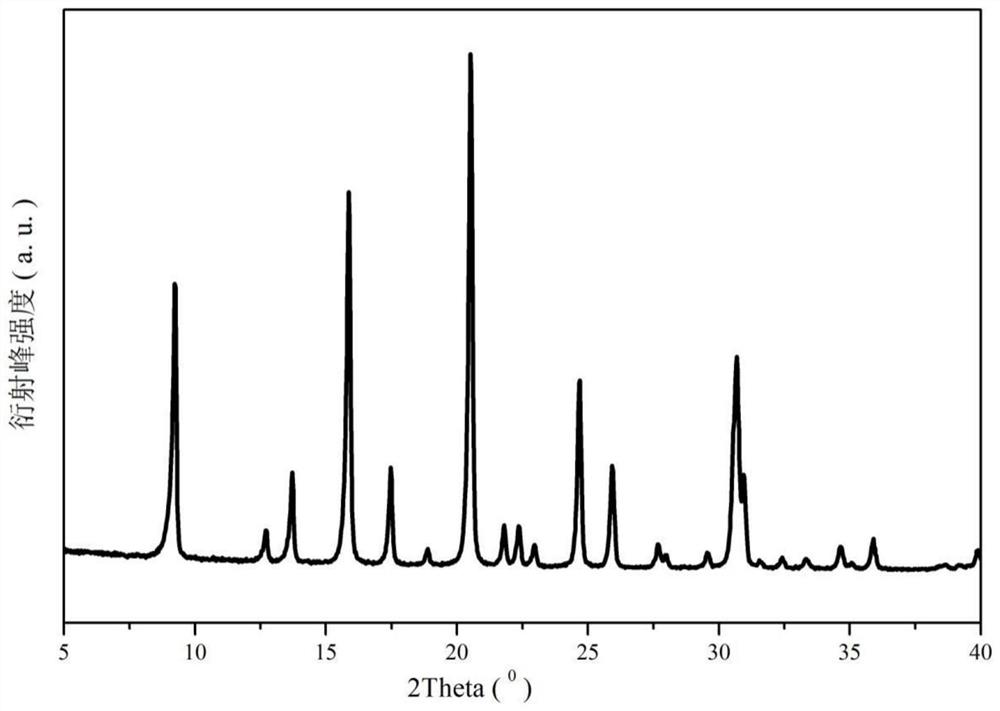
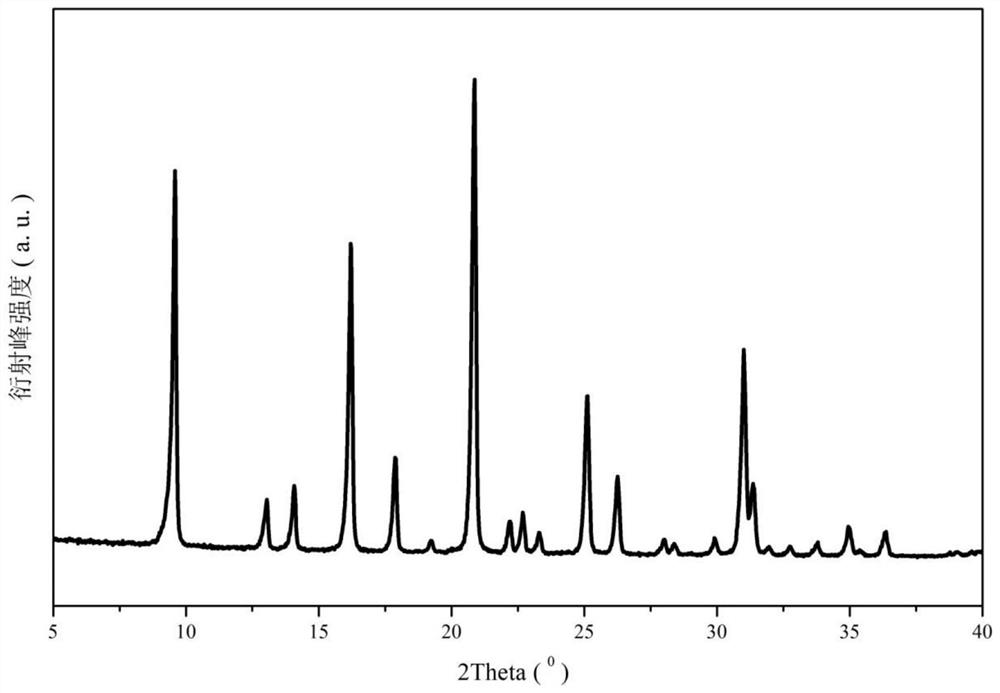
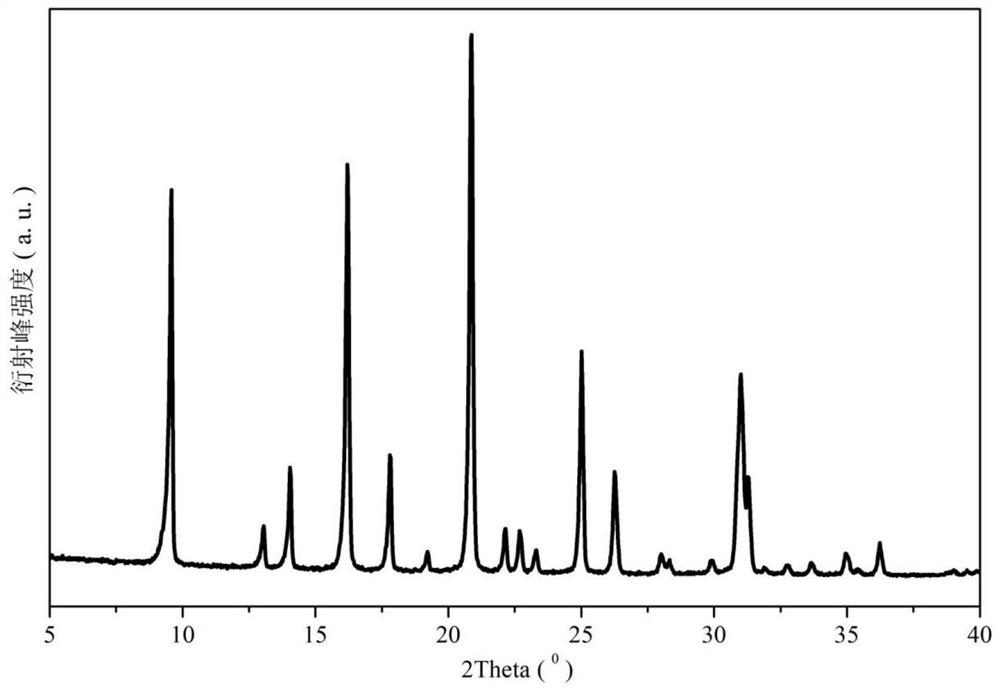
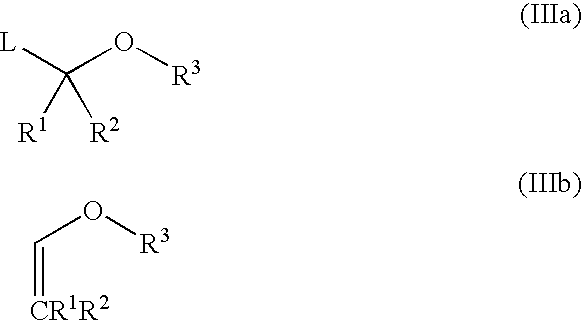
Stereospecific method for the preparation of dioxa-bicyclooctane compounds
This invention relates to a new method for the stereospecific thiocarboxylation of organic compounds for the preparation of compounds according to formula (I):wherein a compound of formula (II):is reacted with a compound of formula (IIIa) or (IIIb):then treating the obtained product with a thiocarboxylic acid or a salt thereof, and subsequently carrying out a nitration reaction.
Owner:LACER SA
Liquid crystal orientating agent
ActiveCN102020994AExcellent display propertiesImprove reliabilityLiquid crystal compositionsNon-linear opticsCrystallographyNorbornene
The invention provides a liquid crystal orientating agent, which can obtain good pretilt angle property through a light orientation method, and also can provide a liquid crystal orientating film which cannot cause property difference in long time continuous operation. The liquid crystal orientating agent comprises at least one polymer selected from a group consisting of polyamic acid prepared by reaction of tetracarboxylic dianhydride and diamine, and polyimide formed by closing-ring dehydration of the polyamic acid, wherein the tetracarboxylic dianhydride comprises at least one item selected from a group consisting of 3, 5, 6-3 carboxyl-2-carboxymethyl norbornene alkyl-2: 3, 5: 6-dianhydride and 2, 4, 6, 8-tetra-carboxyl bicyclo [3, 3, 0] octane-2: 4, 6: 8-dianhydride, and the diamine includes a diamine having a photoreactive structure.
Owner:JSR CORPORATIOON
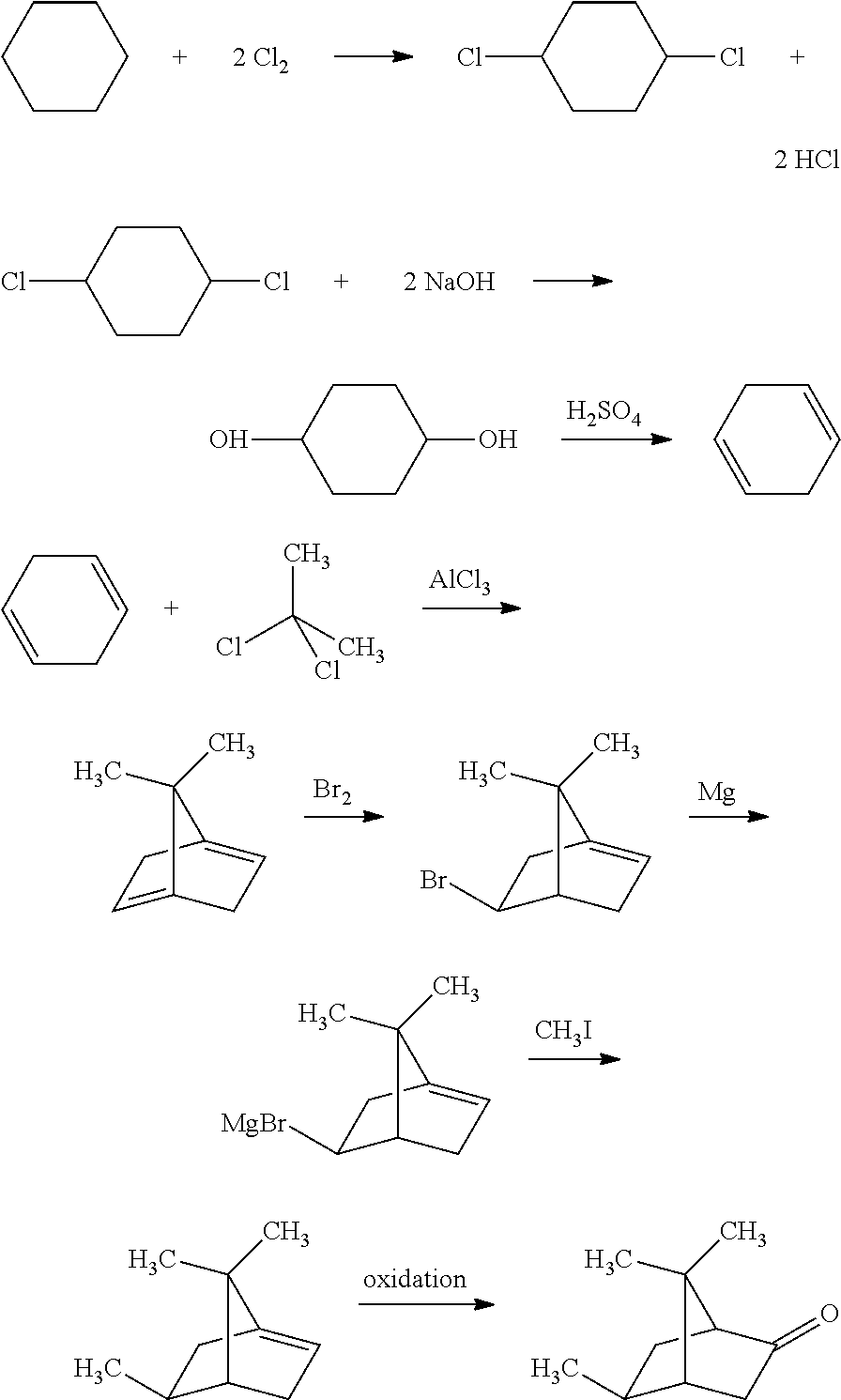
Liquid fuel composition and method for enhancing combustion efficiency of a liquid fuel
InactiveUS20120110900A1Improve combustion efficiencySolid fuelsLiquid carbonaceous fuelsCombustionCyclobutane
A liquid fuel composition includes: a liquid fuel; and a sublimatable organic compound that has a sublimation point lower than 45° C. at atmospheric pressure, and that includes a skeleton selected from bicycloheptane, indane, pyrone, cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and benzene.
Owner:TSAI KAI SHON +1
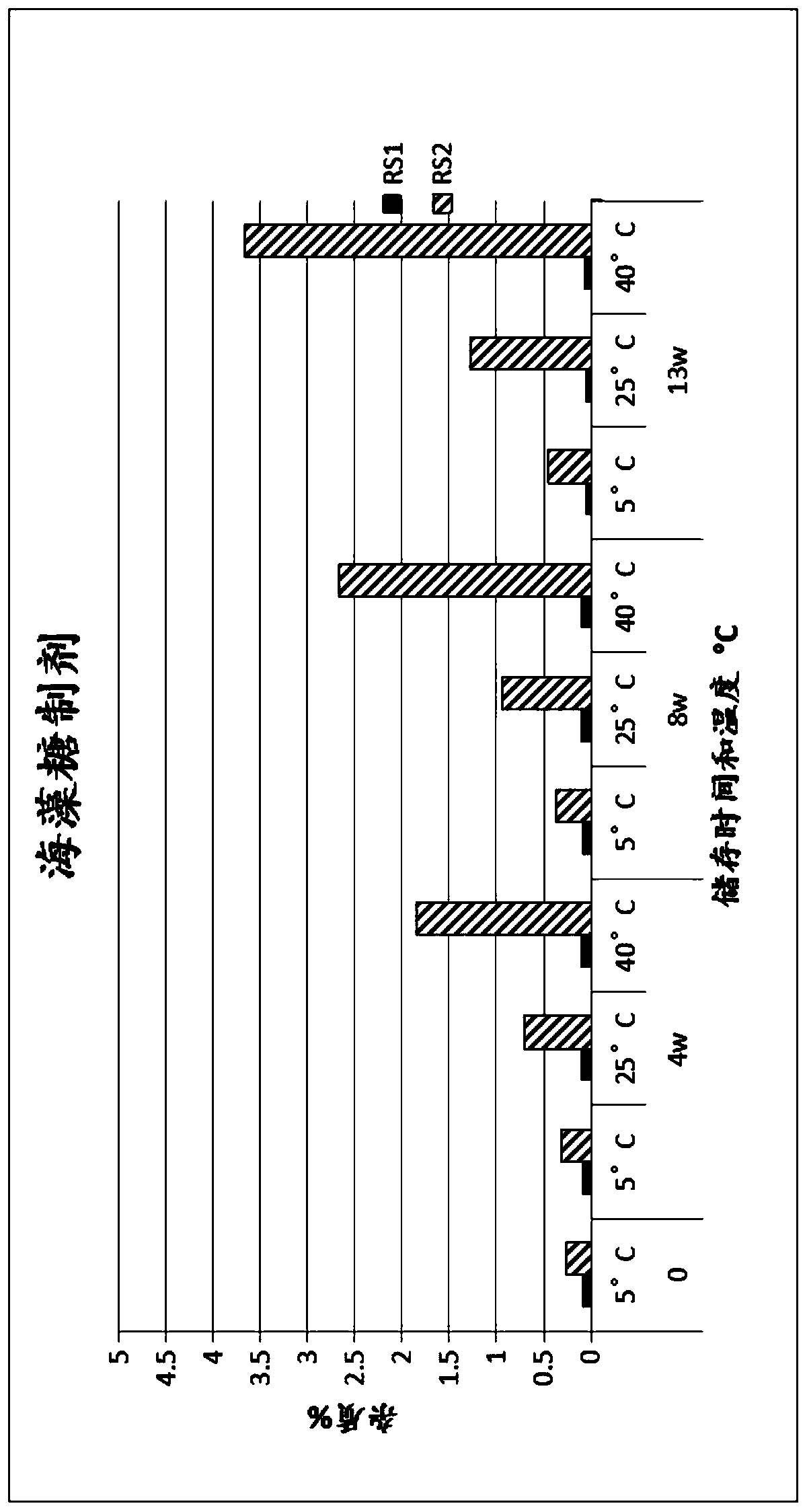
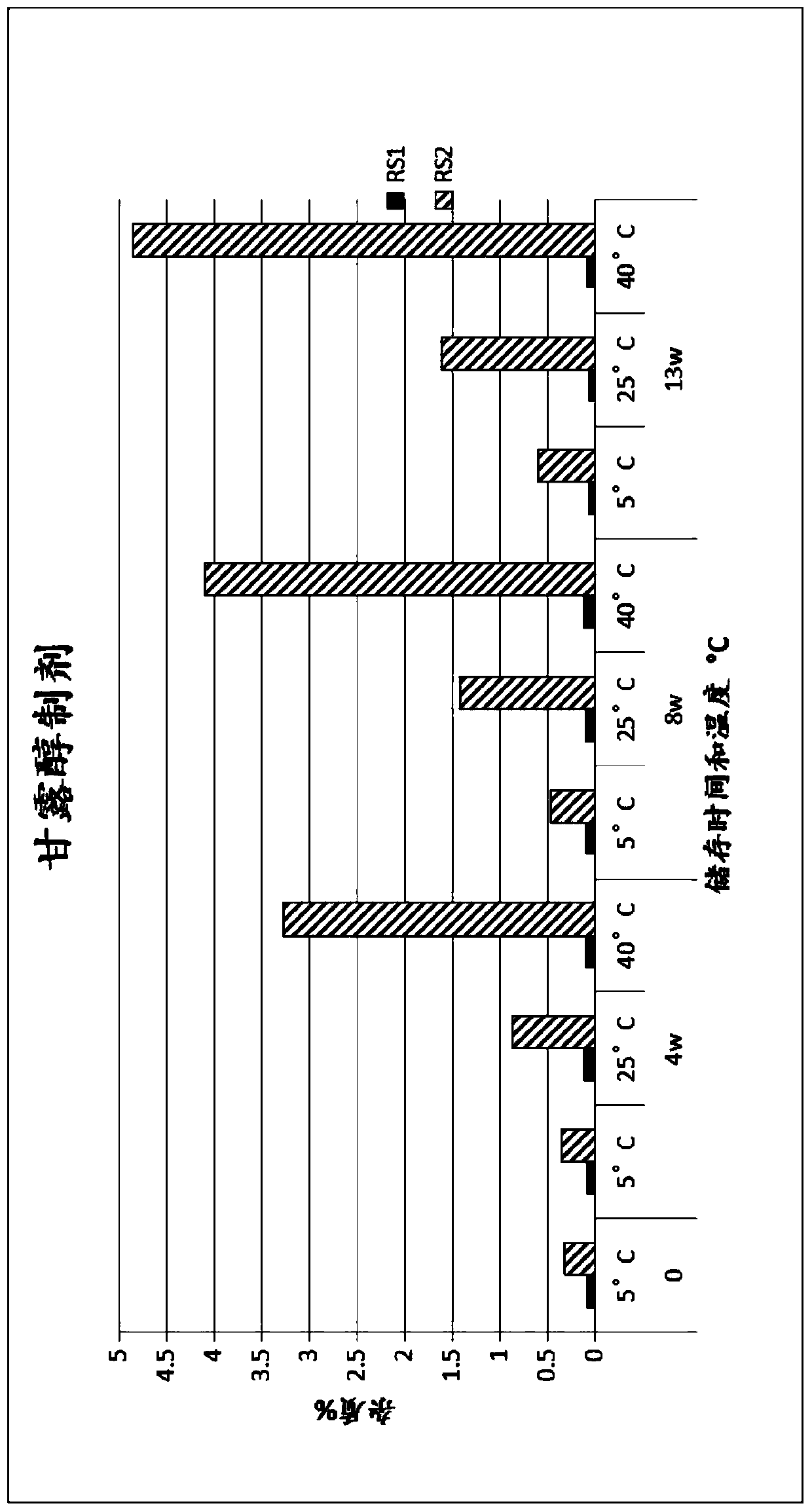
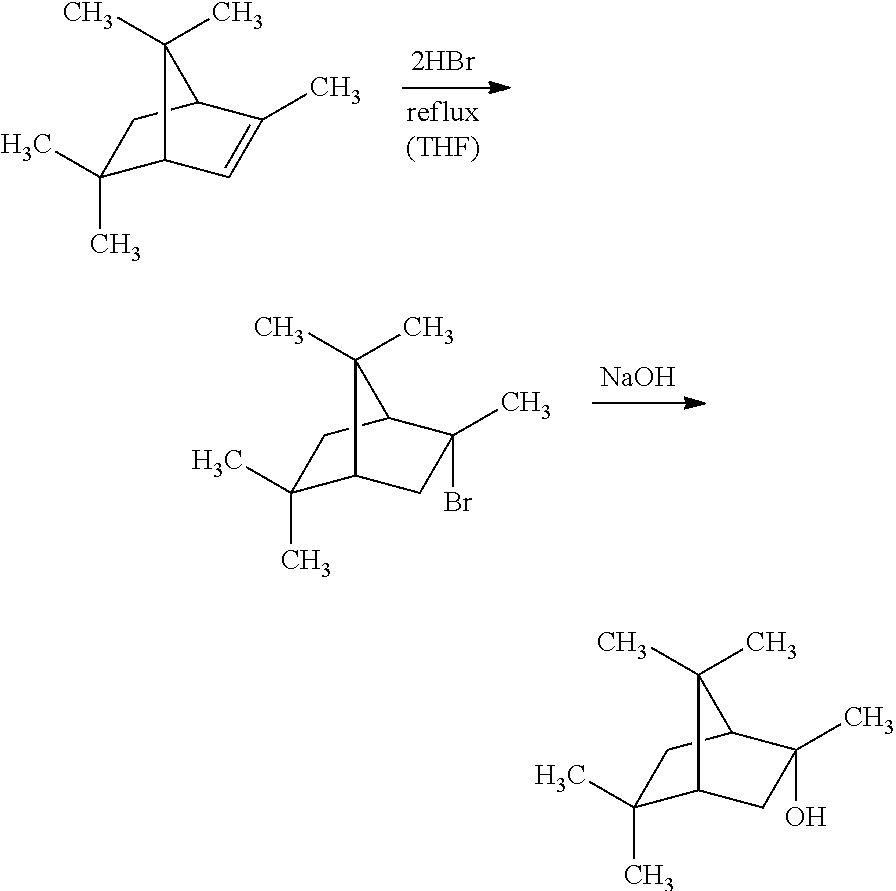
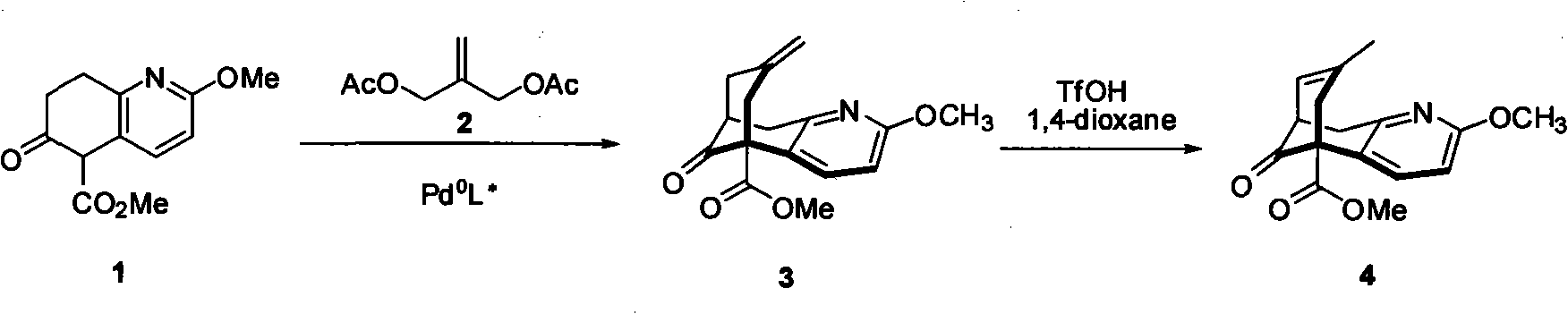
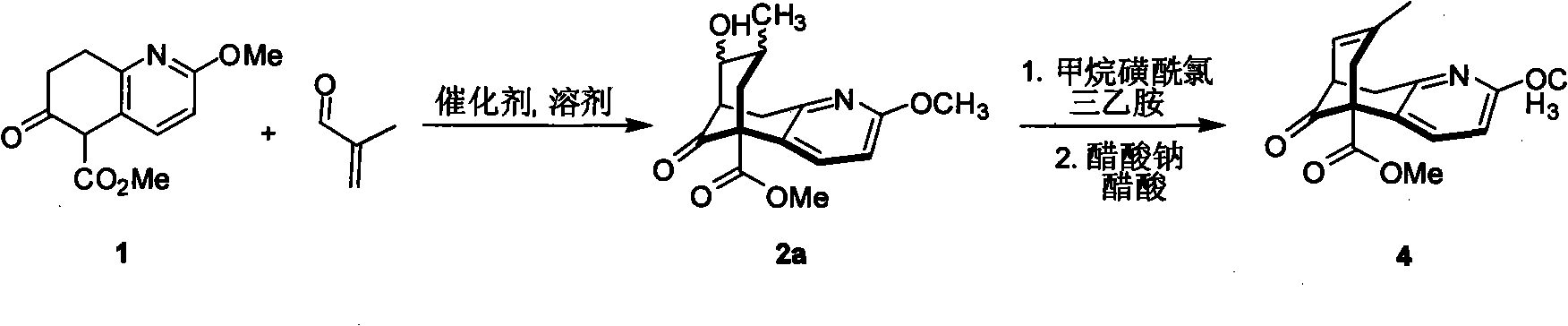
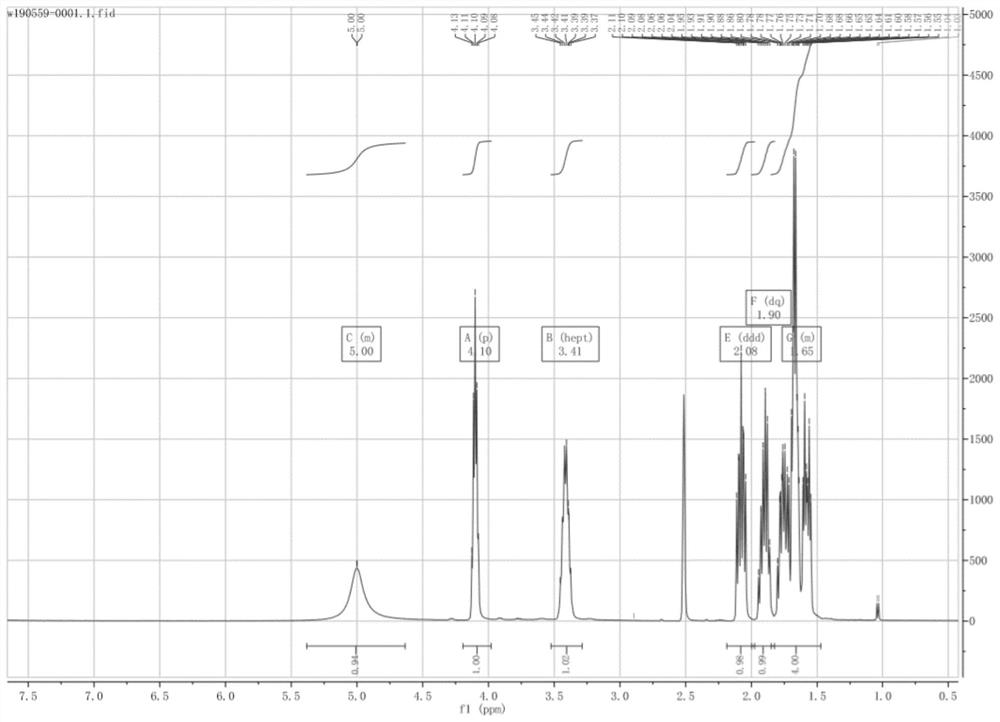
Method for preparing (2s, 3s)-3-amino-bicyclo [2.2. 2] octane-2-formate
ActiveCN111454166ANovel process routeMild reaction conditionsOrganic compound preparationOrganic chemistry methodsFormateBiochemical engineering
The invention discloses a method for synthesizing (2S,3S)-3-amino-bicyclo[2.2.2] octane-2-formate and belongs to the field of pharmaceutical intermediate synthesis. The purposes of the invention are to solve the problems of high preparation cost, low material safety and the like of (2S, 3S)-3-amino-bicyclo[2.2.2] octane-2-formate, further improve the productivity and reduce the production cost. According to the method disclosed by the invention, 3-carbonyl-bicyclo[2.2.2] octane-2-formate is used as a starting material, and reductive amination, basic configuration inversion, hydrogenation protection group removal and the like are sequentially carried out, so that the target product is obtained. According to the invention, the (2S, 3S)-3-amino-bicyclo [2.2. 2] octane-2-formate is synthesizedby using the synthesis method, the novel process route is used, the yield is more than 65%, and the method has characteristics of novel route, mild reaction condition, low cost and the like.
Owner:RAFFLES PHAMRMATECH CO LTD
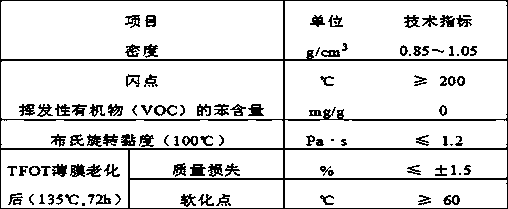
Ultrathin wearing layer normal-temperature asphalt modifier and preparation method thereof
The invention discloses an ultrathin wearing layer normal-temperature asphalt modifier and a preparation method thereof. The ultrathin wearing layer normal-temperature asphalt modifier is prepared from the following raw materials in parts by mass: 55-65 parts of cyclooctane, 30-35 parts of modified epoxy resin glue, 0.6-1 part of a dispersant and 5-10 parts of a coupling agent. The preparation method comprises the following steps: putting the cyclooctane, the modified epoxy resin glue and the dispersant into a stirring kettle according to a ratio, heating to 100+ / -5 DEG C, mixing, and uniformly stirring for 4-6 hours; further putting a coupling agent into the materials in the stirring kettle, and continuously stirring for 4-6 hours, thereby obtaining the product. By adopting the modifier,the rotary viscosity of asphalt at 60 DEG C can be reduced to 2.2-3.0Pa*s, and a mixed asphalt material can be spread for 1.0-2.0cm by using a common spreading machine within 50-60 DEG C; the construction temperature can be greatly reduced when being compared with a conventional construction temperature, and both the construction cost is reduced and the construction quality is ensured.
Owner:BUREAU OF TRAFFIC CONSTR ENG QUALITY SUPERVISE OF INNER MONGOLIA AUTONOMOUS REGION
Method for removing ethanol, isopropanol and octyl alcohol from amino resin workshop wastewater
InactiveCN105084495AStrong complexing abilityFast precipitationWater contaminantsNature of treatment waterOctanolAcetophenone
The invention relates to a method for removing ethanol, isopropanol and octyl alcohol from amino resin workshop wastewater. The components adopted by the method comprises 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane, 1,8-dihydroxy-3-methoxy-6-methylanthraquinone, S(-)-2-amino-6-n-propyle-4,5,6,7-tetrahydrobenzothiazole dihydrochloride, 4-octylphenol ethoxylate, 3',4'-dihydoxy-2-(methylamino)acetophenone hydrochloride, 4-acetoxy-3-methoxy-(2-propenyl) benzene, 4-methoxybenzyl acetate, 6,6,10-trimethyl bicyclo-3,1,1-hept-2-ene, and 2-[[1-(3-acetylthio-2-methylpropionyl)pyrrolidine-2-formyl]amino]-3-phenylpropionic acid. The components adopted by the method have strong complexing capability with target substances, high speed of forming complex precipitates, and high removal rate up to 99.9%.
Owner:李海兰
Pharmaceutical uses and synthesis of benzobicyclooctanes
Benzobicyclooctane compounds, their use in inhibiting cellular events involving TNF-α and IL-8, and in the treatment of inflammation events in general; a combinatorial library of diverse bicyclooctanes and process for their synthesis as a library and as individual compounds.
Owner:CELLTECH R & D LTD
Liquid crystal aligning agent, liquid crystal alignment film, and liquid crystal display element
InactiveCN107090301AImprove friction resistanceExcellent voltage retention characteristicsLiquid crystal compositionsNon-linear opticsPolymer sciencePolyamide
A liquid crystal aligning agent which contains a specific polymer (A) and a specific polymer (B) described below. Specific polymer (A): a polyamic acid which is obtained by reacting a tetracarboxylic acid dianhydride component that contains bicyclo[3,3,0]octane-2,4,6,8-tetracarboxylic acid dianhydride with a diamine component that contains at least one diamine compound as typified by 4,4'-diaminodiphenyl amine and 3,6-diaminocarbazole. Specific polymer (B): a polyimide precursor which has a structural unit represented by formula (7). (In formula (7), R represents a hydrogen atom or an alkyl group; Y represents a tetravalent organic group; and X represents a divalent organic group, with 10-100% by mole of X being a divalent organic group having one of the moieties represented by formulae (8)-(10) in the structure or a paraphenylene group.) (In formula (8), m1 represents an integer of 2-18.) (In formula (9), one or more arbitrary hydrogen atoms on the benzene rings may be substituted by monovalent organic groups other than primary amino groups; and m2 represents an integer of 1-8.) (In formula (10), one or more arbitrary hydrogen atoms on the benzene rings may be substituted by monovalent organic groups other than primary amino groups; and m3 represents an integer of 1-4.
Owner:NISSAN CHEM IND LTD
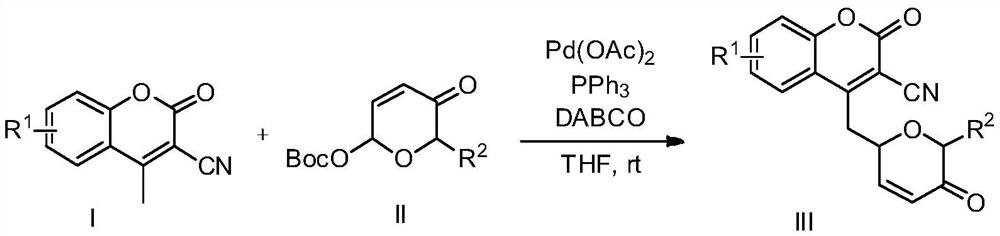
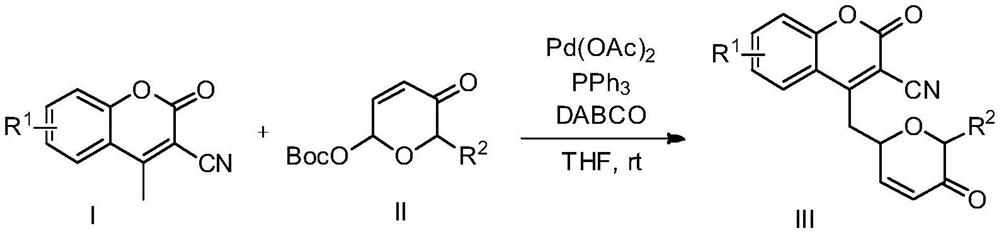
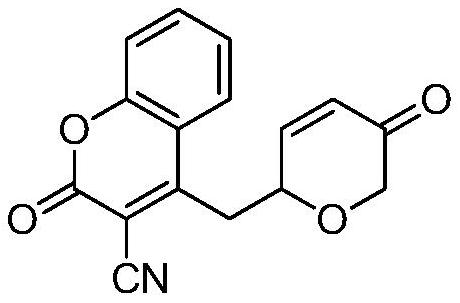
Preparation method of novel dihydropyrone coumarin compound
InactiveCN112409345AMild reaction conditionsHigh regional selectivityOrganic chemistryOrganic synthesisCatalytic effect
The invention belongs to the technical field of organic synthesis, and relates to a preparation method of a novel dihydropyrone coumarin compound. The preparation method comprises the following steps:in tetrahydrofuran, takingtriphenyl phosphine as a ligand and1,4-diazabicyclo[2.2.2] octane as an alkali, and under the catalytic action of Pd(OAc)2, reacting a dihydropyrone compound with a 3-cyano-4-methyl coumarin compound to prepare the 3-cyano-4-(6-dihydropyrone) methyl coumarin. The preparation method is mild in reaction condition, and the product yield is not lower than 52%. The inventionprovides a preparation method for obtaining dihydropyrone coumarin with excellent regioselectivity and high stereoselectivity by a simple and effective method. The preparation method is mild in reaction condition, green, high in reaction efficiency and more suitable for large-scale production requirements, and the prepared dihydropyrone coumarin compound is a compound with potential physiologicalactivity.
Owner:DALIAN UNIV OF TECH
Bicyclo[3,3,0]octane polymerizable compound
InactiveCN105801414AGood miscibilityImproves UV resistanceLiquid crystal compositionsOrganic chemistrySolubilityLiquid-crystal display
The invention discloses a bicyclo[3,3,0]octane polymerizable compound. The general structural formula of the bicyclo[3,3,0]octane polymerizable compound is as shown in a formula I which is described in the specification. The compound as shown in the formula I has the advantages of good intersolubility with other monomers, good ultraviolet ray endurance capability, etc. As an RM, the bicyclo[3,3,0]octane polymerizable compound has the advantages of good intersolubility, a high voltage holding rate (VHR), high polymerization activity (few monomer residuals), etc., and is especially applicable as the RM to liquid crystal mixtures of a PSA (polymer-supported alignment) mode and a PS (polymer stabilization) mode. Meanwhile, the compound can be applied to a liquid crystal display including a polymer-stabilized blue phase and has good dissolvability and polymerisability; and a final product has no or tiny retardation effect.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
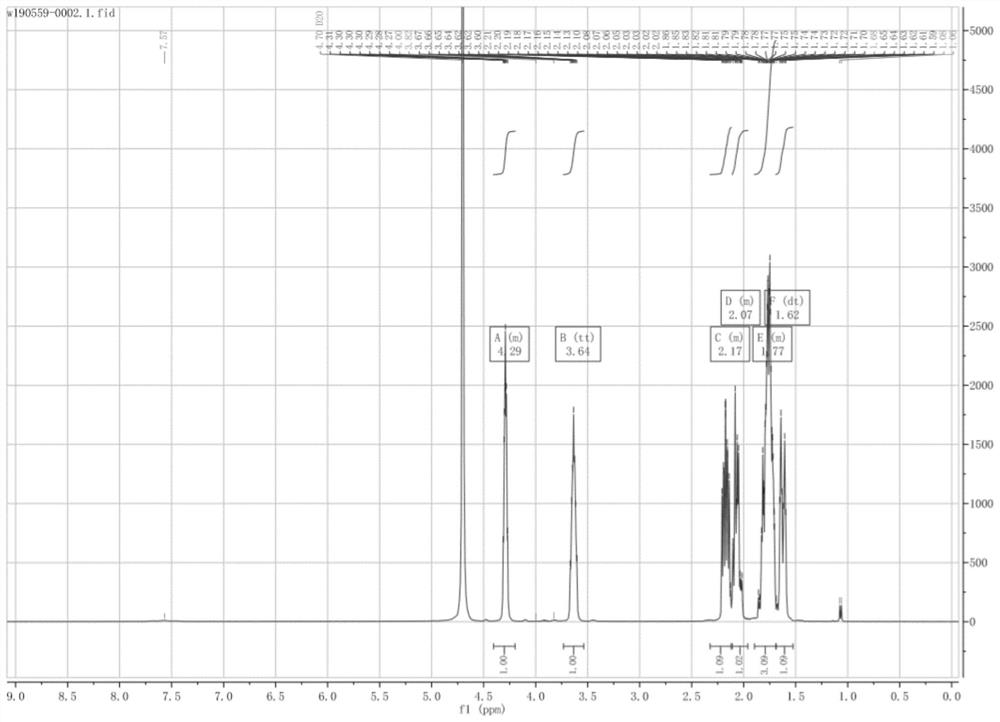
Method for preparing (2S, 3S)-3-amino-bicyclo [2.2.2]octane-2-formate
ActiveCN111620788AShort synthetic routeMild reaction conditionsOrganic compound preparationOrganic chemistry methodsFormateCyclooctanes
The invention discloses a method for preparing (2S,3S)-3-amino-bicyclo [2.2.2]octane-2-formate. The method comprises the following steps: 3-carbonyl-bicyclo [2.2. 2] octane-2-formate is used as a rawmaterial, condensation, reduction, basic configuration inversion and acidic protection group removal are sequentially performed to obtain the (2S, 3S)-3-amino-bicyclo [2.2. 2] octane-2-formate, and Ris an organic substituent group. The synthetic route is short, the reaction conditions are mild, and the total yield is greater than 80%; through a chiral induction mode, an intermediate with high chiral purity can be obtained, and the chiral purity of a target product is improved to 99.5% or above; the used raw materials are easily available bulk raw materials, the equipment requirement is low, and the method is suitable for large-scale industrial production.
Owner:RAFFLES PHAMRMATECH CO LTD
Asymmetric synthetic method of (-)-huperzine key intermediate
InactiveCN101935302AOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesHydrogenGram
The invention belongs to the field of asymmetric catalysis of organic chemistry, and in particular relates to an asymmetric synthetic method of a (-)-huperzine key intermediate, comprising the following step of: synthesizing a huperzine key intermediate by using an asymmetric Michael / Adol cascade reaction of organic micromolecule catalysis. The asymmetric Michael / Adol cascade reaction is characterized in that the key intermediate (-)-(5S, 9R)-9,10-dihydro-2-methoxyl-7-methyl-11-oxo-5,9-methylene cyclooctane [b] pyridine-5(6H)-methyl carbonate is prepared by using high yield, high optical purity in a gram level.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method for removing methanol, n-butanol and isobutanol from wastewater of amino resin workshop
InactiveCN105060442AStrong complexing abilityFast precipitationWater contaminantsNature of treatment waterIsobutanolN-Butanol
The invention relates to a method for removing methanol, n-butanol and isobutanol from wastewater of an amino resin workshop. The method comprises the steps of adding 1,3,3-trimethyl-2-oxa bicyclo[2.2.2]octane, flavanone glycoside, S(-)-2-amino-6-n-propyl amino-4,5,6,7-tetrahydro benzothiazole di-hydrochloride, 4-octyl phenol ethoxylate, 3',4'-dihydroxy-2-(methyl amino)acetophenone hydrochloride, 4-acetyloxy-3-methoxy-(2-propenyl)benzene, acet-4-methoxy-benzyl ester, 6,6,10-trimethyl bicyclo-3,1,1-heptyl-2-alkene, 2-[[1-(3-acetylthio-2-methyl propionyl)pyrrolidine-2-formyl]amino]-3- phenylpropionic acid and the like. The method has the advantages that complex capabilities to target objects are high; the deposition velocities of complex compounds are fast; the removal rate can reach 99.9 percent; the dose is low; hazard to a water body is avoided.
Owner:李海兰
Dibenzocyclooctane carboxylic ether compounds with herbicidal activity
A dibenzocyclooctalkyl carboxylate compound with herbiciding activity and its composition can be used to effectively control the mono-and di-cotyledonous weeds.
Owner:中国中化股份有限公司 +1
A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method
ActiveCN111217826BEfficient synthesis methodThe synthesis method is simpleOrganic chemistryTetrafluoroborateMethyl palmoxirate
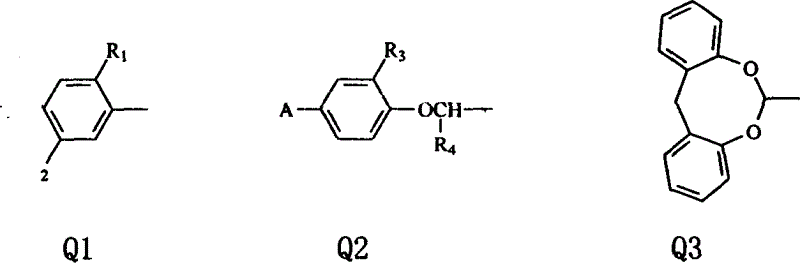
The invention discloses a 2,6-dioxobicyclo[3.3.2]octane derivative and a synthesis method thereof. The synthesis method of the derivative is based on (E)-2-hydroxyl-1-naphthalene vinyl derivative The substrate is under the action of 1-chloromethyl-4-fluoro-1, 4-diazotized bicyclo[2.2.2]octane bis(tetrafluoroborate) salt and cuprous chloride, and the reaction obtains a novel 2,6-Dioxobicyclo[3.3.2]octane skeleton derivative compound. The method of the invention is simple to operate, and the reaction conditions are mild, and a novel 3D skeleton, two rings, three new chemical bonds (two C-O, one C-C) and four chiral centers are constructed in a one-pot reaction And a single stereo configuration was obtained, and a series of 2,6-dioxobicyclo[3.3.2]octane derivatives were constructed.
Owner:NORTHWEST UNIV
A kind of molybdenum-based supramolecular phase change crystal material and preparation method thereof
The invention discloses a molybdenum-based supramolecular phase transition crystal material and a preparation method thereof. In the present invention, a solution 1 with a concentration of 0.2 to 0.4 mol / L is obtained by dissolving the "Mo=O-based" molybdenum metal compound in pure water, adding hydrochloric acid with a mass concentration of 36% to 38% into the solution 1 and letting it stand still, pumping Collect the filtrate after filtering the precipitate; wherein, the volume ratio of solution 1 to hydrochloric acid is (2-5): 10; 1,4-diazabicyclo[2.2.2]octane is dissolved in acetone to obtain a concentration of 0.05- 0.1mol / L solution 2, add the filtrate described in step (1) to the solution 2, mix uniformly, and let stand for 14 to 16 days to obtain a novel molybdenum-based supramolecular phase change crystal material; wherein, solution 2 and the filtrate The volume ratio is (5-8):10. The preparation process of the invention can be completed at room temperature, and compared with the synthesis process of the existing molybdenum-based supramolecular compound, the invention has better energy consumption and is safer.
Owner:XINJIANG AGRI UNIV
A divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application
InactiveCN106083841BUniform particlesHigh thermal decomposition temperature pointSolid-state devicesSemiconductor/solid-state device manufacturingCrystal systemAmine derivatives
The invention discloses a divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application, belonging to molecular ion-based Divalent manganese fluorescent material. The chemical formula of the fluorescent material is C12H26N2Cl4Mn. At the temperature of 296K, the crystal belongs to the orthorhombic system, and the chiral space group is P212121. The product is prepared by mixing soluble salts containing Mn2+ and triethylenediamine derivatives and self-assembling with natural volatile solvents of the solution. The material preparation process adopted by the fluorescent material of the present invention is simple, easy to operate, sufficient source of raw materials, low production cost, high yield and good repeatability; the thermal decomposition temperature point is relatively high, and the crystal particles are uniform.
Owner:JIANGSU UNIV OF SCI & TECH
Method for preparing (2s,3s)-3-amino-bicyclo[2.2.2]octane-2-carboxylate
PendingUS20220033344A1High chiral purityReadily availableOrganic compound preparationOrganic chemistry methodsCyclooctanesCarboxylic acid
A method for preparing (2S,3S)-3-amino-bicyclo[2.2.2]octane-2-carboxylate is in the field of pharmaceutical intermediate synthesis. The method uses 3-carbonyl-bicyclo[2.2.2]octane-2-carboxylate as the starting material and performs reductive amination, alkalinity configuration flip, and hydrogenation to remove the protecting group in sequence to obtain the target product. This synthesis method of (2S,3S)-3-amino-bicyclo[2.2.2]octane-2-carboxylate is characterized by a novel route, mild reaction conditions and low cost, with a yield of more than 65%.
Owner:RAFFLES PHAMRMATECH CO LTD
Preparation method of SGLTs inhibitor and key intermediate thereof
InactiveCN113004349AAddressing Accessibility IssuesFew synthetic stepsSaccharide with carbocyclic radicalsSugar derivativesEthyl groupPharmaceutical drug
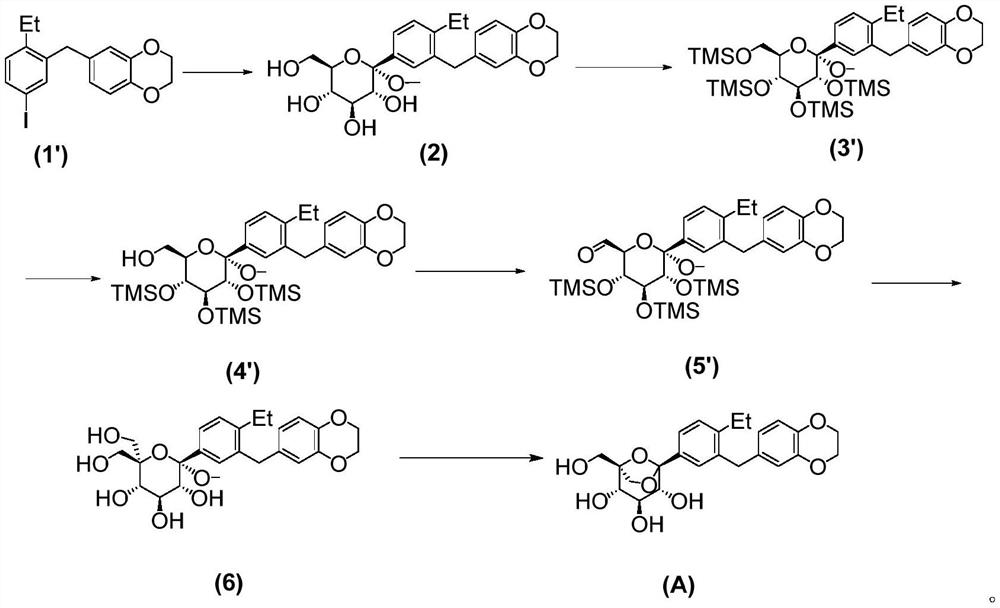
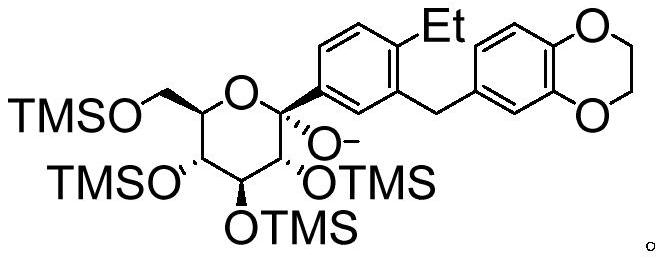
The invention relates to a preparation method of an SGLTs inhibitor and a key intermediate of the SGLTs inhibitor. The SGLTs inhibitor is (1S, 2S, 3S, 4R, 5S)-5-(3-((2, 3-dihydrobenzo [b] [1, 4] dioxin-6-yl)methyl)-4-ethyl phenyl)-1-(hydroxymethyl)-6, 8-dioxabicyclo [3.2.1] octane-2, 3, 4-triol. According to the preparation method, 6-(2-ethyl-5-iodobenzyl)-2, 3-dihydrobenzo [b] [1, 4] dioxin is taken as a raw material, silyl is taken as a protecting group, and six-step reaction is carried out to prepare the product. The preparation method disclosed by the invention is simple to operate, high in yield of each step and stable in process, can adapt to industrial production requirements, solves the problem of drug accessibility, and is beneficial to acceleration of clinical development of the SGLTs inhibitor and drug marketing.
Owner:YOUNGENE THERAPEUTICS CO LTD
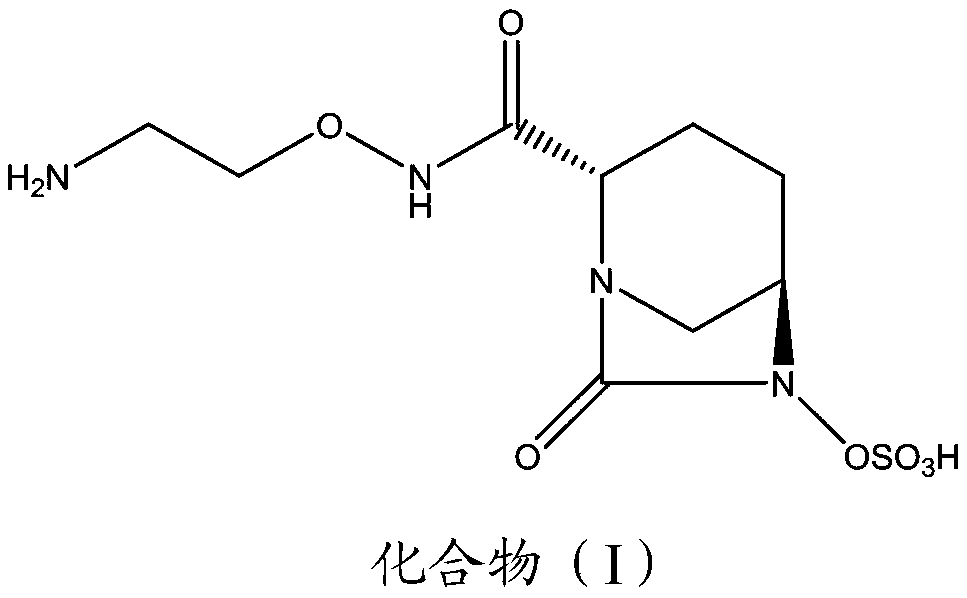
Preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid
ActiveCN105294700AReasonable reaction process designLower synthesis costOrganic chemistryMetaclazepamTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid, and mainly solves the technical problems that in an existing synthetic process, the yield is low, the reaction is not easy to control, the experimental operation is inconvenient and the like. By taking t-butyloxycarboryl methyl pyroglutamate as an initial raw material, the tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid is prepared by five-step reactions. The tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid obtained by the method provided by the invention is a useful intermediate or an intermediate product for synthesis of multiple drugs.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +2
Cross-linked polybenzimidazole basic anion exchange membrane and its preparation and application
ActiveCN109390617BHigh mechanical strengthGood dimensional stabilityOrganic chemistryFuel cellsAlkanePolyvinylbenzyl chloride
The invention relates to an alkaline anion-exchange membrane fuel cell, in particular to a cross-linked polybenzimidazole alkaline anion-exchange membrane and its preparation and application. The method includes the synthesis of N1-long-chain alkane-substituted-1,4-diazabicyclo[2.2.2]octane, N1-long-chain alkane-substituted-1,4-diazabicyclo[2.2.2] Preparation of Octane-functionalized Polybenzimidazole‑Polyvinylbenzyl Chloride Crosslinked Basic Anion Exchange Membranes. The invention adopts the homogeneous method to prepare the crosslinked anion exchange membrane, and the crosslinking and quaternization are completed in one step, which is simple and efficient, not only improves the mechanical strength and dimensional stability of the crosslinked membrane, but also effectively improves the homogeneous reaction used in the preparation process. The efficiency of quaternization is improved, and the microscopic phase separation structure in the membrane makes the crosslinked membrane have high conductivity, and has the advantages of excellent dimensional stability and chemical stability, etc., and has potential in alkaline anion exchange membrane fuel cells. application prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of synthetic method of bictegravir intermediate
ActiveCN110092726BChiralityHigh yieldOrganic compound preparationCarboxylic compound preparationGrignard reagentHydrogen pressure
The invention discloses a method for synthesizing a Bictegravir intermediate. The starting material 3-carbonyl cyclopentanecarboxylic acid (formula I) undergoes an asymmetric reduction reaction under the condition of an enzyme to generate (3R)-3-hydroxycyclopentane Carboxylic acid (Formula II); Formula II reacts with diphenylphosphoryl azide (DPPA) to generate (1R,5S)‑2‑Oxy‑4‑azabicyclo[3.2.1]octane -3-ketone (formula III); formula III is hydrolyzed in hydrochloric acid to directly obtain the Bictegravir intermediate (1R,3S)-3-aminocyclopentanol hydrochloride. The raw materials used in the present invention are cheap and easy to obtain, and the cost is low; the reaction selectivity is high, the by-products are few, the yield is high, and the total yield reaches 63.5%; Hydrogen pressurized reduction and Grignard reagent reaction are adopted, which is safe and environmentally friendly, and suitable for industrial production.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Preparation method and application of chiral 3, 6-diazabicyclo [3.2.1] octane derivative
ActiveCN113527295AThe preparation method is simple and easyRaw materials are cheap and easy to getGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsAzomethine ylideCycloaddition
The invention discloses a preparation method of a chiral 3, 6-diazabicyclo [3.2.1] octane derivative. According to the chiral 3, 6-diazabicyclo [3.2.1] octane derivative, monovalent silver salt is combined with different chiral phosphine-amide ligands to synergistically catalyze 1, 3-dipolar cycloaddition reaction between different series of azomethine ylide and alpha-substituted terminal olefin amide, and the chiral 3, 6-diazabicyclo [3.2.1] octane derivative is directly obtained through intramolecular cyclization under the treatment of strong base. The preparation method disclosed by the invention is simple in steps, relatively cheap and easily available in raw materials, low in corrosion degree and relatively low in toxicity, and simple and easily-realized in reaction conditions. The 3, 6-diazabicyclo [3.2.1] octane derivative obtained according to the preparation method of the chiral 3, 6-diazabicyclo [3.2.1] octane derivative can be applied to a drug inhibitor or a drug intermediate.
Owner:HUNAN UNIV OF TECH
Preparation method and application of a chiral 3,6-diazabicyclo[3.2.1]octane derivative
ActiveCN113527295BThe preparation method is simple and easyRaw materials are cheap and easy to getGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsAzomethine ylideCycloaddition
The invention discloses a method for preparing chiral 3,6-diazabicyclo[3.2.1]octane derivatives. The chiral 3,6-diazabicyclo[3.2.1]octane The derivatives synergistically catalyze the 1,3-dipolar cycloaddition reaction between different series of azomethine ylides and α-substituted terminal olefin amides through monovalent silver salts combined with different chiral phosphine-amide ligands. Chiral 3,6‑diazabicyclo[3.2.1]octane derivatives were directly obtained by intramolecular cyclization under strong base treatment. The preparation method of the invention has simple steps, relatively cheap and easy-to-obtain raw materials, low corrosion and low toxicity, and simple and easy-to-realize reaction conditions. The 3,6-diazabicyclo[3.2.1]octane derivative obtained according to the preparation method of the chiral 3,6-diazabicyclo[3.2.1]octane derivative can be applied to medicine Inhibitors or drug intermediates.
Owner:湖南九维生物医药有限公司
Pharmaceutical forms of diazabicyclooctane derivatives and process for producing the same
PendingCN111447924AAntibacterial agentsOrganic active ingredientsCyclooctanesCombinatorial chemistry
Owner:MEIJI SEIKA KAISHA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists 8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/43cf5f19-9dc4-434c-b9d8-21b418be5f30/US08263618-20120911-D00001.png)
![8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists 8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/43cf5f19-9dc4-434c-b9d8-21b418be5f30/US08263618-20120911-D00002.png)
![8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists 8-azabicyclo[3.2.1]octane compounds as MU opioid receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/43cf5f19-9dc4-434c-b9d8-21b418be5f30/US08263618-20120911-C00001.png)
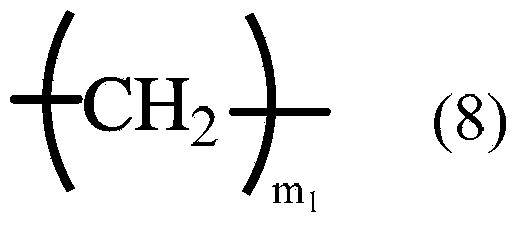


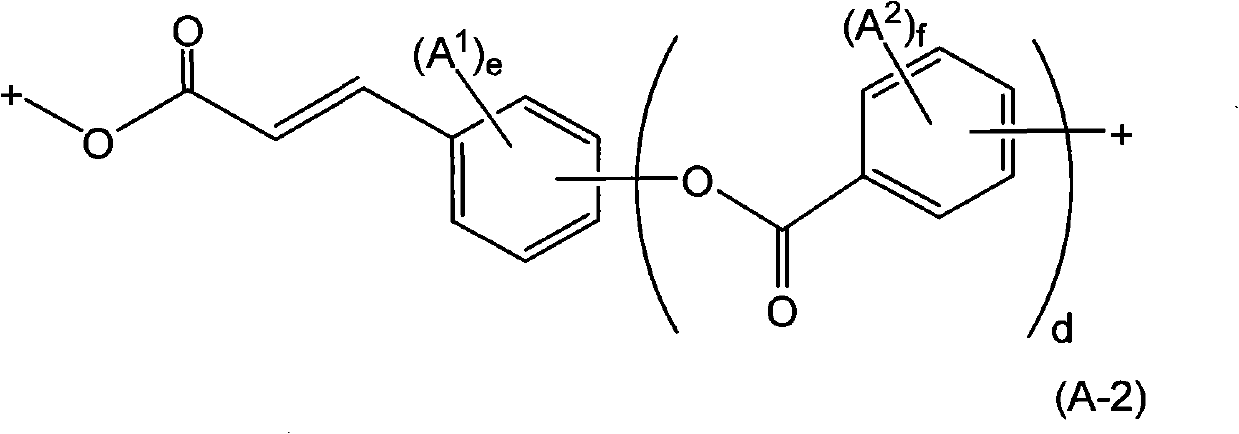
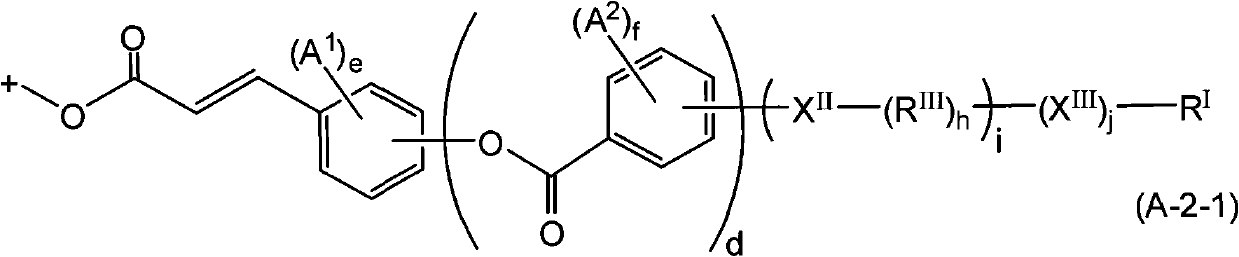
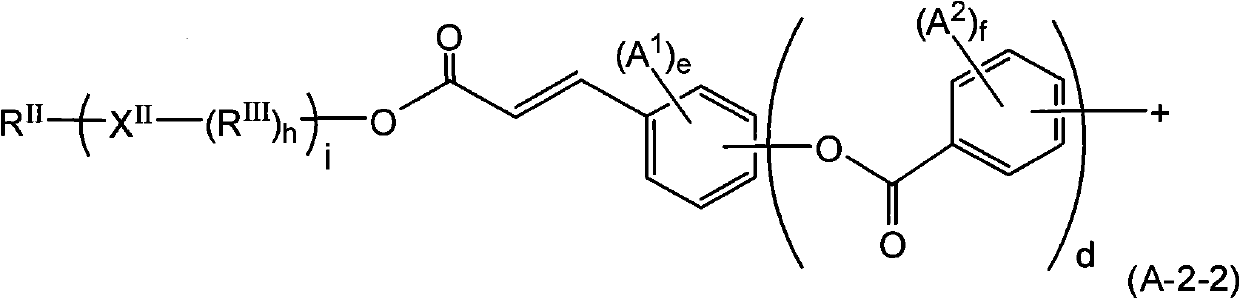
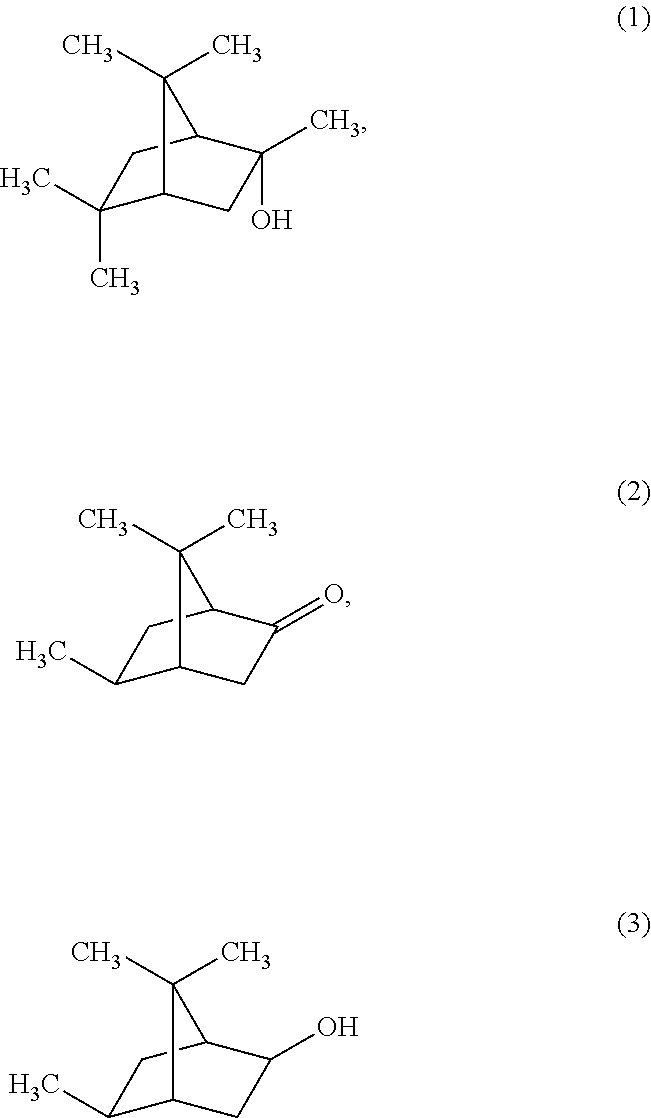
![Method for preparing (2s, 3s)-3-amino-bicyclo [2.2. 2] octane-2-formate Method for preparing (2s, 3s)-3-amino-bicyclo [2.2. 2] octane-2-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5748ab0f-4e7f-4c7e-9b82-6f7b3d1014a5/FDA0002348060530000031.png)
![Method for preparing (2s, 3s)-3-amino-bicyclo [2.2. 2] octane-2-formate Method for preparing (2s, 3s)-3-amino-bicyclo [2.2. 2] octane-2-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5748ab0f-4e7f-4c7e-9b82-6f7b3d1014a5/FDA0002348060530000032.png)
![Method for preparing (2s, 3s)-3-amino-bicyclo [2.2. 2] octane-2-formate Method for preparing (2s, 3s)-3-amino-bicyclo [2.2. 2] octane-2-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5748ab0f-4e7f-4c7e-9b82-6f7b3d1014a5/BDA0002348060540000011.png)
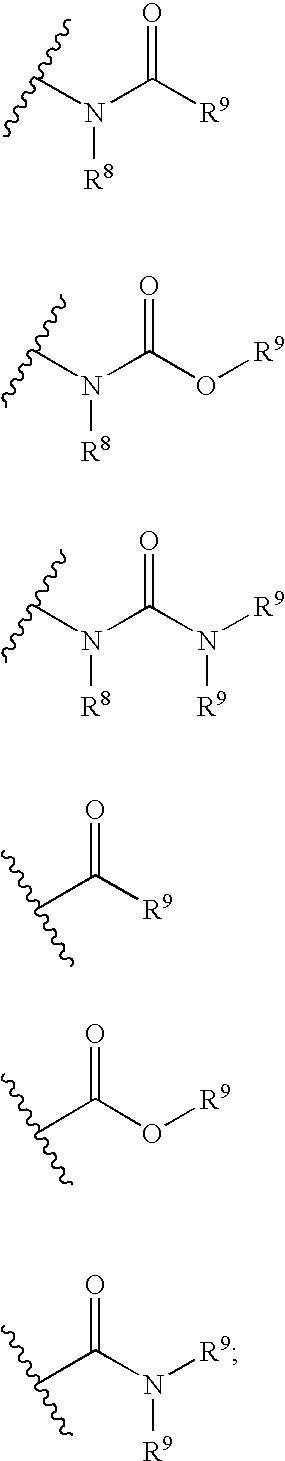
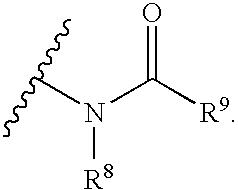
![Bicyclo[3,3,0]octane polymerizable compound Bicyclo[3,3,0]octane polymerizable compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c1c22b2-6d06-4b59-ac3f-9b5979522149/BDA0000648179170000011.PNG)
![Bicyclo[3,3,0]octane polymerizable compound Bicyclo[3,3,0]octane polymerizable compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c1c22b2-6d06-4b59-ac3f-9b5979522149/BDA0000648179170000021.PNG)
![Bicyclo[3,3,0]octane polymerizable compound Bicyclo[3,3,0]octane polymerizable compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c1c22b2-6d06-4b59-ac3f-9b5979522149/BDA0000648179170000031.PNG)
![Method for preparing (2S, 3S)-3-amino-bicyclo [2.2.2]octane-2-formate Method for preparing (2S, 3S)-3-amino-bicyclo [2.2.2]octane-2-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a19c479d-97d8-4c3b-b462-8ec5cc4987aa/FDA0002457705890000011.png)
![Method for preparing (2S, 3S)-3-amino-bicyclo [2.2.2]octane-2-formate Method for preparing (2S, 3S)-3-amino-bicyclo [2.2.2]octane-2-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a19c479d-97d8-4c3b-b462-8ec5cc4987aa/BDA0002457705900000011.png)
![Method for preparing (2S, 3S)-3-amino-bicyclo [2.2.2]octane-2-formate Method for preparing (2S, 3S)-3-amino-bicyclo [2.2.2]octane-2-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a19c479d-97d8-4c3b-b462-8ec5cc4987aa/BDA0002457705900000021.png)

![A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08b7210a-a23a-4304-adc9-1fb6a380f08b/BDA0002265038610000021.png)
![A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08b7210a-a23a-4304-adc9-1fb6a380f08b/BDA0002265038610000022.png)
![A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08b7210a-a23a-4304-adc9-1fb6a380f08b/BDA0002265038610000041.png)
![A divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application A divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18d35b47-3fd0-45d2-8f1e-4b907d838104/HDA0001025437650000011.png)
![A divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application A divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18d35b47-3fd0-45d2-8f1e-4b907d838104/HDA0001025437650000012.png)
![A divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application A divalent manganese fluorescent material based on dibromo-1,4-dipropyl-1,4-diazabicyclo[2.2.2]octane and its preparation method and application](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/18d35b47-3fd0-45d2-8f1e-4b907d838104/HDA0001025437650000021.png)
![Method for preparing (2s,3s)-3-amino-bicyclo[2.2.2]octane-2-carboxylate Method for preparing (2s,3s)-3-amino-bicyclo[2.2.2]octane-2-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/334b0e07-578e-4400-9d7a-84d8726e5a95/US20220033344A1-C00001.png)
![Method for preparing (2s,3s)-3-amino-bicyclo[2.2.2]octane-2-carboxylate Method for preparing (2s,3s)-3-amino-bicyclo[2.2.2]octane-2-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/334b0e07-578e-4400-9d7a-84d8726e5a95/US20220033344A1-C00002.png)
![Method for preparing (2s,3s)-3-amino-bicyclo[2.2.2]octane-2-carboxylate Method for preparing (2s,3s)-3-amino-bicyclo[2.2.2]octane-2-carboxylate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/334b0e07-578e-4400-9d7a-84d8726e5a95/US20220033344A1-C00003.png)

![Preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid Preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3b795afa-7e43-454f-8949-e5bd2f0bd1b1/171856DEST_PATH_IMAGE006.PNG)
![Preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid Preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3b795afa-7e43-454f-8949-e5bd2f0bd1b1/2014103053706100002DEST_PATH_IMAGE003.PNG)
![Preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid Preparation method for tert-butyl dioxy-3,8-diazabicyclo[3.2.1]octane-8-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3b795afa-7e43-454f-8949-e5bd2f0bd1b1/2014103053706100002DEST_PATH_IMAGE005.PNG)
![Preparation method and application of chiral 3, 6-diazabicyclo [3.2.1] octane derivative Preparation method and application of chiral 3, 6-diazabicyclo [3.2.1] octane derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4df623c1-6e20-4834-815d-ea5f9dadae5b/RE-HDA0003236358730000011.png)
![Preparation method and application of chiral 3, 6-diazabicyclo [3.2.1] octane derivative Preparation method and application of chiral 3, 6-diazabicyclo [3.2.1] octane derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4df623c1-6e20-4834-815d-ea5f9dadae5b/RE-HDA0003236358730000021.png)
![Preparation method and application of chiral 3, 6-diazabicyclo [3.2.1] octane derivative Preparation method and application of chiral 3, 6-diazabicyclo [3.2.1] octane derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4df623c1-6e20-4834-815d-ea5f9dadae5b/RE-HDA0003236358730000031.png)
![Preparation method and application of a chiral 3,6-diazabicyclo[3.2.1]octane derivative Preparation method and application of a chiral 3,6-diazabicyclo[3.2.1]octane derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/500ad3a9-e17f-4b40-8ced-2ffa5ee3ef5b/RE-HDA0003236358730000011.png)
![Preparation method and application of a chiral 3,6-diazabicyclo[3.2.1]octane derivative Preparation method and application of a chiral 3,6-diazabicyclo[3.2.1]octane derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/500ad3a9-e17f-4b40-8ced-2ffa5ee3ef5b/RE-HDA0003236358730000021.png)
![Preparation method and application of a chiral 3,6-diazabicyclo[3.2.1]octane derivative Preparation method and application of a chiral 3,6-diazabicyclo[3.2.1]octane derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/500ad3a9-e17f-4b40-8ced-2ffa5ee3ef5b/RE-HDA0003236358730000031.png)