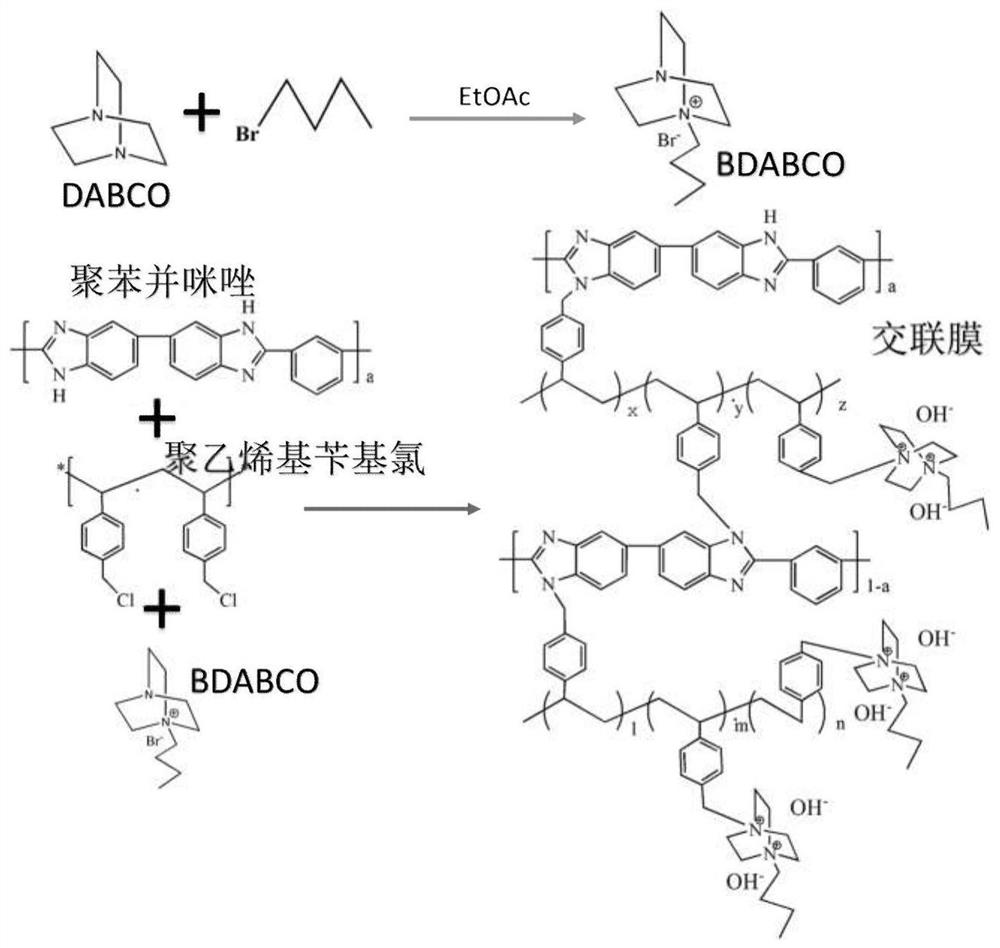
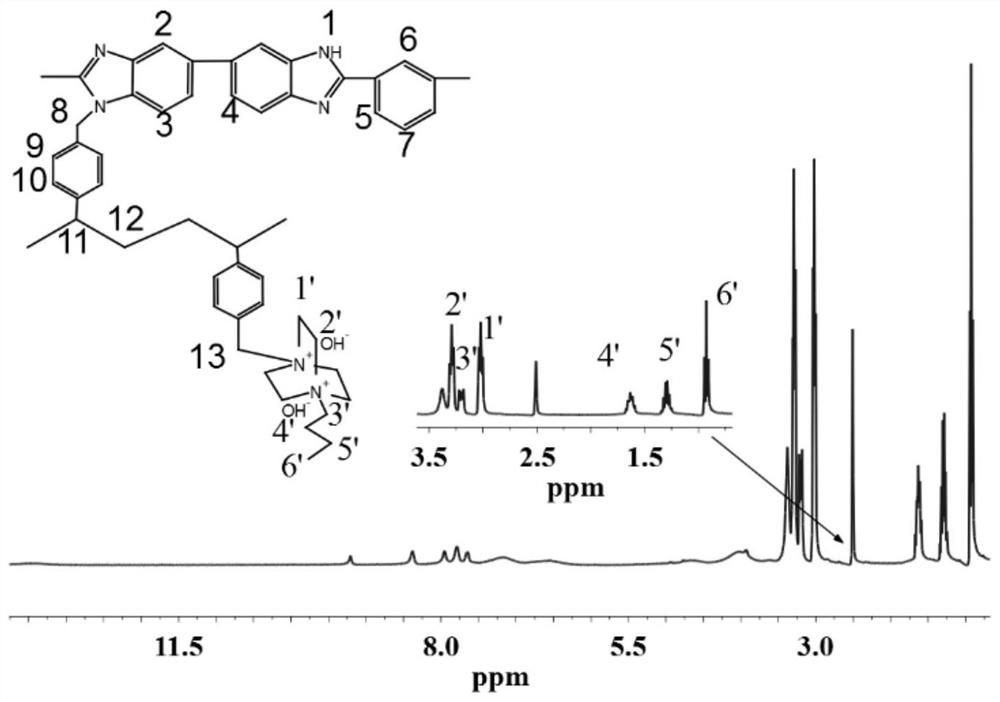
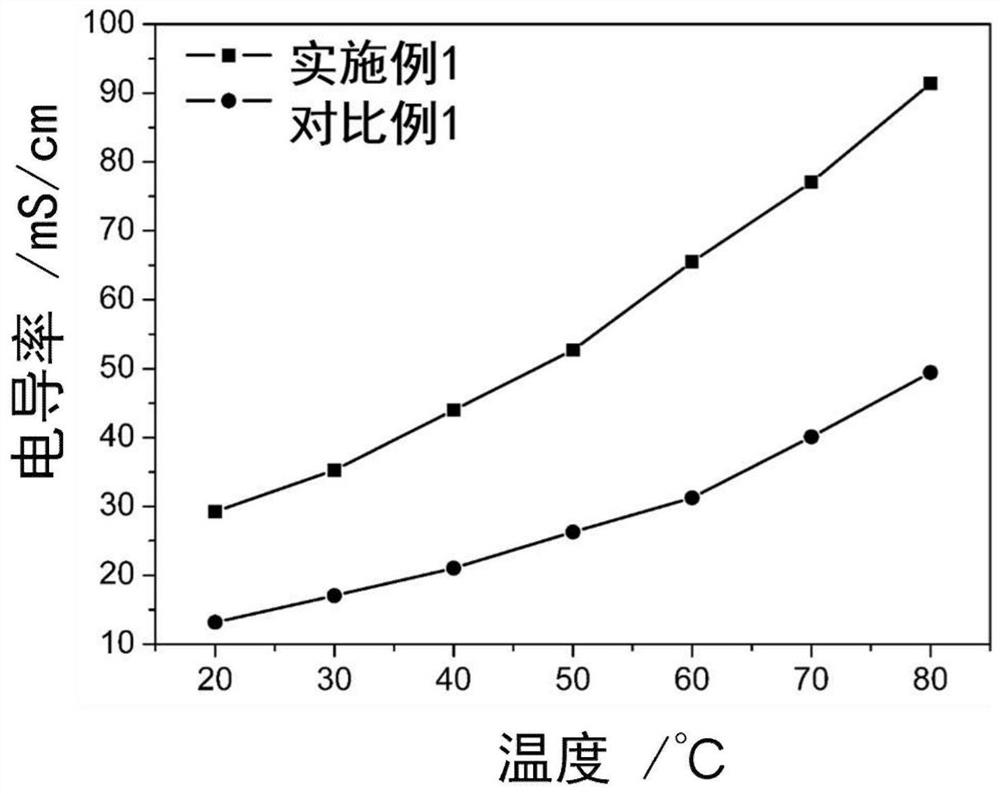
Cross-linked polybenzimidazole basic anion exchange membrane and its preparation and application
A polybenzimidazole and alkaline anion technology, applied in the field of alkaline anion exchange membrane fuel cells, can solve the problems of poor membrane dimensional stability and low conductivity of alkaline anion exchange membranes, achieve stable oxidation resistance, improve mechanical Strength, dimensional stability, and efficiency-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis process of 1-butyl-1,4-diazabicyclo[2.2.2]octane is as follows:
[0038] 1) Add 10g of 1,4-diazabicyclo[2.2.2]octane to 100 mL of ethyl acetate, stir and dissolve at room temperature, and then add n-butane bromide, and the number of moles added is 1,4-di 1.2 times of azabicyclo[2.2.2]octane, stirred at room temperature overnight;
[0039] 2) The white suspension was filtered, washed with ethyl acetate for several times, and immediately dried in a vacuum drying oven at 60 °C to obtain a white powder as 1-butyl-1,4-diazabicyclo[2.2.2] Octane.
[0040] A method for preparing a 1-butyl-1,4-diazabicyclo[2.2.2]octane-functionalized cross-linked polybenzimidazole basic anion exchange membrane, comprising the following steps,
[0041] 1) Prepare a NMP solvent solution of polybenzimidazole with a concentration of 1wt%, and add polyvinylbenzyl chloride at room temperature, wherein the molar ratio of polybenzimidazole:polyvinylbenzyl chloride is: 3:2 , and stirred...
Embodiment 2
[0057] The synthesis process of 1-butyl-1,4-diazabicyclo[2.2.2]octane is as follows:
[0058] 1) Add 15g of 1,4-diazabicyclo[2.2.2]octane into 150 mL of ethyl acetate, stir and dissolve at room temperature, and then add bromo-n-butane, and the number of moles added is 1,4-diol 1.3 times of azabicyclo[2.2.2]octane, stirred at room temperature overnight;
[0059] 2) The white suspension was filtered, washed with ethyl acetate for several times, and immediately dried in a vacuum drying oven at 60 °C to obtain a white powder as 1-butyl-1,4-diazabicyclo[2.2.2] Octane.
[0060] A method for preparing a 1-butyl-1,4-diazabicyclo[2.2.2]octane-functionalized cross-linked polybenzimidazole basic anion exchange membrane, comprising the following steps,
[0061] 1) Prepare a NMP solvent solution of polybenzimidazole with a concentration of 2wt%, and add polyvinylbenzyl chloride at room temperature, wherein the molar ratio of polybenzimidazole:polyvinylbenzyl chloride is: 1:1 , and stirr...
Embodiment 3
[0068] The synthesis process of 1-hexyl-1,4-diazabicyclo[2.2.2]octane is as follows:
[0069] 1) Add 20g of 1,4-diazabicyclo[2.2.2]octane to 300 mL of ethyl acetate, stir and dissolve at room temperature, and then add bromo-n-hexane. The moles added is 1,4-diazo 1.5 times of heterobicyclo[2.2.2]octane, stirred at room temperature overnight;
[0070] 2) The white suspension was filtered, washed several times with ethyl acetate, and immediately dried in a vacuum drying oven at 60 °C to obtain a white powder as 1-hexyl-1,4-diazabicyclo[2.2.2]octane alkyl.
[0071] A method for preparing a 1-hexyl-1,4-diazabicyclo[2.2.2]octane-functionalized cross-linked polybenzimidazole basic anion exchange membrane, comprising the following steps,
[0072] 1) Prepare a DMAc solvent solution of polybenzimidazole with a concentration of 2wt%, at room temperature, add polyvinylbenzyl chloride, wherein the molar ratio of polybenzimidazole:polyvinylbenzyl chloride is: 3:2 , and stirred at room te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com