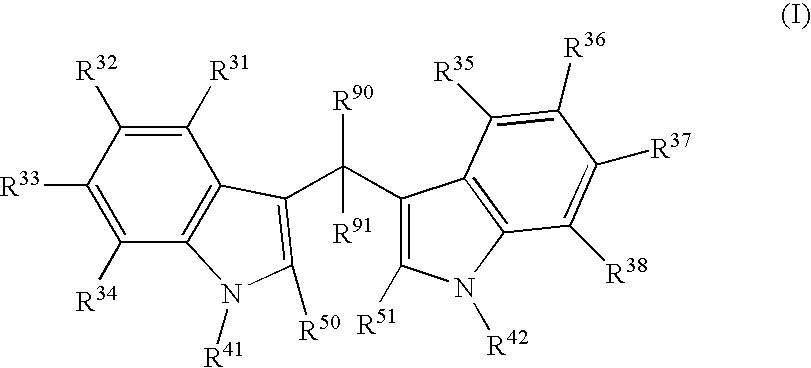
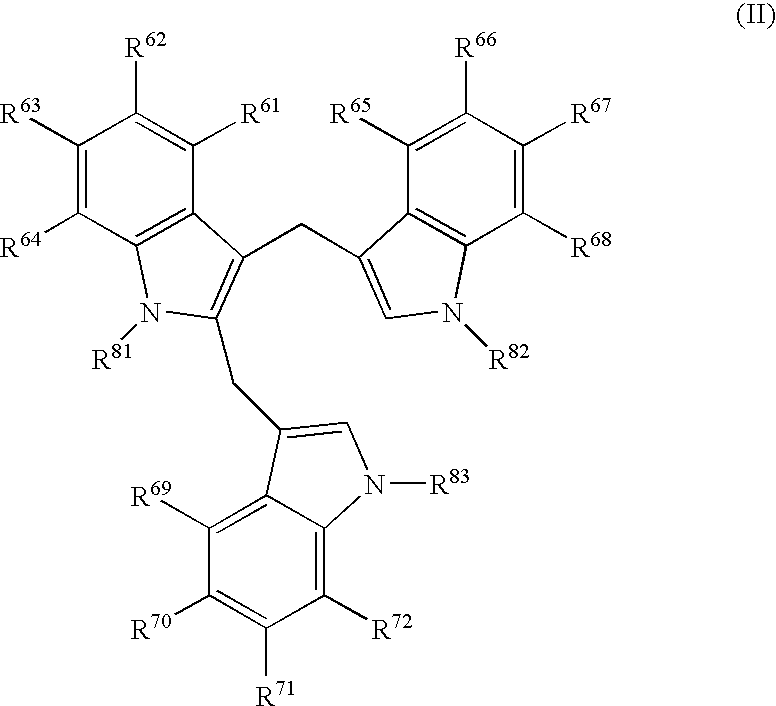
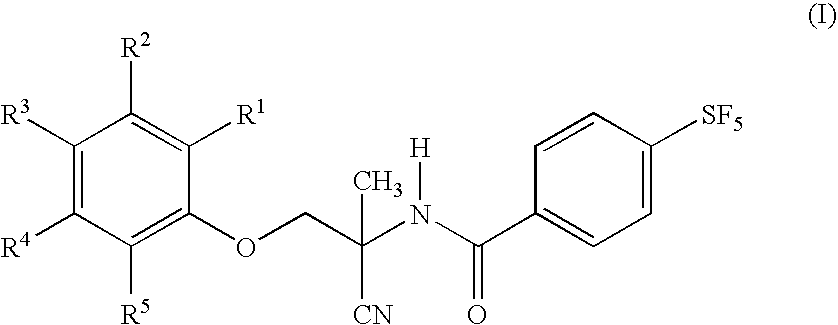
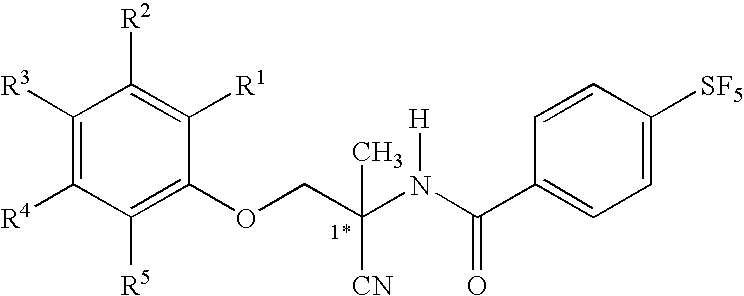
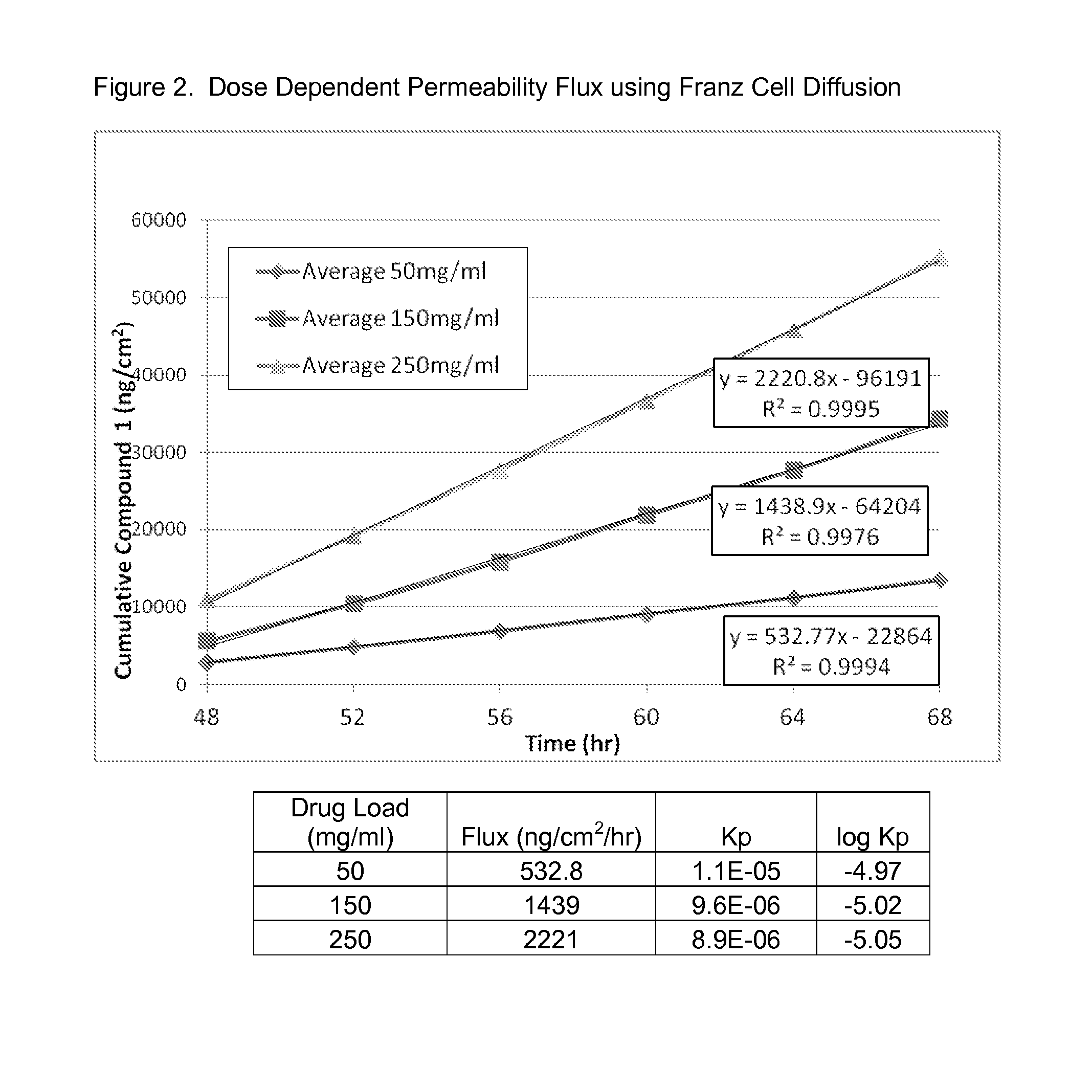
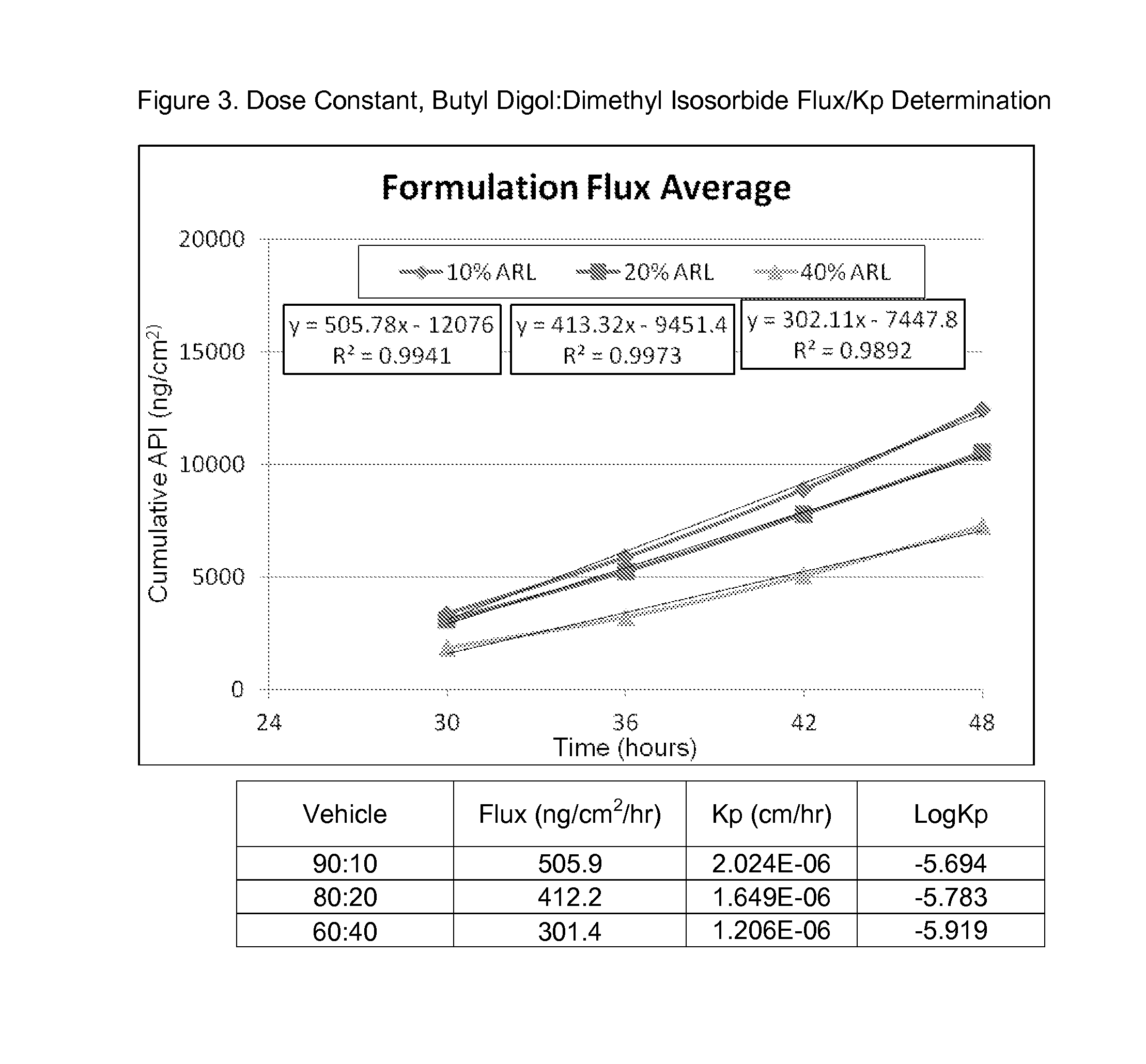
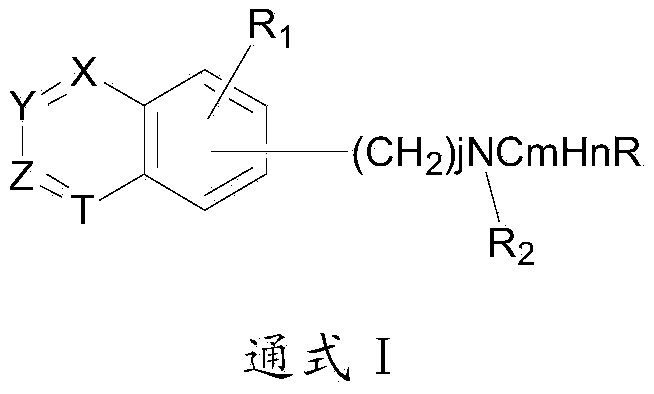
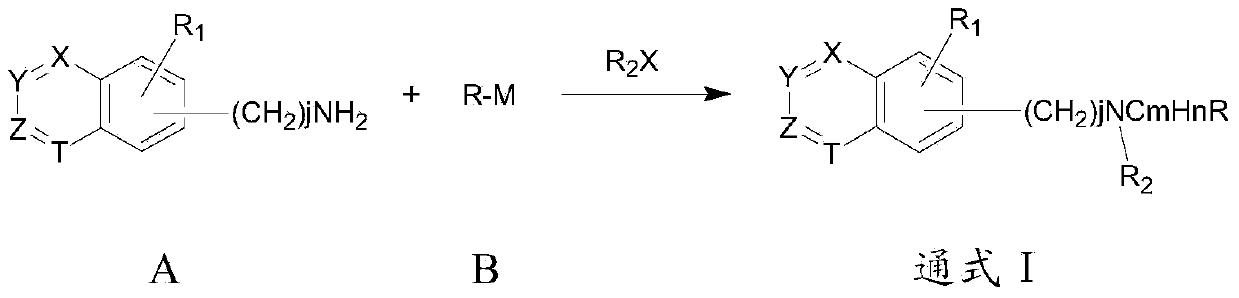
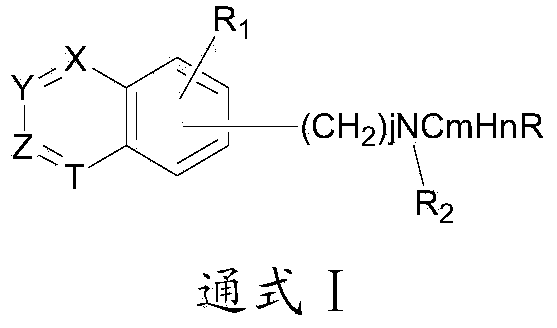
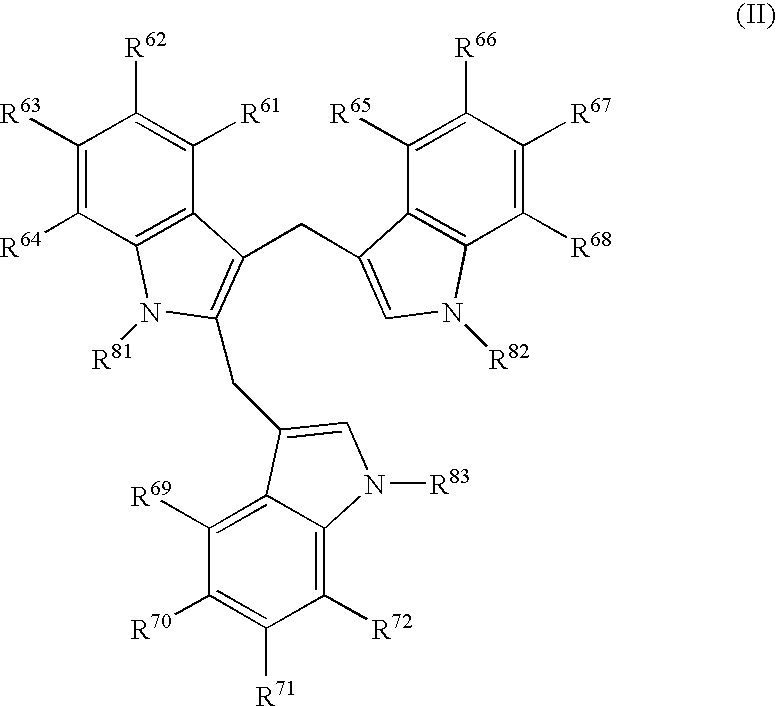
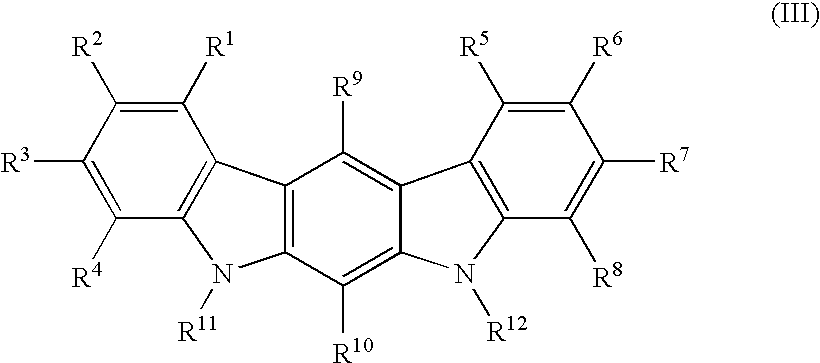
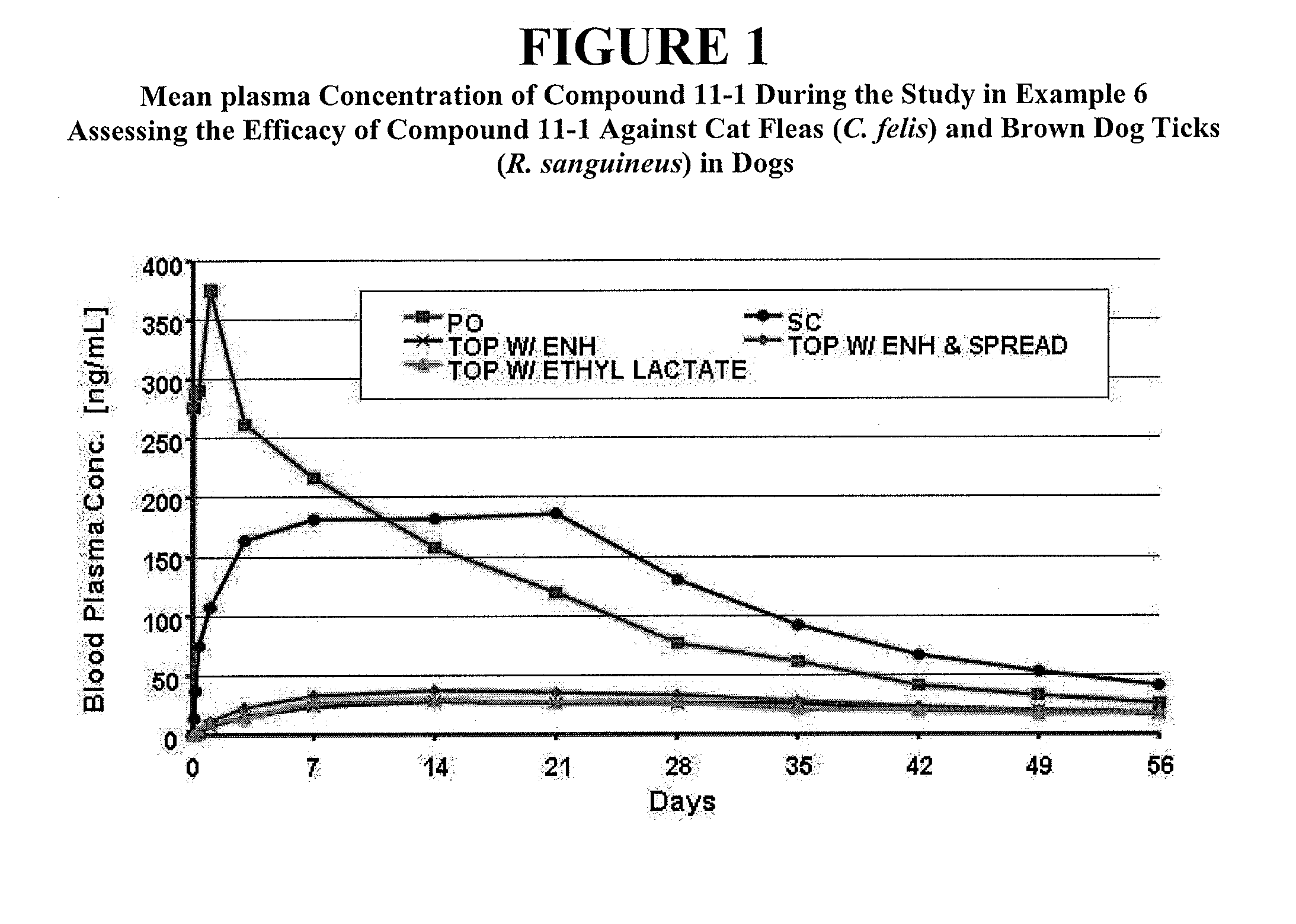
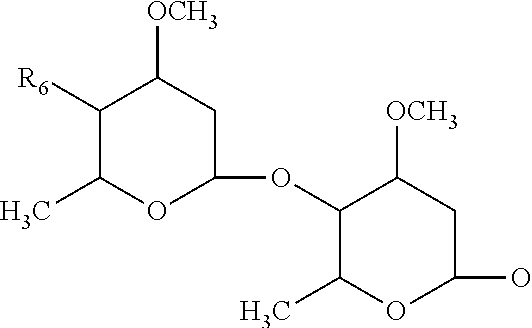
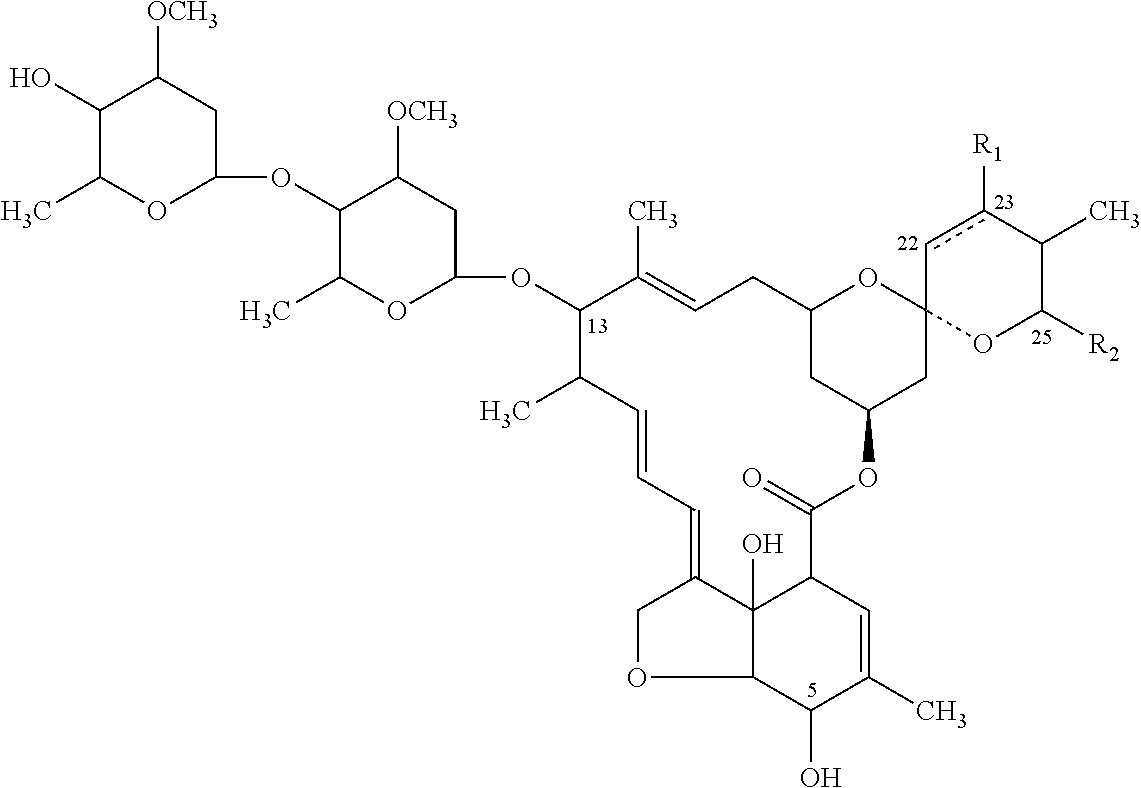
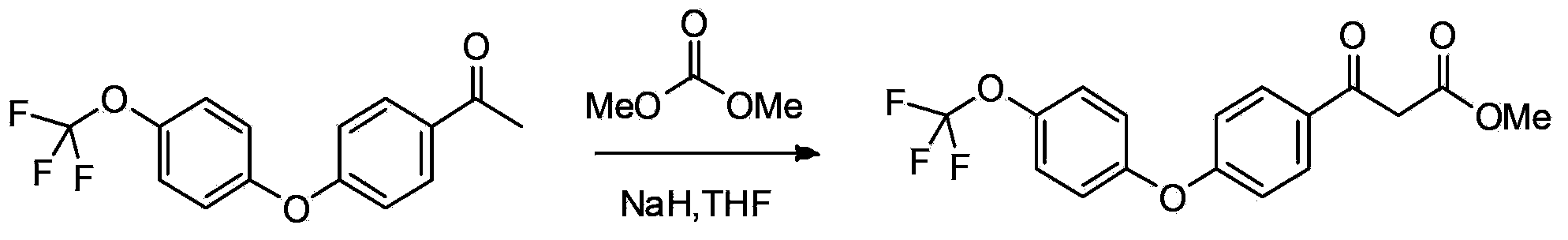
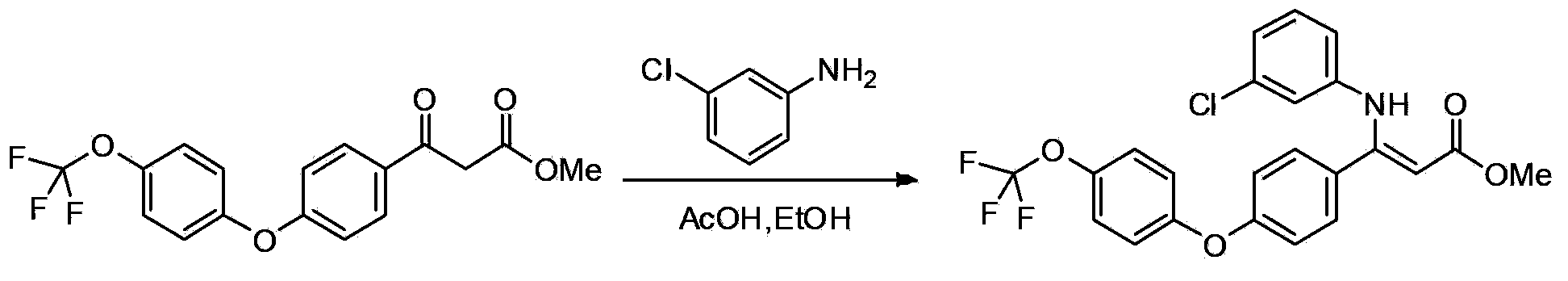
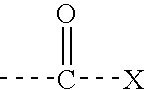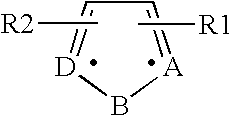Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
129 results about "Antiparasitic Drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Broad-Spectrum antiparasitics, analogous to broad-spectrum antibiotics for bacteria, are antiparasitic drugs with efficacy in treating a wide range of parasitic infections caused by parasites from different classes.
Spot-on formulations for combating parasites
InactiveUS6998131B2Effective and lasting destructionProphylaxis of parasite infestationsBiocideDead animal preservationAntiparasiticMammal
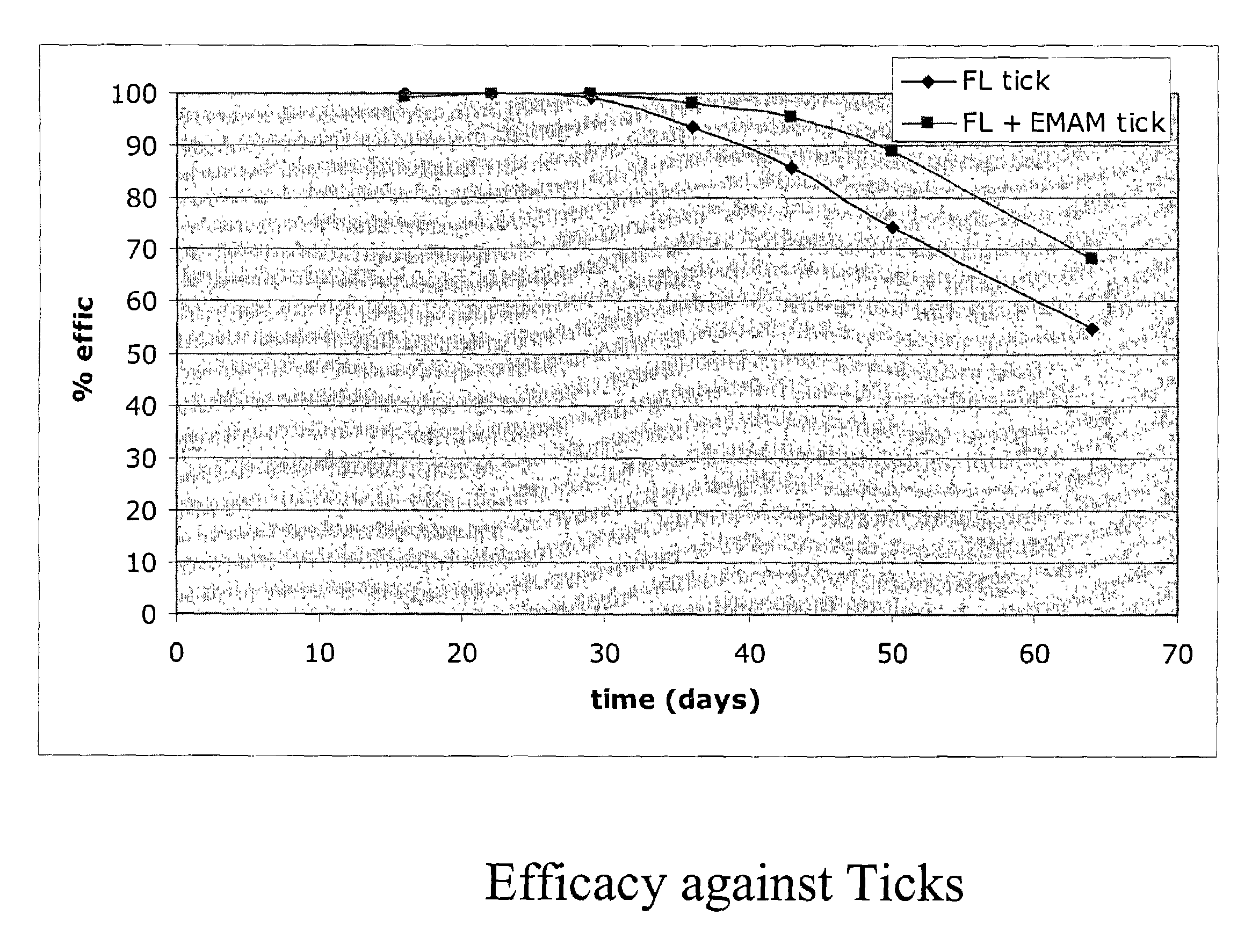
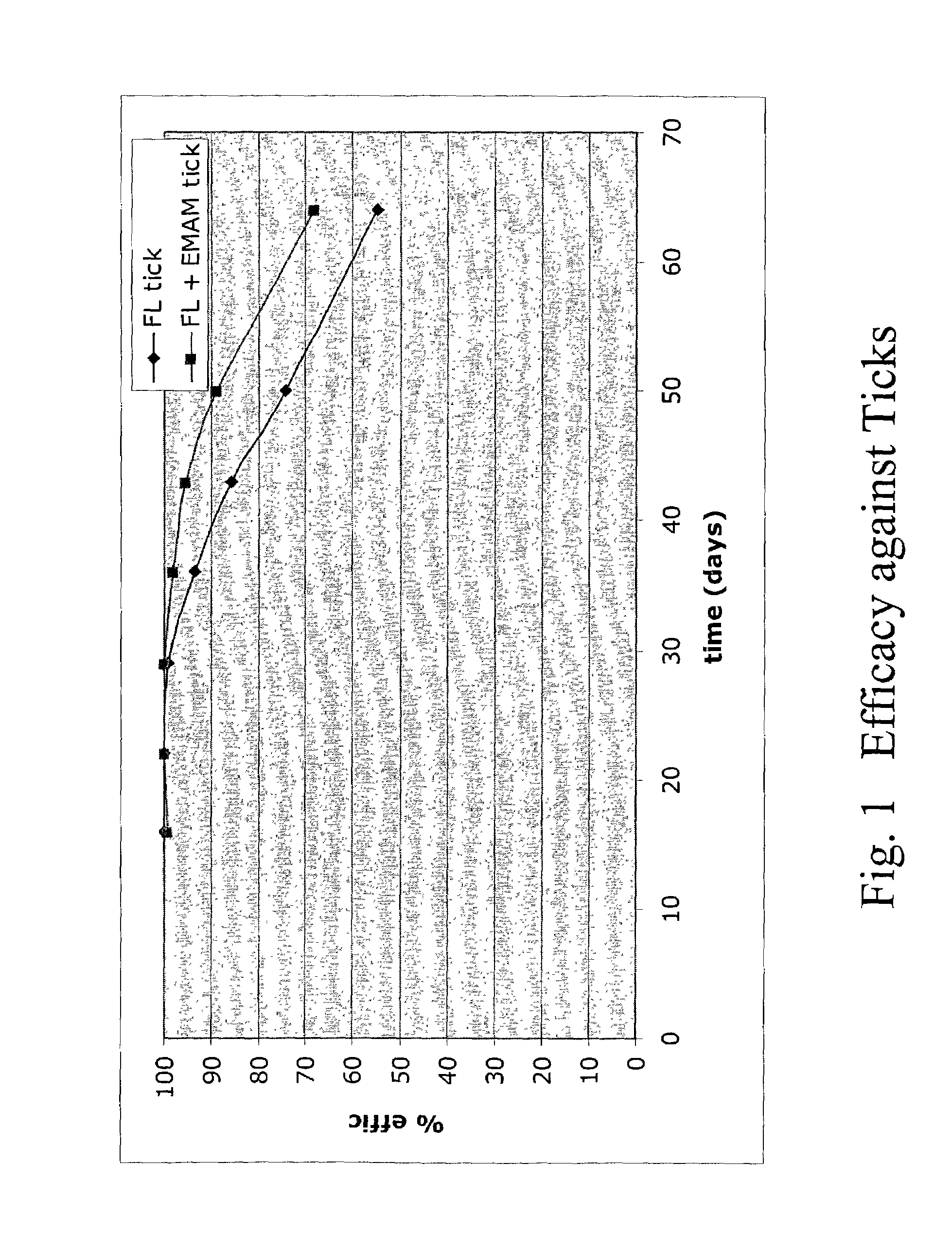
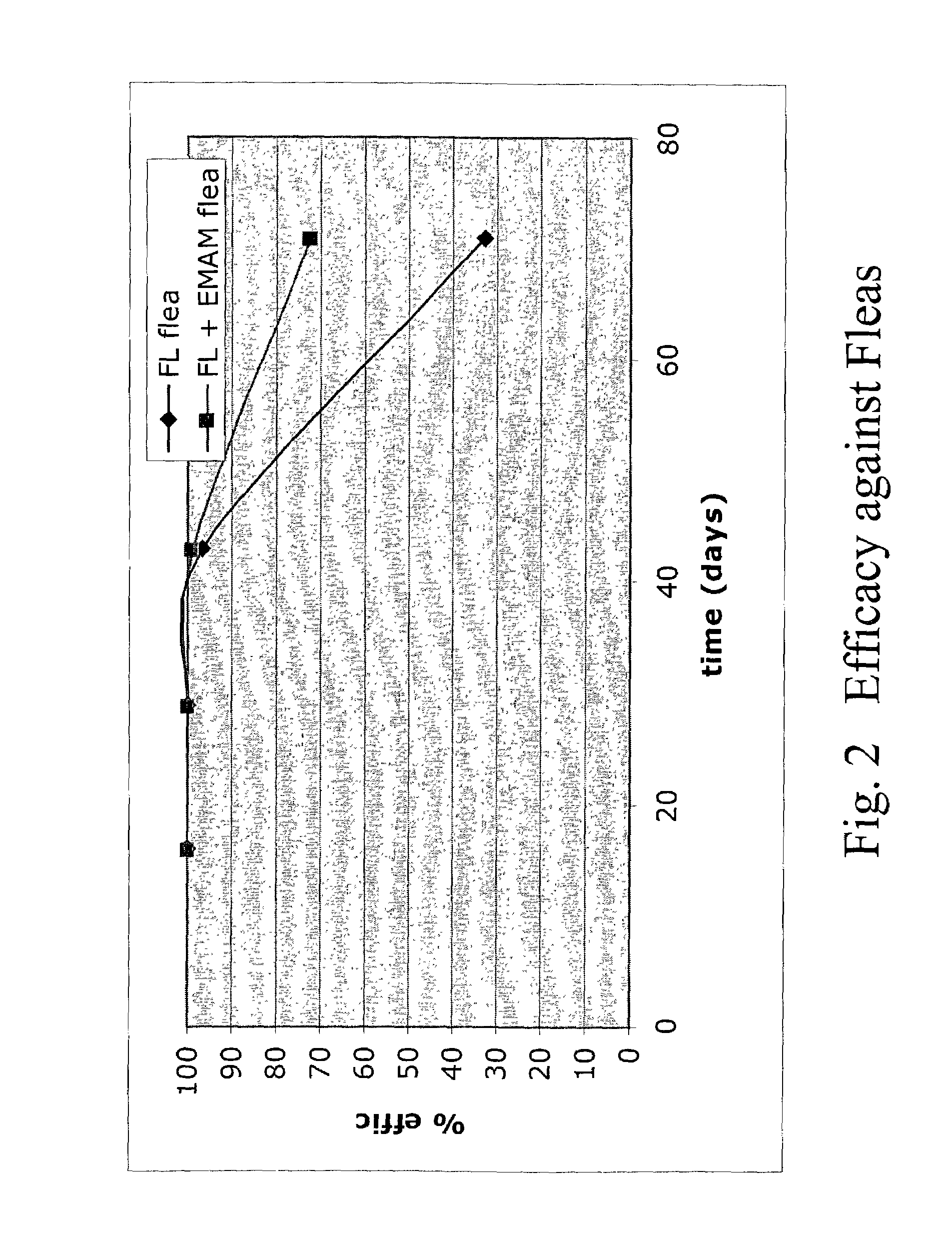
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and(B) an effective amount of emamectin;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL LTD
Pesticidal and antiparasitic compositions
This invention relates to pesticide and antiparasitic compositions for the control of pests, diseases and parasites attacking plants and animals. The compositions include, at least one chitinolytic agent or a chitinolytic activity-inducing agent, and sulfide or a sulfide-producing agent from microorganisms or chemical compounds, wherein the chitinolytic agent or the chitinolytic activity-inducing agent and sulfur or a sulfur-producing agent obtaining from microorganisms or chemical compounds are concurrently applied at a range significantly lower than any of the above-mentioned compounds, when they are individually to attain effective control.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Use of levo-ornidazole for preparing anti-parasitic-infectious drug
The present invention provides an application of levo-orinidazole in preparation of medicine for resisting parasitization. The tests show that the levo-orinidazole is superior to dextro-orinidazole and racemic orinidazole in therapeutic action for curing parasitization (special for curing infection of trichomonas vaginalis and cecal amebic infection). Said invention also relates to the conventional dosage forms for clinical application, including oral preparation, venous administration preparation and vaginal medication preparation, etc.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Pharmacological agents and methods of treatment that inactivate pathogenic prokaryotic and eukaryotic cells and viruses by attacking highly conserved domains in structural metalloprotein and metalloenzyme targets
The invention relates to the treatment of viral, bacterial, parasitic, proliferative diseases, neurodegenerative diseases, inflammatory diseases, immunological diseases, transplanted organ rejection, and diseases produced by intoxication with heavy metals. The invention relates to the use of specific metal chelating agents including, furoic acid, 2-thiophenecarboxylic acid and their derivatives, analogs and structurally related chemicals as pharmacological agents that can be used effectively to disrupt and inactivate specific transition metal ion containing zinc finger structural motifs in metalloproteins and specific transition metal ion containing catalytic sites in metalloproteinases, which in turn, inactivate the pathogenic virus, pathogenic prokaryotic or eukaryotic cells which produces disease conditions. The preparations can be administered topically or for systemic use. The preparations are novel wide-spectrum antibiotics which have antiviral, antiproliferative, antineoplastic, antiangiogenic, antibacterial, antiparasitic, antiinfective, and anti-inflammatory effects and can be used in the treatment and prevention of diseases such as AIDS, cancers, untoward angiogenesis, pulmonary anthrax, malaria, inflammatory responses, Alzheimer's disease and other diseases.
Owner:POLS LAB BIOTECH
Anti-parasitic methods and compositions utilizing diindolylmethane-related indoles
ActiveUS20100055201A1Improve developmentShorten the counting processHeavy metal active ingredientsBiocideCoccidiosisTrypanosomiasis
The present invention includes methods and compositions for the treatment and prevention of protozoal parasitic infections utilizing Diindolylmethane-related indoles. Additive and synergistic interaction of Diindolylmethane-related indoles with other known anti-parasitic and pro-apoptotic agents is believed to permit more effective therapy and prevention of protozoal parasitic infections. The methods and compositions described provide new treatment of protozoal parasitic diseases of mammals and birds including malaria, leishmaniasis, trypanosomiasis, trichomoniasis, neosporosis and coccidiosis.
Owner:BIORESPONSE
Tenvermectin derivatives and anti-parasitic function of tenvermectin derivatives
The invention relates to a series of tenvermectin derivatives obtained by modification of tenvermectin A and / or tenvermectin B show in a following formula and a purpose of the tenvermectin derivatives on an anti-parasitic aspect. The control objects of the tenvermectin derivatives relate to parasites on agriculture and forestry crops, people, birds and beasts, and aquatic products. The derivatives have the advantages of good effect, low toxicity, and environment friendliness.
Owner:深圳市天维生物药业有限公司
Anti-parasitic compounds and methods of their use
Owner:RGT UNIV OF CALIFORNIA +1
Anti-parasitic ivermectin transdermal solution used for livestock and preparation method thereof
InactiveCN101579309AFully reflect the advantages of preparationsPromote percutaneous absorptionOrganic active ingredientsPharmaceutical delivery mechanismSide effectThird generation
The invention relates to an anti-parasitic ivermectin transdermal solution used for livestock and a preparation method thereof. The anti-parasitic ivermectin transdermal solution comprises the following components respectively according to parts by volume: 5 to 15 parts of ethyl acetate, 20 to 30 parts of dimethyl sulfoxide, 2 to 6 parts of azone, 1 to 3 parts of isopropanol and 50 to 70 parts of propanediol; and the content of ivermectin is 0.3g to 1g in a solution of 100ml, and the finished product is prepared by the processes of dissolving, mixing, filtering and filling. The azone related in the prescription of the invention is a novel skin penetration enhancer, can increase the transdermal absorption of the skin to various medicines of different types, effectively avoids the pass effect of hepar, avoids peak and valley phenomena in the absorption process of the medicine, reduces the toxic or side effect caused by the correlative dosage in the medicine, prolongs the effective effect time, changes the administration area, effectively regulates the administration dosage and reduces differences among individuals. The invention has the characteristic of simple, convenient and fast use, and is more suitable for the anti-parasitic prevention and treatment of a pasturing area compared with other preparation forms.
Owner:TIANJIN BIJIA PHARMA CO LTD
A nanoemulsion medicine of eugenol and preparation method thereof
InactiveCN1875960AHigh thermodynamic stabilityGood storage stabilityAntibacterial agentsAntipyreticAntibiosisEugenol
The invention discloses the eugenic nanometer drug, comprising isopropyl microstate, croton oil, vitamin E oil, tuwen-80, spans-80 and eugenic. The drug has the advantages of improving the antibiosis, anti-inflammation, disinfection, analgesic, and ant parasitic. So the drug is green, safe and highly effective ant parasitic agent.
Owner:NORTHWEST A & F UNIV
Antiparasitic agents
Owner:PFIZER ANIMAL HEALTH UK 1 LIMITED
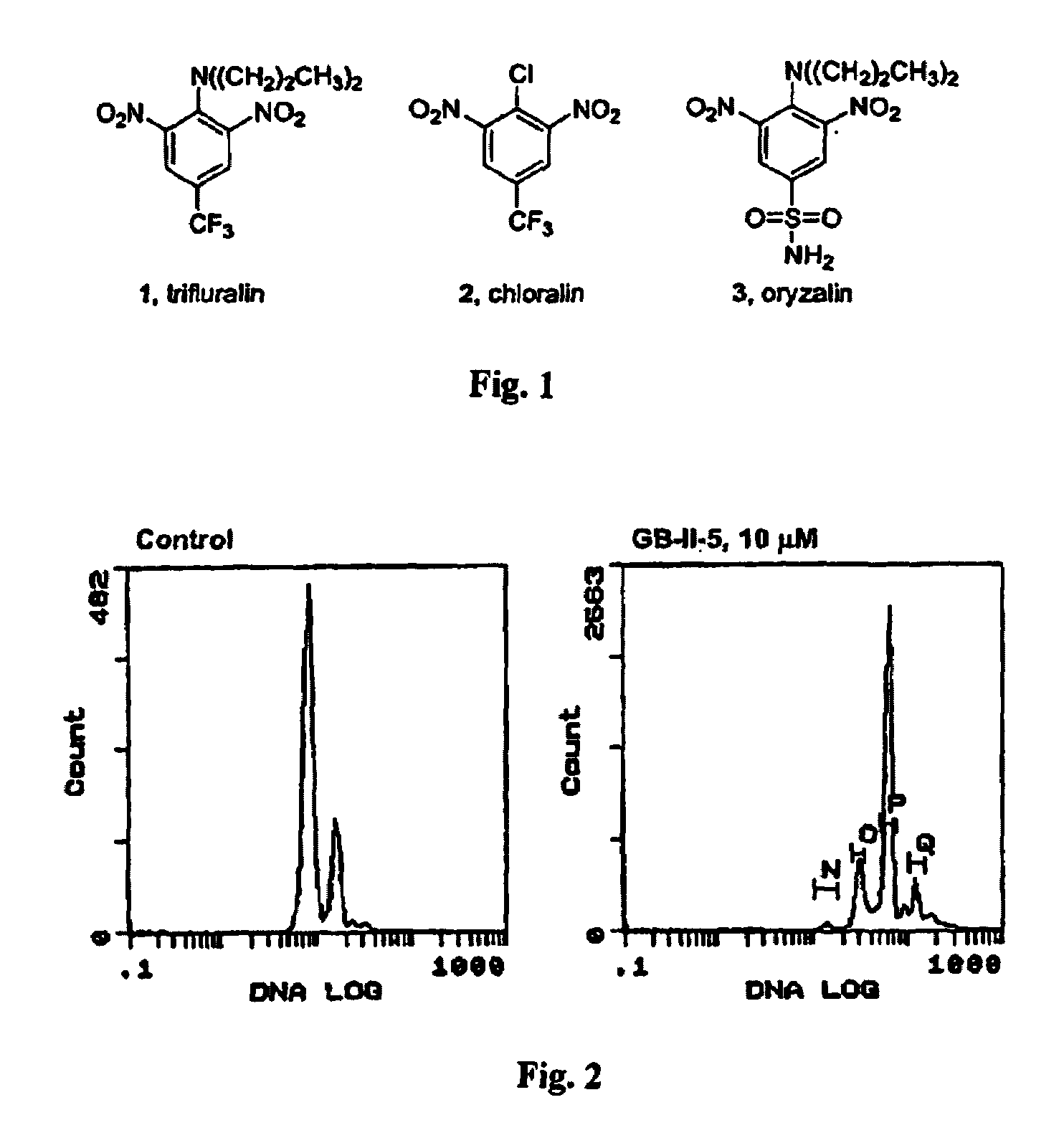
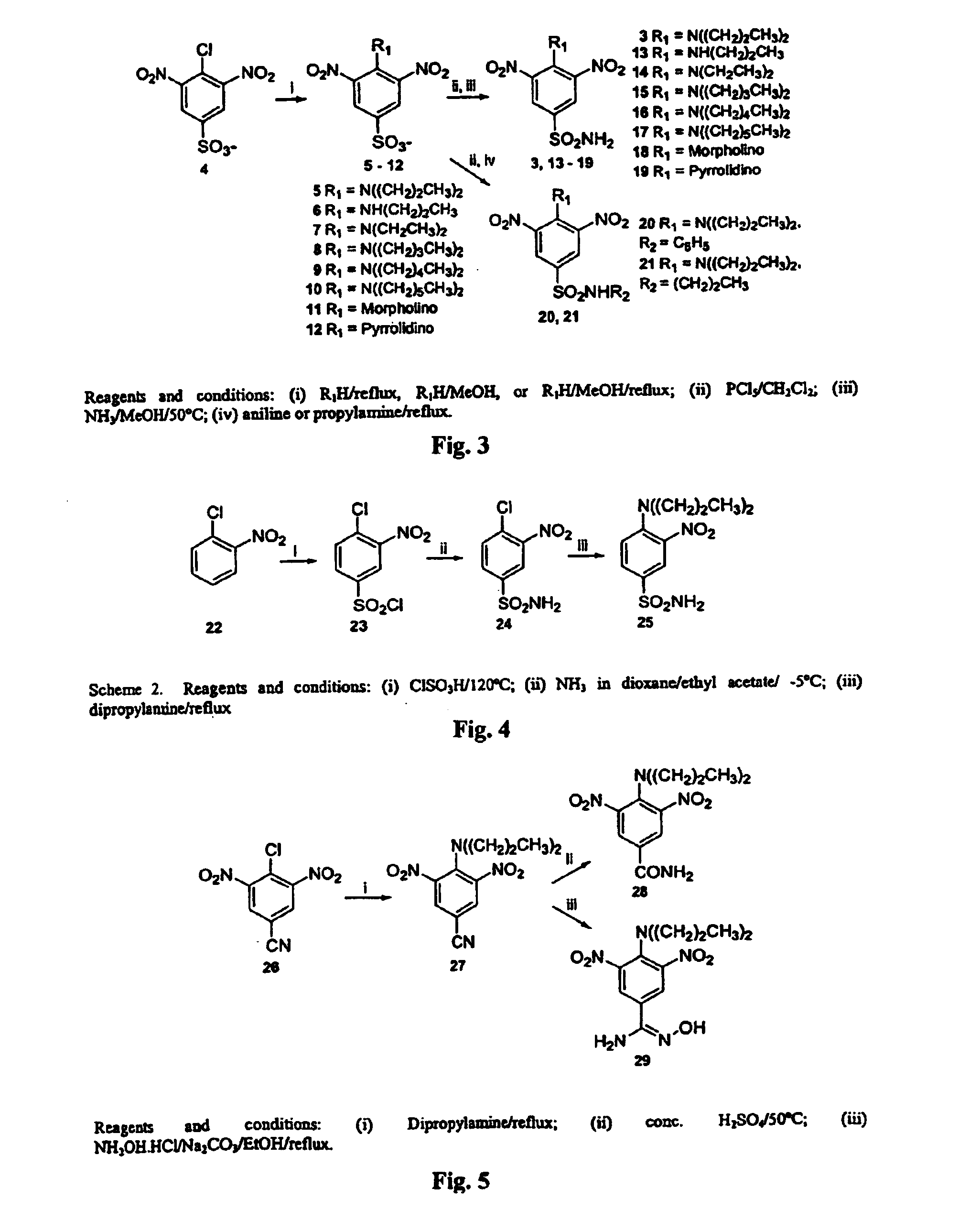
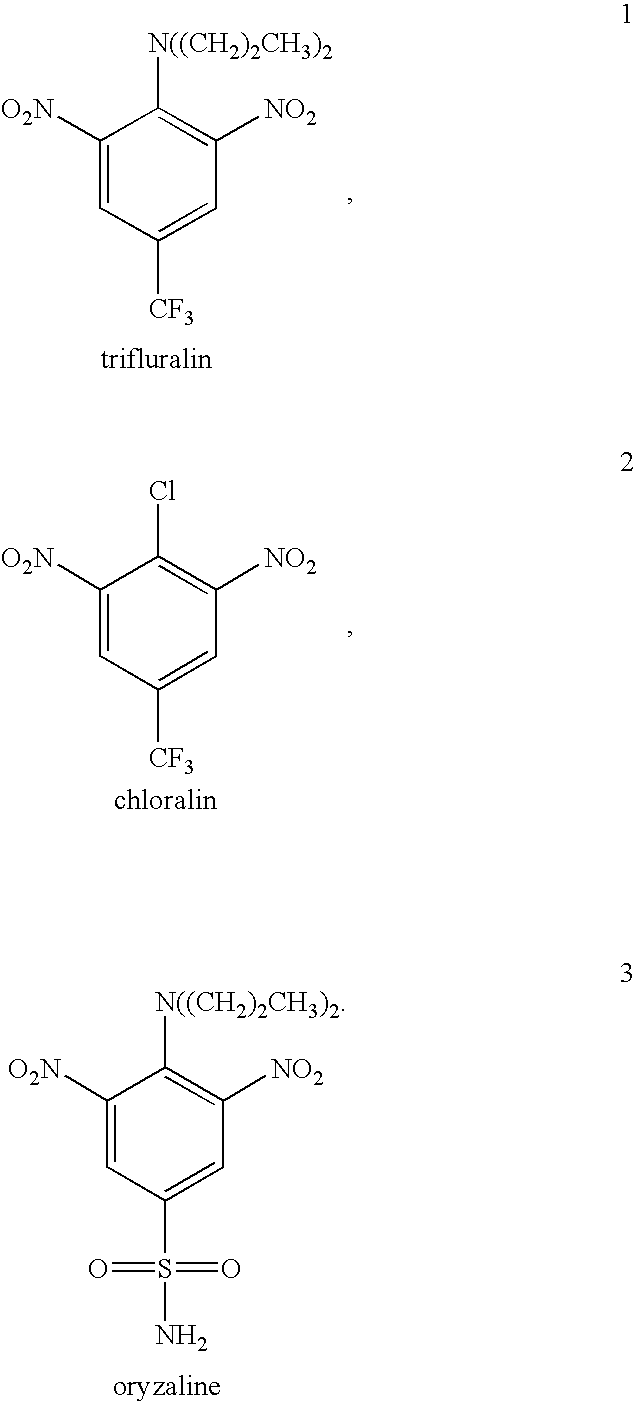
Antileishmanial dinitroaniline sulfanomides with activity against parasite tubulin
Dinitroaniline compounds useful for the treatment of diseases caused by parasitic protozoa in subjects in need of such treatment. The compounds are particularly useful in the treatment of leishmaniasis. The compounds are preferably less cytotoxic to normal cells than oryzalin. Also provided are methods of treating subjects having diseases caused by parasitic protozoa, preferably humans. The method comprising administering a therapeutically effective amount of a dinitroaniline compound of the present invention to a subject in need of such treatment
Owner:THE OHIO STATES UNIV +1
Dog and cat pill containing antiparasitic drug and attractant
The present invention provides a dog and cat pill containing an antiparasitic drug and an attractant. The pill has a coating layer structure, and cannot have a coating layer, wherein the pill with the coating layer structure comprises a drug-containing pill core and a drug-free coating layer coated on the surface of the pill core, one or a plurality of the coating layers can exist, the coating layer at least contains an attractant or a flavoring agent, the pill at least contains an antiparasitic drug or at least contains a microcapsule containing an antiparasitic drug, the antiparasitic drug accounts for 0.05-20% of the weight of the pill, the attractant accounts for 1-65% of the weight of the pill, the pill at least contains an adhesive, the adhesive accounts for 4-45% of the weight of the pill, and the pill can further contain a disintegrant, a preservative, an antioxidant, a coloring agent, a wetting agent and other additives. According to the present invention, the pill has characteristics of easy preparation, low cost and good palatability, wherein the active feeding rate of dog and cat on the pill can achieve more than 90%.
Owner:中农华威生物制药(湖北)有限公司
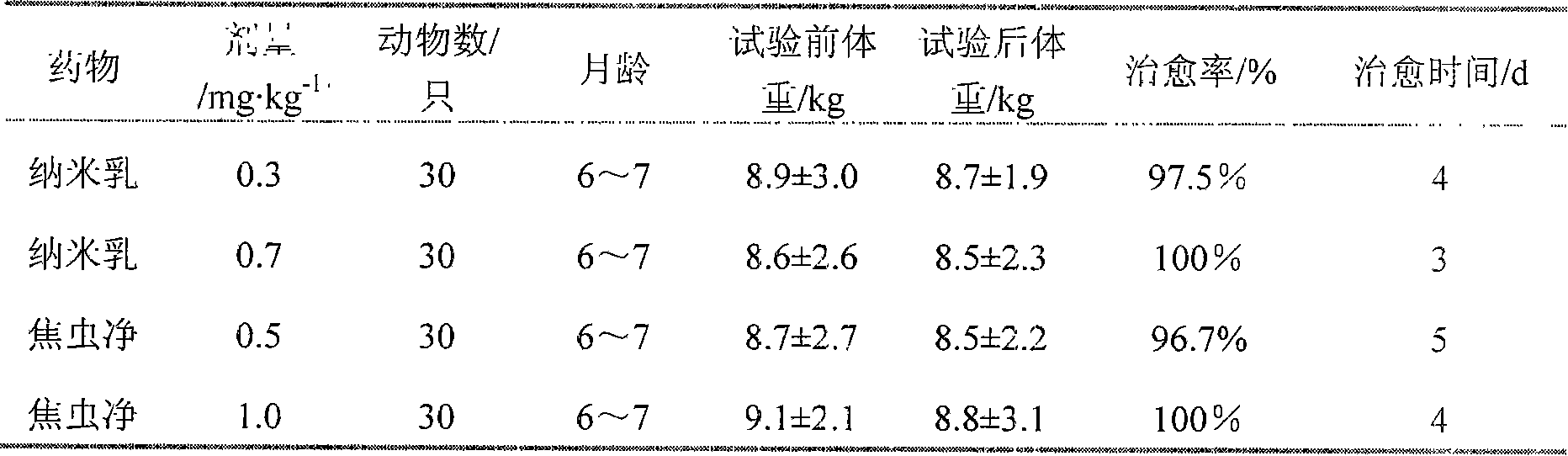
Broad-spectrum antiparasitic drug nano-emulsion and preparation method thereof
InactiveCN105853454AHigh thermodynamic stabilityGood storage stabilityOrganic active ingredientsPharmaceutical non-active ingredientsFenbendazolePreservative
The invention provides a broad-spectrum antiparasitic drug nanoemulsion and a preparation method thereof, which comprises the following components by weight percentage: 5-20% of fenbendazole, 0.3%-3% of ivermectin, and 0.5% of oil phase %-7%, co-surfactant 2%-12%, co-solvent 2%-5%, surfactant 10%-30%, deionized water 3-40%. The antiseptic property obtained by the invention is better, and it will not be mildewed or discolored after being placed at room temperature for three months. No additional preservatives are required, and the shelf life is long.
Owner:高艳春
Artesunate nanoemulsion drug composition and preparation method thereof
InactiveCN101623255AHigh thermodynamic stabilityGood storage stabilityElcosanoid active ingredientsPharmaceutical non-active ingredientsSide effectAcute toxicity testing
The invention discloses an Artesunate nanoemulsion drug that is an O / W (oil-in-water type) nanoemulsion system consisting of ethyl oleate, Tween-80, normal butanol, ultrapure water and Artesunate. The nanoemulsion drug effectively overcomes the first-pass effect of traditional tablets in liver, causes no sense of pain in injection and greatly improves the insecticidal and helminthic effect of original Artesunate. Meanwhile, the nanoemulsion system has high drug-loading rate, is stable when stored, has certain slow release function compared with merchant Artesunate injection, and further is convenient for administration, thus greatly improving the bioavailability. Besides, shown by acute toxicity tests and clinical drug effect tests on mice, the nanoemulsion has no obvious toxic or side effect and is a safe, reliable and high-efficiency nano level antiparasitic drug.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Suspension containing albendazole, and preparation method thereof
The invention belongs to the technical fields of antiparasitic drugs for animals, and preparation methods thereof, and concretely relates to a suspension containing albendazole, and a preparation method thereof. The above preparation comprises an albendazole bulk drug, a suspending aid, a wetting agent, an antiseptic and a flocculating agent. The suspension containing albendazole is prepared through a dispersion process, and is an off-white sticky water suspension. The suspension containing albendazole developed in the invention has the advantages of good stability, good palatability, long elimination half life, and high bioavailability, and can be used by animals as an oral preparation.
Owner:QINGDAO KDN BIOTECH
Molecules that influence pathogen resistance
The functions of IFNgamma-induced GTPases of the IGTP-family as strong anti-infective agents, and more particularly a strong anti-parasite and / or anti-bacterial agents, are disclosed. These molecules (in both protein and nucleic acid forms) are effective to modify anti-microbial e.g., anti-bacterial and / or anti-parasitic) immune responses in a subject, to prevent or inhibit replication or infectivity of microbe, to treat microbial diseases, and to detect susceptibility of a subject to microbial infection. This invention also provides kits and compounds useful in such methods. Also provided are transgenic non-human animals in which IGTP-family member gene expression has been altered, and the use of such animals to screen for anti-microbial agents.
Owner:THE GOVERNMENT OF THE US ASREPRESENTED BY THE SEC OF THE DEPARMENT OF HEALTH & HUMAN SERVICES
Compounds and application thereof in preparation of anti-parasitosis drugs
InactiveCN104761524AEnhanced inhibitory effectEasy to separate and purifyOrganic chemistryAntiparasitic agentsSide effectParasitic disease
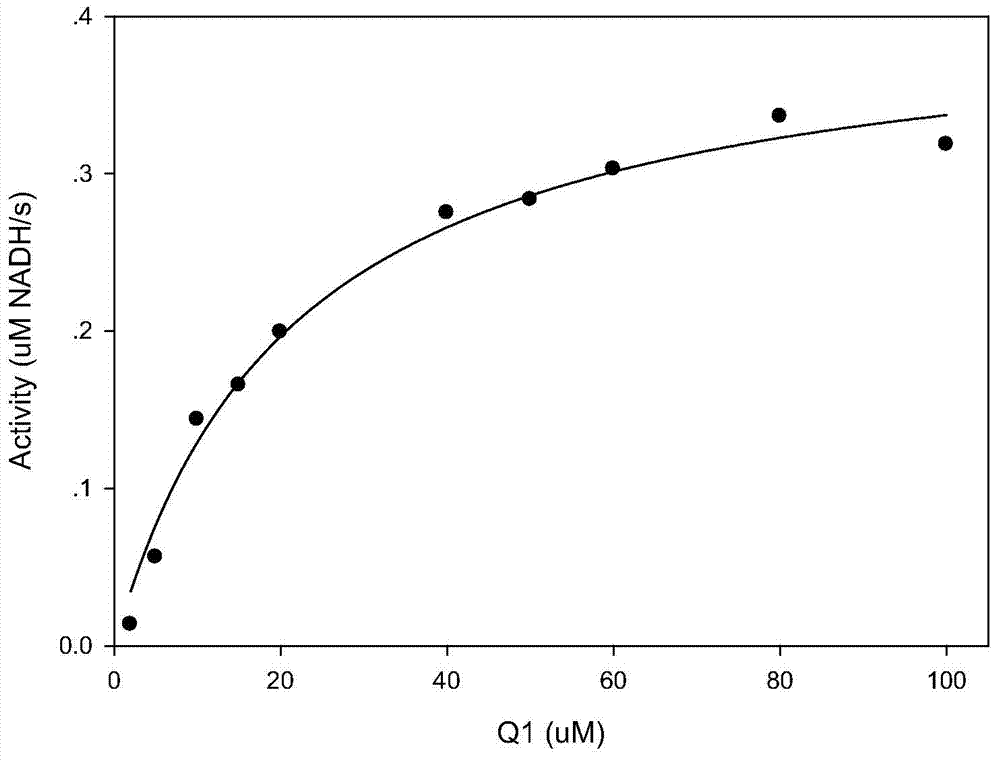
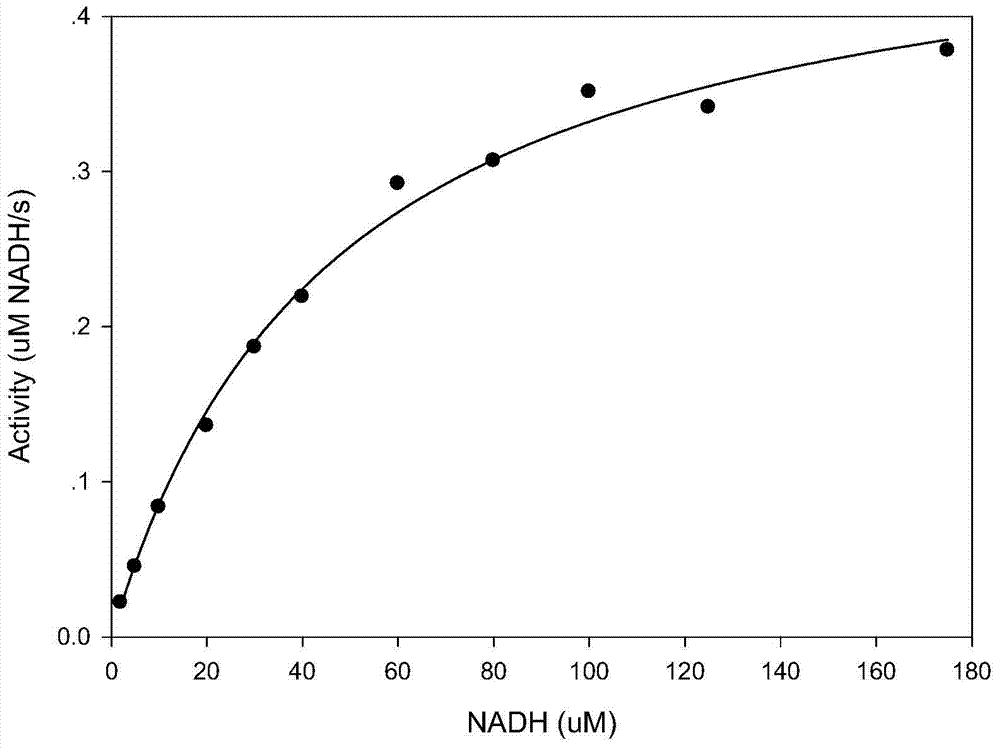
The invention discloses compounds and an application thereof in preparation of anti-parasitosis drugs. The compounds have a structural formula represented by the formula I or the formula II. The compounds provided by the invention have rich functional group diversity and modificability, and the products are relatively easy to separate and purify. The compounds provided by the invention have quite good inhibitory effect on pernicious malaria NDH protein activity and are new anti-malarial action targets and mechanisms, and thus the compounds also have quite good inhibitory effect on many plasmodium species and strains generating resistance to traditional drugs, thereby providing a new breakthrough for current increasingly-serious plasmodium drug resistance aspect. At the same time, the plasmodium NDH and human NADH oxidordeuctase belong to different species, so that the compounds can be expected to produce fewer side effects on human. In summary, the compounds have broad development and application prospects.
Owner:TSINGHUA UNIV
Immunization and/or treatment of parasites and infectious agents by live bacteria
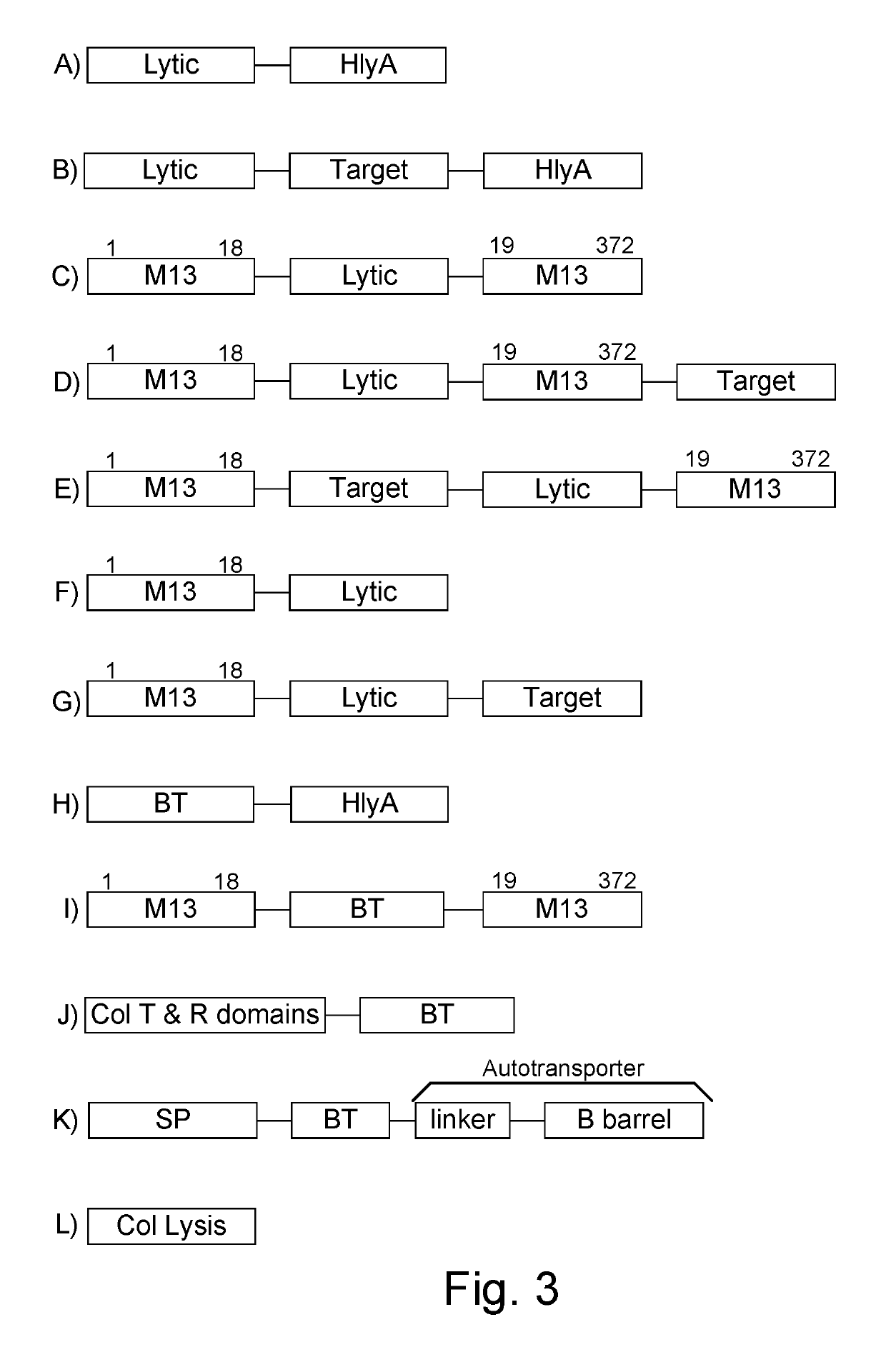
ActiveUS10364435B1Reducing or eliminating the targeted parasite, infectious diseaseSsRNA viruses negative-sensePeptide/protein ingredientsBacteroidesLytic peptide
Chimeric proteins are expressed, secreted or released by a bacterium to immunize against or treat a parasite, infectious disease or malignancy. The delivery vector may also be attenuated, non-pathogenic, low pathogenic, or a probiotic bacterium. The chimeric proteins include chimeras of, e.g., phage coat and / or colicin proteins, bacterial toxins and / or enzymes, autotransporter peptides, lytic peptides, multimerization domains, and / or membrane transducing (ferry) peptides. The active portion of the immunogenic chimeric proteins can include antigens against a wide range of parasites and infectious agents, cancers, Alzheimer's and Huntington's diseases, and have enhanced activity when secreted or released by the bacteria, and / or have direct anti-parasite or infectious agent activity. The activity of the secreted proteins is further increased by co-expression of a protease inhibitor that prevents degradation of the effector peptides. Addition of an antibody binding or antibody-degrading protein further prevents the premature elimination of the vector and enhances the immune response.
Owner:BERMUDES DAVID GORDON
Preparation method and application of polyclonal antibody against CCR12 of Epinephelus coioides
ActiveCN107312092AImmunoglobulins against cell receptors/antigens/surface-determinantsReceptors for cytokines/lymphoines/interferonsPolyclonal antibodiesEpinephelus bruneus
The invention provides a preparation method and application of a polyclonal antibody against CCR12 of Epinephelus coioides. Currently, three XCR1-like chemokine receptors are discovered in fish, i.e., XCR1, XCR1L and CCR12, wherein XCR1L and CCR12 are only discovered in the genomes of fish, but little research is conducted on the functions of XCR1L and CCR12; and so far, in-vitro expression of CCR12 from fish has not been reported, no one has prepared a polyclonal antibody against CCR12 of Epinephelus coioides, and application of the polyclonal antibody against CCR12 has not been reported, either. To further reveal the migration or propagation of Epinephelus coioides CCR12 positive cells in tissue with parasitic infections and to identify the types of the CCR12 positive cells, a recombinant protein of the N-terminal region and three extracellular regions the CCR12 is expressed via a prokaryotic system and purified, and a rabbit anti-CCR12 recombinant protein polyclonal antibody is eventually prepared for the first time; and the antibody can specific recognize the CCR12 protein of Epinephelus coioides and mark cells in blood, so a foundation is laid for further marking of the CCR12 positive cells and research on parasitic infection resistance of the CCR12 positive cells.
Owner:SOUTH CHINA AGRI UNIV
Preparation method for emulsifiable oily injection containing abamectin drugs
ActiveCN104095812APromote absorptionResidue reductionAntibacterial agentsOrganic active ingredientsAvermectinFast release
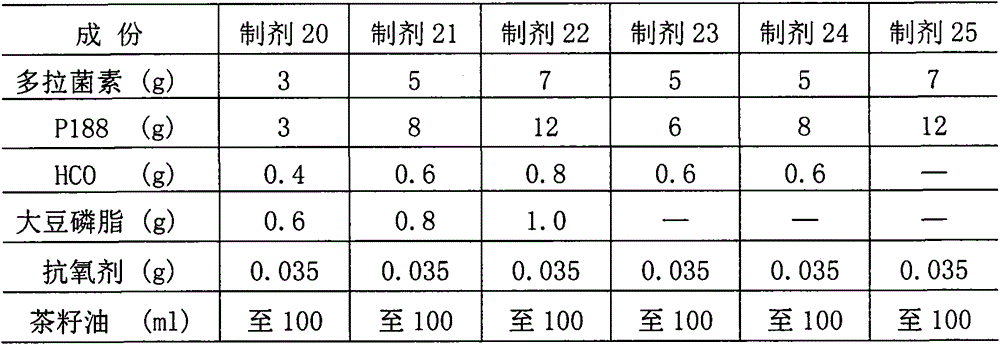
The invention relates to a preparation method for an emulsifiable oily injection containing abamectin drugs. According to the preparation method, poloxamer 188 is combined with an oily medium for preparing an injection containing abamectin antiparasitic drugs; the injection further comprises balanced amounts of hydrogenated castor oil, soya bean lecithin, a local analgesic and an antioxygen, and is mainly characterized in that the injection is emulsified in water (or body fluids); an emulsion droplet-oil mixed system is formed at the injection part. The injection has both the fast-release effect and the long-term effect, and is regarded as the emulsifiable oily injection.
Owner:中农华威制药股份有限公司
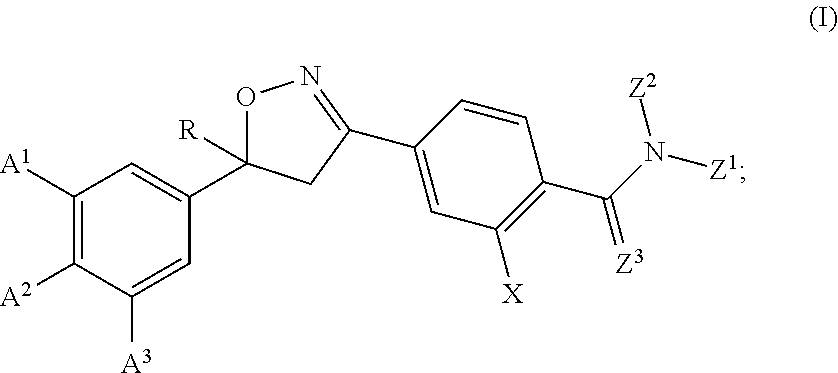
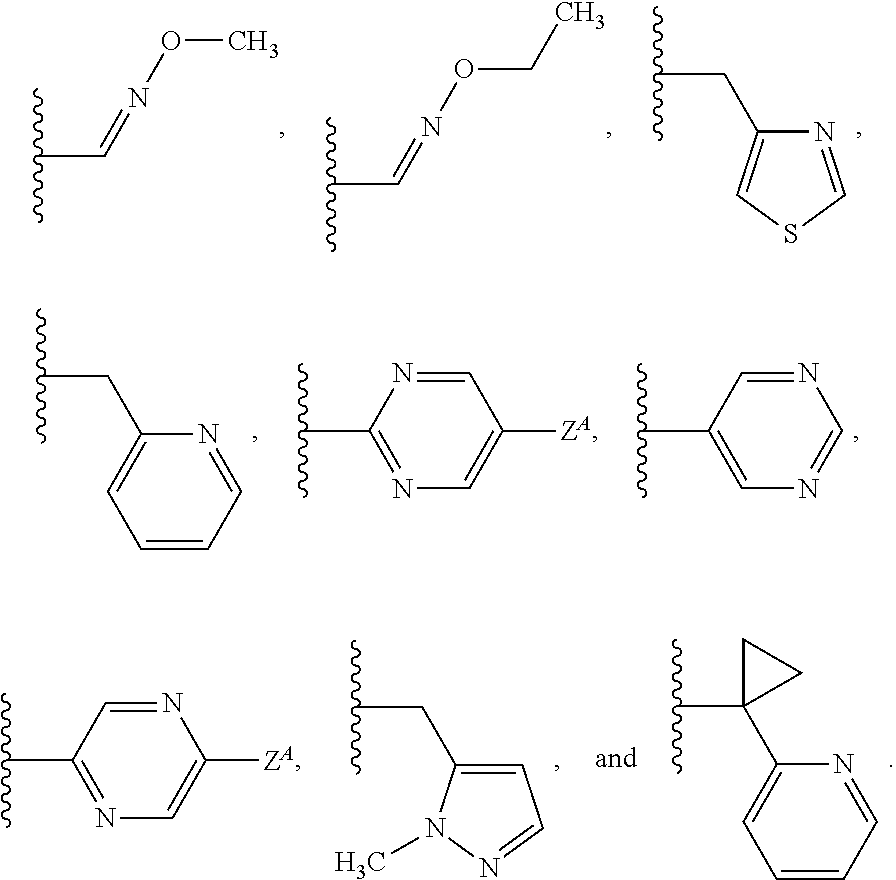
Long-acting spiro-isoxazoline antiparasitic compositions
InactiveUS20160081986A1Improved long-acting topical compositionOrganic active ingredientsBiocideAntiparasiticAntioxidant
The invention describes a long-acting composition comprising a spiro-azetidine isoxazoline of Formula (1) or (2) wherein R1a, R1b, R1c and R2 are as described herein, and stereoisomers thereof. The composition is a veterinary composition and also comprises a glycol ether and at least one veterinarily acceptable solvent, and optionally, at least one precipitation inhibitor, antioxidant and additional veterinary agent, and any mixture thereof. The invention also includes a method of treating an animal with a parasitic infestation by administering the long-acting composition to the animal in need thereof.
Owner:ZOETIS SERVICE LLC
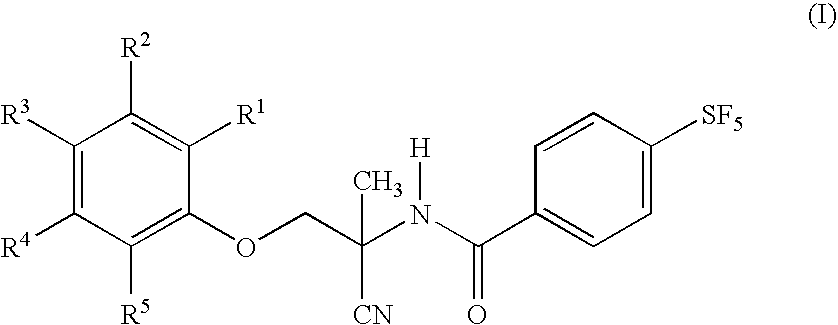
Group of substituted benzoheterocycle amine derivatives and preparation method and related application thereof as IMPDH (inosine monophosphate dehydrogenase) inhibitor
ActiveCN103992310AOrganic active ingredientsOrganic chemistryInosine-5′-monophosphate dehydrogenaseScreening study
The invention discloses a group of substituted benzoheterocycle amine derivatives and a preparation method and related application thereof as an IMPDH (inosine monophosphate dehydrogenase) inhibitor. The IMPDH inhibitor has good application prospects in virus resistance, immunosuppression, tumor resistance, bacterium resistance, parasite resistance and the like. In the invention, a new-structure IMPDH inhibitor shown by the formula (I) is obtained through the design, synthesis and activity screening study on an active compound targeting IMPDH, and a foundation is laid for the development and application of the compounds as the medicines and medicinal compositions thereof with related effects such as virus resistance, tumor resistance, immunosuppression and the like.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Anti-parasitic methods and compositions utilizing diindolylmethane-related indoles
ActiveUS8586621B2Shorten the counting processHeavy metal active ingredientsBiocideCoccidiosisTrypanosomiasis
The present invention includes methods and compositions for the treatment and prevention of protozoal parasitic infections utilizing Diindolylmethane-related indoles. Additive and synergistic interaction of Diindolylmethane-related indoles with other known anti-parasitic and pro-apoptotic agents is believed to permit more effective therapy and prevention of protozoal parasitic infections. The methods and compositions described provide new treatment of protozoal parasitic diseases of mammals and birds including malaria, leishmaniasis, trypanosomiasis, trichomoniasis, neosporosis and coccidiosis.
Owner:BIORESPONSE
Isoxazoline compositions and their use as antiparasitics
Owner:INTERVET INC
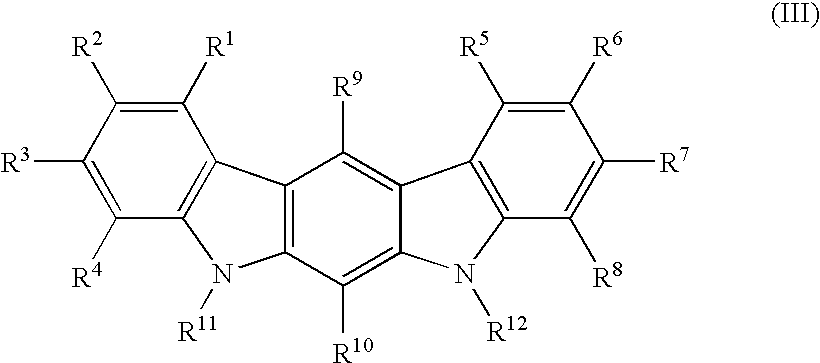
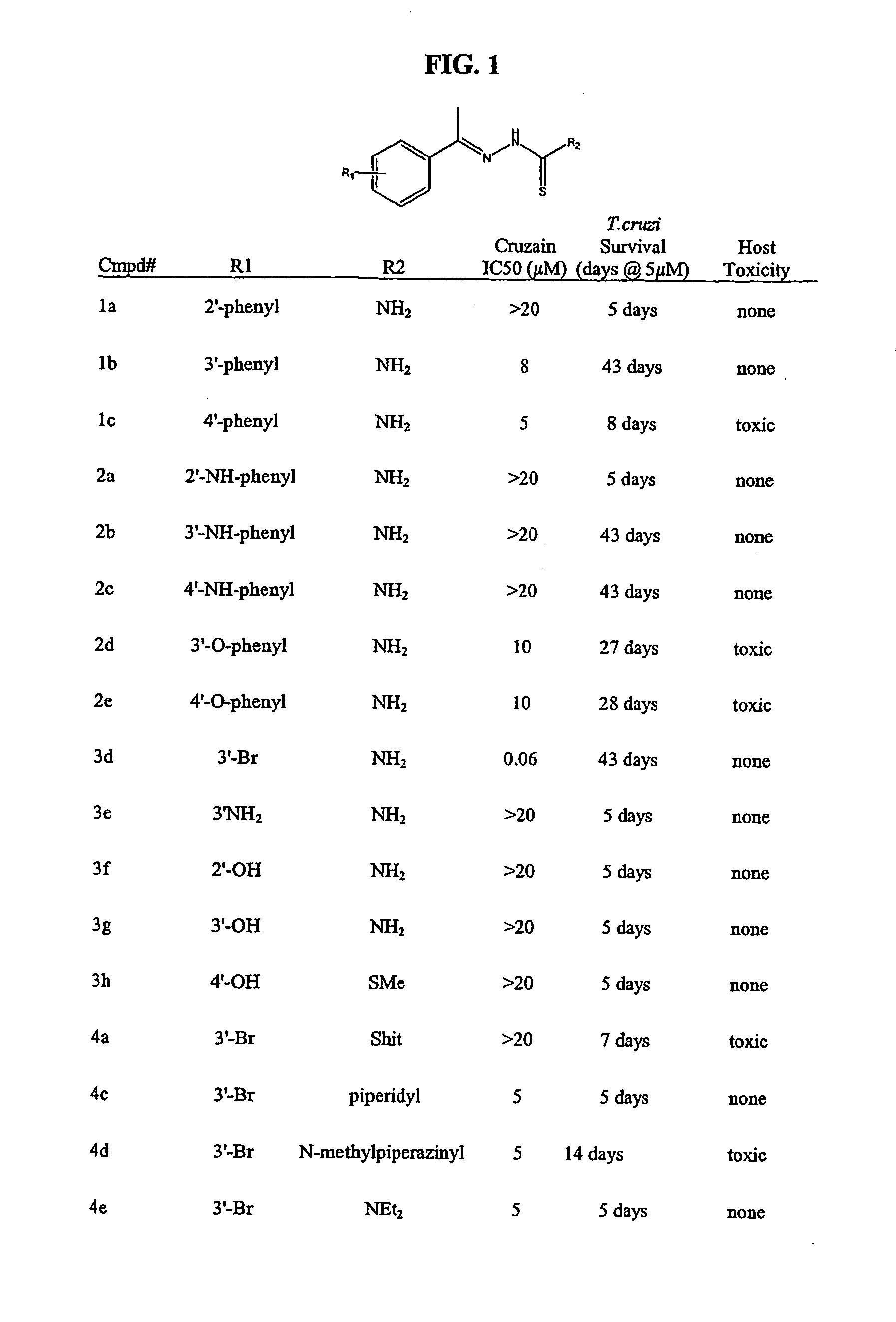
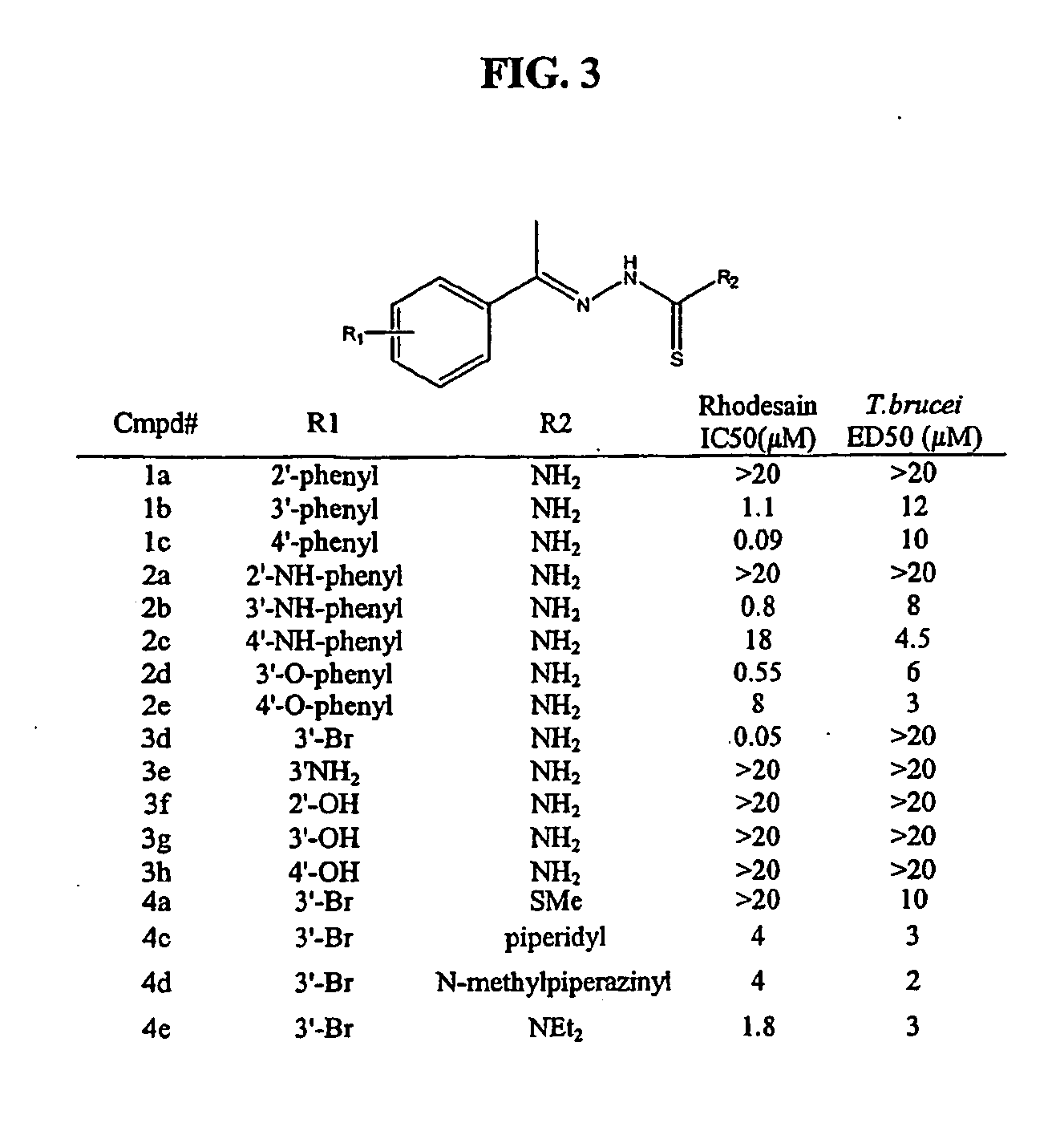
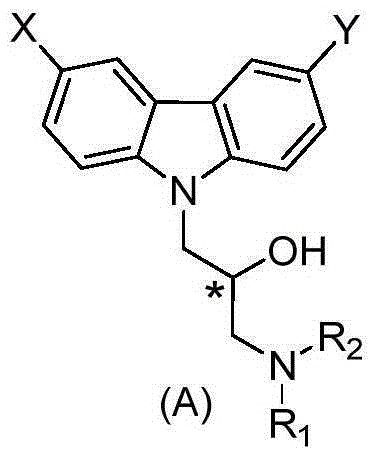
Carbazole alkamine compound and preparation method thereof and application for parasitic disease resistance
ActiveCN106518750AReasonable designRaw materials are easy to getOrganic active ingredientsOrganic chemistryCarbazoleEnantiomer
The invention provides a carbazole alkamine compound and a preparation method thereof and application for a parasitic disease resistance aspect. Specifically, the invention provides a racemic modification of the compound shown as type (A) and an enantiomer or salt capable of being accepted by pharmacy of the enantiomer. The compound has excellent anti-schistosoma and anti-hydatid cyst activity. According to the carbazole alkamine compound and the preparation method thereof and the application for the parasitic disease resistance aspect, the design is reasonable, the source of used raw materials is wide, the preparation method is easy and convenient and suitable for utility and can be applied to parasitic disease resistance drug preparation. Type A (please see the specifications for the chemical structural formula)
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT +1
Anthelminthic formulations
The present invention relates to veterinary or pharmaceutical antiparasitic formulations which may comprise a macrocyclic lactone, one or more alcohol cosolvents and an oil wherein the crystallization of the macrocyclic lactone is minimalized. This invention also provides for, inter alia, antiparasitic formulations for the treating, controlling and preventing of endo- and ectoparasite infections in warm-blooded animals, such as livestock.
Owner:MERIAL LTD
Compound and application of compound in preparation of drugs for resisting parasitic diseases
ActiveCN103787966AInhibitory activityRelieve clinical symptomsOrganic chemistryAntiparasitic agentsQuinoloneHalogen
The invention discloses a compound and application of the compound in preparation of drugs for resisting parasitic diseases. The compound has the chemical formula (VII), in the chemical formula (VII), L is O or CH2, one of R1 and R2 is a halogen element, and the other is H. The compound provided by the invention belongs to a quinolones compound; through research, an inventor finds that the compound can inhibit activity of NDH-2 protein of plasmodium, and therefore, the compound can be used for slowing down the clinical symptoms at asexual reproduction stage and also can be used for stopping diseases spreading at the sexual reproduction stage of life cycle of plasmodium. The compound has great significances for treating the plasmodium diseases. The chemical formula (VII) is as shown in the specification.
Owner:TSINGHUA UNIV
Chinese herbal medicine feed for grass carps
The invention provides a Chinese herbal medicine feed for grass carps. The Chinese herbal medicine feed comprises the following Chinese herbal medicine components in percentage by weight: 20-30% of fish meal, 20-30% of corn, 13-18% of astragalus membranaceus, 8-12% of radix isatidis, 10-15% of licorice, 5-8% of folium isatidis, 6-8% of codonopsis pilosula, 3-5% of rheum officinale, 5-8% of rhizoma phragmitis, 10-15% of tribulusterrestris, 8-12% of white poria and 5-10% of pericarpium granati. According to the Chinese herbal medicine feed provided by the invention, the Chinese herbal medicine contains a variety of immune competent matters, and thus the capacity of promoting the ingestion of the grass carps to increase the food ration of the grass carps is realized, an antiparasitic drug component is contained, and thus the immunity of the grass carps is improved, the somaticsubstances of the grass carps is enhanced, the quality of sperm eggs of the grass carps is enhanced to increase the sperm egg combination rate of the grass carps in a multiplicative process, further the hatchability of fish fries is high and the quality of the fish fries is good; the Chinese herbal medicine feed is simple in production process, and low in cost and raw materials are convenient to obtain.
Owner:TIANJIN WANDA JIAHE FEED
Parasiticidal composition
ActiveUS20060142216A1Improve bioavailabilitySuitable for topical administrationBiocideSalicyclic acid active ingredientsPolyethylene glycolBlood plasma
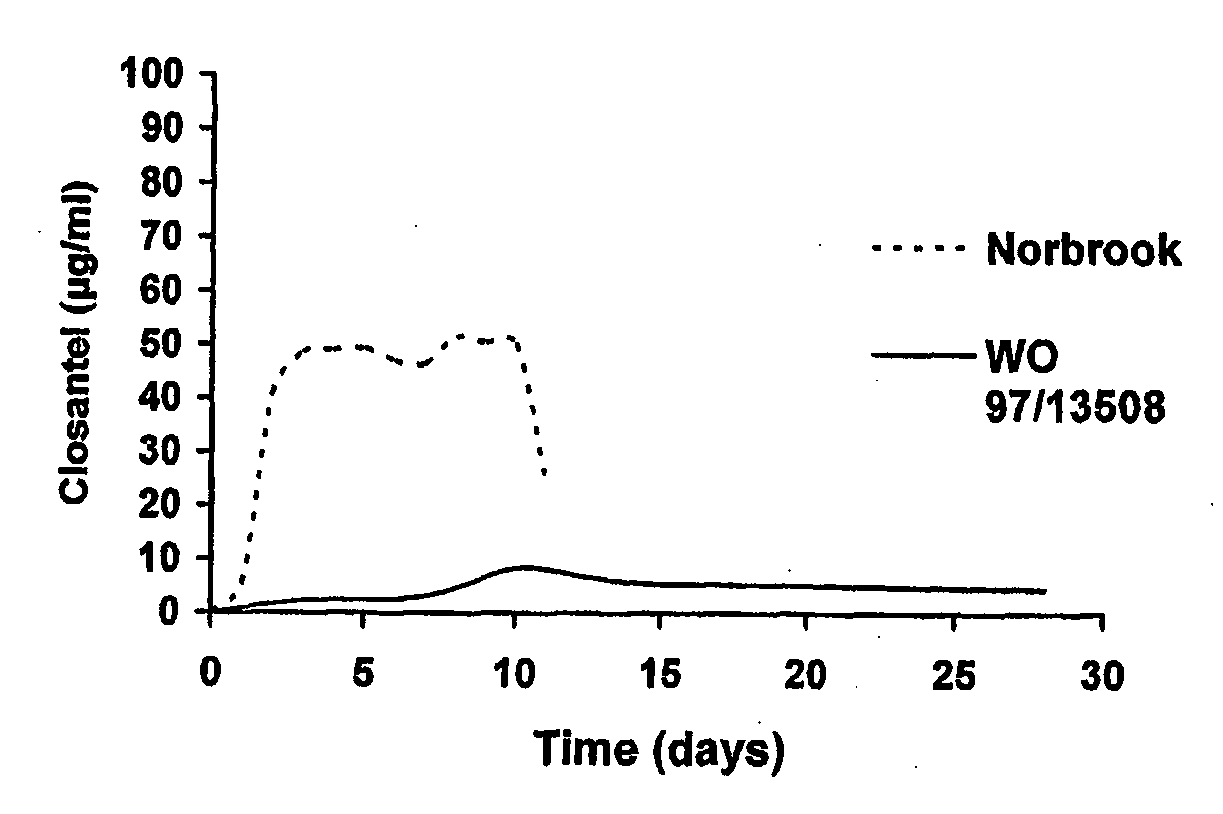
An anti-parasiticidal composition presented as a topical “pour-on” product for treating animals infected by parasites which are known to be susceptible to salicylanilides, especially closantel, alone or together with at least one other anti parasitic compound of the avermectin or milbemycin type and offers enhanced bioavailability of the salicylanilide by provision of a delivery system comprising at least 20% (v / v) of one or more alcohols, and optionally including a polymeric moiety selected from the group consisting of polyvinylpyrrolidone (PVP), polyoxypropylene / polyoxyethylene block copolymers (poloxamer), and polyethylene glycols (PEG), thereby improving the bioavailability of e.g. closantel (as assessed with respect to blood plasma levels of closantel).
Owner:NORBROOK LABORATORIES LIMITED
Use of Levo-Ornidazole For Preparing Anti-Parasitic Infection Drug
PendingUS20080177083A1Low toxicityImprove securityOrganic active ingredientsOrganic chemistryDrugs preparationsTrichomonas vaginalis
The use of levo-ornidazole in the preparation of anti-parasitic infection drug is provided. It is demonstrated that levo-ornidazole is superior to dextro-ornidazole and racemic ornidazole in the therapeutic action against parasitization (especially trichomonas vaginalis infection and cecal amoeba infection), and thus it is more practicable to formulate L-ornidazole as anti-parasitic infection drugs, and particularly as drug preparations which are suitable for clinical uses, including oral preparation, intravenous preparation and vaginal preparation.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com