Preparation method for emulsifiable oily injection containing abamectin drugs
A technology of abamectin and base abamectin, which is applied in the directions of medical preparations, antibacterial drugs, and pharmaceutical formulations containing active ingredients, can solve the problems that the immediate release effect is not as good as that of emulsions and microemulsions, etc., and achieve optimal emulsification. Performance and safety, improving effective utilization, and the effect of high peak plasma concentration levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、4
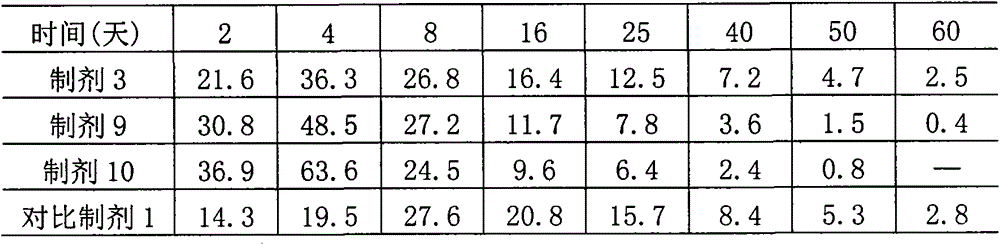
[0026] Composition, preparation and in vitro emulsification performance experiment of embodiment 1,4% abamectin injection
[0027] (1) Preparation composition: Based on the preparation of 100ml, the ingredients of preparations 1 to 10 and the comparative preparation are shown in the table below.
[0028] Preparation number
Abamectin (g)
P188(g)
HCO (g)
Soy Lecithin (g)
Preparation 1
4
5
0.3
0.4
Preparation 2
4
4
0.6
0.8
Preparation 3
4
3
0.9
0.4
Preparation 4
4
9
0.3
0.4
Preparation 5
4
8
0.6
0.8
Preparation 6
4
7
0.9
1.1
Preparation 7
4
12
0.6
0.8
Preparation 8
4
3
0.9
1.1
Preparation 9
4
3
-
-
Preparation 10
4
9
-
-
Comparative preparation 1
4
-
0.9
0.4
Comparative preparation 2
4
-
- ...
Embodiment 2
[0041] Embodiment 2, preparation of different content ivermectin injections and in vitro emulsification performance experiment
[0042] (1) Preparation composition: Based on the preparation of 100ml, the ingredients of preparations 11 to 19 are shown in the table below.
[0043] Preparation number
Ivermectin (g)
P188(g)
HCO (g)
Soy Lecithin (g)
Benzyl Benzoate (ml)
Preparation 11
3
7
0.8
0.4
35
Preparation 12
4.5
5
0.8
0.4
50
Preparation 13
6
3
0.8
0.4
65
[0044] Preparation 14
3
7
0.8
-
35
Preparation 15
4.5
5
0.8
-
50
Preparation 16
6
3
0.8
-
65
Preparation 17
3
3
-
-
65
Preparation 18
4.5
5
-
-
65
Preparation 19
6
7
-
-
65
[0045] Note: All preparations in the table are added with antioxid...
Embodiment 3
[0051] Embodiment 3, composition and preparation of doramectin injection
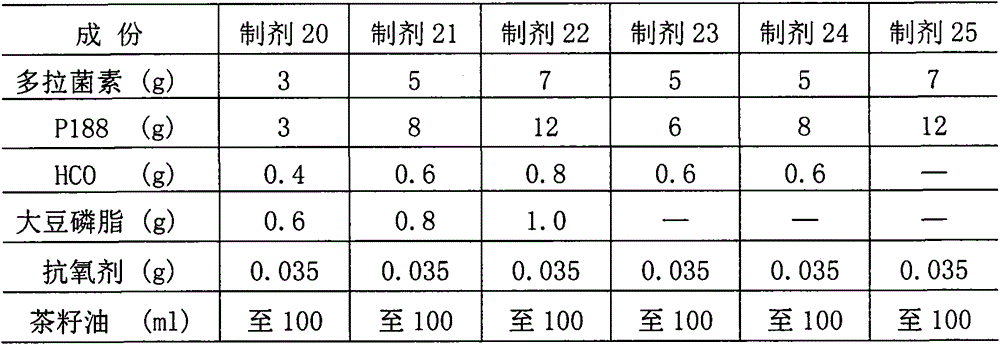
[0052] (1) Preparation composition: Based on the preparation of 100ml, the ingredients of preparations 20 to 25 are shown in the table below.
[0053]
[0054] Note: Antioxidant is BHT+BHA+PG=0.02+0.01+0.005=0.035
[0055] (2) Preparation method
[0056] aPut doramectin and P188 into a reflux bottle, add 4-6 times the amount of absolute ethanol equivalent to doramectin, dissolve in a water bath at 80-85°C under reflux, and then distill under reduced pressure to remove most of the solvent , cooled (5-10°C) to make it solidify, take out the solidified product, dry it to constant weight in a vacuum drying oven at 35-38°C, -0.09 to -0.1MPa, crush it, and pass through a 40-mesh sieve to obtain drug-loaded particles;
[0057] b Mix the drug-loaded particles, HCO, soybean lecithin, antioxidant and 2 / 3 of tea seed oil, and repeatedly shear them with a high-shear homogenizer until the particle size of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com