Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Parasitic Infestation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
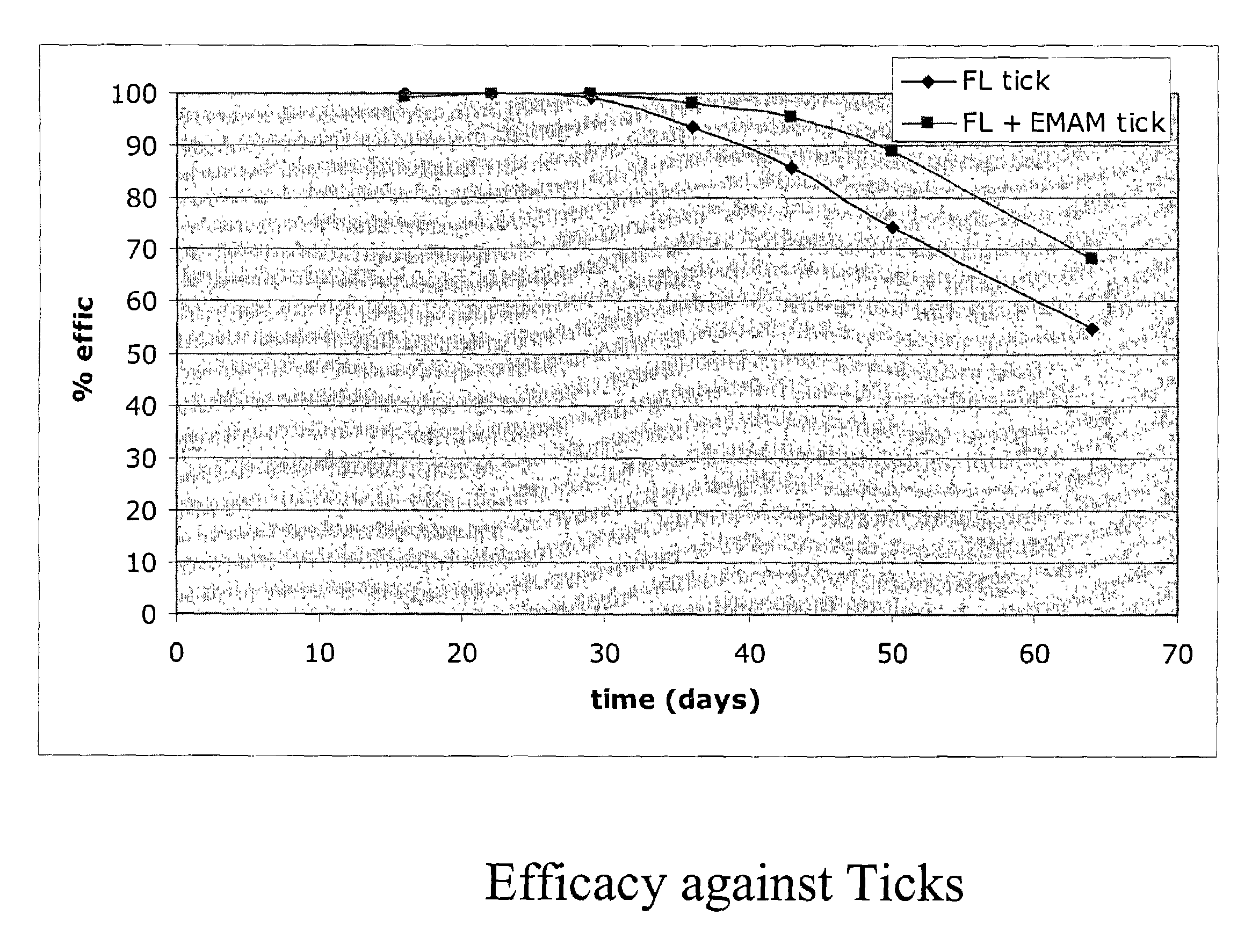
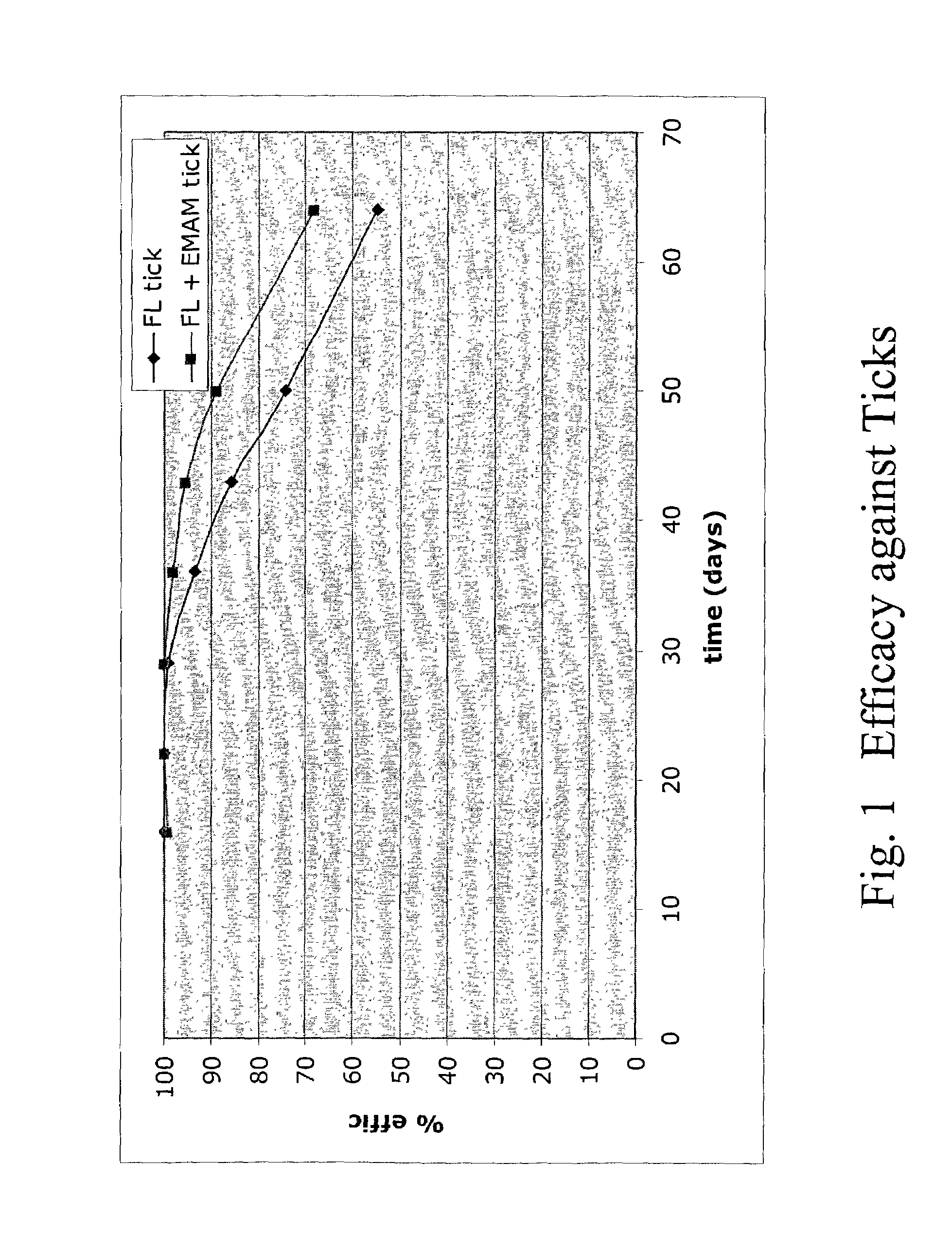
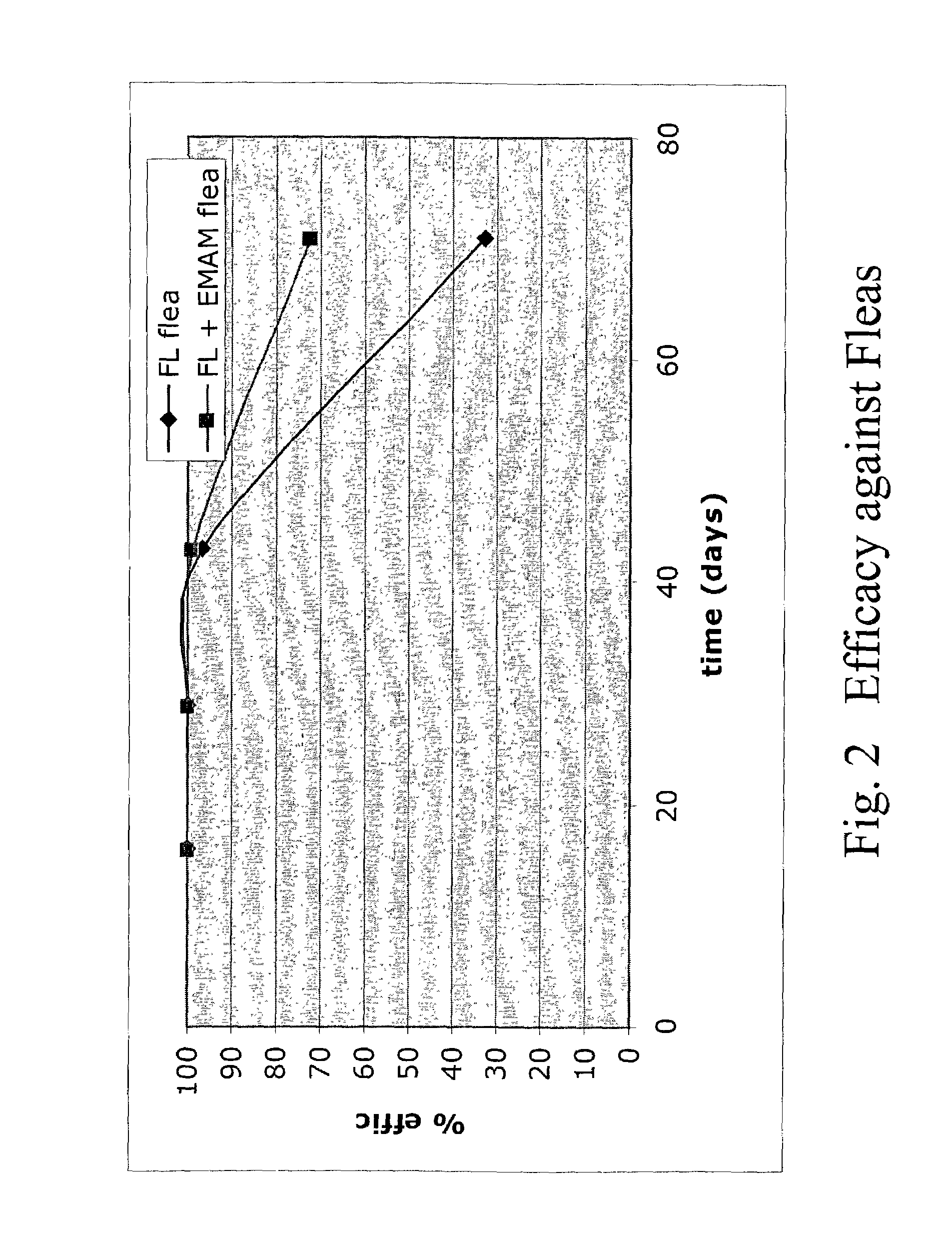
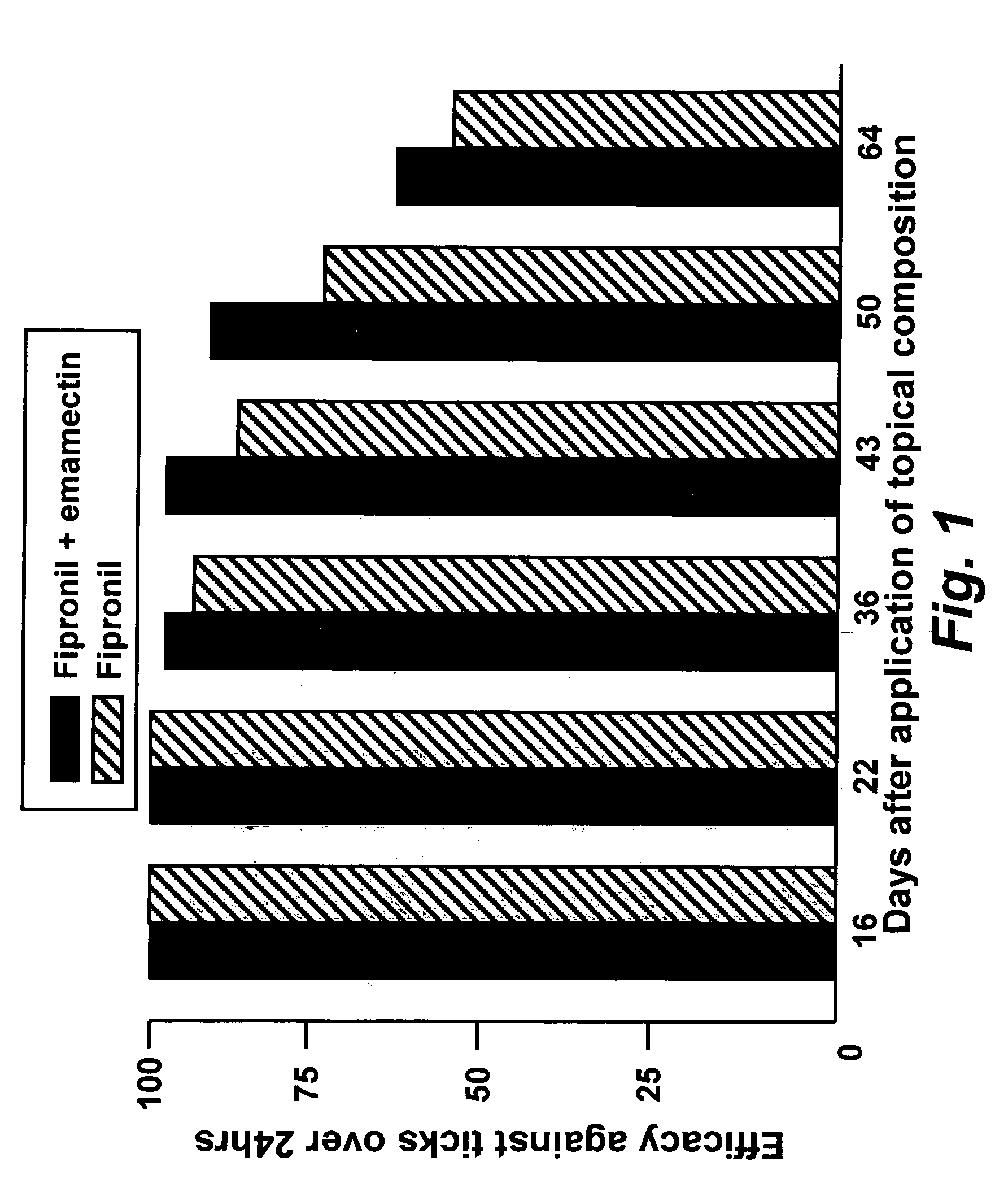
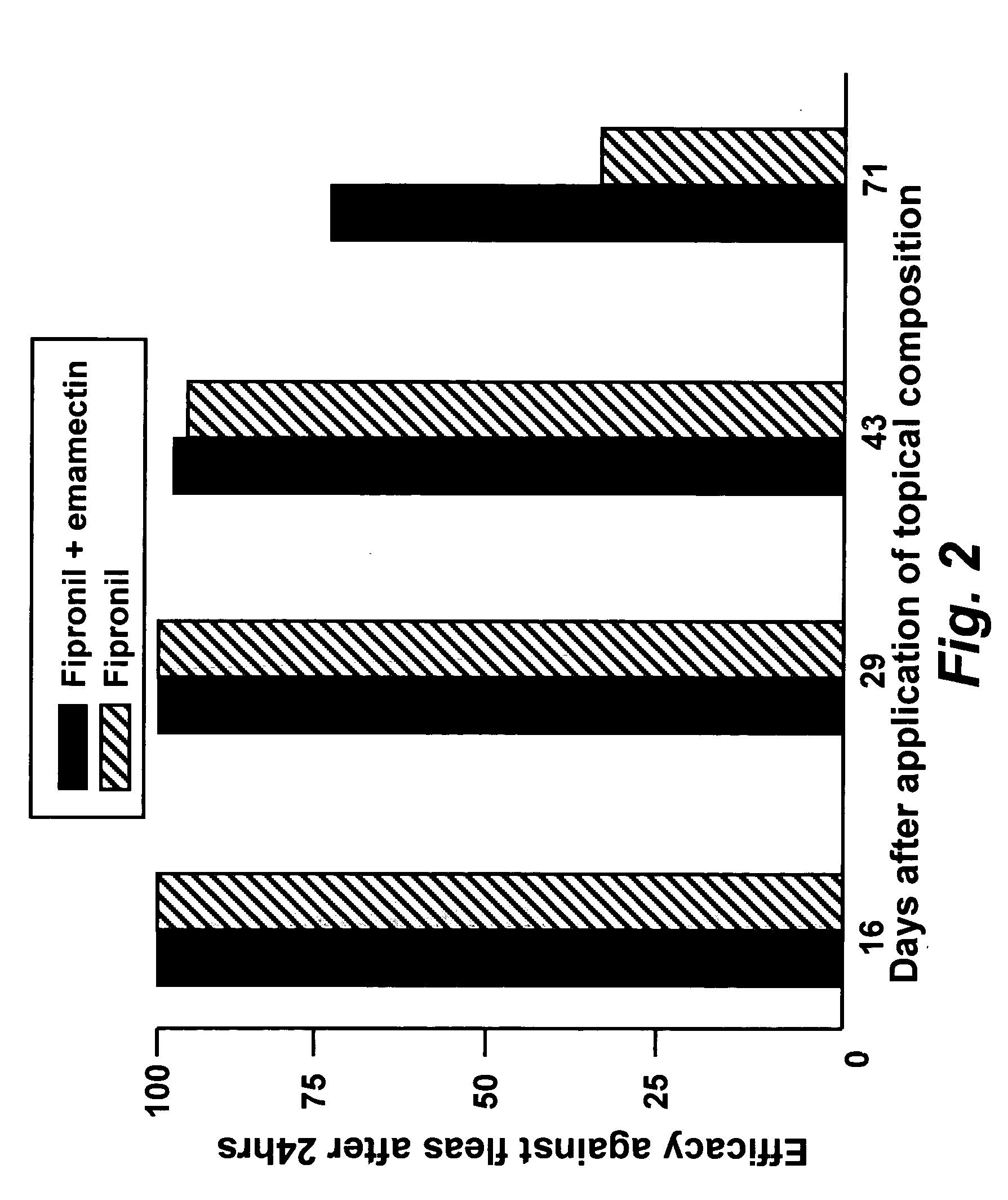
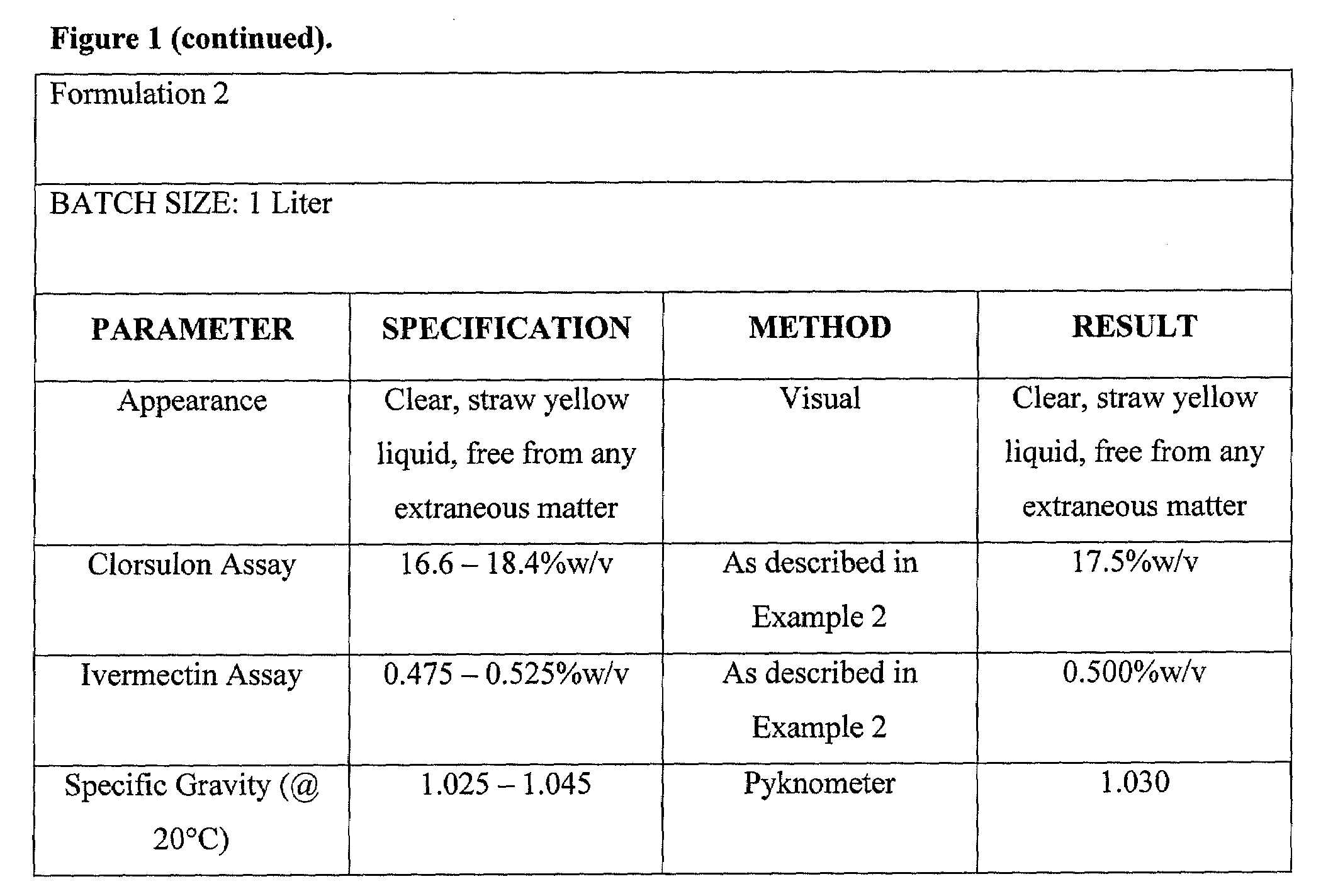
Spot-on formulations for combating parasites
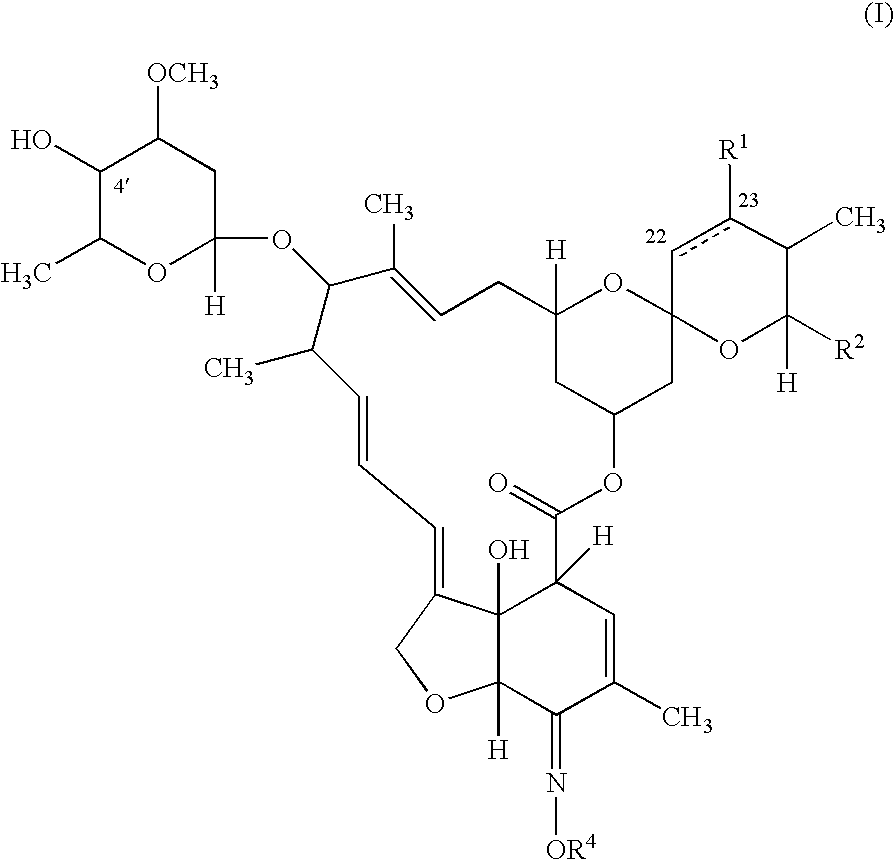
InactiveUS6998131B2Effective and lasting destructionProphylaxis of parasite infestationsBiocideDead animal preservationAntiparasiticMammal
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and(B) an effective amount of emamectin;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL LTD
Spot-on formulations for combating parasites
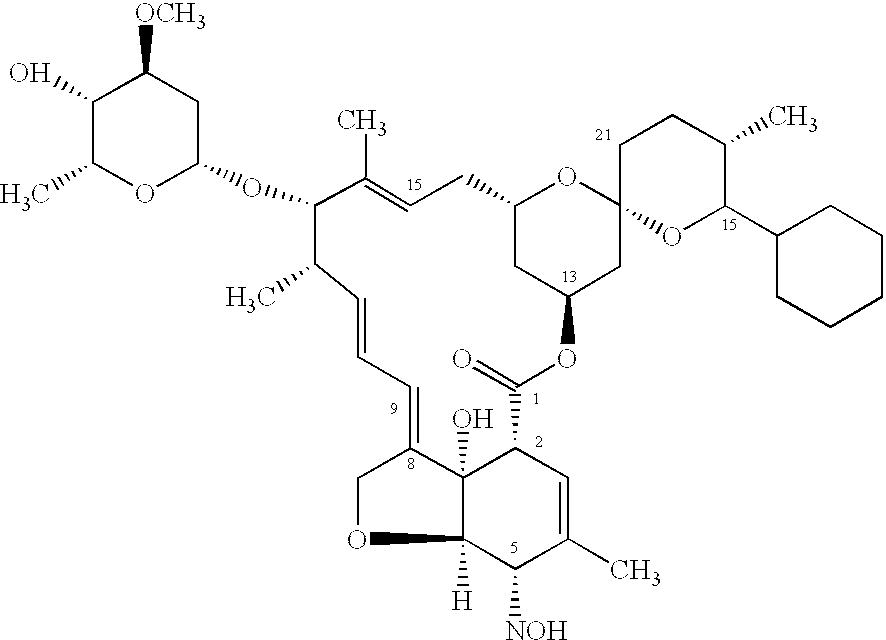
InactiveUS6962713B2Toxic effectsEffective and lasting destructionBiocideDead animal preservationAntiparasiticAntiparasite agent
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and / or(B) an effective amount of a macrocyclic lactone antihelmintic or antiparasitic agent;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL SAS
Spot-on formulations for combating parasites
InactiveUS20050192319A1Effective and lasting destructionProphylaxis of parasite infestationsBiocidePharmaceutical delivery mechanismAntiparasiticAnimal science
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise: (1) a composition comprising (A) an effective amount of a 1-phenylpyrazole derivative; and (B) an effective amount of emamectin, latidectin or lepimectin; (2) an acceptable liquid carrier vehicle; and (3) optionally, a crystallization inhibitor. The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL LTD
Anthelmintic combination
ActiveUS20100197624A1Reduce frequencyBiocideCarbohydrate active ingredientsAnthelmintic drugPhysiology
A method is provided for the treatment of parasitic infestations in mammals, comprising the step of simultaneously or sequentially administering to the mammal effective amounts of (a) 2-desoxoparaherquamide; and (b) abamectin. Also provided is an anthelmintic composition comprising 2-desoxoparaherquamide and abamectin.
Owner:ZOETIS SERVICE LLC
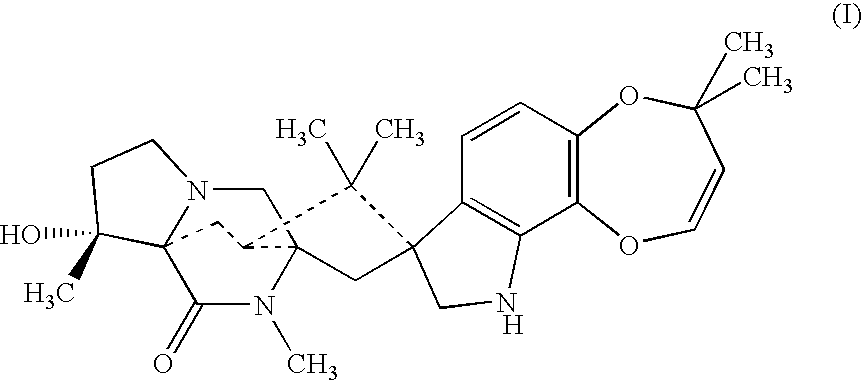
Combination
Compounds of formula (I) are used in combination with a second antiparasitic agent for the treatment of parasitic infestations in a host animal.
Owner:PFIZER LTD +1
Combination
Compounds of formula (I) are used in combination with a second antiparasitic agent for the treatment of parasitic infestations in a host animal.
Owner:PFIZER LTD
Curcuminoid solid dispersion formulation
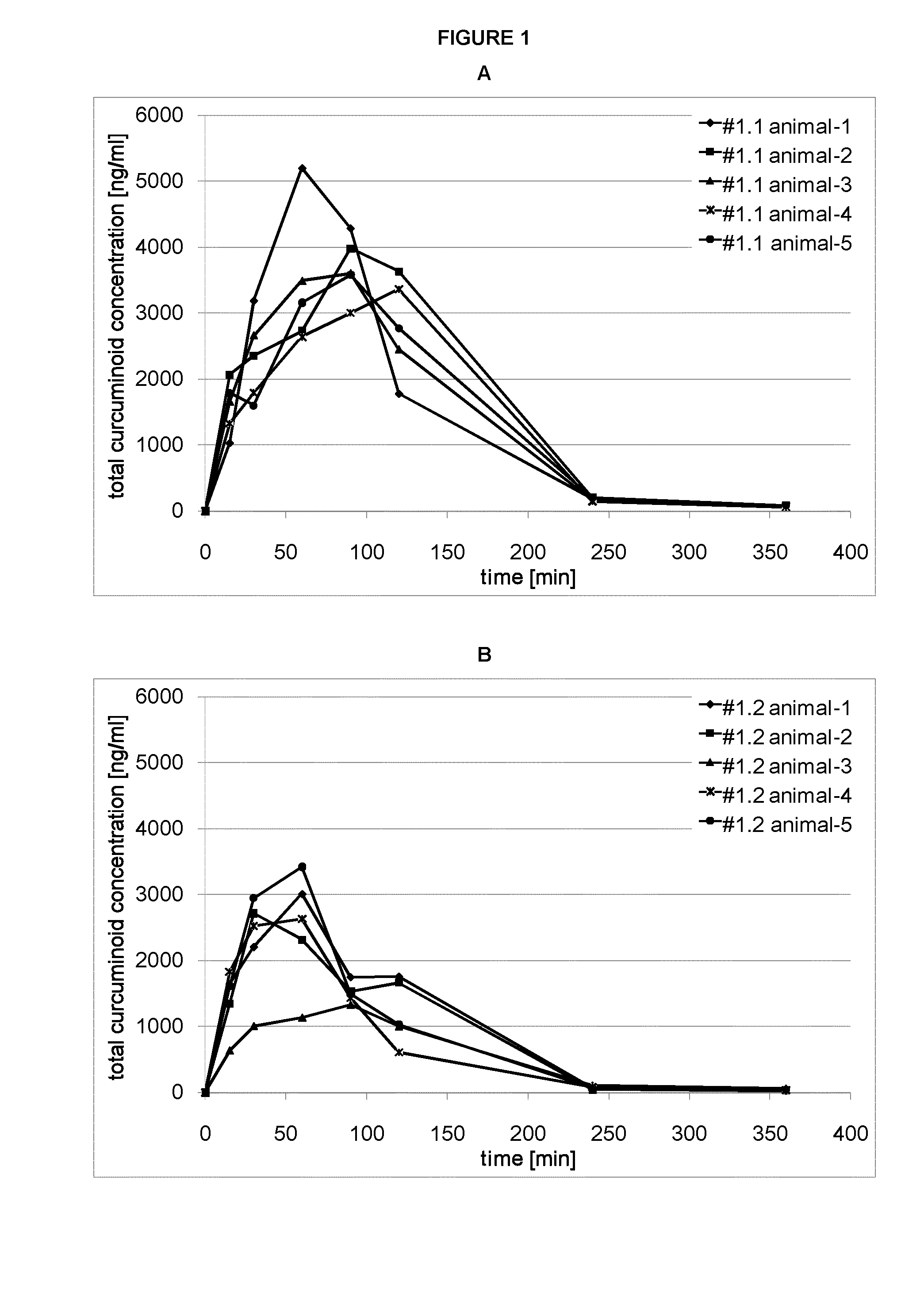
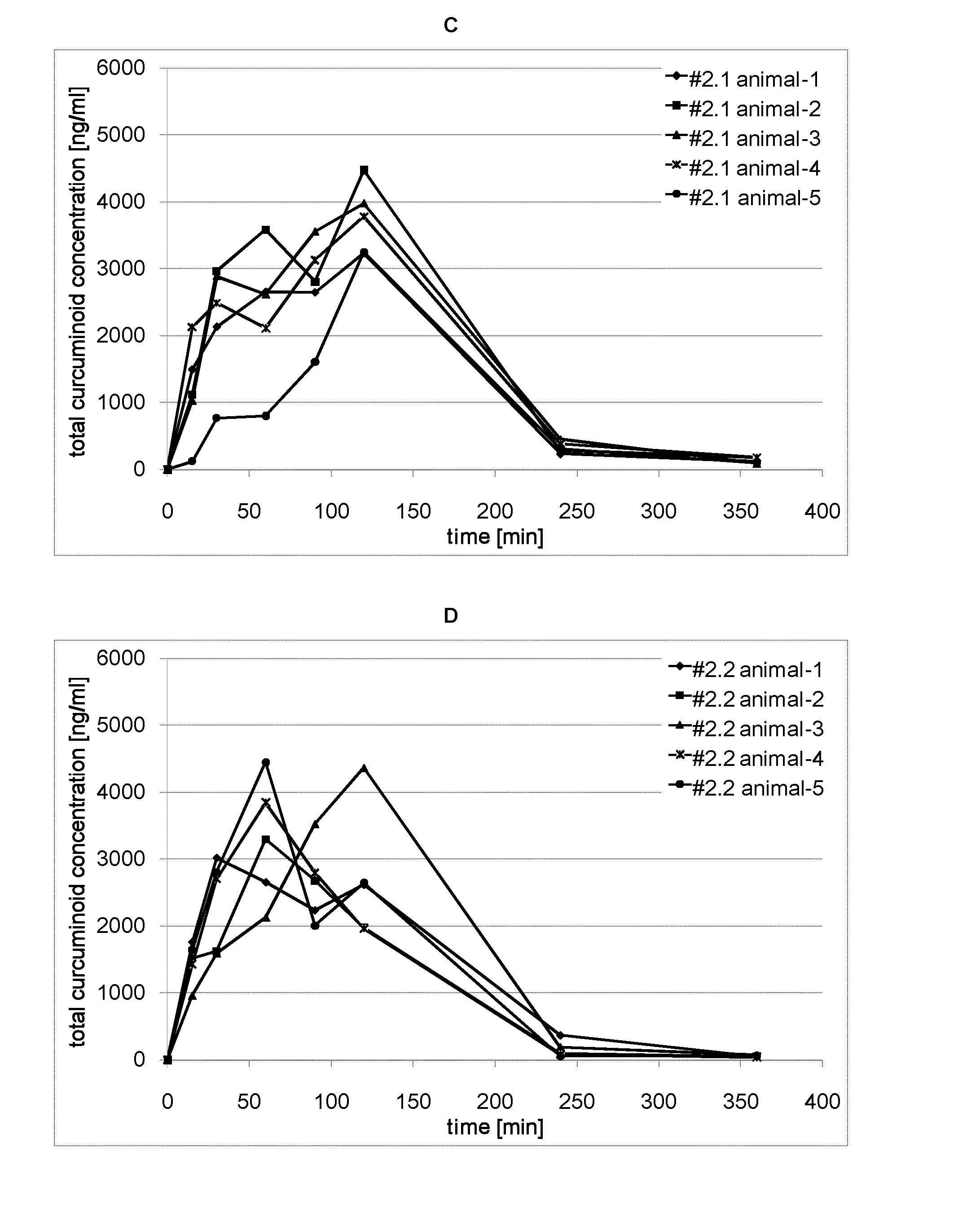
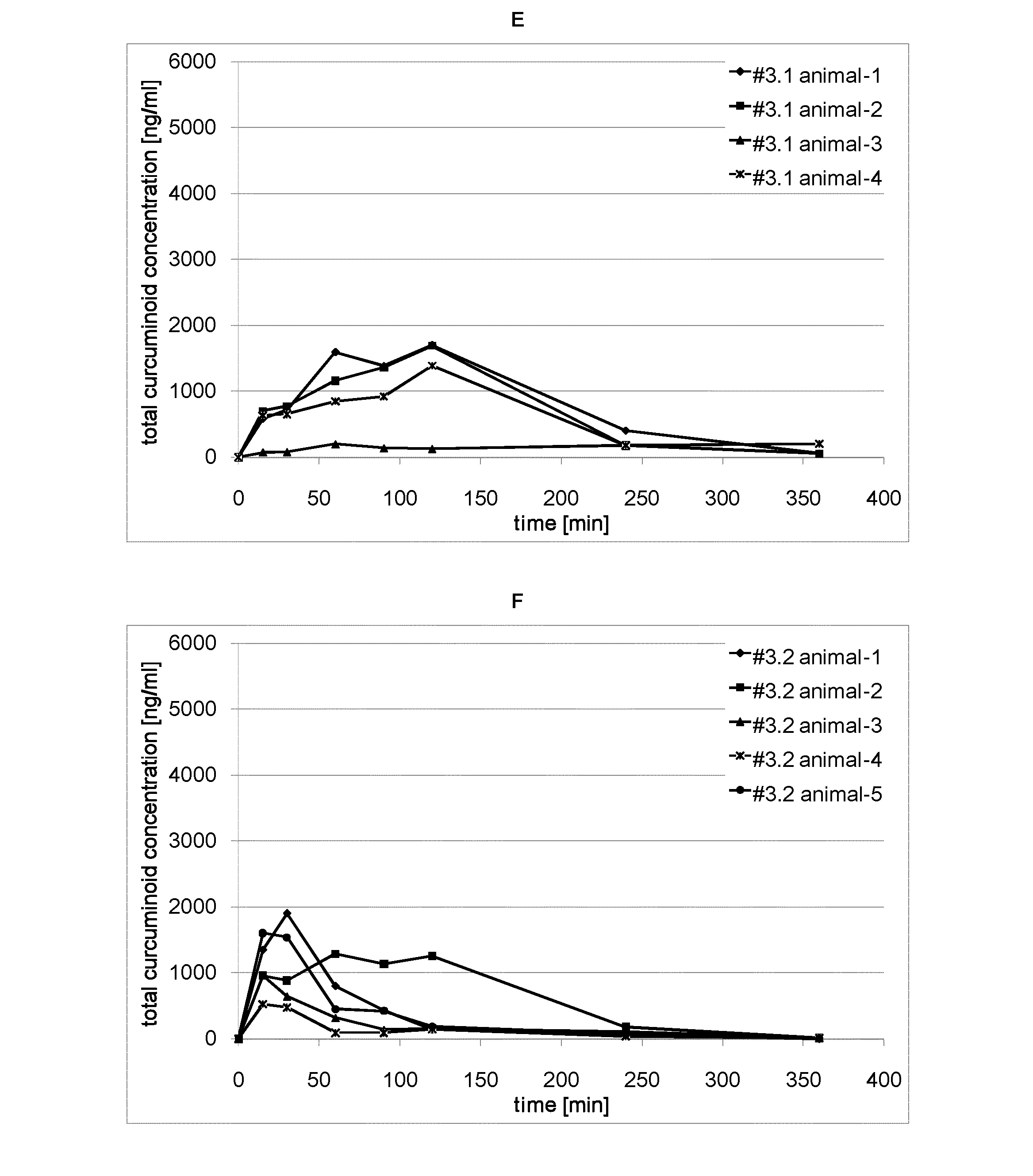
A curcuminoid formulation, comprising a melt-processed solid dispersion product comprising one or more curcuminoids, a nutritionally acceptable thermoplastic polymer, and a phosphatide; providing an improved oral bioavailability compared to non-formulated crystalline curcuminoid. A method for producing said formulation. A nutritional product fortified with said formulation. Said formulation for use in the treatment or prophylaxis of cancer, conditions involving an inflammatory reaction, neurological disorders, cardiovascular disease, pulmonary disease, the formation of cholesterol gallstones, and parasitic infestation.
Owner:ABBOTT LAB INC +1
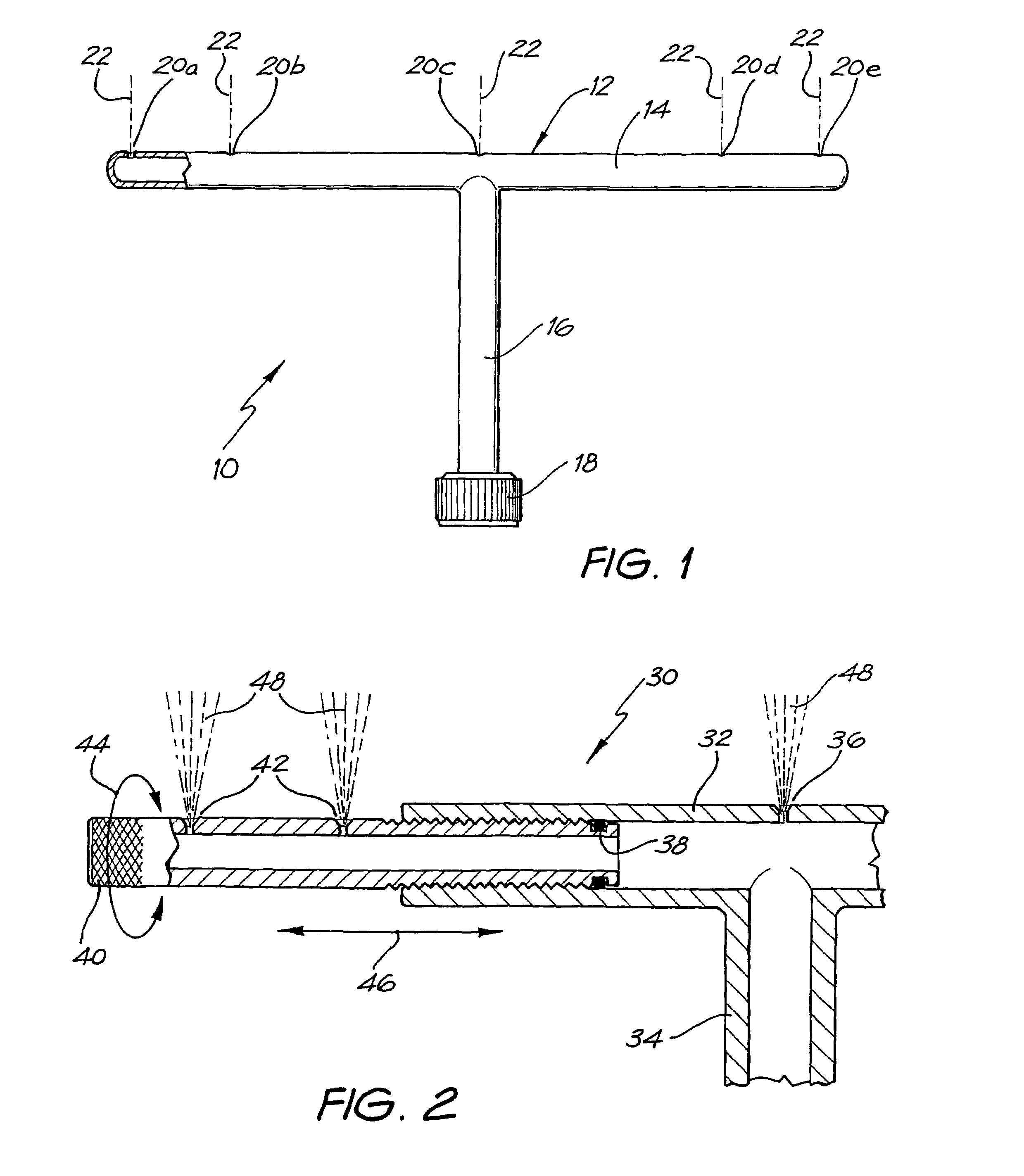
Pour-on application method and devices
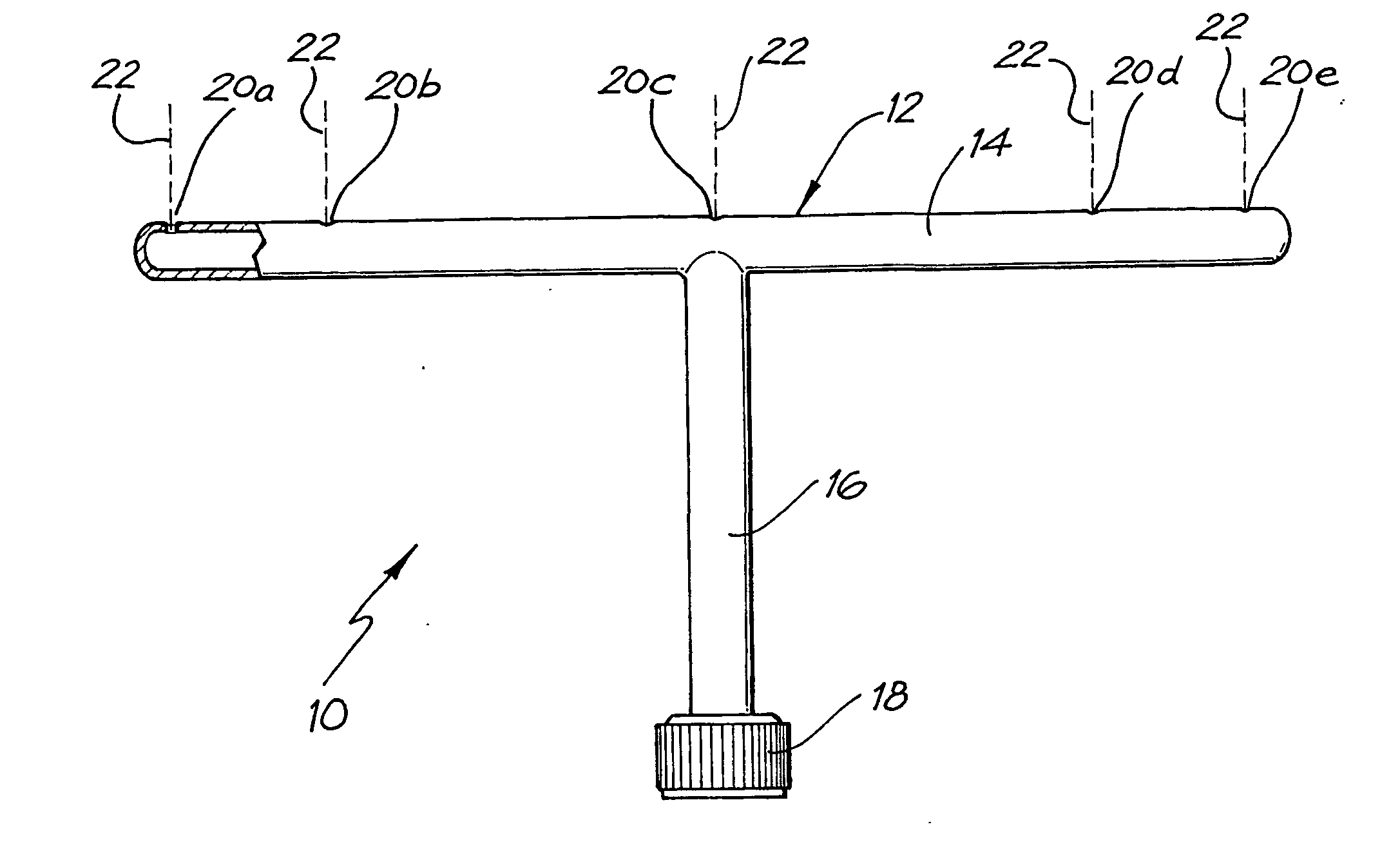
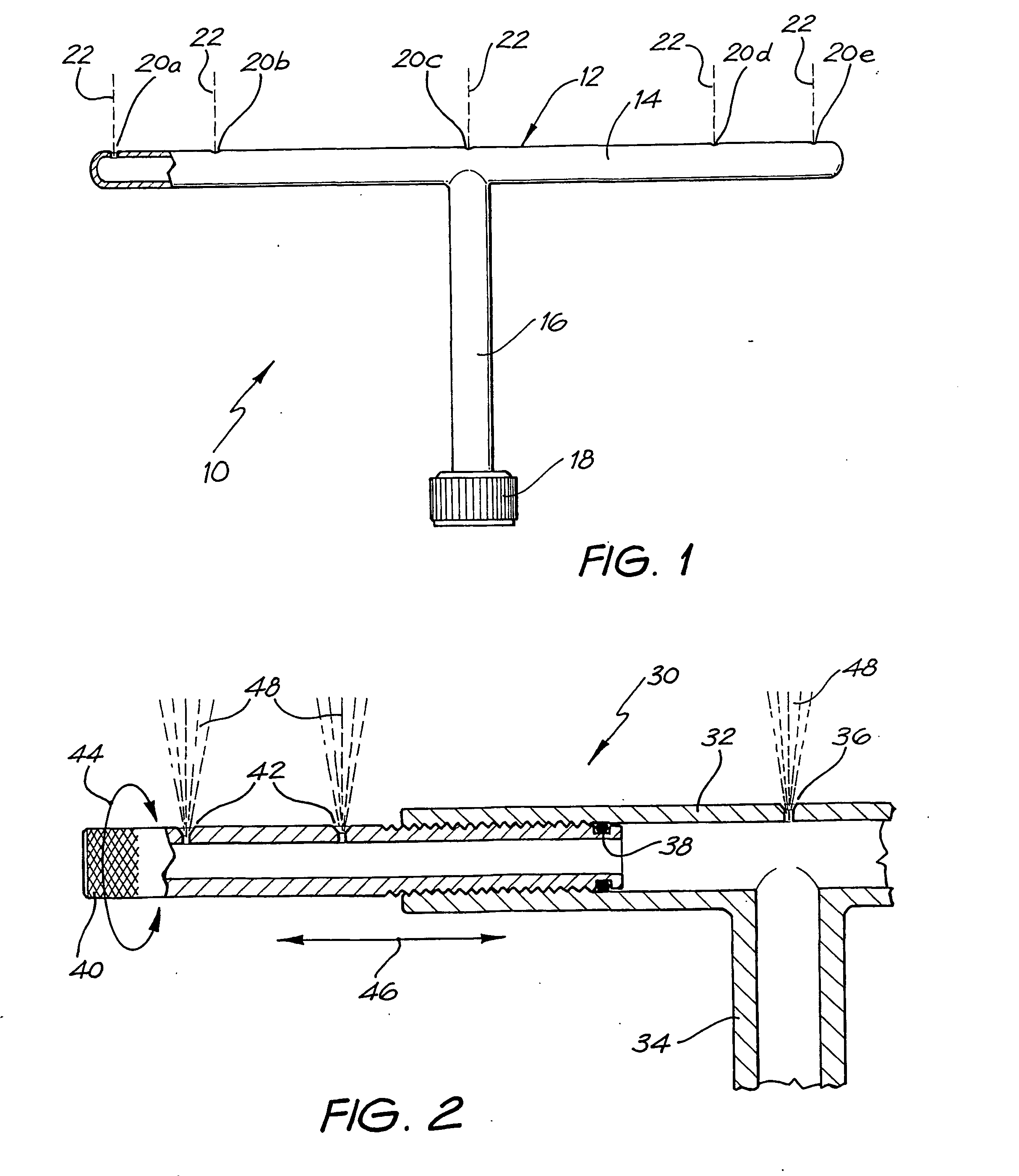
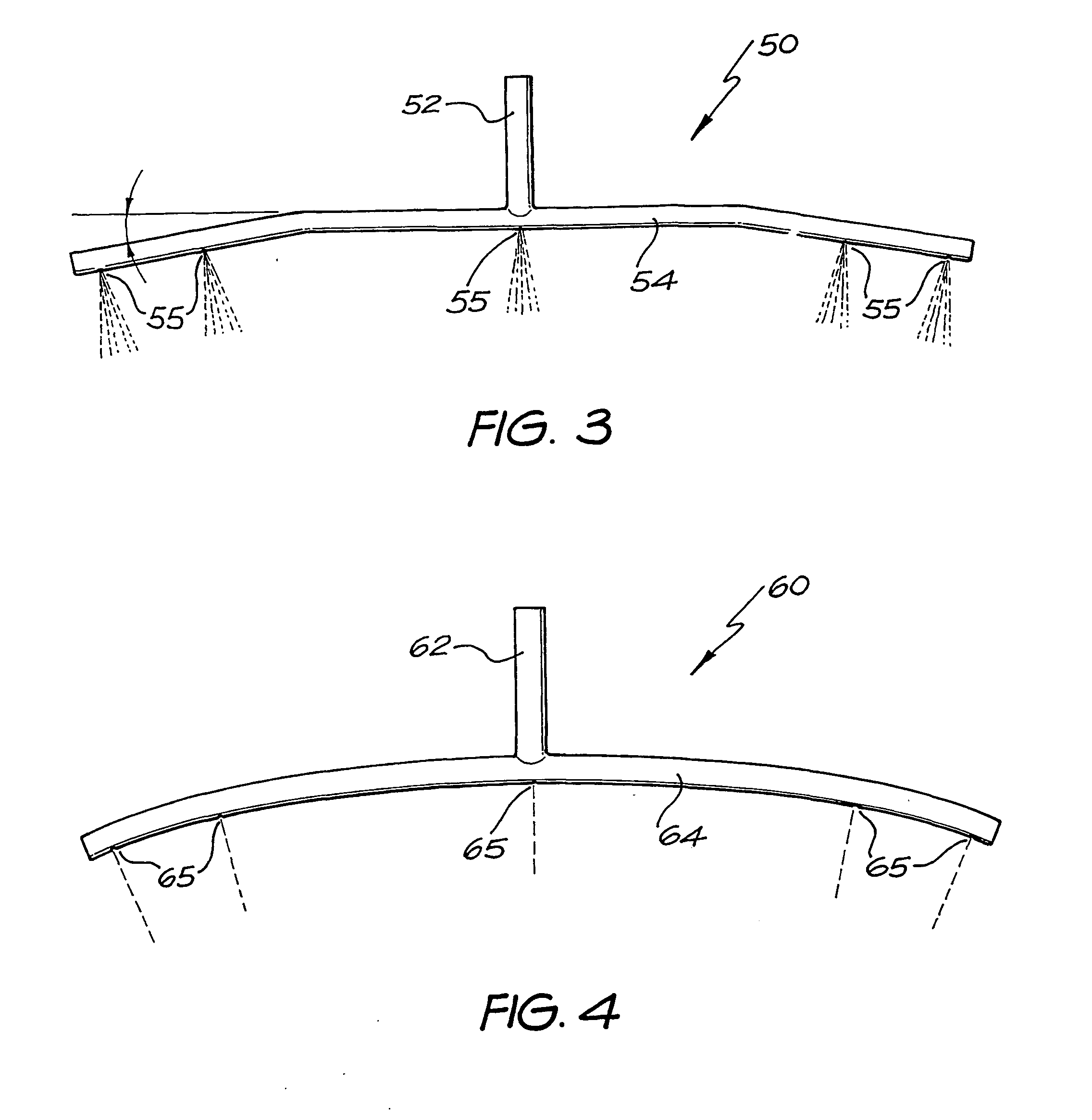
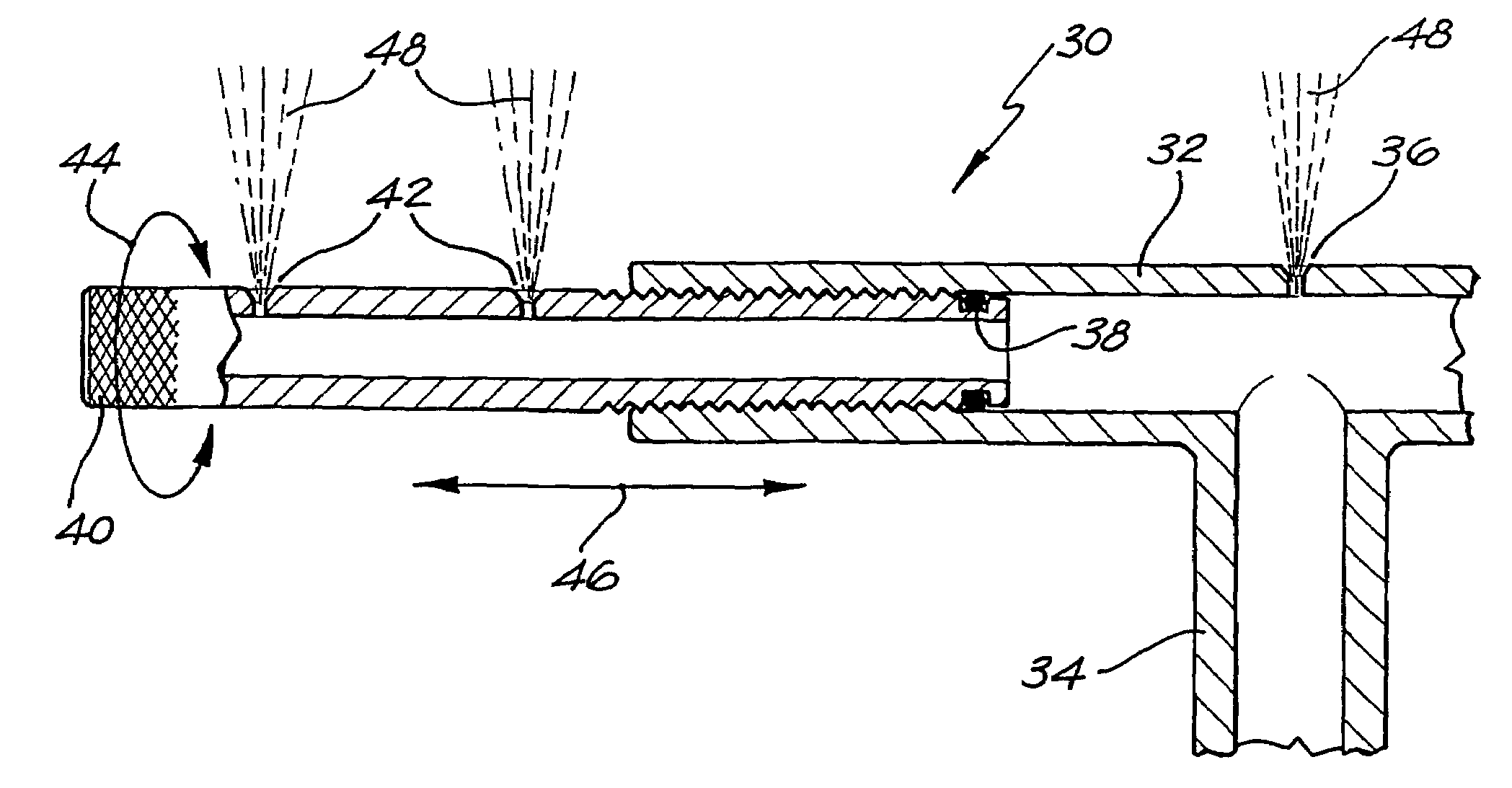
InactiveUS20040040518A1Improve gripSpray nozzlesVeterinary washing devicesParasitic InfestationDomestic animal
The present invention relates to a device for applying a liquid pesticidal formulation to an external surface of a domestic animal, the device comprising an inlet and a plurality of spaced apart outlets, wherein the device is adapted such that when the middle of the device is positioned substantially above the spine of the animal, at least one outlet is positioned substantially above each flank of the animal. The invention also relates to a method for the treatment or prophylaxis of parasitic infestations, including ectoparasitic and / or endoparasitic infestations, of domestic animals comprising topically administering to said animal a pour-on pesticidal formulation to a region of the external surface of an animal extending from one flank to the opposing flank.
Owner:ELI LILLY & CO
Pour-on application method and devices
InactiveUS7140325B2Improve mobilityMaximizing movementSpray nozzlesVeterinary washing devicesParasitic InfestationDomestic animal
The present invention relates to a device for applying a liquid pesticidal formulation to an external surface of a domestic animal, the device comprising an inlet and a plurality of spaced apart outlets, wherein the device is adapted such that when the middle of the device is positioned substantially above the spine of the animal, at least one outlet is positioned substantially above each flank of the animal. The invention also relates to a method for the treatment or prophylaxis of parasitic infestations, including ectoparasitic and / or endoparasitic infestations, of domestic animals comprising topically administering to said animal a pour-on pesticidal formulation to a region of the external surface of an animal extending from one flank to the opposing flank.
Owner:ELI LILLY & CO
Long-Acting Spiro-Isoxazoline Formulations
ActiveUS20160235720A1Simple compositionOrganic active ingredientsPharmaceutical non-active ingredientsBiopolymerParasitic Infestation
The invention describes a long-acting injectable veterinary composition comprising a spirocyclic isoxazoline, at least one biopolymer, and optionally, at least one veterinary acceptable-carrier, -solvent, -excipient, or any mixture thereof. The invention also includes a method of treating an animal with a parasitic infestation by administering the biopolymeric composition to the animal in need thereof, and a process for preparing the biopolymeric composition.
Owner:ZOETIS SERVICE LLC
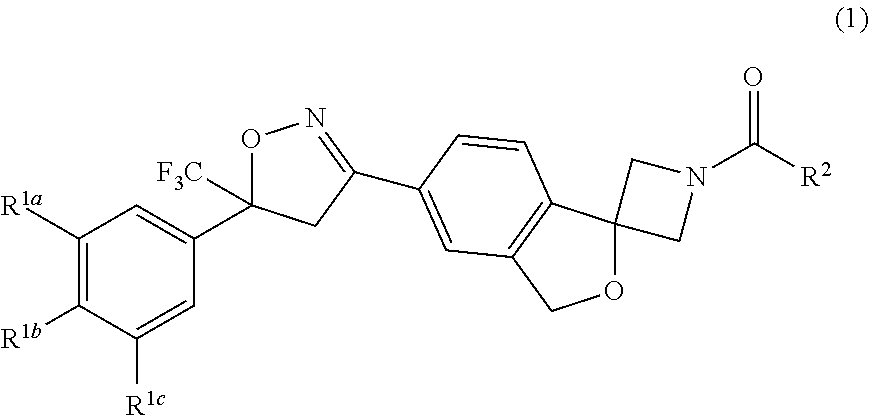
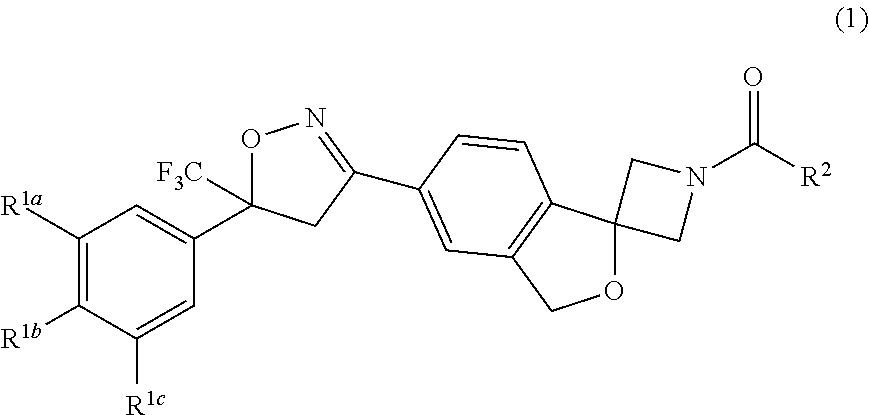
Long-acting spiro-isoxazoline antiparasitic compositions
InactiveUS20160081986A1Improved long-acting topical compositionOrganic active ingredientsBiocideAntiparasiticAntioxidant
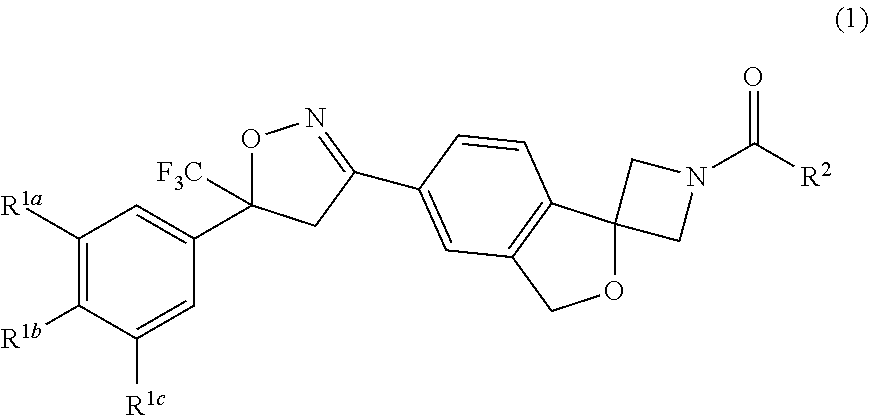
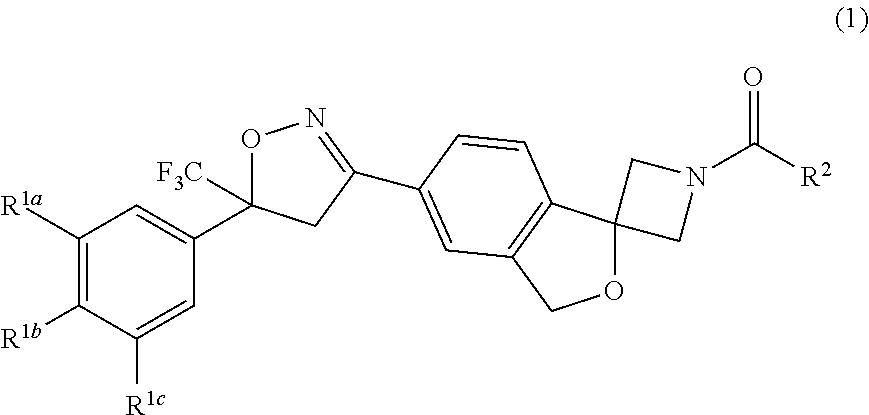
The invention describes a long-acting composition comprising a spiro-azetidine isoxazoline of Formula (1) or (2) wherein R1a, R1b, R1c and R2 are as described herein, and stereoisomers thereof. The composition is a veterinary composition and also comprises a glycol ether and at least one veterinarily acceptable solvent, and optionally, at least one precipitation inhibitor, antioxidant and additional veterinary agent, and any mixture thereof. The invention also includes a method of treating an animal with a parasitic infestation by administering the long-acting composition to the animal in need thereof.
Owner:ZOETIS SERVICE LLC
Isoxazoline compositions and their use as antiparasitics
Owner:INTERVET INC
Pure traditional Chinese medicine preparation infantile malnutrition pills
InactiveCN103100004AWide range of indicationsCan cure indigestionDigestive systemMammal material medical ingredientsOfficinalisParasitic Infestation
The invention discloses pure traditional Chinese medicine preparation infantile malnutrition pills, comprising 120 g of hawthorn, 120 g of fried malt, 140 g of medicated leaven, 120 g of fried endothelium corneum gigeriae galli, 100 g of mangnolia officinalis stir-baked with ginger juice, 100 g of snokeweed processed with salt, 80 g of fructus aurantii fried with bran, 80 g of immature bitter orange fried with bran, 70 g of fried pharbitis seed and 70 g of areca-nut. The raw materials are cut and prepared, classified and dried, crushed, filtered, mixed and processed with honey into pills; and then the pills are lightened and sterilized, tested and packaged; and as a result, the pure traditional Chinese medicine preparation infantile malnutrition pills are obtained. The pure traditional Chinese medicine preparation infantile malnutrition pills have the functions of helping digestion and tonifying spleen and stomach, promoting the circulation of qi and moving qi stagnation, and expelling parasite, and are mainly used for treating diseases such as dyspepsia, dyspepsia, abdominal distension and constipation, abdominal pain due to parasitic infestation, marked emaciation and the like. The pure traditional Chinese medicine preparation infantile malnutrition pills are wide in application range, suitable for the old and the young, numerous in treatment functions and good in effect; and observed through clinical application, the effective rate is 92.3%.
Owner:HUBEI LYUWEIKANG PHARMA CO LTD
Chinese medicament for treating malnutrition and indigestion syndrome in children due to parasitic infestation
InactiveCN101584834ARetentionTo achieve the purpose of treatmentDigestive systemAntiparasitic agentsSide effectRegimen
The invention discloses a Chinese medicament for treating malnutrition and indigestion syndrome in children due to parasitic infestation, which is characterized by comprising the following main components: 3 g of almond, 6 g of winter melon seed, 15 g of coix seed, 6 g of common reed rhizome, 6 g of hawthorn, 6 g of talc, 6 g of fructus amomi, 6 g of pinelliatuber, 6 g of officinal magnolia bark, 12 g of kudzuvine root, 1 dark plum fruit, 6 g of stone-like omphalia, 6 g of grand torreya seed, 6 g of liquorice and the like. The preparation method comprises the following steps: soaking the main components in a container by water for 30 minutes; decocting by slow fire for 30 minutes; and filtering dregs and taking 250 milliliters of decoction. The 250 milliliters of decoction is divided into 6 parts to be taken, one dose is taken each day, and five doses are a period of treatment. The Chinese medicament is prepared from pure Chinese medicaments by a conventional preparation method, maintains medicinal nature of the medicaments, has effects of regulating qi activity, strengthening the spleen and eliminating dampness, regenerating fluid and benefiting stomach, and calming and eliminating parasite, achieves the aim of treatment and has no side effect.
Owner:彭洋法
Avian feed composition
The invention herein contemplates an avian feed composition and a method for administering and customizing the feed composition for one or more birds. The feed composition aids in preventing infection and illness including parasitic infestation.
Owner:BUREK SUSAN
Closed zero-emission factory-based aquaculture method for penaeus vannamei
InactiveCN108522381AAvoid Poor Water ColorPromote growthWater treatment parameter controlWater contaminantsDiseaseTurbidity
The invention relates to a closed zero-emission factory-based aquaculture method for penaeus vannamei. Before shrimp seeds are put into a culture water body, the salinity, pH, total alkalinity and total hardness are of the culture water body adjusted to required ranges, denitrification type biological floc specie, and then the shrimp seeds are put into the culture water body for management. Penaeus vannamei cultured by the method grows well, and the culture problems of poor color caused by imbalance of carbon and nitrogen, long-term standard exceeding of ammonia nitrogen and nitrite, a high pHvalue or sharp changes, breeding of blue-green algae, parasitic infestation, long-term turbidity of water bodies, serious pollution, frequent diseases and the like are avoided.
Owner:广州普麟生物制品有限公司 +1
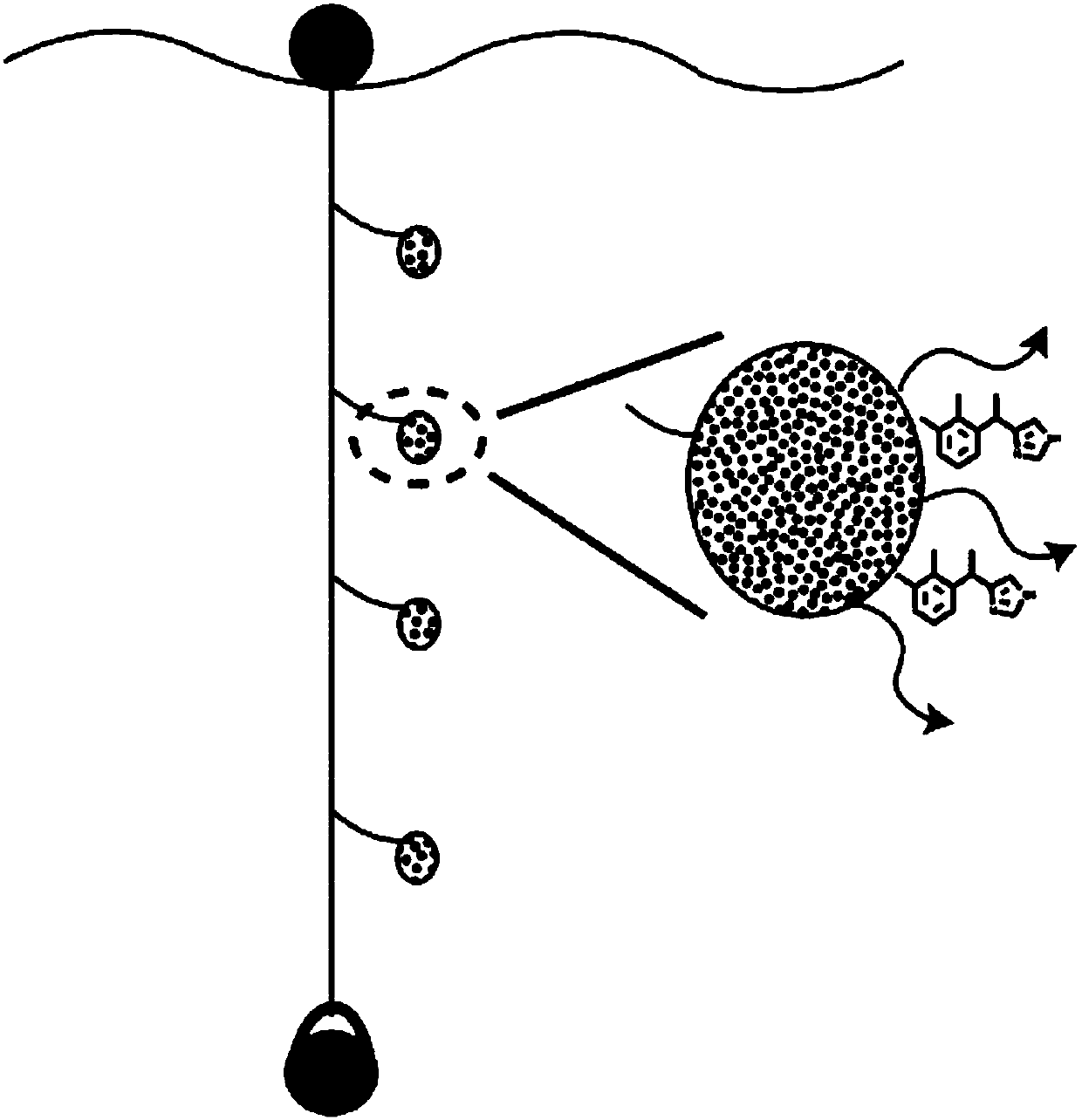
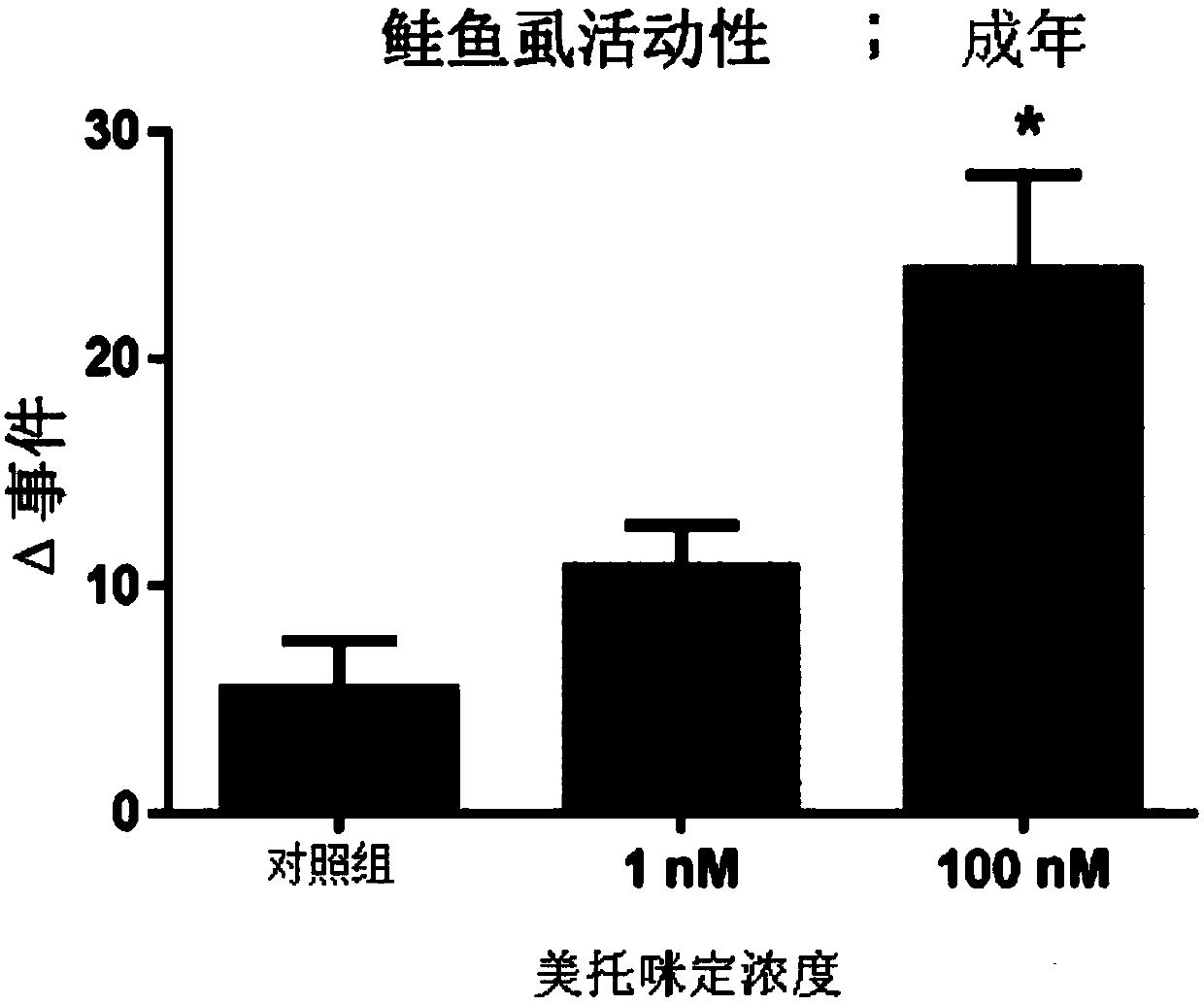
Medetomidine for use in controlling parasitic crustaceans on fish
InactiveUS20180146647A1Reduce and prevent marine biofoulingIncrease water flowBiocideOrganic active ingredientsCrustaceanWater flow
Medetomidine or a salt thereof for use in controlling parasitic crustaceans, such as sea lice, on fish, e.g. salmon. A method of improving water flow into and out of a cage or net for fish farming, by providing said cage or net with a surface coating containing medetomidine or a salt thereof in an amount effective to reduce biofouling of said cage or net. The coating is capable of releasing medetomidine or the salt thereof into the water in the cage or net in an amount effective to reduce or prevent parasitic infestation of the fish in the cage or net.
Owner:I TECH
Daimeton (sodium) suspension injection and preparation thereof
InactiveCN101322686AConvenient clinical administrationReduce stress responseAntibacterial agentsOrganic active ingredientsAdditive ingredientSulfamonomethoxine
The invention relates to a new veterinary medicine preparation (sulfamonomethoxine suspension injection or sulfamonomethoxine sodium suspension injection) and a preparation method thereof; the new veterinary medicine preparation is prepared by taking sulfamonomethoxine or sulfamonomethoxine sodium as main drug and other excipients for assist. The percentage of the main ingredients in the suspension is as follows: 1%-50% (W / V) of sulfamonomethoxine or sulfamonomethoxine sodium and the rest of drug excipients; the excipients include a suspending agent and a disperse medium. The invention takes oil solvent as the disperse medium to prepare sulfamonomethoxine or sulfamonomethoxine sodium into a long-acting and sustained-release preparation; by adopting intramuscular injection for drug administration, the in vivo acting time of the drug can be significantly prolonged, the times of drug administration are reduced and the blood concentration of diseased livestock can keep stable. The new veterinary medicine preparation of the invention is safe, stable, effective and controllable in quality, thus being widely applicable to the treatment of bacterial infection and parasitic infestation in livestock.
Owner:CHONGQING FANGTONG ANIMAL PHARMA
Long-acting spiro-isoxazoline formulations
ActiveUS10350196B2Simple compositionOrganic active ingredientsPharmaceutical non-active ingredientsBiopolymerParasitic Infestation
The invention describes a long-acting injectable veterinary composition comprising a spirocyclic isoxazoline, at least one biopolymer, and optionally, at least one veterinary acceptable-carrier, -solvent, -excipient, or any mixture thereof. The invention also includes a method of treating an animal with a parasitic infestation by administering the biopolymeric composition to the animal in need thereof, and a process for preparing the biopolymeric composition.
Owner:ZOETIS SERVICE LLC
Methods and compositions for rapid treatment of otitis externa
Methods for treating and preventing otitis externa with a course of treatment consisting of as little as a single dose are provided. The methods are practiced by topical administration of compositions having a lipid carrier, such as liposomes and non-vesicular lipids, to the outer ear canal. Such compositions lack viscocity-enhancing celluloses or adhesives, and are preferably not in the form of a gel. Active agents useful for treating pain, inflammation, fungal or parasitic infestation and / or infections in the outer ear are co-administered in or with the composition.
Owner:ELANCO US INC
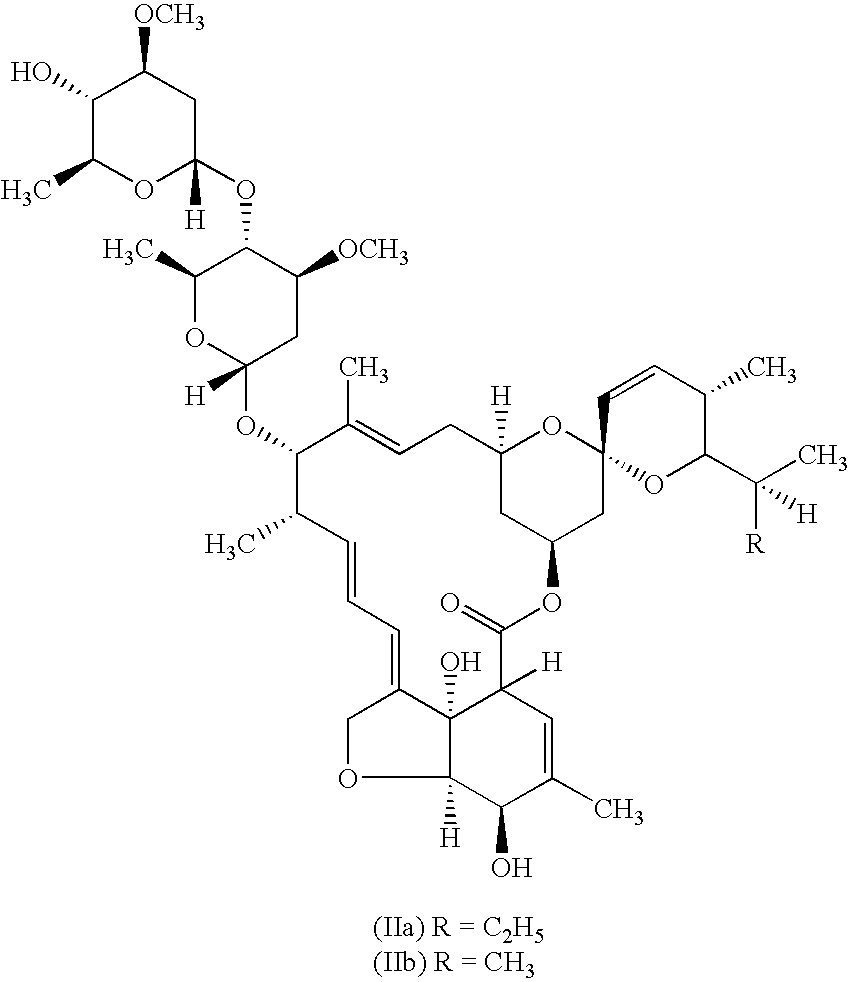
Anti-parasitic composition comprising a macrocyclic lactone and levamisole and method of treatment of parasitic infestation
InactiveUS9198430B2Obvious advantagesBiocidePharmaceutical delivery mechanismAntioxidantParasitic Infestation
Owner:ELANCO NEW ZEALAND
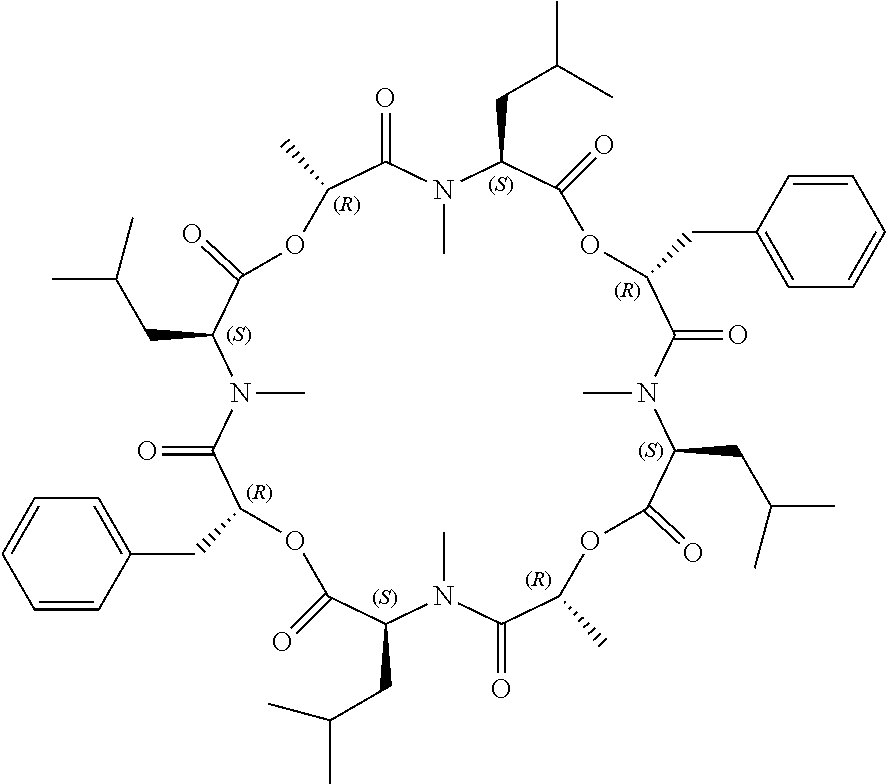
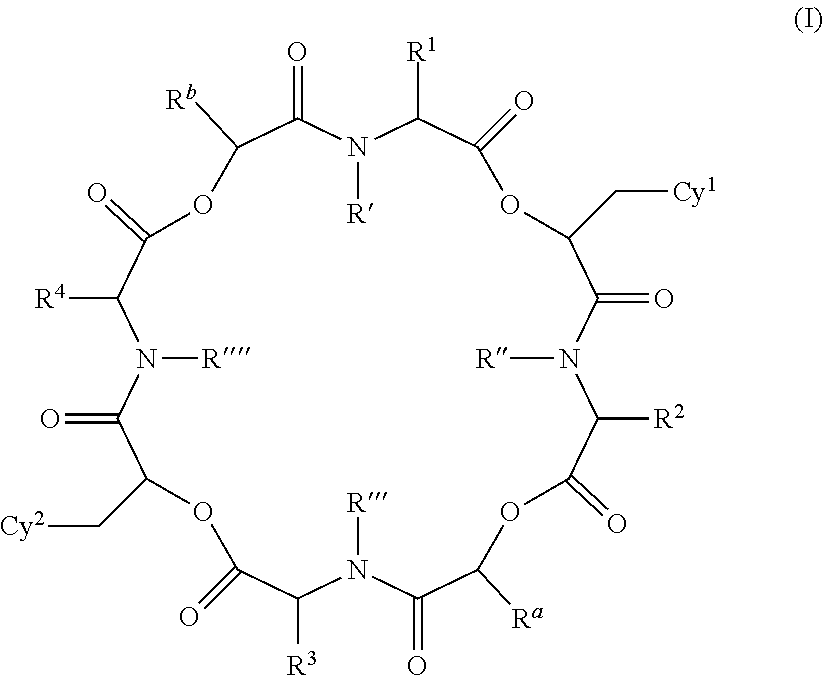
Anthelmintic depsipeptide compounds
The present invention provides cyclic depsipeptide compounds of formula (I) wherein the stereochemical configuration of at least one carbon atom bearing the groups Cy1, Cy2, R1, R2, R3, R4, Ra and Rb is inverted compared with the naturally occurring cyclic depsipeptide PF1022A. The invention also provides compositions comprising the compounds that are effective against parasites that harm animals. The compounds and compositions may be used for combating parasites in or on mammals and birds. The invention also provides for an improved method for eradicating, controlling and preventing parasite infestation in birds and mammals.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Medetomidine for use in controlling parasitic crustaceans on fish
Medetomidine or a salt thereof for use in controlling parasitic crustaceans, such as sea lice, on fish, e.g. salmon. A method of improving water flow into and out of a cage or net for fish farming, byproviding said cage or net with a surface coating containing medetomidine or a salt thereof in an amount effective to reduce biofouling of said cage or net. The coating is capable of releasing medetomidine or the salt thereof into the water in the cage or net in an amount effective to reduce or prevent parasitic infestation of the fish in the cage or net.
Owner:I TECH A B (SE)
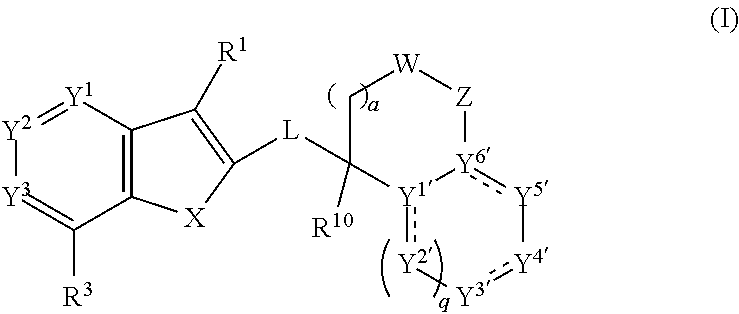
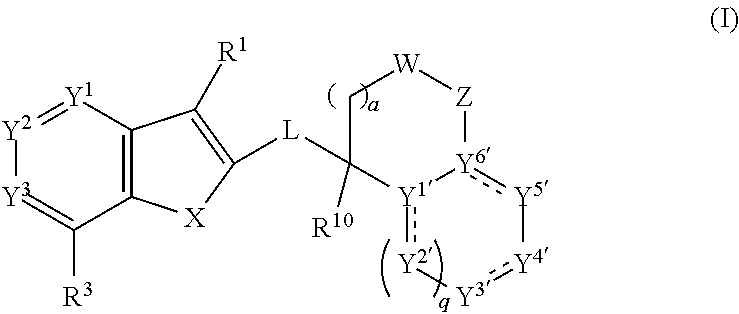
Anthelmintic aza-benzothiophene and aza-benzofuran compounds
ActiveUS20200299304A1Effective treatmentEffective prophylaxisOrganic active ingredientsOrganic chemistryAnthelmintic drugChemical compound
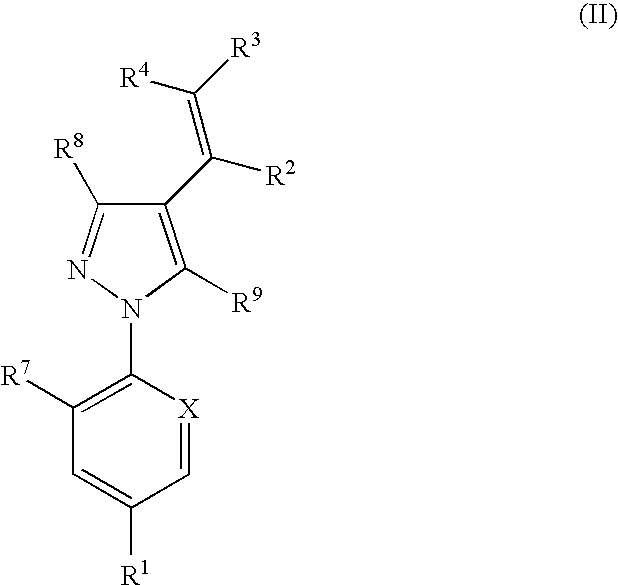
This invention provides for compounds of the formula:wherein the variables are defined herein, or a stereoisomer, tautomer, N-oxide, hydrate, solvate, or salt thereof, compositions comprising these compounds, and method for the treatment, control or prevention of a parasitic infestation or infection in an animal in need thereof by administering an effective amount of these compounds to said animal.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH +1
Methods and compositions for rapid treatment of otitis externa
Methods for treating and preventing otitis externa with a course of treatment consisting of as little as a single dose are provided. The methods are practiced by topical administration of compositions having a lipid carrier, such as liposomes and non-vesicular lipids, to the outer ear canal. Such compositions lack viscocity-enhancing celluloses or adhesives, and are preferably not in the form of a gel. Active agents useful for treating pain, inflammation, fungal or parasitic infestation and / or infections in the outer ear are co-administered in or with the composition.
Owner:ELANCO US INC
Anthelmintic combination
ActiveUS8440633B2Reduce frequencyBiocideCarbohydrate active ingredientsAvermectinParasitic Infestation
A composition comprising 2-desoxoparaherquamide and abamectin for the treatment of a parasitic infestation in mammals.
Owner:ZOETIS SERVICE LLC
Methods for identification of inhibitors of enzyme activity
InactiveUS20090215080A1Inhibitory activityCompound screeningApoptosis detectionParasitic InfestationInfective disorder
The invention discloses compositions and methods of synthesis to create novel ligands and drugs and identifying such compounds as inhibitors of enzyme targets for use in the treatment of clinical disorders, including cancer, infectious diseases, parasitic infestations, neurological disorders, reproductive disorders, inflammatory disorders, circulatory disorders, and metabolic disorders.
Owner:ACTIVESITE PHARMA INC
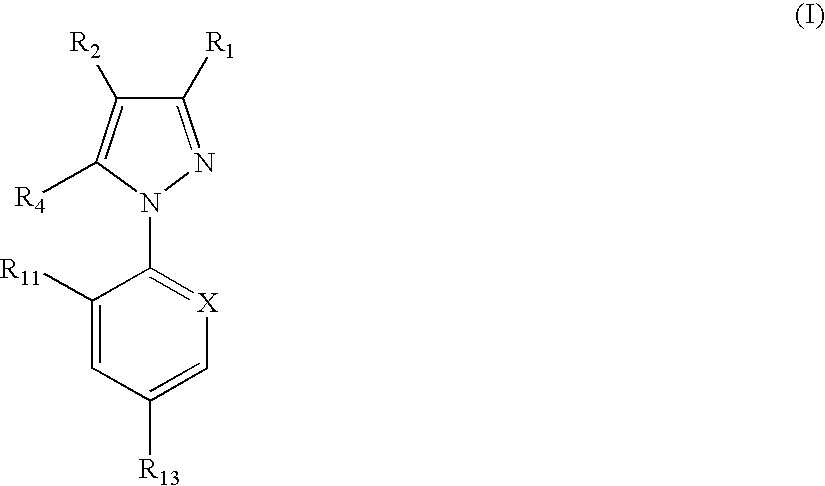
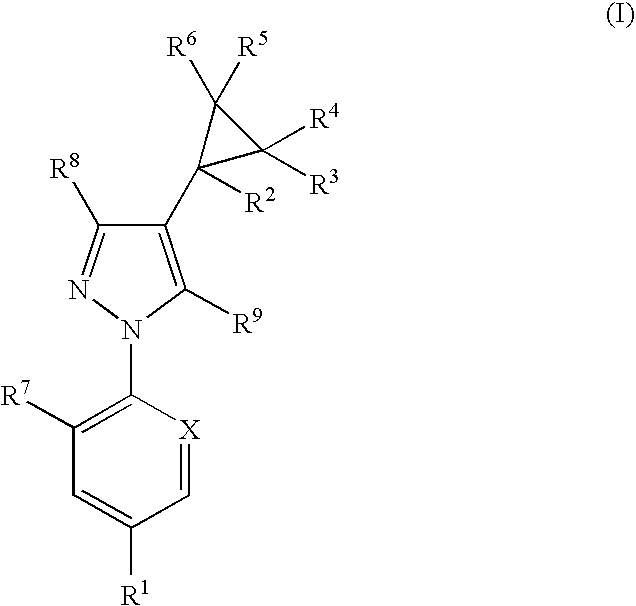
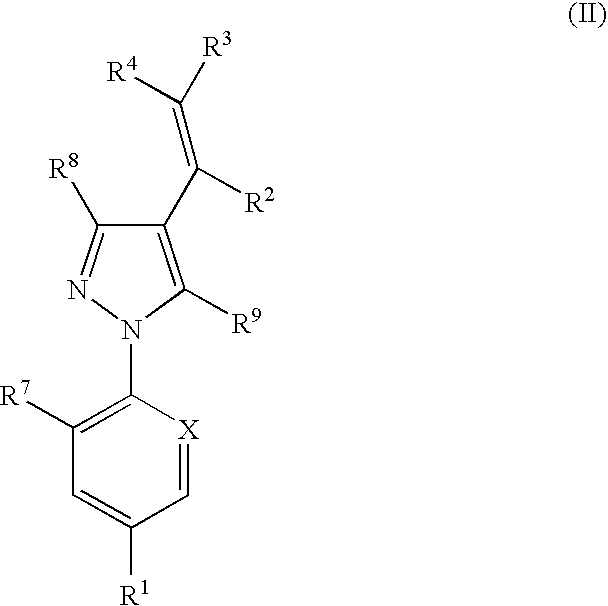
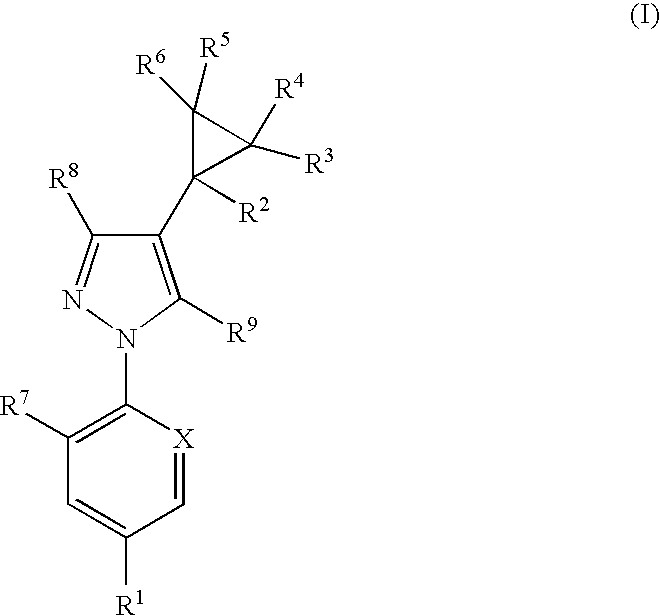
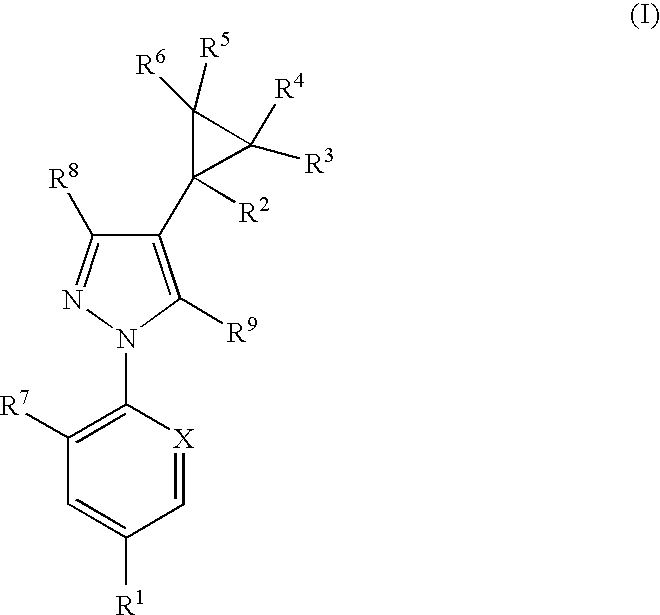
Phenylpyrazole injectable compositions
This invention relates to a veterinary composition comprising a fluorinated cyclopropylphenylpyrazole. The veterinary composition also comprises a veterinary acceptable carrier including a triglyceride, a solvating agent and optionally an antioxidant. The compositions are injectable. The invention also relates to a method of treating an animal with a parasitic infestation by administering the veterinary composition to the animal in need thereof.
Owner:ZOETIS SERVICE LLC
Solvent systems for pour-on formulations for combating parasites
ActiveUS8097266B2Effective and lasting destructionTreatment or prophylaxisBiocidePharmaceutical delivery mechanismBenzeneActive agent
This invention relates to pharmaceutical and veterinary formulations providing enhanced solvency and stability for pharmaceutical and veterinary agents for administration to animals, especially ruminants. In addition, the invention relates to pour-on formulations for combating parasites in animals, such as cattle and sheep. In some embodiments, this invention provides glycol-ether-based pour-on formulations comprising a composition comprising a flukicide, such as, for example, clorsulon (4-amino-6-trichloroethenyl-1,3-benzene disulfonamide) and / or a macrolide anthelmintic or antiparasitic agent. In other embodiments, the invention provides pour-on formulations comprising at least one active agent, a glycol ether, and a stability enhancer. This invention also provides for methods for eradicating, controlling, and / or preventing parasite infestation in animals, such as cattle and sheep.
Owner:MERIAL INC
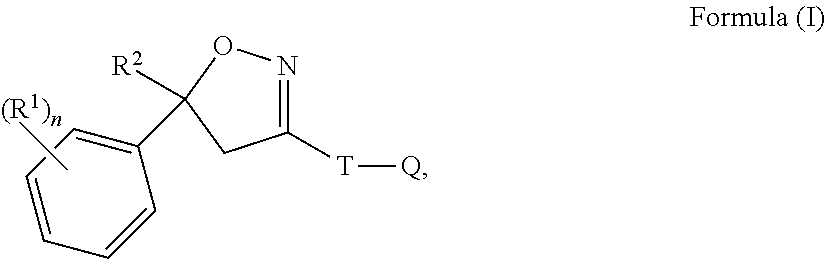
Isoxazoline compositions and use thereof in the prevention or treatment of parasite infestations in animals
This invention is directed to a pharmaceutical composition for drinking water administration comprising isoxazoline compounds of formula (I) and a polysorbate surfactant and diethylene glycol monoethyl ether (transcutol); and the use of the composition to treat or prevent parasite infestations of animals.
Owner:INTERVET INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com