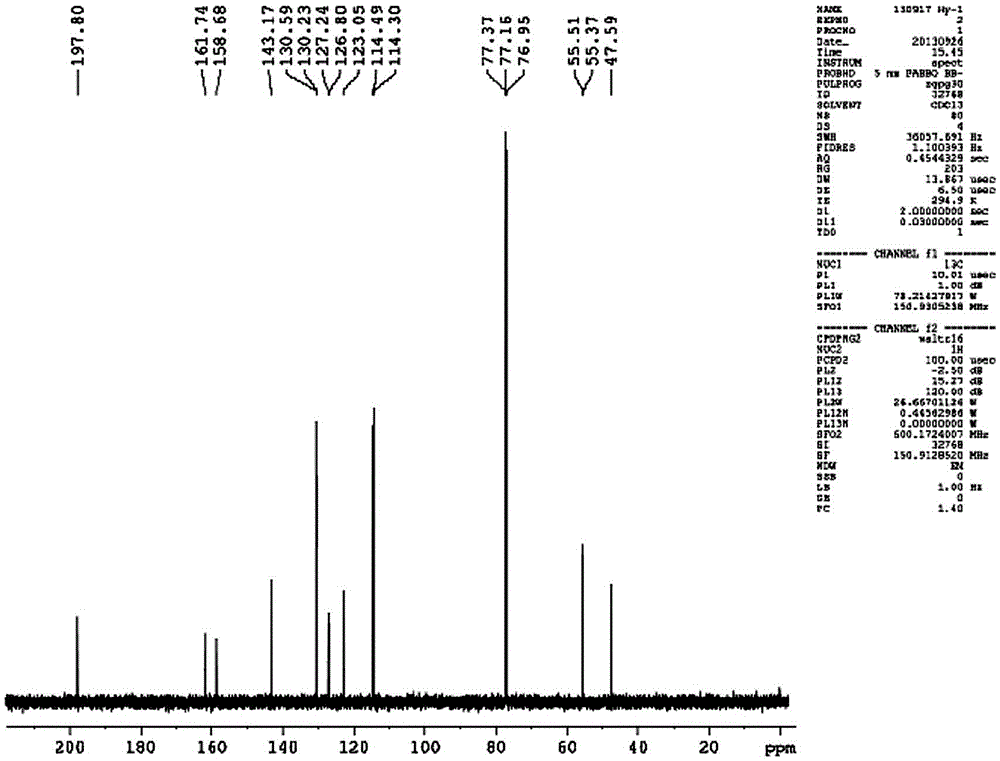
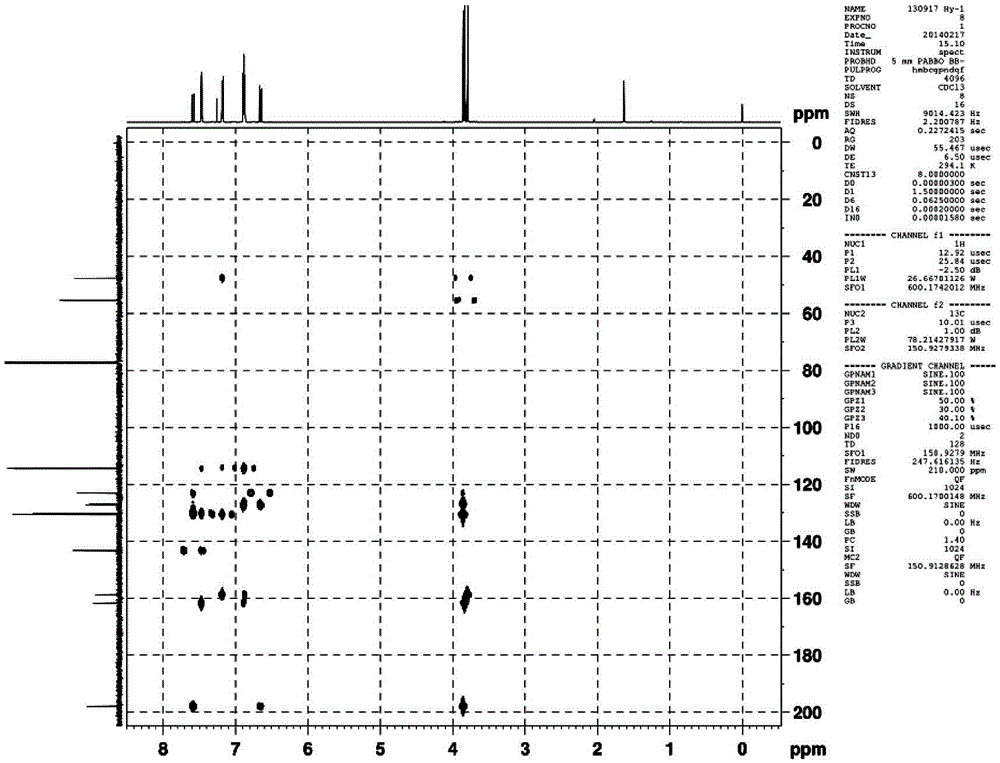
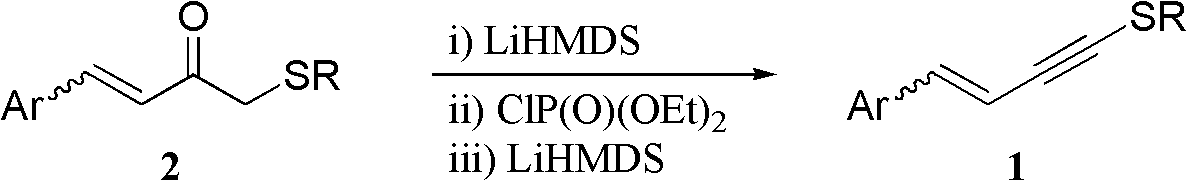Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "3-buten-2-one" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
3-Buten-2-one, also called methyl vinyl ketone, is the organic compound with the formula CH3CCH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof.
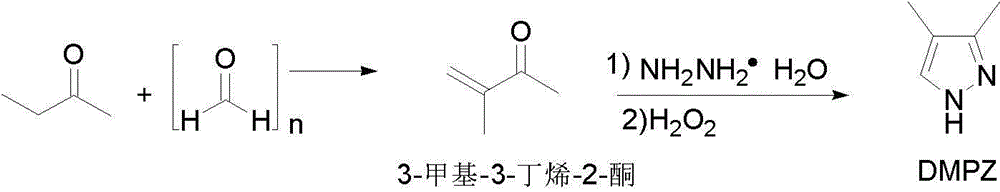
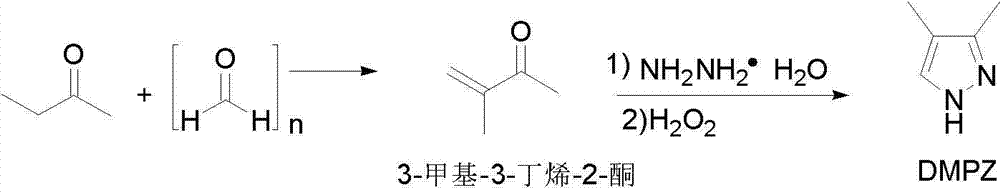
Preparation methods of 3,4-dimethyl pyrazole and 3,4-dimethyl pyrazole phosphate
ActiveCN102911119AReduce generationRaw materials are cheap and easy to getOrganic chemistryPhosphateHydrazine compound
The invention discloses preparation methods of 3,4-dimethyl pyrazole and 3,4-dimethyl pyrazole phosphate. The preparation method of the 3,4-dimethyl pyrazole comprises the following steps of: (1) reacting 2-butanone and paraformaldehyde at the temperature of 30-60 DEG C under the action of protonic acid for 4-20 hours in an organic solvent A, adjusting pH (Potential of Hydrogen) to be 7-8 after the reaction is finished, and performing atmospheric distillation to obtain 3-methyl-3-butylene-2-ketone; and (2) thermally reacting the 3-methyl-3-butylene-2-ketone prepared by the step (1) with hydrazine hydrate at the temperature of 40-50 DEG C for 2-8 hours, cooling reaction solution to 25-30 DEG C, adding an alkaline compound and hydrogen peroxide aqueous solution, and performing thermal reaction at the temperature of 30-60 DEG C for 3-10 hours after the addition is finished, wherein the obtained reaction solution is post-treated to obtain the 3,4-dimethyl pyrazole. The 3,4-dimethyl pyrazole is then reacted with phosphoric acid to form a salt so as to obtain the 3,4-dimethyl pyrazole phosphate. The method has the advantages of cheap and readily available raw materials, easiness in operation, high reaction yield, high product purity, capabilities of removing a large amount of concentrated sulfuric acid in a technological process and thus reducing the production of three wastes and high suitability for industrial large-scale production.
Owner:ZHEJIANG UNIV OF TECH +1
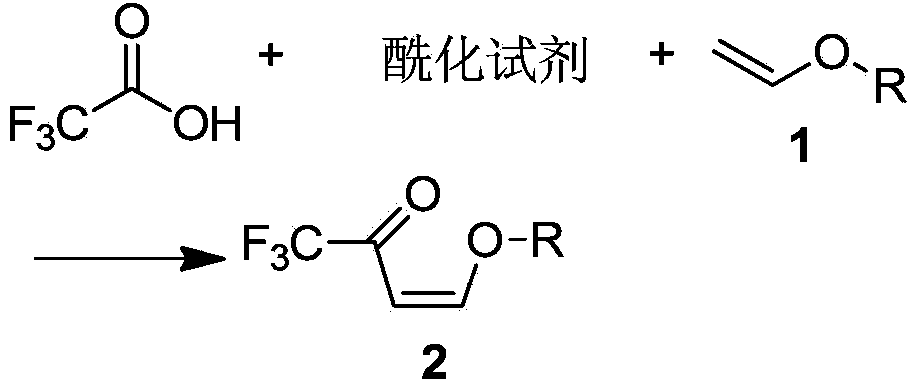
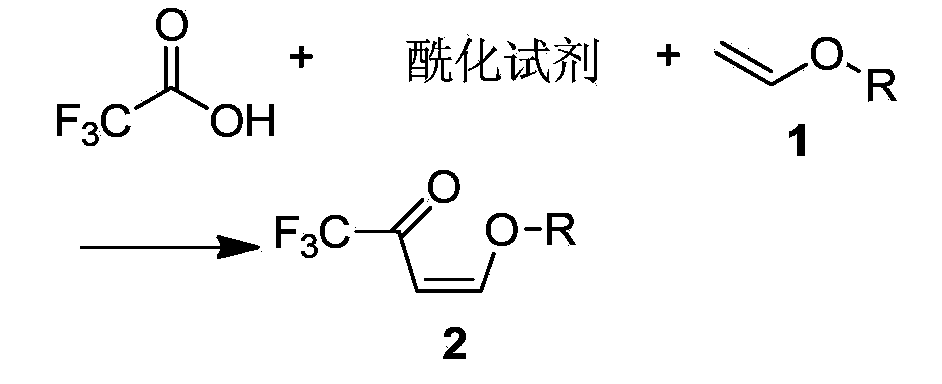
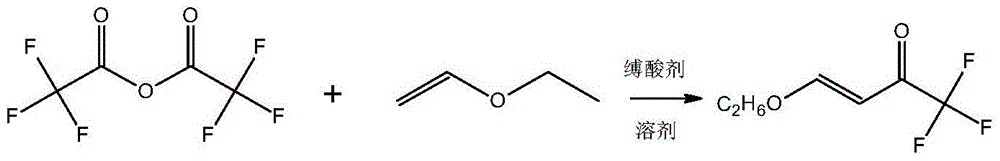
Preparation method of 3-trifluoromethylpyrazole intermediate
InactiveCN104326891AFew reaction stepsReduce manufacturing costCarbonyl compound preparation by condensationChemical recyclingKetoneReaction step
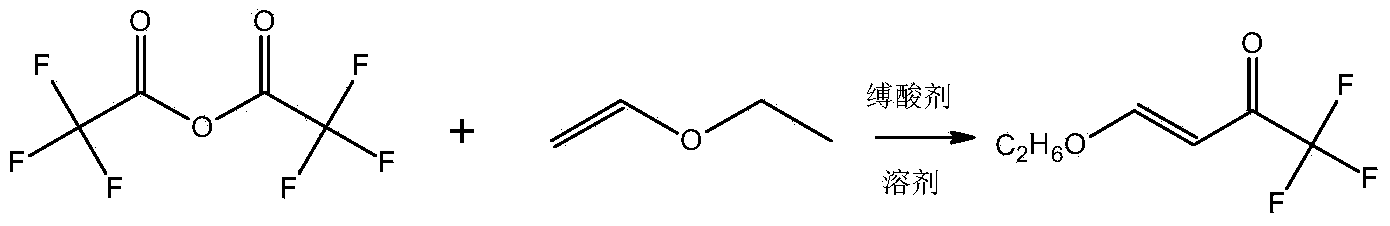
The invention discloses a preparation method of a 3-trifluoromethylpyrazole intermediate. The preparation method is characterized by comprising the following step: carrying out reaction on trifluoroacetic acid, an acylation reagent and vinyl alkyl ether disclosed as Formula 1 in an organic solvent in the presence of an acid-binding agent to obtain 4-alkoxy-1,1,1-trifluoro-3-butenyl-2-one, wherein the acylation reagent is one or more of phosgene, diphosgene and triphosgene, and R is C1-C6 alkyl group. The preparation method has the advantages of fewer reaction steps, mild reaction conditions, environment friendliness, simple after-treatment steps, high reaction yield, high product purity and low production cost, and is suitable for industrial production.
Owner:联化科技(上海)有限公司 +2
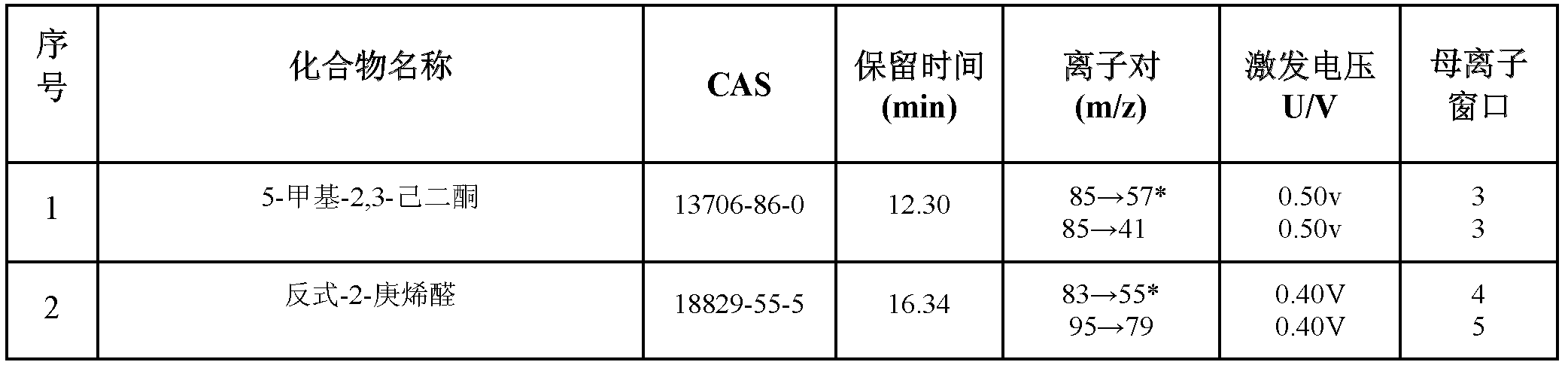
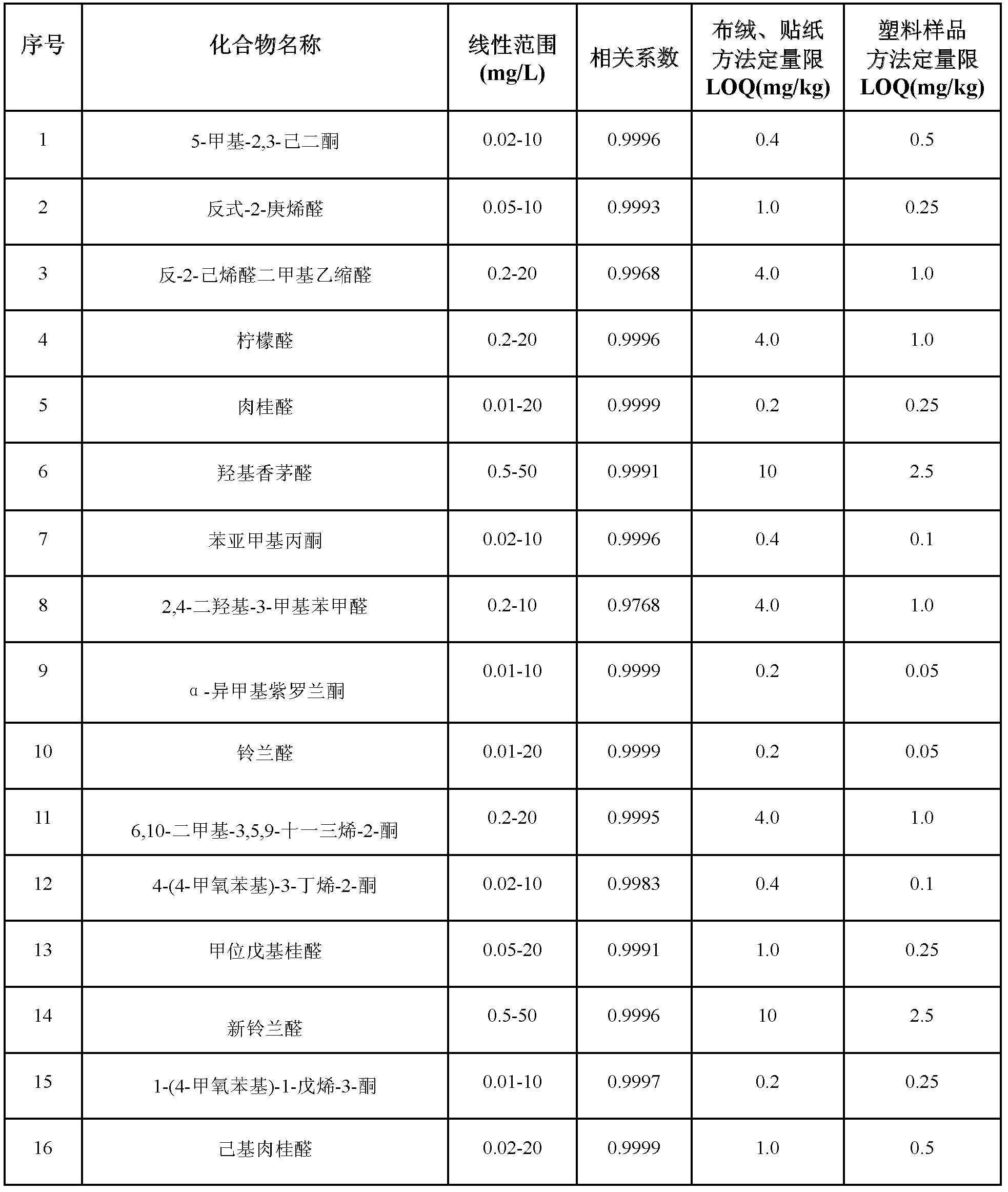
Method for simultaneously determining residual quantities of sixteen sensitized aldehyde and ketone perfumes in toy
The invention relates to a method for simultaneously determining residual quantities of sixteen sensitized aldehyde and ketone perfumes in a toy, and firstly discloses a method for simultaneously determining the residual quantities of the sixteen sensitized aldehyde and ketone perfumes consisting of 5-methyl-2,3-hexanedione, trans-2-heptenal, trans-2-hexenaldimethylacetal, citral, cinnaldehyde, hydroxycitronellal, benzalacetone, 2,4-dihydroxy-3-methylbenzaldehyde, alpha-isomethylionone, lilial, 6,10-dimethyl-3,5,9-undecatrien-2-one, 4-(4-methoxyphenyl)-3-butylene-2-one, alpha-amyl cinnamic aldehyde, lyral, 1-(4-methoxyphenyl)-1-pentene-3-one, and hexylcinnamaldehyde in the toy to fill the technological blank. The method which allows cloth toys, paster toys, and plastic toys (the ABS material, the PVC material and the PS material) to be detected has the advantages of wide coverage and strong applicability. The method which adopts the ion trap-mass spectrum in a qualitative and quantitative secondary mass spectrum MS / MS mode has a good qualitative and quantitative capability to complex matrix toy samples.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
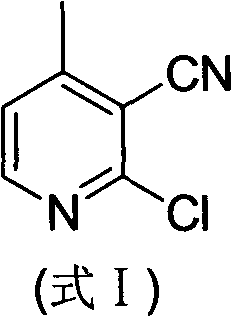
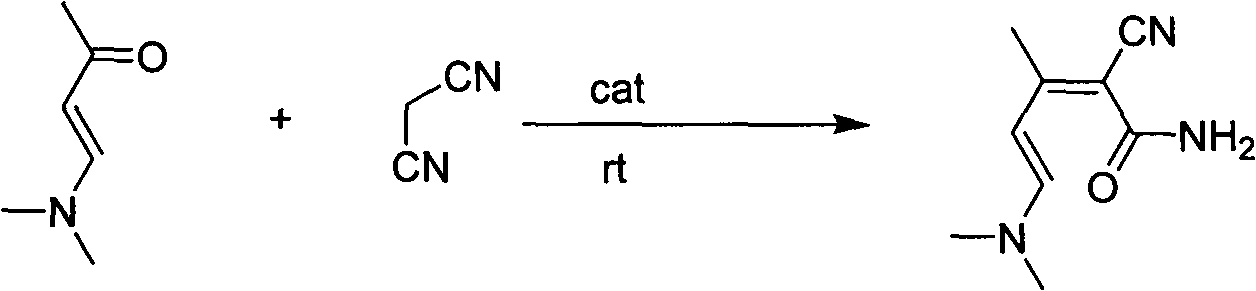
Preparation method of 2-chloro-4-methyl nicotinonitrile
The invention discloses a preparation method of 2-chloro-4-methyl nicotinonitrile. The preparation method disclosed by the invention comprises the following steps: with (E)-4-(dimethylamine) yl-3-butene-2-ketone and malononitrile as raw materials, catalyzing and condensing; and finally, chlorinating closed-loops to obtain 2-chloro-4-methyl nicotinonitrile under the effect of phosphorus oxychloride and phosphorus pentachloride. The total yield of the two reactions can reach 55.7%. The preparation method is simple in process, convenient to operate, low in equipment demand and very applicable to industrial production.
Owner:上海品沃化工有限公司
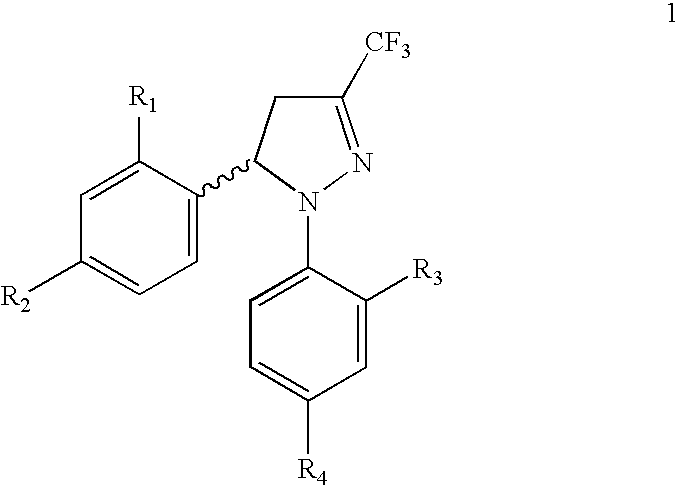
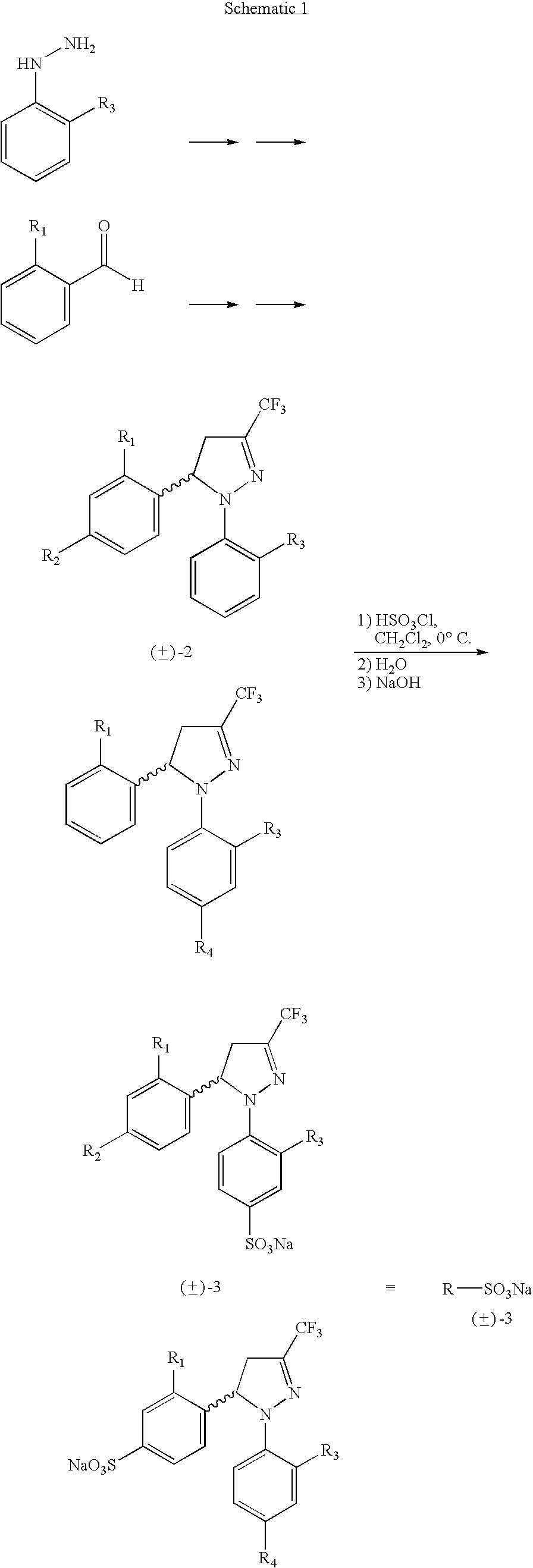
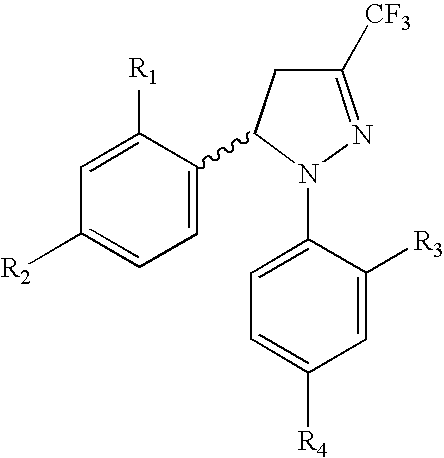
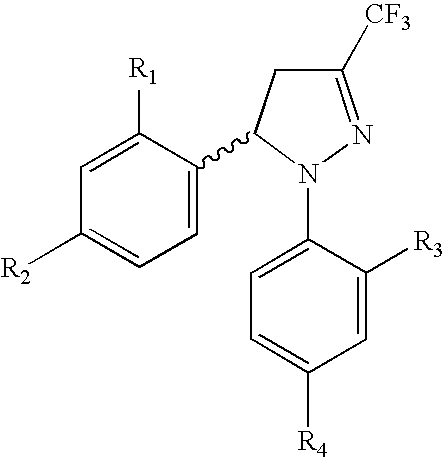
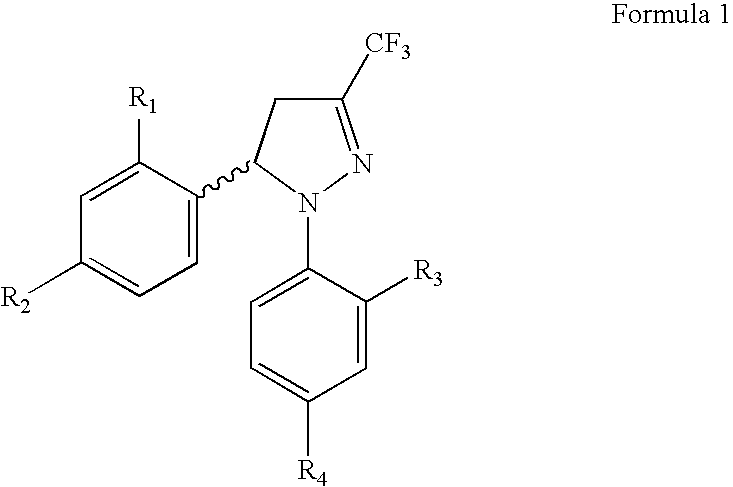
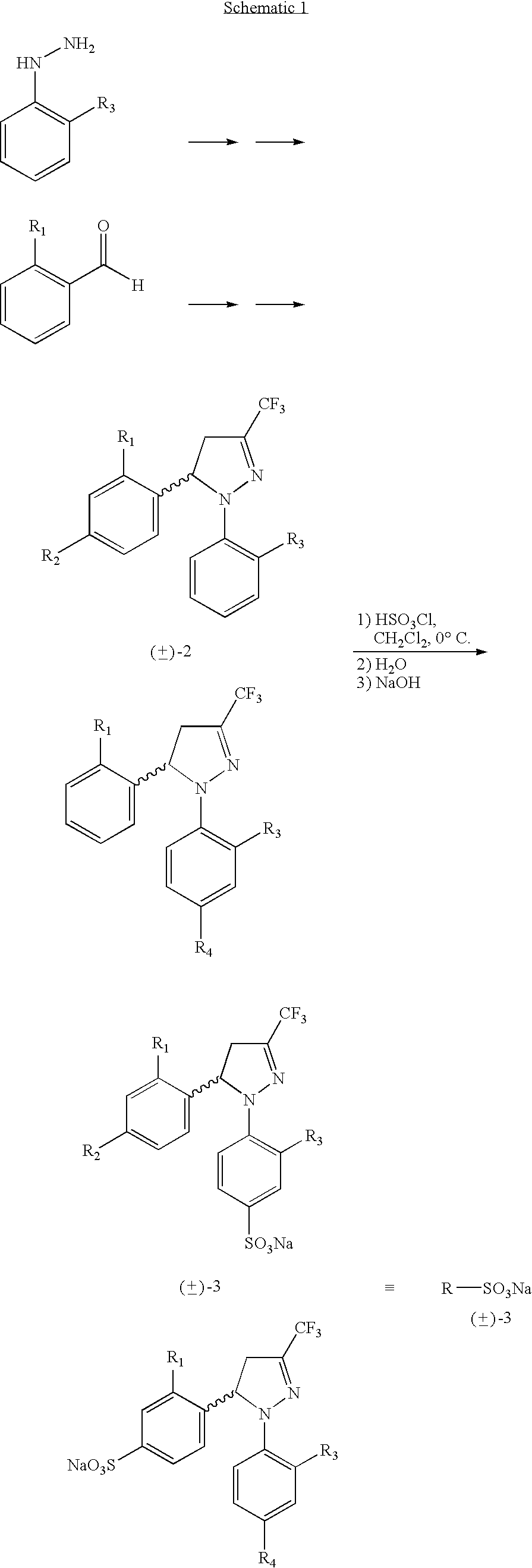
Procedure for the preparation of racemic and enantionmerically pure derivatives of 1,5-diaryl-3-trifluoromethyl-Delta2-pyrazolines
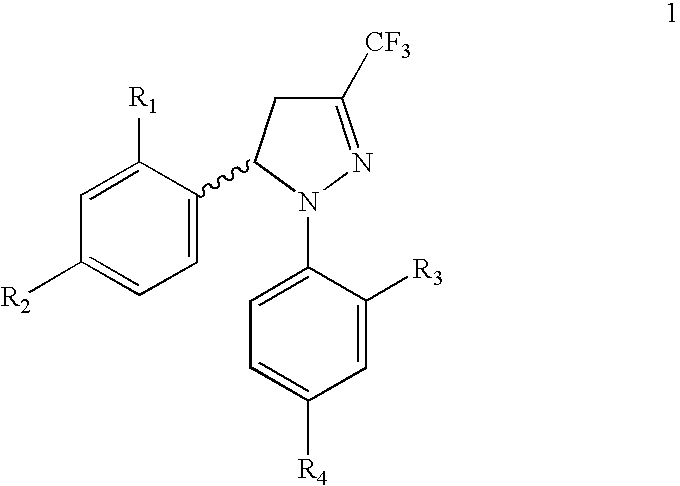
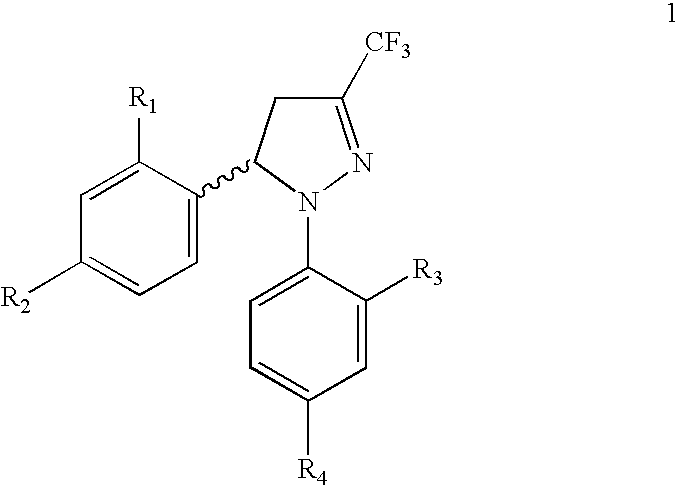
Procedure for preparation of compounds with the general formula 1, which include the racemic mixtures (±)-1, and the enantiomeric ally pure compounds (−)-1 and (+)-1, wherein R1 and R3, like or different, represent an atom of hydrogen, chlorine, fluorine, a methyl, trifluoromethyl or methoxy group; R2 represents an atom of hydrogen, chlorine, fluorine, a methyl, trifluoromethyl, methoxy, trifluoromethoxy, methylsulphonyl or aminosulphonyl group; R4 represents an atom of hydrogen, chlorine, fluorine, a methyl, trifluoromethyl, methoxy, trifluoromethoxy, methylsulphonyl or aminosulphonyl group, with the condition that one of the substituents R2 or R4 is a methylsulphonyl or aminosulphonyl group; which involves obtaining the racemic mixture with the general formula (±)-1 by reacting an (E)-1,1,1-trifluoro-4-aryl-3-buten-2-one with a phenylhydrazine, followed by a treatment with chlorosulphonic acid, or by reacting with chlorosulphonic acid followed by a reaction with sodium hydroxide and, finally, with thionyl chloride. The product obtained by either of these methods is made to react with ammonium carbonate or ammonia, or with sodium sulphite and methyl iodide or methyl sulphate. In addition, to obtain the enantiomerically pure compounds with the general formula 1 by resolving the racemic mixture with the general formula (±)-1, a reaction is effected with optically active ephedrine, followed by formation of the sodium salt of each enantiomer, reaction with thionyl chloride and ammonium carbonate or ammonia, or instead with thionyl chloride followed by sodium sulphite and methyl iodide or methyl sulphate to thereby obtain separately the enantiomerically pure compounds with the general formulae (−)-1 and (+)-1.
Owner:LAB DEL DR ESTEVE SA
Production method of beta-ionone epoxide
InactiveCN104557792AImprove economyLess corrosiveOrganic chemistryChemical recyclingFood flavorNitrogen gas
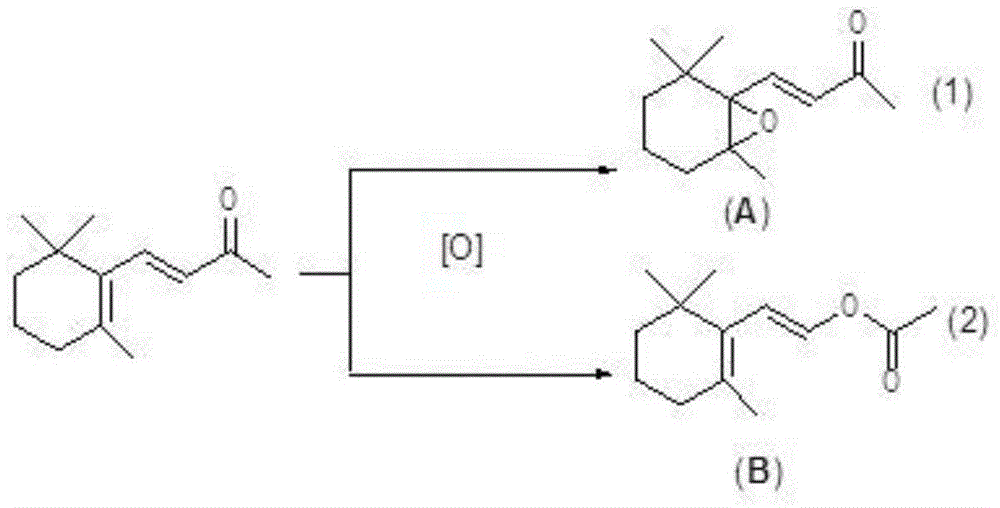
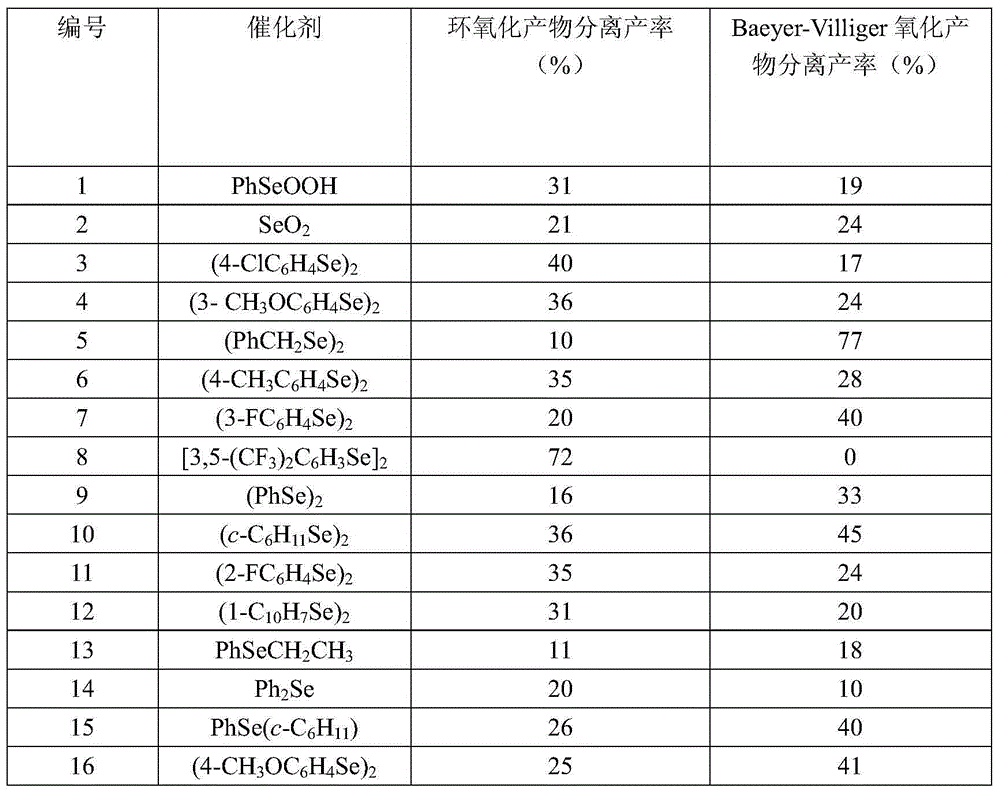
The invention discloses a production method of beta-ionone epoxide in the field of flavor production, which comprises the following steps: in a nitrogen protective atmosphere, oxidizing beta-ionone in a solvent by using di(3,5-ditrifluoromethylphenyl)diselenide as a catalyst and hydrogen peroxide as an oxidizer while controlling the temperature of the reaction system at 0-40 DEG C to obtain the epoxidation product 4-[2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptyl-1-yl]-3-butenyl-2-one. The method can synthesize the 4-[2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptyl-1-yl]-3-butenyl-2-one at high selectivity. The oxidizer is clean, has high economical efficiency, and is harmless to the environment since the reduction byproduct of the oxidizer is only water. The method does not use any metal catalyst, and the used organic selenium catalyst is ecologically friendly. The reaction is performed under mild conditions in a neutral environment, and has small corrosivity for equipment, so the equipment is durable; and the catalyst is recoverable, and thus, is more suitable for industrial production.
Owner:YANGZHOU UNIV
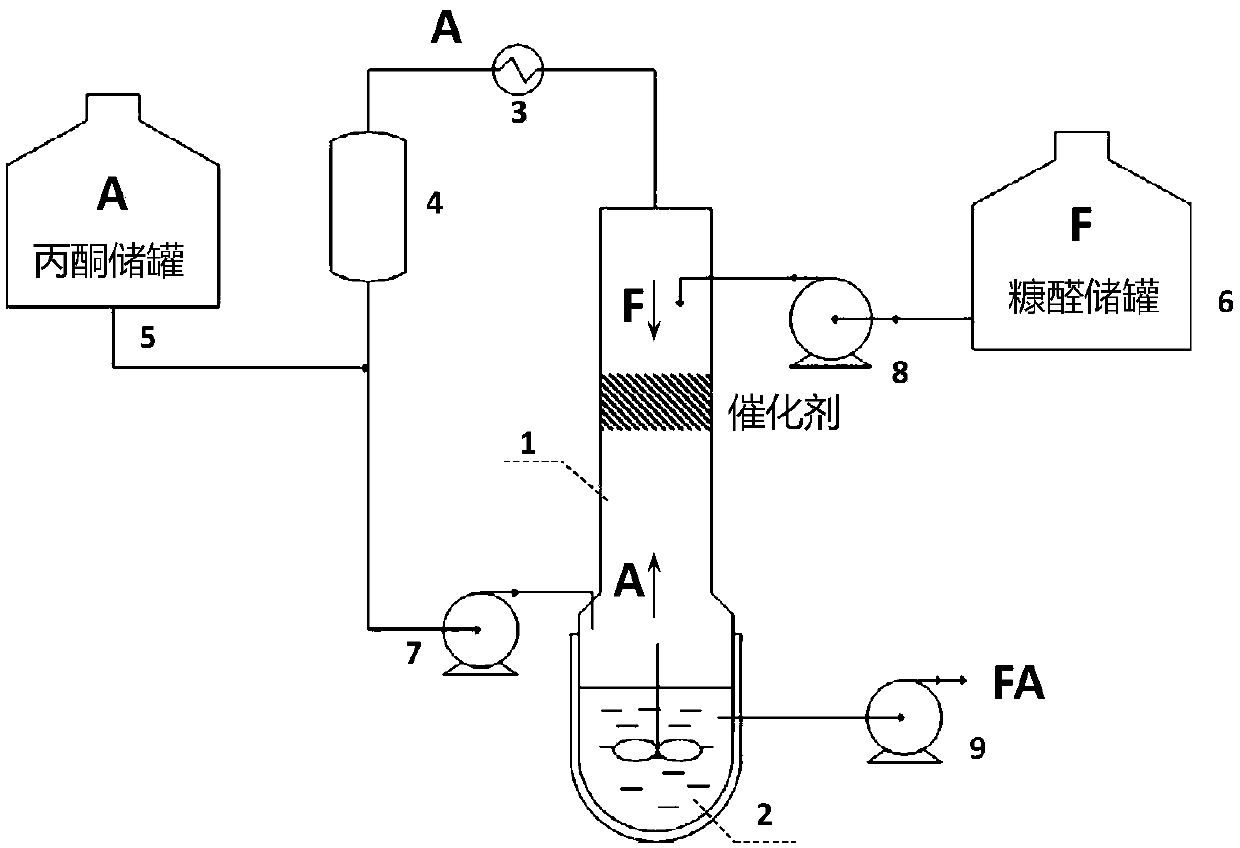
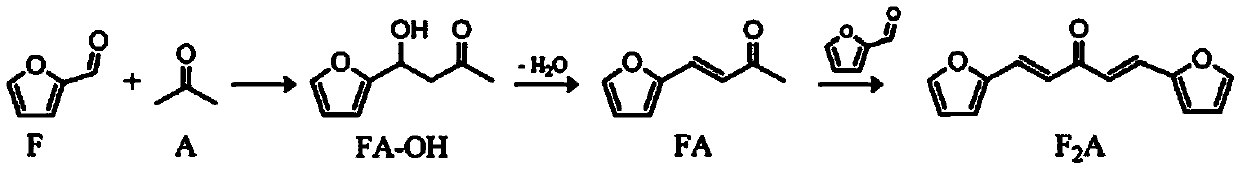
Preparation method of 4-(2-furyl)-3-butylene-2-ketone
The invention discloses a preparation method of 4-(2-furyl)-3-butylene-2-ketone, which comprises the following steps of using anion exchange resin as a catalyst and acetone and furfural as raw materials; dripping a certain amount of furfural at the temperature below 20 DEG C to 40 DEG C; keeping the temperature for a certain time after dripping; filtering and removing the catalyst; and steaming unreacted raw materials to obtain a solid, namely 4-(2-furyl)-3-butylene-2-ketone. The invention has the advantages of high yield rate, less three wastes and simple operation.
Owner:崔磊
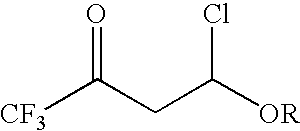
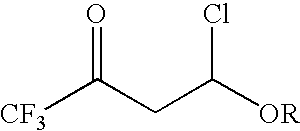
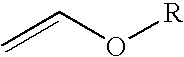
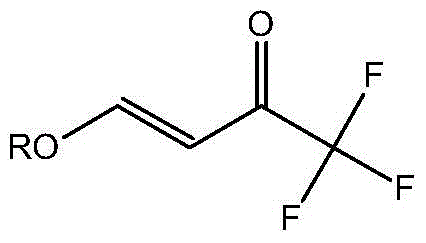
Preparation method for 4- alkoxy-1,1,1- trifluoro-3- butane-2-ketone
ActiveCN104072347ASimple processEasy to operateCarbonyl compound preparation by condensationChemical reactionTrifluoroacetic acid
The invention discloses a method for synthesizing 4-alkoxy-1,1,1-trifluoro-3-butane-2-ketone by taking trifluoroacetic acid, vinyl alkyl ether and phosgene as raw materials in the presence of a solvent and an acid-binding agent. A chemical reaction formula is as shown in the specification, wherein R is methyl, ethyl, n-propyl, isopropyl and normal-butyl. The preparation method disclosed by the invention is simple and convenient in process and simple to operate, has reaction yield of 85.6% (in terms of trifluoroacetic acid), and product purity of 98%, and is low in production cost and suitable for industrial production.
Owner:HUNAN HAILI CHEM IND
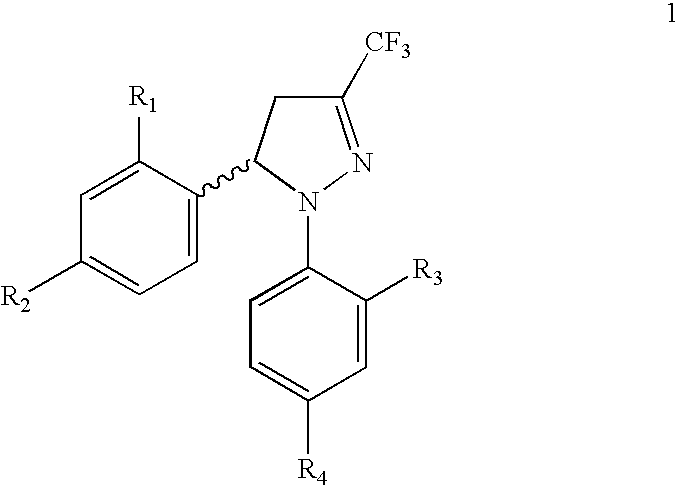
Procedure for the preparation of racemic and enantiomerically pure derivatives O F 1,5 diaryl-3-trifluorromethyl-delta2-pyrazolines
InactiveUS20050096373A1Simple and inexpensive methodEasy to useBiocideOrganic active ingredientsEphedrineChlorosulfuric acid
Owner:LAB DEL DR ESTEVE SA
Preparation method of leaching liquid for repairing heavy metal polluted soil
InactiveCN105542779ALow costEfficient removalOrganic fertilisersSoil conditioning compositionsKetoneNitrogen gas
The invention relates to a preparation method of a leaching liquid for repairing heavy metal polluted soil; the method comprises the steps: putting dioctyl sebacate, 4-(2,6,6-trimethyl-2-cyclooctene-1-yl)-3-butene-2-one, 1,4-dimethyl piperazine, 2-pyridinemethanol, oleanolic aldehyde, salicin, 4-isoquinolinamine and alkyl polyoxyethylene ether acetate into a flask provided with a stirrer, a thermometer and a reflux condensing tube, adding deionized water and polycarboxylic acid salt, heating up to 850 DEG C, introducing nitrogen for protection, and stirring for 45-75 min; and then slowly dropwise adding ammonium citrate, after carrying out heat preservation for 2 h, adding sodium alginate, sodium ethoxide, polyoxypropylene diol and fatty alcohol polyoxyethylene ether, then carrying out a reaction for 4-7 h, cooling to room temperature, and thus obtaining the leaching liquid for repairing the heavy metal polluted soil. The leaching liquid provided by the invention can effectively remove organic pollutants in the soil while repairing the heavy metal contaminated soil, thereby greatly reducing the soil repairing cost.
Owner:缪琼华
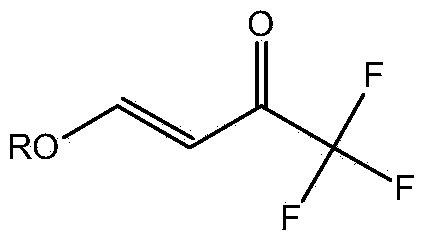
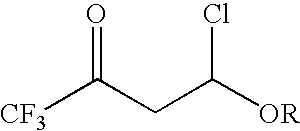
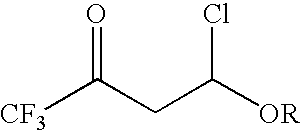
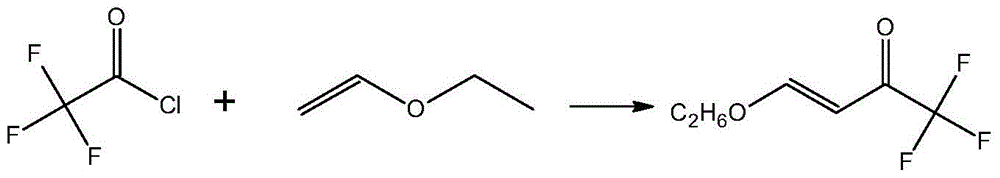
4-chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, their preparation and their use in preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones
ActiveUS20090005603A1Organic compound preparationPreparation by hydrogenolysisVinyl etherAlkoxy group
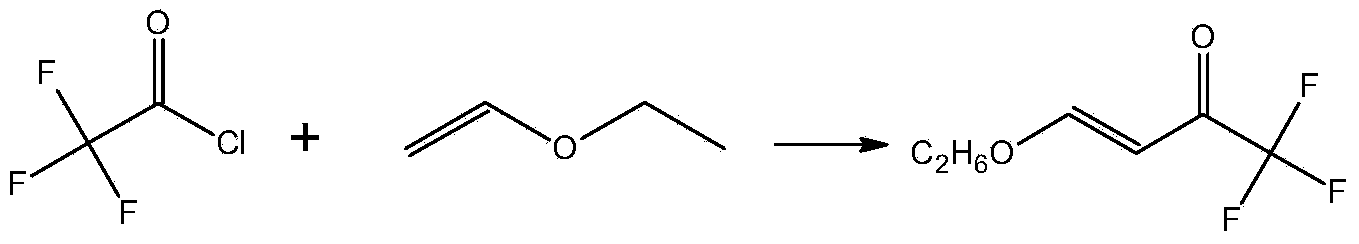
4-Chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, prepared by reacting alkyl vinyl ethers with trifluoroacetyl chloride, are useful for preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones.
Owner:CORTEVA AGRISCIENCE LLC
Solid base catalyst and preparation process of 4-(2-furyl)-3-buten-2-one
ActiveCN109603795AImprove stabilityReduce manufacturing costOrganic chemistryMolecular sieve catalystsSolid base3-buten-2-one
The invention provides a solid base catalyst and a preparation process for preparing 4-(2-furyl)-3-buten-2-one. The catalyst comprises an alkaline carrier, an activated auxiliary agent and a water-resistant auxiliary agent. The catalyst has the characteristics of easy preparation and low preparation cost. The continuous production process is particularly suitable for condensation of furfural withacetone to prepare 4-(2-furyl)-3-buten-2-one, can effectively inhibit occurrence of multi-condensation side reactions, and has a high product yield. At the same time, the catalyst under the process has the characteristics of high activity, high product selectivity, good water resistance and uneasiness to deactivate.
Owner:WANHUA CHEM GRP CO LTD
Benzofuro[2,3-c]pyridine compound and its synthesis method
The invention relates to a benzofuran [2, 3-c] pyridine compound and a synthetic method thereof. The benzofuran [2, 3-c] pyridine compound has a general formula shown in the specification, wherein R1 is phenyl, 3-bromo-phenyl, 4-bromo-phenyl, 4-chloro-phenyl, 4-methoxy-phenyl, 3-methyl-phenyl or 4-methyl-phenyl; R2 is hydrogen or 5-chlorine, 5-bromine, 5-tert-butyl, 3,5-ditert-butyl, 4-methoxy, 5-methoxy, 5-methyl, 3,5-dimethyl and 4,5-dimethyl; R3 is methyl or phenyl, 4-bromo-phenyl, 3-bromo-phenyl, 2-chloro-phenyl, 4-chloro-phenyl, 4-methoxy-phenyl and 4-methyl-phenyl. According to the synthetic method disclosed by the invention, alpha-bromoacetophenone or a derivative thereof as well as 4-(2-hydroxyphenyl)-3-butylene-2-ketone or a derivative thereof are used as reactants, and a one-pot method is adopted to synthesize the benzofuran [2, 3-c] pyridine compound. The synthetic method is simple and convenient in step, mild in condition and high in yield.
Owner:WUHAN UNIV OF TECH
Tar-containing wastewater treatment method
InactiveCN105540919AReduce dosageHigh oil removal rateFatty/oily/floating substances removal devicesWater contaminantsHigh concentrationFatty alcohol
The invention relates to a tar-containing wastewater treatment method. The method comprises orderly adding a de-emulsifier, 4-(2, 6, 6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one, N, N-(2-hydroxytrimethylene)bis(trimethylammonium) diiodide, salicin, n-butyllithium, ammonium citrate, oleanic aldehyde, fatty alcohol-polyoxyethylene ether, polyoxypropylene diol, nanometer titanium dioxide, polycarboxylate, sodium alginate, sodium ethoxide and aluminum trichloride. The tar-containing wastewater treatment method synchronously realizes demulsification, flocculation, bridge formation and foaming, has a small use amount and a high oil removal rate and is especially suitable for high-concentration tar-containing sewage treatment.
Owner:HANGZHOU FUYANG WEIWEN ENVIRONMENTAL PROTECTION TECH CO LTD
Method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione
InactiveUS10329267B2Produced inexpensively and safelyHigh yieldOrganic active ingredientsOrganic compounds purification/separation/stabilisationFuranAlcohol
The invention addresses the problem of providing a method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione that is suited to industrial production. The invention provides a method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione by reacting 3-bromo-3-buten-2-one and 2-hydroxy-1,4-naphthoquinone in the presence of a solvent, then obtaining crystals of 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione by adding an alcohol-based solvent and / or water to the reaction system, and treating the crystals by using a specific adsorbent in the presence of a solvent.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Water treatment agent for removing organic pesticide in wastewater
InactiveCN105600987AFast precipitationStrong complexing abilityWater contaminantsTreatment involving filtrationDistarch glycerolKetone
The invention relates to a water treatment agent for removing an organic pesticide in wastewater. The water treatment agent is prepared by compounding the following raw materials: alpha-humulene, calcium carboxymethylcellulose, 5,5-dimethylimidazolidine-2,4-diketone, 3,5,7-trihydroxy-4`-methoxyflavone, (3beta)-8,24-lanostadiene-3-alcohol, 4-(2,6,6-trimethyl-2-cyclooctene-1-yl)-3-butene-2-ketone, crosslinked polyvinyl pyrrolidone, alpha-furfural, alpha-methyl-4-(1-methyl ethyl)benzenepropanal, 1,2-dyhydroxyl-1,2,3-propanetriformic acid, ethyl myristate, distarch glycerol, and 7-hydroxyl-3,7-dimethyl-octanal. The water treatment agent is strong in complexing power with the organic pesticide, is fast in sedimentation speed of formed complex, has the removal rate up to 99 percent, is low in toxicity, is small in dosage, does not generate harm to a water body, is small in occupation area, and is low in treatment cost.
Owner:杨俊锋
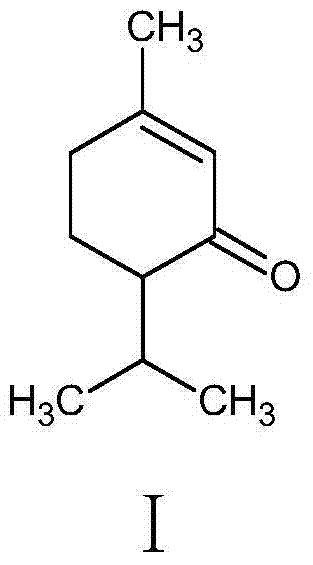
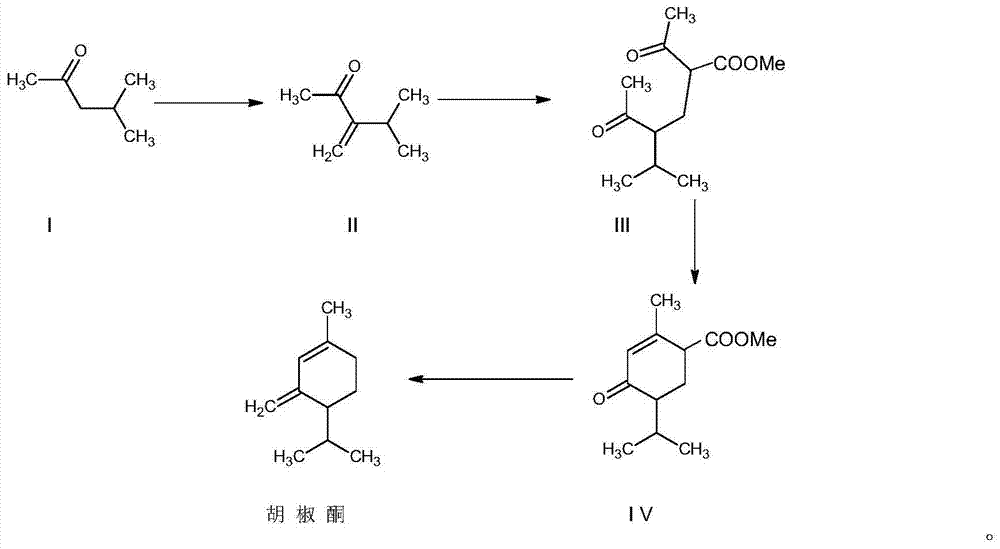
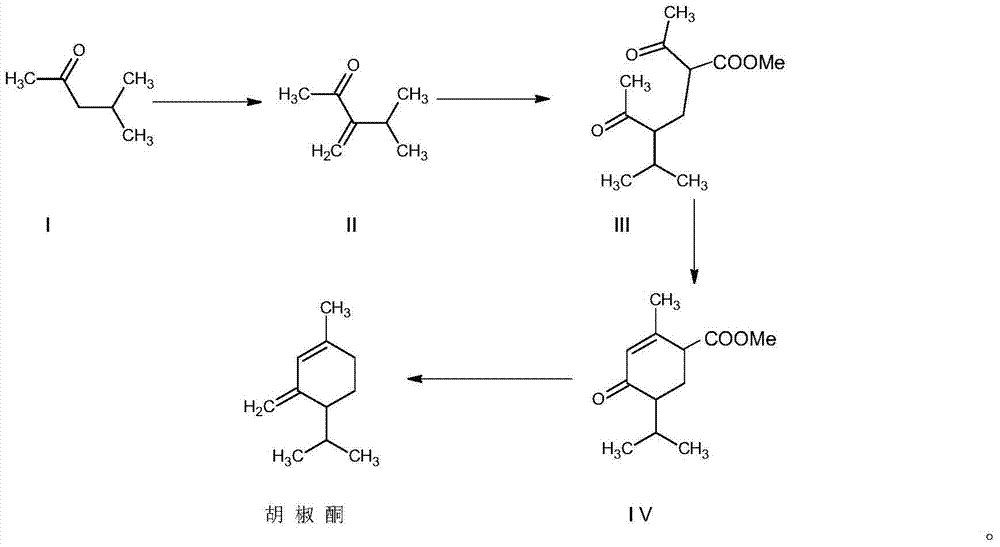
Synthesis method for piperitone
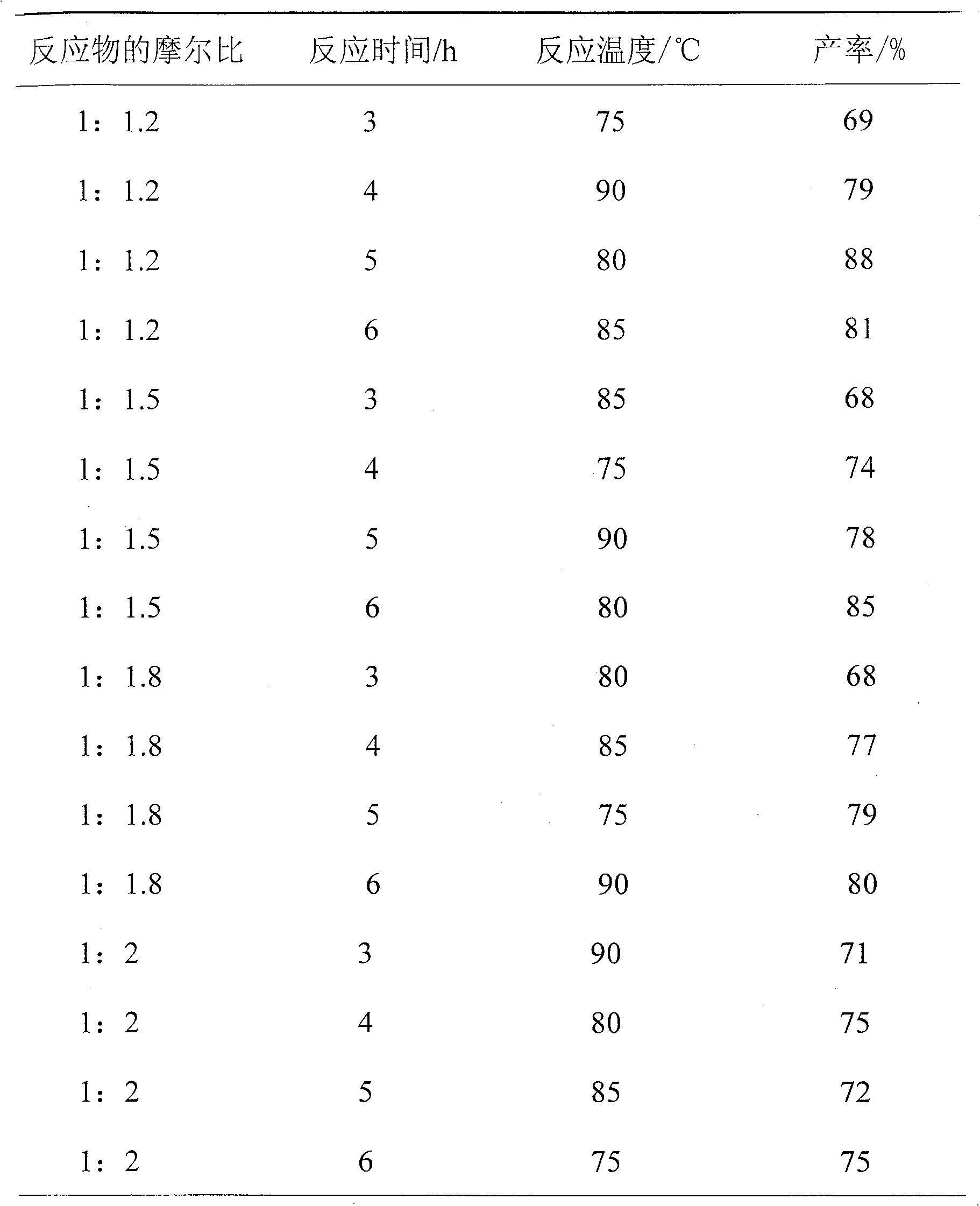
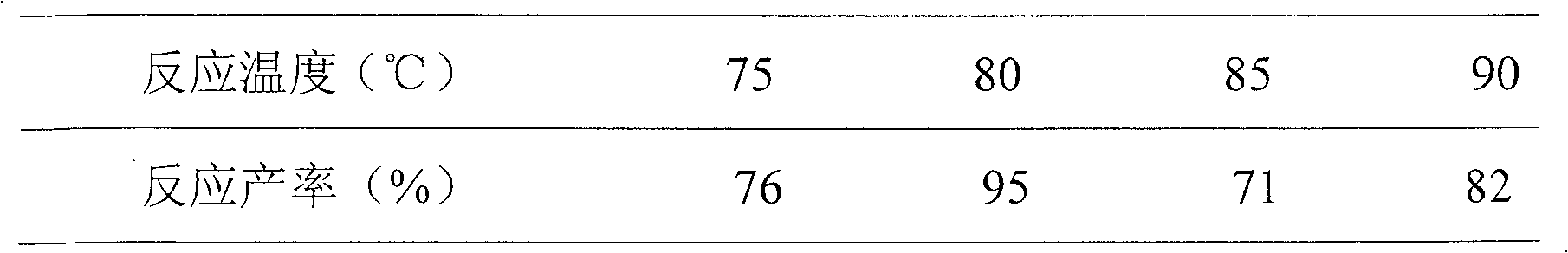
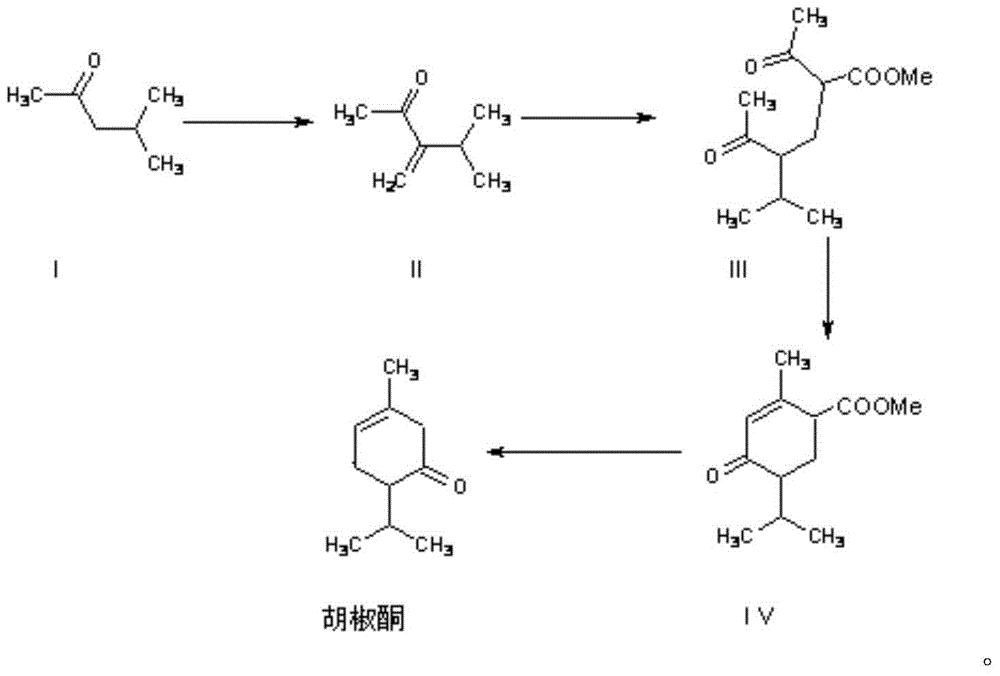
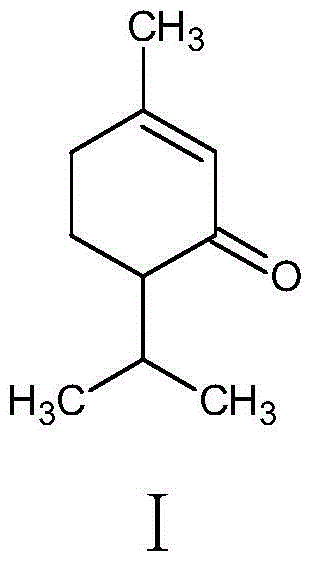
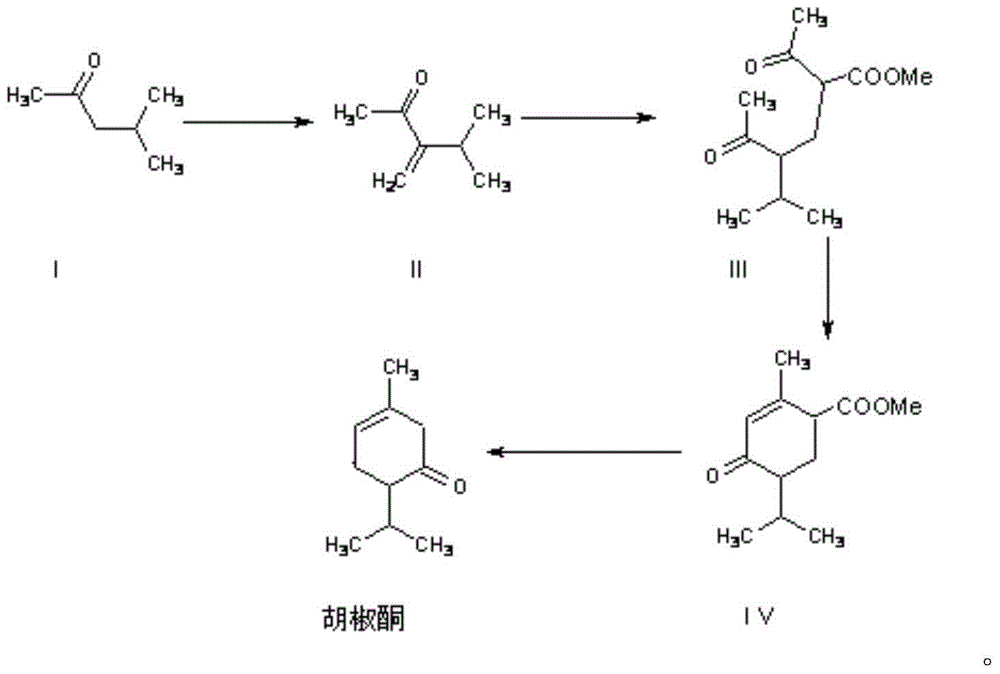
ActiveCN103755539AMild reaction conditionsGood conditionOrganic compound preparationCarboxylic acid esters preparationAcetoacetatesSynthesis methods
The invention discloses a synthesis method for piperitone. The synthesis method comprises the following steps: A: taking methyl isobutyl ketone as a raw material to react with formaldehyde under the catalysis of hydrogen chloride to obtain 3-isopropyl-3-butene-2-ketone; B: carrying out Michael addition on the 3-isopropyl-3-butene-2-ketone and acetyl acetate under the effect of an alkaline catalyst to obtain a 2-acetyl-4-isopropyl-5-oxo-methyl caproate intermediate; C: carrying out intramolecular aldol condensation on the 2-acetyl-4-isopropyl-5-oxo-methyl caproate intermediate under an alkaline condition to obtain a carboxylic ester of the piperitone; D: carrying out hydrolysis and acidification decarboxylation on the carboxylic ester of the piperitone under the alkaline condition to obtain the piperitone. According to the synthesis method, a synthesis route uses conventional chemical raw materials and a reaction condition is moderate; related synthesis steps are classic methods. The three-waste condition and the operation condition are greatly improved so that the synthesis method is a novel synthesis route.
Owner:SHANGHAI WANXIANG FLAVORS & FRAGRANCES
Benzofuran [2, 3-c] pyridine compound and synthetic method thereof
The invention relates to a benzofuran [2, 3-c] pyridine compound and a synthetic method thereof. The benzofuran [2, 3-c] pyridine compound has a general formula shown in the specification, wherein R1 is phenyl, 3-bromo-phenyl, 4-bromo-phenyl, 4-chloro-phenyl, 4-methoxy-phenyl, 3-methyl-phenyl or 4-methyl-phenyl; R2 is hydrogen or 5-chlorine, 5-bromine, 5-tert-butyl, 3,5-ditert-butyl, 4-methoxy, 5-methoxy, 5-methyl, 3,5-dimethyl and 4,5-dimethyl; R3 is methyl or phenyl, 4-bromo-phenyl, 3-bromo-phenyl, 2-chloro-phenyl, 4-chloro-phenyl, 4-methoxy-phenyl and 4-methyl-phenyl. According to the synthetic method disclosed by the invention, alpha-bromoacetophenone or a derivative thereof as well as 4-(2-hydroxyphenyl)-3-butylene-2-ketone or a derivative thereof are used as reactants, and a one-pot method is adopted to synthesize the benzofuran [2, 3-c] pyridine compound. The synthetic method is simple and convenient in step, mild in condition and high in yield.
Owner:WUHAN UNIV OF TECH
4-Chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, their preparation and their use in preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones
4-Chloro-4-alkoxy-1,1,1-trifluoro-2-butanones, prepared by reacting alkyl vinyl ethers with trifluoroacetyl chloride, are useful for preparing 4-alkoxy-1,1,1-trifluoro-3-buten-2-ones.
Owner:CORTEVA AGRISCIENCE LLC
Preparation methods of 3,4-dimethyl pyrazole and 3,4-dimethyl pyrazole phosphate
ActiveCN102911119BReduce generationRaw materials are cheap and easy to getOrganic chemistryDistillationHydrazine compound
The invention discloses preparation methods of 3,4-dimethyl pyrazole and 3,4-dimethyl pyrazole phosphate. The preparation method of the 3,4-dimethyl pyrazole comprises the following steps of: (1) reacting 2-butanone and paraformaldehyde at the temperature of 30-60 DEG C under the action of protonic acid for 4-20 hours in an organic solvent A, adjusting pH (Potential of Hydrogen) to be 7-8 after the reaction is finished, and performing atmospheric distillation to obtain 3-methyl-3-butylene-2-ketone; and (2) thermally reacting the 3-methyl-3-butylene-2-ketone prepared by the step (1) with hydrazine hydrate at the temperature of 40-50 DEG C for 2-8 hours, cooling reaction solution to 25-30 DEG C, adding an alkaline compound and hydrogen peroxide aqueous solution, and performing thermal reaction at the temperature of 30-60 DEG C for 3-10 hours after the addition is finished, wherein the obtained reaction solution is post-treated to obtain the 3,4-dimethyl pyrazole. The 3,4-dimethyl pyrazole is then reacted with phosphoric acid to form a salt so as to obtain the 3,4-dimethyl pyrazole phosphate. The method has the advantages of cheap and readily available raw materials, easiness in operation, high reaction yield, high product purity, capabilities of removing a large amount of concentrated sulfuric acid in a technological process and thus reducing the production of three wastes and high suitability for industrial large-scale production.
Owner:ZHEJIANG UNIV OF TECH +1
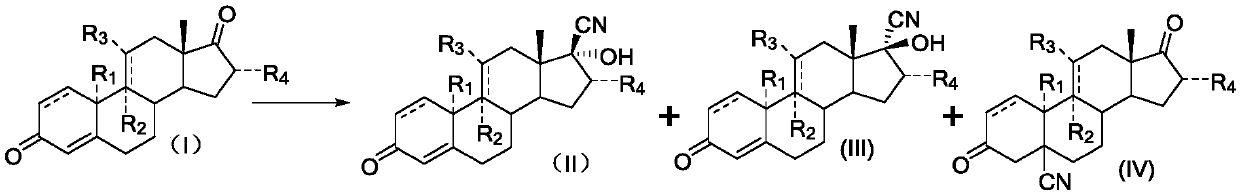
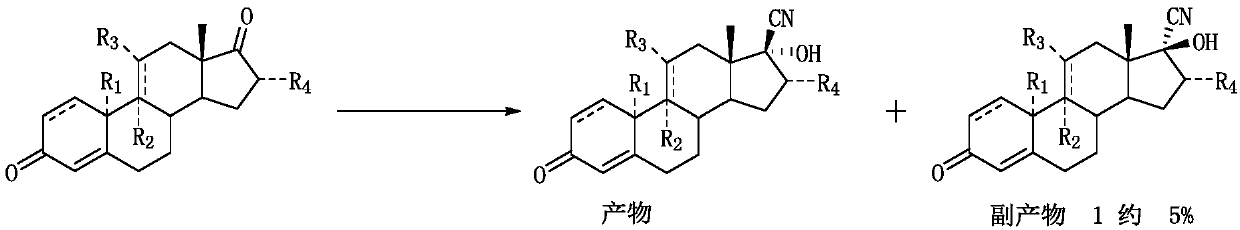
A synthetic method of 17alpha-hydroxy-17beta-cyano-steroid compounds
A synthetic method of 17alpha-hydroxy-17beta-cyano-steroid compounds is disclosed. The method includes a) dispersing a compound of a formula (I) into methanol, heating the mixture to 30-32 DEG C, adding 3-buten-2-one into the mixture, then adding acetone cyanohydrin into the mixture, fully stirring the mixture, then adding dropwise a prepared aqueous potassium carbonate solution, and maintaining the temperature until the reaction is completed; and 2) cooling the reaction solution to 0-5 DEG C after the reaction is finished, then stirring the reaction solution until a product is completely precipitated, performing suction filtration, washing filter cake with a small amount of water until neutrality, and drying the product to obtain a compound of a formula (II), and a small amount of a compound of a formula (III) and a compound of a formula (IV) which are adopted as byproducts. The weight ratio of the compound of the formula (I), the methanol, the 3-buten-2-one and the acetone cyanohydrin is 100:90-110:0.9-1.1:80-90. The weight-to-volume ratio of the compound of the formula (I) to the potassium carbonate solution is 100:90-110. The weight percentage of the potassium carbonate solution is 2.5-3.5%.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD
Preparation method of 4-alkoxy-1,1,1-trifluoro-3-buten-2-one
ActiveCN104072347BSimple processEasy to operateCarbonyl compound preparation by condensationChemical reactionAlkoxy group
The invention discloses a method for synthesizing 4-alkoxy-1,1,1-trifluoro-3-butane-2-ketone by taking trifluoroacetic acid, vinyl alkyl ether and phosgene as raw materials in the presence of a solvent and an acid-binding agent. A chemical reaction formula is as shown in the specification, wherein R is methyl, ethyl, n-propyl, isopropyl and normal-butyl. The preparation method disclosed by the invention is simple and convenient in process and simple to operate, has reaction yield of 85.6% (in terms of trifluoroacetic acid), and product purity of 98%, and is low in production cost and suitable for industrial production.
Owner:HUNAN HAILI CHEM IND
Method for producing 2-acetyl-4h,9h-naphtho[2,3-b]furan-4,9-dione
InactiveUS20180201597A1High yieldProduced inexpensively and safelyOrganic active ingredientsOrganic compounds purification/separation/stabilisationFuranAlcohol
The invention addresses the problem of providing a method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione that is suited to industrial production. The invention provides a method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione by reacting 3-bromo-3-buten-2-one and 2-hydroxy-1,4-naphthoquinone in the presence of a solvent, then obtaining crystals of 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione by adding an alcohol-based solvent and / or water to the reaction system, and treating the crystals by using a specific adsorbent in the presence of a solvent.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Procedure for the preparation of racemic derivatives of 1,5-diaryl-3-trifluoromethyl-delta2-pyrazolines
InactiveUS20050096474A1Simple and inexpensive methodEasy to useOrganic chemistryEphedrineChlorosulfuric acid
Procedure for preparation of compounds with the general formula 1, which include the racemic mixtures (±)-1, and the enantiomerically pure compounds (−)-1 and (+)-1, wherein R1 and R3, like or different, represent an atom of hydrogen, chlorine, fluorine, a methyl, trifluoromethyl or methoxy group; R2 represents an atom of hydrogen, chlorine, fluorine, a methyl, trifluoromethyl, methoxy, trifluoromethoxy, methylsulphonyl or aminosulphonyl group; R4 represents an atom of hydrogen, chlorine, fluorine, a methyl, trifluoromethyl, methoxy, trifluoromethoxy, methylsulphonyl or aminosulphonyl group, with the condition that one of the substituents R2 or R4 is a methylsulphonyl or aminosulphonyl group; which involves obtaining the racemic mixture with the general formula (±)-1 by reacting an (E)-1,1,1-trifluoro-4-aryl-3-buten-2-one with a phenylhydrazine, followed by a treatment with chlorosulphonic acid, or by reacting with chlorosulphonic acid followed by a reaction with sodium hydroxide and, finally, with thionyl chloride. The product obtained by either of these methods is made to react with ammonium carbonate or ammonia, or with sodium sulphite and methyl iodide or methyl sulphate. In addition, to obtain the enantiomerically pure compounds with the general formula 1 by resolving the racemic mixture with the general formula (±)-1, a reaction is effected with optically active ephedrine, followed by formation of the sodium salt of each enantiomer, reaction with thionyl chloride and ammonium carbonate or ammonia, or instead with thionyl chloride followed by sodium sulphite and methyl iodide or methyl sulphate to thereby obtain separately the enantiomerically pure compounds with the general formulae (−)-1 and (+)-1.
Owner:LAB DEL DR ESTEVE SA
Alpha/beta-unsaturated ketone and application thereof
InactiveCN105315144ATitian effect is goodRaise the gradeAntibacterial agentsAntimycoticsButene3-buten-2-one
The invention provides alpha / beta-unsaturated ketone and application thereof. The alpha / beta-unsaturated ketone is a compound obtained by recovering useful substances from a waste material produced by extracting aromas from natural plants. The compound is 1,4-bi(4-methoxyphenyl)-3-butene-2-ketone. The industrial waste material is further extracted and utilized, and useful substances alpha / beta-unsaturated ketone are separated out and are used as cigarette aroma-improving additives or antibacterial medicine or antihistamine medicine.
Owner:黎芷杉
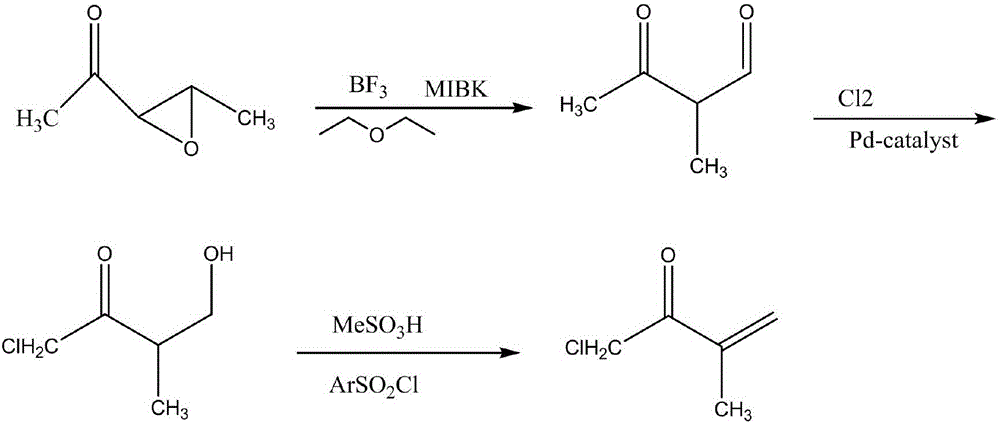
Synthetic method for 1-chloro-3-methyl-3-buten-2-one
InactiveCN105837424AEasy to makeEasy to operateOrganic compound preparationPreparation from heterocyclic compoundsOrganic solventOrganic synthesis
The invention discloses a synthetic method for 1-chloro-3-methyl-3-buten-2-one, which belongs to the technical field of organic synthesis. The synthetic method of the invention has the advantages of high yield and short synthetic route and overcomes the problems of long synthetic route, low total yield of less than 32.5%, usage of hazardous organic solvents, great risks and difficulties in large-scale production and purification of traditional synthetic methods for 1-chloro-3-methyl-3-buten-2-one.
Owner:CHANGZHOU UNIV
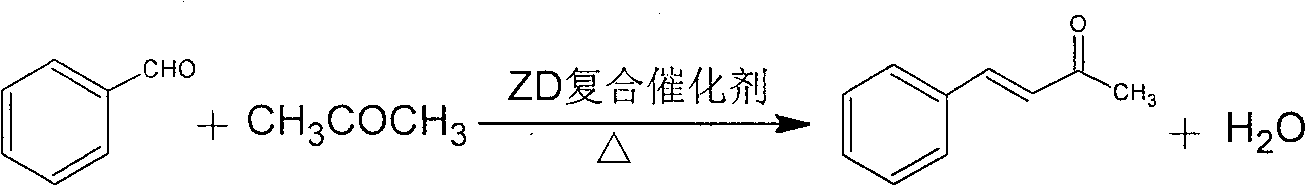
Aldol condensation catalyst and use thereof
The invention discloses an aldol condensation catalyst and application thereof in synthetization of 4-phenyl-3- butane-2-ketone. The invention is prepared by the weight percent of the following raw materials: 5-15: CaO percent, 3-5: MgO percent, 1-2: La2O3 percent, 0.01 to 0.02: Pt percent, 0.01 to 0.02: Pd percent, and the rest is Al2O3 percent. The aldol condensation catalyst has simple formula, is easy to be prepared and convenient in use, has good catalyst performance; in particular in aldol condensation preparation of the 4-phenyl-3- butane-2-ketone, the yield of the invention can reach above 95 percent, and the post-processing is simple; in addition, the invention has moderate response time, simple process, low production cost and is good for mass production.
Owner:ZHENGZHOU UNIV +1
A kind of synthetic method of piperonone
ActiveCN103755539BMild reaction conditionsGood conditionOrganic compound preparationCarboxylic acid esters preparationAcetoacetatesSynthesis methods
The invention discloses a synthesis method for piperitone. The synthesis method comprises the following steps: A: taking methyl isobutyl ketone as a raw material to react with formaldehyde under the catalysis of hydrogen chloride to obtain 3-isopropyl-3-butene-2-ketone; B: carrying out Michael addition on the 3-isopropyl-3-butene-2-ketone and acetyl acetate under the effect of an alkaline catalyst to obtain a 2-acetyl-4-isopropyl-5-oxo-methyl caproate intermediate; C: carrying out intramolecular aldol condensation on the 2-acetyl-4-isopropyl-5-oxo-methyl caproate intermediate under an alkaline condition to obtain a carboxylic ester of the piperitone; D: carrying out hydrolysis and acidification decarboxylation on the carboxylic ester of the piperitone under the alkaline condition to obtain the piperitone. According to the synthesis method, a synthesis route uses conventional chemical raw materials and a reaction condition is moderate; related synthesis steps are classic methods. The three-waste condition and the operation condition are greatly improved so that the synthesis method is a novel synthesis route.
Owner:SHANGHAI WANXIANG FLAVORS & FRAGRANCES
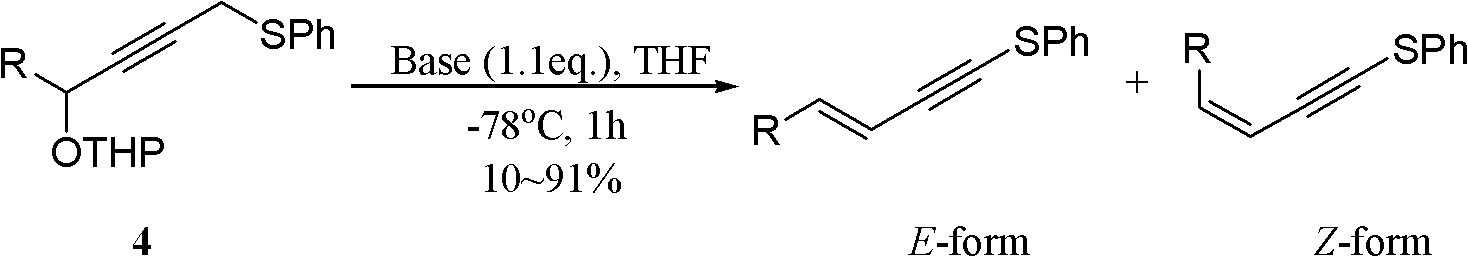
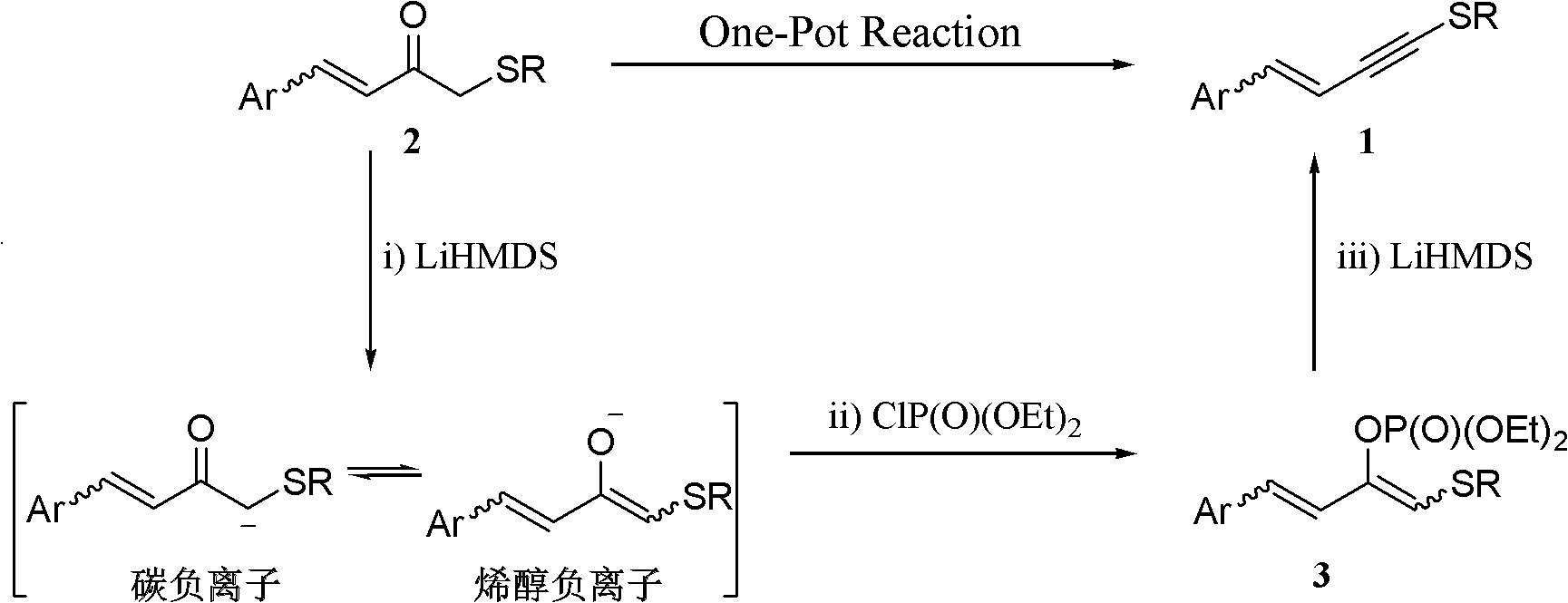
One-pot synthesis method of conjugate eneyne thioether
ActiveCN102617419BEasy to prepareImprove versatilitySulfide preparationChromatographic separationPhosphoric acid
The invention provides a one-pot synthesis method of conjugate eneyne thioether based on 1-hydrocarbon sulfenyl-4-aryl-butene-2-ketone substrate. The method includes feeding Lithium Hexamethyldisilazide (LiHMDS) to a tetrahydrofuran (THF) solution of 1-hydrocarbon sulfenyl-4-aryl-butene-2-ketone under protection of low-temperature nitrogen; stirring; adding [C1P(O) (OEt)2] dropwisely to the reaction system, increasing the temperature to a room temperature after the addition and continuing to stir; after the reaction system cools again to a low temperature, dripping the LiHMDS to the reaction system and stirring for reaction under the temperature; and obtaining the conjugate eneyne thioether from the reaction mixture after normal aftertreatment and column chromatographic separation. The method is a convenient and economical processing method for the conjugate eneyne thioether.
Owner:HUNAN UNIV
Preparation method for dodecanedioic acid purification material
InactiveCN108192028AProcess stabilityHigh mechanical strengthOther chemical processesBenzoyl peroxideCarboxylic acid
The invention provides a preparation method for a dodecanedioic acid purification material. The invention provides a preparation method for a dodecanedioic acid purification material. The preparationmethod comprises the following steps: adding poly(1-decene)hydride, 1,4-butanediol vinyl diether, tetrabutylammonium difluorotriphenylstannate, 3-trimethylsilyl-3-buten-2-one, 5-norbornene-2-methylvinyl ether, methyl-6-bromobenzo[D][1,3]dioxole-5-carboxylic acid methyl ester, benzoyl peroxide, nanometer calcium carbonate, water and gelatin into a reaction vessel, carrying out heating after uniformmixing under stirring, carrying out a reaction, and subjecting a reaction product to filtering, washing with water and drying successively so as to obtain the dodecanedioic acid purification material.
Owner:孝感市锐思新材科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
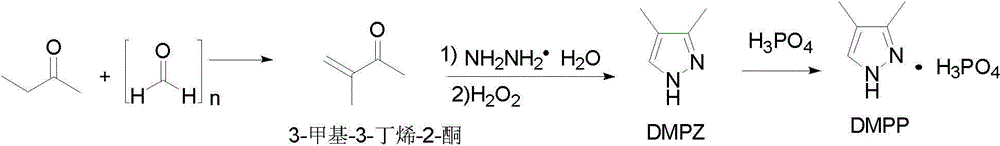

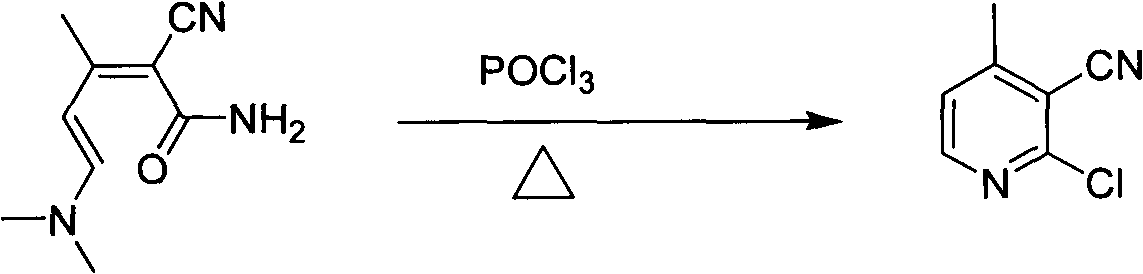
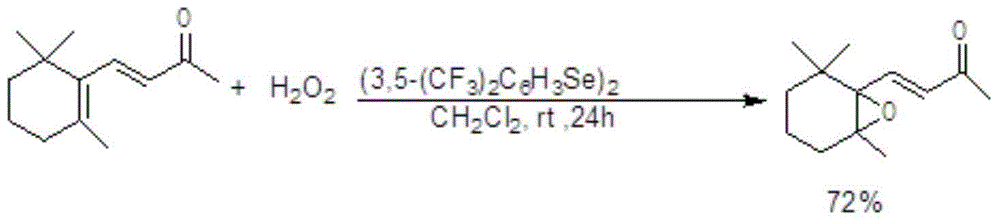

![Benzofuro[2,3-c]pyridine compound and its synthesis method Benzofuro[2,3-c]pyridine compound and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d8a91ff-360e-4783-80d3-9eff21627f8a/HDA0000446809740000011.PNG)
![Benzofuro[2,3-c]pyridine compound and its synthesis method Benzofuro[2,3-c]pyridine compound and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d8a91ff-360e-4783-80d3-9eff21627f8a/HDA0000446809740000012.PNG)
![Benzofuro[2,3-c]pyridine compound and its synthesis method Benzofuro[2,3-c]pyridine compound and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6d8a91ff-360e-4783-80d3-9eff21627f8a/HDA0000446809740000021.PNG)
![Method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione Method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fa321d45-9449-461c-b9f0-68d0a2a46706/US10329267-C00001.png)
![Method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione Method for producing 2-acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fa321d45-9449-461c-b9f0-68d0a2a46706/US10329267-C00002.png)
![Benzofuran [2, 3-c] pyridine compound and synthetic method thereof Benzofuran [2, 3-c] pyridine compound and synthetic method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/29e971f4-2134-4ae5-9c33-2100a58d4dd1/HDA0000446809740000011.PNG)
![Benzofuran [2, 3-c] pyridine compound and synthetic method thereof Benzofuran [2, 3-c] pyridine compound and synthetic method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/29e971f4-2134-4ae5-9c33-2100a58d4dd1/HDA0000446809740000012.PNG)
![Benzofuran [2, 3-c] pyridine compound and synthetic method thereof Benzofuran [2, 3-c] pyridine compound and synthetic method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/29e971f4-2134-4ae5-9c33-2100a58d4dd1/HDA0000446809740000021.PNG)
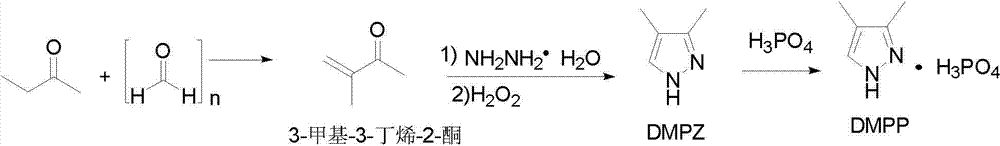

![Method for producing 2-acetyl-4h,9h-naphtho[2,3-b]furan-4,9-dione Method for producing 2-acetyl-4h,9h-naphtho[2,3-b]furan-4,9-dione](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0a284f27-707e-4e33-b413-afe257a2b698/US20180201597A1-20180719-C00001.png)
![Method for producing 2-acetyl-4h,9h-naphtho[2,3-b]furan-4,9-dione Method for producing 2-acetyl-4h,9h-naphtho[2,3-b]furan-4,9-dione](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0a284f27-707e-4e33-b413-afe257a2b698/US20180201597A1-20180719-C00002.png)