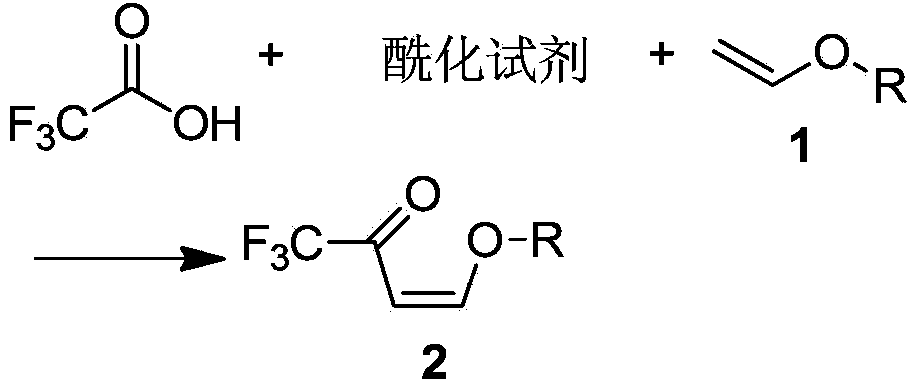
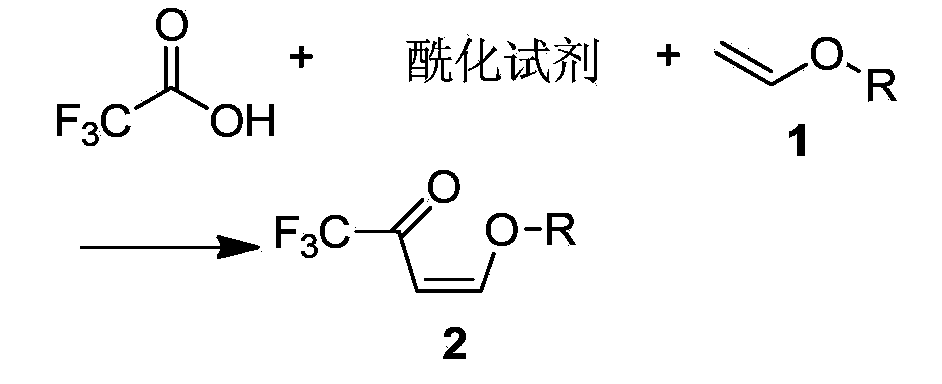
Preparation method of 3-trifluoromethylpyrazole intermediate
A technology for trifluoromethylpyrazole and intermediates, which is applied in the field of preparation of 3-trifluoromethylpyrazole intermediates, can solve the problems of unfavorable industrial production, cumbersome post-processing steps, and harsh reaction conditions, and achieve production costs Low, simple after-treatment steps, less reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 26.56g (0.368mol) of vinyl ethyl ether, 450g toluene, and 53.25g (0.526mol) of triethylamine into a 1000mL four-necked flask equipped with a thermometer, a condenser, and an exhaust gas absorption device. 30g (0.263mol) of fluoroacetic acid is controlled at a temperature lower than 20°C, the dropwise addition is completed in 0.5 hours, the temperature is lowered to 0°C, and it takes about 1 hour to feed 31.5g (0.316mol) of phosgene; the temperature is slowly raised to 0°C and kept for 5 hours. Add 100g of water dropwise, stir and keep warm at 10°C for 0.5 hours, let stand, separate layers, add 100g of NaHCO with a mass percentage of 5% to the organic layer 3 The aqueous solution was stirred and incubated for 0.5 hours (the mass percentage refers to the percentage of the mass of sodium bicarbonate in the total mass of the sodium bicarbonate aqueous solution), allowed to stand, and layered; the organic layer was dehydrated to obtain 4-ethoxyl-1,1,1 -Toluene solution o...
Embodiment 2
[0041] Add 20.87g (0.289mol) of vinyl ethyl ether, 150g xylene, and 52.03g (0.658mol) of pyridine into a 500mL four-neck flask equipped with a thermometer, condenser, and tail gas absorption device, cool down in an ice bath to 0°C, and drop trifluoro 30g (0.263mol) of acetic acid is controlled at a temperature lower than 20°C, the dropwise addition is completed in 0.5 hours, the temperature is lowered to 0°C, and it takes about 1 hour to introduce 34.36g (0.347mol) of phosgene; the temperature is slowly raised to 10°C, and the temperature is kept for 3 hours. Add 100g of water dropwise, stir and keep warm at 10°C for 0.5 hours, let stand, separate layers, add 100g to the organic layer with a mass percentage of 5% NaHCO 3 The aqueous solution was stirred and incubated for 0.5 hours (the mass percentage refers to the percentage of the mass of sodium bicarbonate in the total mass of the sodium bicarbonate aqueous solution), allowed to stand, and layered; the organic layer was dehy...
Embodiment 3
[0043] Add 19g (0.263mol) of vinyl ether, 600g of dichloromethane, and 83.25g (1.052mol) of pyridine into a 1000mL four-neck flask equipped with a thermometer, condenser, and tail gas absorption device, cool down in an ice bath to 0°C, and dropwise add trifluoro 30g (0.263mol) of acetic acid is controlled at a temperature lower than 20°C, and the dropwise addition is completed in 0.5 hours. After cooling down to 0°C, it takes about 1 hour to feed 54.65g (0.526mol) of phosgene; Add 100g of water, stir and keep warm at 10°C for 0.5 hours, let stand, separate layers, add 100g of NaHCO with a mass percentage of 5% to the organic layer 3 The aqueous solution was stirred and incubated for 0.5 hours (the mass percentage refers to the percentage of the mass of sodium bicarbonate in the total mass of the sodium bicarbonate aqueous solution), allowed to stand, and layered; the organic layer was dehydrated to obtain 4-ethoxyl-1,1,1 - Dichloromethane solution of trifluoro-3-buten-2-one, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com