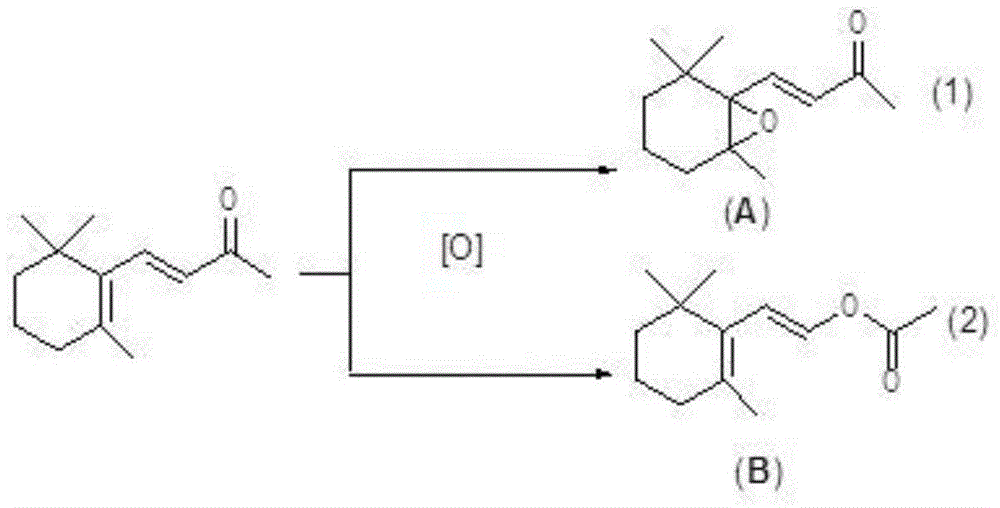
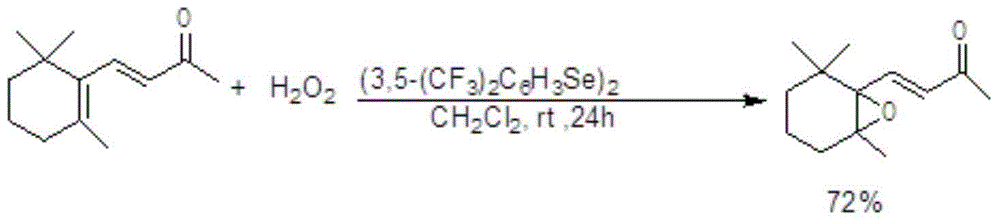Production method of beta-ionone epoxide
A technology of ionone ring and production method, which is applied in the field of production of β-ionone epoxides, can solve problems such as corrosion, high cost, and large energy consumption, and achieve high economic efficiency and low equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Take 1 mmol of β-ionone, under the protection of nitrogen, control the temperature of the reaction system at 0-40°C, use hydrogen peroxide as the oxidant in different solvents, and catalyze the oxidation of β-ionone, as listed in the table below, using different dose and temperature conditions. The solvent can be one of ethanol, N,N-dimethylformamide, water, dichloromethane, acetonitrile, acetone, and tetrahydrofuran. The reaction time was 24 hours. After the reaction, the solvent was evaporated to dryness and separated by preparative thin layer chromatography to obtain the epoxidized product 4-[2,2,6-trimethyl-7-oxabicyclo[4.1.0 ]hept-1-yl]-3-buten-2-one.
[0022] Its reaction formula is as follows:
[0023]
[0024] Table 1 shows the reactions at different temperatures
[0025] Numbering
temperature(℃)
Yield(%)
1
0
54
2
10
66
3
25
72
4
30
70
5
40
63
[0026] Fro...
Embodiment 2
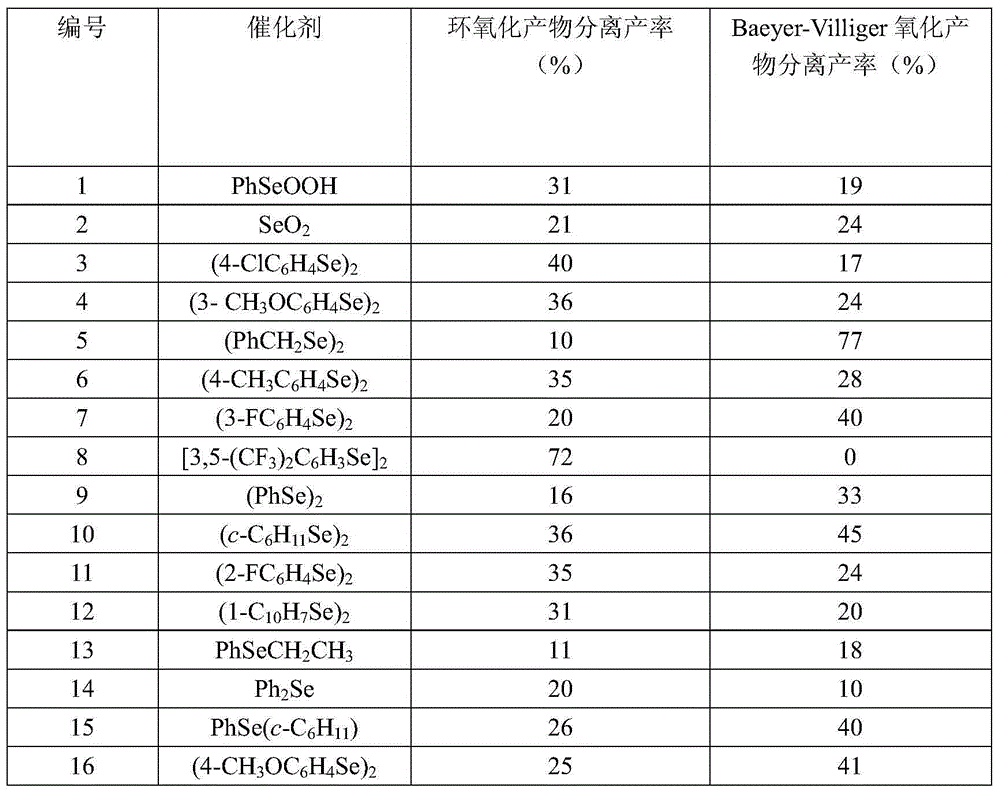
[0045] Under the protection of nitrogen, 50 mmol of β-ionone, 150 mmol of hydrogen peroxide (30w / w% aqueous solution), 0.5 mmol of bis(3,5-bistrifluoromethylphenyl)diselenide were dissolved in 50 mL of diselenide Stir in methyl chloride at room temperature (25°C) for 24 hours. The solvent was evaporated to dryness, and the epoxidized product 4-[2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl]-3- Buten-2-one. The catalyst residue is put into the next round of use, and the effect of catalyst recovery and use is checked, and the results are shown in Table 7
[0046] Numbering
[0047] It can be seen from the above results that the catalyst is stable and can be recycled and used many times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com