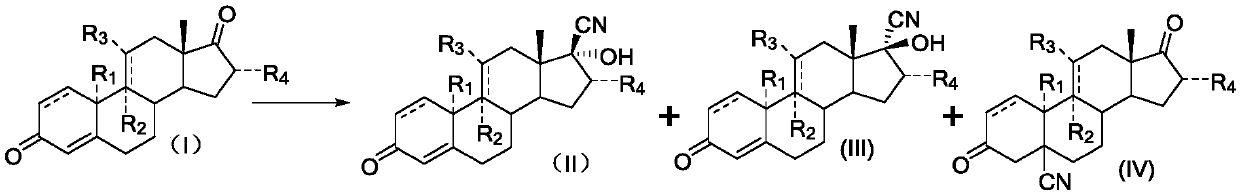
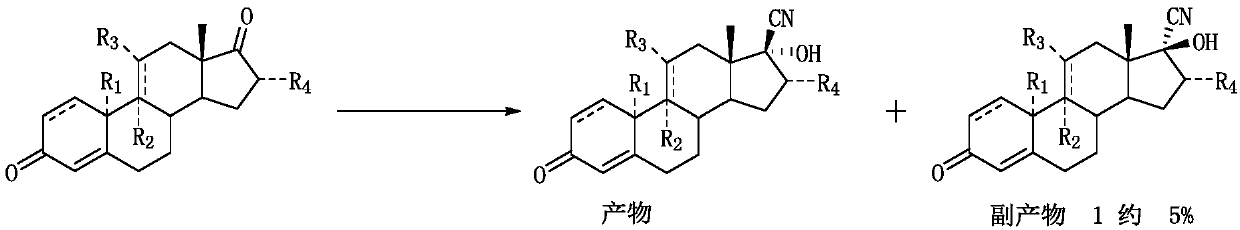
A synthetic method of 17alpha-hydroxy-17beta-cyano-steroid compounds
A technology of steroidal compounds and synthetic methods, which is applied in the field of synthesis of 17αhydroxyl-17βnitrile-steroidal compounds, can solve problems such as incomplete reaction and long reaction time, and achieve product quality improvement, quality and yield improvement, The effect of solving environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Take a three-necked reaction flask, add 100g of compound (I-1), add 90g of methanol, heat up to 30-32°C, add 1g of 3-butene-2-ketone, then add 80g of acetone cyanohydrin and stir for 3min, add the prepared 3 % potassium carbonate aqueous solution 200ml. Insulated for 30 hours, the raw materials were basically completely reacted by sampling. After cooling down to 0-5°C, stir for 30 minutes. Suction filtration, wash the filter cake with a small amount of water until neutral, and dry to obtain 104.9 g of the product, with a mass yield of 104.9%. HPLC content: compound (II-1) 98.2%, compound (III-1) 0.8%, compound (IV-1) 0.5%.
Embodiment 2
[0025]
[0026] Take a three-necked reaction flask, add 100g of compound (I-2), add 100g of methanol, heat up to 29-31°C, add 1g of 3-butene-2 ketone, then add 80g of acetone cyanohydrin and stir for 3min, add dropwise 3% 200ml of sodium carbonate aqueous solution. Insulated for 32 hours, the raw materials were basically completely reacted by sampling. After cooling down to 0-5°C, stir for 30 minutes. Suction filtration, wash the filter cake with a small amount of water until neutral, and dry to obtain 105.1 g of the product, with a mass yield of 105.1%. HPLC content: compound (II-2) 98.4%, compound (III-1) 0.6%, compound (IV-1) 0.5%.
Embodiment 3
[0028]
[0029] Take a three-necked reaction flask, add 100g of compound (I-3), add 110g of methanol, heat up to 32-33°C, add 1g of 3-buten-2-ketone, then add 90g of acetone cyanohydrin and stir for 3min, add the prepared 3% 200ml of sodium carbonate aqueous solution. Insulated for 31 hours, the raw materials were basically completely reacted by sampling. After cooling down to 0-5°C, stir for 30 minutes. Suction filtration, wash the filter cake with a small amount of water until neutral, and dry to obtain 105.3 g of the product, with a mass yield of 105.3%. HPLC content: compound (II-3) 98.3%, compound (III-3): 0.7%, compound (IV-3) 0.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com