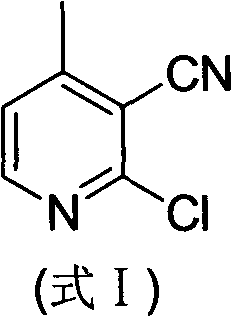
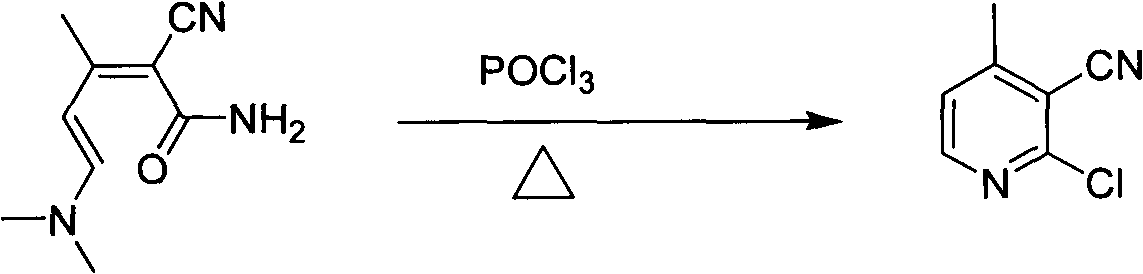Preparation method of 2-chloro-4-methyl nicotinonitrile
A technology of methylnicotinonitrile and cyano group is applied in the field of preparation of 2-chloro-4-methylnicotinonitrile, and can solve the problems of low yield, triethyl orthoformate acetic anhydride solution reaction is not easy to carry out, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
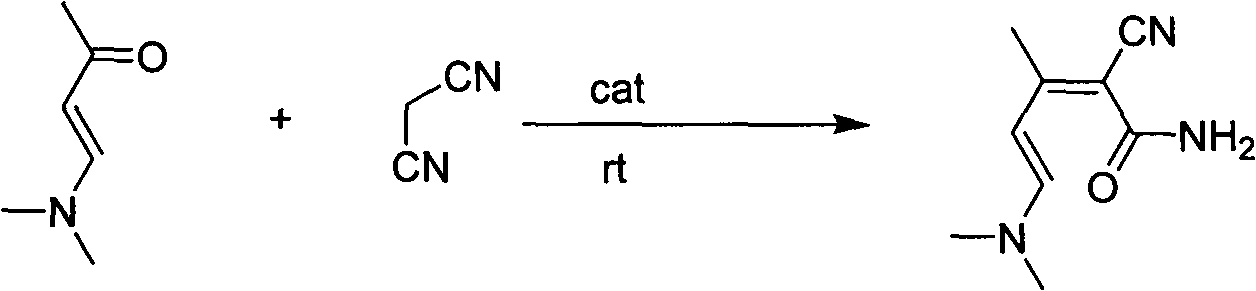
[0018] (1) Preparation of 2-cyano-5-(dimethylamino)-3-methyl-2,4-pentadienamide
[0019]
[0020] Add 100mL of methanol to a 250mL four-necked flask with mechanical stirring and a thermometer, then add 3g (0.05mol) of glacial acetic acid, 0.2g of β-alanine and 23.1g (0.204mol) of (E)-4-(dimethyl Amino) base-3-en-2-butanone, stir well and cool in a water bath. At room temperature, 13.2 g (0.2 mol) of malononitrile was added dropwise for about 1 to 2 hours. After the addition of malononitrile, the reaction was stirred at room temperature for 24h. After the reaction is completed, cool to 5-10°C with an ice-water bath. Suction filtration, the filter cake was washed with 10 mL ice methanol, and the filter cake was dried to obtain 47.5 g orange solid, namely 2-cyano-5-(dimethylamino)-3-methyl-2,4-dipentenamide . Yield: 88.4%; Purity: 98%.
[0021] (2) Preparation of 2-chloro-4-methylnicotinonitrile
[0022]
[0023] Add 17.9 g (0.1 mol) of 2-cyano-5-(dimethylamino)-3-met...
Embodiment 2
[0025] (1) Preparation of 2-cyano-5-(dimethylamino)-3-methyl-2,4-pentadienamide
[0026] Synthesize as in the method and conditions of Example 1 (1), only the catalyst is changed to piperidine acetate to obtain 2-cyano-5-(dimethylamino)-3-methyl-2,4-di Pentenamide, yield 75.5%, purity 98.3%.
[0027] (2) Preparation of 2-chloro-4-methylnicotinonitrile
[0028] Synthesize as in the method and condition of embodiment one (2), only change the mixture of phosphorus oxychloride (1.5eq) and phosphorus pentachloride (0.5eq) into the mixture of chlorination reagent phosphorus oxychloride, obtain 2-chloro- 4-Methylnicotinonitrile, yield 62%, purity 93%.
Embodiment 3
[0030] (1) Preparation of 2-cyano-5-(dimethylamino)-3-methyl-2,4-pentadienamide
[0031] Synthesized as in the method and conditions of Example 1 (1), only changing the catalyst to piperidine to obtain 2-cyano-5-(dimethylamino)-3-methyl-2,4-dipentenamide , yield 63%, purity 93.2%.
[0032] (2) Preparation of 2-chloro-4-methylnicotinonitrile
[0033] Synthesized as in the method and conditions of Example 1 (2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com