Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
285 results about "Phosphorus pentachloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphorus pentachloride is the chemical compound with the formula PCl₅. It is one of the most important phosphorus chlorides, others being PCl₃ and POCl₃. PCl₅ finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.
Preparation method of lithium hexafluorophosphate
ActiveCN102009972ARich sourcesReduce manufacturing costPhosphorus compoundsPhosphoric acidNitrogen gas
The invention relates to a preparation method of lithium hexafluorophosphate. The preparation method comprises the following steps of: (1) distilling to obtain hydrogen fluoride liquid of which the purity is over 99.99 weight percent; (2) reacting the high-purity hydrogen fluoride liquid with phosphorus pentachloride to obtain mixed gas of the phosphorus pentafluoride and hydrogen chloride; (3) introducing the mixed gas of the phosphorus pentachloride and the hydrogen chloride into hydrogen fluoride and lithium fluoride, reacting at a certain temperature and under certain pressure to obtain solution of lithium hexafluorophosphate, exhausting hydrogen chloride gas at regular time, and absorbing by using water to prepare byproduct hydrochloric acid; and (4) crystallizing and separating, namely filtering the solution of lithium hexafluorophosphate, delivering filtrate into a crystallizing slot, separating the lithium hexafluorophosphate out at the temperature of between -70 and 80 DEG C, filtering, and performing primary drying and secondary drying to obtain a lithium hexafluorophosphate product, wherein the residual hydrogen fluoride gas is displaced by nitrogen. The preparation method has readily available raw materials and is easy to operate, the purity of the obtained lithium hexafluorophosphate product is over 99.9 percent, the moisture is lower than 10ppm, and the production requirements of lithium ion electrolytic cells are met.
Owner:MORITA NEW ENERGY MATERIALS ZHANGJIAGANG CO LTD
Methods for preparing phosphorus pentafluoride gas and preparing lithium hexafluorophosphate using the gas
ActiveCN101353161AEasily hydrolyzedStrong moisture absorptionPhosphorus halides/oxyhalidesLithiumPhysical chemistry
A preparation method of phosphorus pentafluoride gas comprises a step of causing phosphorus pentachloride to react with anhydrous hydrogen fluoride, wherein, the reaction occurs in the presence of a solvent. A preparation method of lithium hexaflourophosphate comprises a contact reaction between solid lithium fluoride and phosphorus pentafluoride gas, wherein the phosphorus pentafluoride gas is prepared by the method of the invention. Compared with the preparation method of the lithium hexaflourophosphate with the phosphorus pentafluoride gas as the raw material in the prior art, the phosphorus pentafluoride gas prepared by the preparation method of the invention has higher purity and lower cost. The yield of the lithium hexaflourophosphate prepared by the method of the invention is higher than 93%, and the purity thereof is up to 99.95%.
Owner:BYD CO LTD
Method for improving solar battery diffusion
InactiveCN101237010AImprove electrical performanceEasy to operateFinal product manufactureSemiconductor devicesHigh resistanceChemical reaction
The present invention discloses a method for improving the diffusion of a solar cell, comprising the following steps: A. adopting silicon material and P conductive type; B. diffusion: a silicon wafer chemically reacts with oxygen and phosphorus oxychloride at high temperature to produce phosphor, phosphorus pentachloride, phosphorus pentoxide and chlorine; C. feeding oxygen to reallocate: feeding oxygen to continue to react with unexhausted PCl5 to produce phosphorus; D. a phosphor atom Drive in process which is combined with the reallocation; E. the surface block resistance is 28-38 omega after the diffusion, the reallocation and Drive in processes. The method is capable of improving the conversion efficiency of high resistance rate single crystal silicon solar cells, the diffusion of the diffused silicon wafer surface is uniform; the method is easily implemented, the operation is convenient and the cost is low.
Owner:JIAWEI SOLAR WUHAN
Method for preparing potassium hexafluoro phosphate
The invention relates to Li ion battery technique, a lithium hexafluoro- phosphate preparing method, characterized in comprising the process steps of: using easy sublimation property of phosphorus pentachloride, heating and sublimating it to remove nonvolatile components, refining industrial anhydrous hydrogen fluoride to remove water and heavy metal impurities; reacting them with each other to prepare mixed gas of phosphorus pentachloride and hydrogen chloride; leading the mixed gas into anhydrous hydrogen fluoride of lithium fluoride, reacting, crystallizing and separating and drying to obtain pure lithium hexafluoro-phosphate.
Owner:TIANJIN CHEM RES & DESIGN INST
Method for producing lithium hexafluorophosphate
InactiveCN101570327ARich sourcesReduce manufacturing costLead-acid accumulatorsPhosphorus compoundsReaction rateMoisture
The invention relates to a method for producing lithium hexafluorophosphate. The method comprises the following steps: (1), rectifying and purifying industrial anhydrous hydrogen fluoride and removing moisture and heavy metal impurities therein; (2), enabling the rectified anhydrous hydrogen fluoride and phosphorus pentachloride to react to prepare the mixed gas of phosphorus pentafluoride and chlorine hydride; (3), dissolving high-pure lithium fluoride in an anhydrous hydrogen fluoride solution to form an anhydrous hydrogen fluoride solution containing the lithium fluoride; (4), cooling and guiding the mixed gas of the phosphorus pentafluoride and the chlorine hydride to the anhydrous hydrogen fluoride solution containing the lithium fluoride, reacting, crystallizing, separating and drying to obtain a pure lithium hexafluorophosphate product; and (5), continuously guiding the unreacted gas of the phosphorus pentafluoride and the chlorine hydride after a reaction to the other anhydrous hydrogen fluoride solution containing the lithium fluoride and continuously reacting to obtain a lithium hexafluorophosphate finished product. The invention uses the industrial anhydrous hydrogen fluoride, the phosphorus pentachloride and the high-pure lithium fluoride as raw materials to prepare the lithium hexafluorophosphate product, has rich raw material resources, low production cost, high reaction rate, high product quality and thorough reaction and can realize semi-continuous production.
Owner:DO FLUORIDE CHEM CO LTD
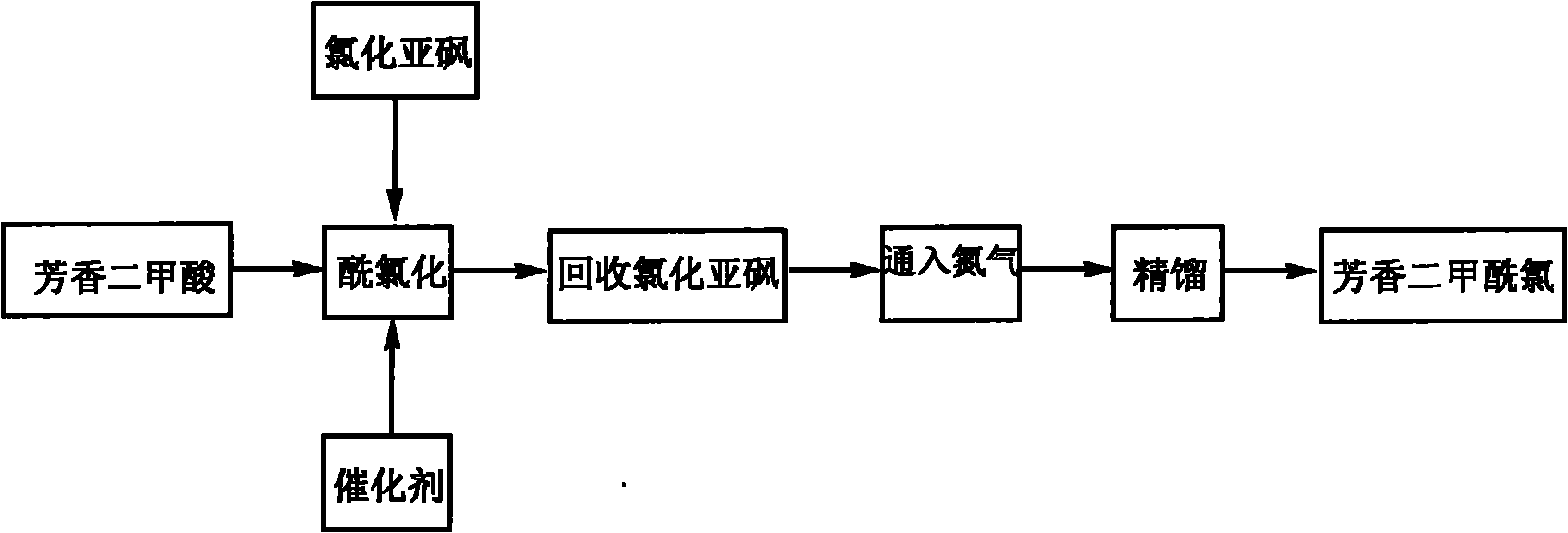
Method for preparing aromatic dimethyl chloride
InactiveCN102093209AHigh yieldHigh purityOrganic compound preparationCarboxylic compound preparationPyridinePhosphorus pentachloride
The invention discloses a method for preparing aromatic dimethyl chloride, which relates to the technical field of preparation methods of aromatic chloride. The method comprises the following steps of: reacting aromatic dioctyl phthalate with a chlorinating agent to generate the aromatic dimethyl chloride; evaporating the chlorinating agent; exhausting tail gas; and distilling the obtained aromatic dimethyl chloride, wherein the reaction of the aromatic dioctyl phthalate and the chlorinating agent is performed in the presence of a catalyst, namely pyridine or N-methyl pyrolidone; and the chlorinating agent is one or more of chlorine, phosphorus trichloride, phosphorus pentachloride and thionyl chloride. Compared with the prior art, the method for preparing the aromatic dimethyl chloride has the advantages that: the yield and the purity of the aromatic dimethyl chloride are increased, transformation rate is up to 95 percent, and the purity is up to 99.9 percent.
Owner:YANTAI TAYHO ADVANCED MATERIALS CO LTD
Preparation method of lithium hexafluorophosphate
ActiveCN101723346AHigh yieldImprove securityFinal product manufactureElectrolyte accumulators manufactureHydrogen fluoridePhysical chemistry
The invention provides a preparation method of a lithium hexafluorophosphate, which comprises the following steps: firstly, reacting a phosphorus pentachloride with an anhydrous hydrogen fluoride to form mixed liquid of the phosphorus pentachloride and the anhydrous hydrogen fluoride; secondly, preparing anhydrous hydrogen fluoride solution of lithium fluoride; and finally, adding the anhydrous hydrogen fluoride solution of the lithium fluoride into the mixed liquid of the phosphorus pentachloride and the anhydrous hydrogen fluoride, and sequentially performing reaction, crystallization, separation and drying to obtain a pure lithium hexafluorophosphate product. The preparation method of the invention has the advantages of mild reaction, high safety and purity of the lithium hexafluorophosphate product of over 99.9 percent; and the mother liquor can be reclaimed and reused so that the cost is reduced.
Owner:DO FLUORIDE CHEM CO LTD
Synthesis method for pyridone
InactiveCN104030972AOptimize the synthetic routeReduce manufacturing costOrganic chemistrySynthesis methodsMorpholine
The invention discloses a synthesis method for pyridone, and relates to the technical field of synthesis of a heterocyclic compound containing three heterocyclic rings one of which takes nitrogen and oxygen as the only one heteroatom. The synthesis method comprises the following steps: reacting tetrahydrofuran, ursol, triethylamine and 5-chlorin valeryl chloride which are taken as raw materials, and after the reaction, adding potassium tert-butoxide for reacting again, thereby obtaining a monomer 1 after reaction; reacting the monomer 1 with phosphorus pentachloride in a dichloromethane solvent, thereby obtaining a monomer 2 after reaction; reacting the monomer 2 with morpholine to obtain a final product I. The synthesis method has the advantages that the raw materials are cheap and easily available, and the reaction process is greatly shortened in comparison with that of the prior art, and the synthesis method is mild and safe in reaction conditions, good in reaction reproducibility, low in cost, and high in efficiency, and has simple and easy operations in the reaction.
Owner:河北序能生物技术有限公司
Method for synthesizing p-acetamido benzene sulfonyl chloride by phosphorus pentachloride
InactiveCN101613308AHigh yieldReduce dosageSulfonic acid preparationSulfonyl chlorideChlorosulfuric acid
The invention relates to a method for synthesizing p-acetamido benzene sulfonyl chloride by phosphorus pentachloride, relating to the preparation method for sterilization and mould inhibition midbody of sulfonamides. The invention takes acetanilide and chlorosulfonic acid as raw materials, uses phosphorus pentachloride as chlorinating agent; under the action of organic dissolvent, the raw materials are sulfonated, chloridized, separated, and washed to obtain the product. The invention has the effects of little chlorosulfonic acid usage, high product yield, few generated waste acid,, completely cycling dissolvent, recycled by-products, low manufacturing cost, being convenient to popularize and apply, and the like. The products prepared by the method can be widely applied to the preparation of the sterilization and mould inhibition of sulfonamides and industries such as coating, plastics, pesticides, etc.
Owner:CHONGQING UNIV
Pyroxasulfone synthesis method
ActiveCN111393427AStabilizes and strengthens oxidationConducive to mutual changeOrganic chemistryPtru catalystThiourea
The invention provides a pyroxasulfone synthesis method. The method comprises the steps of: adopting a compound I as a starting raw material, carrying out cyclization reaction to synthesize an intermediate II, and carrying out chlorination reaction on the intermediate II under the action of a chlorination reagent to obtain an intermediate III; reacting the intermediate III with thiourea to obtaina hydrochloride intermediate IV, wherein the chlorination reagent is phosphorus pentachloride and / or phosphorus oxychloride; reacting the hydrochloride intermediate IV and a compound VI with formaldehyde to obtain an intermediate VII, carrying out difluoromethoxylation reaction on the intermediate VII to obtain an intermediate VIII, and oxidizing the intermediate VIII with hydrogen peroxide in thepresence of a catalyst to obtain pyroxasulfone IX, wherein the catalyst is sodium tungstate and acid. According to the method, the total yield is increased to 31-38%, the raw materials are simple andeasily available, the reaction process is simple and safe, the yield is high, and the method has certain significance for industrial production.
Owner:HEFEI JIUYI AGRI DEV
Lithium tetrafluoro oxalate-phosphate preparation method
ActiveCN105218348AEasy to prepareEasy to makeOrganic compound preparationCarboxylic acid salt preparationLithiumHydrogen fluoride
The invention provides a lithium tetrafluoro oxalate-phosphate preparation method which is simple and practical and can be applied to large-scale industrial production. The lithium tetrafluoro oxalate-phosphate preparation method provided by the invention is characterized by comprising the specific steps: firstly, weighing lithium oxalate, putting weighed lithium oxalate into a 316L stainless-steel reactor A with a jacket and a filtrating device, and thoroughly stirring weighed lithium oxalate for 2-6 hours, so as to fully dissolve weighed lithium oxalate into anhydrous HF; and then, adding phosphorus pentachloride and hydrogen fluoride into another 316L stainless-steel reactor B with a jacket and a filtrating device, and carrying out reaction. The lithium tetrafluoro oxalate-phosphate preparation method has the advantages that lithium tetrafluoro oxalate-phosphate is prepared from low-cost raw materials, the preparation method is simple, the disadvantages of other methods that the number of reaction steps is large, the consumption of reaction is high and impurities in the final product are too many are overcome, and thus the cost can be greatly reduced.
Owner:TIANJIN JINNIU POWER SOURCES MATERIAL +1
Preparation method of lithium hexafluorophosphate of lithium ion battery electrolyte
InactiveCN102515133AMeet the needs of productionReduce manufacturing costPhosphorus compoundsNew energySodium-ion battery
The invention relates to the technical field of lithium batteries, in particular to a preparation method of lithium hexafluorophosphate of lithium ion battery electrolyte. The preparation method is characterized in that hydrogen fluoride, phosphorus pentachloride and lithium fluoride, which are adopted as raw materials, are refined and react under the conditions of proper temperature, pressure and time, and then high-purity lithium hexafluorophosphate is prepared by adopting the technical route of low-temperature crystallization and vacuum drying. The raw materials used in the method are cheap and easy to obtain; the production cost is low; the technological process is simple; the operation is convenient to control; excellent economical and social values in extensive use of energy-saving environment-friendly new energies are achieved; moisture, free acid and other impurities in a product are effectively reduced, so that the product yield is high, and the product quality is high; and the purity of the lithium hexafluorophosphate product reaches 99.95 percent, so as to satisfy the production requirements of the lithium ion batteries.
Owner:DONGGUAN DONGJUN NEW ENERGY TECH
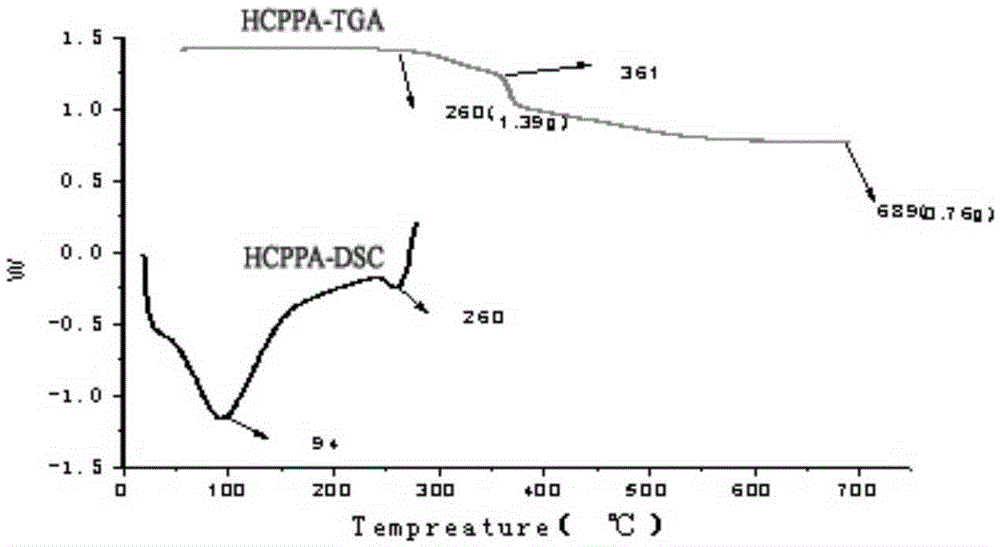
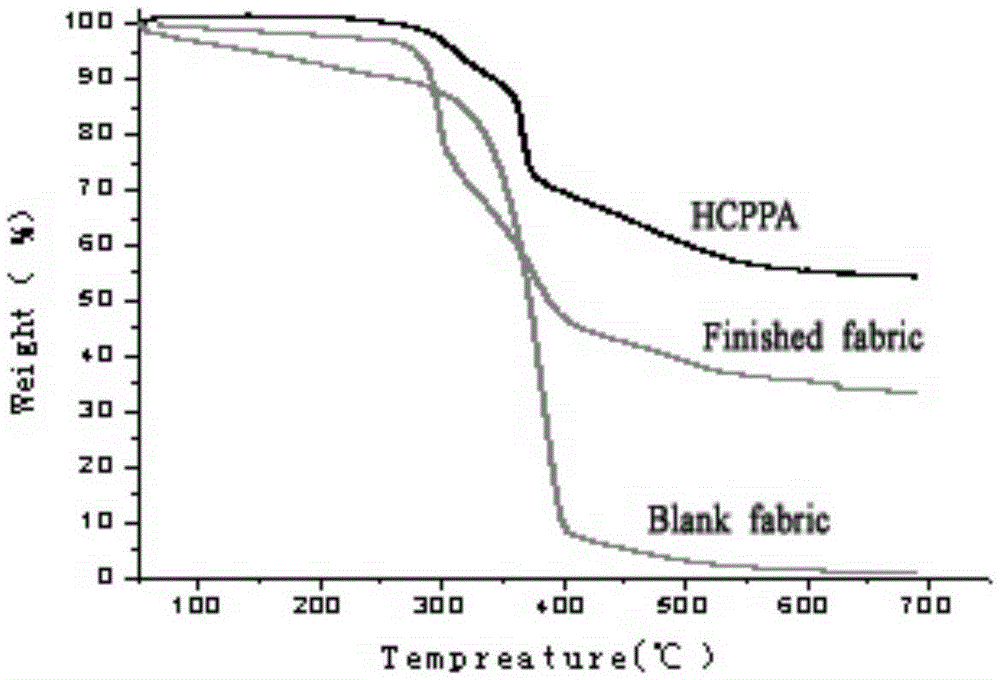
N-P flameresistant material and preparation method thereof and application in textiles
ActiveCN105348326ARapid responseFast processGroup 5/15 element organic compoundsHeat resistant fibresFire retardantHydroxymethyl
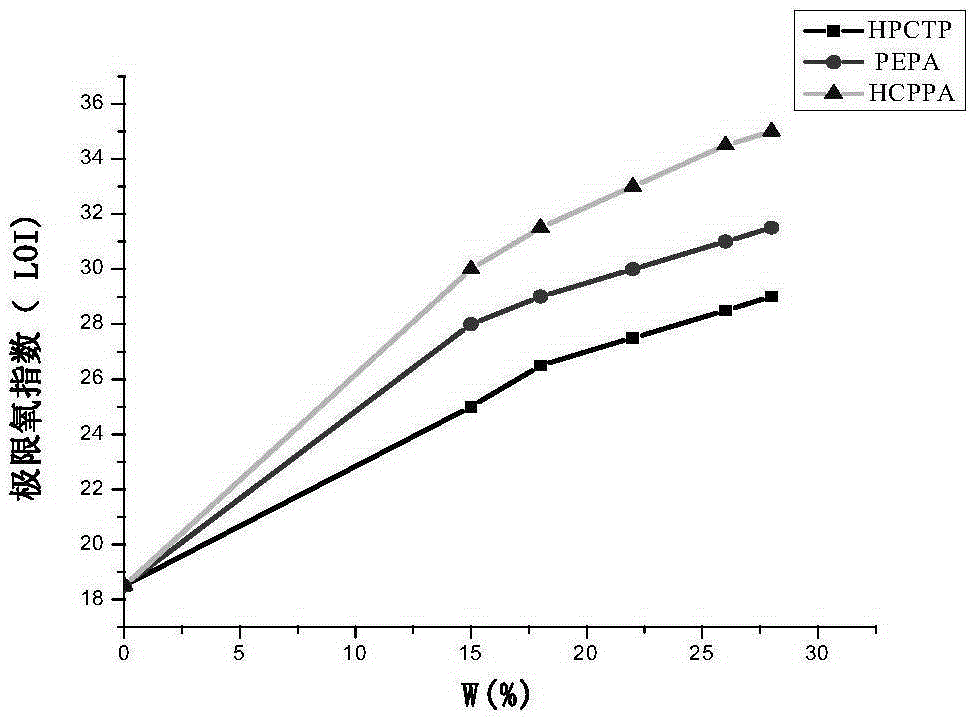
The invention discloses an N-P flameresistant material and a preparation method thereof and application in textiles. The chemical name of a flame retardant of the material is hexa(1-oxo-phospha-2,6,7-trioxabicyclo[2,2,2]octane-4-methylenedioxy)cyclotriphosphazene (HCPPA); the preparation method of the material comprises the steps of synthesizing hexachlorocyclotriphosphazene (HCPP) by reacting ammonium chloride with phosphorus pentachloride, wherein a catalyst is pyridine and ZnO; then synthesizing 1-oxo-phospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2,2,2]octane (PEPA) by reacting pentaerythritol with phosphorus oxychloride; finally synthesizing the HCPPA by reacting the HCPP with the PEPA. According to the preparation method, NaH is used as a catalyst, so that the synthesis reactions can be performed quickly, the reaction time is greatly shortened, and the product yield is improved. When the N-P flame retardant is used for retarding a flame of a cotton fabric, high limit oxygen index and char yield are achieved, and the wash durability is good.
Owner:HUNAN INSTITUTE OF ENGINEERING
Green synthesis method of methyl phosphorus dichloride
InactiveCN106117267AImprove conversion rateImprove protectionGroup 5/15 element organic compoundsPhosphorus pentachloridePhosphorus trichloride
The invention discloses a green synthesis method of methyl phosphorus dichloride. The method comprises the following steps: by taking phosphorus pentachloride as a catalyst, heating phosphorus trichloride and phosphorus pentachloride into vapor, and preheating to 150-250 DEG C and mixing with methane and then entering a tubular reactor, and reacting for 0.1-1.0s under the conditions that the temperature is at 400-500 DEG C and the pressure is 0.3-1.2MPa, to obtain methyl phosphorus dichloride. After the catalyst is applied to methane and phosphorus trichloride to synthesize methyl phosphorus dichloride, the conversion rate of the phosphorus trichloride is effectively improved to 40%-50%, and the yield is 90%-95%; the adopted catalyst phosphorus pentachloride is decomposed into phosphorus trichloride and chlorine gas, the catalyst and the product is not required for separation, three wastes are not generated during the whole preparation, and all the materials are recycled, so that the resources are saved, the environment is beneficially protected, and the synthesis method is green.
Owner:ANHUI COSTAR BIOCHEM CO LTD
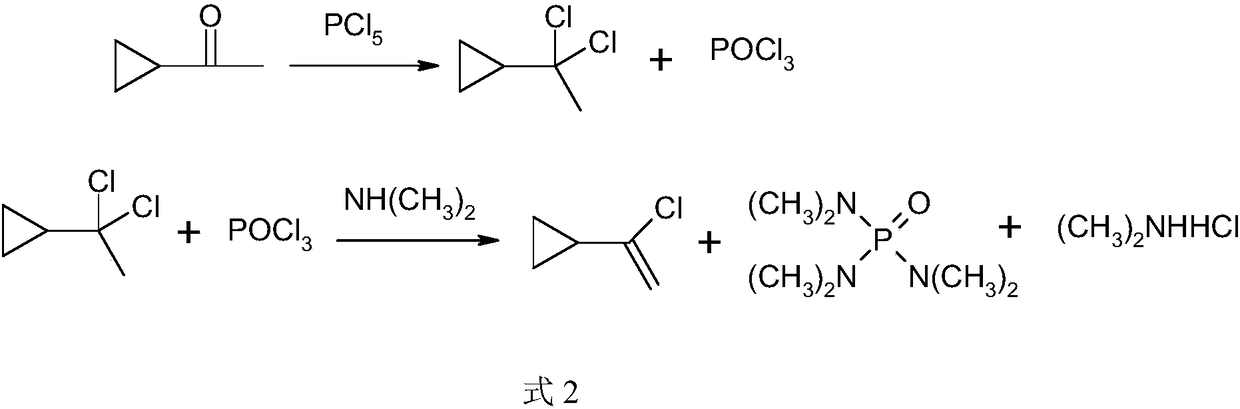
Method for efficiently preparing cylopropyl ethylnen
InactiveCN105152846AHigh yieldAtom utilization is highHydrocarbon from halogen organic compoundsOrganic solventDistillation
The invention discloses a method for effectively preparing cylopropyl ethylnen as shown in the formula (I). The method comprises the steps that firstly, cylopropyl methyl ketone shown in the formula (II) is taken as a raw material and fully reacts in an organic solvent under the effect of phosphorus pentachloride and a catalyst, a reaction solution is subjected to reduced pressure distillation to obtain 1,1-dichloro-1-cylopropyl ethane as shown in the formula (III), an elimination reaction is then carried out under the effect of alkali, and a reaction solution is subjected to rectification and purification to obtain the product cylopropyl ethylnen. The method for effectively preparing the cylopropyl ethylnen has the advantages of being mild in reaction condition, high in yield, low in cost, little in three-waste and the like, and is suitable for industrial production.
Owner:JIUJIANG ZHONGTIAN PHARMA +1
Method for preparing antithrombotic medicament apixaban
InactiveCN101967145BReasonable designThe reaction steps are simpleOrganic chemistryPhysical/chemical process catalystsMorpholineP-Nitroaniline
The invention discloses a method for preparing antithrombotic medicament apixaban. The method comprises the following steps of: obtaining a compound V by performing an amidation-cyclization two-step one-pot reaction on paranitroaniline serving as a raw material and a general purpose reagent 5-chlorine valeryl chloride under an alkaline condition; performing di-chlorination on the alpha-hydrogen of the V by using phosphorus pentachloride; performing a condensation-elimination reaction with excess morpholine to obtain a compound VI; reducing the VI into a compound VII by using sodium sulfide; performing the amidation-cyclization two-step one-pot reaction on the VII and the 5-chlorine valeryl chloride to obtain a key intermediate III; obtaining II by performing a [3+2] cyclization-elimination reaction on the III and another intermediate IV; and finally, obtaining I by performing aminolysis on the II. The method has the advantages that: process design is reasonable, expensive reagent is not used, reaction yield is high, raw material cost is low, and the method is simple and convenient to operate, has no harsh reaction condition, and is easy to perform large-scale production.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Preparation method of intermediate cyclopropyl acetylene of anti-AIDS (acquired immune deficiency syndrome) drug efavirenz
InactiveCN108440229AAvoid hydrolysisLow costPreparation by hydrogen halide split-offPhosphorus halides/oxyhalidesIce waterHydrolysis
The invention discloses a preparation method of intermediate cyclopropyl acetylene of anti-AIDS (acquired immune deficiency syndrome) drug efavirenz. The preparation method comprises the steps of performing a reaction in an organic solvent by taking cyclopropyl methyl ketone as a raw material and phosphorus pentachloride as a chlorinating agent by the action of a catalyst to generate alpha, alpha-dichloroethyl cyclopropane and phosphorus oxychloride, performing decompressed rectification to remove phosphorus oxychloride, removing part of hydrogen chloride from alpha, alpha-dichloroethyl cyclopropane by the action of triethylamine to generate alpha-chlorovinyl cyclopropane, and further removing part of hydrogen chloride by the action of strong base to generate cyclopropyl acetylene. Phosphorus oxychloride is removed by the decompressed rectification; the generation of much phosphorus wastewater due to hydrolysis of phosphorus oxychloride in ice water is avoided; recovered phosphorus oxychloride can be comprehensively utilized by simple rectification; waste gas, wastewater and industrial residue are reduced; the cost is lowered; cyclopropyl acetylene is prepared from alpha-chlorovinyl cyclopropane via a reaction rectification technology; and the method has the advantages of high conversion rate, good selectivity, low energy consumption and the like, and is suitable for industrialproduction.
Owner:JIANGSU YUXIANG CHEM
Preparation method of lithium hexafluorophosphate
ActiveCN101723346BHigh yieldImprove securityFinal product manufactureElectrolyte accumulators manufactureHydrogen fluoridePhysical chemistry
The invention provides a preparation method of a lithium hexafluorophosphate, which comprises the following steps: firstly, reacting a phosphorus pentachloride with an anhydrous hydrogen fluoride to form mixed liquid of the phosphorus pentachloride and the anhydrous hydrogen fluoride; secondly, preparing anhydrous hydrogen fluoride solution of lithium fluoride; and finally, adding the anhydrous hydrogen fluoride solution of the lithium fluoride into the mixed liquid of the phosphorus pentachloride and the anhydrous hydrogen fluoride, and sequentially performing reaction, crystallization, separation and drying to obtain a pure lithium hexafluorophosphate product. The preparation method of the invention has the advantages of mild reaction, high safety and purity of the lithium hexafluorophosphate product of over 99.9 percent; and the mother liquor can be reclaimed and reused so that the cost is reduced.
Owner:DO FLUORIDE CHEM CO LTD
Preparation method of hexaphenoxycyclotriphosphazene
InactiveCN103539820AReduce consumptionReduce energy consumptionGroup 5/15 element organic compoundsChlorobenzenePotassium
The invention discloses a preparation method of hexaphenoxycyclotriphosphazene. The method comprises the following steps of: with phosphorus pentachloride and ammonium chloride as starting raw materials and chlorobenzene as a solvent, carrying out a high-selectivity synthetic reaction, and filtering to obtain high-purity chlorobenzene solution of hexachlorocyclotriphosphazene, and then reacting the high-purity chlorobenzene solution of hexachlorocyclotriphosphazene with potassium phenate to obtain high-purity hexaphenoxycyclotriphosphazene. The preparation method is simple in process and good in reproducibility; and as a result, the problems of low conversion rate and difficult industrialization in the synthesis of hexaphenoxycyclotriphosphazene can be effectively solved.
Owner:威海金威化学工业有限责任公司
Synthetic method of intermediate cyclopropyl acetylene of anti-aids drug efavirenz
InactiveCN103664465AProcess raw materials are easy to getSimple processHydrocarbon from halogen organic compoundsPtru catalystEthyl group
The invention discloses a synthetic method of intermediate cyclopropyl acetylene of an anti-aids drug efavirenz, and relates to the technical field of synthesis of cyclopropyl acetylene. The synthetic method comprises the following steps: reacting in an organic solvent to produce alpha, alpha-ethyl dichloride cyclopropane by taking cyclopropyl methyl ketone as a raw material and taking phosphorus pentachloride as a chlorinating agent; and obtaining the cyclopropyl acetylene by adding the alpha, alpha-ethyl dichloride cyclopropane into strong base aqueous liquor through a phase transfer catalyst. The synthetic method has the beneficial effects that the organic solvent and inorganic strong base are not needed to be adopted, expensive reagents such as the organic solvent and potassium tert-butoxide are avoided; moreover, process raw materials are easily available, process is simple, operation is convenient, cost is lowered and the three wastes are reduced, and therefore, the synthetic method is suitable for industrial production.
Owner:JIUJIANG ZHONGTIAN PHARMA
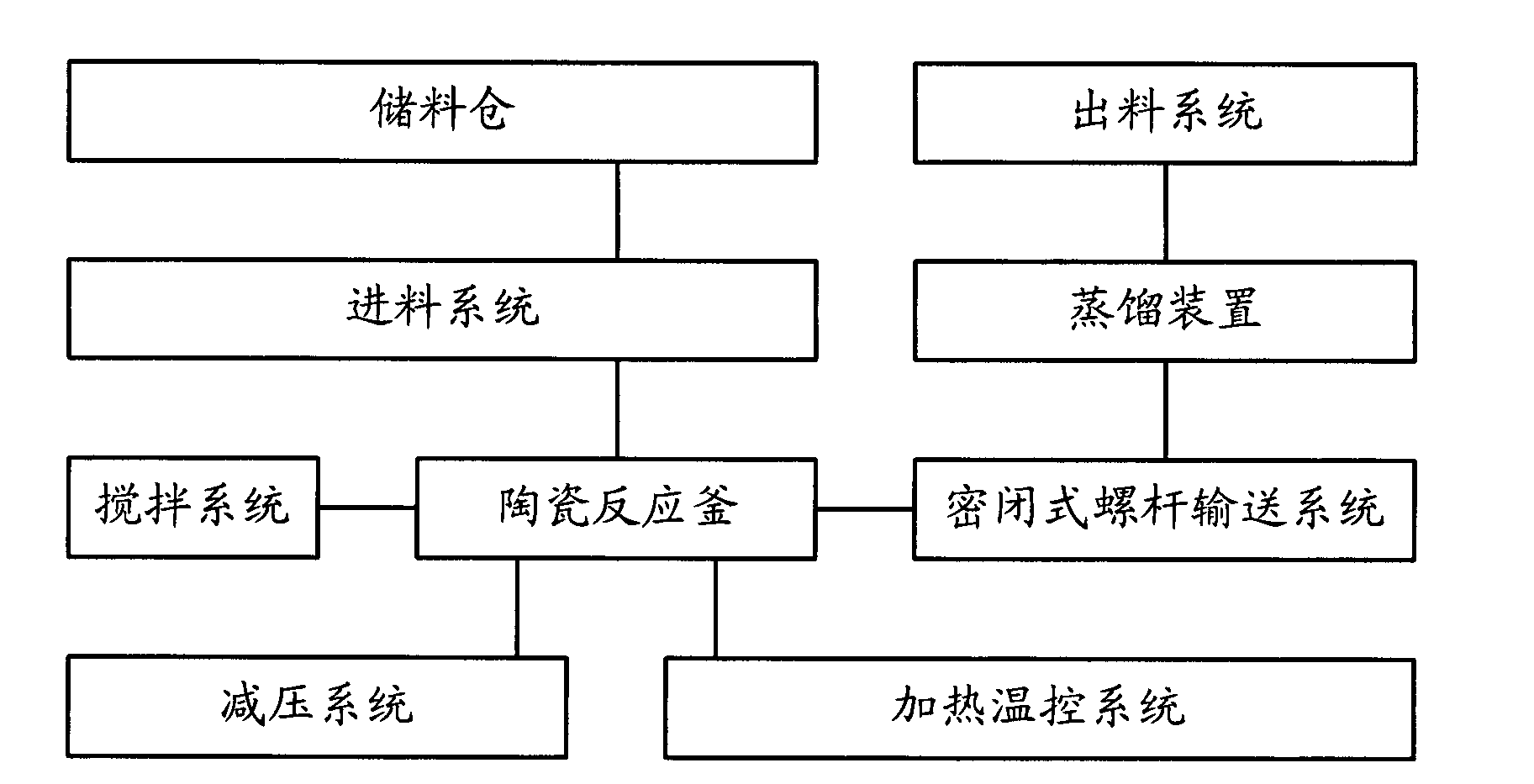
Synthesizing method and device of phosphonitrilic chloride trimer as well as preparation method of terphenyl cycloposphazene
InactiveCN103896985AGood market application valueGroup 5/15 element organic compoundsReaction temperatureChloride
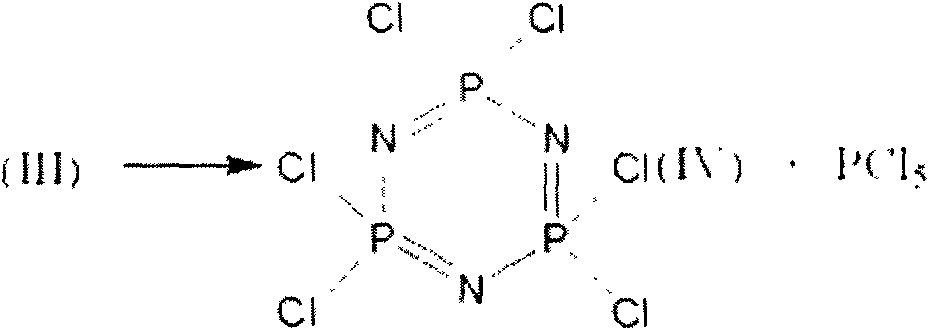
The invention discloses a synthesizing method and a synthesizing device of phosphonitrilic chloride trimer as well as a preparation method of terphenyl cycloposphazene. The synthesizing method of the phosphonitrilic chloride trimer comprises the following steps: A1, adding powdered ammonium chloride and phosphorus pentachloride into a ceramic reaction kettle in a weight ratio of (900-1500):3000; A2, adding zinc chloride, heating and stirring to react, constantly dripping an acid-binding agent during stirring, wherein the weight ratio of zinc chloride to phosphorus pentachloride is (20-35):3000, the weight ratio of the acid-binding agent to the phosphorus pentachloride is (30-50):3000, the reacting temperature is 65-150 DEG C, and the reacting time is 1.5-2.5 hours; and A3, feeding to sublimation purifying equipment by adopting a closed screw rod, heating to 125-135 DEG C, and sublimating to obtain the phosphonitrilic chloride trimer.
Owner:SHENZHEN HALCYON NEW MATERIALS
Preparation method of 2, 6-dichlorobenzaldehyde
ActiveCN103396301AReduce consumptionReduce generationCarbonyl compound preparation by hydrolysisChemical synthesisCatalytic effect
The invention provides a preparation method of 2, 6-dichlorobenzaldehyde and relates to the technical field of chemical synthesis production. The preparation method of the invention comprises the following steps of: performing a chlorination reaction on 2,6-dichlorotoluene and chlorine under a reaction condition of 50-250 DEG C in the catalytic effect of phosphorus pentachloride and light, and performing rectification to prepare 2,6-dichloro dchlorobenzyl; adding the 2,6-dichloro dchlorobenzyl, an acidic solvent and zinc chloride into a hydrolysis nitrilation kettle, and performing a hydrolysis reaction under a heating reflux condition to prepare the 2, 6-dichlorobenzaldehyde. The preparation method provided by the invention has the advantages of ensuring easy production control, little material consumption and low production cost, reducing the generation of solid waste and reducing the emission of nitrogen-containing nitroso group waste, thereby being an energy-saving, emission-reducing, environment-friendly, industrial, practical and clean production process.
Owner:永椿化工新材料有限公司
Preparation method of 4,6-dichloropyrimidine
The invention discloses a preparation method of 4,6-dichloropyrimidine. The preparation method comprises the following steps: 1) mixing 4,6-dyhydroxy pyrimidine, phosphorus oxychloride and phosphorus pentachloride, controlling the temperature to be 50-110 DEG C, reacting, stopping the reaction until the content of 4,6-dyhydroxy pyrimidine is lower than 1%, cooling a reactant mixture to a temperature lower than 30 DEG C; or, mixing 4,6-dyhydroxy pyrimidine with phosphorus oxychloride, controlling the temperature to be 50-110 DEG C, adding phosphorus pentachloride batch by batch, reacting, stopping the reaction until the content of 4,6-dyhydroxy pyrimidine is lower than 1%, cooling the reactant mixture to a temperature lower than 30 DEG C; 2) carrying out reduced pressure distillation on the reactant mixture to recover phosphorus oxychloride; and 3) purifying the reactant mixture from which phosphorus oxychloride is recovered in step 2), thus obtaining 4,6-dichloropyrimidine. Namely, the invention provides the method for preparing 4,6-dichloropyrimidine without utilizing an organic alkali, by which, a tedious process of recovering and recycling the organic alkali is avoided; phosphorus oxychloride can be recycled, therefore the waste of resources is avoided; and furthermore, a large amount of phosphorus-containing waste solution and waste slag is not likely to generate.
Owner:CHONGQING UNISPLENDOUR CHEM
Preparation method of Ni2P-supported Ni-based catalyst, obtained catalyst and application thereof
ActiveCN108796552AReduce usageAvoid high temperature preparationElectrodesWhite PhosphorusPhosphorus pentachloride
The invention discloses a preparation method of a Ni2P-supported Ni-based catalyst, the obtained Ni2P-supported Ni-based catalyst and application thereof. Low temperature phosphating can be successfully achieved by using plasmas for processing elemental nickel, nickel hydroxides and nickel oxides, and Ni2P is obtained. Meanwhile, non-toxic red phosphorus is used as a phosphorus source in the preparation method, so that using of high-toxicity phosphorus (such as PH3) or white phosphorus or phosphorus pentachloride is avoided, and using of expensive organic reagents such as P(SiMe3)3 and tri-n-octylphosphine (TOP) is avoided. In addition, the Ni2P-supported Ni-based catalyst obtained through the preparation method can be directly used for hydrogen evolution through electrolysis of water, sothat using of binding agents in traditional granular type catalysts is avoided.
Owner:PEKING UNIV
Method for producing high-purity lithium hexafluorophosphate
InactiveCN102515132ASimple processImprove performancePhosphorus compoundsPhysical chemistryHydrogen chloride
The invention discloses a method for producing high-purity lithium hexafluorophosphate, comprising the following steps: 1) reacting hydrogen fluoride with phosphorus pentachloride to prepare a mixed gas of phosphorus pentafluoride and hydrogen chloride, removing impurities by letting the mixed gas pass through a washing device; 2) dissolving lithium fluoride in hydrogen fluoride to form a hydrogen fluoride solution of lithium fluoride; and 3) reacting lithium fluoride solution with phosphorus pentafluoride in an absorption reactor to form a lithium hexafluorophosphate solution, carrying out crystallization, separating and drying to obtain a high-purity lithium hexafluorophosphate solid.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Method for preparing 2, 6-dichlorobenzonitrile
ActiveCN103382166AReduce consumptionReduce generationPreparation by nitrogen oxide-organic compound reactionNitrosoChemical synthesis
The invention discloses a method for preparing 2, 6-dichlorobenzonitrile, and relates to the technical field of production of chemically synthesized 2, 6-dichlorobenzonitrile. According to the method, 2, 6-dichlorotoluene is used as a starting material and chlorinated to prepare 2, 6-benzylidene chloride, the 2, 6-benzylidene chloride is hydrolyzed and quaternized to prepare a 2, 6-dichlorobenzonitrile crude product, and the 2, 6-dichlorobenzonitrile crude product is refined to obtain a 2, 6-dichlorobenzonitrile fine product. Production is easily controlled, raw material consumption is less, production cost is low, solid waste is decreased, emission of nitrogenous nitroso-group waste gas is reduced, and the method is an energy-saving, emission-reduction and environment-friendly industrial practical cleaning production technique.
Owner:永椿化工新材料有限公司
Method for catalytically synthesizing hexachlorocyclotriphosphazene
InactiveCN101602780AShort reaction timeReduce yieldGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsMetal chlorideChlorobenzene
The invention discloses a method for catalytically synthesizing hexachlorocyclotriphosphazene. The method is characterized by comprising the following steps: taking raw materials according to a molar ratio that quaternary ammonium salt:anhydrous metal chloride:chlorobenzene:ammonium chloride:phosphorus pentachloride is 1-2:0.1-1:5-10:1-1.4:1; adding the quaternary ammonium salt and anhydrous metal chloride into a reactor to react by stirring for 1 to 2 hours at a temperature of between 80 and 120 DEG C to prepare ionic liquid; adding the chlorobenzene, the ammonium chloride and the phosphorus pentachloride into the reactor to react by stirring for 1 to 5 hours at a temperature of between 100 and 130 DEG C to obtain reaction mixed material; separating or / and filtering the reaction mixed material to separate the ionic liquid from chlorobenzene solution containing the reactant; and washing and distilling the chlorobenzene solution containing the reactant at reduced pressure to obtain the hexachlorocyclotriphosphazene product. The method has simple and safe operation and low cost, and is easy for industrial production and application.
Owner:SICHUAN DONGFANG INSULATING MATERIAL
Method for Producing Electrolyte Solution for Lithium Ion Battery and Battery Using Same
ActiveUS20090081559A1Easy to produceElectrolytic capacitorsOrganic electrolyte cellsHydrogen fluorideLithium chloride
There is provided a method for producing an electrolyte solution for lithium ion battery, which is characterized in that lithium fluoride, lithium chloride, lithium bromide, lithium iodide or a mixture of any of these is reacted with phosphorus pentachloride and hydrogen fluoride in a nonaqueous organic solvent, when an electrolyte solution for lithium ion battery, which contains lithium hexafluorophosphate as an electrolyte, is produced.
Owner:CENT GLASS CO LTD
Methods for preparing phosphorus pentafluoride gas and preparing lithium hexafluorophosphate using the gas
ActiveCN101353161BEasily hydrolyzedStrong moisture absorptionPhosphorus halides/oxyhalidesLithiumPhysical chemistry
A preparation method of phosphorus pentafluoride gas comprises a step of causing phosphorus pentachloride to react with anhydrous hydrogen fluoride, wherein, the reaction occurs in the presence of a solvent. A preparation method of lithium hexaflourophosphate comprises a contact reaction between solid lithium fluoride and phosphorus pentafluoride gas, wherein the phosphorus pentafluoride gas is prepared by the method of the invention. Compared with the preparation method of the lithium hexaflourophosphate with the phosphorus pentafluoride gas as the raw material in the prior art, the phosphorus pentafluoride gas prepared by the preparation method of the invention has higher purity and lower cost. The yield of the lithium hexaflourophosphate prepared by the method of the invention is higher than 93%, and the purity thereof is up to 99.95%.
Owner:BYD CO LTD
Preparation method of phosphonitrile chloride catalyst, and application of phosphonitrile chloride catalyst in preparation of alkoxy-terminated polysiloxane
ActiveCN110655049AHigh catalytic activityHigh activityNitrogen compoundsPtru catalystTetrachloroethane
The invention discloses a preparation method of a phosphonitrile chloride catalyst, and an application of the phosphonitrile chloride catalyst in the preparation of alkoxy-terminated polysiloxane. Thepreparation method comprises the following steps: purging a reaction device with nitrogen to achieve preheating treatment, opening the reaction device to remove condensate water, and sequentially infiltrating the reaction device with dichloromethane and tetrachloroethane; mixing and uniformly stirring tetrachloroethane and ammonium chloride to prepare a tetrachloroethane solution of ammonium chloride for later use; adding phosphorus pentachloride into the reaction device, slowly raising the temperature to 140-160 DEG C under the protection of nitrogen, then dropwise adding the prepared tetrachloroethane solution of ammonium chloride, carrying out a reflux reaction after the drop-by-drop addition is completed, then raising the temperature to 170-175 DEG C, continuously carrying out the reflux reaction, and cooling the reaction device to room temperature after the reaction is completed; and adding anisole into the reaction solution, and performing stirring under a vacuum condition to achieve uniform mixing in order to obtain target crystals which are the phosphonitrile chloride. The prepared phosphonitrile chloride has a high catalytic activity; and when the phosphonitrile chlorideis used for preparing alkoxy-terminated polysiloxane, a polymer with an excellent storage stability can be effectively prepared.
Owner:新纳奇材料科技江苏有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com