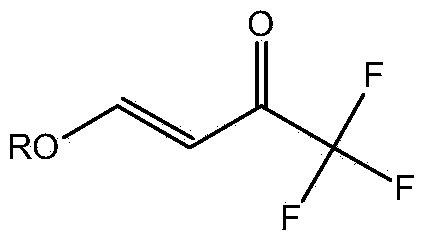
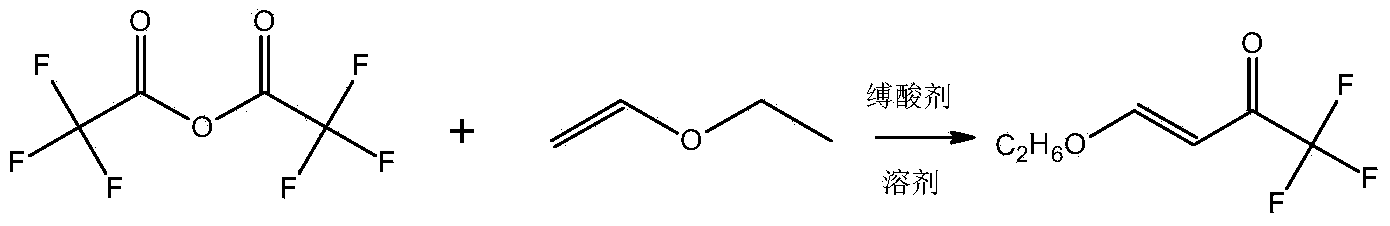
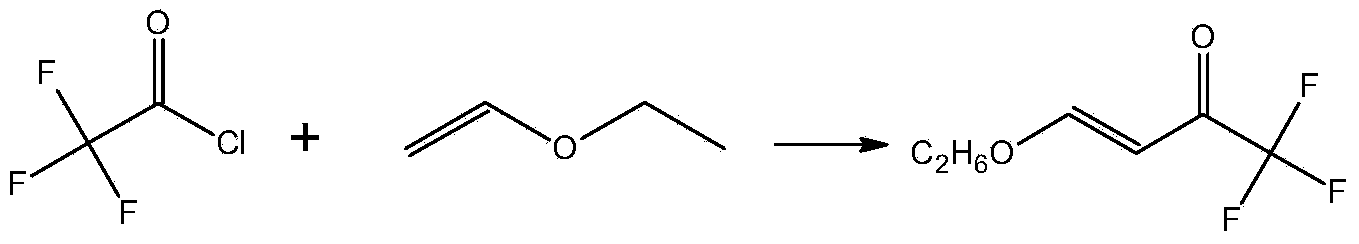
Preparation method for 4- alkoxy-1,1,1- trifluoro-3- butane-2-ketone
A technology of alkoxy and butene, applied in the direction of condensation to prepare carbonyl compounds, organic chemistry, etc., can solve the problems of high molecular weight, high production cost and short reaction steps of trifluoroacetic anhydride, and achieve high reaction yield and low production cost. Low, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a three-necked reaction flask equipped with stirring, a thermometer and a dropping funnel, add 450ml of toluene and 144g (1800mmol)) of pyridine in sequence, and add 102.6g of trifluoroacetic acid (900mmol)) dropwise at 0~-10°C, and the dropping time is about After 1 hour, the addition was completed. After adding 65.5g (900mmol) of vinyl ether at 0 to -10°C, 94.5g (945mmol) of phosgene was introduced into the reaction mixture at 0 to -10°C for about 4 hours. Gas chromatography monitoring after passing through phosgene, vinyl ether is less than 1%, add 220ml of water after the reaction is complete, stir for 60 minutes, separate layers, extract the water layer with 90ml of toluene, combine the organic phases, wash twice with 2*135ml of water, Dry the organic phase with anhydrous magnesium sulfate, filter, wash with 2*40ml toluene, combine the organic phases, detoluene at normal pressure, and distill under reduced pressure to obtain the product 4-ethoxy-1,1,1-trifluoro-3...
Embodiment 2
[0023] In a three-necked reaction flask equipped with stirring, a thermometer and a dropping funnel, add 450ml of dichloromethane and 220.3g (1800mmol)) of N,N-dimethylaniline in sequence, and dropwise add 102.6g of trifluoro Acetic acid (900mmol)), the dropwise addition time is about 1 hour, after the addition is completed, 105.6g (1800mmol) vinyl methyl ether is passed into the reaction mixture at 0~-10°C, and 94.5g ( 945mmol) phosgene, pass through in about 4 hours, monitor by gas chromatography after passing through phosgene, vinyl methyl ether is less than 1%, add 220ml water after the reaction is complete, stir for 60 minutes, separate layers, and extract the water layer with 90ml dichloromethane , combine the organic phases, wash twice with 2*135ml water, dry the organic phases with anhydrous magnesium sulfate, filter, wash with 2*40ml dichloromethane, combine the organic phases, remove dichloromethane at normal pressure, and distill under reduced pressure, 121.1 g of t...
Embodiment 3
[0025] In a three-necked reaction flask equipped with stirring, a thermometer and a dropping funnel, add 900ml of dichloroethane and 271.3g (1800mmol) of N,N-diethylaniline in sequence, and dropwise add 102.6g of trifluoro Acetic acid (900mmol)), the dropwise addition time is about 1 hour, after the addition is completed, 78.3g (900mmol) vinyl n-propyl ether is added at 0~-10°C, and 180.0g ( 1800mmol) phosgene, passed through in about 2 hours, gas chromatographic monitoring after passing through phosgene, vinyl n-propyl ether was less than 1%, after the reaction was complete, add 220ml water, stir for 60 minutes, separate layers, and use 90ml dichloroethyl ether for the water layer Extract with alkane, combine the organic phases, wash twice with 2*135ml water, dry the organic phase with anhydrous magnesium sulfate, filter, wash with 2*40ml dichloroethane, combine the organic phases, and remove dichloroethane under normal pressure, Distilled under reduced pressure to obtain 142...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com