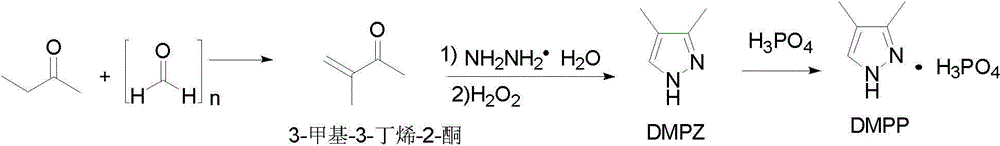Preparation methods of 3,4-dimethyl pyrazole and 3,4-dimethyl pyrazole phosphate
A technology of dimethylpyrazole phosphate and dimethylpyrazole, which is applied in the field of preparation of 3,4-dimethylpyrazole, can solve the problem of high price of 3-methyl-2-butanone and a large amount of strong acidity Waste water, high anti-corrosion requirements, etc., to achieve the effect of simple operation, reduction of three wastes, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
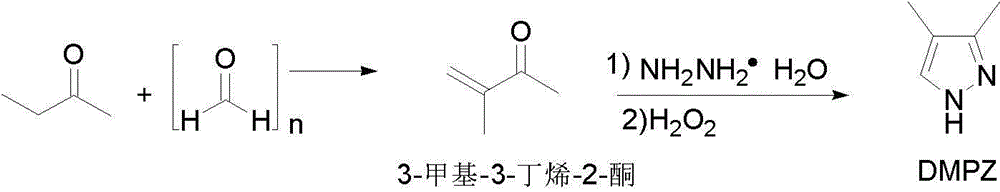
[0036] The synthesis of embodiment 1 3-methyl-3-buten-2-one
[0037] The ratio of the amount of feed material 2-butanone: paraformaldehyde (M=30): protonic acid is 1.0:0.9:0.1, and the protonic acid is sulfuric acid.
[0038]In a 500mL three-necked flask equipped with a thermometer, reflux condenser and mechanical stirring, add 144g (2mol) of 2-butanone, 54g (1.8mol) of paraformaldehyde, and 182mL of methanol. Sulfuric acid solution (concentrated sulfuric acid 20g (0.2mol) diluted with 15mL of methanol), dropwise addition, 30-35 ℃ heat preservation reaction for 20h, after the reaction is completed, adjust the pH to 7-8, and collect 96-98 ℃ fractions by atmospheric distillation to obtain 3- Methyl-3-buten-2-one 136.0g (1.62mol) product yield 81%, content 99.4% (GC). 1 H NMR (400 MHz, CDCl 3 ): δ= 5.96 (s, 1H), 5.80 (s, 1H), 2.37 (s, 3H), 1.90 (s, 3H). MS (EI): m / z= 84 (M + , 100).
Embodiment 2
[0039] Example 2 Synthesis of 3,4-dimethylpyrazole
[0040] The molar ratio of 3-methyl-3-buten-2-one:hydrazine hydrate:hydrogen peroxide:basic compound is 1.0:0.8:1.3:0.1, and the basic compound is triethylamine.
[0041] In a 1000mL three-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, 136g (1.62mol) of 3-methyl-3-buten-2-one prepared in Example 1 was added, and 85% hydrated Hydrazine solution 70.1g (1.3mol), control the reaction temperature not higher than 50°C, keep the reaction at 40-50°C for 2 hours after the dropwise addition, and add 16.3g (0.16mol) of triethylamine when the reaction solution drops to 25-30°C , and slowly add 238.6g (2.1mol) of 30% aqueous hydrogen peroxide solution dropwise. After the dropwise addition, keep the temperature at 30-35°C for 10h, cool to room temperature, add 200mL of toluene, stand and separate to obtain the toluene layer, and first recover under reduced pressure The crude product 3,4-dimethylpyrazole ...
Embodiment 3
[0043] Example 3 Synthesis of 3,4-dimethylpyrazole phosphate
[0044] Dissolve 143g of 3,4-dimethylpyrazole obtained in Example 2 in 100mL of ethanol, add phosphoric acid at room temperature to adjust the pH to 1-3, continue stirring at room temperature for 5h, filter, and dry to obtain 3,4 -Dimethylpyrazole phosphate 277.4g, product yield 96%, content 99.1% (HPLC).
[0045] M.p.168.7~169.6 o C. IR (KBr), ν / cm -1 = 2721, 2375, 1074, 977. 1 H-NMR (400 MHz, D 2 O): δ= 7.67 (s, 1H), 2.24 (s, 3H), 1.96 (s, 3H). 13 C-NMR (100MHz, D 2 O): δ= 143.4, 132.5, 116.0, 8.7, 7.0. MS (ESI): m / z = 217.8 (M + +Na).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com