Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
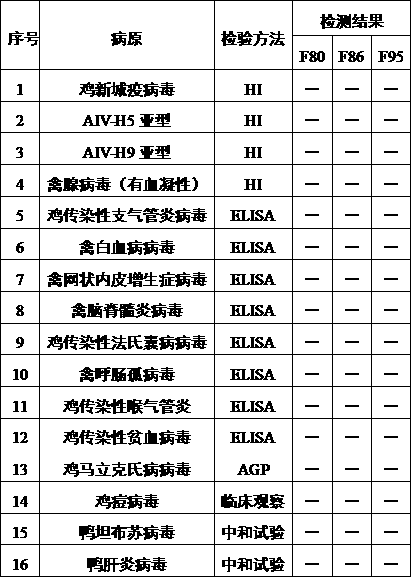
79results about How to "High virus content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
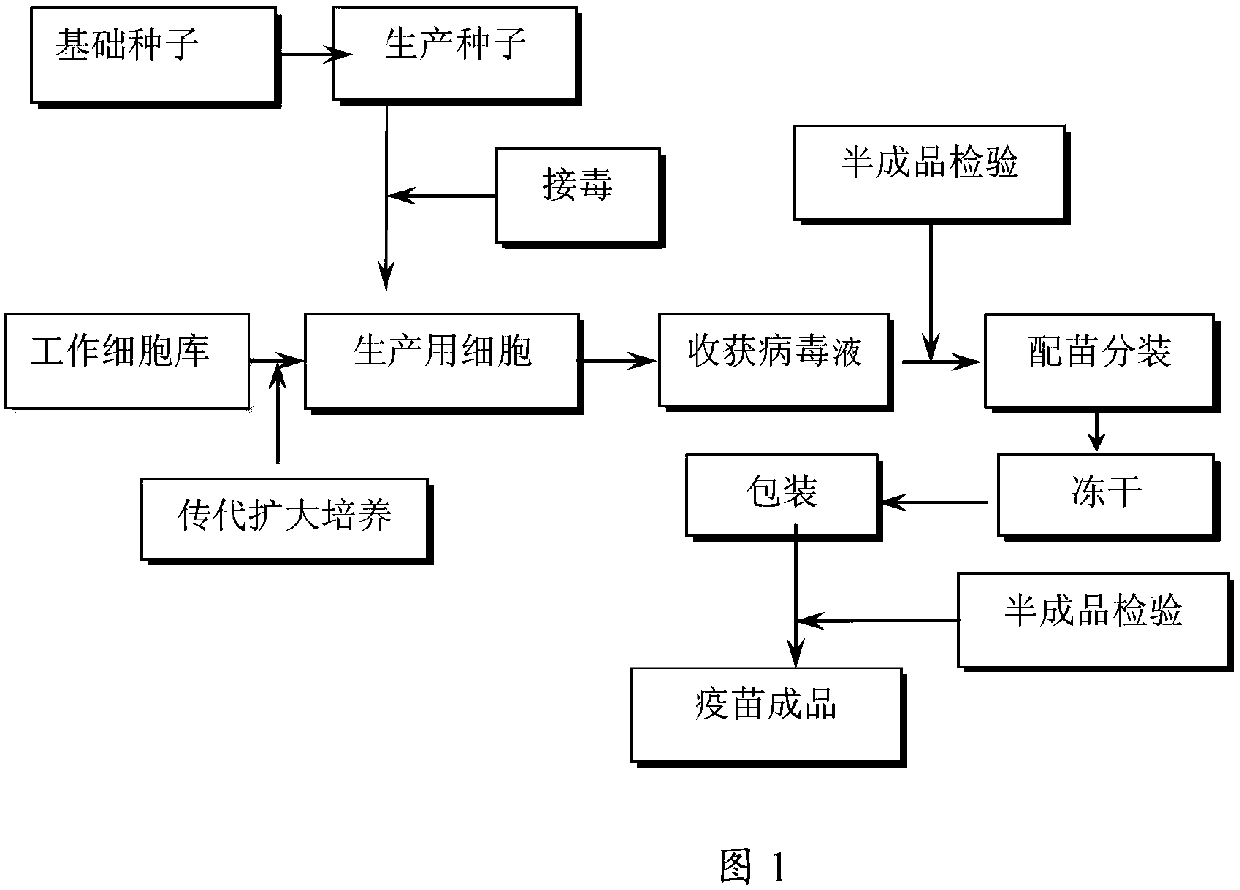
Method for producing swine fever live vaccine with cell line
ActiveCN101181637AGuaranteed to be pureEnsure safetyAntiviralsAntibody medical ingredientsQuality controlSeedling
The invention discloses a method for producing a live swine fever vaccine by using a cell line. The present invention comprises the following technical steps: (1) selecting a cell line as the cells for making seedlings; (2) subculture and cultivation of cells for making seedlings; (3) breeding of cytotoxic species; (4) breeding of venom for making seedlings; 5) Mixing seedlings, subpackaging and freeze-drying. The invention has the advantages of simple and stable production process, easy operation, high virus content, small difference between batches, easy quality control, and can significantly improve the yield and quality of vaccines. The live swine fever vaccine produced by the invention has good safety and high immune efficacy, and has complete immune protection against the virulent attack of swine fever.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
Method, primer group and kit for capturing novel coronavirus whole genome
ActiveCN111118226AEasy to operateShorten the timeMicrobiological testing/measurementAgainst vector-borne diseasesMolecular biologyRNA
The invention relates to a super-sensitivity method, primer group and kit for capturing whole genome of novel coronavirus. The method can conveniently and quickly amplify the whole genome of the novelcoronavirus by using a small amount of RNA, and can directly dock with a second-generation sequencing library-building reagent and a third-generation sequencing platform so as to obtain a whole genome sequence of the novel coronavirus.
Owner:北京微未来科技有限公司
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
The invention provides a method for producing pseudorabies living vaccines by using a subculture cell source and a pseudorabies living vaccine product thereof. The method comprises the following steps: culturing pseudorabies virus low-virulent strains by using subculture cells; harvesting the strains to obtain cell culture venom; and then adding a stabilizing agent and an antibiotic into the cellculture venom, and freezing and vacuum-drying the mixture to obtain the pseudorabies living vaccines of the subculture cell source. The subculture cells are subculture cells ST of pig testicle or subculture cells PK15 or IBRS-2 of pig kidney. The method for producing the pseudorabies living vaccines by using the subculture cell source has the advantages of simple and stable production process, easy operation, high virus content, little batch difference and controllable quality, can remarkably improve the yield and quality of the vaccines and reduce the anaphylactic reaction and the like. The pseudorabies living vaccines obtained by using the production method of the invention have good safety and high immune efficacy, and have better immune protection effect on pseudorabies virulent attack.
Owner:广东永顺生物制药股份有限公司
Method of preparing vaccine through suspension culture of mammal cells and application of the method
ActiveCN105311630AReduce dependenceReduce manufacturing costAntiviralsViruses/bacteriophagesImmune effectsViral Vaccine
The invention provides a method of preparing a vaccine through suspension culture of mammal cells, which includes the following steps: (1) preparing microencapsulated animal cells through a microencapsulation method; (2) inoculating the microencapsulated animal cells in a cell culture system to perform cultivation; and (3) inoculating virus into the cell culture system to culture the virus, and obtaining a virus liquid to prepare the vaccine. By means of the microencapsulation method to culture the animal cells, a problem of large-scale continuous amplification of cells is solved. When the cultured animal cells are used for breeding virus, a high content of virus is achieved, so that the virus liquid, when being used for preparing the viral vaccine, can also achieve a better immune effect. The invention also provides a method of preparing a cell-expression product through the suspension culture of the mammal cells. The method reduces dependency on micro-carriers and is greatly reduced in production cost.
Owner:PU LIKE BIO ENG
Combined live vaccine against porcine reproductive and respiratory syndrome and pseudorabies, and preparation method thereof
ActiveCN102727884AImprove immune efficiencyIncrease productionViral antigen ingredientsGenetic material ingredientsPseudorabiesImmunosuppression
The invention provides a combined live vaccine for preventing porcine reproductive and respiratory syndrome and pseudorabies, and a preparation method and application thereof. According to the invention, no immunosuppression occurs between two vaccines of the combined live vaccine; compared with each single vaccine, the combined live vaccine has no obvious difference in security, immunogenicity, immunity duration and immuno-protective effects and has remarkable immuno-protective effects on preventing porcine reproductive and respiratory syndrome and pseudorabies.
Owner:华威特(江苏)生物制药有限公司
Method for mass production of pseudorabies virus vaccine
InactiveCN101804203ASolve the problems of low production output, high labor intensity and high costExpand production scaleAntiviralsTissue/virus culture apparatusFiberPolyester
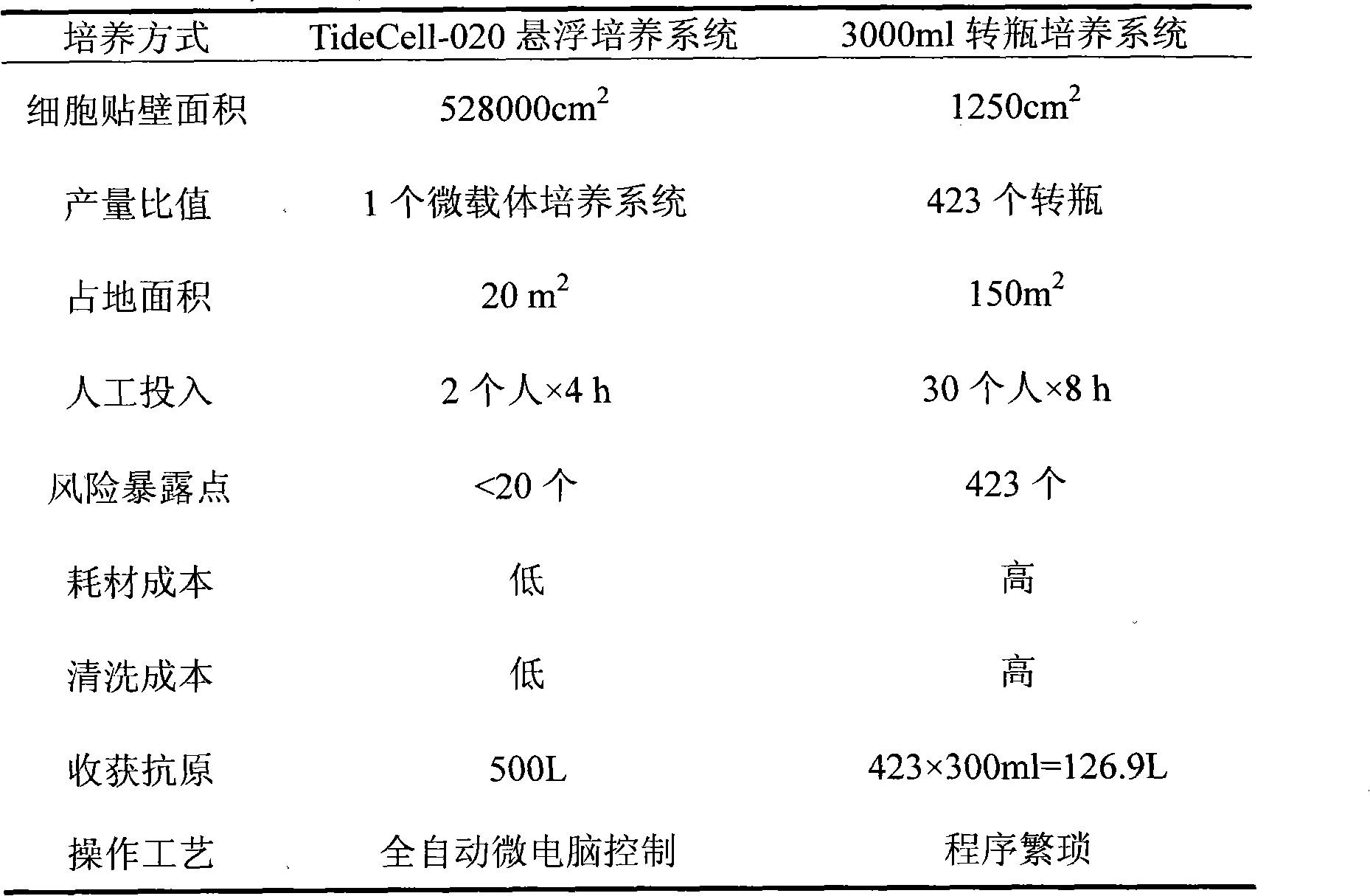
The invention discloses a method for mass production of pseudorabies virus vaccine, comprising the following steps: (a) adding netty polyester fiber which serves as a carrier into a bioreactor provided with a tide type micro-carrier suspension culture system and inoculating cells for producing vaccine; (b) inoculating pseudorabies virus vaccine when the culture cell grows to a certain intensity, so that the cells are infected by the pseudorabies virus vaccine; (c) reproducing the virus in great numbers under appropriate conditions; (d) harvesting the virus when cytopathic rate reaches above 70%; (e) carrying out freeze thawing on the harvested virus for once or twice to lead the cells to completely come off and disperse and then adding freeze-drying protective agent, evenly mixing the mixture, packaging the mixture in fixed volume and freeze-drying.The method of the invention has the advantages of good stability, explicit process control indicators, good controllability, easy operation, large process scale and the like.
Owner:PU LIKE BIO ENG
Method for producing porcine circovirus type 2 antigens in large scale with high density
ActiveCN104004720AIncrease productionQuality improvementViral antigen ingredientsAntiviralsAntigenHigh density
The invention relates to a method for producing porcine circovirus type 2 antigens in large scale with high density. A bioreactor microcarrier suspension culture technology used for replacing an existing spinner bottle culture technology for producing the porcine circovirus type 2 antigens. The method can greatly reduce the production cost and improve the yield. Compared with the spinner bottle technology, the unit antigen cost is reduced by 80% to 90%, the production period is reduced by 7 days, and the yield is improved by 3 times to 10 times; due to the fact that the antigens can be obtained repeatedly, compared with a traditional reactor culture technology, the unit antigen cost is reduced by 40% to 50%, and the yield is improved by 3 times to 5 times. The antigens produced through the method has no serum residues, the produced vaccine is higher in safety and small in batch difference, the quality is stable and easy to control, and the yield and quality of the produced vaccine can be obviously improved.
Owner:JIANGSU NANNONG HI TECH
Method for producing duck tembusu virus inactivated vaccines in large scale
InactiveCN103143009AHigh titerQuality improvementViral antigen ingredientsMicroorganism based processesAutomatic controlVenom
The invention discloses a method for producing duck tembusu virus inactivated vaccines in a large scale. According to the method, high-density culture of cells is performed by using a bioreactor culture technology to produce the duck tembusu virus inactivated vaccines. Compared with a conventional rotating bottle production process, the method for producing duck tembusu virus inactivated vaccines in a large scale has the characteristics that the automated control degree is high, and the production can be monitored in real time to ensure that the product quality is uniform and stable; and manpower and material resources can be saved during production to reduce the production cost; and the produced venom is high in virus content and low in inter-batch difference, and side effects are reduced.
Owner:QILU ANIMAL HEALTH PROD
Method for establishing hog cholera lapinized virus labeled vaccine strain and preparing vaccine
ActiveCN102221618AImprove welfareReduce economic lossAntiviralsViruses/bacteriophagesHigh cellVaccine virus
The invention relates to a method for establishing a hog cholera lapinized virus labeled vaccine strain and preparing a vaccine. The method comprises the following steps: (1) establishing the overall-length infectious clone of the hog cholera lapinized virus strain (the C strain or the Chinese strain); (2) introducing labeled protein genes; (3) rescuing the hog cholera lapinized labeled vaccine virus; (4) breeding the virus; (5) inspecting a virus solution; (6) preparing a vaccine; and (7) inspecting the finished product of the vaccine, comprising safety inspection and efficacy inspection. The hog cholera lapinized virus labeled vaccine established by the invention maintains the characteristics of good safety, excellent immunogenicity and the like of the hog cholera lapinized virus strain; the virus strain has the advantages of good stability and high cell production virus content, can be used for industrial mass production and can generate a labeled protein specificity antibody aftera hog is immunized to distinguish immunization and natural infection and has important significances on hog cholera prevention, control and purification and emergent immunization.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Combined live vaccine against porcine reproductive and respiratory syndrome, swine fever and pseudorabies, and preparation method thereof
ActiveCN102727882AImprove immune efficiencyIncrease productionViral antigen ingredientsAntiviralsDiseaseHIV vaccine
The invention provides a combined live vaccine for preventing porcine reproductive and respiratory syndrome, swine fever and pseudorabies, and a preparation method and application thereof. According to the invention, no immunosuppression occurs among three vaccine strains of the combined live vaccine; the combined live vaccine is identical with each single vaccine in the aspects of security, immunogenicity, immunity duration and immuno-protective effects, but in the aspect of convenience, the combined live vaccine is more convenient than each single vaccine since prevention of three diseases is realized through only one immunization; thus, work load of immunization and inoculation is mitigated, stress on swinery is reduced, and immunological paralysis and immunological failure caused by frequent immunization are avoided, thereby achieving the effect of preventing porcine reproductive and respiratory syndrome, swine fever and pseudorabies.
Owner:华威特(江苏)生物制药有限公司
Newcastle disease (ND) vaccine, and its production method
InactiveCN102600465AGuaranteed to be pureEnsure safetyViral antigen ingredientsAntiviralsEmbryoControl quality
A production method of Newcastle disease (ND) vaccine includes (1) adding virus growth solution to chick-embryo-cultured inoculation subculture cell line of attenuated ND virus, and culturing to obtain cell-adapted vaccine seed virus; adding virus culture maintenance medium to inoculation subculture cell line of cell-adapted vaccine seed virus, and culturing to obtain proliferated virus suspension; (3) determining titer of the proliferated virus suspension, preparing vaccine from qualified suspension, sub-packaging, and lyophilizing. The production method has advantages of simple and stable production process, easy operation, high virus content, small batch difference, easily controlled quality, rapid and accurate titer determination, improved vaccine yield and quality, high vaccine safety, high immune efficacy, and complete immuno-protection effect against ND virus.
Owner:SINOVET BEIJING BIOTECH +1
Preparation method of avian influenza virus H9 subtype inactivated vaccine
ActiveCN106924727AClear backgroundNo foreign pathogensSsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusBiotin
The invention belongs to the technical field of veterinary biological products and particularly relates to a preparation method of an avian influenza virus H9 subtype inactivated vaccine. An LMH passage cell line is adopted to serve as a carrier cell for viral multiplication, a culture medium prepared from glutamine, recombinant human insulin, human serum albumin, transferrin, biotin and a growth factor is adopted for culture, and virus liquid is collected, inactivated and prepared into the vaccine. The LMH passage cell line is adopted to perform avian influenza virus H9 subtype viral multiplication without additional pancreatin adding, and the LMH cell is clear in background, free of extraneous pathogens and easy to multiply. The process can be effectively simplified, and the cost is reduced. In addition, the avian influenza virus H9 subtype inactivated vaccine prepared by adopting the preparation method is high in virus content and good in stability and safety and is a more ideal avian influenza virus H9 subtype inactivated vaccine.
Owner:广州渔跃生物技术有限公司 +2
Method for preparing duck tembusu virus inactivated vaccine and duck tembusu virus inactivated vaccine
ActiveCN108721615AImprove adaptabilityHigh virus contentSsRNA viruses positive-senseViral antigen ingredientsDiseaseSide reaction
The invention discloses a method for preparing a duck tembusu virus inactivated vaccine and the duck tembusu virus inactivated vaccine. A cell line used for virus inoculation is EB66 cell line (a duckembryonic stem cell-derived cell strain), and a bioreactor is adopted for virus culture in a serum-free full suspension manner; and the method comprises the following steps: 1) breeding of virus species; 2) establishment of virus seed batches; 3) preparation of cell venom; 4) inactivation of viruses; and 5) emulsification and other steps to complete the preparation of the vaccine. The method provided by the invention has the characteristics that the prepared cell venom is high in virus content, the production process is stable, intelligent control is achieved, large-scale serum-free suspension culture is realized, the operation is easy, and the cost is low; and the prepared duck tembusu virus inactivated vaccine has the advantages of safety, low side reactions, high immune efficacy, smallbatch-to-batch difference, small number of tests, low cost and the like, and is an ideal vaccine for preventing the occurrence and the prevalence of a duck tembusu virus disease in the waterfowl industry.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Marking vaccine and serological identification method for porcine reproductive and respiratory syndrome high in pathogenicity
ActiveCN105749267AHigh production virus contentHigh virus contentSsRNA viruses positive-senseViral antigen ingredientsHighly pathogenicPathogenicity
The invention relates to a marking vaccine for the porcine reproductive and respiratory syndrome high in pathogenicity and a preparation method thereof.A virus seed produced through the vaccine is a constructed JXA1-RM strain, safety and immunogenicity of the JXA1-RM strain are maintained, in addition, the strain is good in stability, and the content of viruses produced by cells is high; after a pig is immunized through the vaccine, no sequence antibody against the M protein linear list position peptide amino acid sequence of the PRRS virus of an American strain is generated, and the vaccine can be used for differentiating an immunized animal from a naturally-infected animal.By means of a method for identifying and diagnosing the strain, antibodies generated by a field strain and a vaccine strain can be diagnosed, a conventional ELISA detection method is combined, an animal immunized through the vaccine and a naturally-infected animal can be differentiated, and it is beneficial to purification of the porcine reproductive and respiratory syndrome high in pathogenicity.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
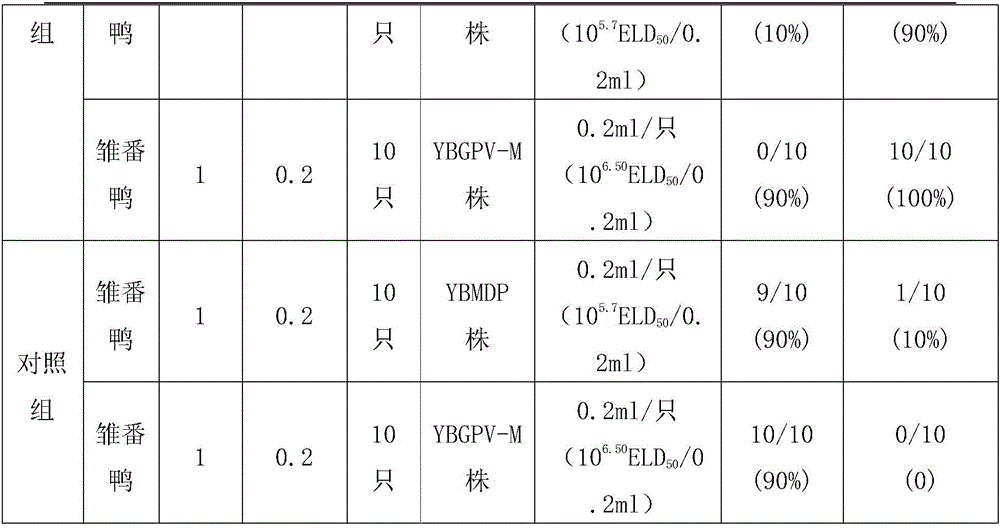
Muscovy duck parvovirus and gosling plague bivalent vaccine
ActiveCN105920596AImproving immunogenicityImprove securityViral antigen ingredientsAntiviralsMaternal antibodyImmunogenicity
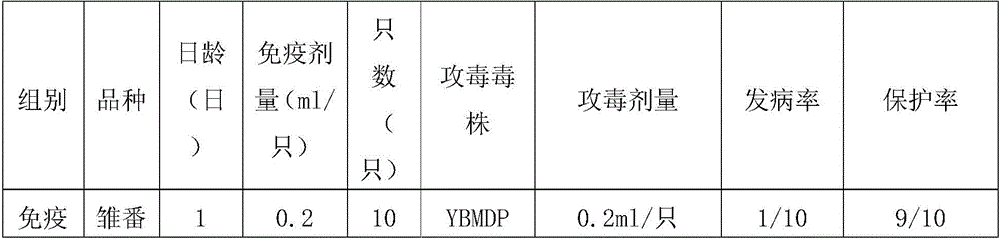
The invention provides a Muscovy duck parvovirus and gosling plague bivalent vaccine. The antigens used by the vaccine is inactivated Muscovy duck parvoviruses and Muscovy duck-source gosling plague viruses, the preservation number of the Muscovy duck parvoviruses is CGMCC No. 8504, and the preservation number of the Muscovy duck-source gosling plague viruses is CCTCC No. V201620. A preparation method of the Muscovy duck parvovirus and gosling plague bivalent vaccine includes: the Muscovy duck parvovirus YBMDP strains and Muscovy duck-source gosling plague virus YBGPV-M strains which are high in virus content and good in immunogenicity are screened, infected embryos and allantoic fluid are collected after duck embryo inoculation, and oil emulsion adjuvant is added for emulsification and mixing to obtain the vaccine after homogenization, ultrafiltration and concentration, and formaldehyde solution inactivation. The prepared vaccine can immunize breeding Muscovy ducks and increase the level of two types of antibodies of the breeding Muscovy ducks at the same time, guarantee the offspring maternal antibody level of the breeding Muscovy ducks, and prevent the young Muscovy duck parvovirus diseases caused by the Muscovy duck parvoviruses and gosling plague virus infection caused by the Muscovy duck-source gosling plague viruses.
Owner:YEBIO BIOENG OF QINGDAO
Preparation method of duck reovirus inactivated vaccine
ActiveCN110354259AHigh virus contentImproving immunogenicityViral antigen ingredientsAntiviralsBiotinViral Inactivation
The invention belongs to the technical field of veterinary biological products, and particularly relates to a preparation method of a duck reovirus inactivated vaccine. In the invention, an LMH passage cell line is used as a carrier cell for virus proliferation; a culture medium A consisting of lysine, recombinant human insulin, human serum albumin, protamine, biotin, polysaccharide sulfate and agrowth factor is used for culture; and the virus fluid is collected and inactivated to prepare a vaccine. The LMH passage cell line is used as a carrier cell for virus proliferation without the need of adding pancreatin, and the LMH cells have a clear background, have no exogenous pathogens and are easy for proliferation, which can effectively simplify the process and reduce costs. Meanwhile, theduck reovirus inactivated vaccine prepared by the invention has high virus content, good stability and high safety, and is an ideal duck reovirus inactivated vaccine.
Owner:广东渔跃生物技术有限公司
Porcine reproductive and respiratory syndrome virus liposome diluent lyophilized product and preparing method thereof
InactiveCN106074404ASmall batch-to-batch variancePromote reproductionPowder deliverySsRNA viruses positive-senseMannitolRespiratory syndrome virus
The invention provides a porcine reproductive and respiratory syndrome virus liposome diluent lyophilized product and a preparing method thereof, and belongs to the technical field of animal biological products. 1000 ml of a PBS solution contains 2-8 g of astragaloside III, 5-10 g of aloe polysaccharide, 2-6 g of curcumin, 10-15 g of lipidosome, 10-15 g of glucose and 10-15 g of mannitol. The prepared liposome diluent lyophilized product can reduce difference between semi-finished product batches and can promote propagation of porcine reproductive and respiratory syndrome viruses, increase virus content and enhance immunogenicity of vaccines.
Owner:浙江美保龙生物技术有限公司
Method for producing porcine pseudorabies gE gene deletion virus inactivated vaccine
ActiveCN107137705AHigh potencyHigh virus contentViral antigen ingredientsCulture processImmune effectsVaccine Production
The invention relates to a method for producing a porcine pseudorabies gE gene deletion virus inactivated vaccine by using a BHK cell line. The method comprises the following steps: thawing BHK21 cells, inoculating the cells into a spinner bottle for culturing until the cells grow into sheets, inoculating a porcine pseudorabies gE gene deletion virus, continuously culturing, collecting the cell venom when cytopathy CPE reaches 90% or higher, and concentrating, inactivating and degerming the collected venom, thereby obtaining a finished product. The porcine pseudorabies gE gene deletion virus liquor collected by the vaccine production method provided by the invention has high virus content of more than or equal to 107.2TCID50 / mL and has relatively high immunogenicity, an excellent immune effect can be achieved without adding any immunopotentiator, in-vivo secretion of neutralizing antibodies can be promoted after immunization, the immune protective rate reaches 100%, and the vaccine efficacy evaluation standard is completely met.
Owner:广东渔跃生物技术有限公司 +1
Vero-E6 suspension cell strain sVero-E6 adapted to porcine epidemic diarrhea virus and application of Vero-E6 suspension cell strain sVero-E6
ActiveCN114276981AStable proliferationHigh virus contentInactivation/attenuationVertebrate cellsDiseaseEpidemic diarrhea
The invention discloses a Vero-E6 suspension cell strain sVero-E6 adapted to a porcine epidemic diarrhea virus and application of the Vero-E6 suspension cell strain sVero-E6, and belongs to the technical field of biology. The sVero-E6 suspension strain provided by the invention can stably subculture and grow, when the sVero-E6 suspension strain is used for culturing PEDV, the cultured PEDV can stably proliferate, and the sVero-E6 suspension strain can be used for continuously proliferating and culturing the PEDV and repeatedly harvesting a virus culture solution, so that a large amount of production cost and culture time can be saved, and the productivity is improved. The inactivated vaccine prepared from the obtained virus culture solution is good in immunogenicity, stable and safe, and can be effectively used for preventing porcine epidemic diarrhea diseases.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Porcine circovirus 2-type lipidosome diluent lyophilized product and preparation method thereof
InactiveCN106109424ASmall batch-to-batch variancePromote reproductionPowder deliveryViral antigen ingredientsCordycepsMANNITOL/SORBITOL
The invention discloses a porcine circovirus 2-type lipidosome diluent lyophilized product and a preparation method thereof, and belongs to the technical field of animal biological products. Each 1,000 ml of PBS solution is prepared from 5 g to 10 g of cordyceps polysaccharide, 5 g to 10 g of lycopene, 1 g to 5 g of ginsenoside Rh2, 6 g to 12 g of lipidosome, 10 g to 15 g of saccharose and 10 g to 15 g of mannitol. According to the prepared lipidosome diluent lyophilized product, the difference between batches of semi-finished products can be reduced. The prepared lipidosome diluent lyophilized product can promote reproduction of porcine circovirus 2 type, the virus content can be increased, and vaccine immunogenicity can be enhanced.
Owner:浙江美保龙生物技术有限公司
Method for preparing inactivated rabies vaccine for animal by bioreactor
InactiveCN102949716AQuality improvementImproved oxygen deliveryViruses/bacteriophagesAntibody medical ingredientsAdjuvantBioreactor
The invention provides a method for preparing inactivated rabies vaccine for animal by a bioreactor. The method includes by the aid of the bioreactor and a Cytodex-1 microcarrier system, subjecting high-density Vero cells (or BHK cells) adaptive to rabies virus CTN-1 strain (aG-strain or PV-strain) to virus infection by utilizing the CTN-1 strain (aG-strain or PV-strain) rabies virus with good antigenicity so as to prolong virus harvest time from 6-9 days to 11-15 days, increase virus titer from 105-7LD50 / ml to 108-9LD50 / ml and increase virus content by 10-1000 times; and inactivating harvest virus liquid, adding additives, and then packing to obtain high-quality vaccine products. The prepared vaccine is safe and effective, production process is stable, and production quality is controllable.
Owner:TANGSHAN YIAN BIOLOGICAL ENG CO LTD
Duck plague live vaccine and preparation method thereof
ActiveCN108815517AImprove securityAvoid Pollution Safety HazardsPowder deliveryViral antigen ingredientsFibroblastEmbryo
The invention provides a duck plague live vaccine and a preparation method thereof, and belongs to the technical field of biological product preparation. A chicken embryo fibroblast line DF-1 is takenas the host of duck plague viruses to produce a duck plague virus solution, and then a heatproof protective agent is added into the duck plague virus solution to prepare the duck plague live vaccine.DF-1 is used to culture duck plague viruses to avoid the safety hazard that the duck plague viruses cultured by (chicken embryo fibroblast) CEF cells are contaminated by exogenous viruses; the DF-1 cells are available at any time, time and labor are saved, and the production cost is greatly reduced. The prepared duck plague live vaccine has the advantages of good safety and high immunity effect,and can protect ducks from strong duck plague viruses. Furthermore, the live vaccine also comprises an improved heatproof protecting agent, thus the duck plague live vaccine can be stored for 24 months at a temperature of 2-8 DEG C, the virus content an titer are not reduced, the storage conditions are lowered, the storage and transportation of the live vaccine become convenient, and the production cost is reduced.
Owner:广东永顺生物制药股份有限公司
Recombinant gene VII type Newcastle disease virus strain and vaccine composition, preparation method and application thereof
ActiveCN110713987AHigh titerHigh HA titerSsRNA viruses negative-senseViral antigen ingredientsAntigenNewcastle disease virus NDV
The invention discloses a recombinant gene VII type Newcastle disease virus attenuated strain rN7a strain, and further discloses a vaccine composition containing the rN7a strain or a culture-inactivated antigen of the rN7a strain. The rN7a strain is the attenuated strain obtained by replacing a P protein gene sequence of a Newcastle disease virus N7a strain with the preservation number of CCTCC NO:V201545 with a P protein gene sequence as shown in SEQ ID No.1. The rN7a strain has high virus titer and high HA titer after culture. The vaccine composition can provide complete protection to a variety of strains.
Owner:LUOYANG HUIZHONG BIOTECH
Method for producing swine fever live vaccine with cell line
ActiveCN100577204CGuaranteed to be pureEnsure safetyAntiviralsAntibody medical ingredientsFreeze-dryingControl quality
The invention discloses a method of using clone for producing swine fever living vaccine. The invention comprises the following technical steps: (1) selecting clone as the cell for producing vaccine; (2) the culture transferring and culturing of the cell for producing vaccine; (3) the breeding of the cell virus; (4) the breeding of the virus liquid for producing vaccine; (5) vaccine preparing, split charging and freeze-drying. The invention has the advantages of simple and stable technique, easy operation, high virus content, small inter-batch difference, easy controlled quality, which can markedly improve the vaccine output and quality. The swine fever living vaccine produced by adopting the invention is good in safety and high in immunity effect, which has complete protecting function against the attack of the swine fever strong virus.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
Method for producing H7 subtype avian influenza virus inactivated vaccine by using low-immunity chick embryo
ActiveCN112143714AEfficient ProliferationReduce manufacturing costSsRNA viruses negative-senseViral antigen ingredientsChick embryosFlu immunization
By improving the seed virus diluent, adding aspartic acid, ZnCl2 and epidermal growth factors in a specific proportion on the basis of a phosphate buffer, so that the H7 subtype avian influenza virusin the low-immunity chick embryo can be efficiently proliferated. The level of virus proliferation and the immune effect of the prepared vaccine are basically close to those of SPF chick embryos and non-immune chick embryos, however, the production cost is effectively reduced, the production threshold is lowered, which has a positive significance in production of H7 subtype avian influenza vaccines.
Owner:广东永顺生物制药股份有限公司
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573BImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
Owner:广东永顺生物制药股份有限公司
Method for preparing pseudorabies live vaccines from DF1 continuous cells and product prepared by method
InactiveCN105521487AIncrease productionQuality improvementAntiviralsAntibody medical ingredientsMicroorganismFreeze-drying
The invention provides a method for preparing pseudorabies live vaccines from DF1 continuous cells and a product prepared by the method. The method includes the steps of culturing a pseudorabies virus attenuated strain by the DF1 continuous cells; obtaining cell culture virus fluid; adding stabilizers and antibiotics and obtaining the pseudorabies DF1 live vaccines after vacuum freeze drying. The method has the advantages that the DF1 continuous cells which are heterogenous continuous cells are used for culturing pseudorabies attenuated viruses, so that the possibility that homologous cells have unknown swine-source microorganisms can be avoided and pure vaccines can be better guaranteed.
Owner:广东永顺生物制药股份有限公司
Method for purifying foot-and-mouth disease virus antigen by utilizing ion exchange chromatography
PendingCN114106114ANot conducive to preservationHigh yieldSsRNA viruses positive-senseVirus peptidesDiseasePolyethylene glycol
The invention provides a method for purifying a foot-and-mouth disease virus antigen by utilizing ion-exchange chromatography, which comprises the following steps: inactivating and filtering a harvested foot-and-mouth disease virus solution, and sequentially carrying out PEG (Polyethylene Glycol) precipitation, redissolution, ion-exchange chromatography and the like. Wherein the ion exchange chromatography comprises the following steps: adopting a flow-through process, enabling a redissolved solution to pass through a chromatographic column, and collecting a flow-through component. By adopting the process, the yield of three subtype inactivated antigens of foot-and-mouth disease AKT-III, O / Mya 98 and OHM02 is more than 90%, the purity is more than 70%, the content of impure protein in the antigens is greatly reduced, more than 50% of total protein can be removed, the process is stable in amplification and short in time consumption, the process can be used for preparing high-quality foot-and-mouth disease vaccines, and the safety and effectiveness of the foot-and-mouth disease vaccines are improved.
Owner:天康生物制药有限公司
Application of antigen causing antibody-dependent enhancement of Tembusu virus
ActiveCN108676078APromote proliferationHigh virus contentSsRNA viruses positive-senseViral antigen ingredientsAntibody-dependent enhancementGenetic enhancement
The present invention discloses application of an antigen causing antibody-dependent enhancement of Tembusu virus. The antigen causing the antibody-dependent enhancement of Tembusu virus has an aminoacid sequence shown in SEQ ID NO.1. The antigen causing the antibody-dependent enhancement of the Tembusu virus is applied to preparation of a Tembusu virus vaccine. According to the invention, the epitope protein expressed by escherichia coli is used for immunizing ducklings, so that high-level specific antibodies can be induced in the ducklings, and the antibodies can promote virus proliferationand increase the titer of the virus in the ducklings. In the future research and development of the Tembusu virus vaccine, this kind of antigen can be removed through molecular design to avoid the antibody-dependent enhancement caused by the vaccine and ensure the protection effect. Therefore, identifying the antigen causing the antibody-dependent enhancement of the Tembusu virus has an importantapplication value in developing effective and safe vaccines and preventing and controlling the epidemic of the Tembusu virus.
Owner:JIANGSU ACAD OF AGRI SCI
Veterinary bovine ephemeral fever inactivated vaccine and large-scale production method thereof
PendingCN113117068AHigh virus contentImprove immune efficiencySsRNA viruses negative-senseViral antigen ingredientsVaccine PotencyVaccine Production
The invention discloses a veterinary bovine ephemeral fever inactivated vaccine and a large-scale production method thereof, and belongs to the technical field of vaccine production. The method provided by the invention comprises the steps of performing cell culture by utilizing a suspension culture process, performing virus suspension culture, performing virus antigen solution clarification, performing concentration and purification, performing inactivation and performing emulsification vaccine preparation, and the method can realize mass proliferation of the bovine ephemeral fever virus, improves the content and yield of the virus antigen, and has a high automation degree; The synergistic effect of all the steps can jointly improve the quality and stability of the vaccine, and the technical problems that the yield of the bovine ephemeral fever virus produced by cell culture through a traditional spinner bottle or microcarrier process is low, the process is tedious, the difference between virus antigen batches is large, the vaccine efficacy is low, the vaccine validity period is short and the like are solved.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com