Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
328results about "Mercury compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
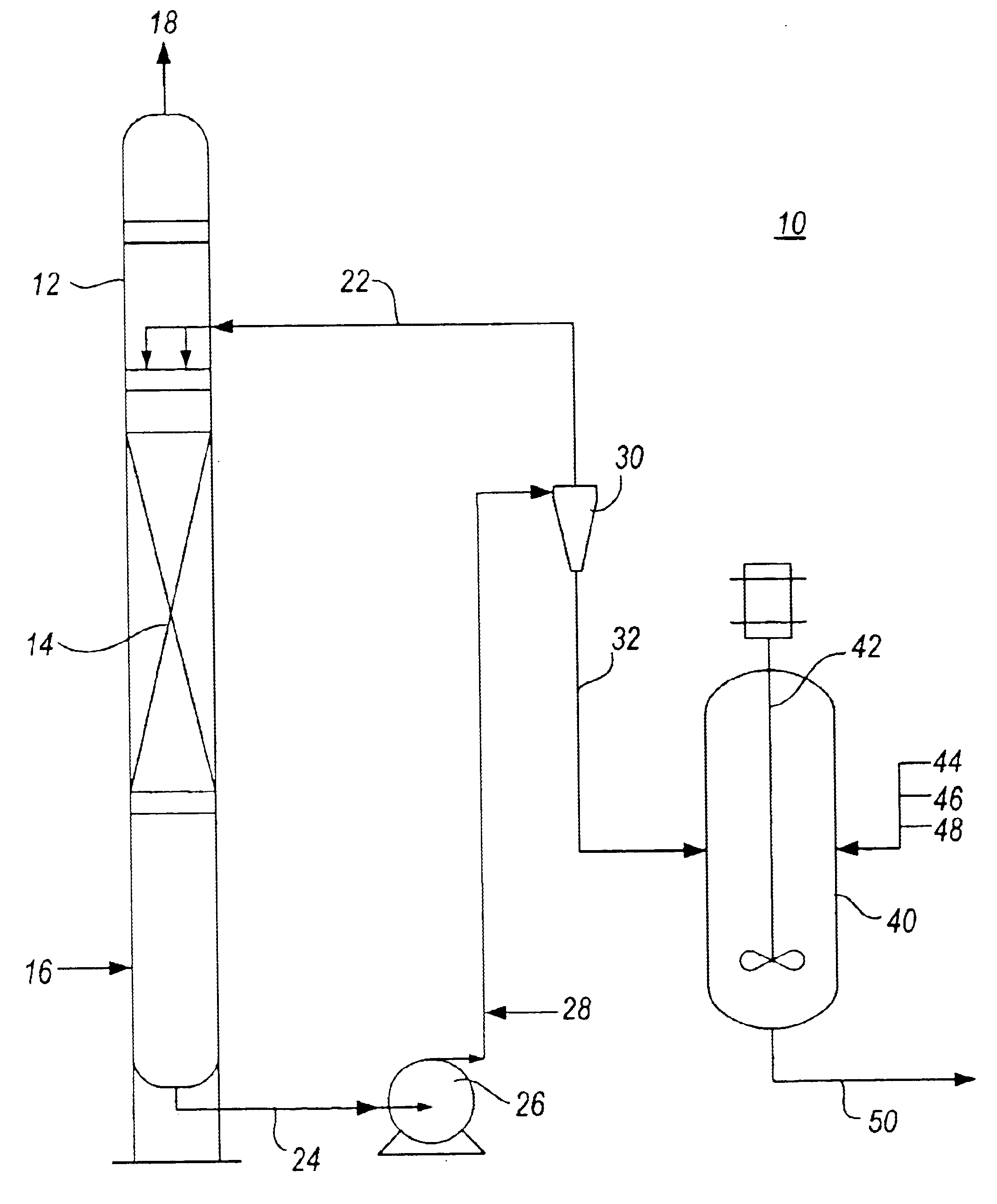
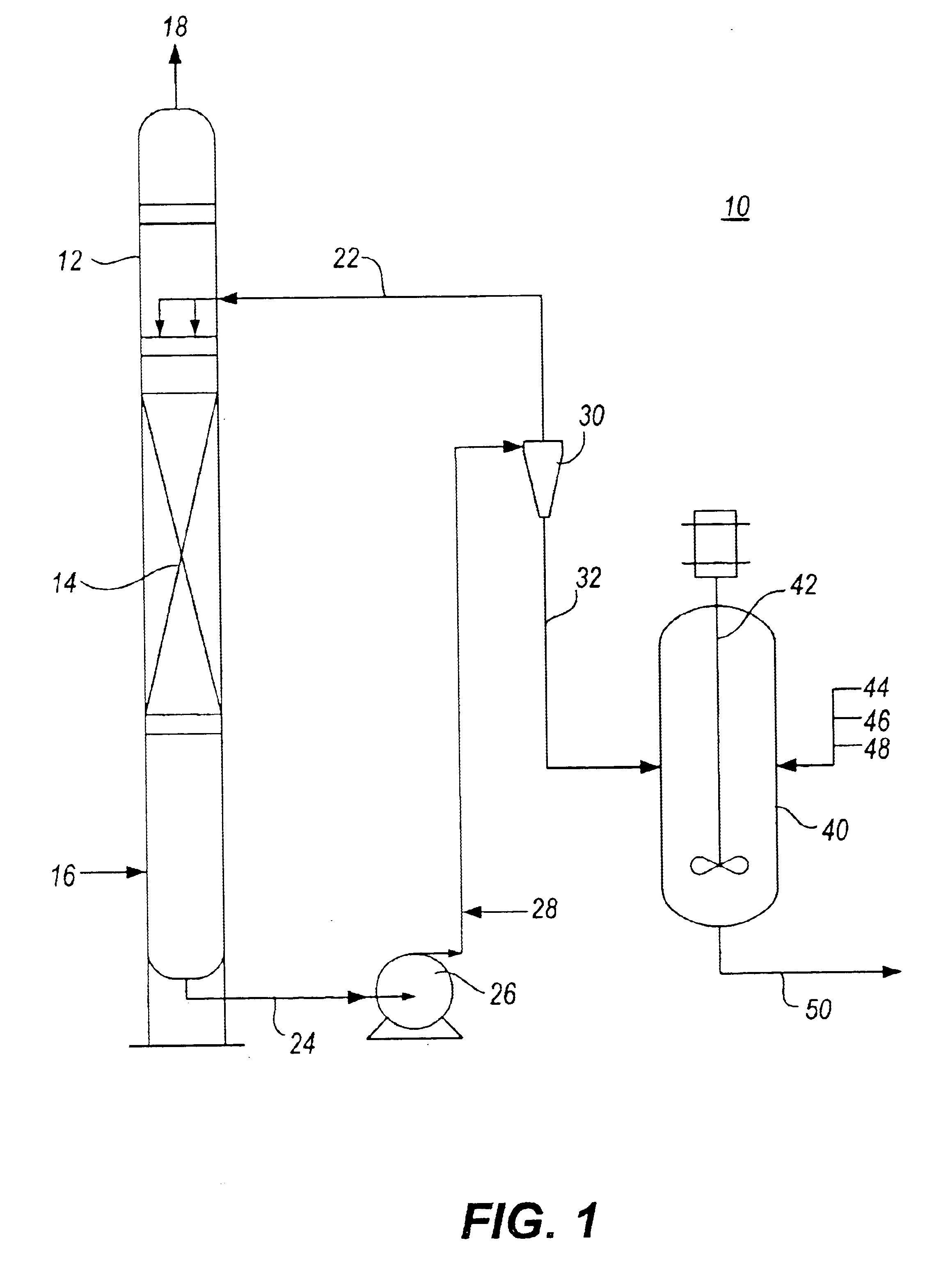
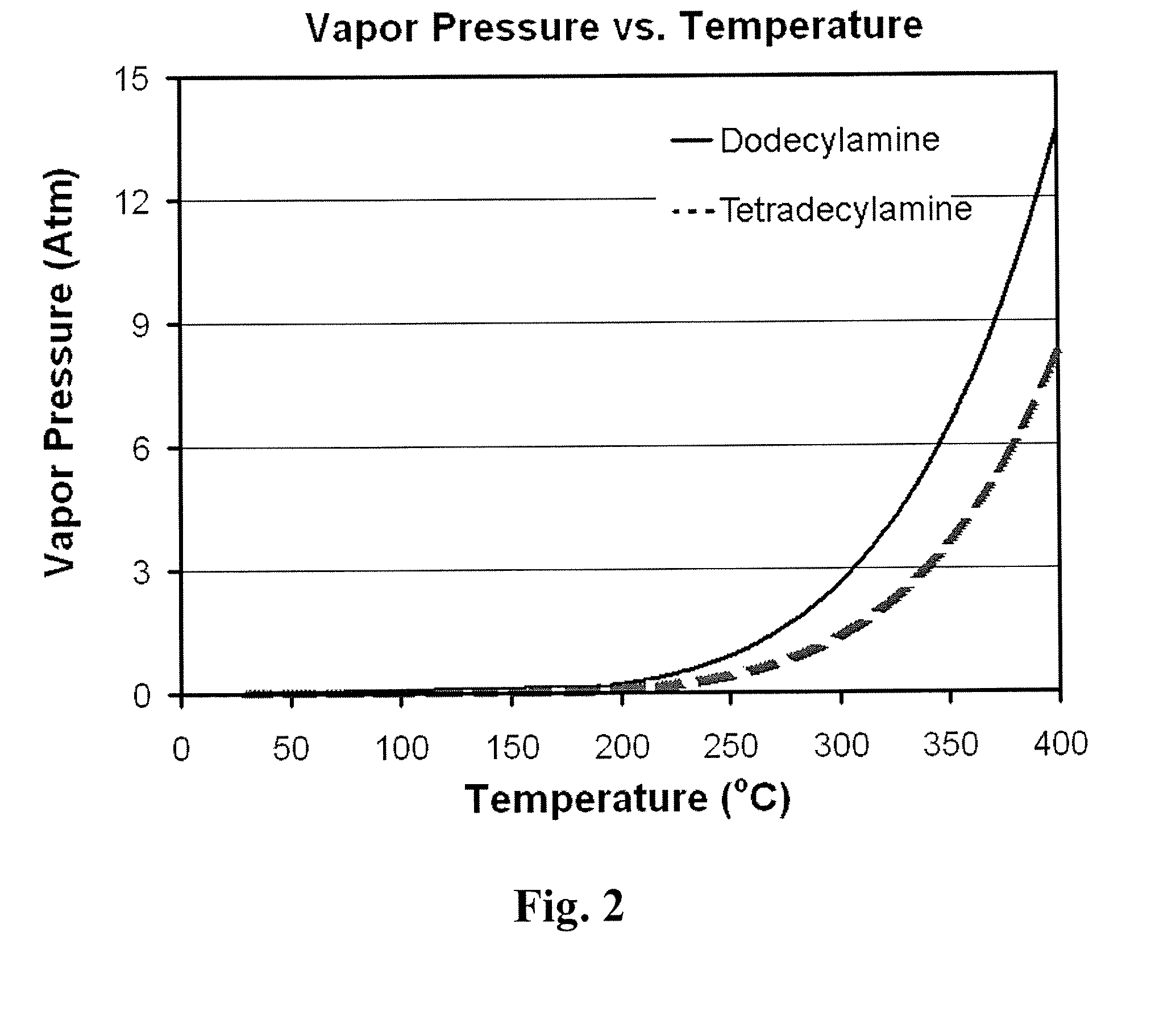
Method for removal and stabilization of mercury in mercury-containing gas streams
InactiveUS6942840B1Improve scalabilityCombination devicesDispersed particle filtrationOxygenVapor phase
The present invention is directed to a process and apparatus for removing and stabilizing mercury from mercury-containing gas streams. A gas stream containing vapor phase elemental and / or speciated mercury is contacted with reagent, such as an oxygen-containing oxidant, in a liquid environment to form a mercury-containing precipitate. The mercury-containing precipitate is kept or placed in solution and reacts with one or more additional reagents to form a solid, stable mercury-containing compound.
Owner:MERCURY CONTROL TECH
Nitride semiconductor crystal and its production method
InactiveUS20110129669A1Easy to produceEfficient productionSemiconductor/solid-state device manufacturingGlass/slag layered productsCrystal growthSeed crystal
A method for efficiently producing a plate-like nitride semiconductor crystal having the desired principal plane in a simple method is provided. A raw material gas is fed to a seed crystal in which a ratio (L / W) of length L in a longitudinal direction and maximum width W, of a plane of projection obtained by projecting a crystal growth face on the seed crystal in a growth direction is from 2 to 400, and the maximum width W is 5 mm or less, thereby growing a plate-like semiconductor crystal on the seed crystal.
Owner:MITSUBISHI CHEM CORP
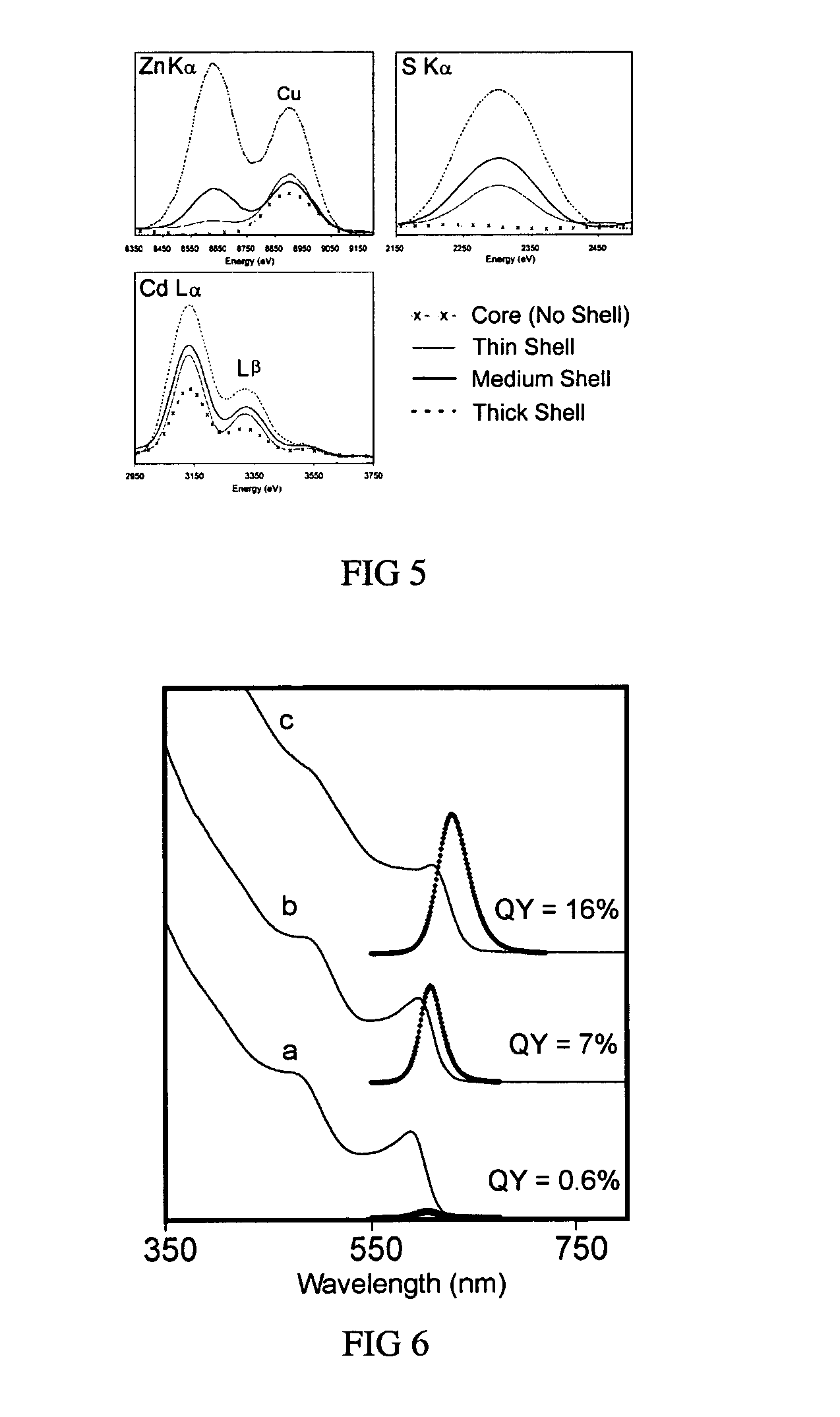
Graded core/shell semiconductor nanorods and nanorod barcodes
InactiveUS20050054004A1Improve quantum efficiencyIncrease in photoluminescence QYMaterial nanotechnologyNanosensorsGroup iiSemiconductor
Disclosed herein is a graded core / shell semiconductor nanorod having at least a first segment of a core of a Group II-VI, Group III-V or a Group IV semiconductor, a graded shell overlying the core, wherein the graded shell comprises at least two monolayers, wherein the at least two monolayers each independently comprise a Group II-VI, Group III-V or a Group IV semiconductor.
Owner:RGT UNIV OF CALIFORNIA
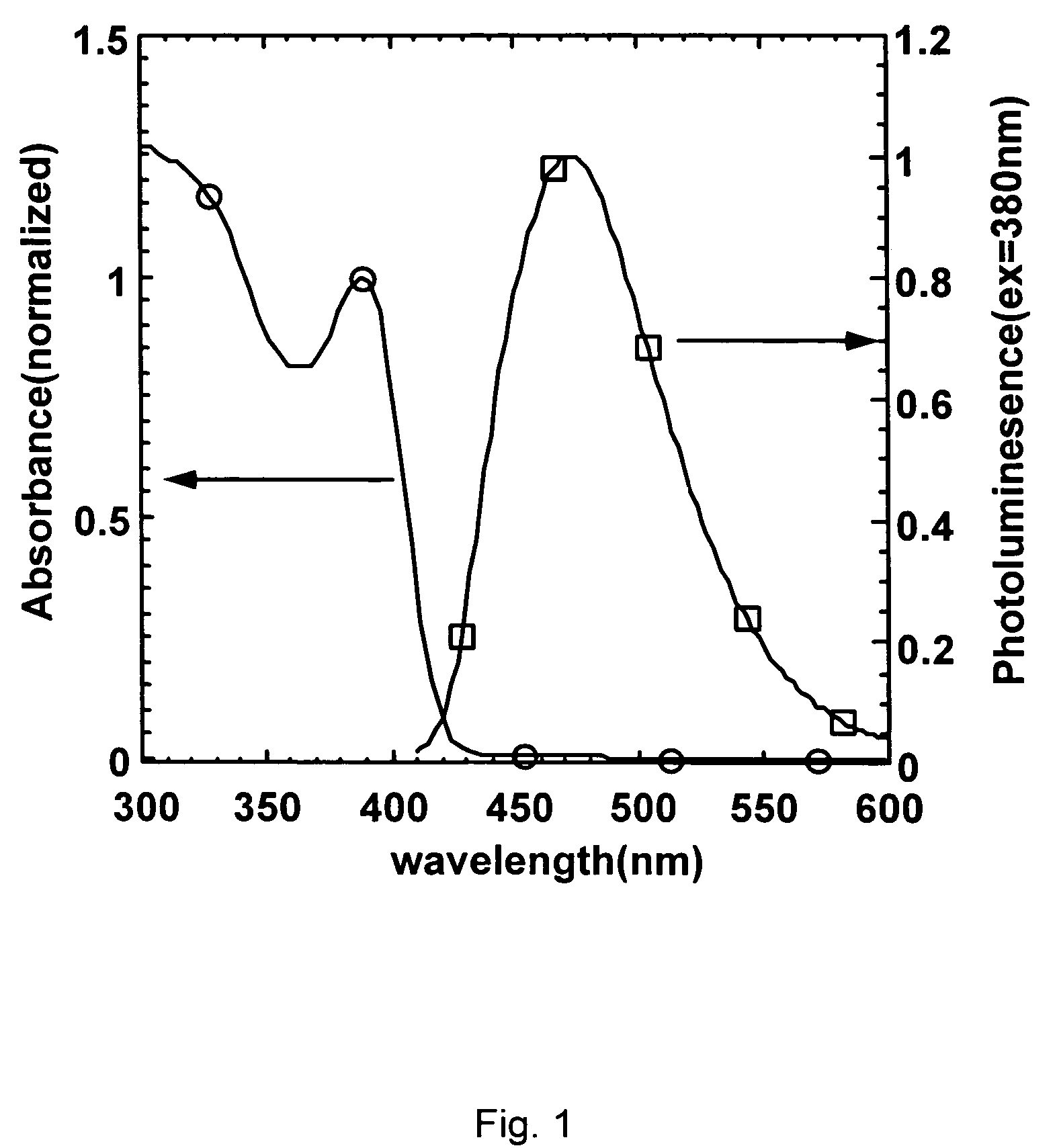
Colloidal nanocrystals with high photoluminescence quantum yields and methods of preparing the same
InactiveUS6869545B2Good monodispersityImprove launch performanceMaterial nanotechnologyNanoopticsQuantum yieldPhotoluminescence
The present invention provides new compositions containing colloidal nanocrystals with high photoluminescence quantum yields, new synthetic methods for the preparation of highly luminescent colloidal nanocrystals, as well as methods to control the photoluminescent properties of colloidal nanocrystals. The new synthetic methods disclosed herein allow photoemission brightness (quantum yield) to be correlated with certain adjustable nanocrystal growth parameters associated with a given synthetic scheme.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Process for the recovery of value metals from base metal sulfide ores
InactiveUS20050118081A1Simple gas/liquidReduce the amount requiredSulfur compoundsSolid sorbent liquid separationSulfideDissolution
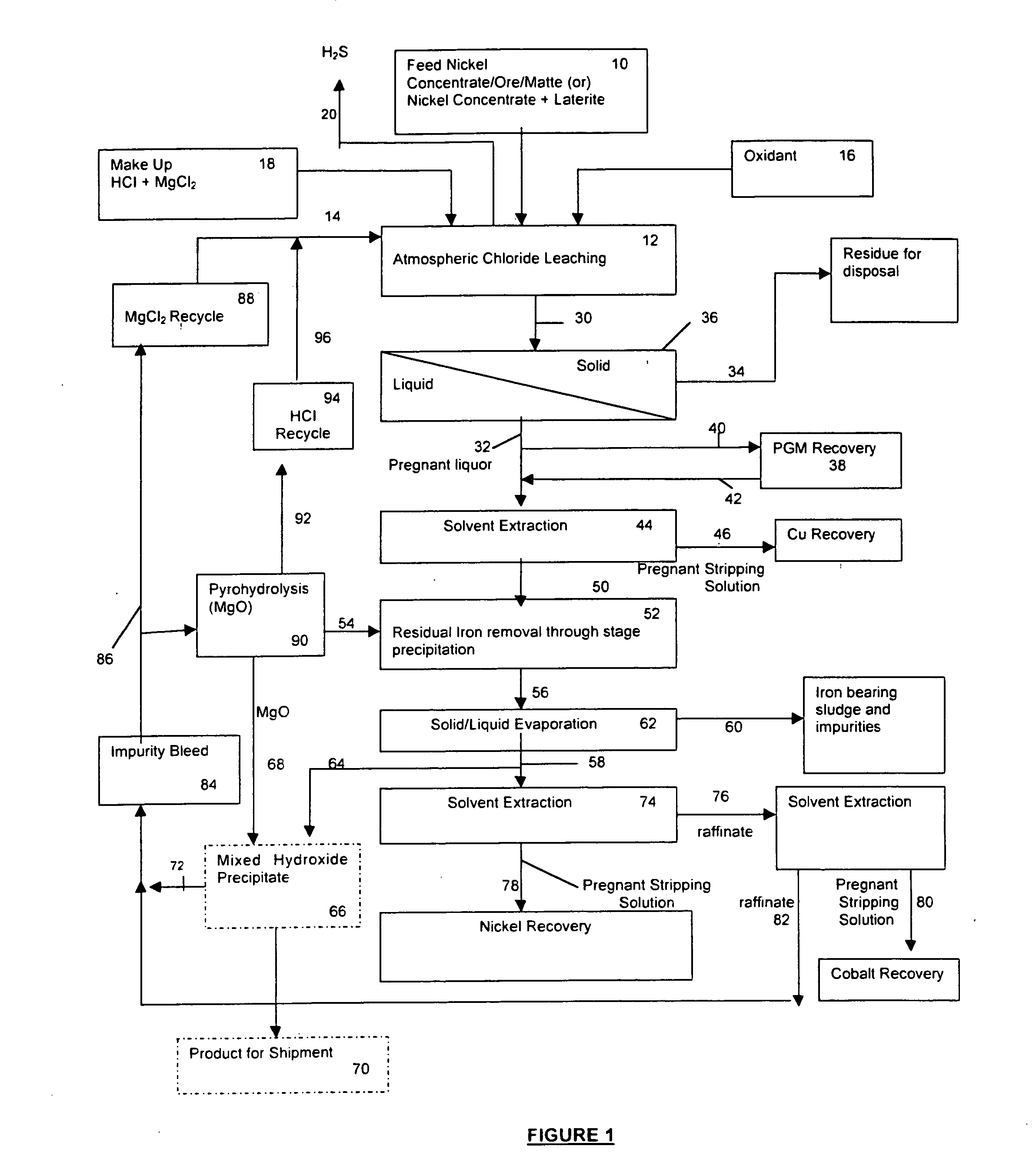
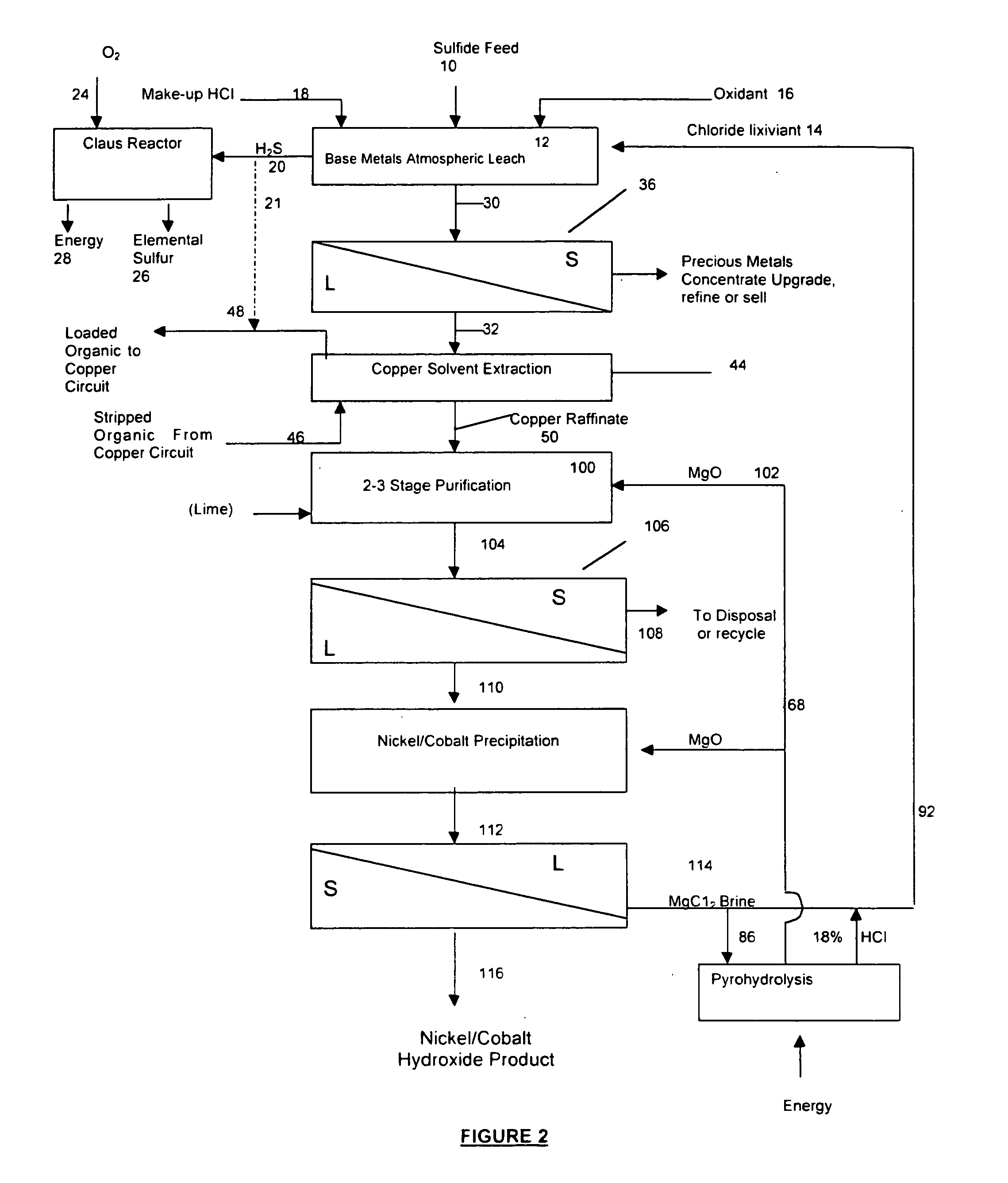
A process for leaching a value metal from a base metal sulfide ore, comprising the step of leaching the ore with a lixiviant comprising a chloride, an oxidant and hydrochloric acid is disclosed. The leaching is controlled, by use of low concentrations of hydrochloric acid and a redox potential, to effect formation of hydrogen sulfide from the base metal sulfide ore. The hydrogen sulfide is stripped from the leach solution, thereby reducing the amount of sulfate generated in the leach to very low levels. The leaching may also be conducted to limit the co-dissolution of platinum group metals and gold with the base value metals. The leach forms a value metal-rich leachate and a solids residue. The solids residue may be subsequently leached to recover the platinum group metals and gold. The value metal-rich leachate can be is oxidized and neutralized to recover the value base metals. In an embodiment, the chloride is magnesium chloride and lixiviant solution is regenerated.
Owner:JAGUAR NICKEL INC
Alloyed semiconductor quantum dots and concentration-gradient alloyed quantum dots, series comprising the same and methods related thereto
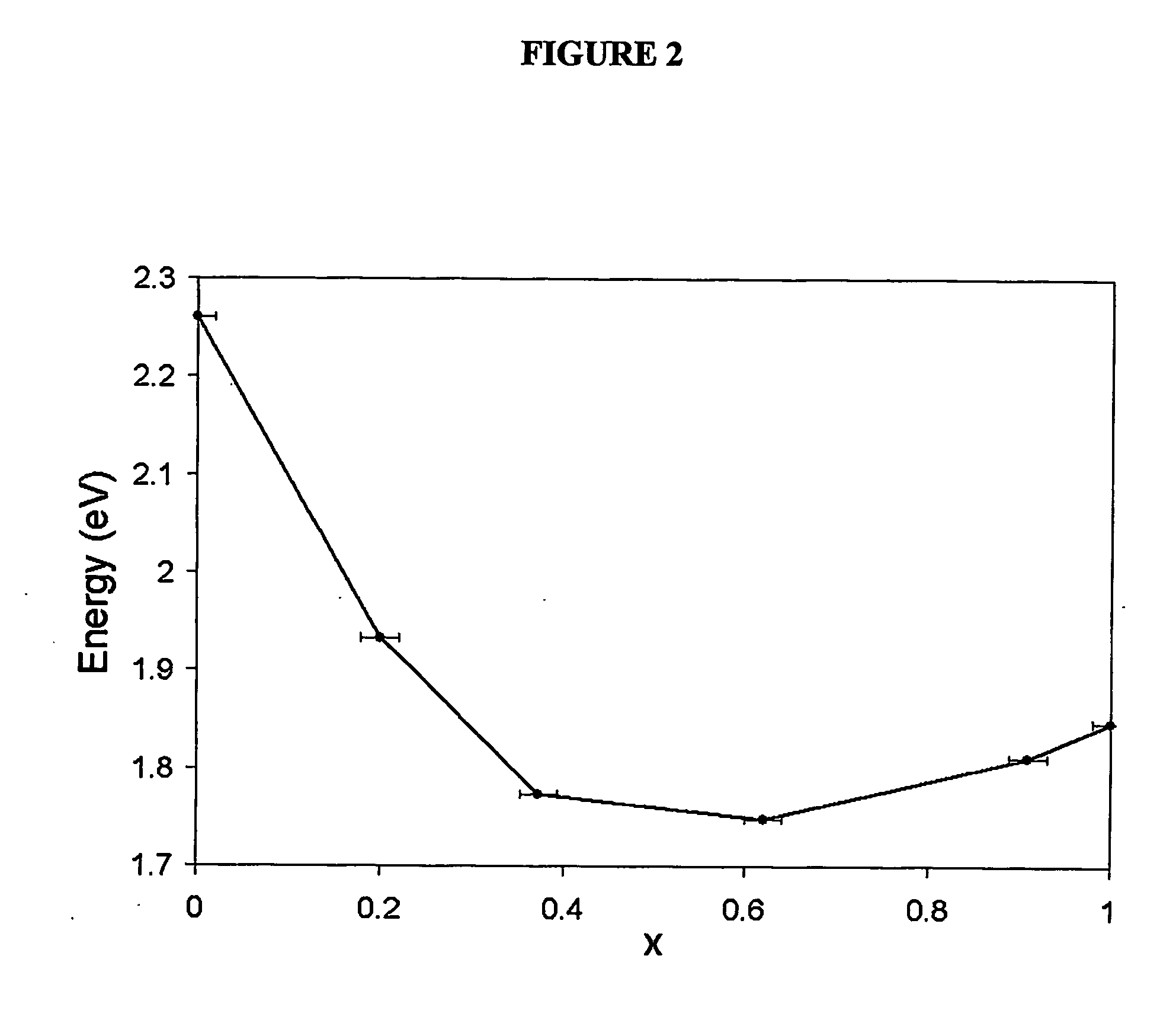
An alloyed semiconductor quantum dot comprising an alloy of at least two semiconductors, wherein the quantum dot has a homogeneous composition and is characterized by a band gap energy that is non-linearly related to the molar ratio of the at least two semiconductors; a series of alloyed semiconductor quantum dots related thereto; a concentration-gradient quantum dot comprising an alloy of a first semiconductor and a second semiconductor, wherein the concentration of the first semiconductor gradually increases from the core of the quantum dot to the surface of the quantum dot and the concentration of the second semiconductor gradually decreases from the core of the quantum dot to the surface of the quantum dot; a series of concentration-gradient quantum dots related thereto; in vitro and in vivo methods of use; and methods of producing the alloyed semiconductor and concentration-gradient quantum dots and the series of quantum dots related thereto.
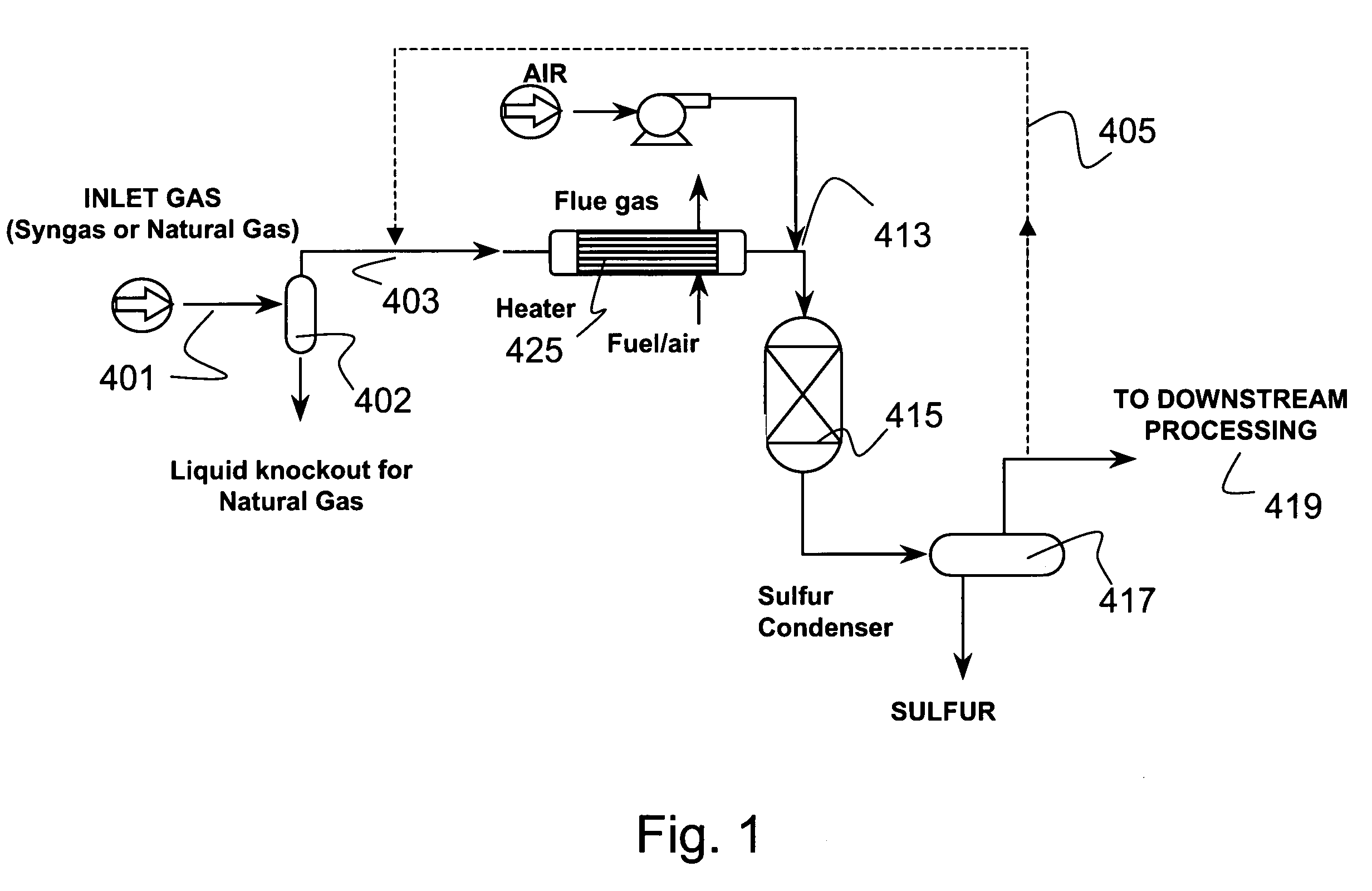
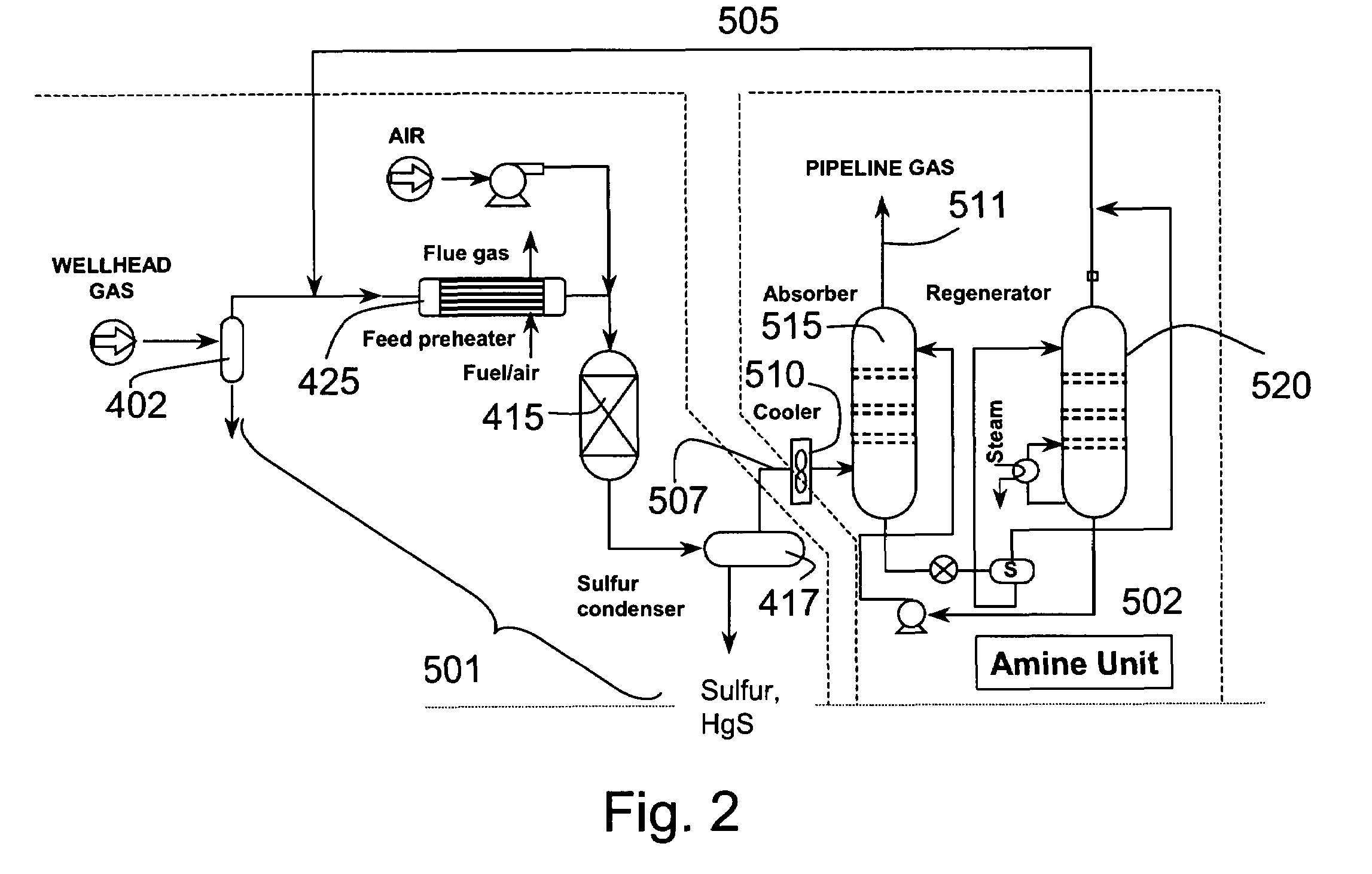
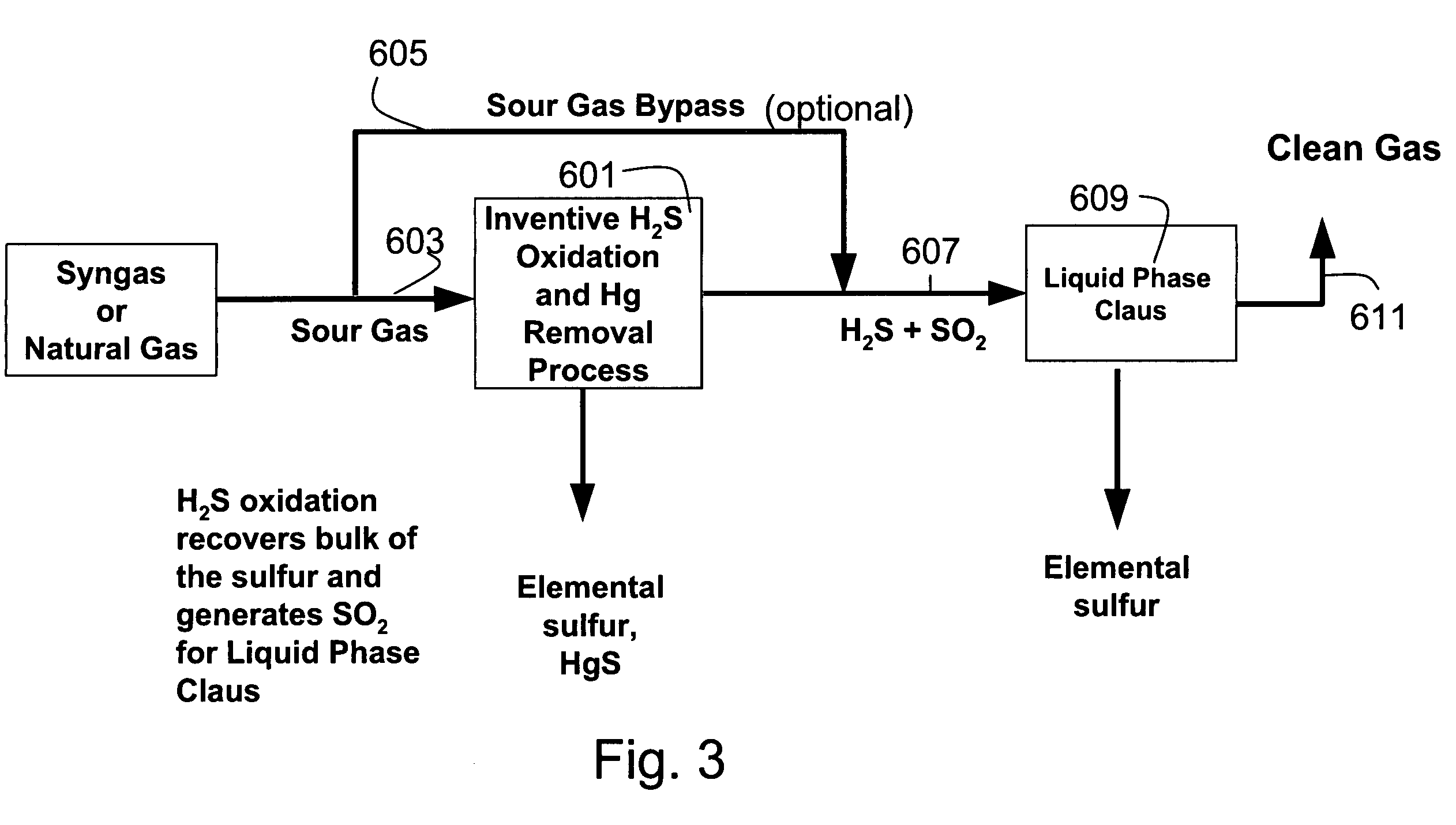
Process for the simultaneous removal of sulfur and mercury
InactiveUS7060233B1Lower capitalReduce operating costsGas treatmentUsing liquid separation agentSulfur containingInorganic sulfide
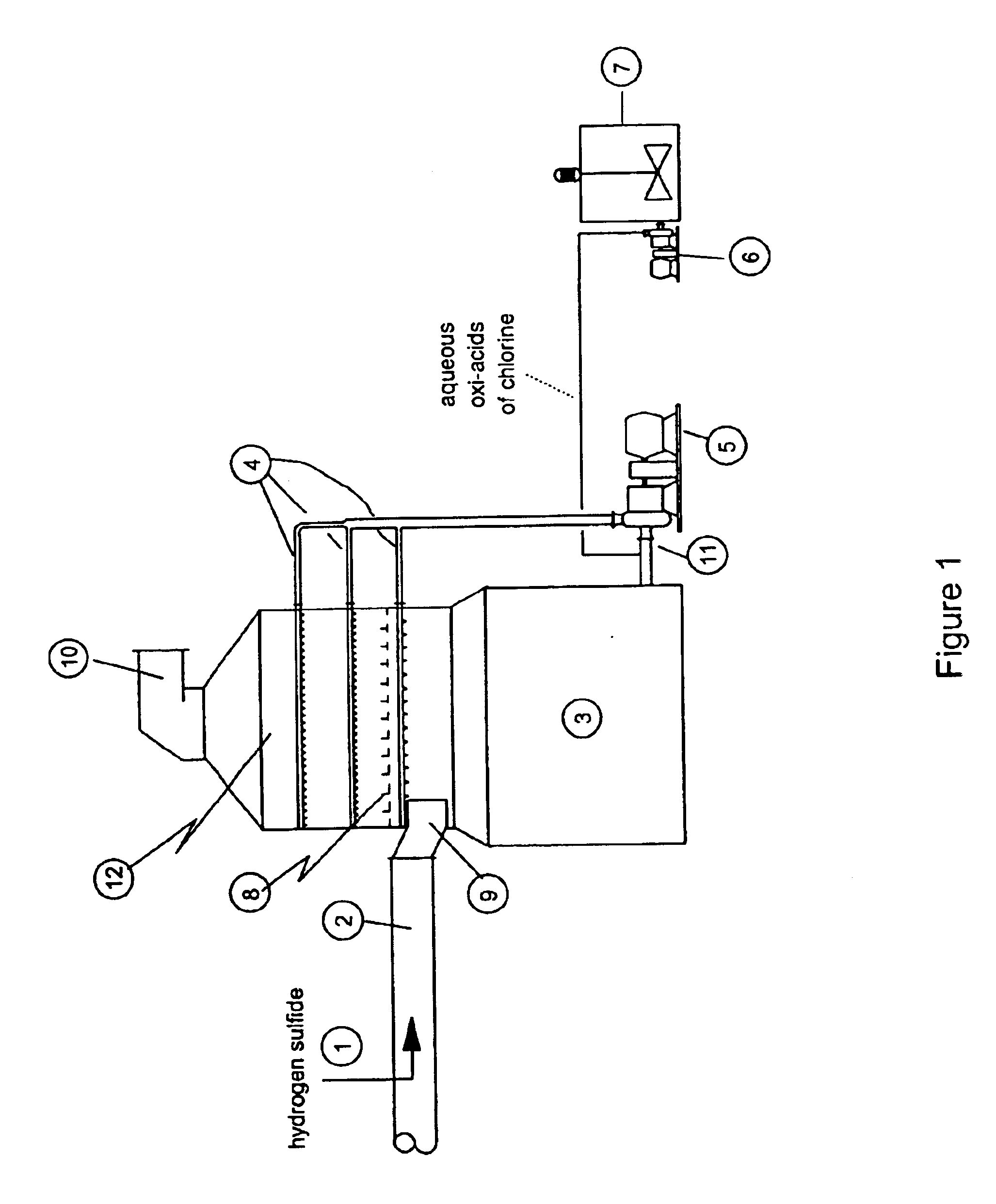
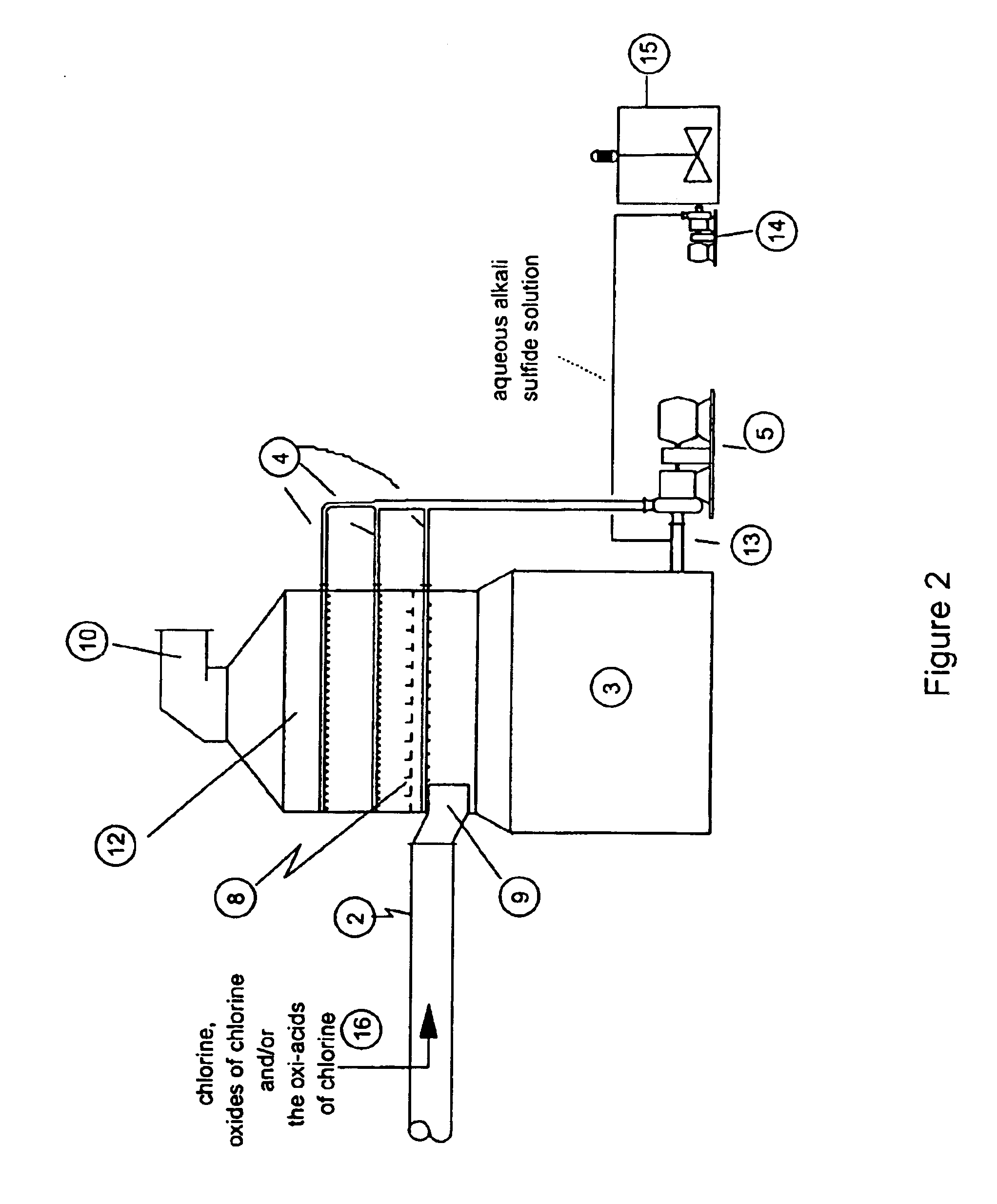
A process for removing hydrogen sulfide, other sulfur-containing compounds and / or sulfur and mercury from a gas stream contaminated with mercury, hydrogen sulfide or both. The method comprises the step of selective oxidation of hydrogen sulfide (H2S) in a gas stream containing one or more oxidizable components other than H2S to generate elemental sulfur (S) or a mixture of sulfur and sulfur dioxide (SO2). The sulfur generated in the gas stream reacts with mercury in the gas stream to generate mercuric sulfide and sulfur and mercuric sulfide are removed from the gas stream by co-condensation.
Owner:TDA RES
Method for controlling elemental mercury emissions
InactiveUS6855859B2Simple equipmentReduce oxidationGas treatmentExhaust apparatusElemental mercuryPrecipitation
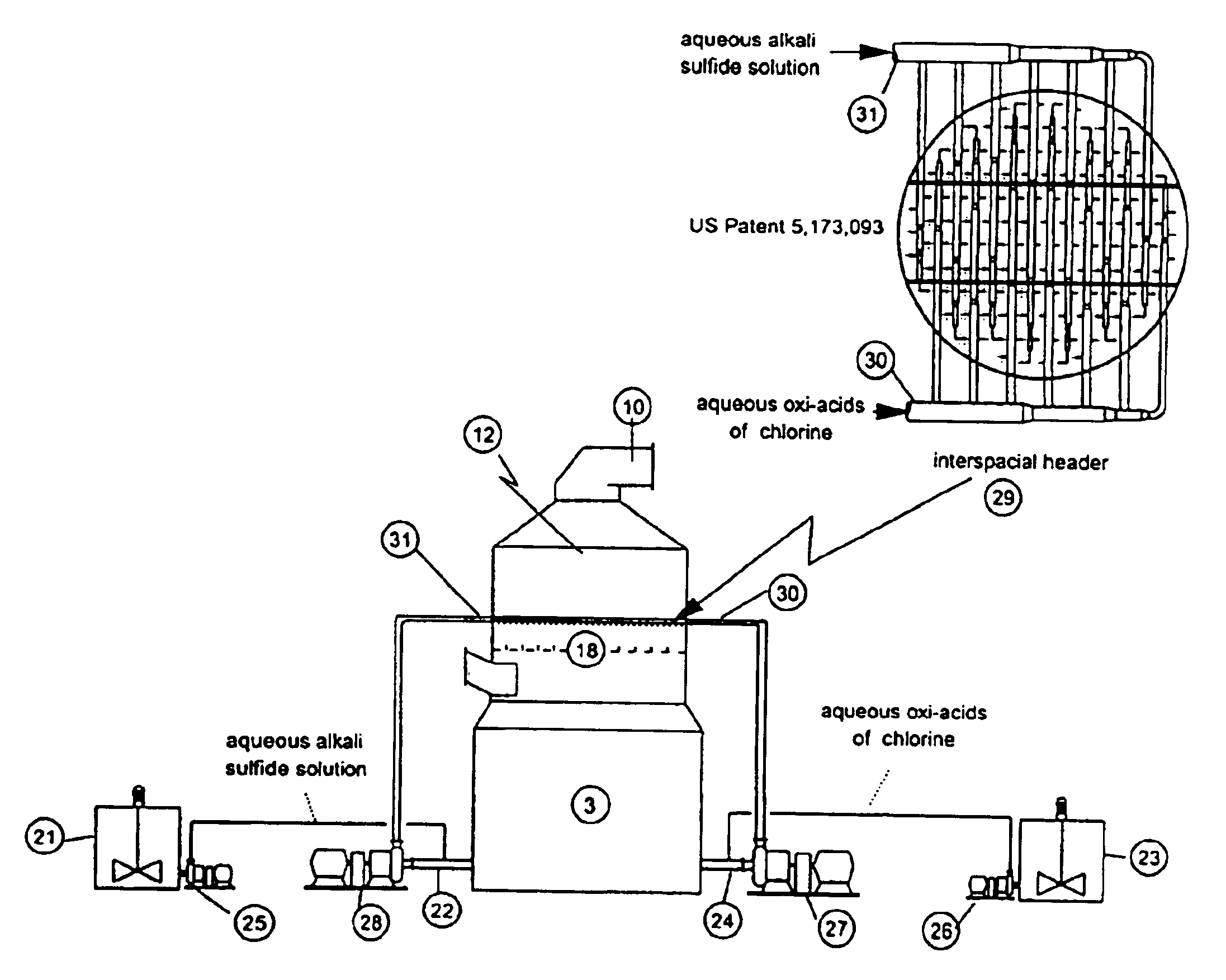
Chlorine and sulfide species are separately introduced to a flue gas passing through a scrubber in order to remove the elemental and oxidized mercury from the gas through the precipitation of mercuric sulfide at near 100% efficiency.
Owner:THE BABCOCK & WILCOX CO
Single-crystal-like materials
InactiveUS7022303B2Improve fracture toughnessReadily apparentMagnesium fluoridesCalcium/strontium/barium fluoridesMetallurgySingle crystal
Polycrystalline materials of macroscopic size exhibiting Single-Crystal-Like properties are formed from a plurality of Single-Crystal Particles, having Self-Aligning morphologies and optionally ling morphology, bonded together and aligned along at least one, and up to three, crystallographic directions.
Owner:RUTGERS THE STATE UNIV
III-V semiconductor nanocrystal complexes and methods of making same
ActiveUS20060001119A1High Luminescence Quantum YieldMaterial nanotechnologyFrom normal temperature solutionsLuminescence quantum yieldSemiconductor nanocrystals
A semiconductor nanocrystal complex that is stable and has high luminescent quantum yield. The semiconductor nanocrystal complex has a semiconductor nanocrystal core of a III-V semiconductor nanocrystal material. A method of making a semiconductor nanocrystal complex is also provided. The method includes synthesizing a semiconductor nanocrystal core of a III-V semiconductor nanocrystal material, and forming a metal layer on the semiconductor nanocrystal core after synthesis of the semiconductor nanocrystal core.
Owner:SAMSUNG ELECTRONICS CO LTD
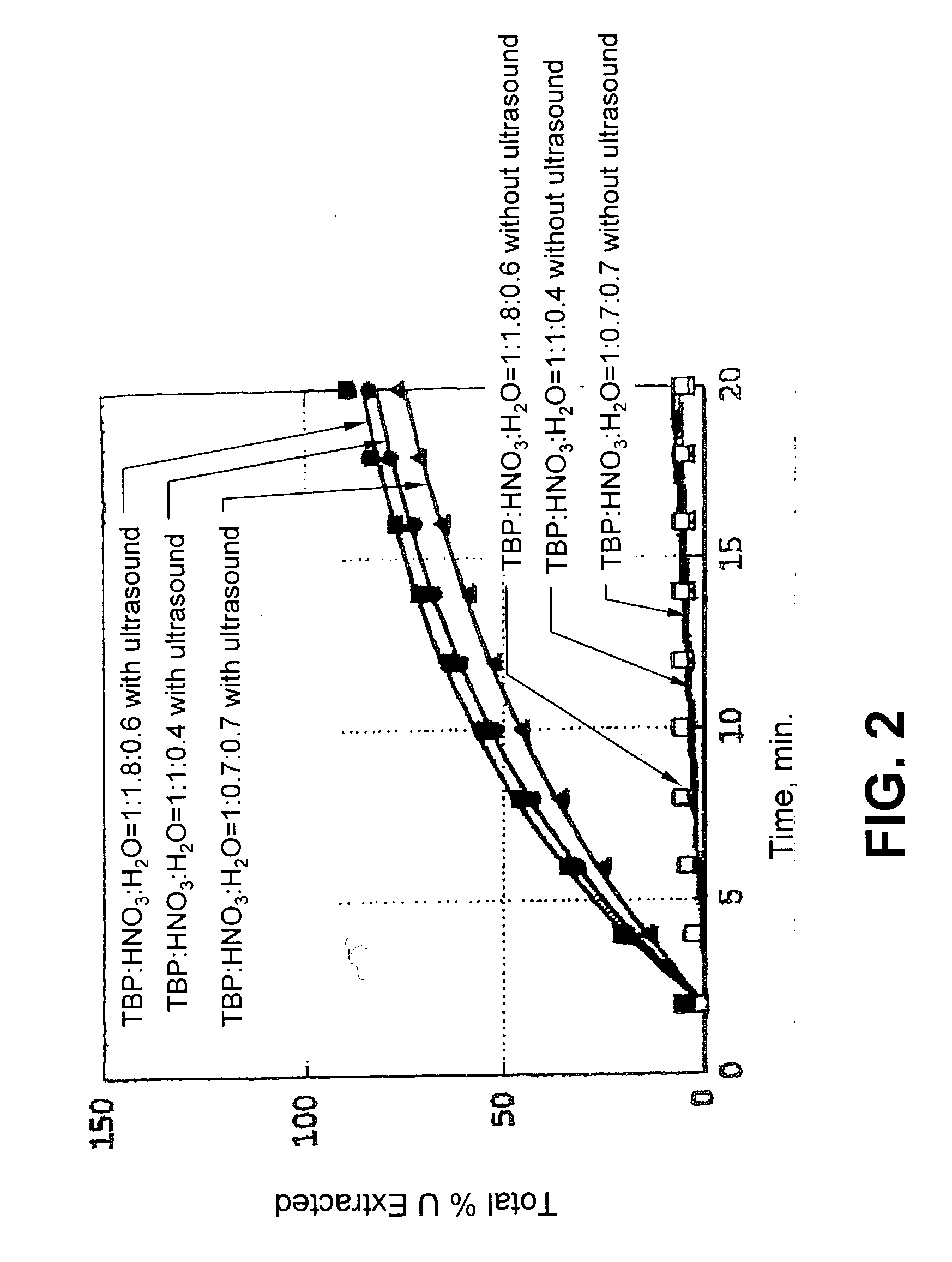
Ultrasound enhanced process for extracting metal species in supercritical fluids
InactiveUS20030183043A1Enhances rate and efficiencyReduce probabilitySolid sorbent liquid separationGold compoundsUranium oxidePresent method
Improved methods for the extraction or dissolution of metals, metalloids or their oxides, especially lanthanides, actinides, uranium or their oxides, into supercritical solvents containing an extractant are disclosed. The disclosed embodiments specifically include enhancing the extraction or dissolution efficiency with ultrasound. The present methods allow the direct, efficient dissolution of UO2 or other uranium oxides without generating any waste stream or by-products.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
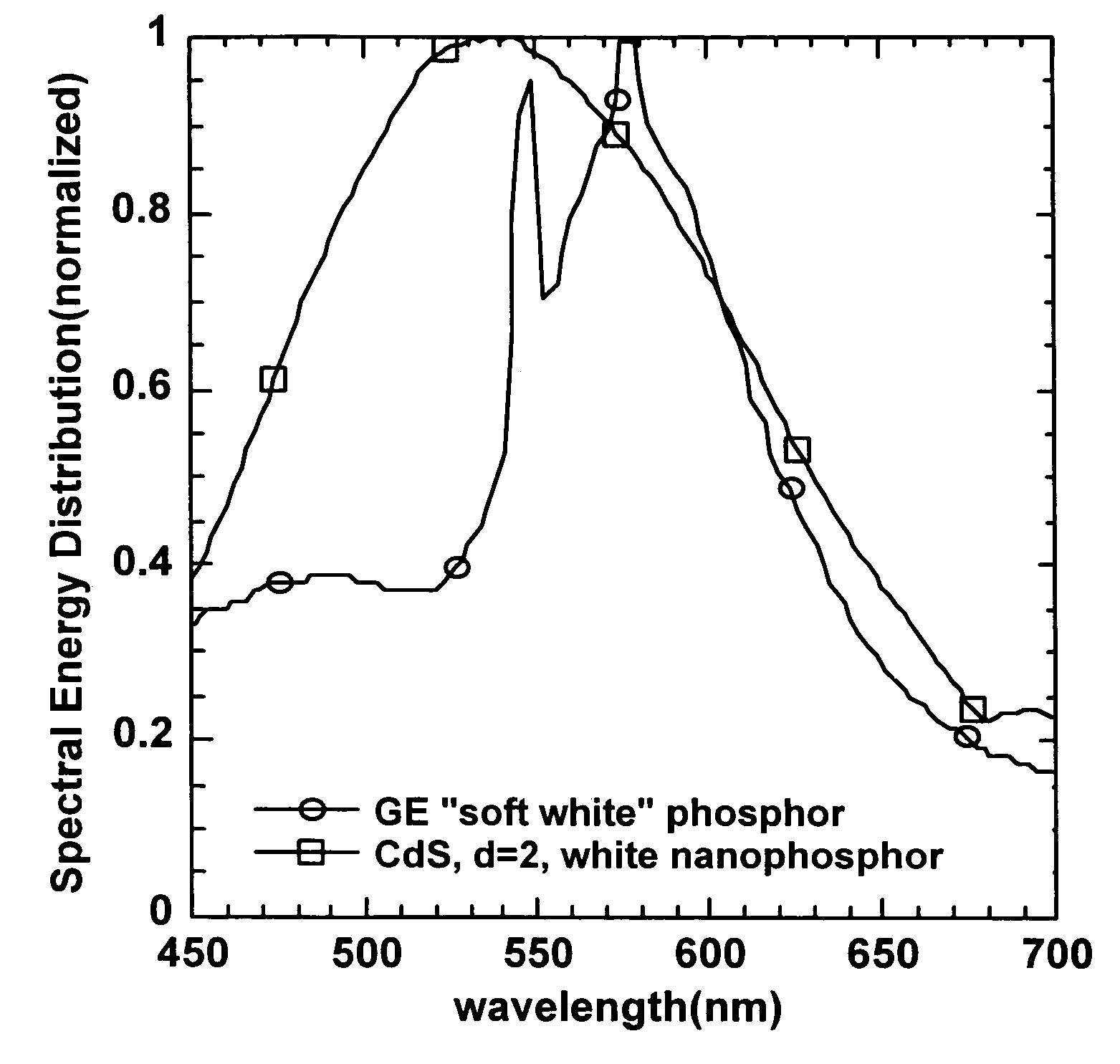
Nanocluster-based white-light-emitting material employing surface tuning
A method for making a nanocrystal-based material capable of emitting light over a sufficiently broad spectral range to appear white. Surface-modifying ligands are used to shift and broaden the emission of semiconductor nanocrystals to produce nanoparticle-based materials that emit white light.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Semiconductor nanocrystal complexes and methods of making same
ActiveUS20060014040A1Material nanotechnologyLiquid surface applicatorsLattice mismatchSemiconductor nanocrystals
A semiconductor nanocrystal complex including a metal layer formed on the outer surface of a semiconductor nanocrystal core after synthesis of the semiconductor nanocrystal core and a method for preparing a nanocrystal complex comprising forming a metal layer on a semiconductor nanocrystal core after synthesis of the semiconductor nanocrystal core. The metal layer may passivate the surface of the semiconductor nanocrystal core and protect the semiconductor nanocrystal core from the effects of oxidation. Also provided is a semiconductor nanocrystal complex with a shell grown onto the metal layer formed on the semiconductor nanocrystal core. In this embodiment, the metal layer may prevent lattice mismatch between the semiconductor shell and the semiconductor nanocrystal core.
Owner:SAMSUNG ELECTRONICS CO LTD
Nanocrystals in ligand boxes exhibiting enhanced chemical, photochemical, and thermal stability, and methods of making the same
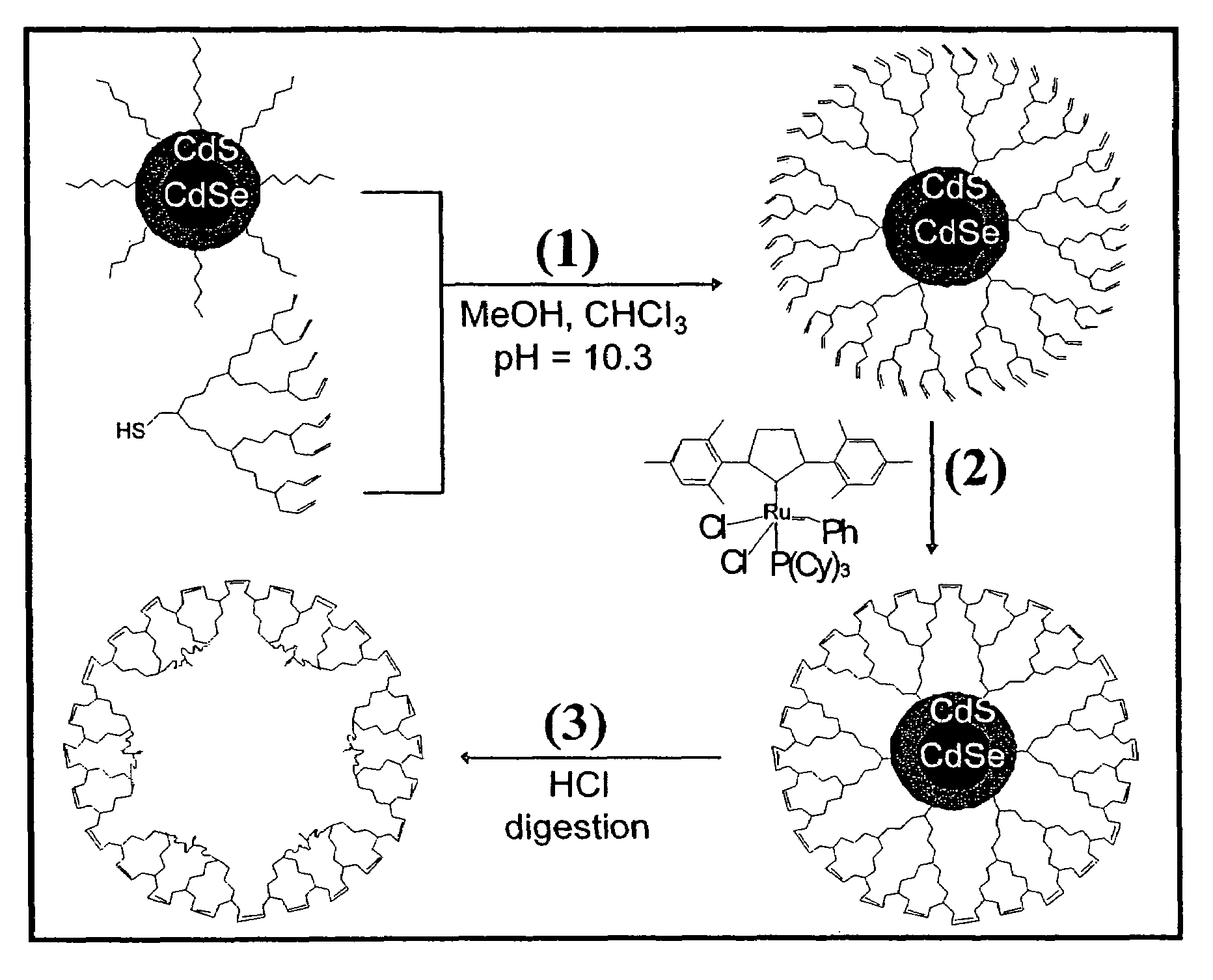
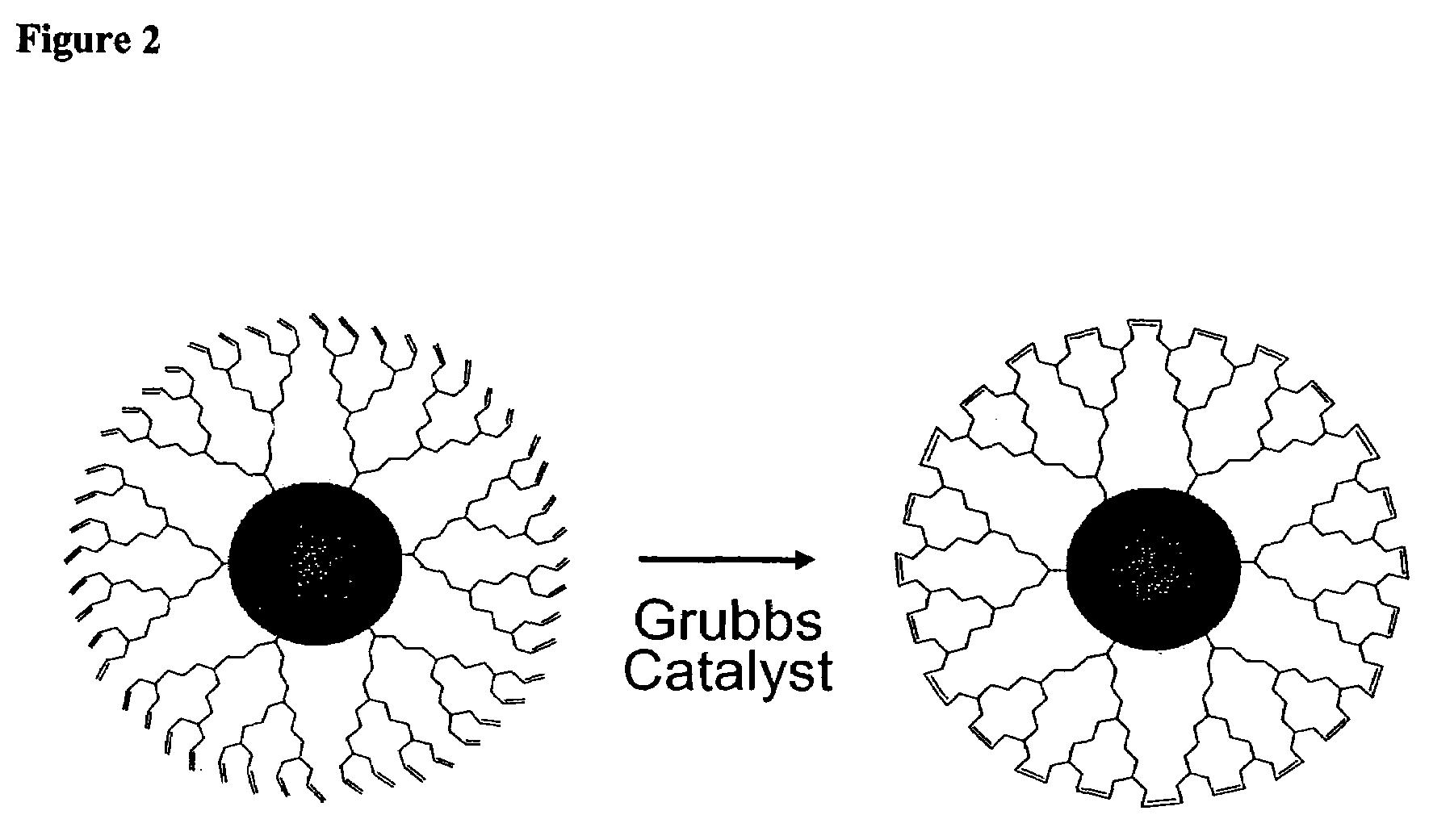
ActiveUS7273904B2Improve stabilityEasy to detectMaterial nanotechnologyFrom normal temperature solutionsDendrimerCross-link
Dendron ligands or other branched ligands with cross-linkable groups were coordinated to colloidal inorganic nanoparticles, including nanocrystals, and substantially globally cross-linked through different strategies, such as ring-closing metathesis (RCM), dendrimer-bridging methods, and the like. This global cross-linking reaction sealed each nanocrystal within a dendron box to yield box-nanocrystals which showed dramatically enhanced stability against chemical, photochemical and thermal treatments in comparison to the non-cross-linked dendron-nanocrystals. Empty dendron boxes possessing a very narrow size distribution were formed by the dissolution of the inorganic nanocrystals contained therein upon acid or other etching treatments.
Owner:BEIJING JINGTAI MEIKANG BIOTECH CO LTC +1
One-pot synthesis of high-quality metal chalcogenide nanocrystals without precursor injection
InactiveUS20060019427A1Material nanotechnologyPolycrystalline material growthSulfurMetal chalcogenides
A method of homogeneously forming metal chalcogenide nanocrystals includes the steps combining a metal source, a chalcogenide source, and at least one solvent at a first temperature to form a liquid comprising assembly, and heating the assembly at a sufficient temperature to initiate nucleation to form a plurality of metal chalcogenide nanocrystals. The plurality of metal chalcogenide nanocrystals are then grown without injection of either the metal source or the chalcogenide source at a temperature at least equal to the sufficient temperature, wherein growth proceeds substantially without nucleation to form a plurality of monodisperse metal chalcogenide nanocrystals. An optional nucleation initiator can help control the final size of the monodisperse crystals. Such synthesis, without the need for precursor injection, is suitable for the industrial preparation of high-quality nanocrystals.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Metal chalcogenide composite nano-particles and layers therewith
A metal chalcogenide composite nano-particle comprising a metal capable of forming p-type semiconducting chalcogenide nano-particles and a metal capable of forming n-type semiconducting chalcogenide nano-particles, wherein at least one of the metal chalcogenides has a band-gap between 1.0 and 2.9 eV and the concentration of the metal capable of forming p-type semiconducting chalcogenide nano-particles is at least 5 atomic percent of the metal and is less than 50 atomic percent of the metal; a dispersion thereof; a layer comprising the nano-particles; and a photovoltaic device comprising the layer.
Owner:AGFA GEVAERT AG
Process for recovery of nickel and cobalt from laterite ore
ActiveUS20060228279A1Efficient separation and recoveryHigh purityCobalt sulfidesSolvent extractionFree solutionSlurry
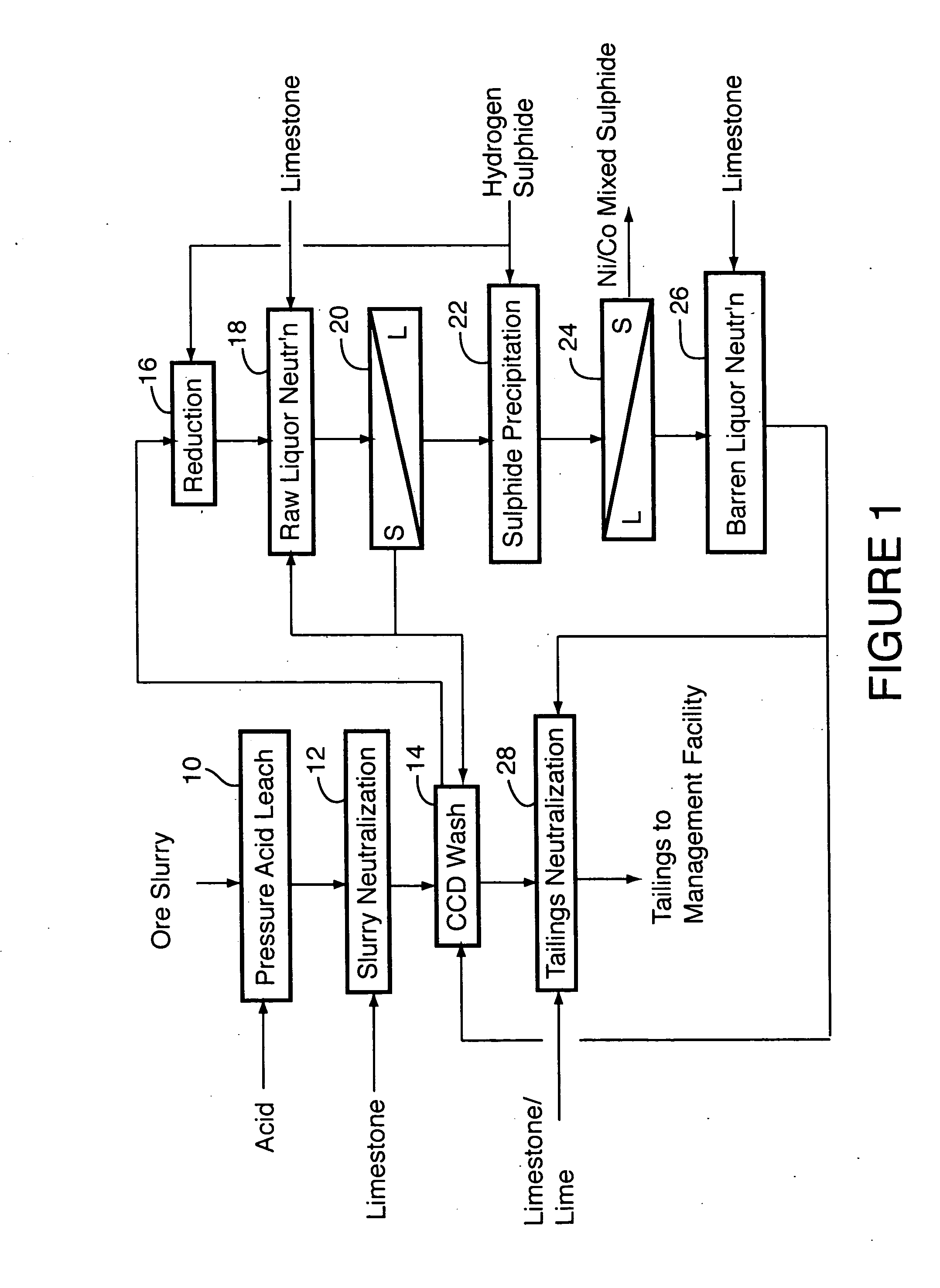
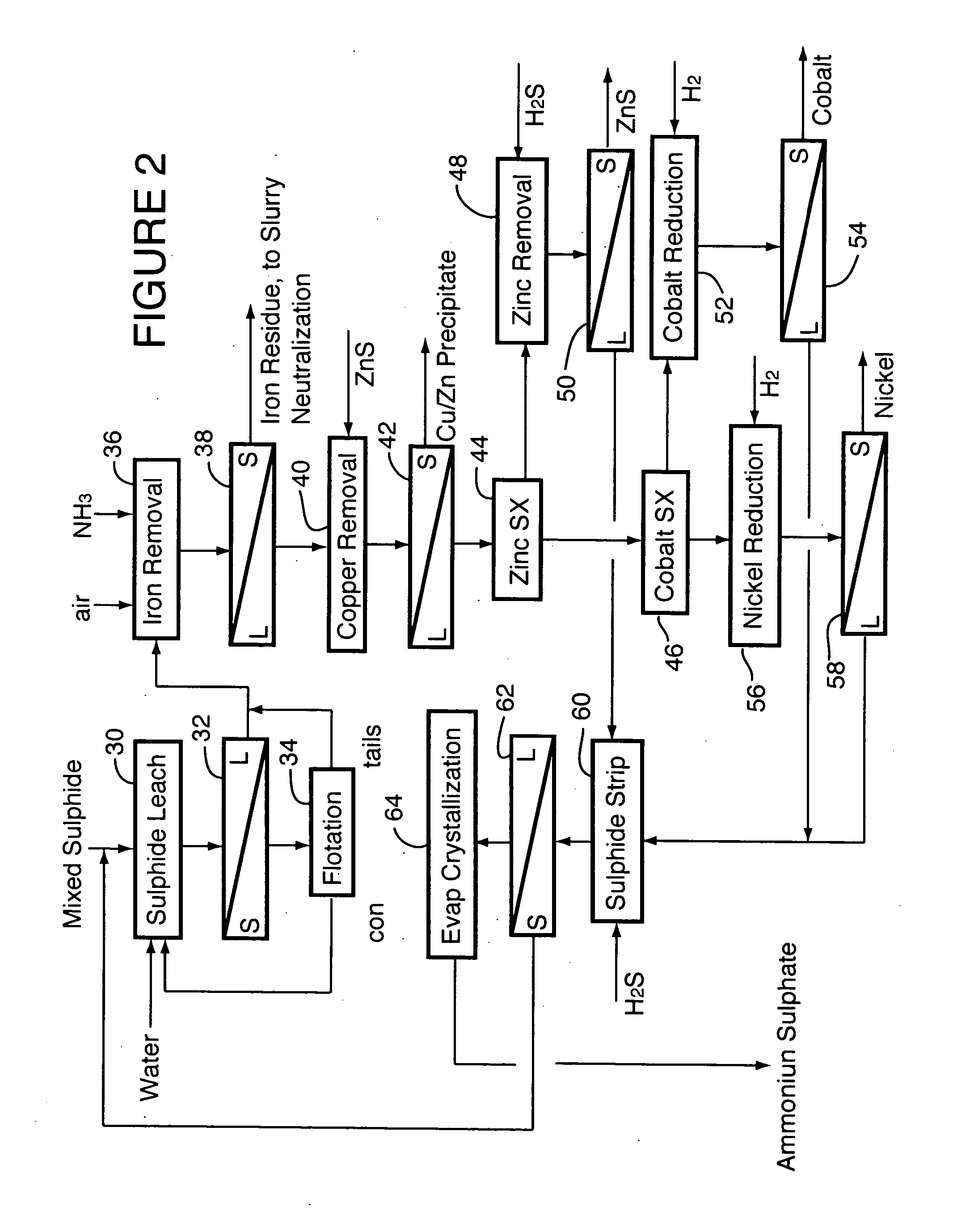
A process for recovering nickel and cobalt values from nickel- and cobalt-containing laterite ores as an enriched mixed nickel and cobalt sulphide intermediate and for producing nickel and cobalt metal from the nickel and cobalt sulphide intermediate. The laterite ore is leached as a slurry in a pressure acid leach containing an excess of aqueous sulphuric acid at high pressure and temperature, excess free acid in the leach slurry is partially neutralized to a range of 5 to 10 g / L residual free H2SO4 and washed to yield a nickel- and cobalt-containing product liquor, the product liquor is subjected to a reductant to reduce any Cr(VI) in solution to Cr(III), the reduced product liquor is neutralized to precipitate ferric iron and silicon at a pH of about 3.5 to 4.0, and the neutralized and reduced product liquor is contacted with hydrogen sulphide gas to precipitate nickel and cobalt sulphides. The precipitated nickel and cobalt sulphides can be leached in a water slurry in a pressure oxidation leach, the leach solution subjected to iron hydrolysis and precipitation, the iron-free solution contacted with zinc sulphide to precipitate copper, the iron- and copper-free solution subjected to zinc and cobalt extraction by solvent extraction to produce a nickel raffinate, the nickel raffinate contacted with hydrogen gas to produce nickel powder and the cobalt strip solution from the solvent extraction step contacted with hydrogen gas to produce cobalt powder.
Owner:SHERRITT INTERNATIONAL
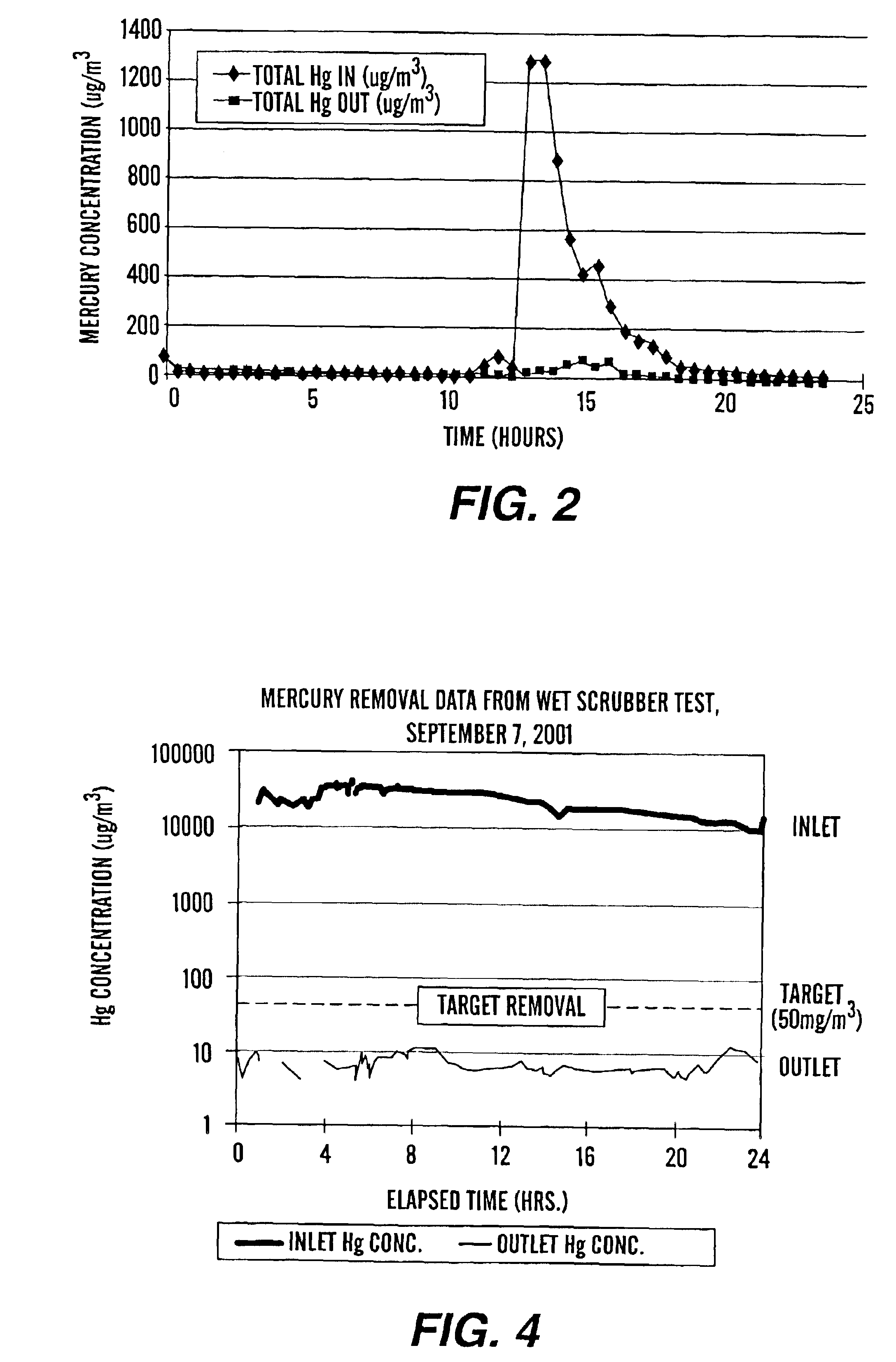
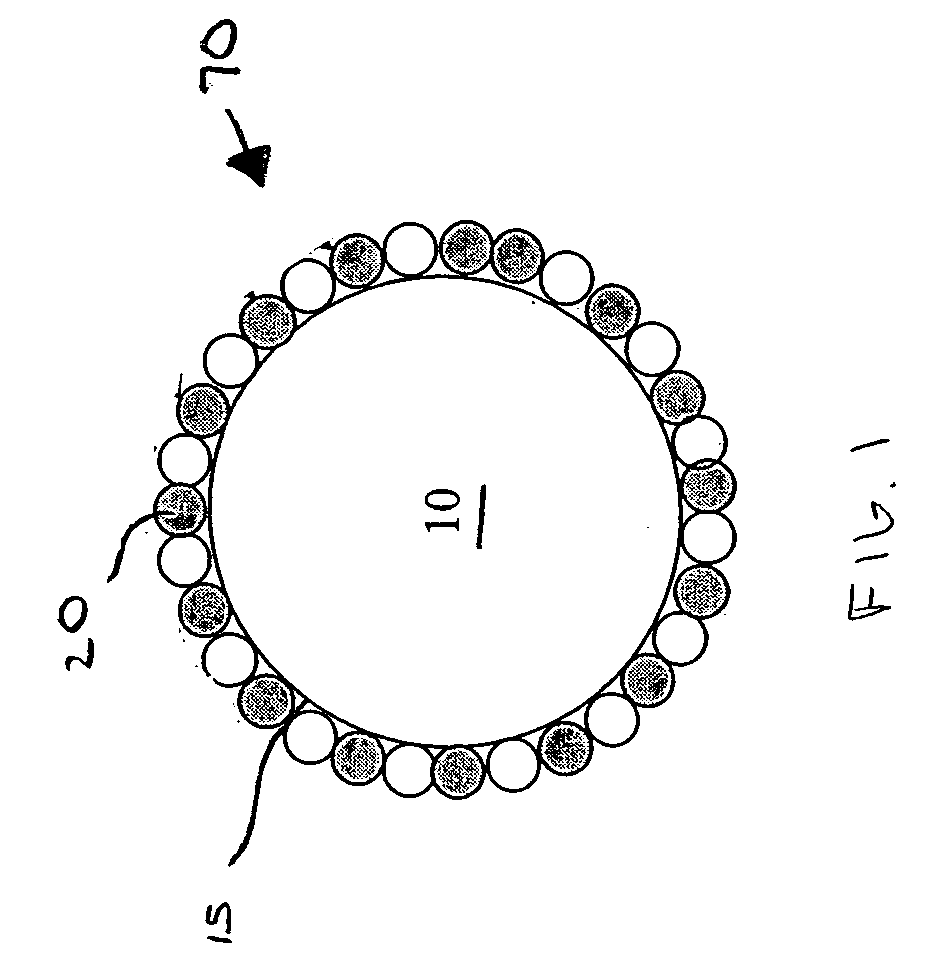
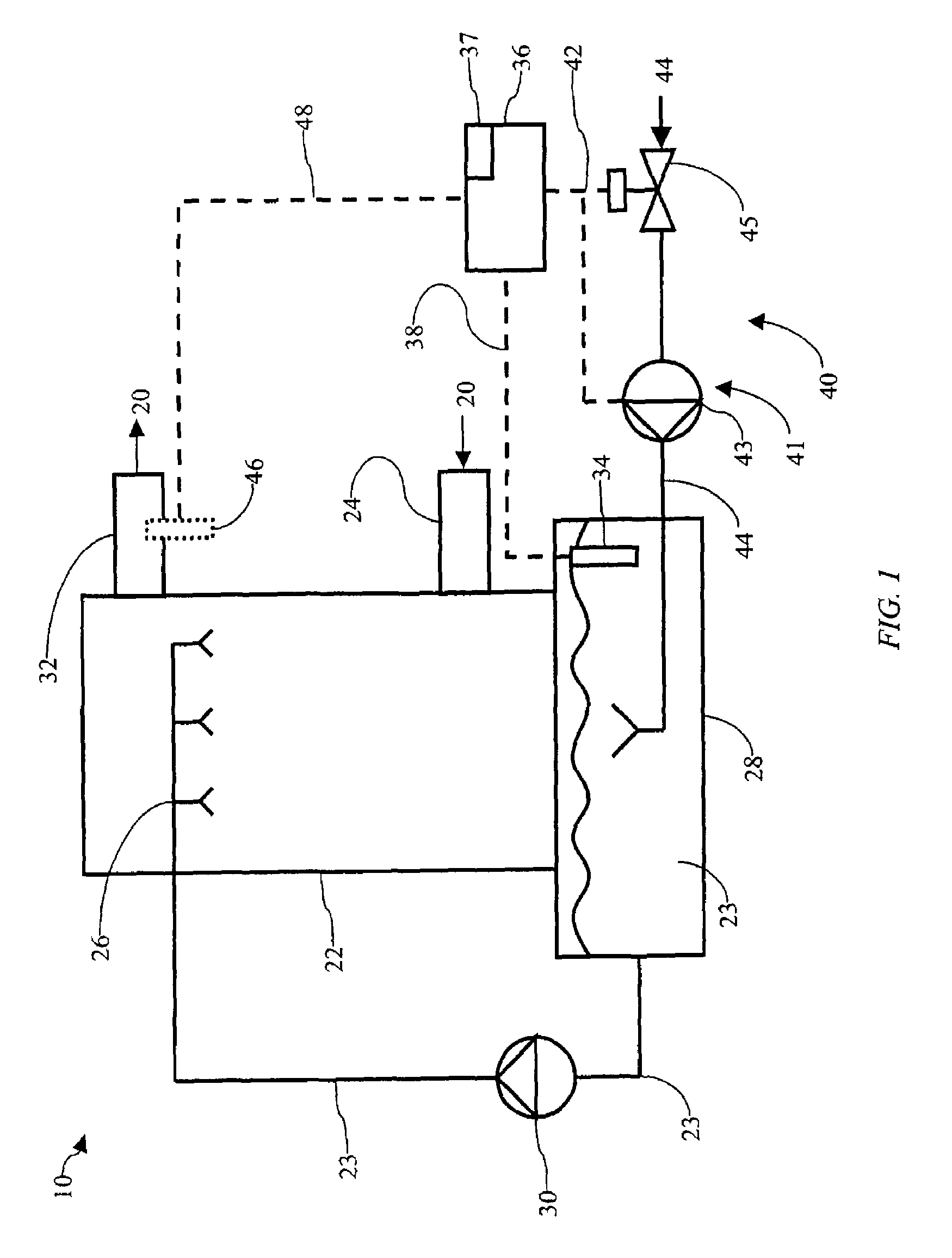
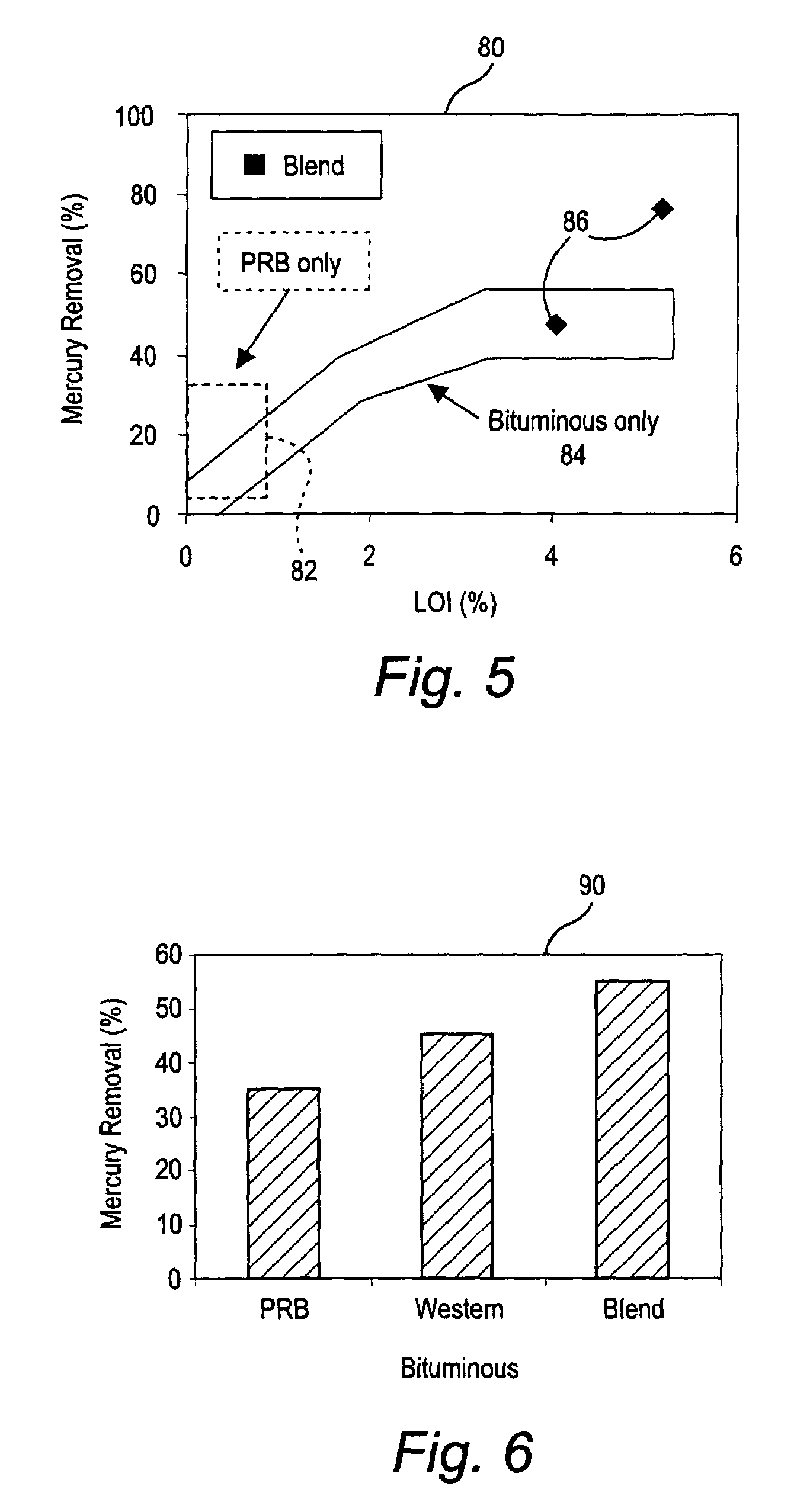
Method of mercury removal in a wet flue gas desulfurization system
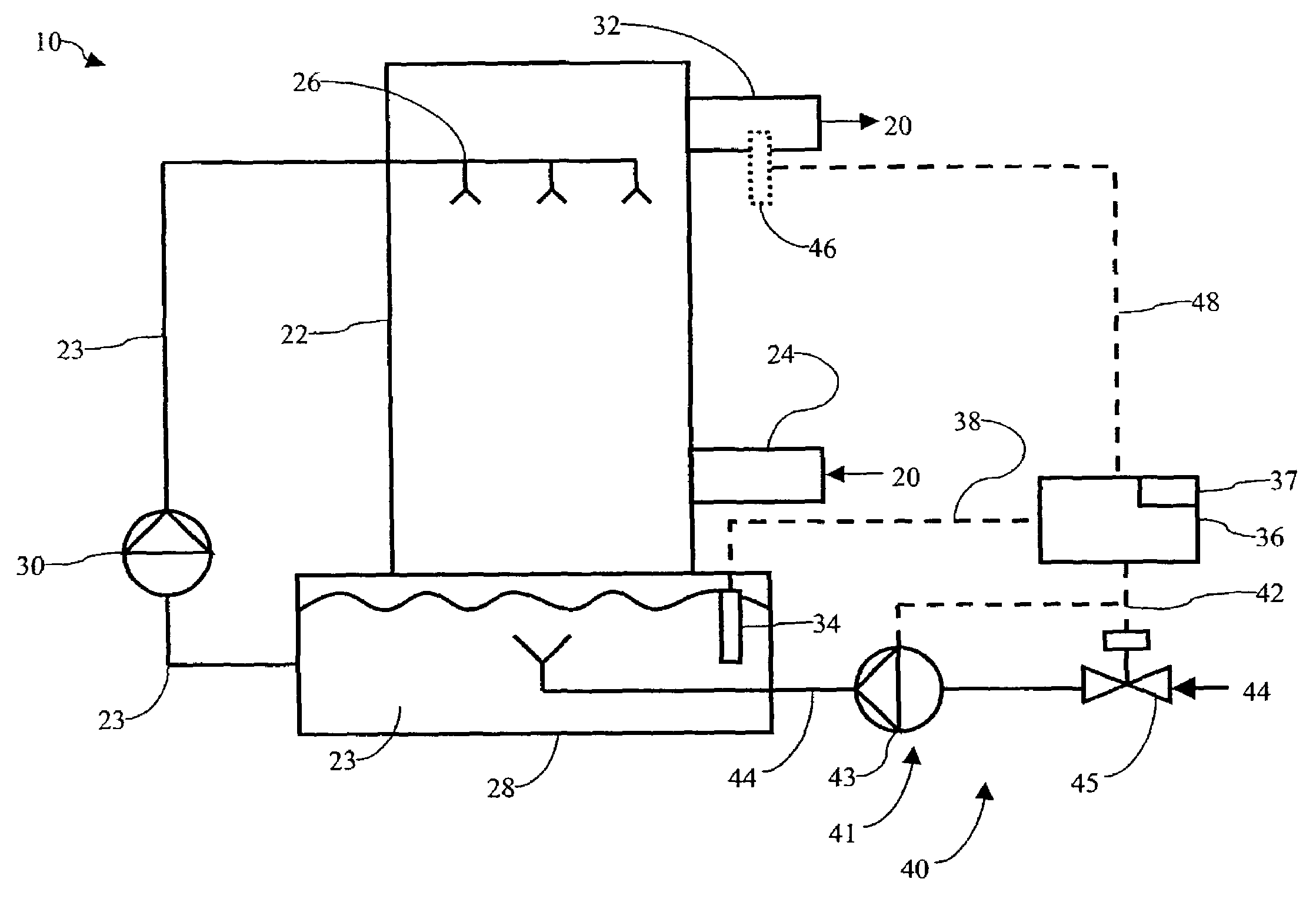
Controlling the reductive capacity of an aqueous alkaline slurry (23) in a wet scrubber makes it possible to accurately control the mercury emission from the scrubber to a desired value. One method of controlling the reductive capacity of the slurry is to measure the reduction-oxidation potential (“redox potential”) of the aqueous alkaline slurry (23) and to add or remove substances that affect the redox potential and thus the reductive capacity of the slurry. In wet scrubbers in which limestone is used for absorption of acid gases and where a gypsum slurry is circulated, it has been found to be an attractive solution to control the amount of oxidation air blown into the scrubber in order to control the redox potential and thereby the mercury emissions.
Owner:GENERAL ELECTRIC TECH GMBH
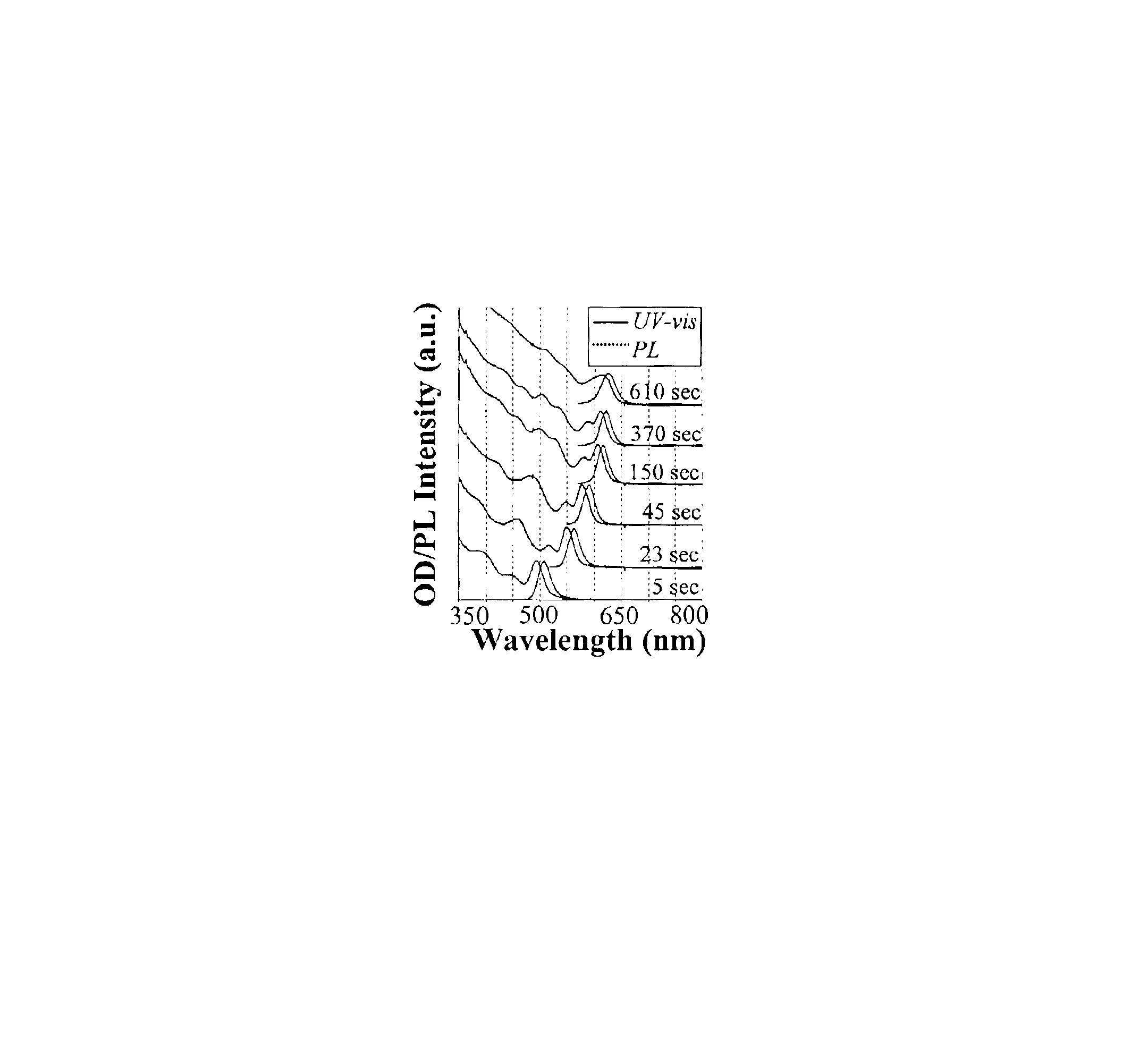
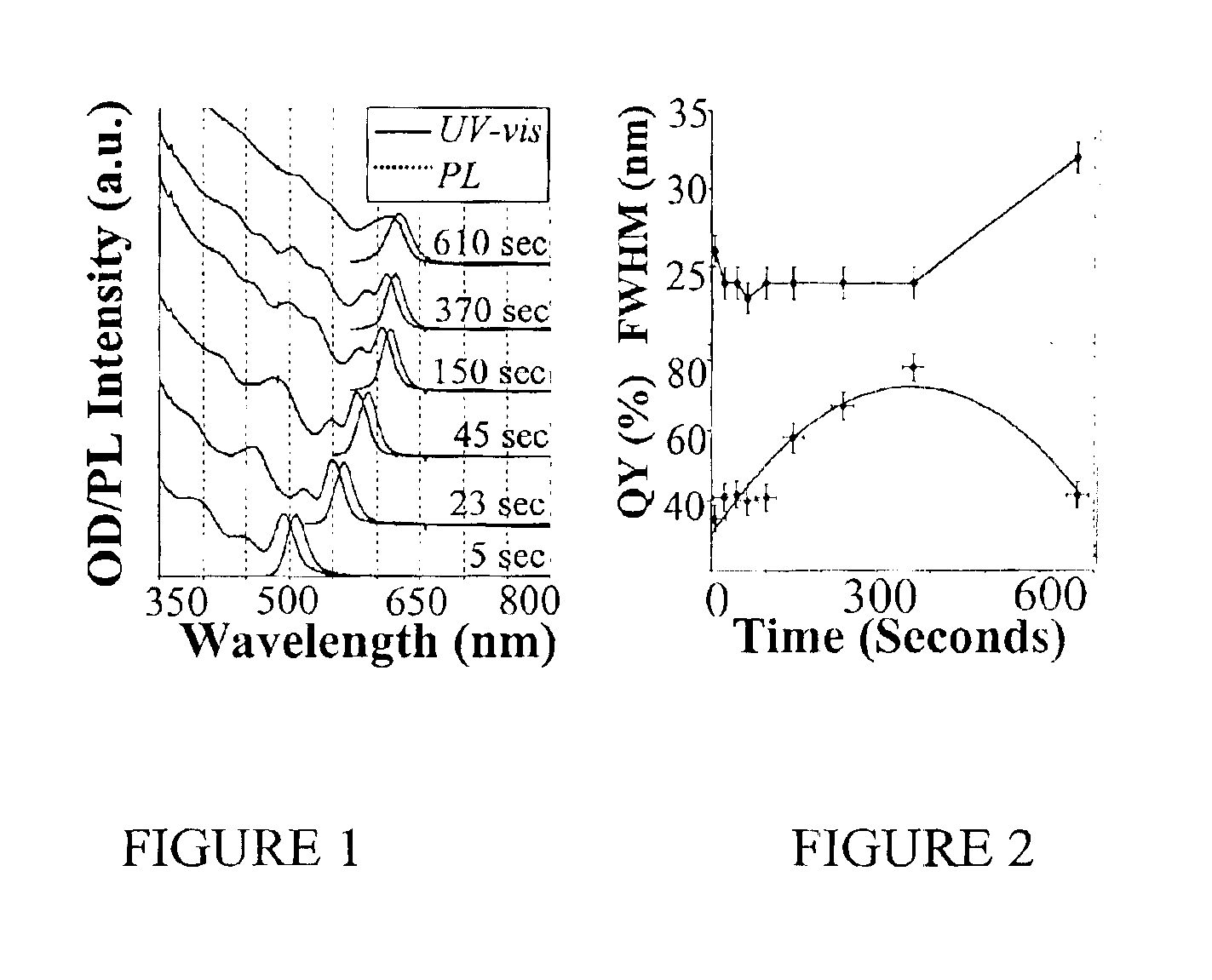
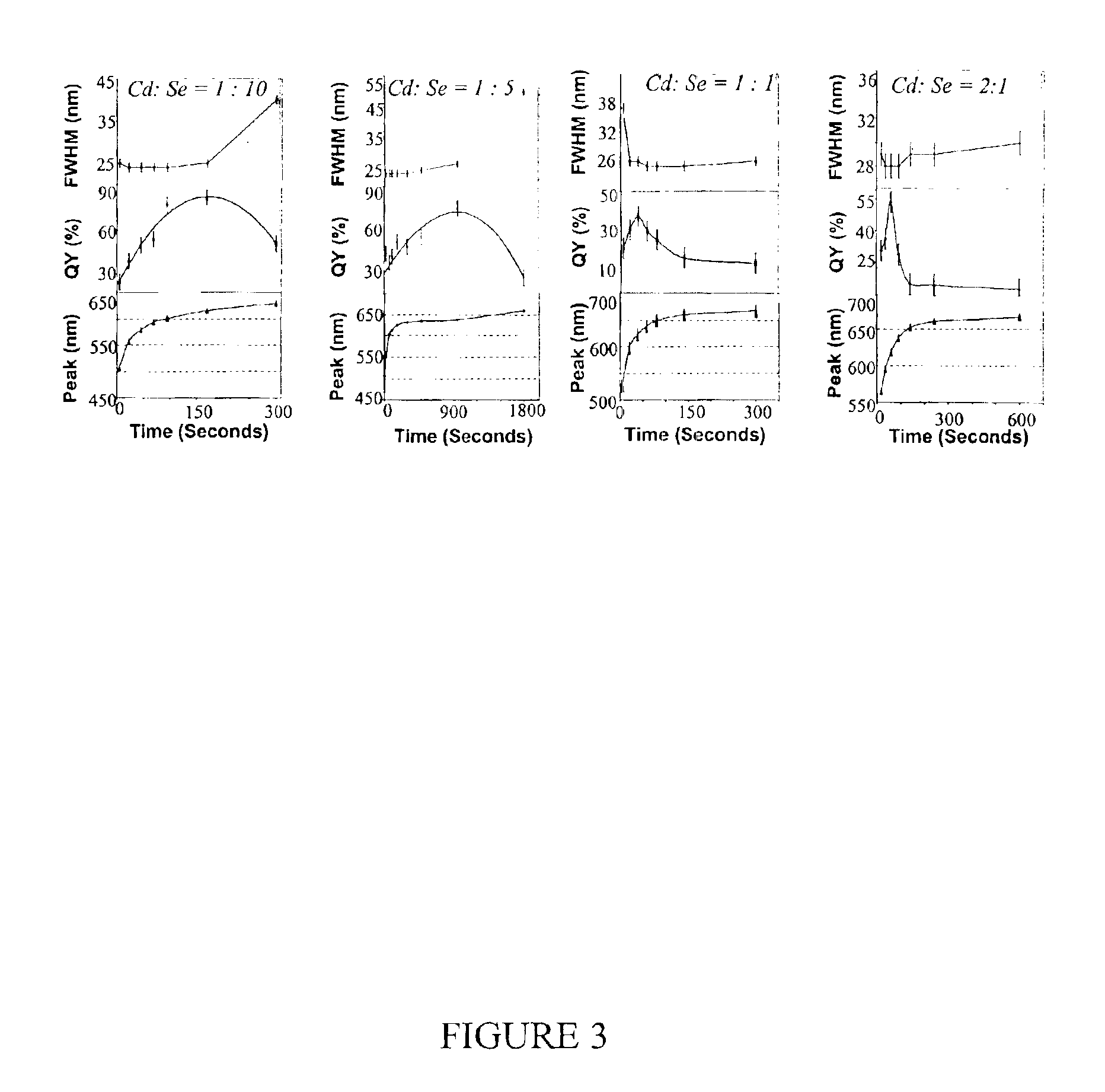
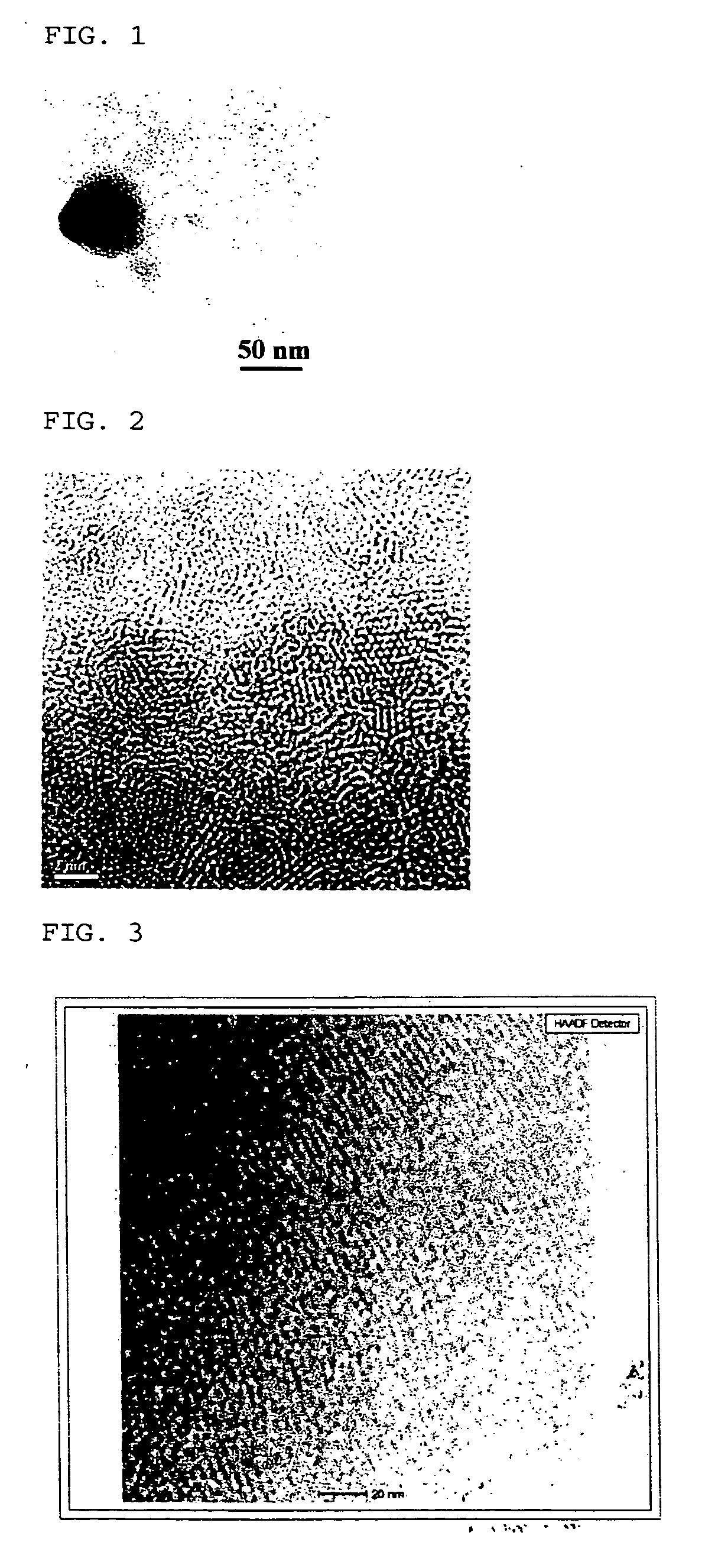
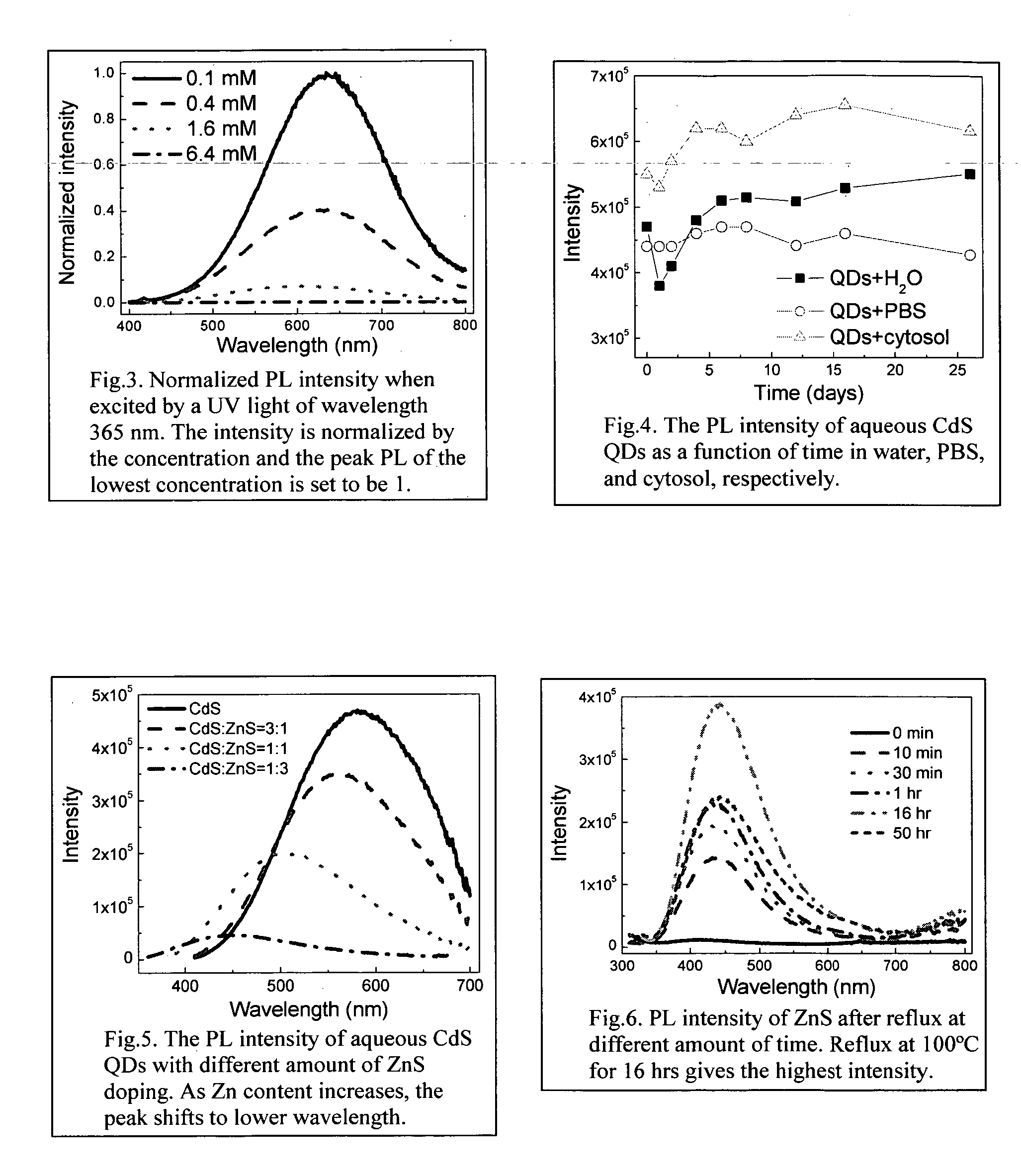
Method of preparing cadmium sulfide nanocrystals emitting light at multiple wavelengths, and cadmium sulfide nanocrystals prepared by the method
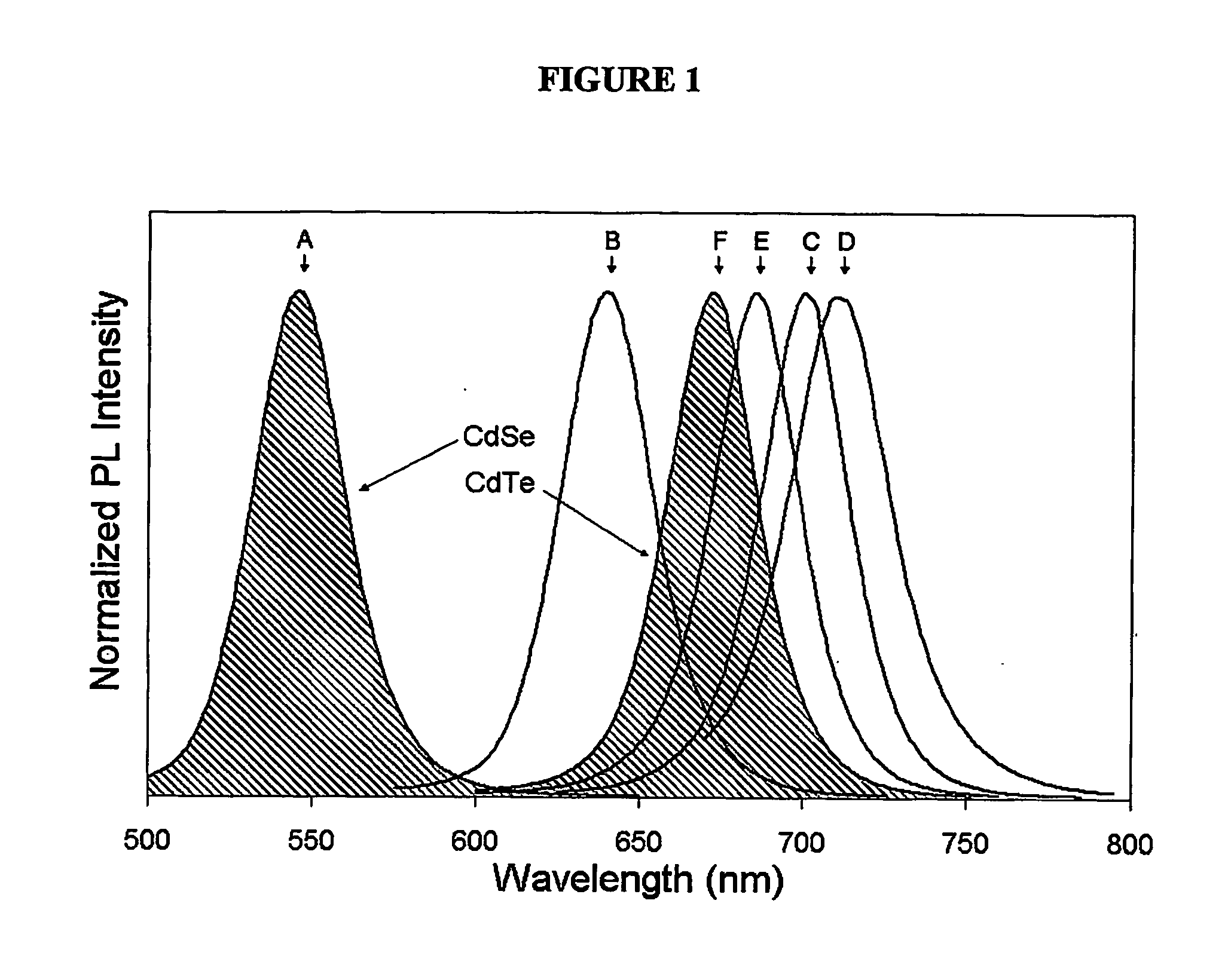
A method for preparing cadmium sulfide nanocrystals emitting light at multiple wavelengths. The method comprises the steps of (a) mixing a cadmium precursor and a dispersant in a solvent that weakly coordinates to the cadmium precursor, and heating the mixture to obtain a cadmium precursor solution, (b) dissolving a sulfur precursor in a solvent that weakly coordinates to the sulfur precursor to obtain a sulfur precursor solution, and (c) feeding the sulfur precursor solution to the heated cadmium precursor solution maintained at a high temperature to prepare cadmium sulfide crystals, and growing the cadmium sulfide crystals. Further, cadmium sulfide nanocrystals prepared by the method. The cadmium sulfide nanocrystals have uniform size and shape and can emit light close to white light simultaneously at different wavelengths upon excitation. Due to these characteristics, the cadmium sulfide nanocrystals can be applied to white light-emitting diode devices.
Owner:SAMSUNG ELECTRONICS CO LTD
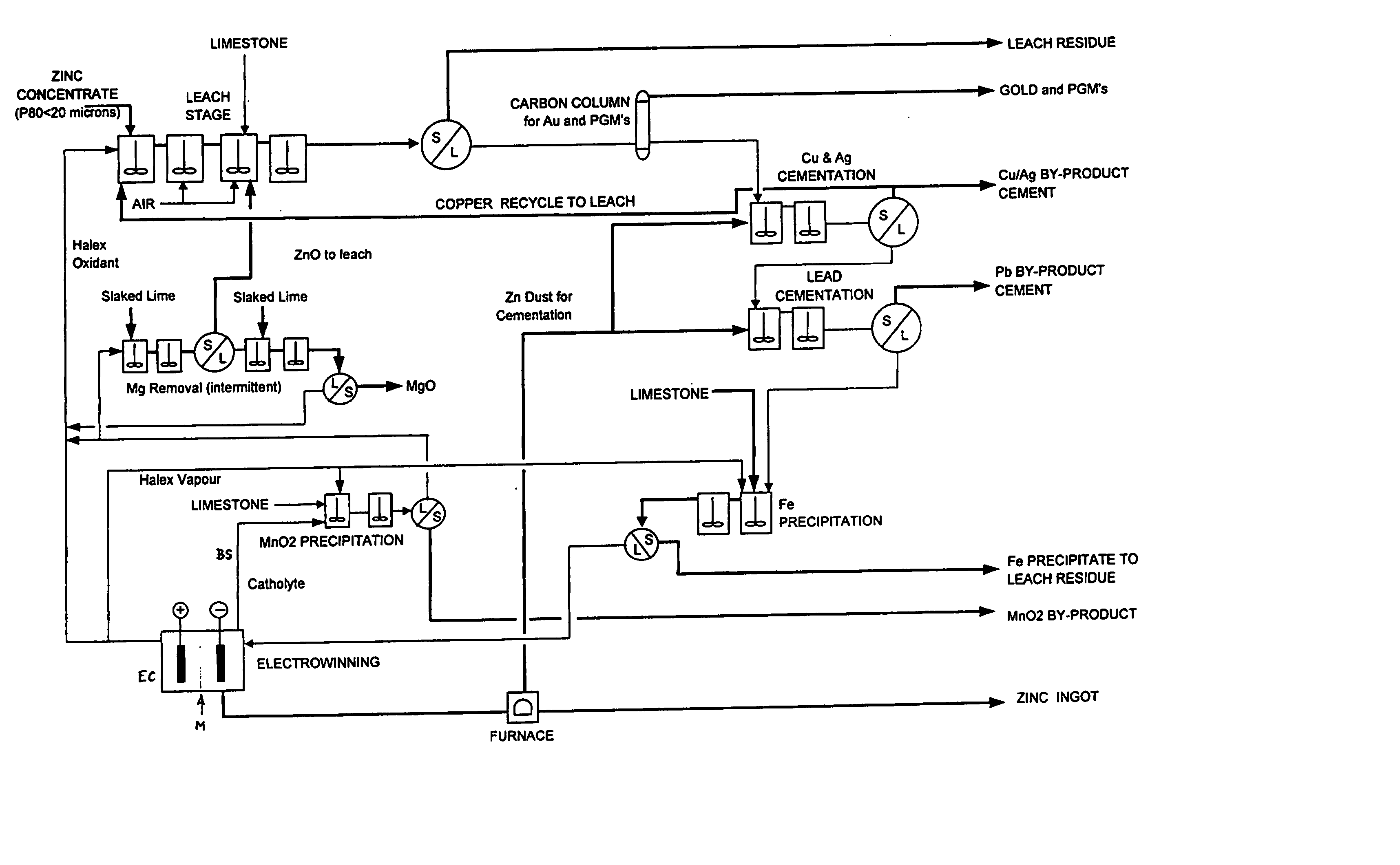
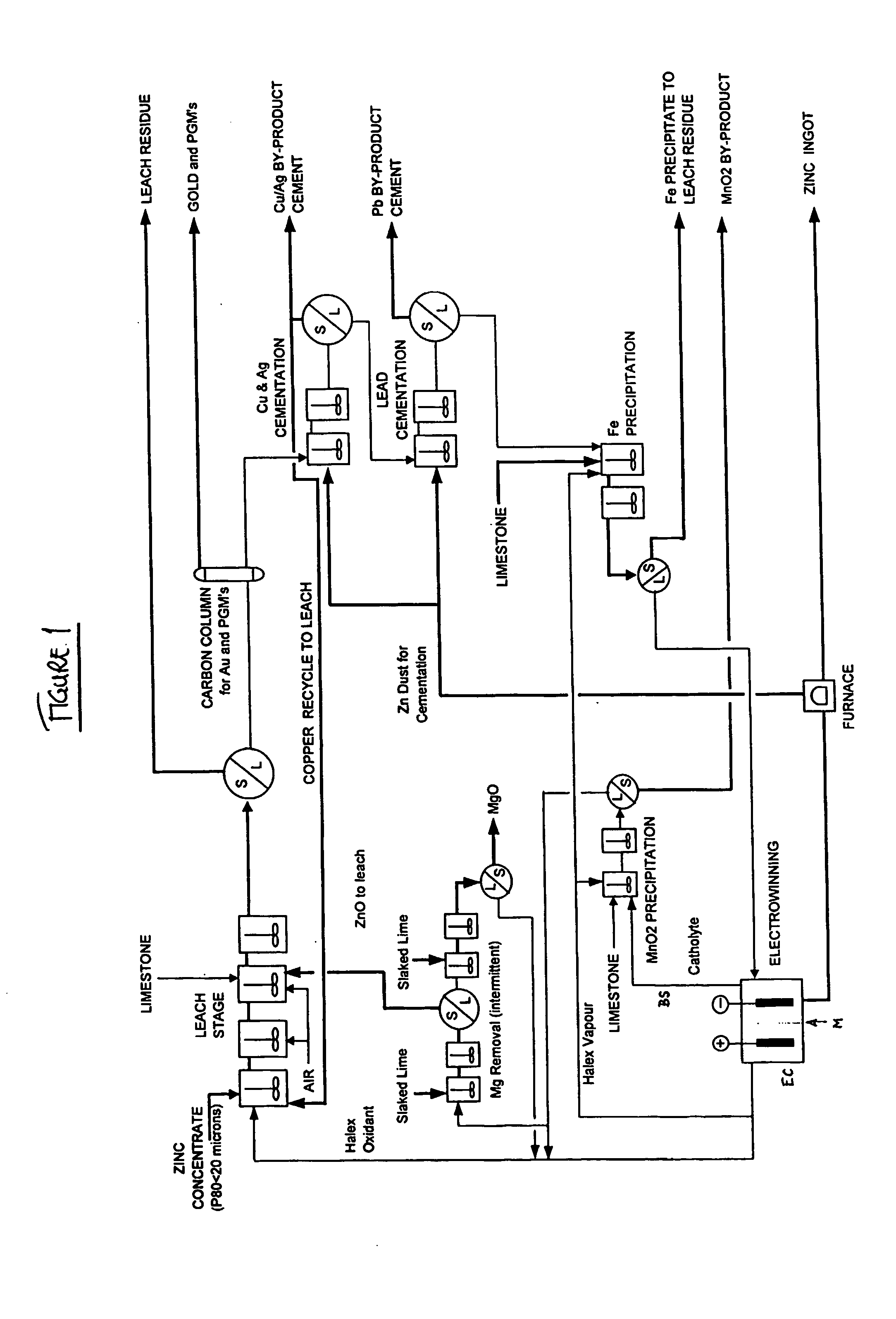
Zinc recovery process
InactiveUS20040237720A1Improve filtering effectEh is decreasedPhotography auxillary processesSolvent extractionZinc metalManganese
A process for the recovery of zinc metal from a zinc mineral includes the steps of leaching the zinc mineral in a solution including a halide species formed from two or more different halides, to leach the zinc into the solution. The zinc-bearing solution is then electrolysed to yield zinc metal and to generate the halide species. The electrolysed solution including the halide species is then returned to the leaching step. A portion of the electrolysed solution can be removed as a bleed stream from a cathode compartment of an electrolytic cell of the electrolysis process and processed to remove manganese as manganese dioxide precipitate by adding thereto limestone, and the halide species from an anode compartment of the electrolysis process. In this regard, the pH and Eh of the solution can regulated in a manner that favours the formation of the manganese dioxide precipitate over the formation of a precipitate of zinc.
Owner:INTEC INT PROJECTS
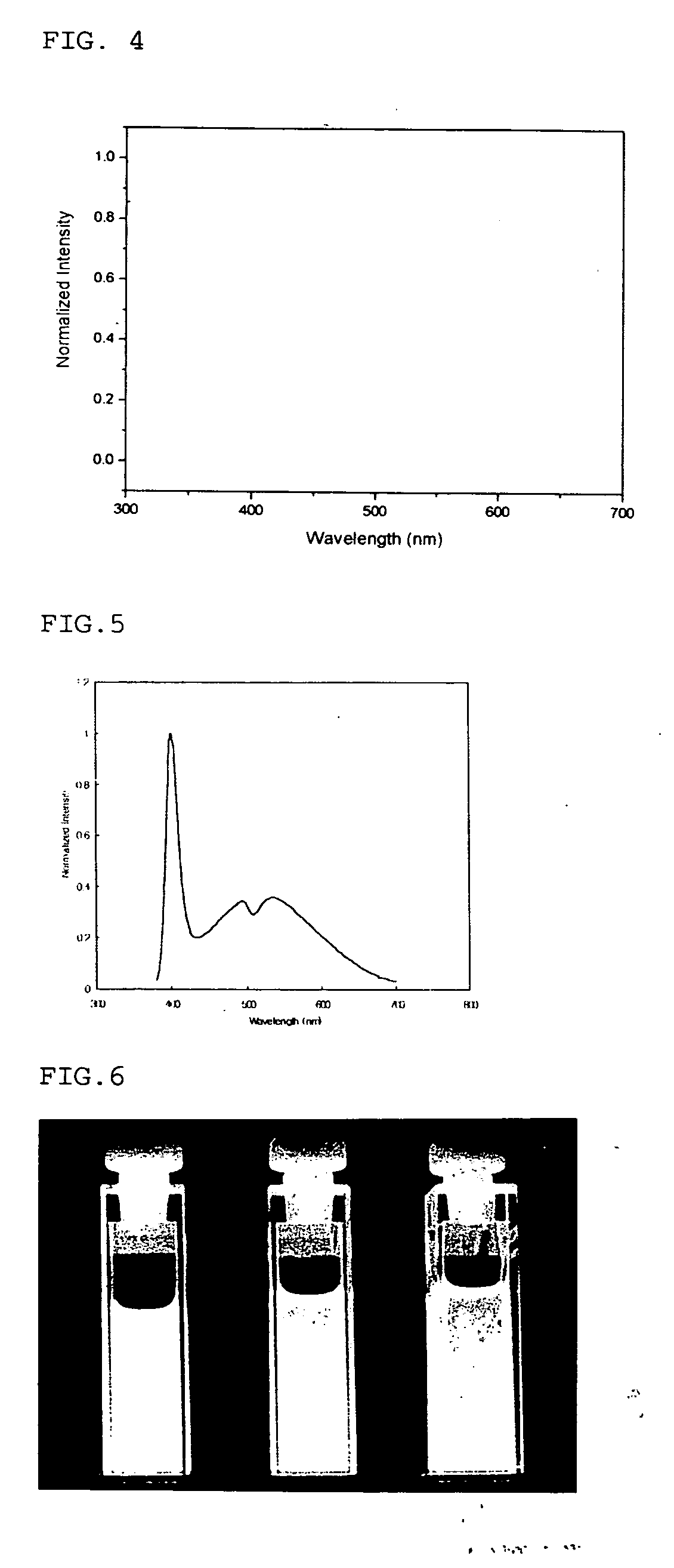
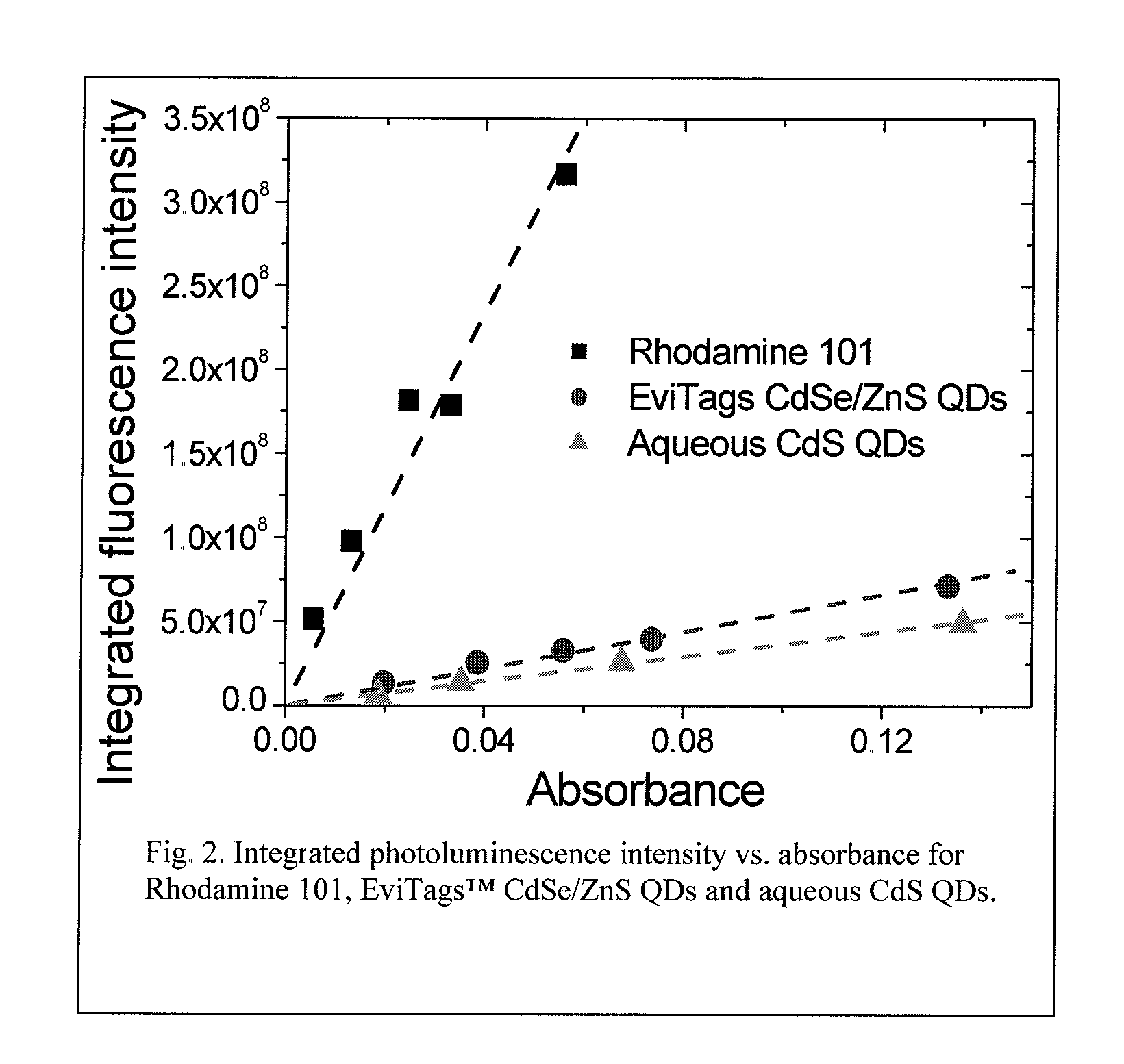
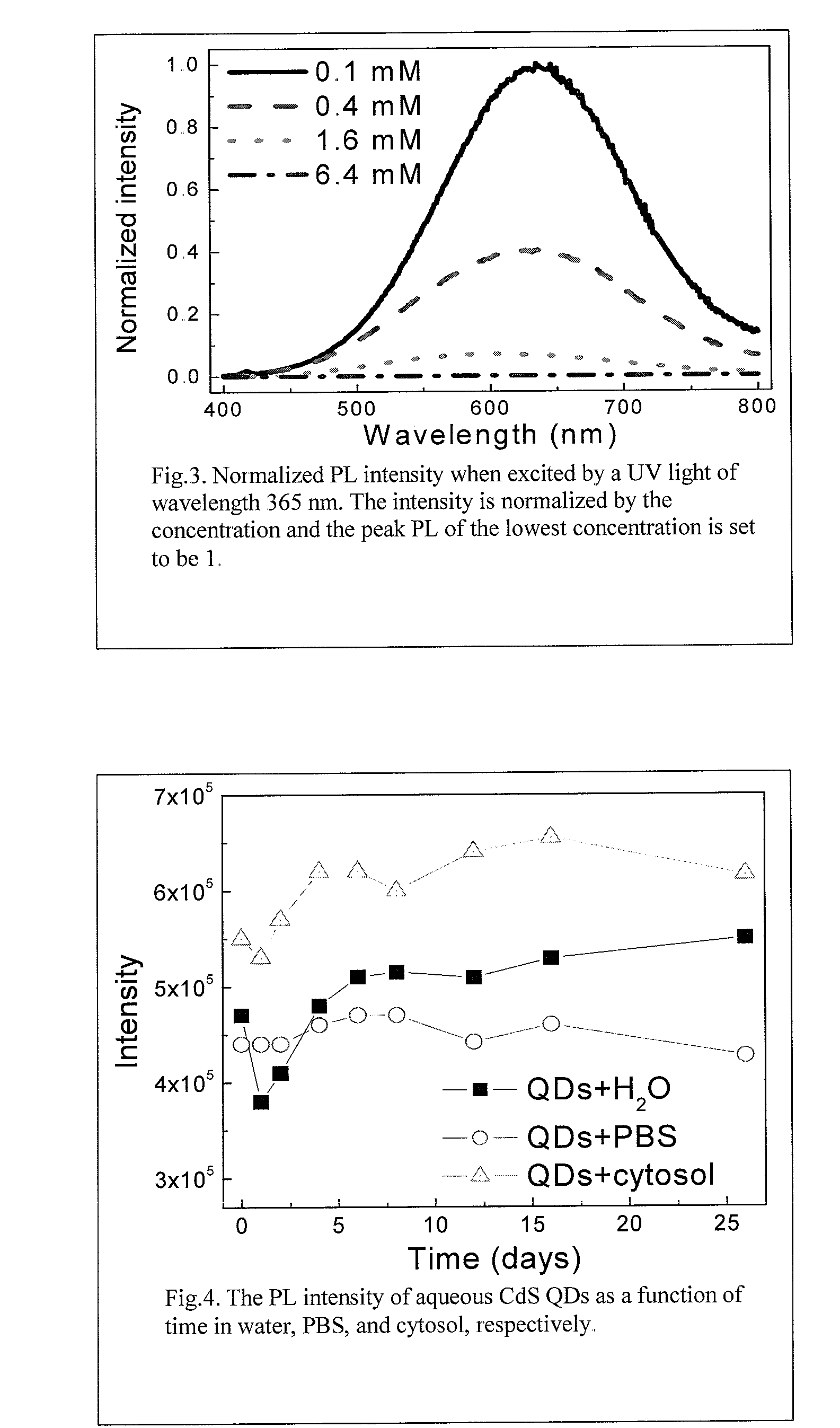
Synthesis of water soluble nanocrystalline quantum dots and uses thereof
InactiveUS20060078490A1Improved emission spectrumAccelerate emissionsMaterial nanotechnologyHybrid immunoglobulinsPhotoluminescenceSynthesis methods
An economic, direct synthetic method for producing water soluble QDs that are ready for bioconjugation is provided. The method can produce aqueous QDs with emission wavelengths varying from 400 nm to 700 nm. Highly luminescent metal sulfide (MS) QDs are produced via an aqueous synthesis route. MS QDs are capped with thiol-containing charged molecules in a single step. The resultant MS QDs exhibit the distinctive excitonic photoluminescence desired of QDs and can be fabricated to avoid undesirable broadband emissions at higher wavelengths. This provides a significant improvement over the present complex and expensive commercial processes for the production of QDs. The aqueous QDs are stable in biological fluids over a long period of time. In addition, nontoxic ZnS QDs have been produced with good photoluminescence properties by refluxing the ZnS QD suspensions over a period of time.
Owner:DREXEL UNIV
Rapid synthesis of ternary, binary and multinary chalcogenide nanoparticles
InactiveUS20100003187A1Rapid responseEasy to handleMaterial nanotechnologyPhosphorus sulfur/selenium/tellurium compoundsOrganic solventSulfur
A method for synthesizing a chalcogenide nanoparticle is provided. The method comprises reacting a metal component with an elemental chalcogen precursor in the presence of an organic solvent. The chalcogenide nanoparticles include ternary, binary and / or multinary chalcogenide nanoparticles and the metal component comprises metal halides or elemental metal precursors. The alkylamine solvent has a normal boiling temperature of above about 220° C. and an average particle size of from about 5 nm to about 1000 nm.
Owner:PURDUE RES FOUND INC
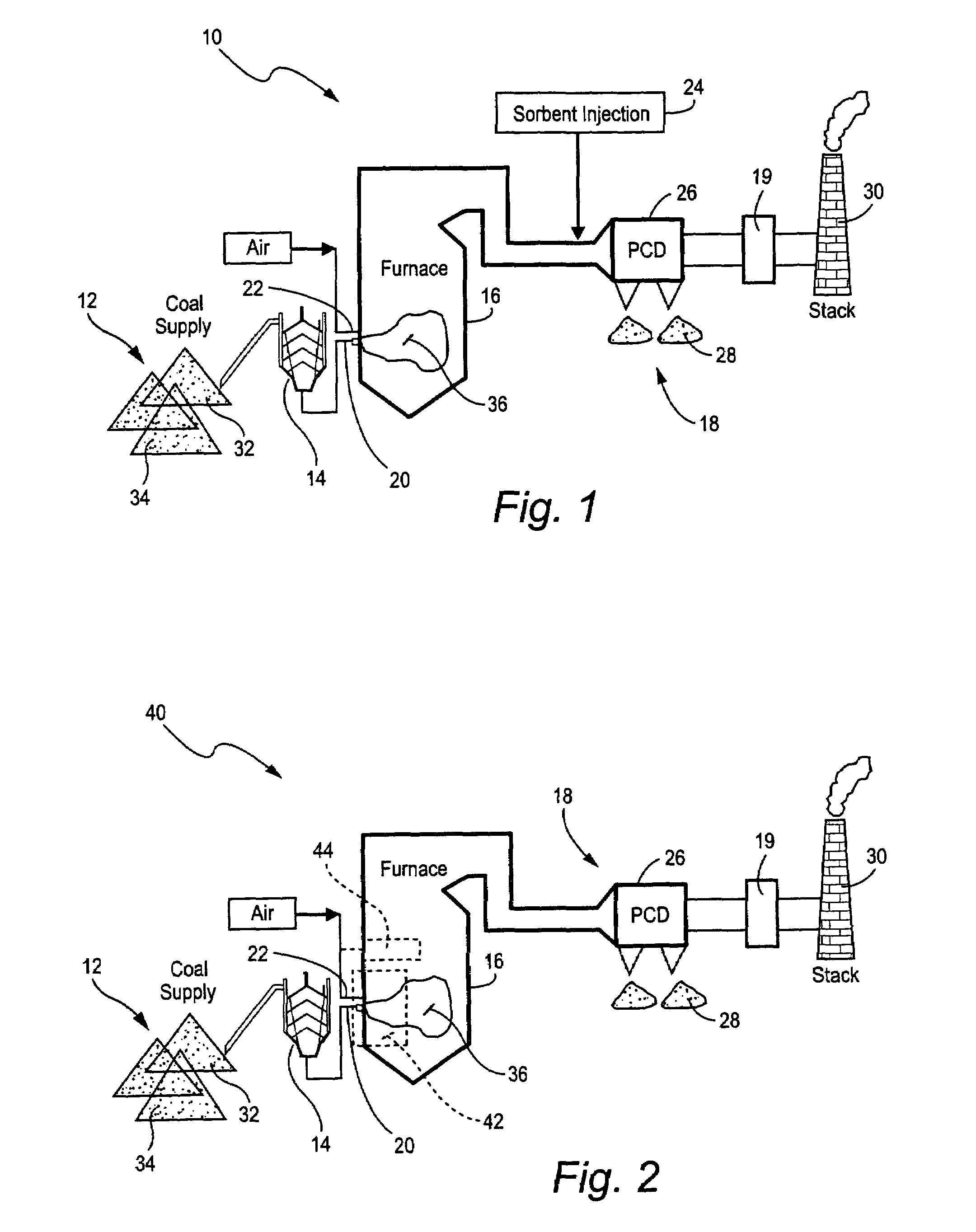
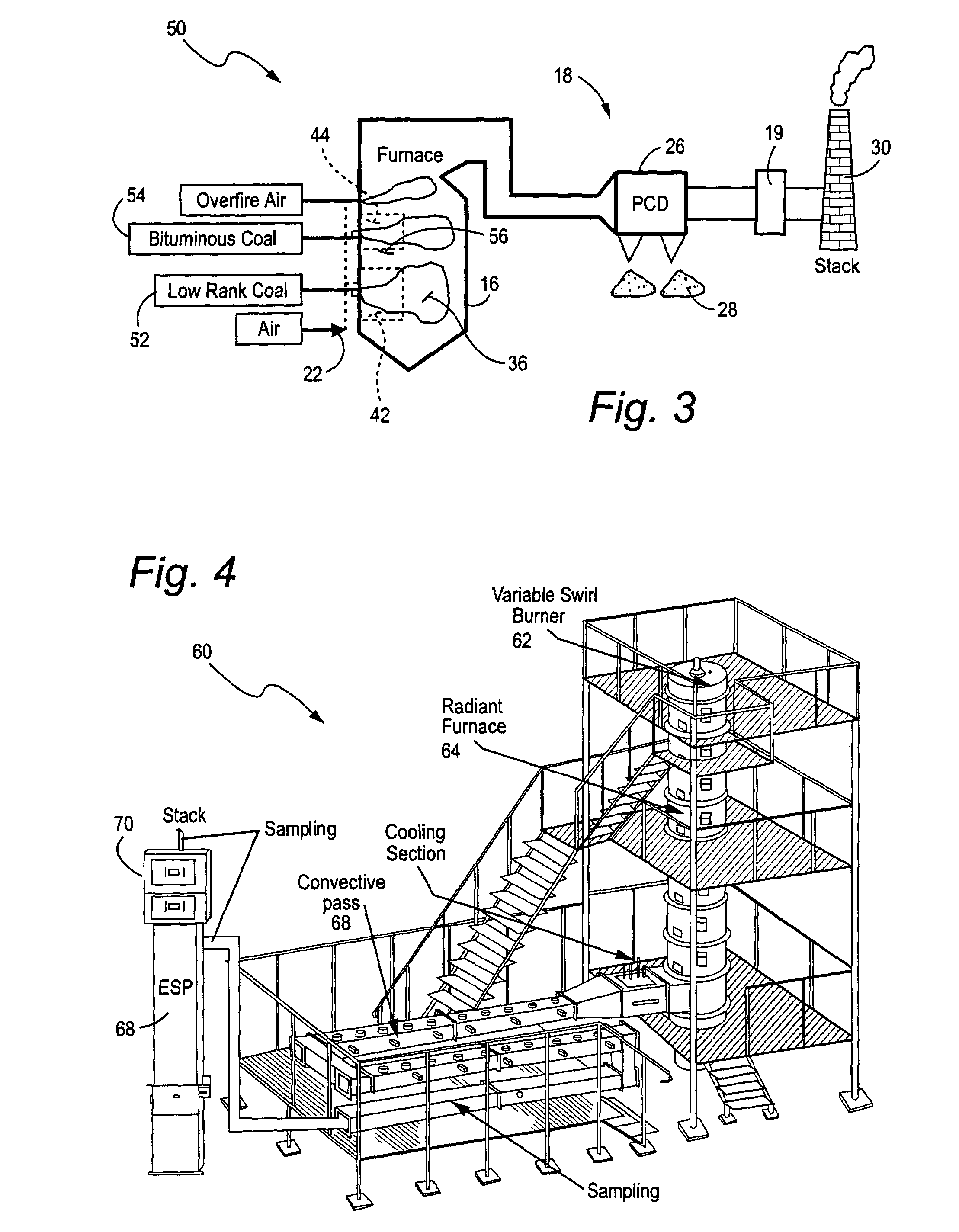
Mercury reduction system and method in combustion flue gas using coal blending
ActiveUS7381387B2Reduce gas emissionsGas treatmentUsing liquid separation agentCombustion systemCombustion
A method to reduce mercury in gas emissions from the combustion of low rank coal in a combustion system including: combusting coal having a low chlorine content in the combustion system, wherein elemental mercury (Hg0) is released in the flue gas produced by the combustion of the low rank coal; releasing chlorine into the flue gas by combusting a coal having a high chlorine in the combustion system; reacting the elemental mercury and released chlorine in the flue gas to oxidize the mercury; adsorbing at least a portion of the oxidized mercury generated by the combustion of the coal with an adsorbent in the flue gas, and collecting the adsorbent with the oxidized mercury in a combustion waste treatment system.
Owner:GENERAL ELECTRIC CO
Synthesis of water soluble non-toxic nanocrystalline quantum dots and uses thereof
An economic, direct synthetic method for producing water soluble ZnS QDs that are ready for bioconjugation is provided. The method can produce aqueous ZnS QDs with emission wavelengths varying from 400 nm to 700 nm. Highly luminescent metal sulfide (MS) QDs are produced via an aqueous synthesis route. MS QDs are capped with thiol-containing charged molecules in a single step. The resultant MS QDs exhibit the distinctive excitonic photoluminescence desired of QDs and can be fabricated to avoid undesirable broadband emissions at higher wavelengths. The aqueous ZnS QDs are stable in biological fluids over a long period of time. In addition, non-toxic ZnS QDs have been produced with good photoluminescence properties.
Owner:DREXEL UNIV
Electrode active material for secondary battery and method for preparing the same
ActiveUS20110311875A1Minimizing dead volumeHigh-capacity batteryMaterial nanotechnologyGallium/indium/thallium compoundsParticulatesLithium oxide
The disclosure relates to an electrode active material including: (a) first particulate of a metal (or metalloid) oxide alloyable with lithium; and (b) second particulate of an oxide containing lithium and the same metal (or metalloid) as that of the metal (or metalloid) oxide, and to a secondary battery including the electrode active material. When the electrode active material is used as an anode active material, reduced amounts of an irreversible phase such as a lithium oxide or a lithium metal oxide are produced during initial charge-discharge of a battery since lithium is already contained in the second particulate before the initial charge-discharge, and thus a dead volume on the side of the cathode can be minimized and a high-capacity battery can be fabricated.
Owner:LG ENERGY SOLUTION LTD
Hydrometallurgical process for the treatment of metal-bearing sulfide mineral concentrates
InactiveUS20070098609A1Improve efficiencyReduce the amount requiredSolvent extractionGold compoundsAmmonium compoundsMetallic sulfide
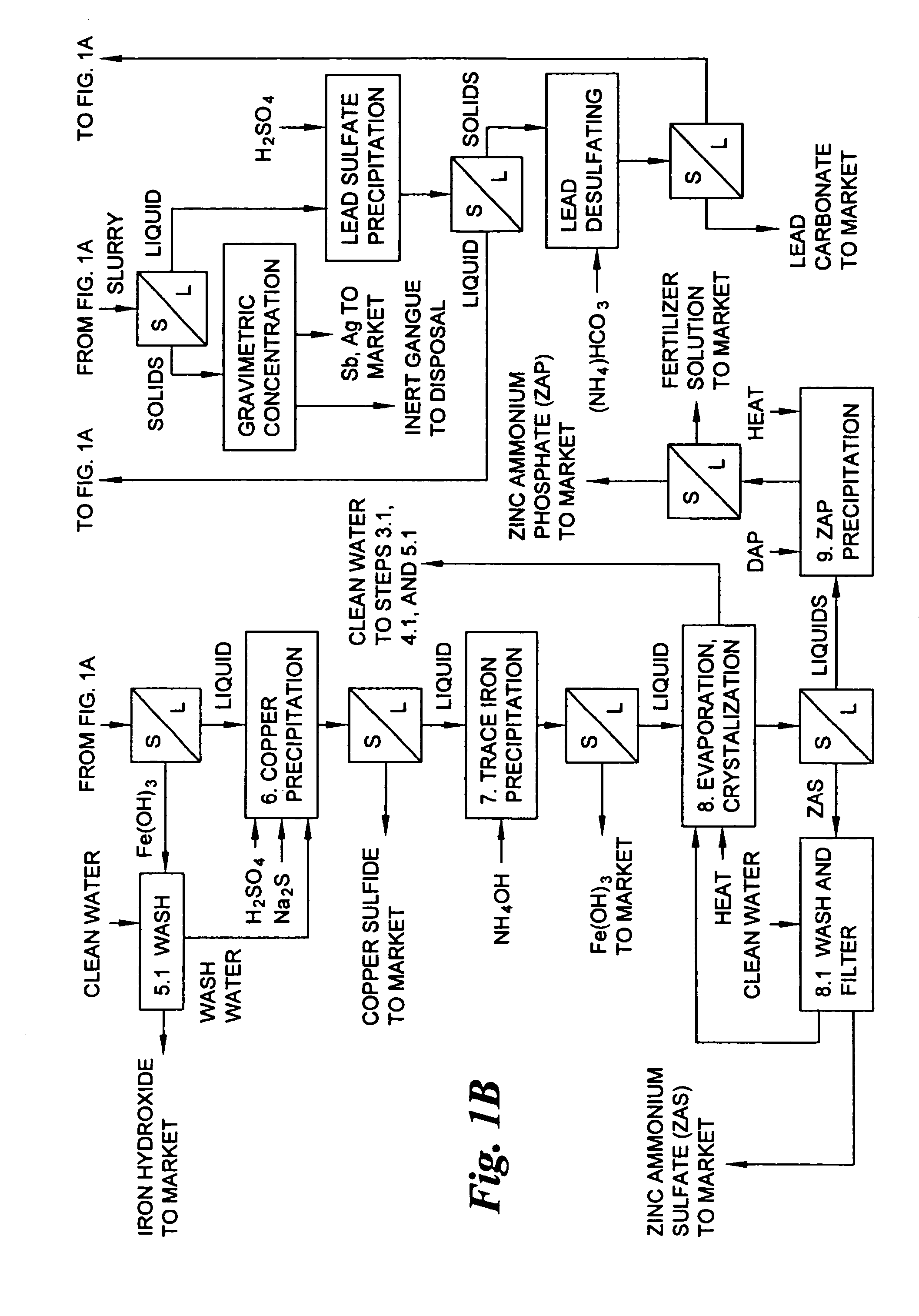
A hydrometallurgical process for the treatment of complex silver-bearing sulfide ores and concentrates that recovers substantially all silver, lead, antimony, zinc, copper and sulfur, along with the chemical reagents utilized during the process. Finely ground ores and concentrates are leached under heat and pressure with water, sulfuric acid, nitric acid, oxygen, and a catalyst, and are further treated to recover silver in the form of silver chloride; iron in the form of iron hydroxide; copper and all traces of soluble toxic metals as sulfides; zinc as zinc ammonium sulfate and specifically nitric acid, sulfuric acid, oxygen, ammonia, and ammonium compounds as valuable fertilizer products.
Owner:ROYAL SILVER PANAMA
In Situ Immobilization of Metals in Contaminated Sites Using Stabilized Nanoparticles
InactiveUS20070203388A1Low costProcess environmental protectionMaterial nanotechnologyWater treatment compoundsCelluloseNanoparticle
A method for preparing a class of highly stabilized and soil-dispersible nanoparticles and using the nanoparticles as a remediation technology for immobilizing toxic metals at toxic metal contaminated sites. The method employs a composition containing select polysaccharides (starch or cellulose) as a stabilizer for the nanoparticles in a liquid carrier, and results in suspensions of nanoparticles of desired size and mobility in water, soils or sediments. The stabilizer can facilitate controlling the dispersibility of the nanoparticles in the liquid carrier. An effective amount of the composition is delivered to a contaminated site so that the nanoparticles can immobilize one or more toxic metals of the contaminated site.
Owner:AUBURN UNIV
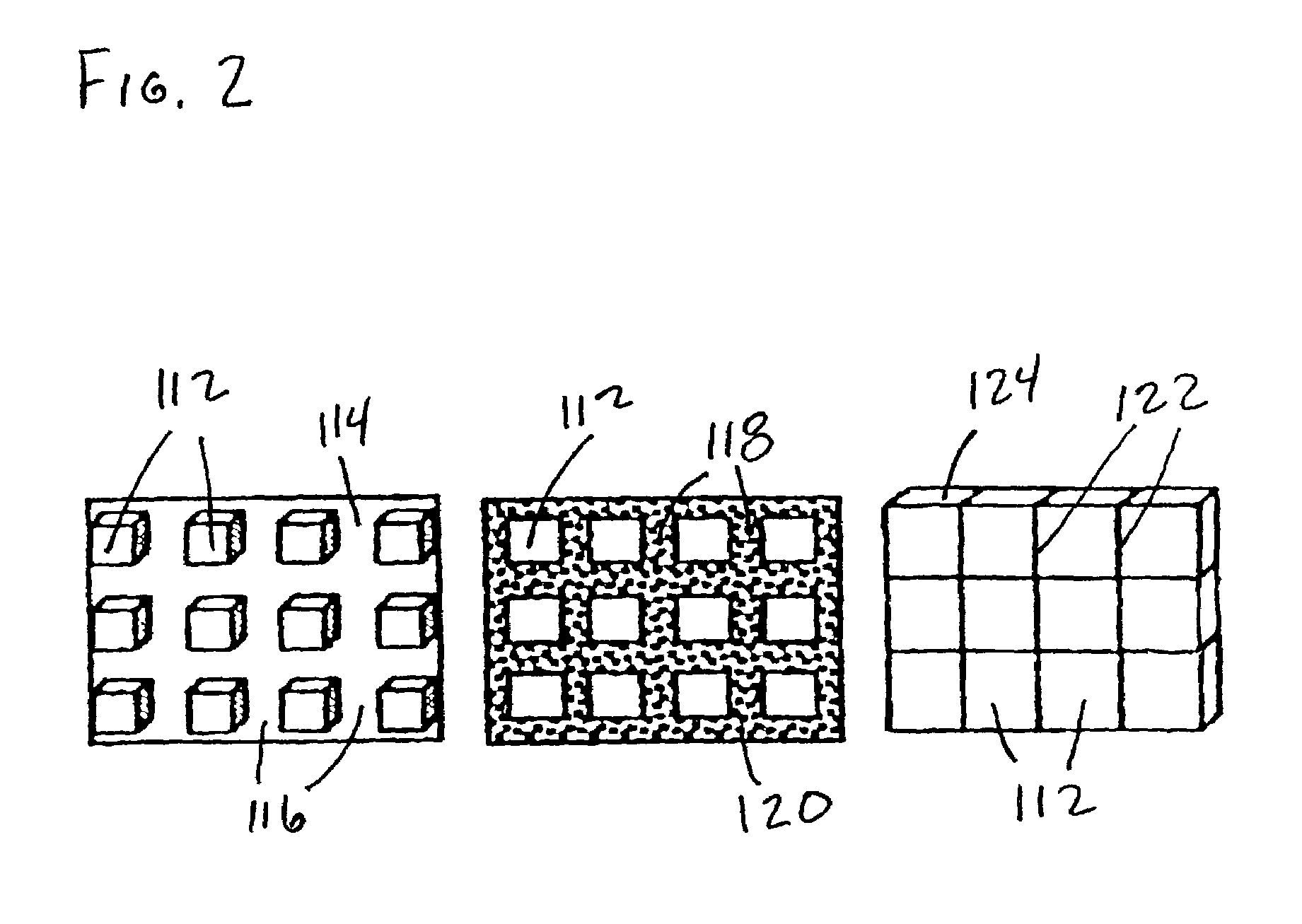
Process for producing nano-powders and powders of nano-particle loose aggregate
InactiveUS7238331B2Speed up the processSimple structureCalcium/strontium/barium carbonatesMaterial nanotechnologyPrillNanoparticle
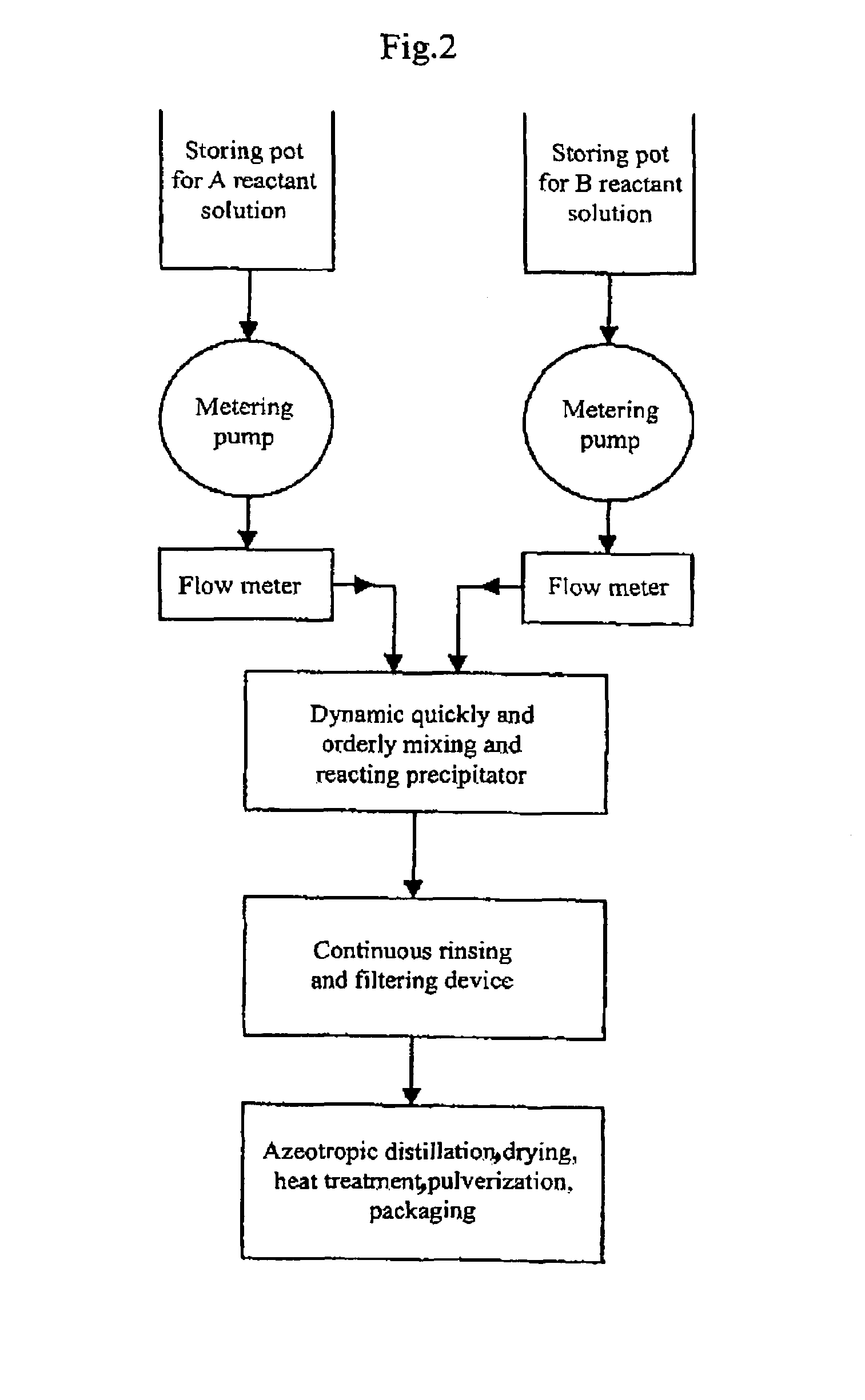
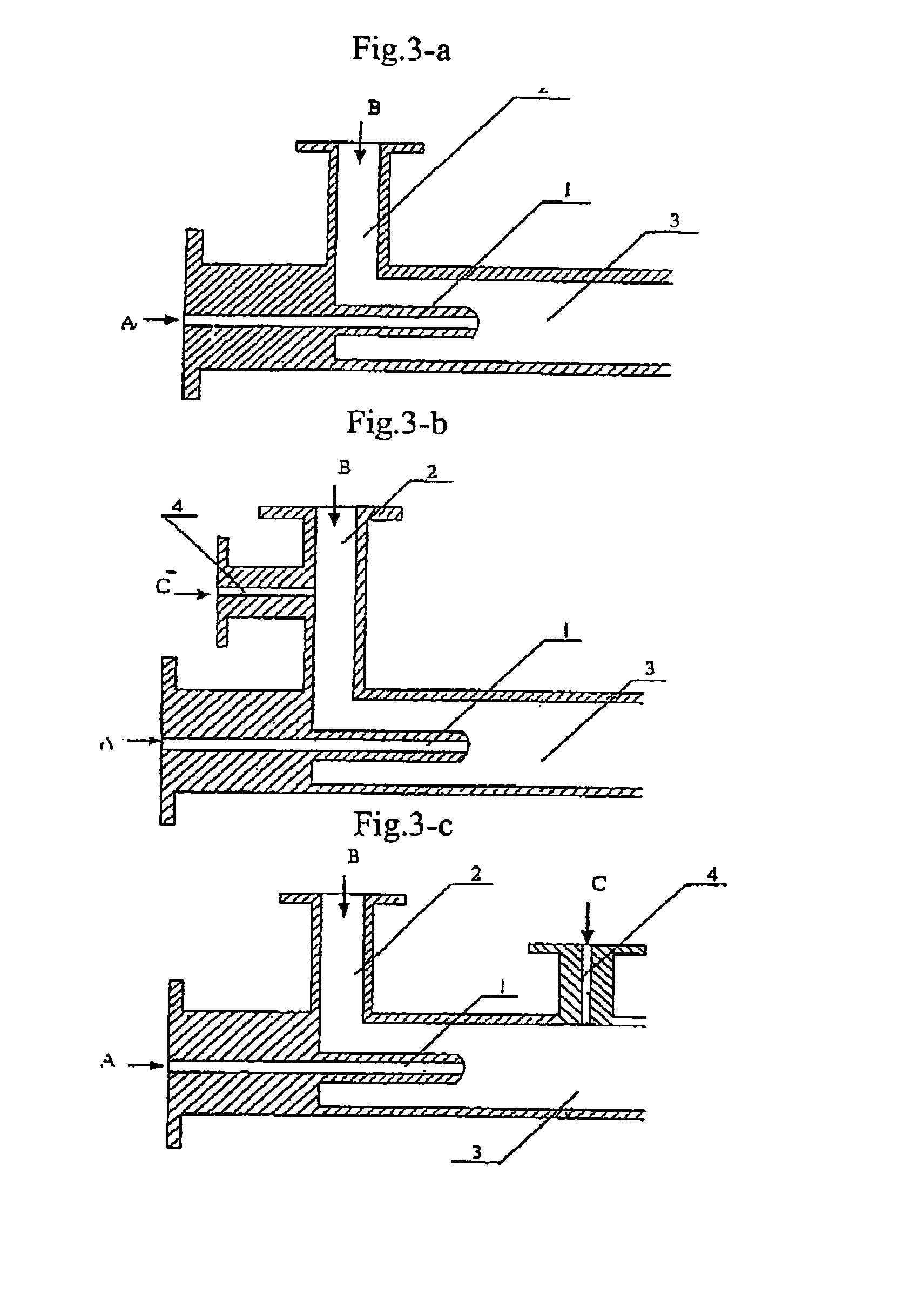
The present invention discloses a process for producing nano-powders and powders of nano-particle loose aggregate, which includes: (a) providing at least two reactant solutions A and B capable of rapidly reacting to form deposits; (b) supplying the at least two reactant solutions A and B at least at the reaction temperature into a mixing and reaction precipitator respectively, in which mixing reaction and precipitation are continuously carried out in sequence, the mixing and reaction precipitator being selected from at least one of a tubular ejection mixing reactor, a tubular static mixing reactor and an atomization mixing reactor; and (c) treating the deposit-containing slurry continuously discharged from the mixing reaction precipitator.
Owner:UNIV OF SCI & TECH LIAONING
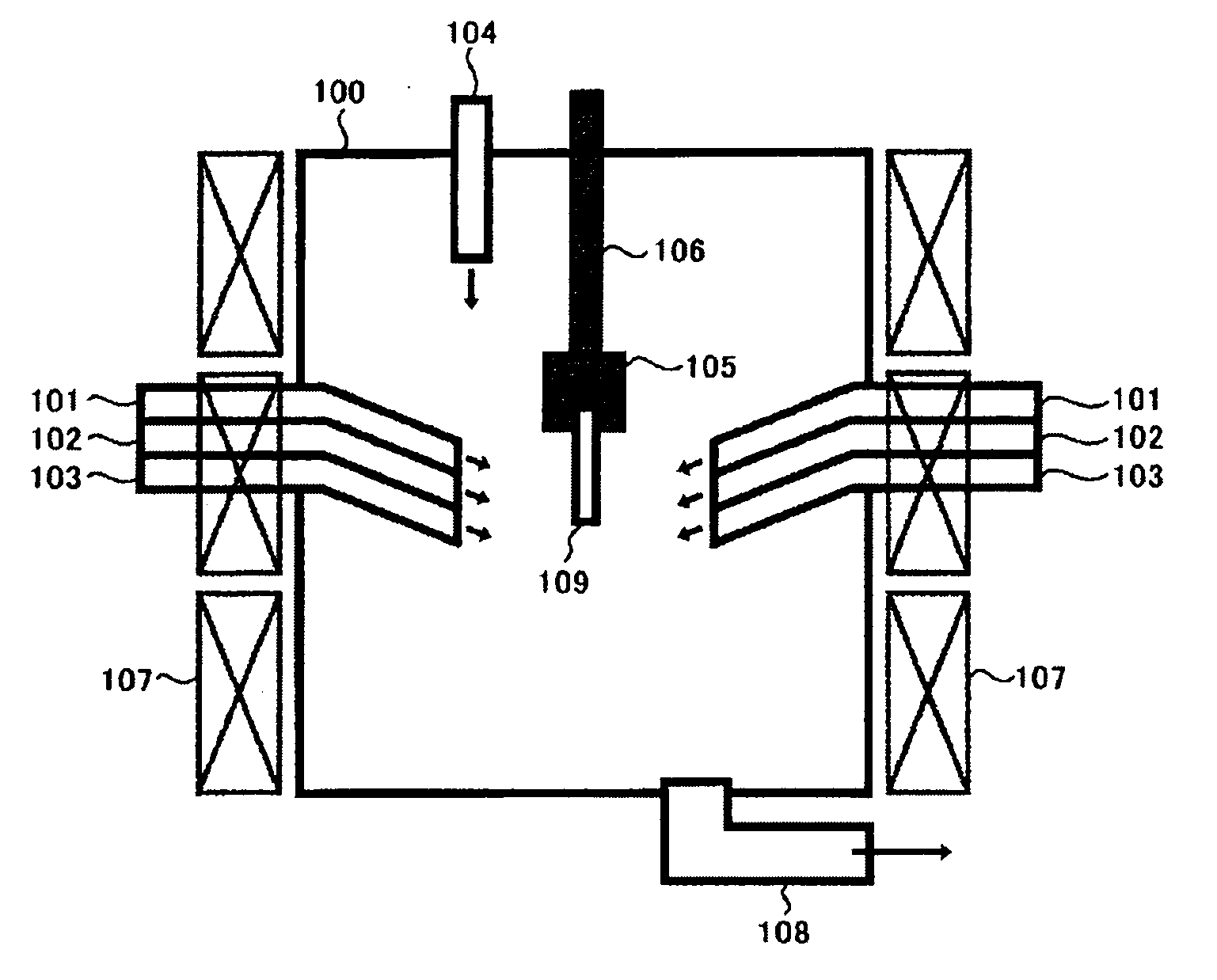
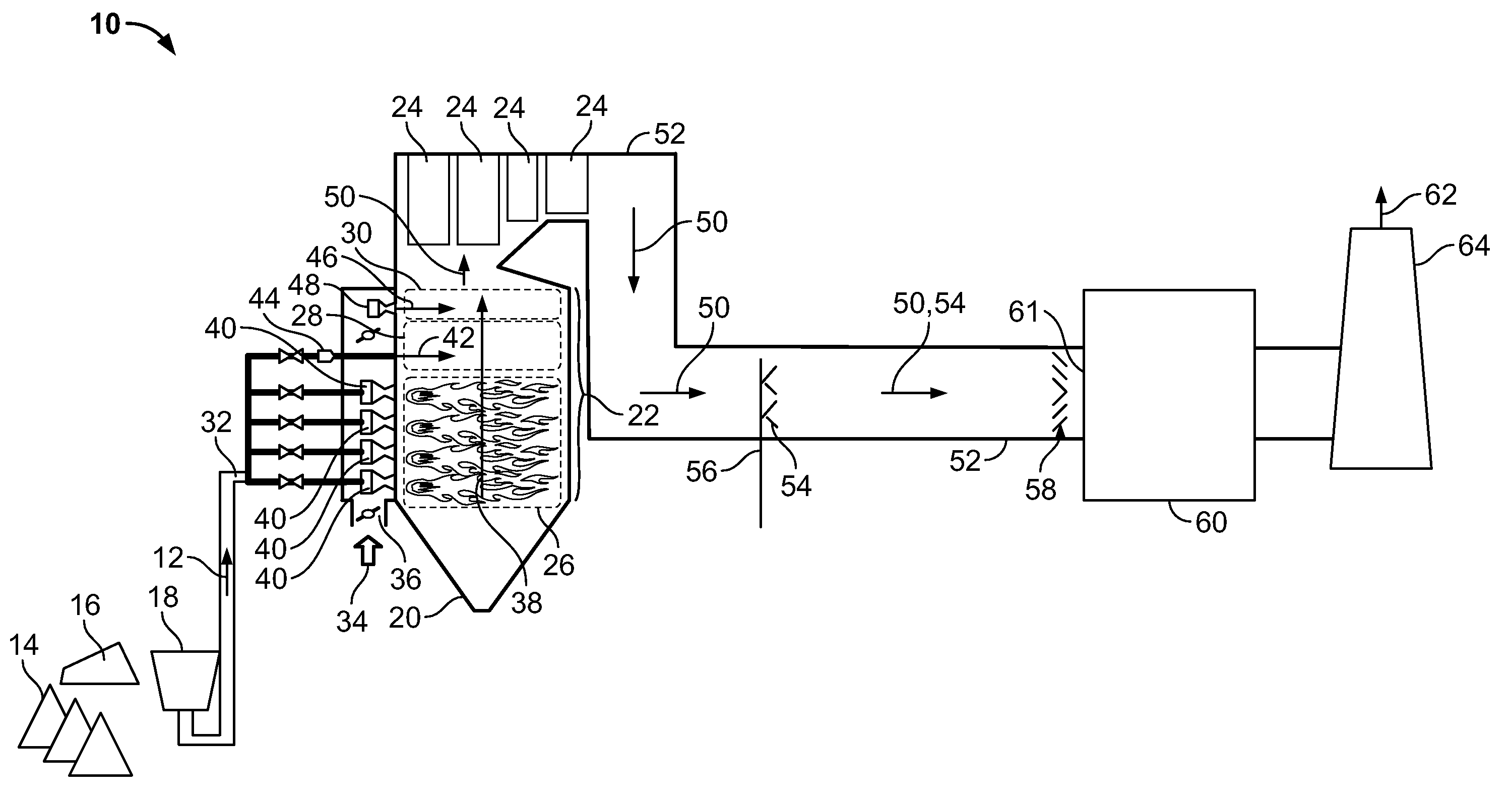
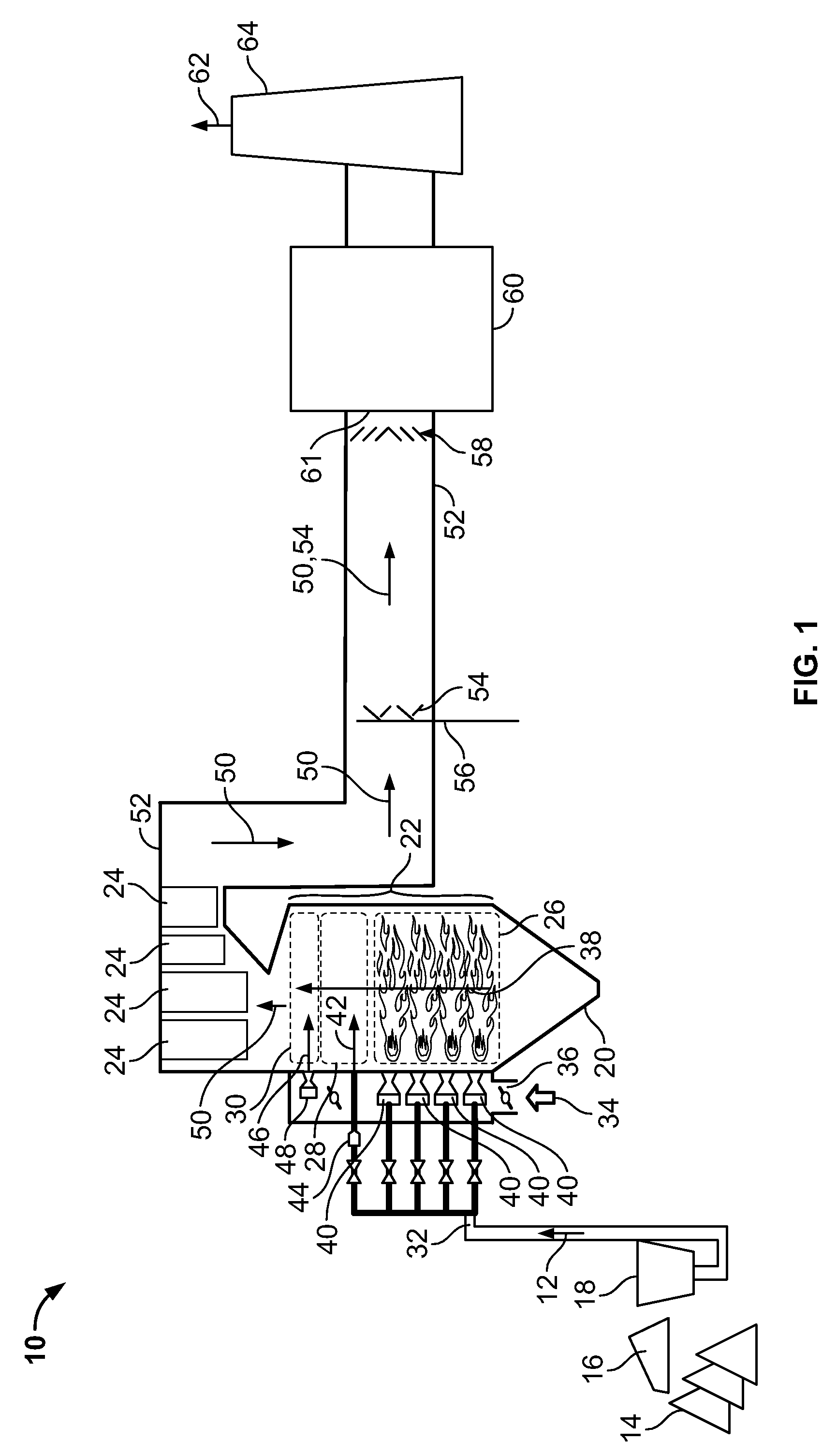
Method and apparatus for removing mercury from combustion exhaust gas
A method for reducing mercury emissions in combustion flue gas is provided. The method includes combusting coal such that a flue gas flow is created. The flue gas flow includes at least mercury and carbon-containing fly ash. The method further includes cooling the flue gas flow within a duct and creating turbulence in the flue gas flow. The mercury is removed from the flue gas flow.
Owner:GENERAL ELECTRIC CO
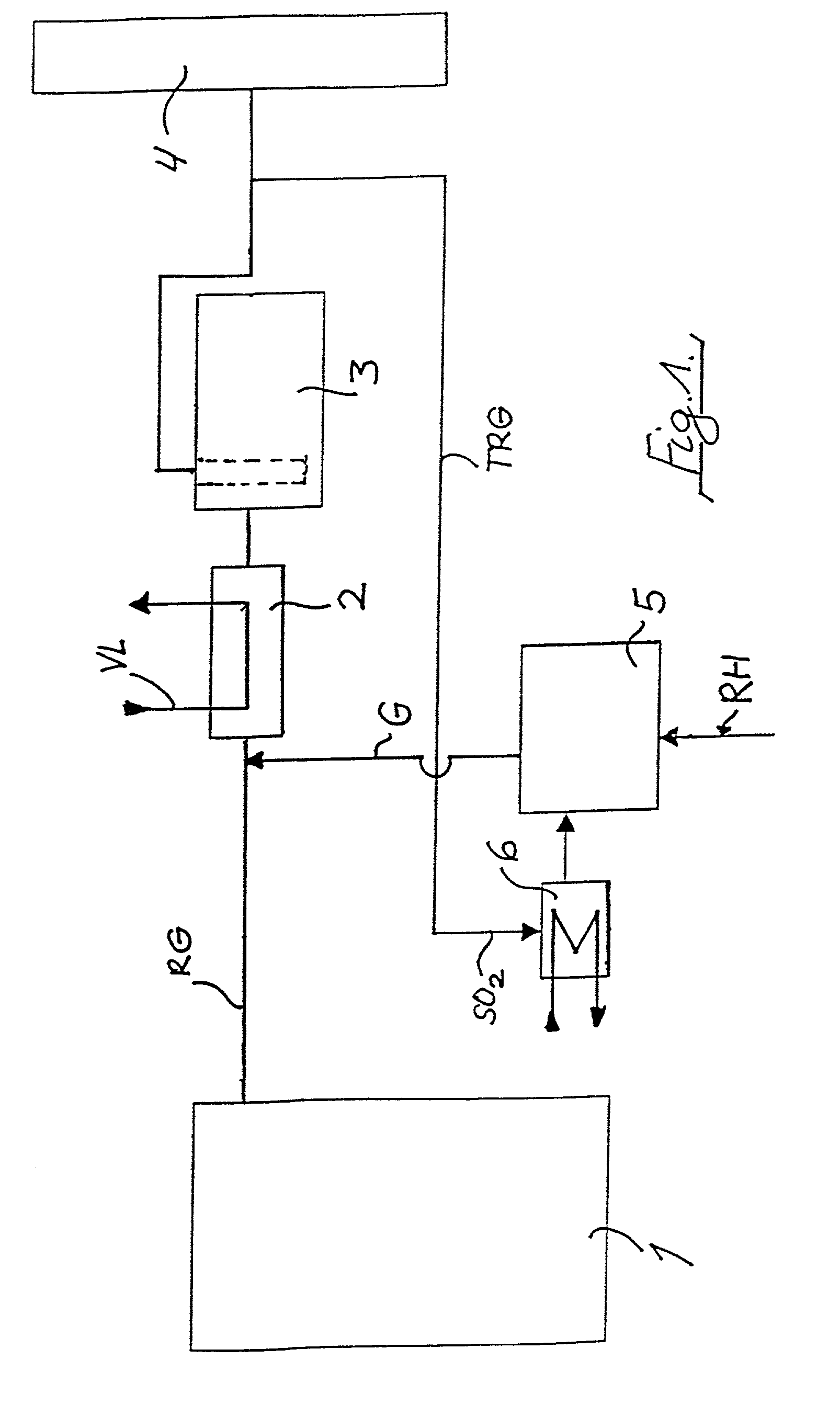
Method of removing mercury from flue gases
A method of removing metallic mercury and ionic mercury from flue gases, especially of a power plant, is provided. A gas that contains sulfur dioxide, or other adequate amounts of sulfur in the form of H2S or COS, and a gas that contains hydrogen, are conveyed to a catalyzer for producing a gas that contains elemental sulfur and hydrogen sulfide. This gas is conveyed to flue gas upstream of a separator, wherein mercury in the flue gas reacts with the sulfur and ionic sulfur in the gas and is separated out in the separator.
Owner:FISIA DEUT GMBH AND +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com