Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69results about "Boron halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
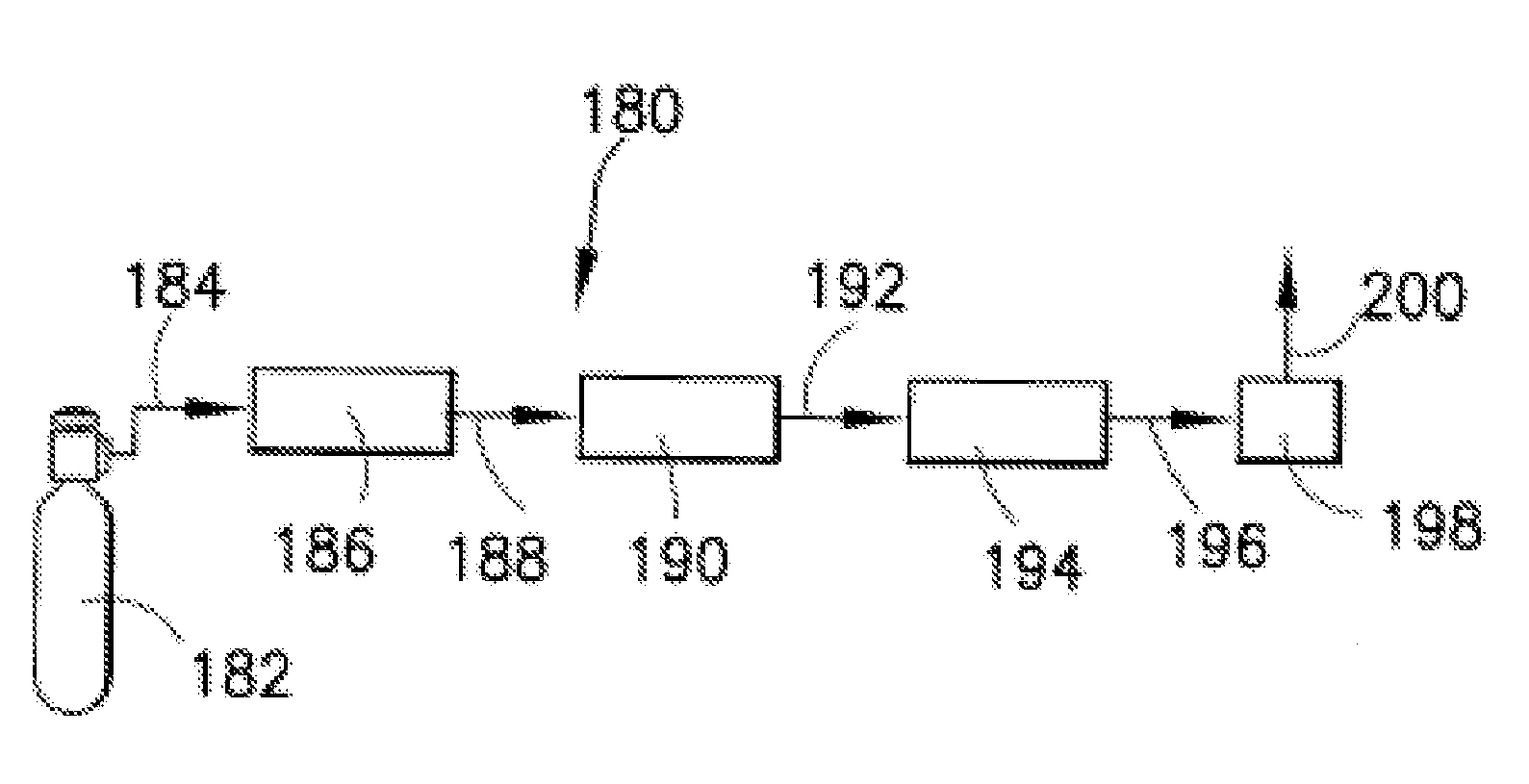
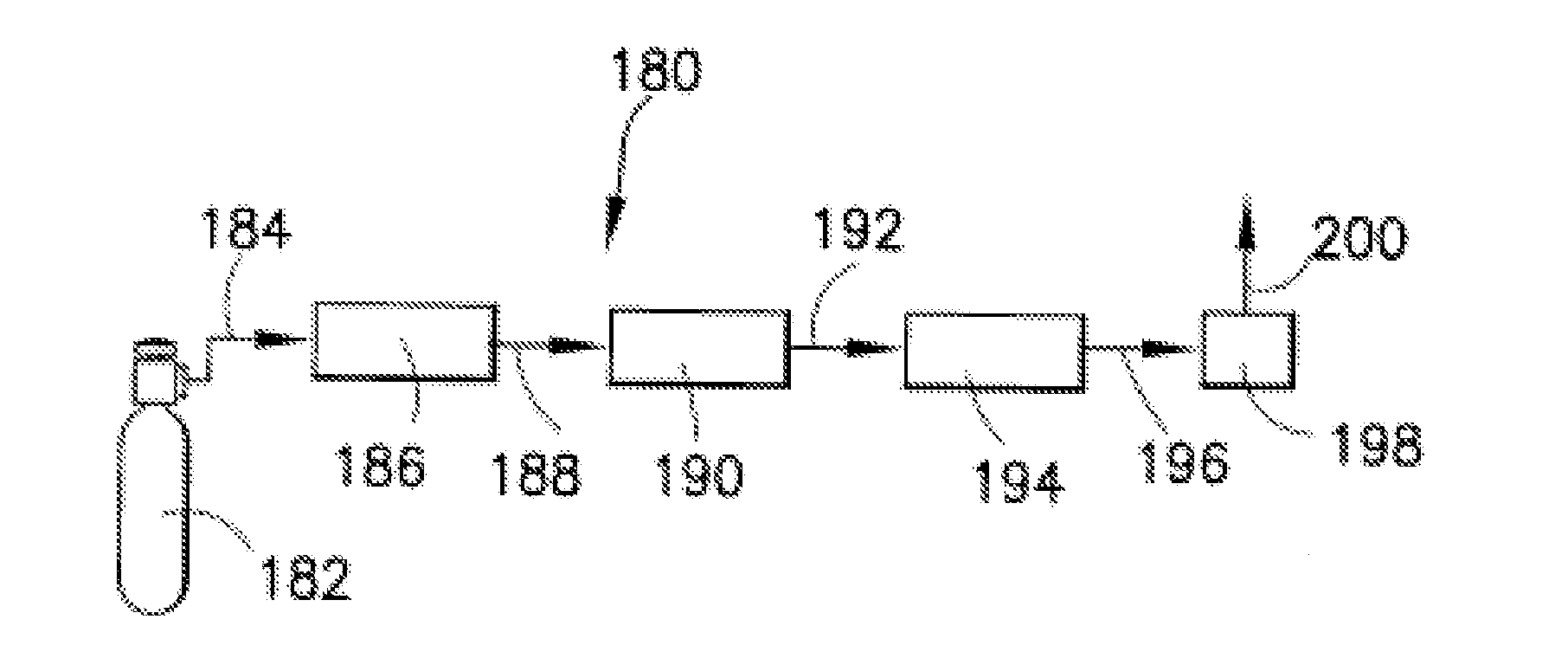
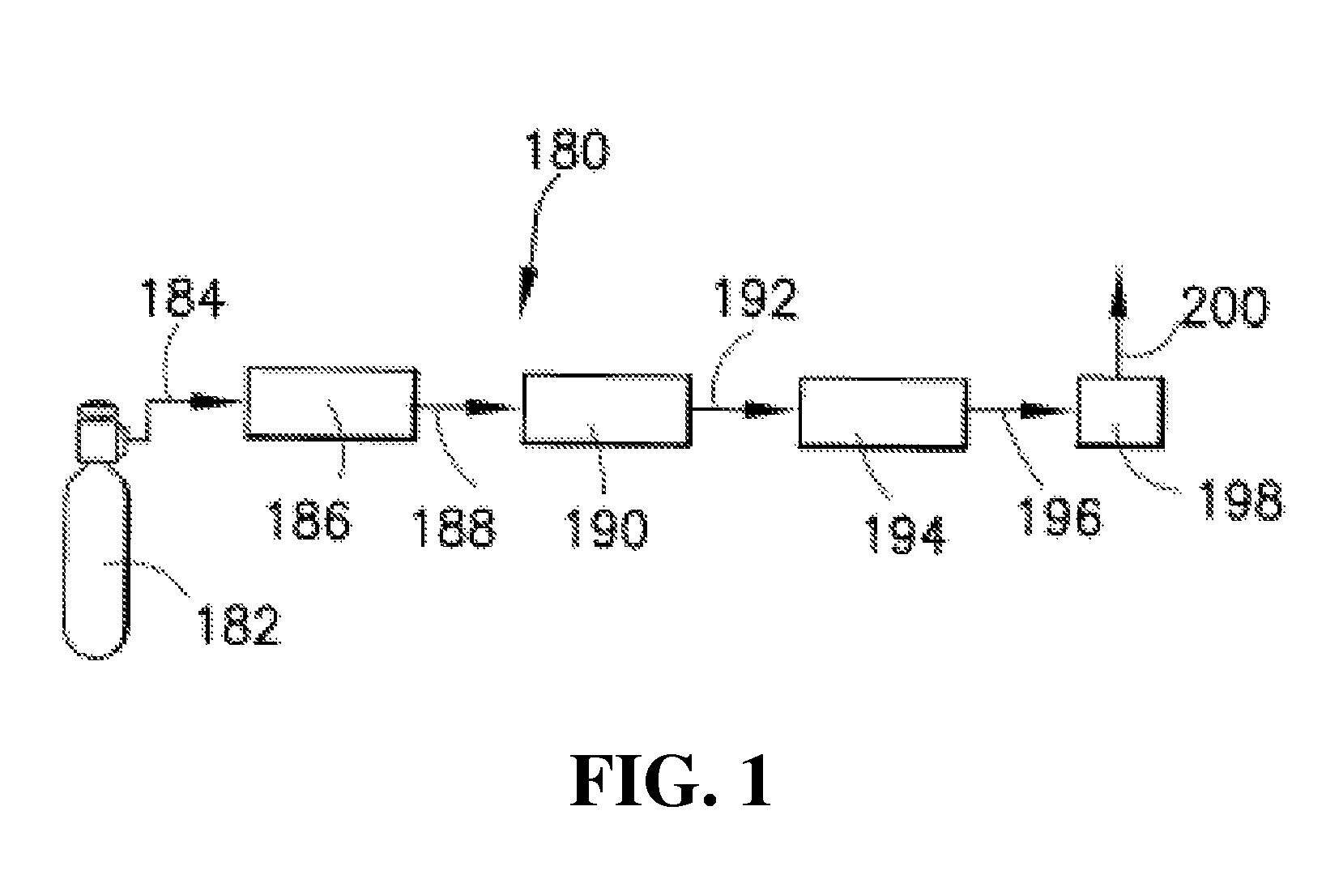
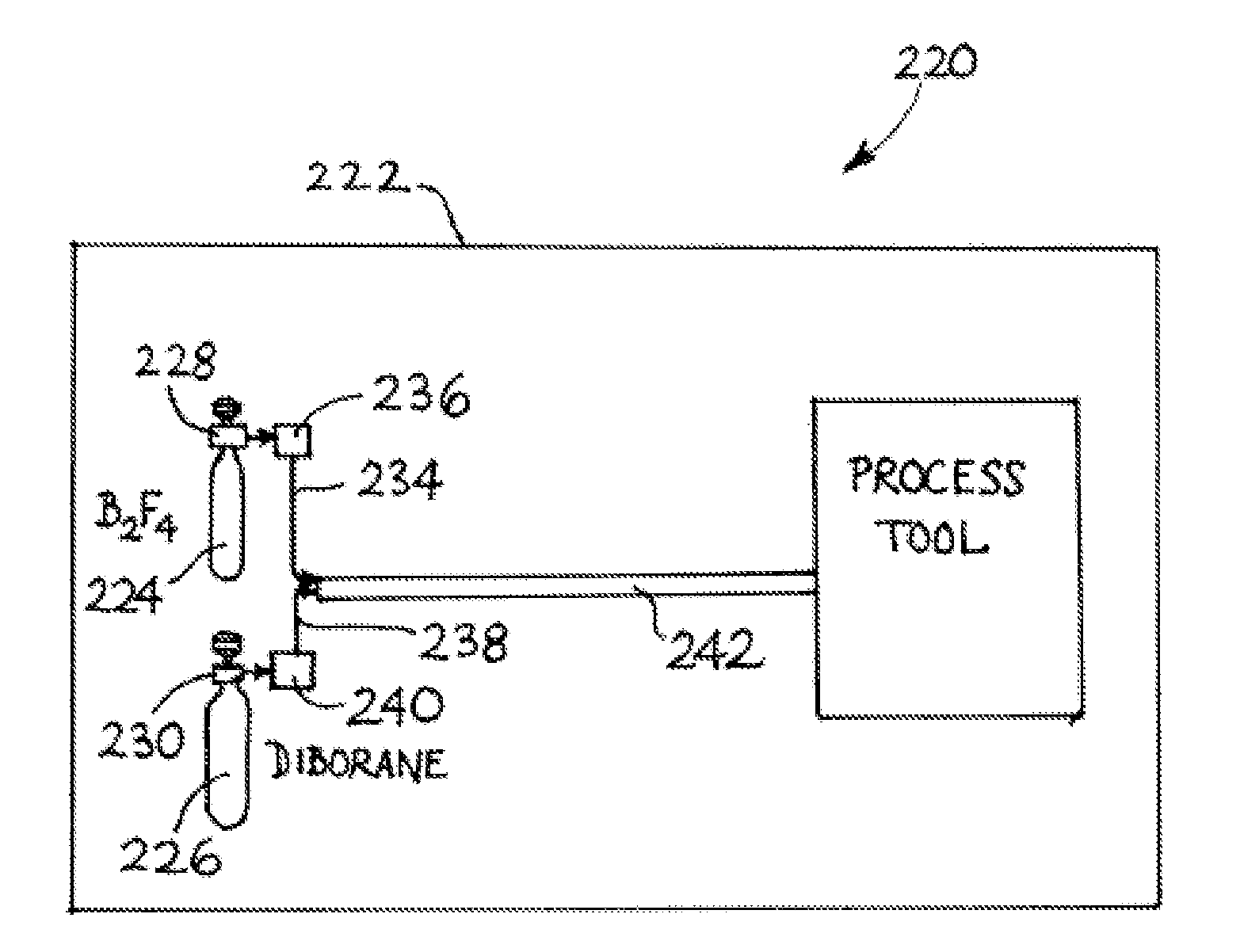
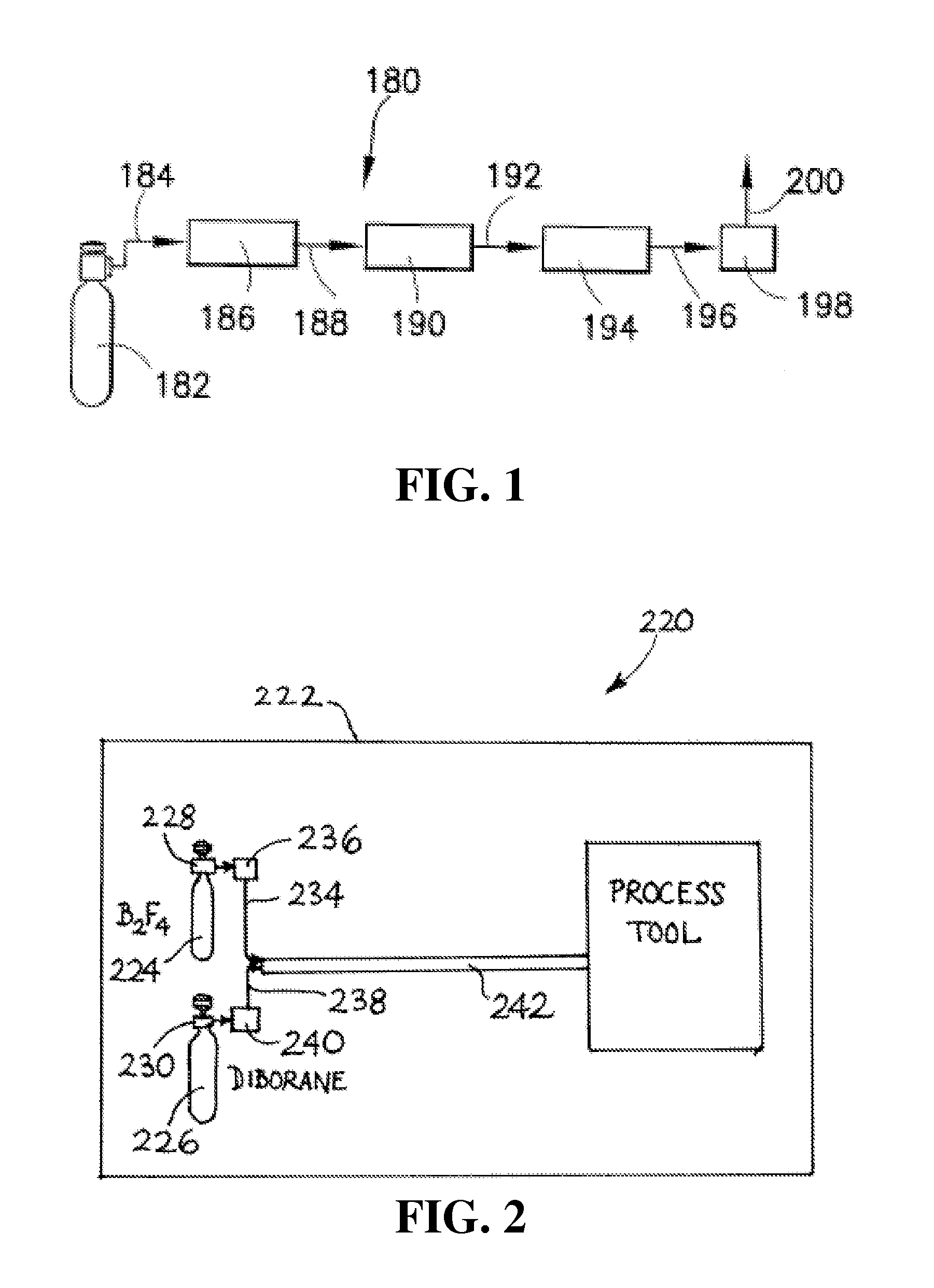
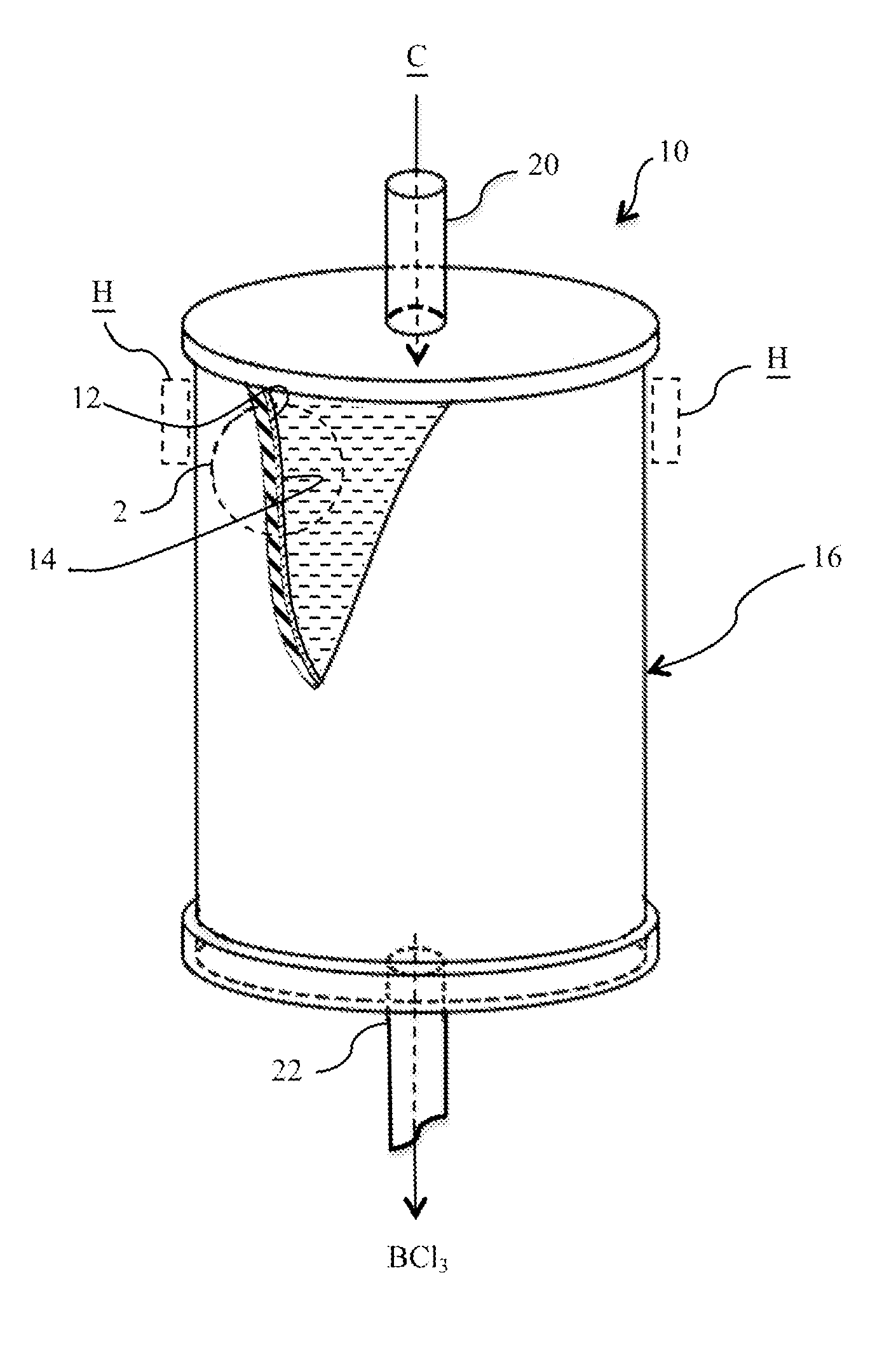
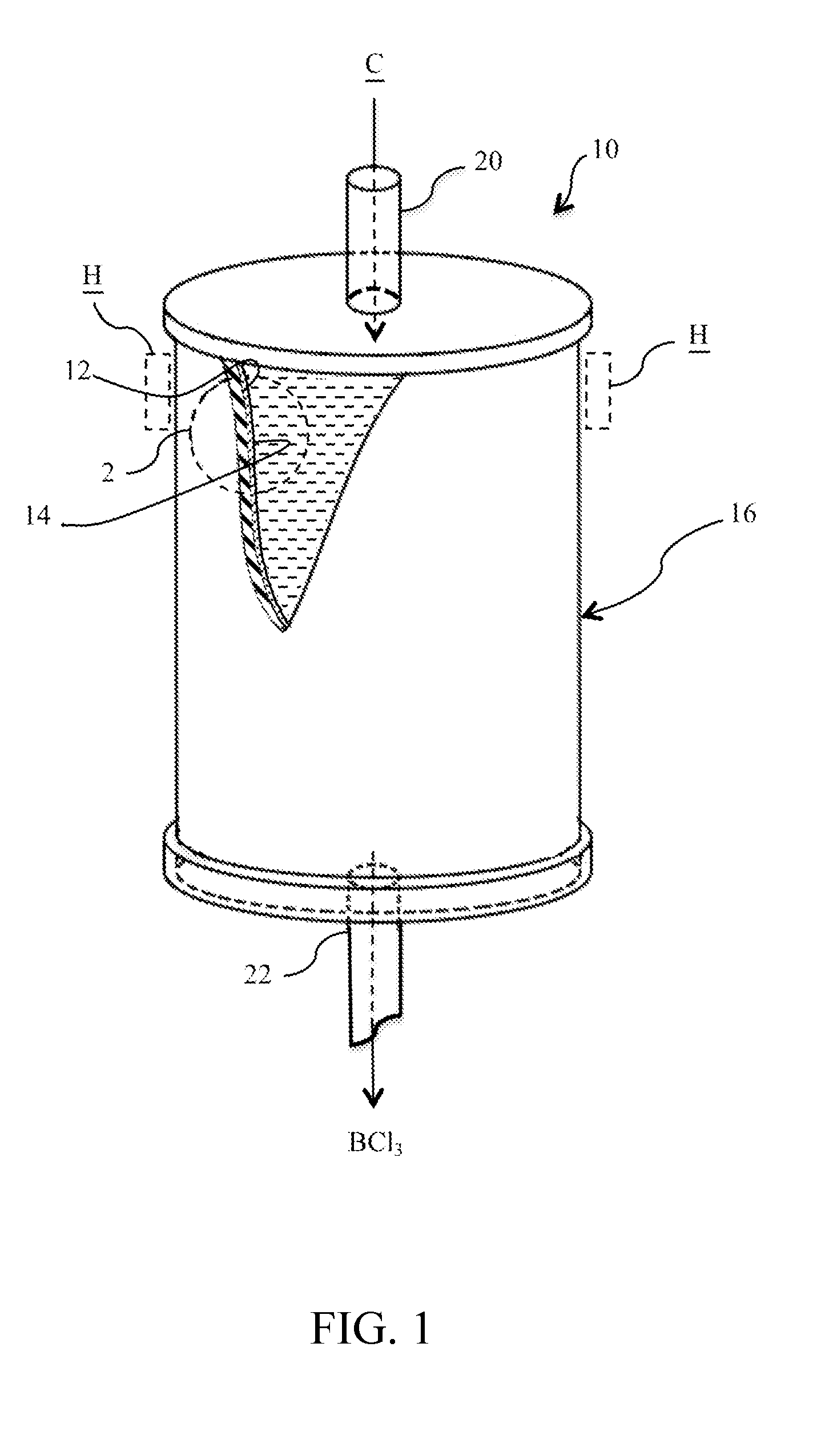
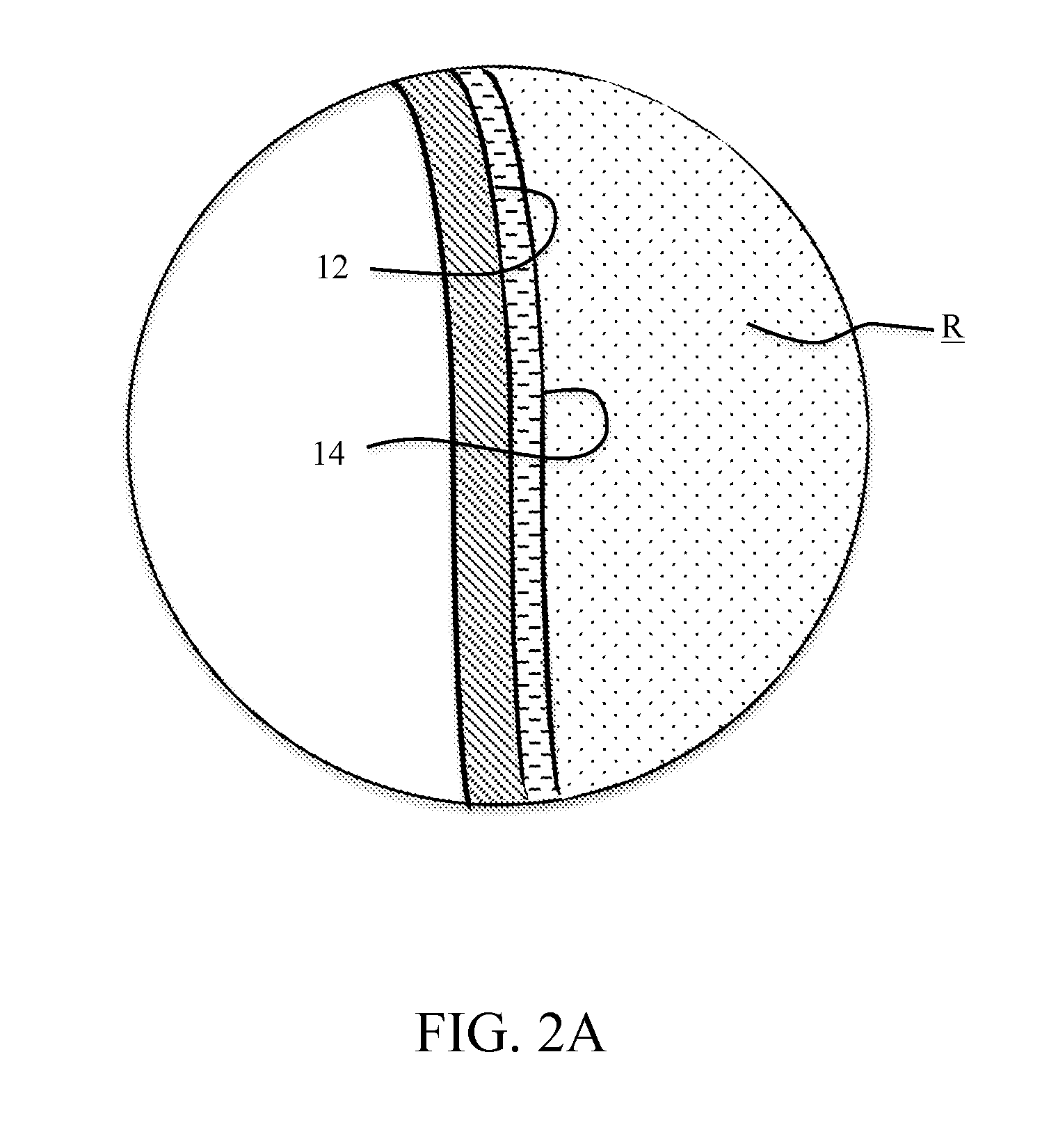
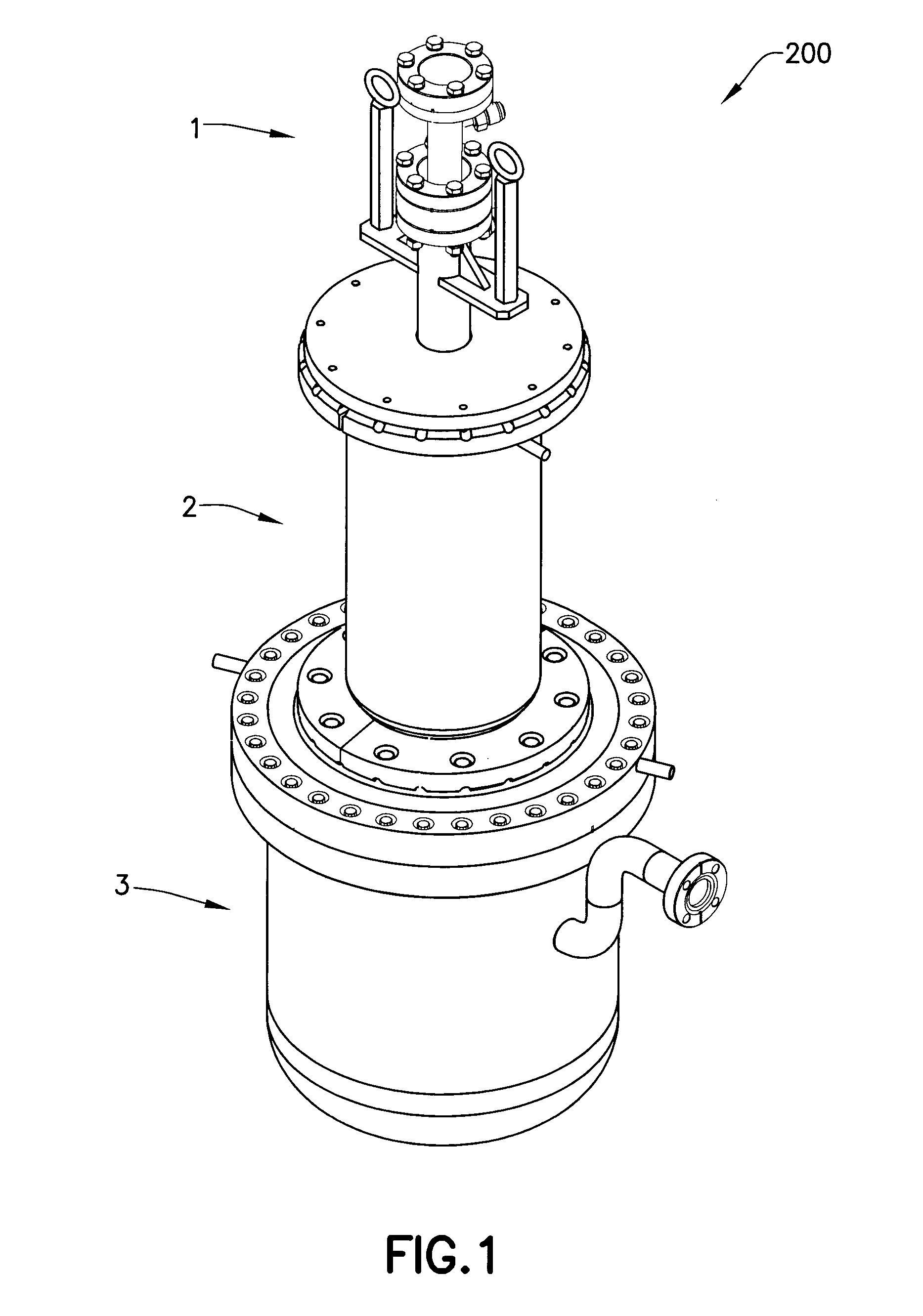
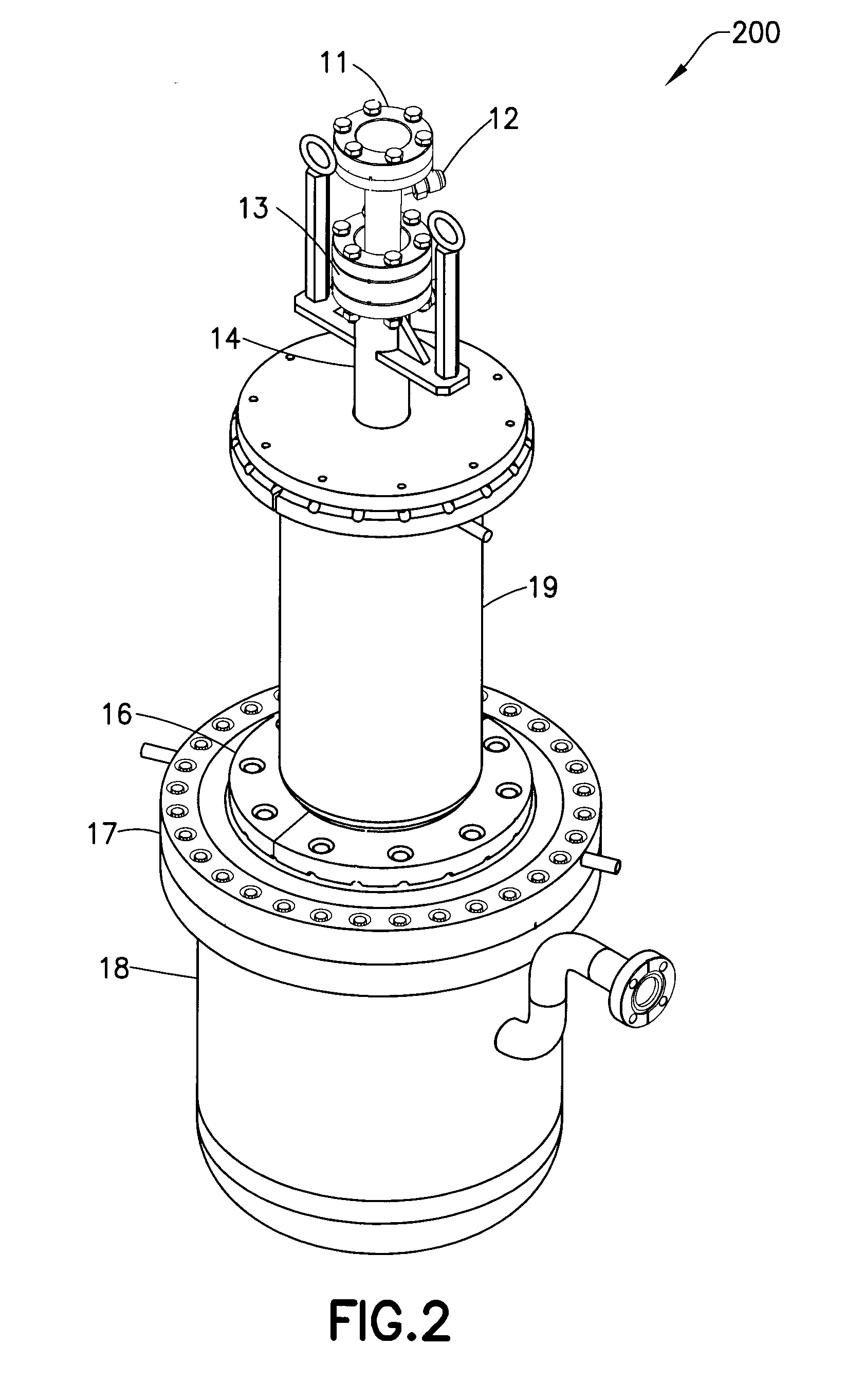
Isotopically-enriched boron-containing compounds, and methods of making and using same
ActiveUS20110159671A1Electric discharge tubesSemiconductor/solid-state device manufacturingSynthesis methodsChemical compound
An isotopically-enriched, boron-containing compound comprising two or more boron atoms and at least one fluorine atom, wherein at least one of the boron atoms contains a desired isotope of boron in a concentration or ratio greater than a natural abundance concentration or ratio thereof. The compound may have a chemical formula of B2F4. Synthesis methods for such compounds, and ion implantation methods using such compounds, are described, as well as storage and dispensing vessels in which the isotopically-enriched, boron-containing compound is advantageously contained for subsequent dispensing use.
Owner:ENTEGRIS INC
Isotopically-enriched boron-containing compounds, and methods of making and using same
ActiveUS20120108044A1Improving beam currentIncrease currentElectric discharge tubesVacuum evaporation coatingSynthesis methodsChemical compound
An isotopically-enriched, boron-containing compound comprising two or more boron atoms and at least one fluorine atom, wherein at least one of the boron atoms contains a desired isotope of boron in a concentration or ratio greater than a natural abundance concentration or ratio thereof. The compound may have a chemical formula of B2F4. Synthesis methods for such compounds, and ion implantation methods using such compounds, are described, as well as storage and dispensing vessels in which the isotopically-enriched, boron-containing compound is advantageously contained for subsequent dispensing use.
Owner:ENTEGRIS INC
Isotopically-enriched boron-containing compounds, and methods of making and using same
An isotopically-enriched, boron-containing compound comprising two or more boron atoms and at least one fluorine atom, wherein at least one of the boron atoms contains a desired isotope of boron in a concentration or ratio greater than a natural abundance concentration or ratio thereof. The compound may have a chemical formula of B2F4. Synthesis methods for such compounds, and ion implantation methods using such compounds, are described, as well as storage and dispensing vessels in which the isotopically-enriched, boron-containing compound is advantageously contained for subsequent dispensing use.
Owner:ENTEGRIS INC
Isotopically-enriched boron-containing compounds, and methods of making and using same
ActiveUS8598022B2Electric discharge tubesVacuum evaporation coatingSynthesis methodsBoron containing
An isotopically-enriched, boron-containing compound comprising two or more boron atoms and at least one fluorine atom, wherein at least one of the boron atoms contains a desired isotope of boron in a concentration or ratio greater than a natural abundance concentration or ratio thereof. The compound may have a chemical formula of B2F4. Synthesis methods for such compounds, and ion implantation methods using such compounds, are described, as well as storage and dispensing vessels in which the isotopically-enriched, boron-containing compound is advantageously contained for subsequent dispensing use.
Owner:ENTEGRIS INC
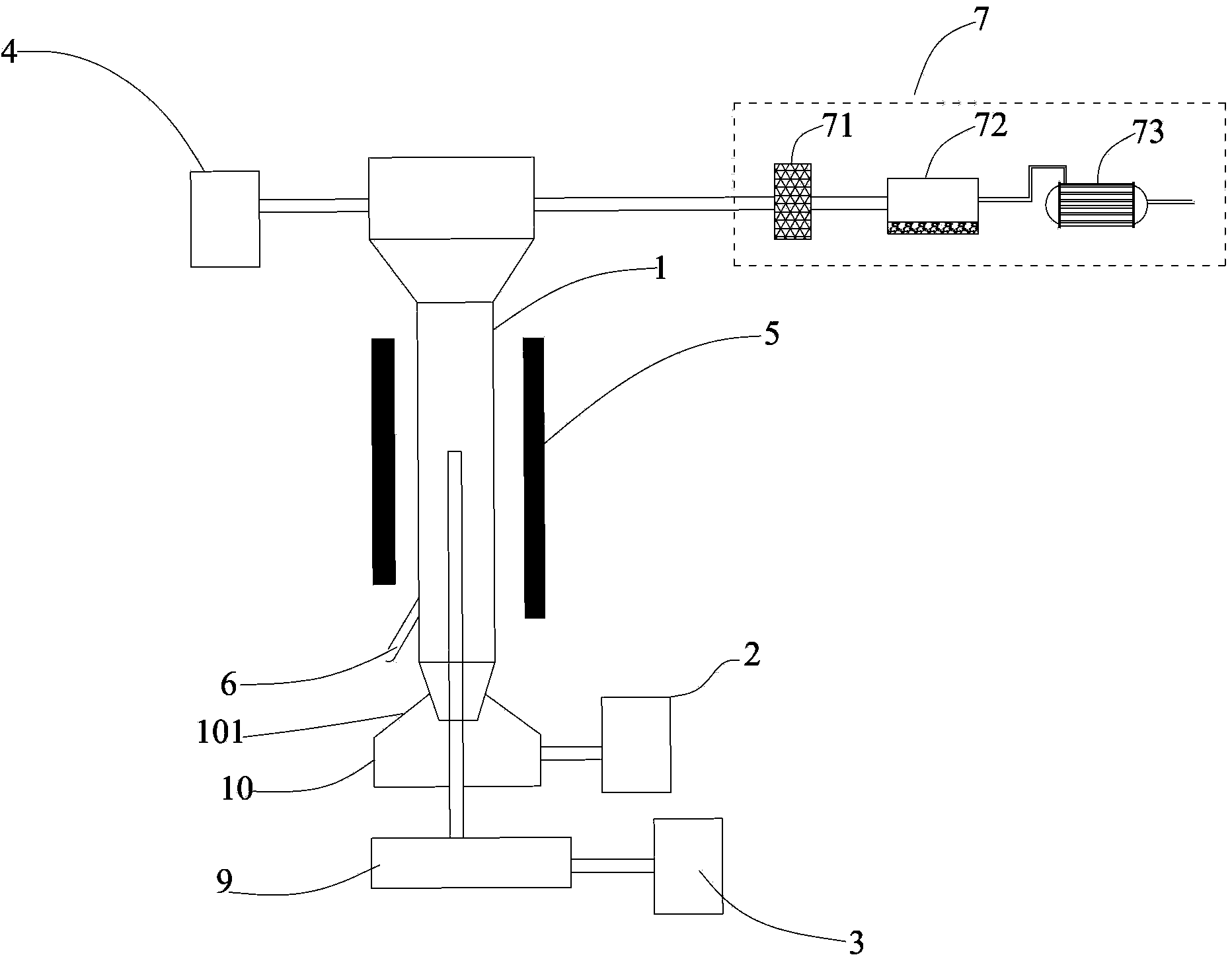
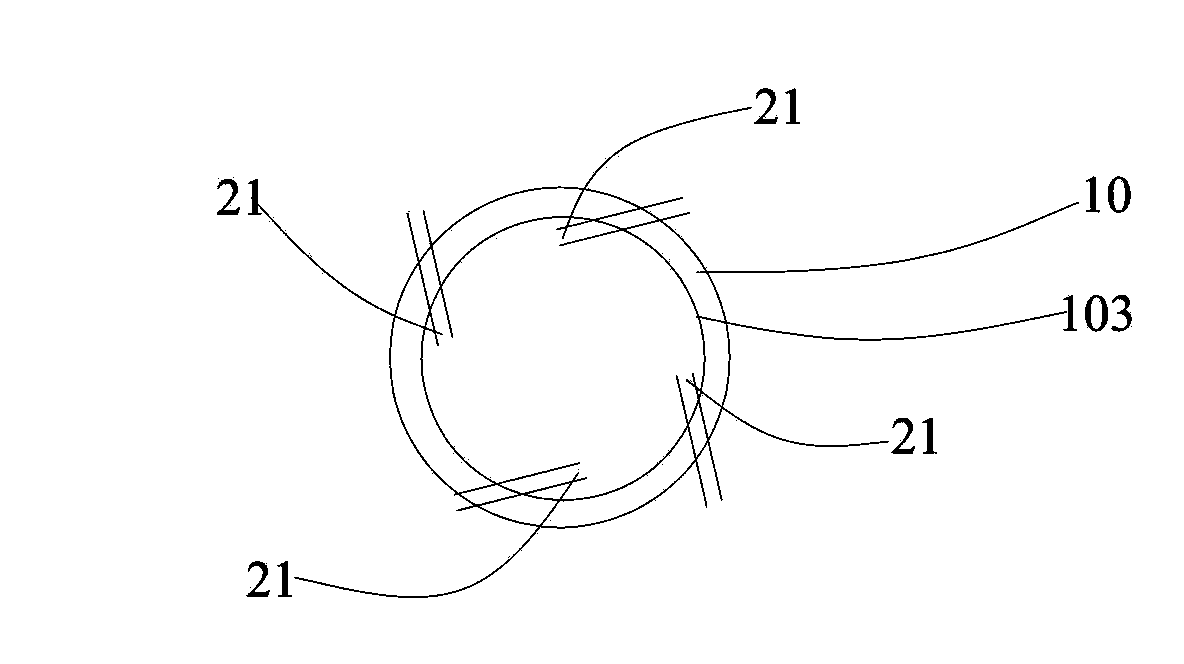
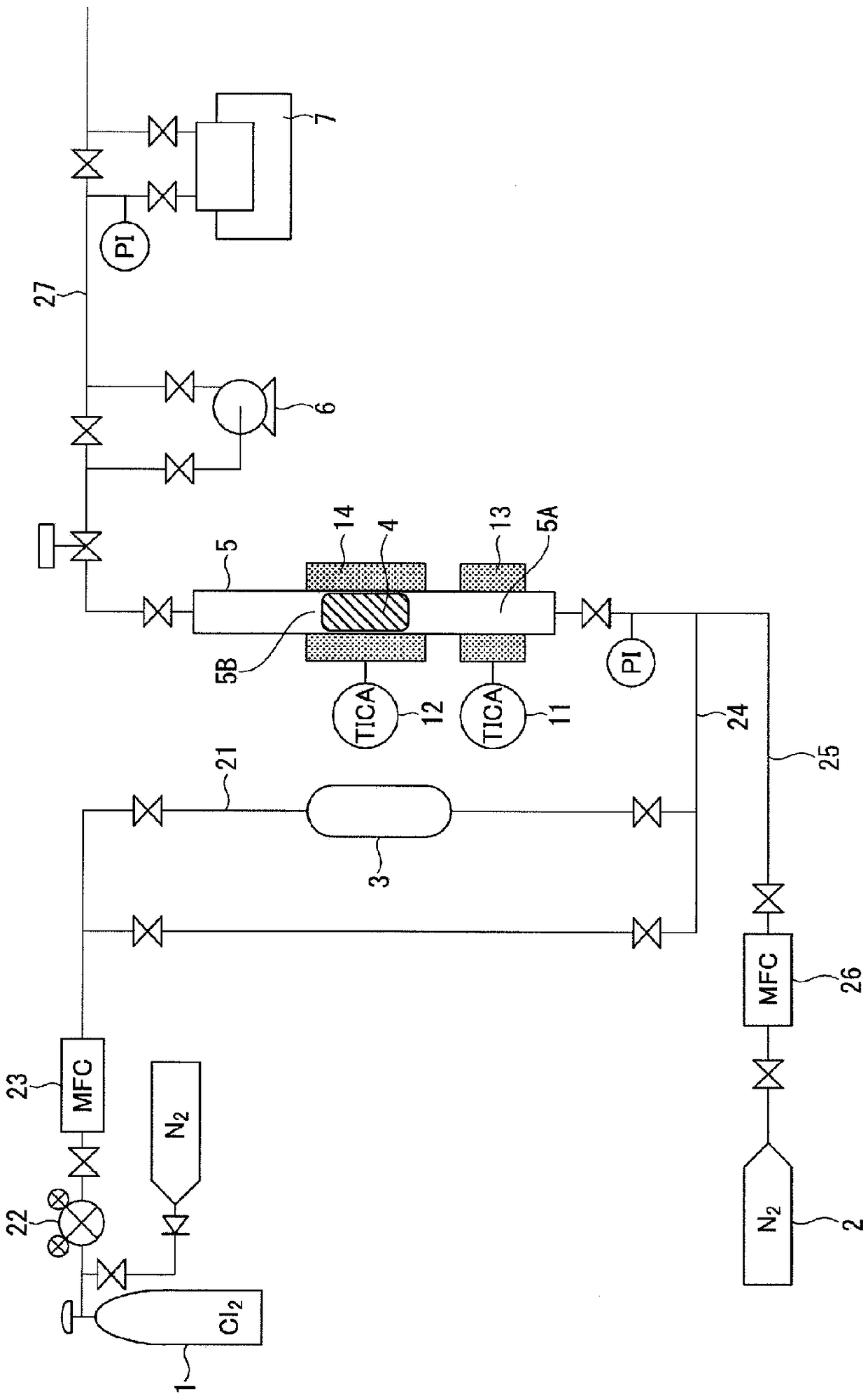
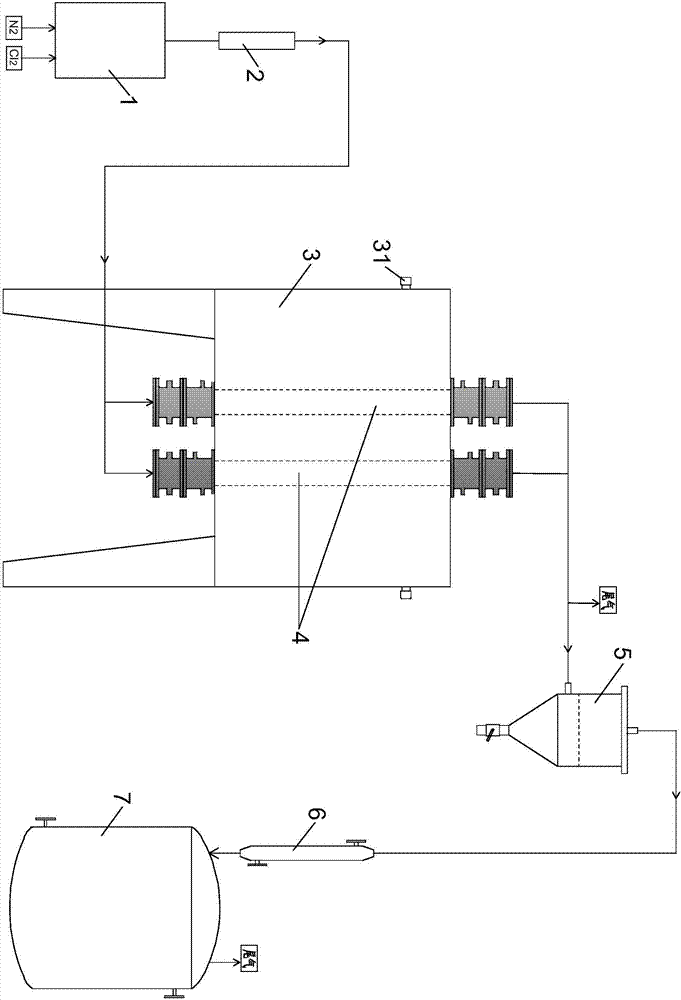
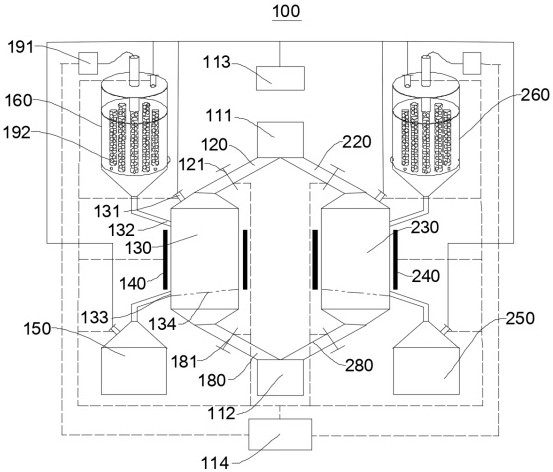
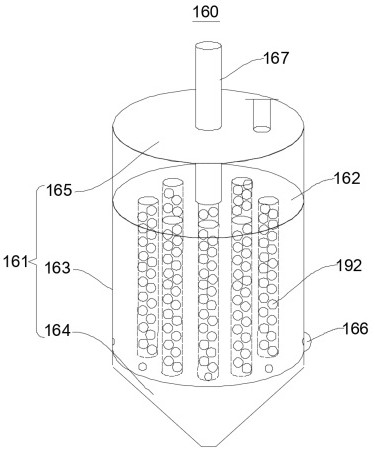
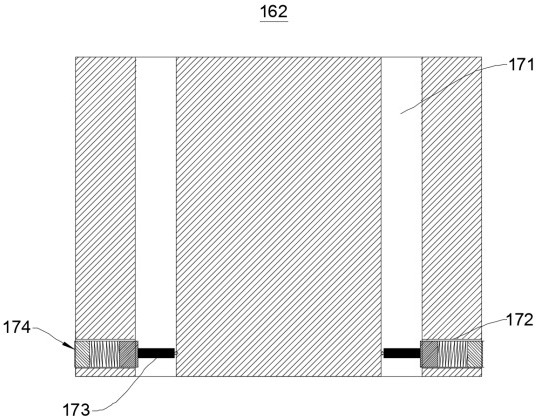
Sieve plate-free fluidized bed as well as preparation method of boron trichloride
ActiveCN103506056AReduce energy consumptionSolve the problem of easy clogging of sieve plate holesBoron halidesChemical/physical processesBorideAlkaline earth metal
Disclosed are a fluidized bed without a sieve plate and a method for preparing boron trichloride using same. The method comprises: filling the bed body (1) of a fluidized bed without a sieve plate with a powder raw material, i.e. an alkaline earth metal boride, via a raw material supplying device (4); opening a carrier gas source (2), feeding the carrier gas into the bed body (1) of the fluidized bed without a sieve plate, so that the powder raw material i.e. the alkaline earth metal boride is in a fluidized state in the bed body (1) of the fluidized bed without a sieve plate; opening a heating device (5) to heat the bed body (1), feeding a reaction gas i.e. anhydrous hydrogen chloride when the temperature reaches the specified temperature; and after the reaction reaches the specified time, opening and starting a separating device (7), so as to obtain the necessary boron trichloride.
Owner:昆明先导新材料科技有限责任公司
Boron trichloride production method
Provided is a production method for boron trichloride whereby high purity boron trichloride can be produced using a simple production method and production line blockages are unlikely to occur. This boron trichloride production method comprises: a metal chlorination step in which a chlorine gas-containing gas is caused to come in contact with a raw material boron carbide being a boron carbide containing a metal other than boron as an impurity, the chlorine gas in the chlorine gas-containing gas is reacted with the metal, a metal chloride is formed, and a boron carbide containing a metal chloride is obtained; a removal step in which the metal chloride is removed from the boron carbide containing the metal chloride obtained in the metal chlorination step; and a generation step in which the chlorine-gas containing gas is caused to come in contact with the boron carbide that has had the metal chloride removed during the removal step, the boron carbide and the chlorine gas in the chlorine-gas containing gas are reacted, and boron trichloride is generated.
Owner:RESONAC CORPORATION
Fluidized bed without sieve plate and preparation method of boron trichloride
ActiveCN103506056BReduce energy consumptionSolve the problem of easy clogging of sieve plate holesBoron halidesChemical/physical processesBorideAlkaline earth metal
Disclosed are a fluidized bed without a sieve plate and a method for preparing boron trichloride using same. The method comprises: filling the bed body (1) of a fluidized bed without a sieve plate with a powder raw material, i.e. an alkaline earth metal boride, via a raw material supplying device (4); opening a carrier gas source (2), feeding the carrier gas into the bed body (1) of the fluidized bed without a sieve plate, so that the powder raw material i.e. the alkaline earth metal boride is in a fluidized state in the bed body (1) of the fluidized bed without a sieve plate; opening a heating device (5) to heat the bed body (1), feeding a reaction gas i.e. anhydrous hydrogen chloride when the temperature reaches the specified temperature; and after the reaction reaches the specified time, opening and starting a separating device (7), so as to obtain the necessary boron trichloride.
Owner:昆明先导新材料科技有限责任公司
REDUCTION OF SiCl4 IN THE PRESENCE OF BCl3
ActiveUS20150265996A1Prevent/minimize silicon tetrachloride (SiCl4) formationGas treatmentSilicon halogen compoundsBoron trichlorideSilicon tetrachloride
The present invention relates, in general, to the purification of boron trichloride (BCl3). More particularly, the invention relates to a process for minimizing silicon tetrachloride (SiCl4) formation in BCl3 production and / or the removal of SiCl4 in BCl3 product stream by preventing / minimizing the silicon source in the reaction chambers. In addition, a hydride material may be used to convert any SiCl4 present to SiH4 which is easier to remove. Lastly freeze separation would replace fractional distillation to remove SiCl4 from BCl3 that has been partially purified to remove light boilers.
Owner:MATHESON TRI GAS
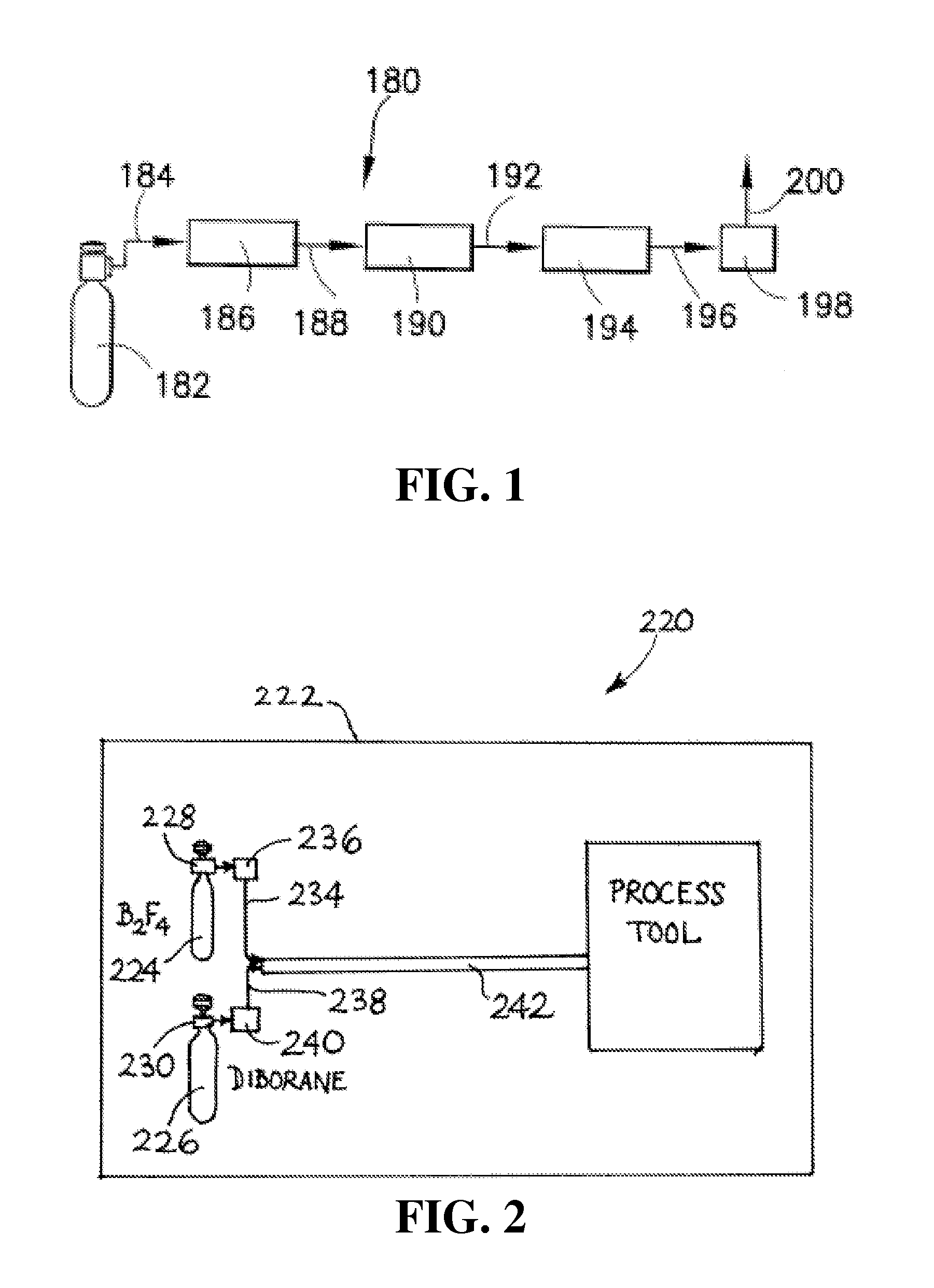
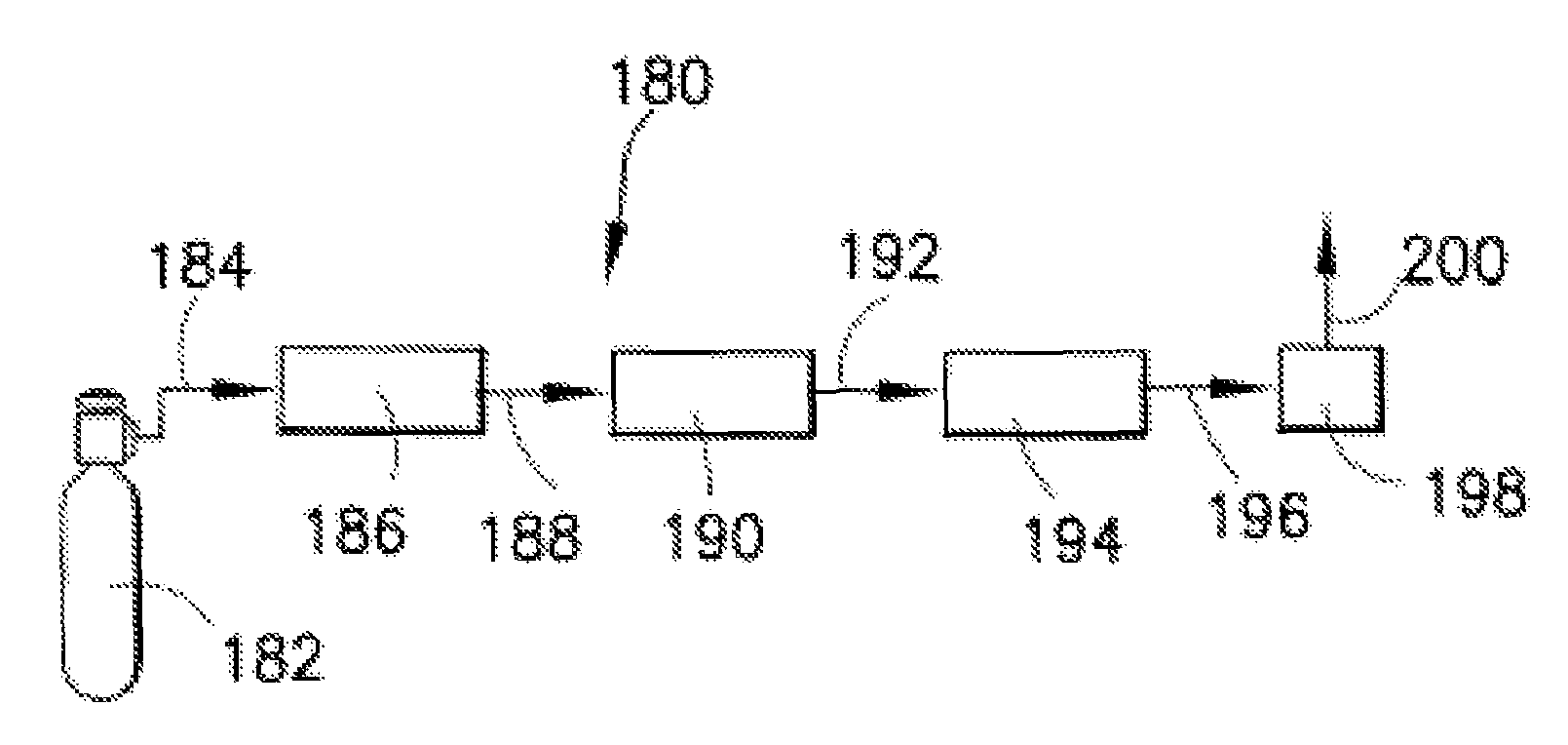
B2f4 manufacturing process
A reaction system and method for preparing compounds or intermediates from solid reactant materials is provided. In a specific aspect, a reaction system and methods are provided for preparation of boron-containing precursor compounds useful as precursors for ion implantation of boron in substrates. In another specific aspect, a reactor system and methods are provided for manufacture of boron precursors such as B2F4.
Owner:ENTEGRIS INC
Energy efficient synthesis of boranes
The reaction of halo-boron compounds (B—X compounds, compounds having one or more boron-halogen bonds) with silanes provides boranes (B—H compounds, compounds having one or more B—H bonds) and halosilanes. Inorganic hydrides, such as surface-bound silane hydrides (Si—H) react with B—X compounds to form B—H compounds and surface-bound halosilanes. The surface bound halosilanes are converted back to surface-bound silanes electrochemically. Halo-boron compounds react with stannanes (tin compounds having a Sn—H bond) to form boranes and halostannanes (tin compounds having a Sn—X bond). The halostannanes are converted back to stannanes electrochemically or by the thermolysis of Sn-formate compounds. When the halo-boron compound is BCl3, the B—H compound is B2H6, and where the reducing potential is provided electrochemically or by the thermolysis of formate.
Owner:RGT UNIV OF CALIFORNIA LOS ALAMOS NAT LAB +1
Production Method of High Purity Silver Tetrafluoroborate
InactiveUS20090274604A1High purityPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesBoron halidesSilver tetrafluoroborateHydrofluoric acid
A production method of high purity silver tetrafluoroborate, capable of producing silver tetrafluoroborate (AgBF4) at purity higher than the conventional, without using an organic solvent. The production method of the present invention is characterized in that the method comprises the step of: reacting silver fluoride with boron trifluoride in the presence of anhydrous hydrofluoric acid. Boron trifluoride is delivered into a solution obtained by dissolving or suspending silver fluoride in an anhydrous hydrofluoric acid solution.
Owner:STELLA CHEMIFA CORP
Method for producing boron trichloride
ActiveCN109195909ASuppress generationEfficient preparationBoron halidesBoron trichlorideBoron carbide
Owner:RESONAC CORPORATION
Low phosgene content boron trichloride production device and method
ActiveCN106957061APrevent overflowReduce residual chlorine impurity contentChemical industryBoron halidesDouble tubeBoron trichloride
The invention relates to a low phosgene content boron trichloride production device. An outlet of a drier is connected with an inlet at the bottom of a vertical reaction furnace; observation holes are formed on the two sides of the vertical reaction furnace; the vertical reaction furnace is internally provided with two fixed double-layer reaction tubes; outer layers of the double-layer reaction tubes are quartz tubes; inner layers of the double-layer reaction tubes are graphite rings internally tangent with the quartz tubes; the graphite rings are filled with boron carbide particles; at the same time, outlets at the upper ends of the double-layer reaction tubes are connected to an inlet of an ash blocker; an outlet of the ash blocker is connected into a condenser; the lower part of the condenser is connected into a storage tank; circulation cooling water is arranged in the condenser and the storage tank; a boron carbide raw material is filled in the double-layer reaction tubes excessively to avoid residual chlorine overflow; at the same time, after boron carbide chlorination reaction, residual carbon is not directly contacted with the quartz tubes; the generation of a phosgene synthesis raw material is avoided; impurity contents of silicon tetrachloride and residual chlorine are reduced; a phosgene content is reduced to below 3ppm; further, the requirement of electronic-grade boron trichloride is met; and a production method is efficient, stable and reliable and can achieve industrial production.
Owner:江西瑞合特种材料有限公司
Processes for the recovery and recycling of boron trifluoride or complexes thereof
InactiveUS20050272597A1Improve efficiencyHigh molecular weightOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst regeneration/reactivationBoron trifluorideOxygen
The invention provides processes for the recovery of a catalyst which are to be applied to the reactions wherein boron trifluoride or a complex thereof is used as the catalyst, that is, (I) a process for the recovery of a catalyst which comprises using as the reaction solvent a solvent containing a hydrofluorocarbon compound and / or an oxygenic hydrofluorocarbon compound and, after the completion of the reaction, separating the reaction solvent from the reaction mixture; and (II) a process for the recovery of a catalyst which comprises extracting boron trifluoride or a complex thereof from the reaction mixture after the completion of the reaction by using a hydrofluorocarbon compound and / or an oxygenic hydrofluorocarbon compound as the extracting solvent. According to the processes, boron trifluoride or complexes thereof used as catalyst can be easily separated from the reaction mixtures and re-used.
Owner:IDEMITSU KOSAN CO LTD
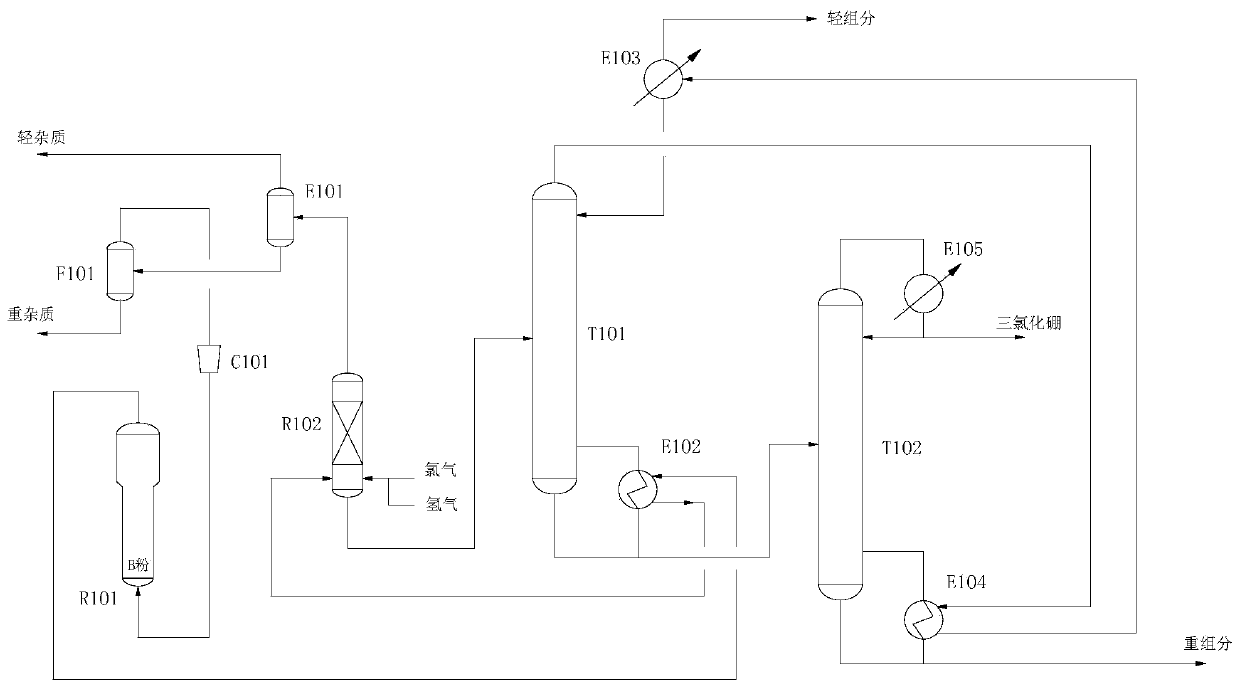
Purification method and device for boron trichloride
PendingCN108821302AReduce energy consumptionImprove the purification effectBoron halidesPurification methodsBoron trichloride
The invention discloses a purification method for boron trichloride, which includes the steps of: S1) decomposition section: feeding a liquid-phase boron trichloride crude product into a carbonyl chloride decomposer, wherein carbonyl chloride impurities in the boron trichloride are subjected to photo-decomposition under ultraviolet irradiation catalysis in the decomposer, pressure in the decomposer being 4.5-5.5 bar and reaction temperature being 30-40 DEG C, in addition, back-blowing is carried out at the bottom of the decomposer with inert gas with pressure being 5.5-6.5 bar; S2) rectification section: after the treatment in the S1), the boron trichloride is compressed into a two-stage rectification section under effect of pressure difference from the bottom of the carbonyl chloride decomposer to successively remove the heavy components and the light components from the boron trichloride crude product to obtain a boron trichloride product, wherein in the first rectification stage, operation pressure is 3-3.5 bar and operation temperature is 43-53 DEG C, and in the second rectification stage, operation pressure is 2.6-3.1 bar and operation temperature is 6-50 DEG C. Compared withthe prior art, the method can increase the purity of purified boron trichloride and reduce energy consumption.
Owner:欧中电子材料(重庆)有限公司
Method and device for producing high-purity boron tribromide
ActiveCN106829989AReduce manufacturing costSuitable for large-scale productionBoron halidesDistillationReaction temperature
The invention provides a method and device for preparing high-purity boron tribromide (BBr3) from boron carbide used as a raw material. The method comprises the following steps: vaporizing industrial grade liquid bromine through a vaporization kettle, introducing the bromine vapor into a drying chamber under the guidance action of a trace of nitrogen gas, removing moisture by means of anhydrous calcium chloride, feeding into a reaction furnace, and reacting with boron carbide particles at a reaction temperature of 650-850 DEG C to obtain boron tribromide containing impurities, dust and colors; performing ash removal on the boron tribromide through an ash blocker, removing residual bromine and iron ions by means of naphthol in a distillation kettle, and distilling to obtain white boron tribromide; and rectifying through a rectification tower, and removing high boiling point and low boiling point impurities to obtain a boron tribromide product of which the purity is greater than 5N, wherein the boron tribromide rectifying temperature is 120 DEG C, and the reflux ratio is 2-3.The high-purity boron tribromide preparation device provided by the invention is composed of the vaporization kettle, a dryer, the reaction furnace, the ash blocker, a condenser, the distillation kettle (for iron and bromine removal), the rectification tower and a finished product kettle. The purity of the boron tribromide produced by the invention can be up to 5N or above; and the method and device are low in cost and high in efficiency, and can realize industrial continuous production.
Owner:江西瑞合特种材料有限公司
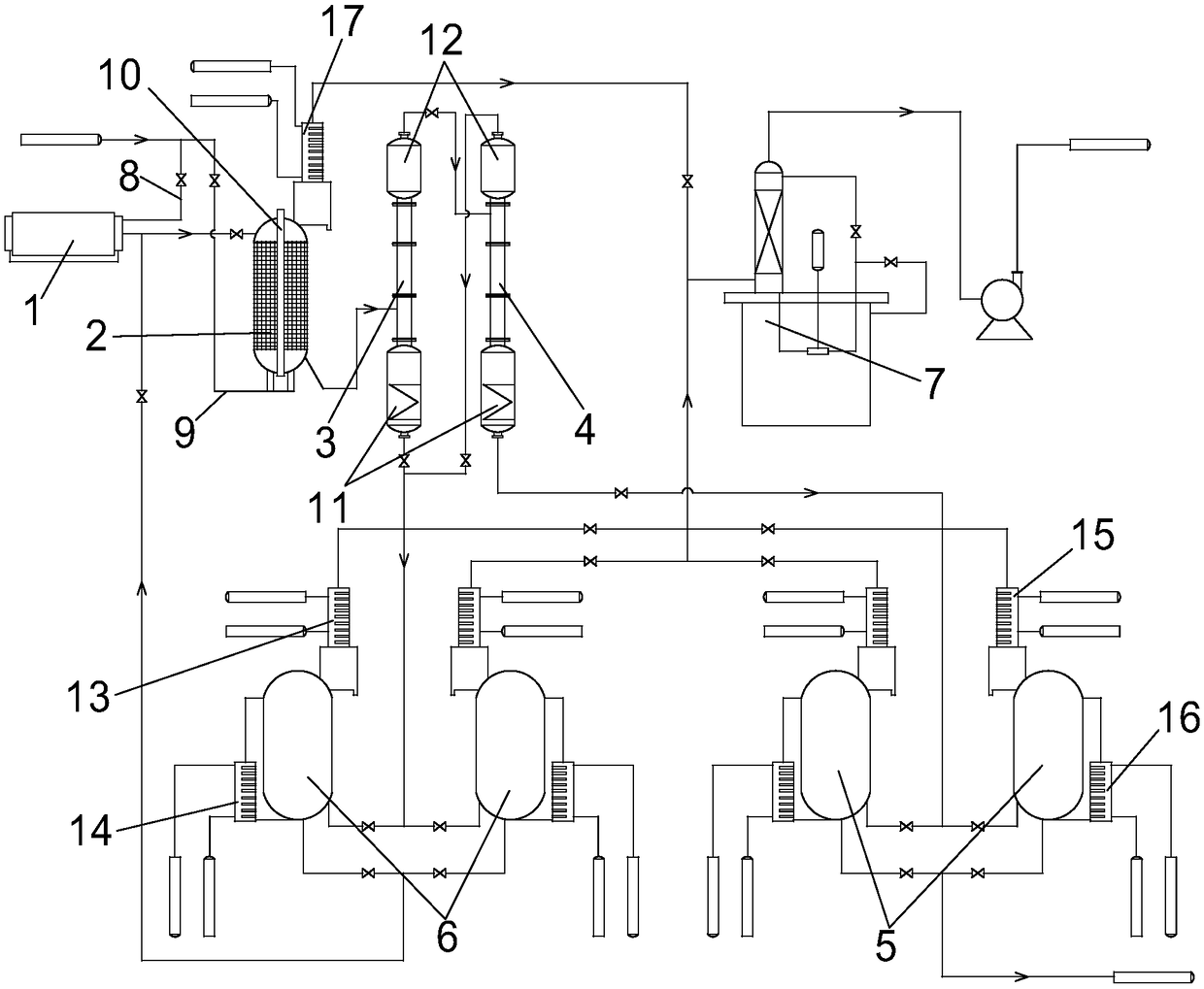
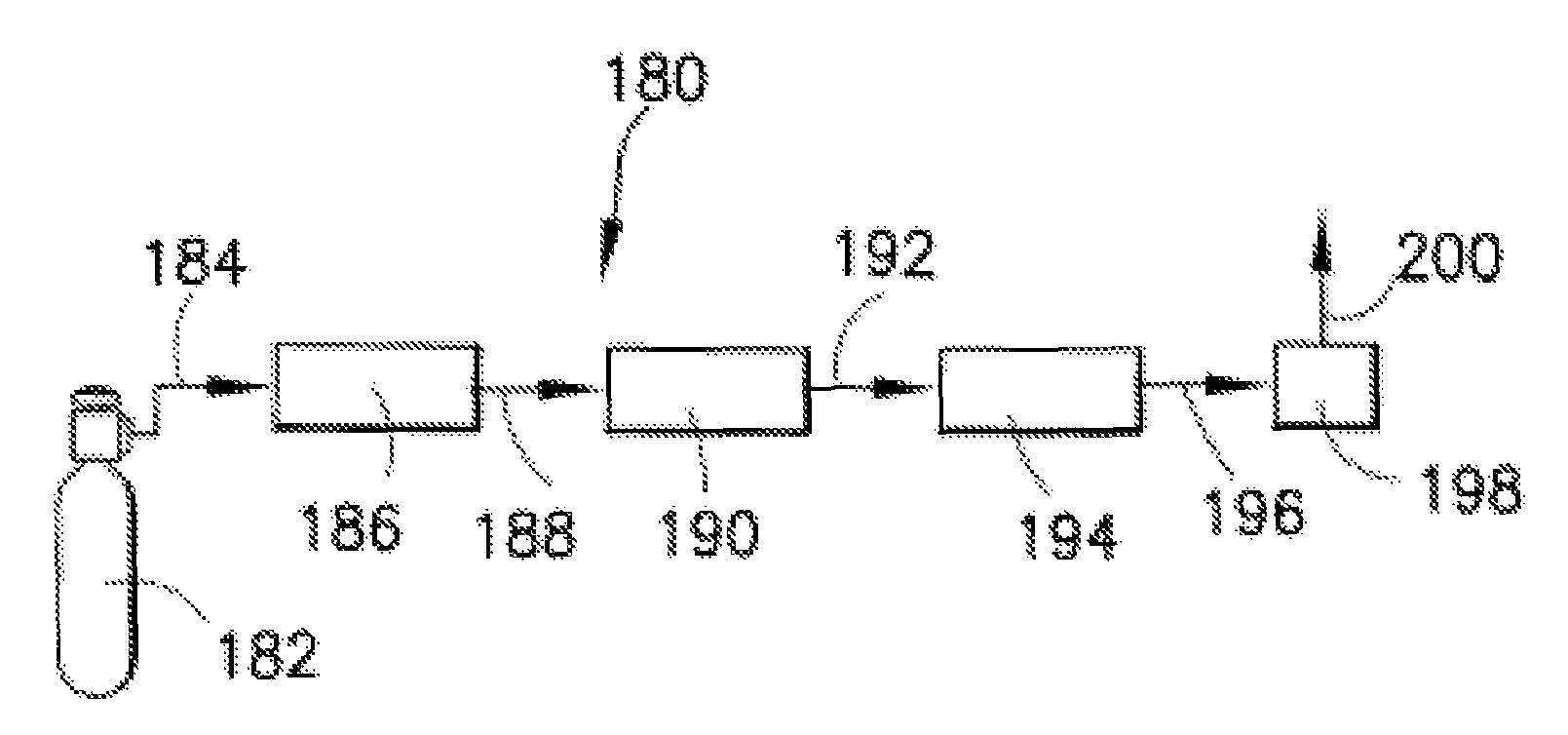
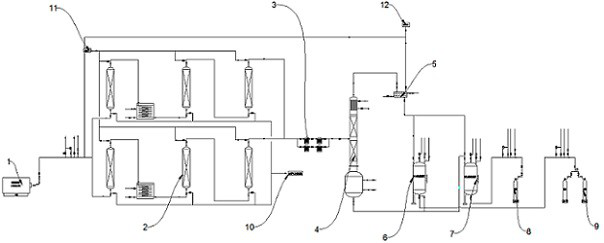
Hydrogen chloride removal device for purifying boron trichloride and boron trichloride purification system
ActiveCN114572992ARealize continuous productionImprove continuityChemical industryBoron halidesData terminalBoride
The invention discloses a hydrogen chloride removal device for purifying boron trichloride and a boron trichloride purification system, and relates to the technical field of boron trichloride purification. The hydrogen chloride removal device for purifying boron trichloride comprises an upper pipe body, at least two reaction beds arranged in parallel, temperature control assemblies in one-to-one correspondence with the reaction beds, a lower pipe body, a waste residue storage tank, a feeding device, a nitrogen device and a data terminal, and a feed port, a nitrogen inlet and a slag outlet of the reaction bed and a switch of the temperature control assembly are connected to a data terminal. The boron trichloride purification system comprises a crude product supply device, a heavy component removal rectifying tower, a light component removal rectifying tower, a hydrogen chloride removal device for purifying boron trichloride, a filter and a condensing device which are connected in sequence. According to the hydrogen chloride removal device for purifying the boron trichloride and the boron trichloride purification system, continuous production is realized by connecting at least two reaction beds in parallel, and automatic replacement of the filler coated with the alkaline earth metal boride is realized through cooperation of the reaction beds, the feeding device and other parts.
Owner:福建福豆新材料有限公司 +1
Isotopically-enriched boron-containing compounds, and methods of making and using same
Owner:ENTEGRIS INC
Method for efficiently preparing borohydride anion [RnH(4-n)B]<->
InactiveCN108676023AWide applicabilityLow costBoron halidesGroup 3/13 element organic compoundsHydrogenSilanes
The invention discloses a method for efficiently preparing borohydride anion [RnH(4-n)B]<->, lewis acid RnX3-nB is taken as a catalyst, hydrogen containing silane is taken as a reducing agent, and various halogenated boron anionic ammonium salts [R'4N]<+>[RnX(4-n)B]<-> are reduced to corresponding borohydride anion ammonium salts [R'4N]<+>[RnH(4-n)B]<->. The method not only can prepare BH4<-> anionic salt and triphenyl borohydride ammonium salt [Ph3HB]<->, but also can synthesize various other types of borohydride anions [RnH(4-n)B]<->, the applicability is wide, and the synthesis method is simple and efficient. The method provides a novel way for the synthesis of the borohydride anion [RnH(4-n)B]<->.
Owner:LESHAN NORMAL UNIV
Preparation method of electronic-grade boron trichloride
InactiveCN110803708ALow reaction temperatureReduce material requirementsBoron halidesPhotocatalytic reactionBoron trichloride
The invention discloses a preparation method of electronic-grade boron trichloride, wherein the preparation method comprises the steps: chlorine and hydrogen are introduced into a photocatalytic reactor from the bottom, phosgene in a reaction product of a fluidized bed reactor is decomposed into carbon monoxide and chlorine under illumination, the chlorine and hydrogen are subjected to a reactionunder ultraviolet irradiation to generate hydrogen chloride, and the hydrogen chloride is extracted from the top of the photocatalytic reactor; hydrogen chloride is subjected to heat exchange and vaporization treatment and then subjected to a reaction with boron powder in the fluidized bed reactor, and a reaction product is extracted from the top of the fluidized bed reactor, then enters a light component removal tower reboiler for heat exchange and next enters a photocatalytic reactor; a boron trichloride crude product extracted from the bottom of the photocatalytic reactor enters the light component removal tower, a product extracted from the top of the light component removal tower enters a heavy component removal tower reboiler for heat exchange, and then enters a fractional condenserfor condensation and cooling, and a liquid phase finally flows back to the light component removal tower; a material of the tower kettle of the light component removal tower enters a heavy component removal tower, and a 6N boron trichloride product is extracted after the tower top of the heavy component removal tower is condensed by a condenser. The method is simple, reliable, low in energy consumption, easy to implement and convenient for industrial production.
Owner:TIANJIN ZHONGKE TUOXIN TECH CO LTD
Boron trichloride purification device
PendingCN113582192AEasy to purifyHigh purityBoron halidesChemical recyclingBoron trichloridePhysical chemistry
The invention discloses a boron trichloride purification device, and relates to the technical field of boron trichloride. The device comprises a raw material flow convergence channel, a boron trichloride adsorption system, a filter, a rectifying tower, a liquefier, a finished product storage mechanism, a recovery storage mechanism, a recovery filling mechanism, a finished product filling mechanism, a tail gas removal system, a hot nitrogen access port and a non-condensable steam discharge mechanism. Through cooperation of all accessories, boron trichloride can be well purified, high-purity boron trichloride is obtained, unqualified boron trichloride can be automatically recycled, the production cost is reduced, one-time forming is achieved in the purification process, continuity of the purification technology is guaranteed, boron trichloride is prevented from being affected by external impurities, the purity of boron trichloride is guaranteed, and the method is easy to operate, high in efficiency and suitable for large-scale popularization.
Owner:CHONGQING TONGHUI GAS
Composition for Preparing Molded Polymeric Article
The present invention is directed to an organic polymerizable composition for producing a molded polymeric article. The composition includes a mold release agent of ionic fluoride and / or ionic fluoride precursor present in an amount sufficient to effect at least partial demolding of the polymeric article from a mold. Molded articles also are provided.
Owner:PPG IND OHIO INC
Fluorination device for treating neodymium iron boron waste powder
InactiveCN108326279AReduce flow rateAvoid blowing awayFluoride preparationTransportation and packagingEngineeringInductor
The invention discloses a fluorination device for treating neodymium iron boron waste powder, and belongs to the technical field of neodymium iron boron waste treatment. The fluorination device comprises a fluorine gas generating device, and a top outlet of the fluorine gas generating device is connected with a corrugated pipe. The right side wall of the fluorine gas generating device is connectedwith a reaction pipe through a screw, and the top of the left end of the reaction pipe is connected with a gas inlet. The gas inlet is connected with the bottom of the right end of the corrugated pipe, and spoilers are evenly mounted on the left side wall of an inner cavity of the reaction pipe. The upper side of the middle of the reaction pipe is connected with a charging opening, and a protection cover is mounted in the middle of the upper side wall of the inner cavity of the reaction pipe. The protection cover is connected with the bottom of the charging opening, and the bottom of the reaction pipe is connected with an arc-shaped reaction bin. According to the fluorination device for treating the neodymium iron boron waste powder, by arranging the spoilers, the flow velocity of fluorine gas can be reduced; by arranging the protection cover, gas flow is prevented from blowing away the raw material powder; and by arranging a gas pressure inductor and a PLC, the intelligent control degree is higher.
Owner:TIANJIN NIBBOH MAGNETS
Method and device for recovering boron trifluoride in wastewater containing boron trifluoride
PendingCN112850731AReduce the impactReduce wasteBoron halidesGroup 3/13 element organic compoundsSodium tetrafluoroborateBoron trifluoride
The invention belongs to the technical field of wastewater treatment, and particularly relates to a method and device for recovering boron trifluoride in wastewater containing boron trifluoride. The recovery method provided by the invention comprises the following steps: mixing wastewater containing boron trifluoride with strong base to carry out acid-base neutralization reaction to obtain a neutralization reaction product, wherein the boron trifluoride contained in the wastewater is hydrolyzed into fluoboric acid and boric acid; and mixing the neutralization reaction product with fuming sulfuric acid, and carrying out a replacement reaction to obtain boron trifluoride gas. According to the method, by adding the strong base into the boron trifluoride-containing wastewater and carrying out acid-base neutralization reaction on the strong base and fluoboric acid in the wastewater, sodium fluoborate is obtained; and sodium fluoborate, boric acid and fuming sulfuric acid react to obtain the boron trifluoride gas. According to the method, boron trifluoride in the wastewater is recycled by utilizing a simple chemical reaction. The recovery method provided by the invention is simple and easy to implement; the influence of boron trifluoride on the environment and an existing wastewater treatment system is reduced; and the waste of resources is reduced.
Owner:SHANDONG HEYI GAS CO LTD DONGYING CITY
A process for purifying boron trifluoride gas by low-temperature rectification
The invention discloses a process for purifying boron trifluoride gas through rectification at the low temperature and belongs to the technical field of high-purity boron trifluoride production. According to the process, boron trifluoride gas containing impurities is treated through steps including compression, liquification, low-temperature rectification, filling and the like, and high-purity boron trifluoride gas with the purity of 99.9%-99.99% is finally obtained. Gas is compressed to 2.0-4.0 MPa and liquified at the temperature of subzero 50 DEG C to subzero 30 DEG C to form a liquid, and the liquid enters a rectification tower for rectification. The operation conditions of the rectification tower are shown as follows: the temperature of the top of the tower ranges from subzero 40 DEG C to subzero 30 DEG C, the temperature of the bottom of the tower ranges from 10 DEG C to 15 DEG C, the pressure ranges from 3.0 MPa to 4.0 MPa, and rectification is operated continuously. The process is simple and easy to implement, high requirements of ultralow temperature rectification for equipment are avoided, the equipment investment is low, the yield is high, and the product purity is high. The process is suitable for industrial continuous production.
Owner:中昊光明化工研究设计院有限公司 +1
Method for comprehensively recycling valuable components in paigeite
InactiveCN108893572AReduce tensionEfficient separationBoron halidesProcess efficiency improvementHigh magnesiumTunnel kiln
The invention discloses a method for comprehensively recycling valuable components in paigeite, and belongs to the field of metallurgy. The method adopts microwave heating, tunnel kiln smelting and chloride separation. According to the method, iron, boron and magnesium elements in the paigeite can be effectively separated, the grade and yield of iron and boron can be improved, high-magnesium tailings can be recycled, utilization of the composite paigeite is promoted, the situation of iron mine, boron mine and magnesium mine resource shortage can be effectively relieved, the ecological featureof resource and energy can be taken into consideration, and comprehensive utilization of paigeite resource can be realized. The method has the advantages of simple process, low production cost, energyconservation, emission reduction and the like.
Owner:HEBEI UNIV OF ENG +1
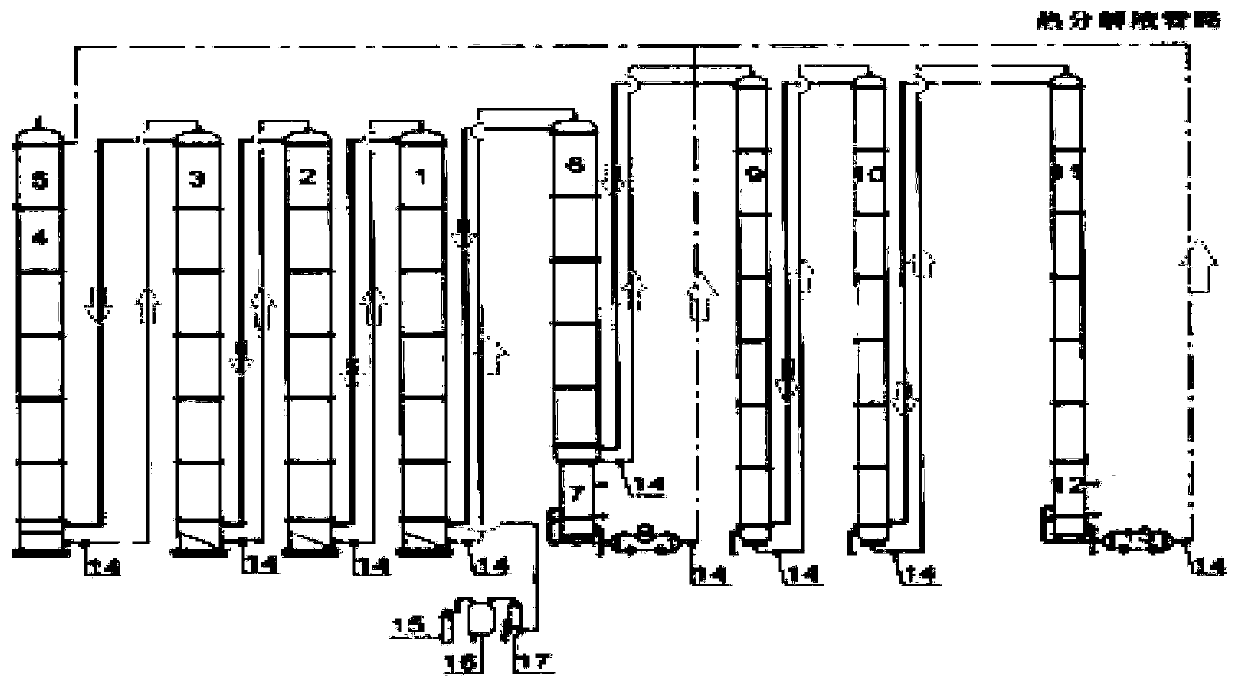
A method and system for simultaneously producing high-concentration boron-10 boron trifluoride and high-concentration boron-11 boron trifluoride
ActiveCN108275691BExchangeRealize energy saving and zero emissionBoron halidesBoron trifluoridePhysical chemistry
Owner:大方元素(广东)科技有限公司
Hydrogenated isotopically enriched boront trifluoride dopant source gas composition
The invention relates to a hydrogenated isotopically enriched boron trifluoride (BF3) dopant source gas composition. The composition contains (i) boron trifluoride isotopically enriched above naturalabundance in boron of atomic mass 11 (UB), and (ii) hydrogen in an amount of from 2 to 6.99 vol.%, based on total volume of boron trifluoride and hydrogen in the composition. Also described are methods of use of such dopant source gas composition, and associated apparatus therefor.
Owner:ENTEGRIS INC
Production method of high purity silver tetrafluoroborate
InactiveUS8501138B2High purityPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesBoron halidesHydrofluoric acidSilver tetrafluoroborate
A production method of high purity silver tetrafluoroborate, capable of producing silver tetrafluoroborate (AgBF4) at purity higher than the conventional, without using an organic solvent. The production method of the present invention is characterized in that the method comprises the step of: reacting silver fluoride with boron trifluoride in the presence of anhydrous hydrofluoric acid. Boron trifluoride is delivered into a solution obtained by dissolving or suspending silver fluoride in an anhydrous hydrofluoric acid solution.
Owner:STELLA CHEMIFA CORP
Processes for the recovery and recycling of boron trifluoride or complexes thereof
InactiveUS7235509B2Improve efficiencyHigh molecular weightOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst regeneration/reactivationBoron trifluorideSolvent
The invention provides processes for the recovery of a catalyst which are to be applied to the reactions wherein boron trifluoride or a complex thereof is used as the catalyst, that is, (I) a process for the recovery of a catalyst which comprises using as the reaction solvent a solvent containing a hydrofluorocarbon compound and / or an oxygenic hydrofluorocarbon compound and, after the completion of the reaction, separating the reaction solvent from the reaction mixture; and (II) a process for the recovery of a catalyst which comprises extracting boron trifluoride or a complex thereof from the reaction mixture after the completion of the reaction by using a hydrofluorocarbon compound and / or an oxygenic hydrofluorocarbon compound as the extracting solvent. According to the processes, boron trifluoride or complexes thereof used as catalyst can be easily separated from the reaction mixtures and re-used.
Owner:IDEMITSU KOSAN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com