Method for producing boron trichloride
A boron trichloride and manufacturing method technology, applied in the direction of boron halide compounds, boron halides, etc., can solve the problems of reduced manufacturing efficiency, blockage of manufacturing pipelines, etc., and achieve the effects of efficient manufacturing and suppression of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
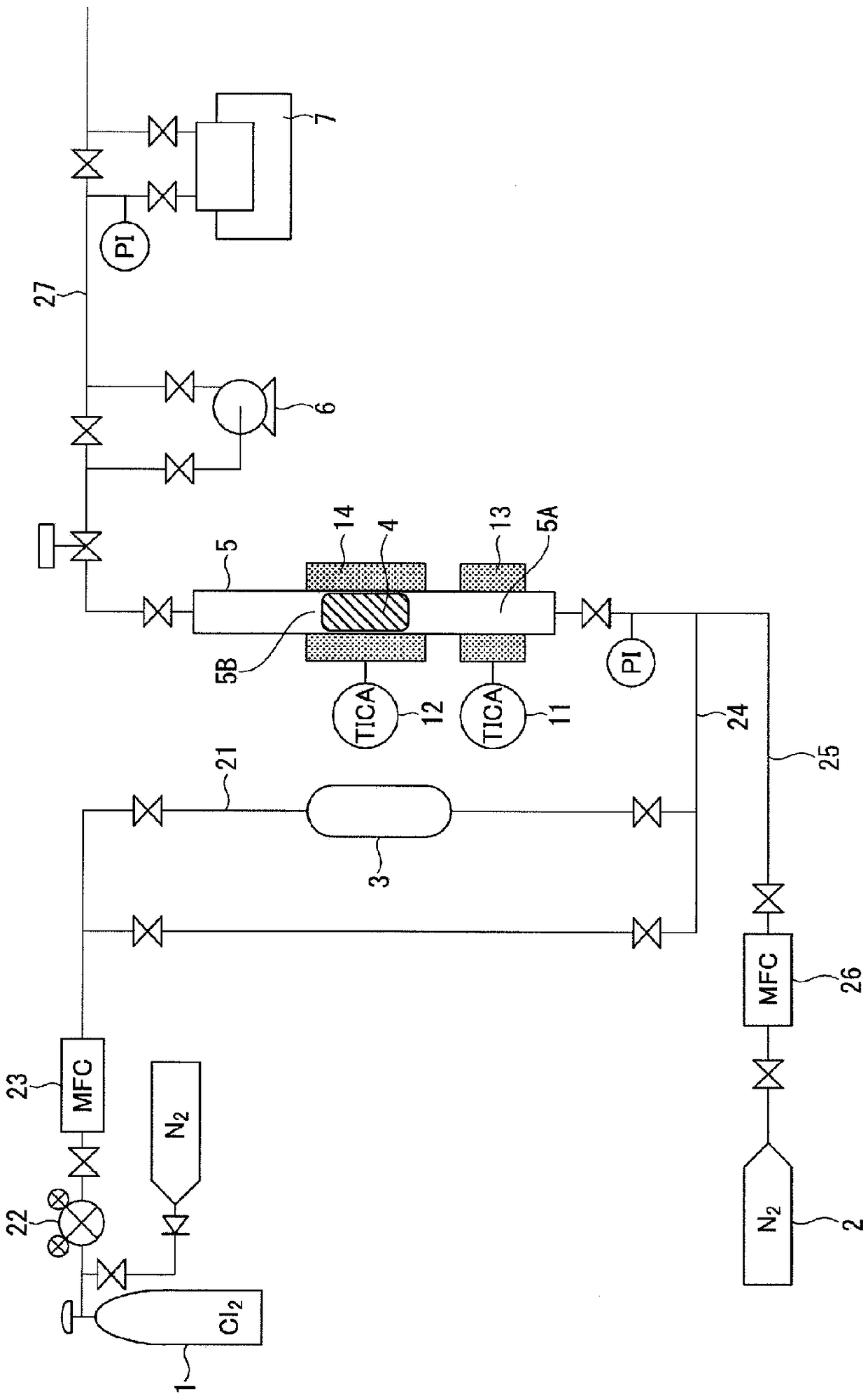
[0073] use with figure 1 The same boron trichloride manufacturing apparatus as the boron trichloride manufacturing apparatus operates in the same manner as in the above embodiment to react boron carbide and chlorine gas to manufacture boron trichloride. As the chlorine-containing gas, commercially available high-purity chlorine gas having a purity of 99.999% by volume and a water content of 0.9 ppm by volume was used. For boron carbide, the following powder is used: when the particle size is measured through a dry sieve, the undersize of a dry sieve with an opening of 5.60 mm is 100% by mass, and a sieve that cannot pass through a dry sieve with an opening of 1 mm The upper amount is 65% by mass or more.
[0074] Pack 20 g of this boron carbide powder into a tubular reaction vessel made of graphite (with an inner diameter of 22 mm, a height of 700 mm, and a volume of a reaction part filled with boron carbide of 19 cm 3 ), nitrogen with a flow rate of 500ccm was introduced in...
Embodiment 2
[0079] The reaction was carried out in the same manner as in Example 1, except that the following chlorine gas was used instead of the high-purity chlorine gas as the chlorine-containing gas used in the dehydration treatment of boron carbide. That is, commercially available industrial chlorine gas with a purity of 99.9% by volume and a moisture content of 5 ppm by volume is passed through a 1 L SUS cartridge filled with molecular sieve 3A to reduce the moisture content to 1 ppm by volume or less, and the resulting chlorine gas is referred to as chlorine-containing gas. gas use.
[0080] As a result, the reaction was completed in 6 hours, the amount of boron trichloride obtained was 167 g, and the yield was 99% by mass. In addition, the amount of by-generated hydrogen chloride was 1 mg.
Embodiment 3
[0082] The reaction was carried out in the same manner as in Example 1, except that the following gas was used instead of the high-purity chlorine gas as the chlorine-containing gas used in the dehydration treatment of boron carbide. That is, as the chlorine-containing gas, a mixed gas obtained by mixing equal amounts of commercially available high-purity chlorine gas and nitrogen gas and having a water content of 1 volume ppm or less was used.
[0083] As a result, the reaction was completed in 6 hours, the amount of boron trichloride obtained was 167 g, and the yield was 99% by mass. In addition, the amount of by-generated hydrogen chloride was 1 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com