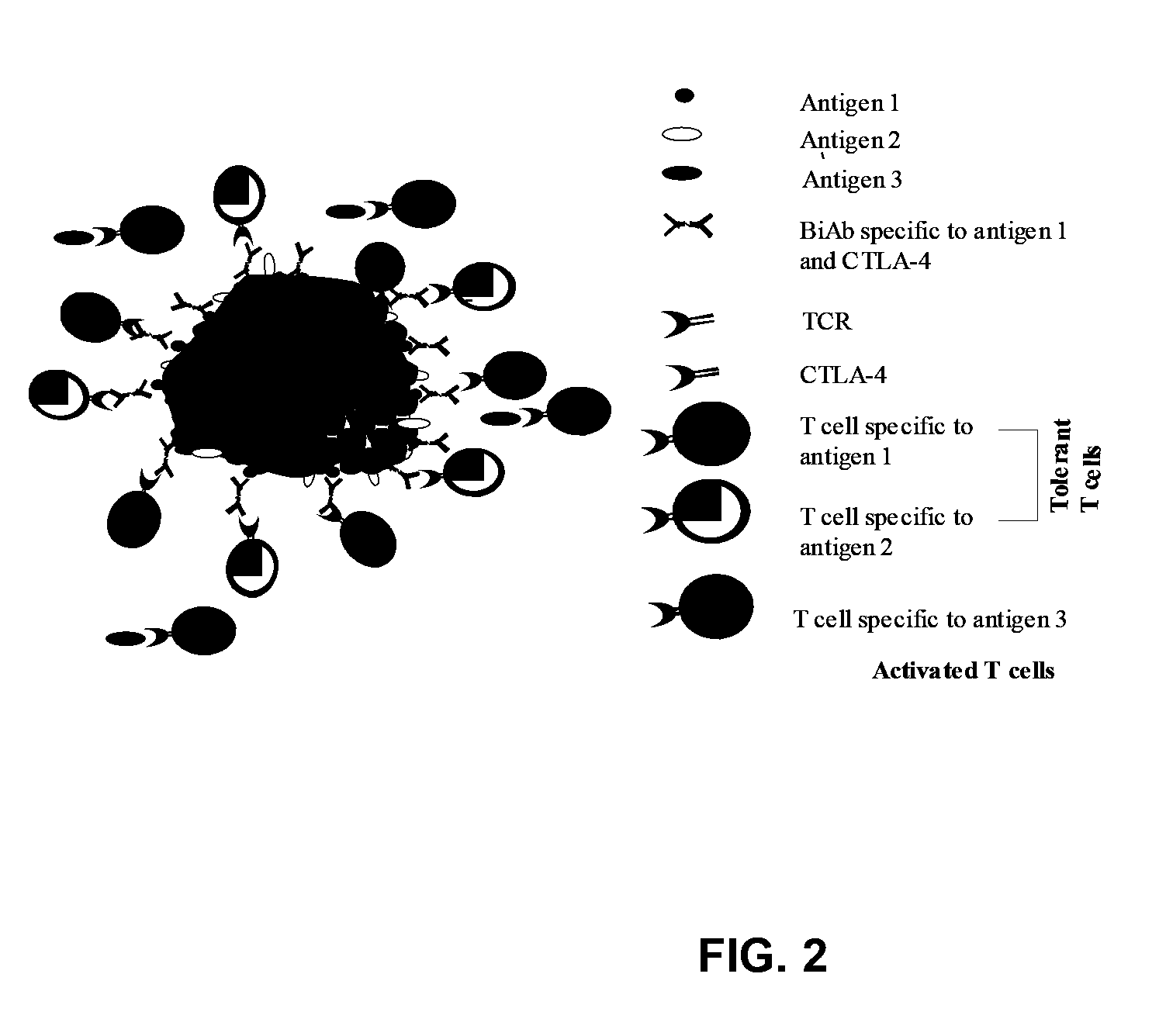
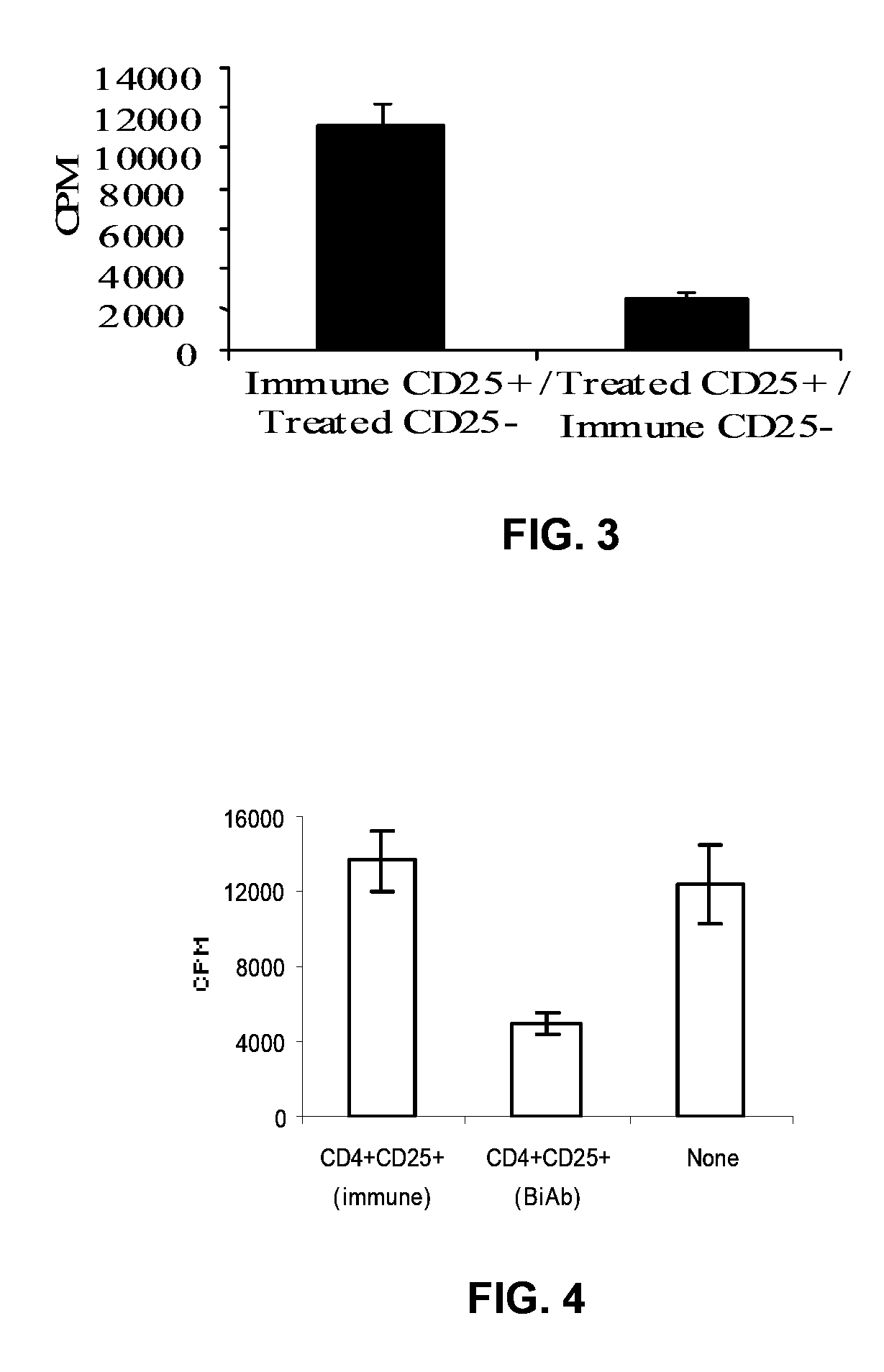
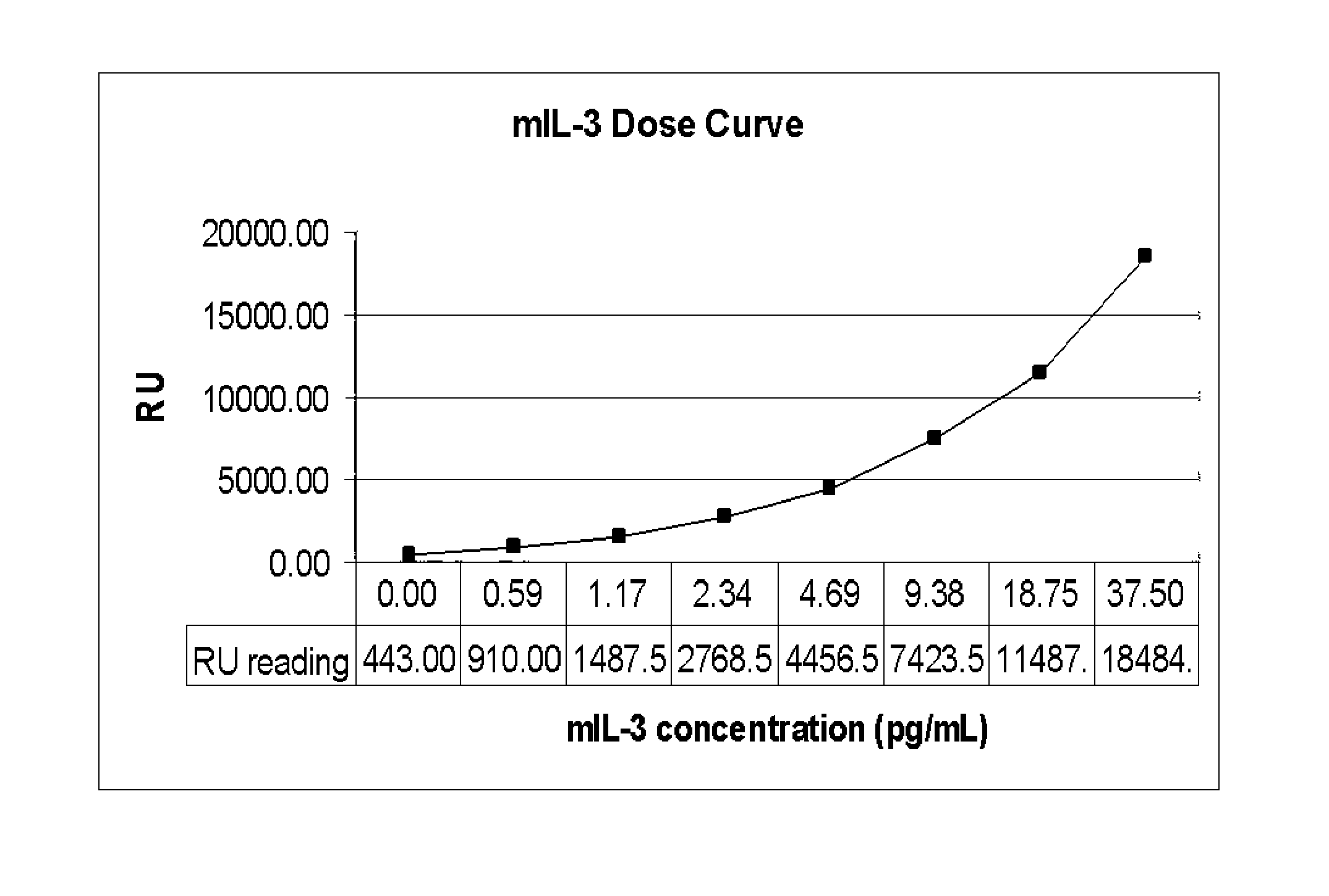
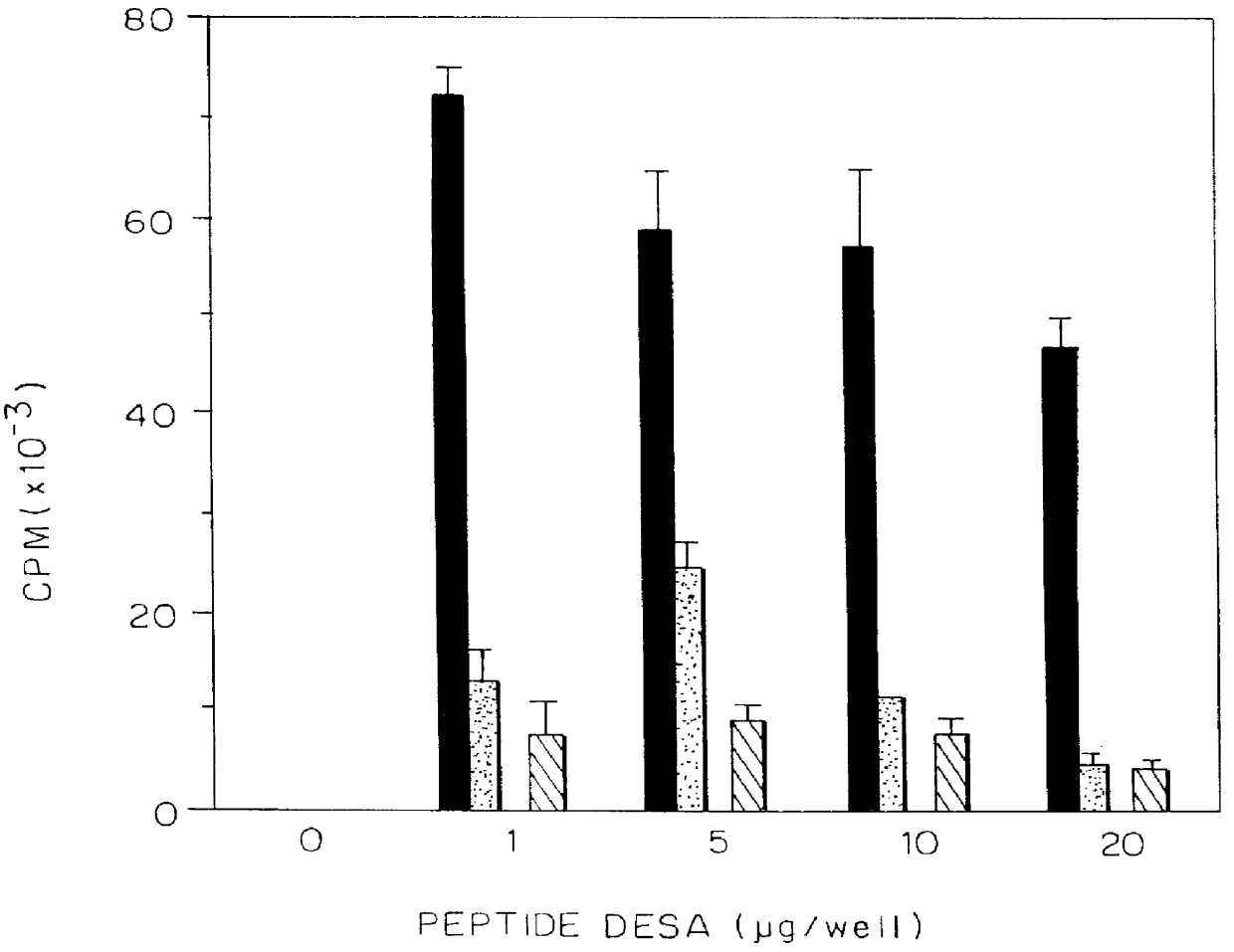
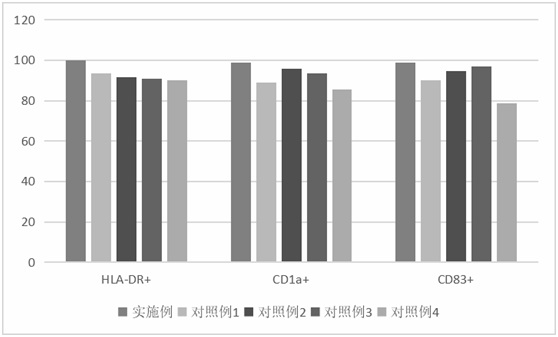
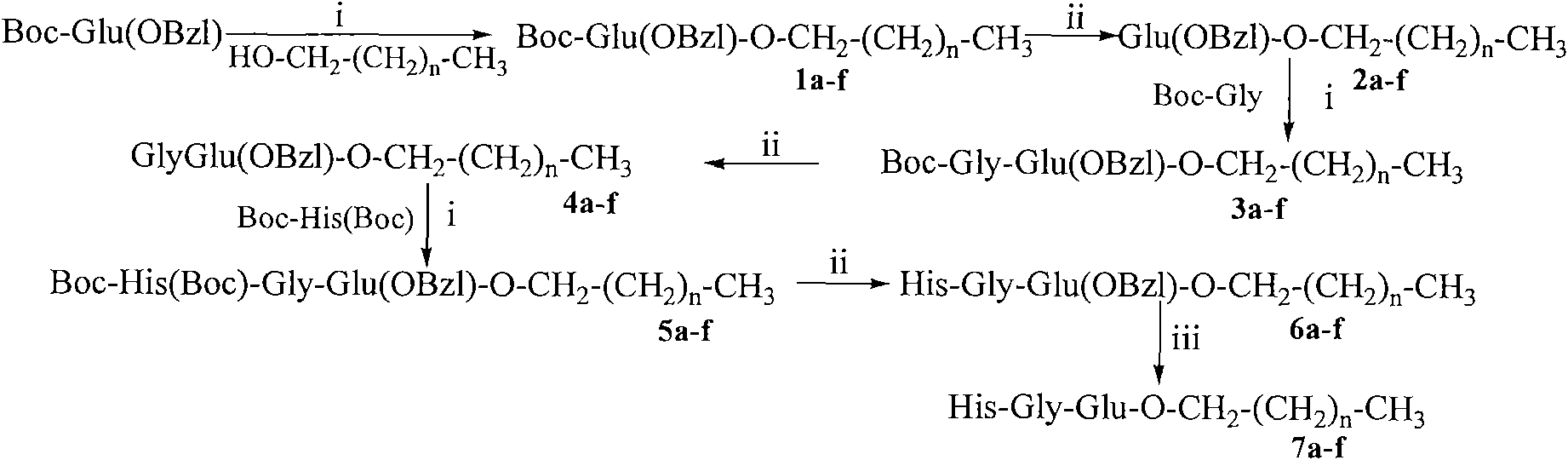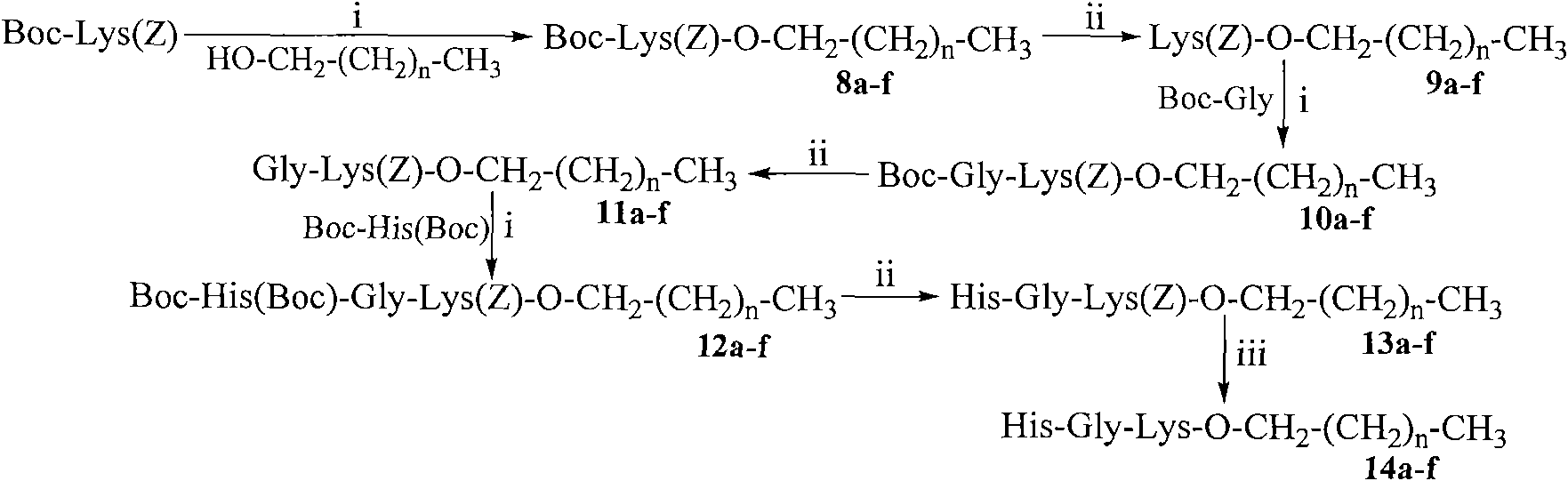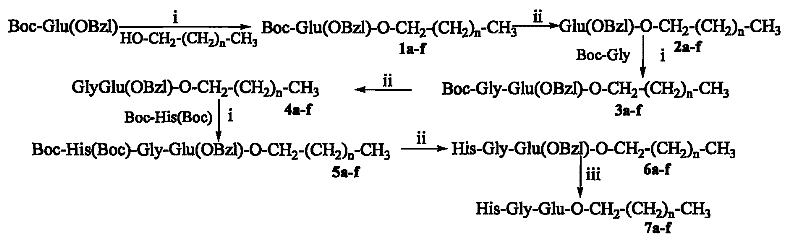Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Proliferative response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Supression of allergic reactions by transdermal administration of allergens conjugated to cholera toxin or fragments thereof
InactiveUS20050074462A1Suppress allergic reactionsBacterial antigen ingredientsPeptide/protein ingredientsAdjuvantCo administration
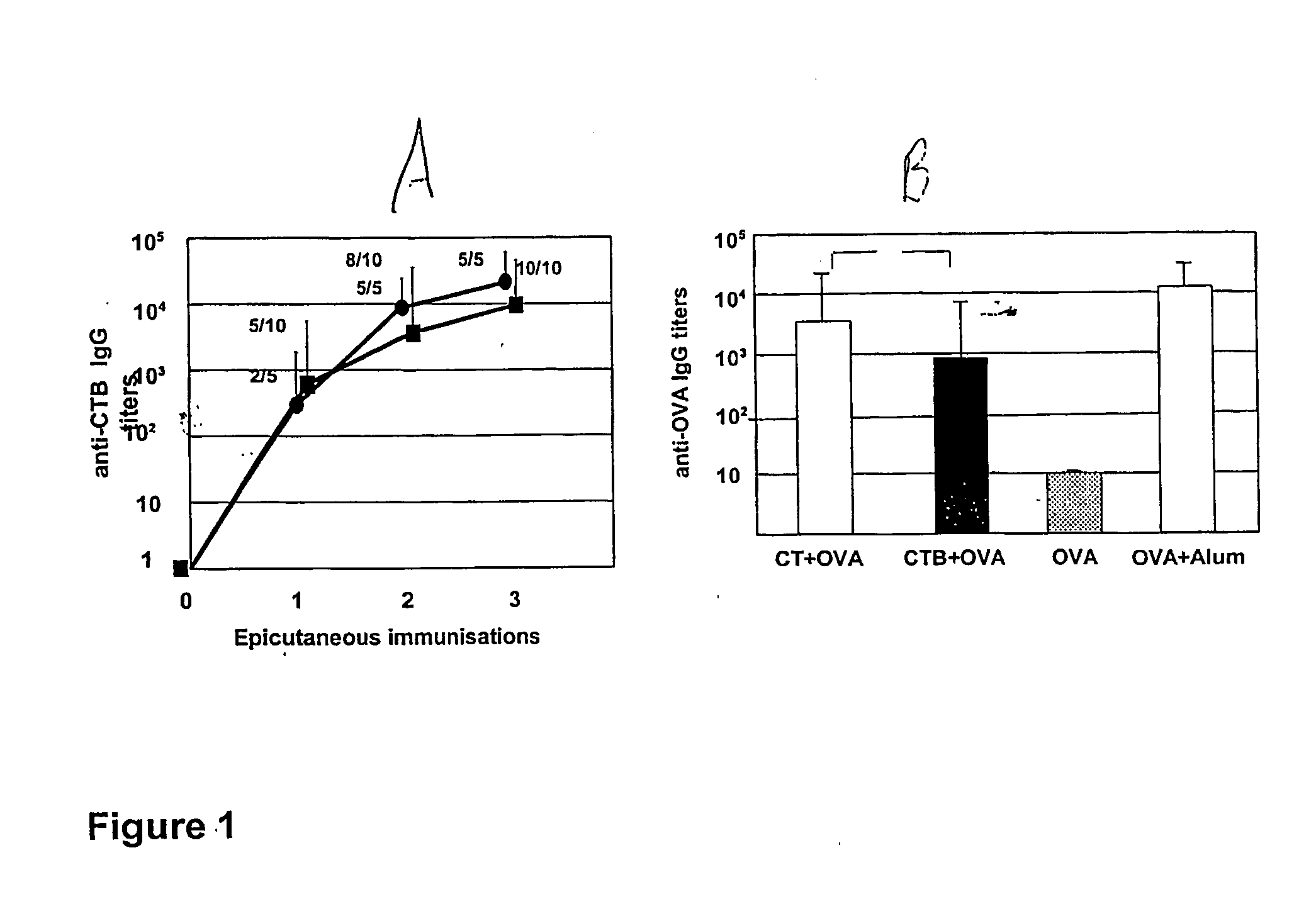
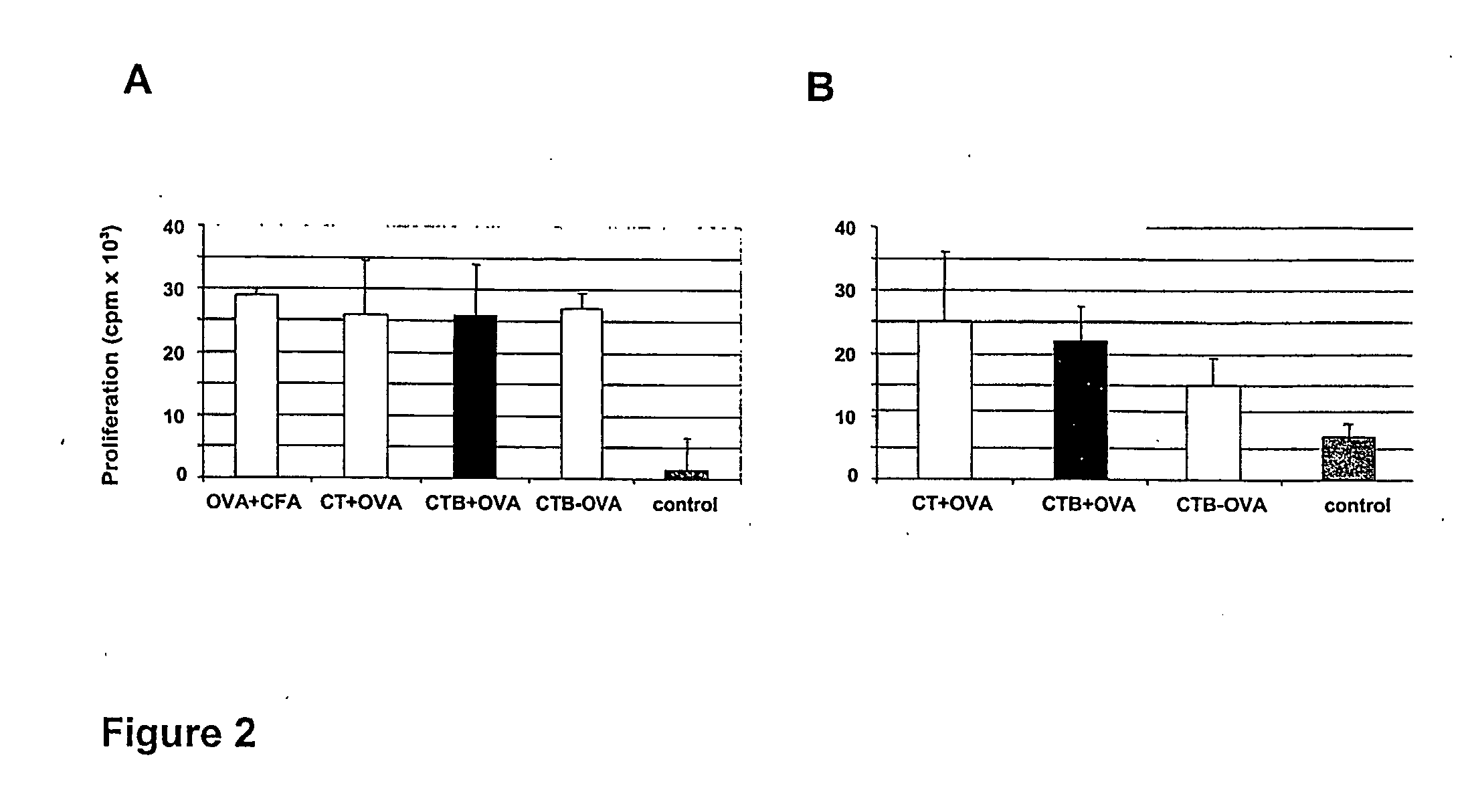
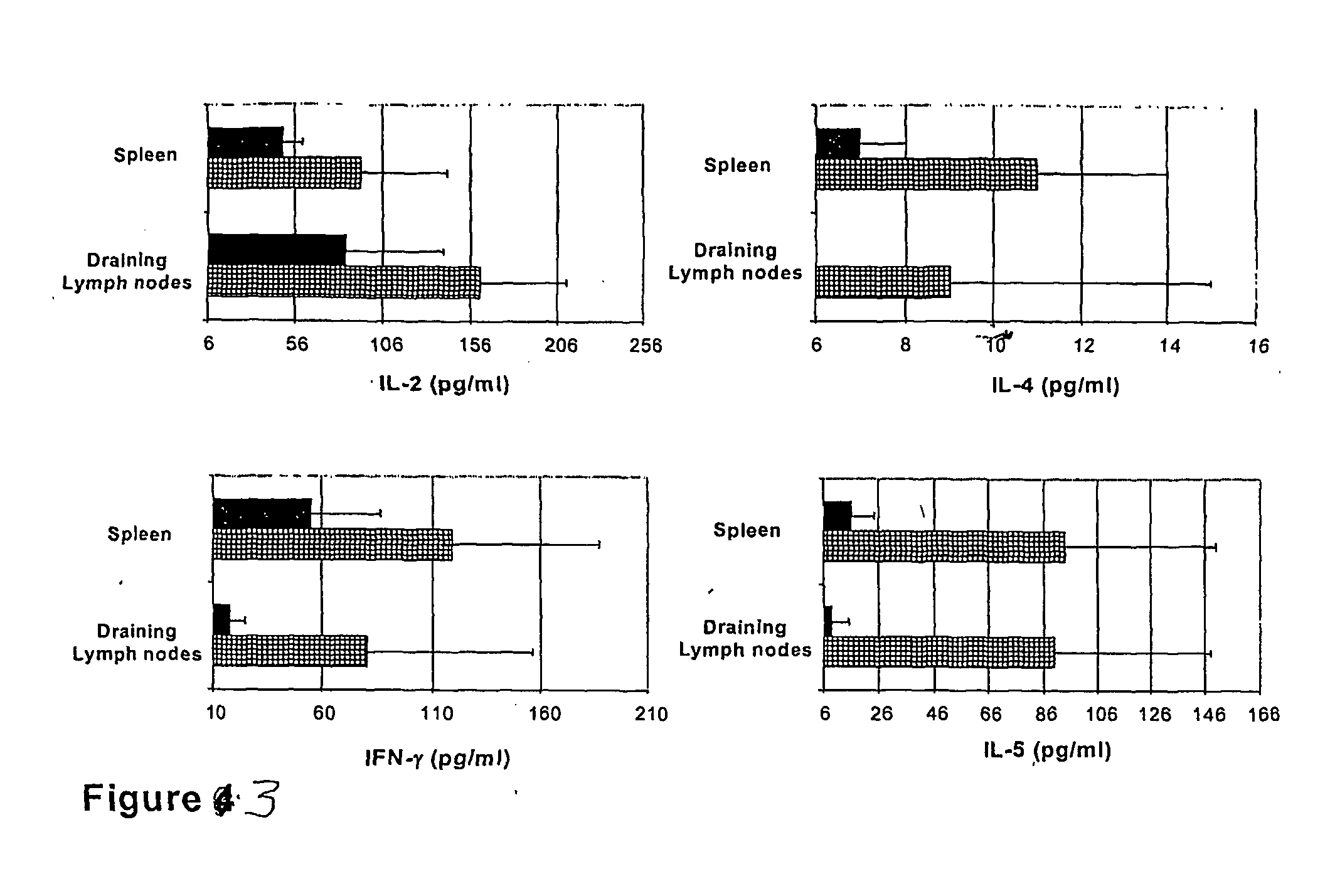
The present invention discloses the use of the non-toxic cell-binding B subunit of CT (CTB), and holotoxin CT that is devoid of ADP-ribosylating activity, as adjuvants for enhancing transcutaneous immune response to a co-administered protein allergen. It was found that topical administration of CTB to mice induced serum antibody response against itself comparable to those evoked by CT, but was inefficient at promoting systemic antibody responses against an admixed prototype protein allergen. To the contrary co-administration of either CT or CTB with allergen led to vigorous antigen-specific T cell proliferative responses in lymph nodes draining the cutaneous site of administration and at distant systemic sites. Consistent with these observations, it was found that CTB selectively potentiated Th1-driven responses without affecting Th2-dependent responses. Cutaneously applied CT enhanced serum IgE responses to a co-administered allergen, while CTB partially suppressed epicutaneously induced IgE responses to the same allergen.
Owner:DUOTOL
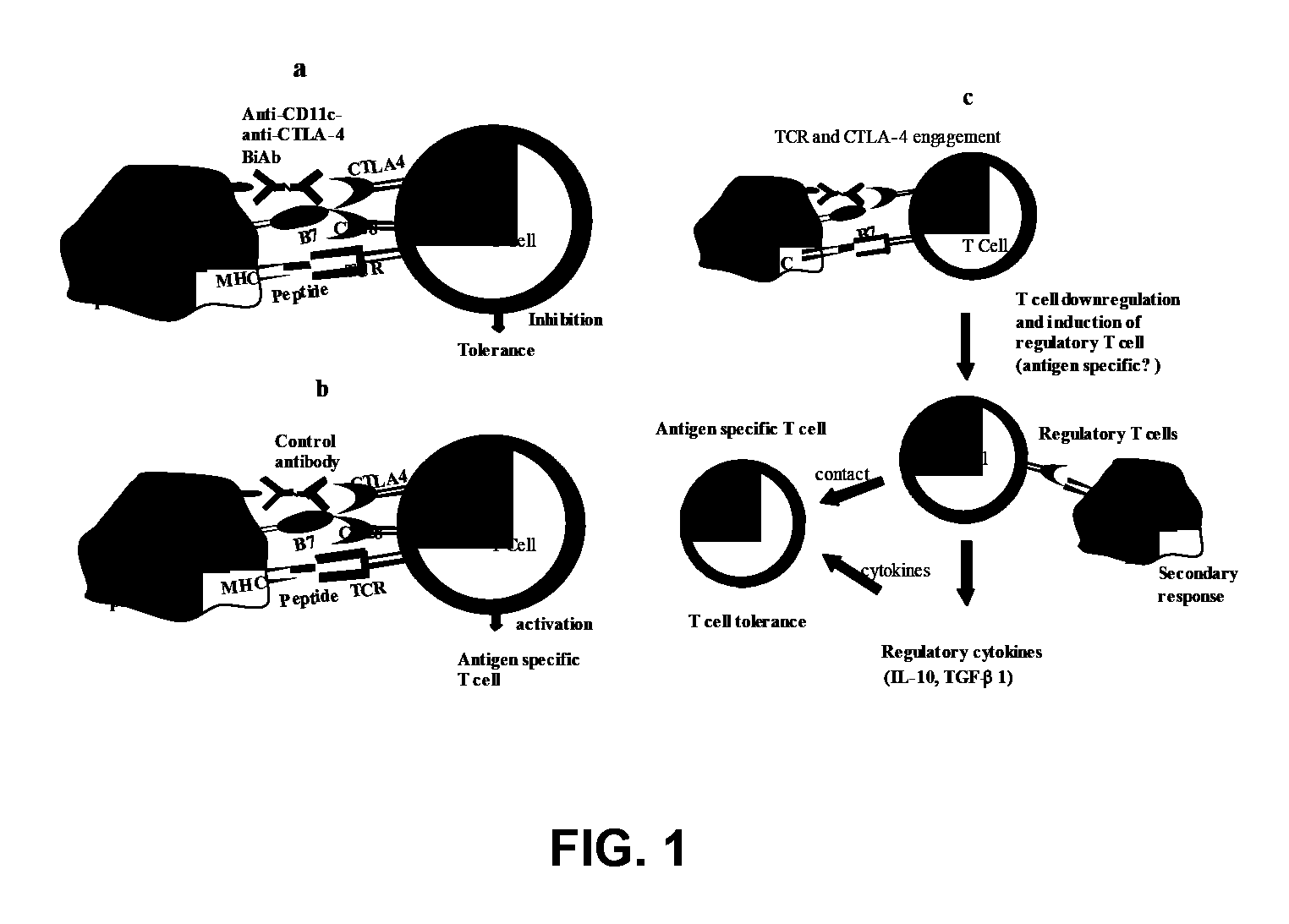
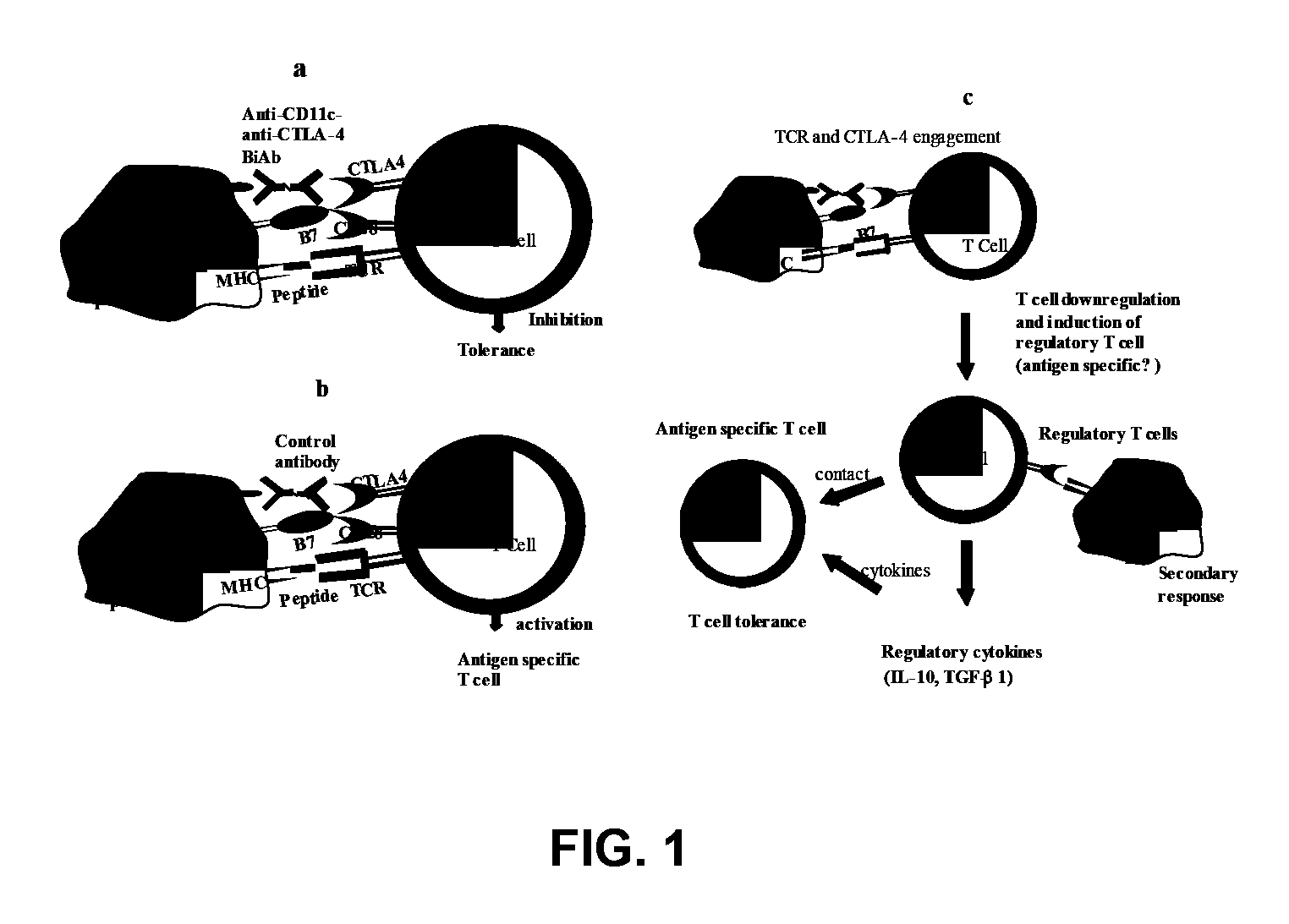
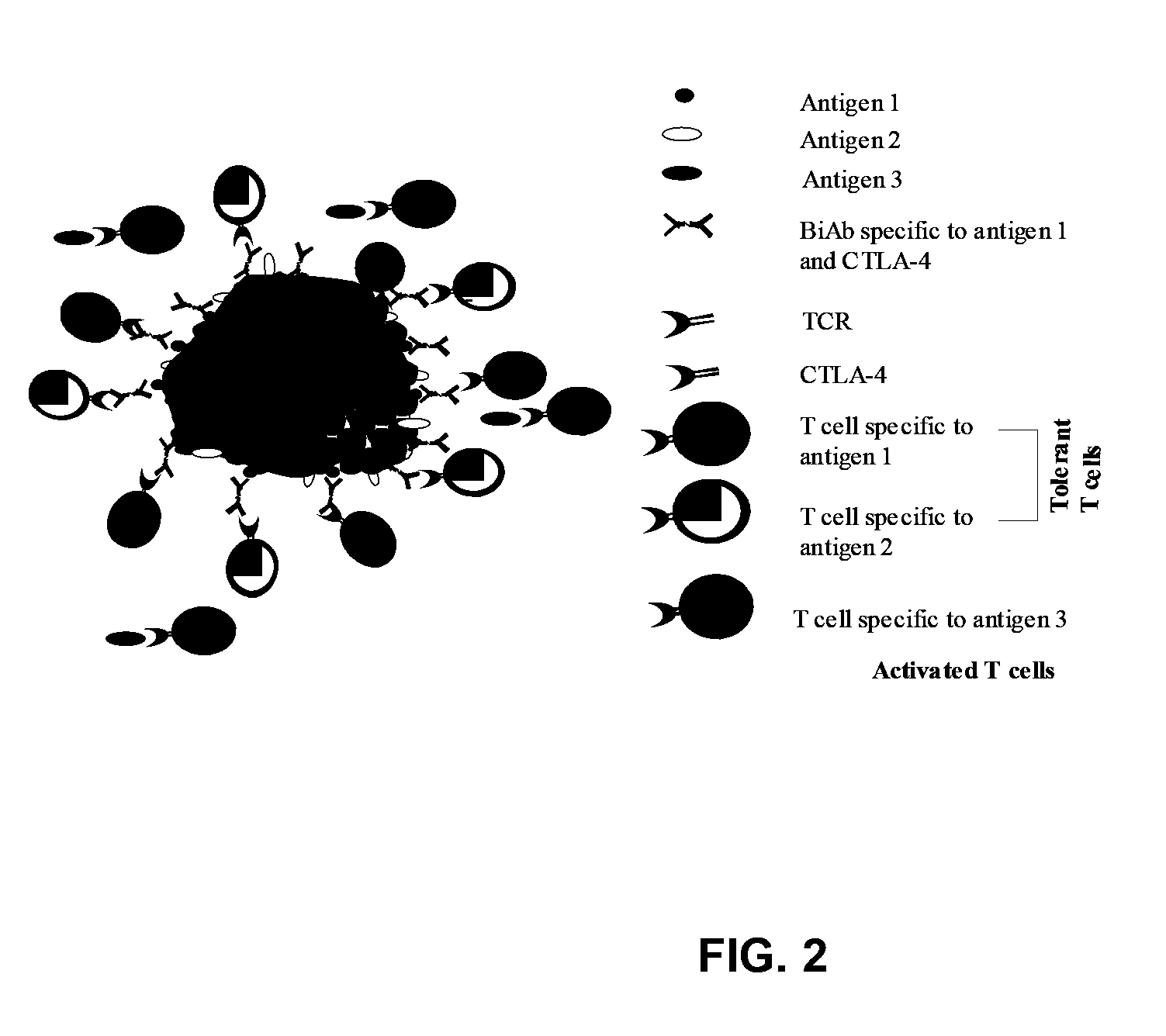
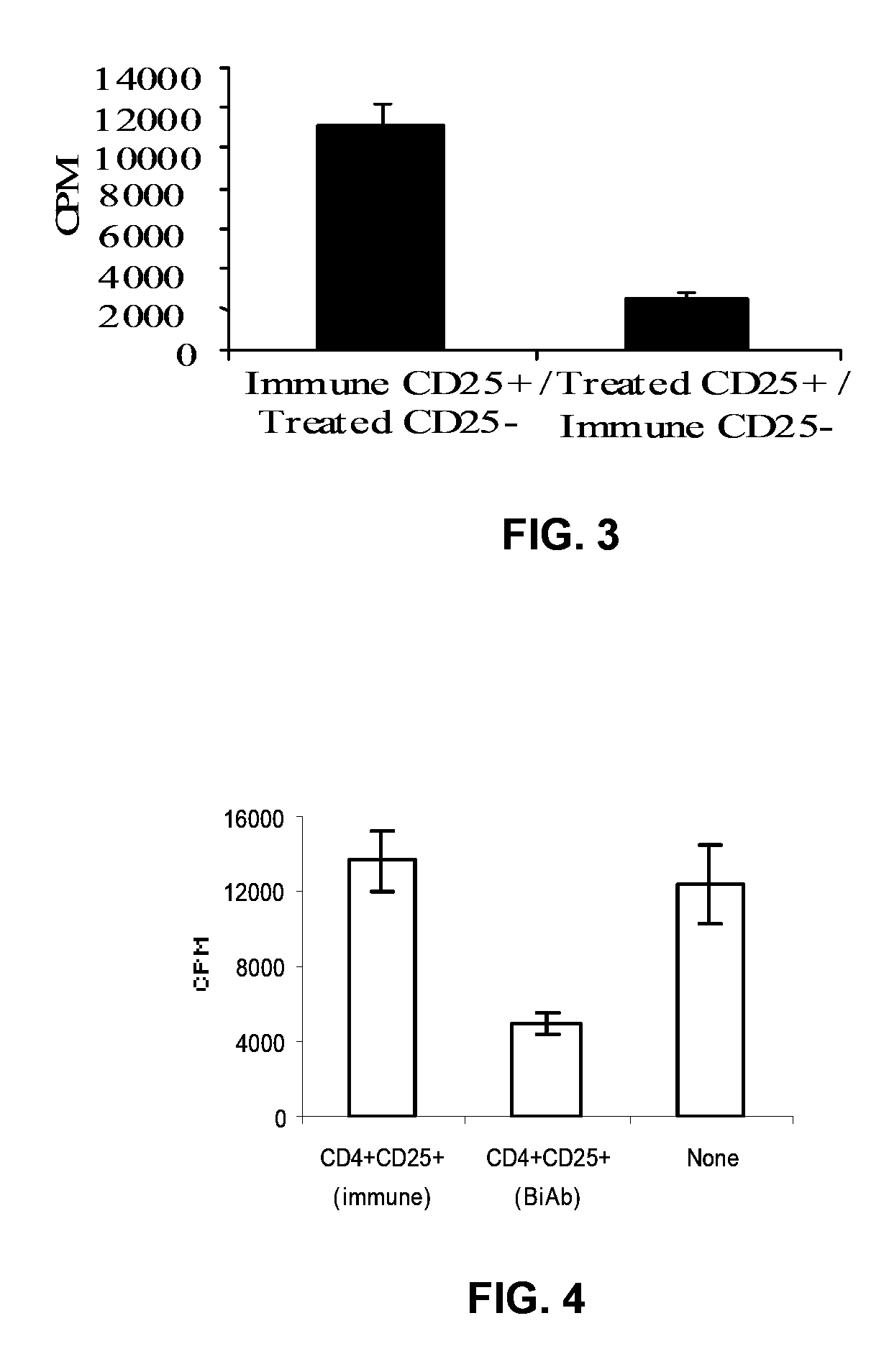
Uses of bispecific antibody coated dendritic cells pulsed with antigens and gm-CSF in immune regulation
GM-CSF administered before immunization exerted a sustained suppressive effect against the induction of myasthenia gravis (MG). This suppression was associated with lowered serum autoantibody levels, reduced T cell proliferative responses to AChR, and an expansion in the population of FoxP3+ regulatory T cells. Manipulating DCs to expand regulatory T cells is useful for the control of autoimmune diseases such as myasthenia gravis MG.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Cell systems and methods for detecting proliferation acitvity
The invention provides proliferative response indicator cell having a vertebrate cell having a luciferase encoding nucleic acid and a heterologous proliferation factor receptor encoding nucleic acid, wherein each of the encoding nucleic acids are operationally linked to expression elements for co-expression of a luciferase polypeptide and a heterologous proliferation factor receptor. The invention also provides a method of determining a cell proliferative response to a proliferation factor. The method includes: (a) contacting a vertebrate cell expressing luciferase and a proliferation factor receptor with a proliferation factor for sufficient time for the proliferation factor to bind to the proliferation factor receptor; (b) culturing the contacted cell expressing luciferase for at least one generation, and (c) measuring the amount of light emission, wherein the luciferase expression is driven from a promoter non-responsive to the proliferation factor and the light emission directly correlates with proliferation factor-mediated cell proliferation. The proliferation factor can be a growth factor, a cytokine or a hormone or an agonist or antagonist thereof. The methods of the invention additionally include determining the effect of an inhibitor of the proliferation factor. Cells used in the method are contacted with a proliferation factor in the presence of a sample suspected of containing an inhibitor of the proliferation factor. The inhibitor can be neutralizing antibody, or binding fragment thereof, to the proliferation factor. The methods of the invention also are applicable as an indicator of cell health or viability. The invention further provides a diagnostic system. The diagnostic system includes a plurality of different vertebrate cell lines each encoding a luciferase gene and a different proliferation factor receptor, the luciferase gene being operationally linked to a promoter non-responsive to a proliferation factor bound by the proliferation factor receptor, wherein light emission from each of the different cell lines being characterized as directly correlating with proliferation factor-mediated cell proliferation.
Owner:AMGEN INC
Synthetic peptides for the treatment of myasthenia gravis
Peptides having at least nine amino acid residues each including an amino acid sequence which corresponds to position p200-208 or p262-266 of the human acetylcholine receptor alpha -subunit, but differing therefrom by one or more amino acid substitutions, are disclosed. These peptides inhibit the proliferative response of human peripheral blood lymphocytes to the myasthenogenic peptides p195-212 and p259-271 and are suitable for treatment of subjects afflicted with myasthenia gravis.
Owner:YEDA RES & DEV CO LTD
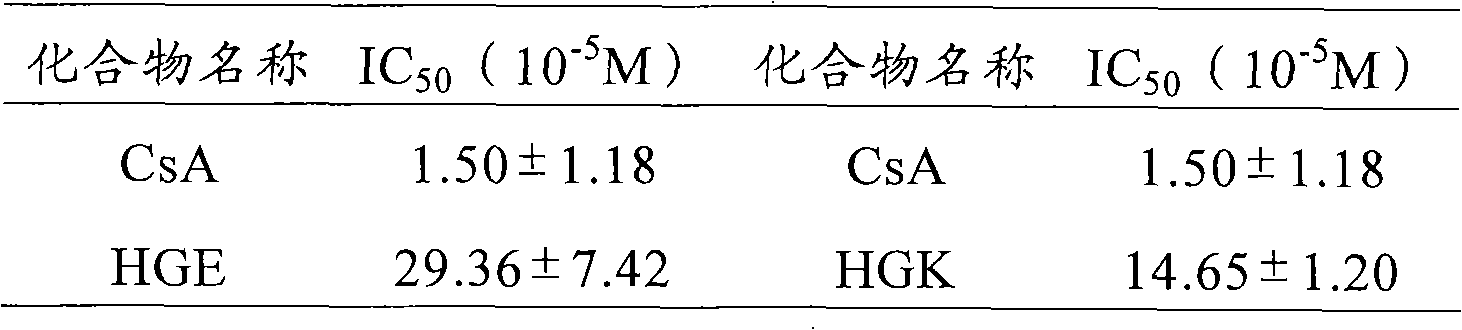
Saturated fatty chain alcohol His-Gly-AA tripeptide ester, synthetic method and application thereof
InactiveCN101899090AExcellent immunosuppressive effectTripeptide ingredientsPeptide preparation methodsChemistrySplenic lymphocyte
The invention relates to 12 saturated fatty chain alcohol His-Gly-AA tripeptide ester conjugates with immunosuppressive activity in a general formula His-Gly-AA-O-CH2-(CH2)nCH3 I, wherein AA stands for Glu or Lys. In saturated fatty chain alcohol, n is equal to 6, 8, 10, 12, 14 or 16. the invention also relates to a preparation method and application of the conjugates as an immunosuppressant. An experimental result of the saturated fatty chain alcohol His-Gly-AA tripeptide ester which has the inhibition effects of splenic lymphocyte mitogen breeder reaction and macrophage phagocytosis activity indicates that the conjugates of the invention have excellent immunosuppressive action and can be clinically used as an immunosuppressant.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Saturated fatty chain alcohol Glu-Asp-Gly tripeptide ester, synthetic method and application thereof
InactiveCN101899085AExcellent immunosuppressive effectTripeptide ingredientsPeptide preparation methodsAlcoholImmunosuppressive effect
The invention relates to 6 saturated fatty chain alcohol Glu-Asp-Gly tripeptide ester conjugates with immunosuppressive activity in a general formula Glu-Asp-Gly-O-CH2-(CH2)nCH3 I. In saturated fatty chain alcohol, n is equal to 6, 8, 10, 12, 14 or 16. The invention also relates to a preparation method and application of the conjugates as an immunosuppressant. An experimental result of the saturated fatty chain alcohol Glu-Asp-Gly tripeptide ester has the inhibition effects of splenic lymphocyte mitogen breeder reaction and macrophage phagocytosis activity indicates that the conjugates of the invention have excellent immunosuppressive action and can be clinically used as an immunosuppressant.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Conjugates of saturated aliphatic chain alcohol, dexamethasone, and Glu-Asp-Gly, preparation, nano structure, and applications thereof
InactiveCN104211760AExcellent immunosuppressive effectAntipyreticAnalgesicsNano structuringSide effect
The invention relates to conjugates of saturated aliphatic chain alcohol, dexamethasone, and Glu-Asp-Gly, preparation, a nano structure, and applications thereof. The invention discloses 6 saturated aliphatic chain alcohol modified dexamethasone-Glu-Asp-Gly conjugates represented by the formula 10 a-f, wherein in the formula the n represents 7, 9, 11, 13, 15, or 17. The invention also discloses a preparation method, a nano structure, immunity inhibition activity, inflammation inhibition activity, and pain relieving activity of the conjugates. The invention also finds that the conjugates do not have any side effect leading to osteoporosis. The researches on the inhibition effect of the conjugates on splenic lymphocyte mitogen proliferation reactions and the survival time after mouse retroauricular cardiac muscle transplant show that the conjugates have an excellent immunity inhibition effect. The researches on the effect of the conjugates on the swelling degree of mouse ears which are inflamed due to xylene show that the conjugates have an excellent anti-inflammation effect. The researches on the effect of the conjugates on mouse heat radiation tail flick time show that the conjugates have an excellent pain-relieving effect. The researches on the effect of the conjugates on the mouse thigh bones show that the conjugates cannot cause osteoporosis. So the conjugates have a wide application prospect in the preparation of immunity inhibition drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Antigen-antibody complex for preventing and/or treating avian influenza
ActiveCN101732716AChange submission pathEnhance immune functionAntiviralsAntibody ingredientsAvian influenza virusOrganism
The invention provides an antigen-antibody complex for preventing and / or treating avian influenza, which comprises inactivation avian influenza totiviruses which are used as antigen and an immune body thereof, and the mass concentration ratio of the antigen and the immune body is more than 1. After entering organisms to perform initial immunization, the antigen-antibody complex stimulates the organisms again to induce immunoreaction, and immune response is quick in speed and strong in reaction; the antigen-antibody complex used as a carrier is more favorable for capturing and presenting antigen presenting cells and can also strengthen a breeder reaction of T cells effectively; purified totiviruses used as the antigen increase the molecular weight of the complex, are more favorable for ingestion of immunocyte of the organisms on the antigen, cause the more extensive immunoreaction quickly, and have a significance for preventing avian influenza viruses of which the antigen is easy for variation. A preparation of the antigen-antibody complex does not need to use solid carriers, does not cause intense stimulus on the organisms or initiates the organisms to generate an adverse reaction, and is simple to prepare and safe and convenient to use.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Conjugates of saturated aliphatic chain alcohol, dexamethasone, and His-Gly-Glu, preparation, nano structure, and applications thereof
The invention relates to conjugates of saturated aliphatic chain alcohol, dexamethasone, and His-Gly-Glu, preparation, a nano structure, and applications thereof. The invention discloses 6 saturated aliphatic chain alcohol modified dexamethasone-His-Gly-Glu conjugates represented by the formula 10 a-f, wherein in the formula the n represents 7, 9, 11, 13, 15, or 17. The invention also discloses a preparation method, a nano structure, immunity inhibition activity, inflammation inhibition activity, and pain relieving activity of the conjugates. The invention also finds that the conjugates do not have any side effect leading to osteoporosis. The researches on the inhibition effect of the conjugates on splenic lymphocyte mitogen proliferation reactions and the survival time after mouse retroauricular cardiac muscle transplant show that the conjugates have an excellent immunity inhibition effect. The researches on the effect of the conjugates on the swelling degree of mouse ears which are inflamed due to xylene show that the conjugates have an excellent anti-inflammation effect. The researches on the effect of the conjugates on mouse heat radiation tail flick time show that the conjugates have an excellent pain-relieving effect. The researches on the effect of the conjugates on the mouse thigh bones show that the conjugates cannot cause osteoporosis. So the conjugates have a wide application prospect in the preparation of immunity inhibition drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Nitrogenatd trans-stilbene analogs, method for the obtention and medical applications thereof
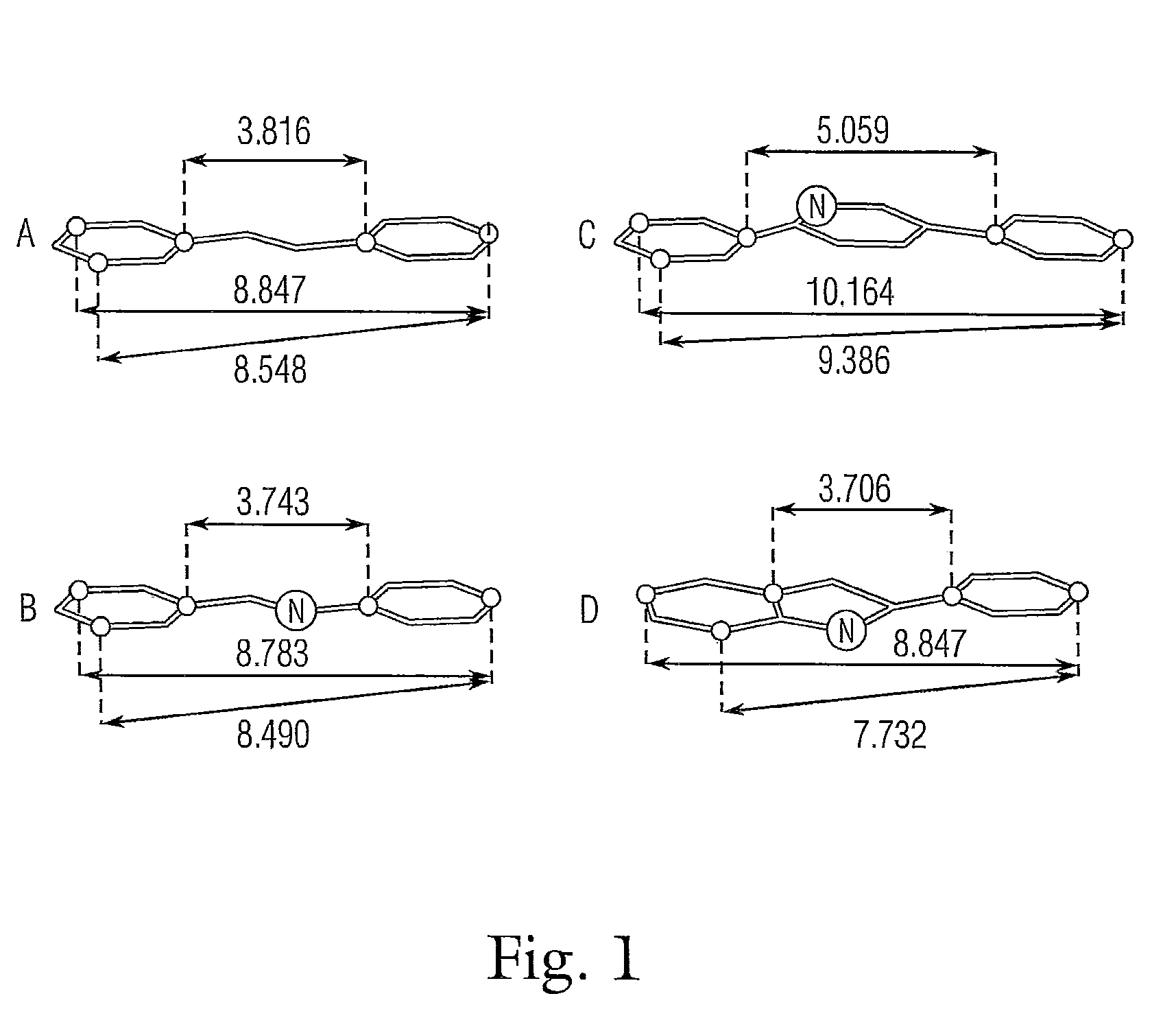
This invention is related to new nitrogenated trans-stilbene analog compounds, more specifically, imine, pyrrol and Indole derivatives, with procedures for the preparation and use thereof as pharmaceutical compositions for the treatment and / or chemoprevention of those mammalian diseases such as cancer, fibrosclerosis and acute / chronic inflammation, graft-versus-host reaction, ischemic-reperfusion tissue injury in stroke and heart attack, neurodegeneration, and during organ transplantation, whose pathogenic and pathophysiological mechanisms depend on or are significantly contributed by undesirable oxidative stress, angiogenic and proliferative responses.
Owner:DOMINION PHARMAKINE +1
Porcine circovirus 2 immune protection polypeptide and vaccine comprising same
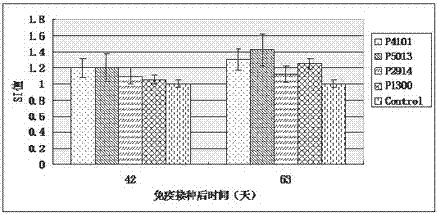
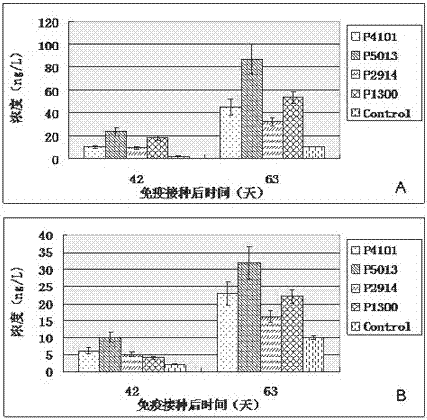
ActiveCN104098657AHigh titerRaise antibody levelsViral antigen ingredientsVirus peptidesPeptide vaccineCytokine
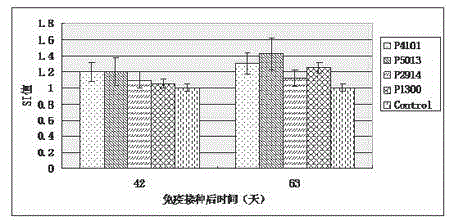
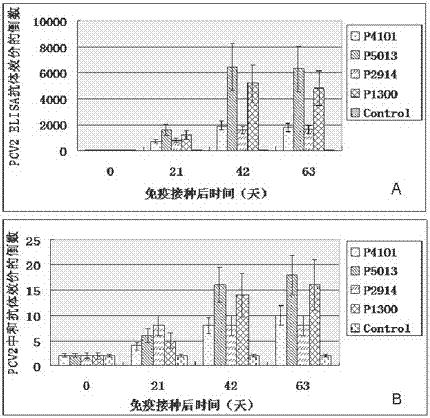
The invention relates to the technical field of porcine circovirus, in particular to a porcine circovirus 2 (PCV2) immune protection polypeptide, and a vaccine containing the PCV2 immune protection polypeptide. The amino acid sequence of the PCV2 immune protection polypeptide is shown in the sequence table 1-4. According to the vaccine, tests show that after 42-63 days of first immune of a vaccine of P5013 and a vaccine of P1300, the antibody titer and the neutralizing antibody titer of an ELISA antibody are remarkably increased, the lymphocyte proliferative response and IL-4 and IFN-Gamma cytokines are significantly higher than those of a control group, and the antibody level and the cellular immunity level of the vaccine group of P5013 are the highest; mice tests show that PCV2 immune response induced by the synthetic peptide vaccine of P5013 is the highest and the strongest; pig tests find that the synthetic polypeptide vaccine of P5013 can induce pigs to generate a PCV2-specific immune response, can reduce viremia caused by PCV2 virulent virus attack, alleviate the clinical symptoms and pathological lesions of viremia, and has immune protection effect on PCV2.
Owner:NANJING AGRICULTURAL UNIVERSITY
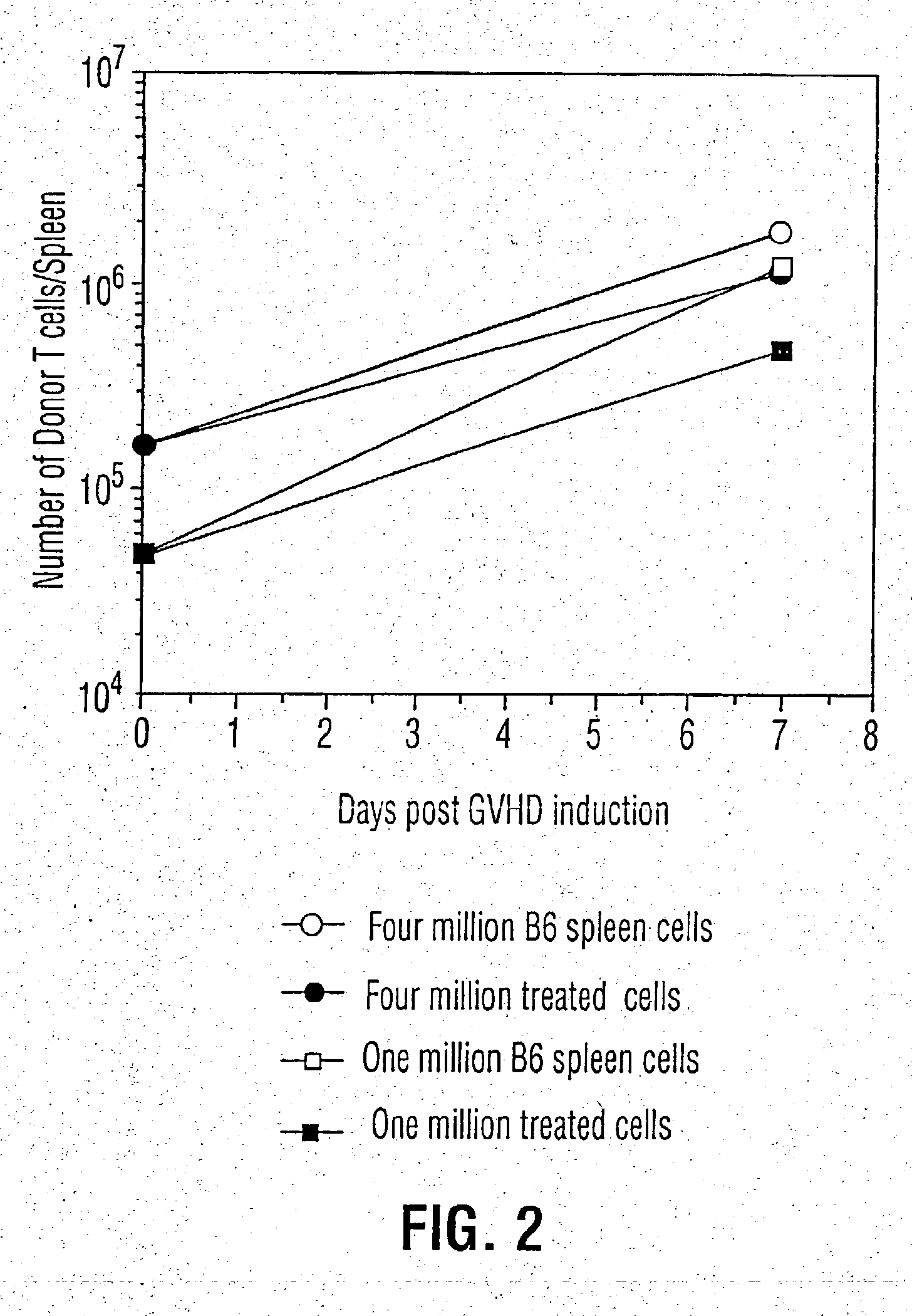
Inhibition of graft versus host disease
The development of graft versus host disease in a mammalian patient undergoing cell transplantation therapy for treatment of a bone marrow mediated disease, is prevented or alleviated by subjecting at least the T-cells of the allogeneic cell transplantation composition, extracorporeally, to oxidative stress, in appropriate dosage amounts, such as bubbling a gaseous mixture of ozone and oxygen through a suspension of the T-cells. The process may also include irradiation of the cells with UV light, simultaneously with the application of the oxidative stress. The oxidative stress induces reduced inflammatory cytokine production and a reduced proliferative response in the T-cells.
Owner:VASOGEN IRELAND LTD
Clone, expression and application of BALF4 polypeptide
InactiveCN107163109ASimple purification methodStimulate the immune systemFungiViral antigen ingredientsYeastLymphocyte proliferation
The invention provides clone, expression and application of BALF4 polypeptide and relates to the technical field of molecular biology. According to the clone, the expression and the application, through gene optimization, an optimized nucleotide sequence encoding the BALF4 polypeptide is obtained, high-expression yeast cells for recombining the BALF4 polypeptide are constructed, a purification method is optimized, and the BALF4 polypeptide is obtained finally. The BALF4 polypeptide provided by the invention can stimulate production of a high-level antigen specific antibody, induces mouse splenic lymphocyte proliferative response, can effectively stimulate a body immune system, has stronger immunogenicity, can be used as an immune adjuvant and is used for improving the prevention effect on an EB virus vaccine so as to reach the purpose of effectively preventing EB virus infection.
Owner:QINGDAO UNIV
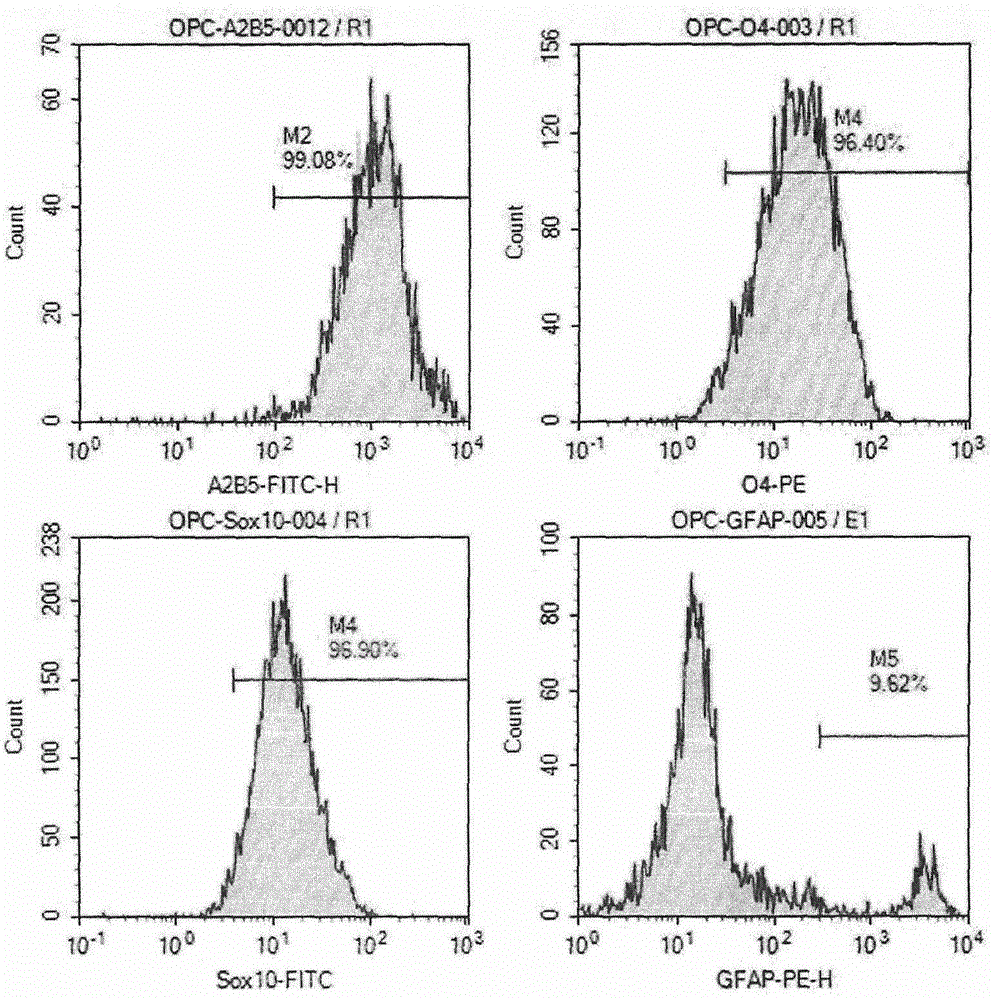
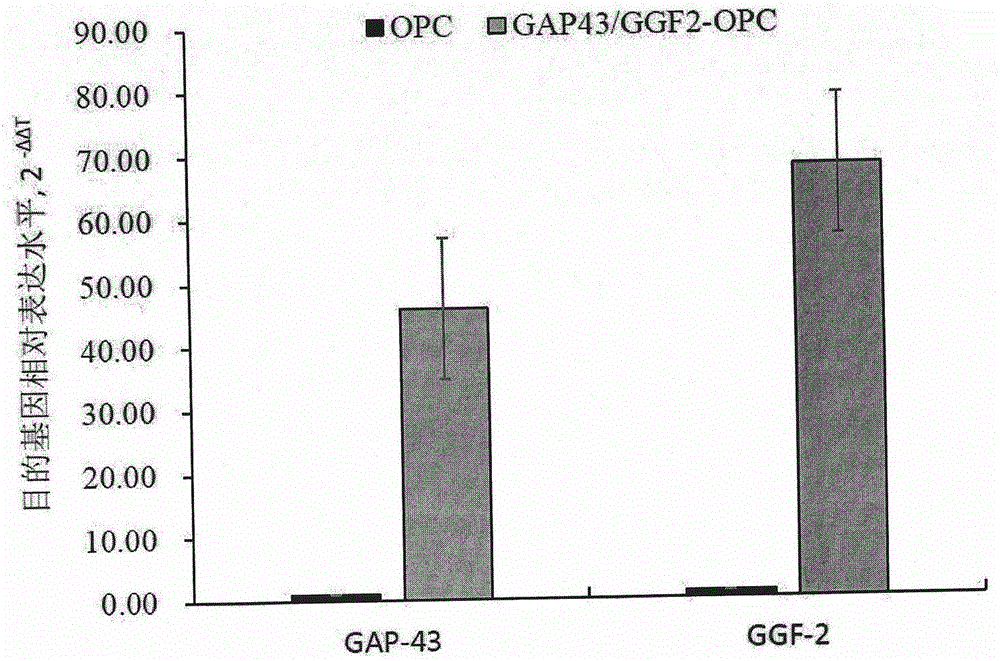
Preparation method of human oligodendrocyte precursor cell inhibiting nerve secondary injury, kit and application thereof
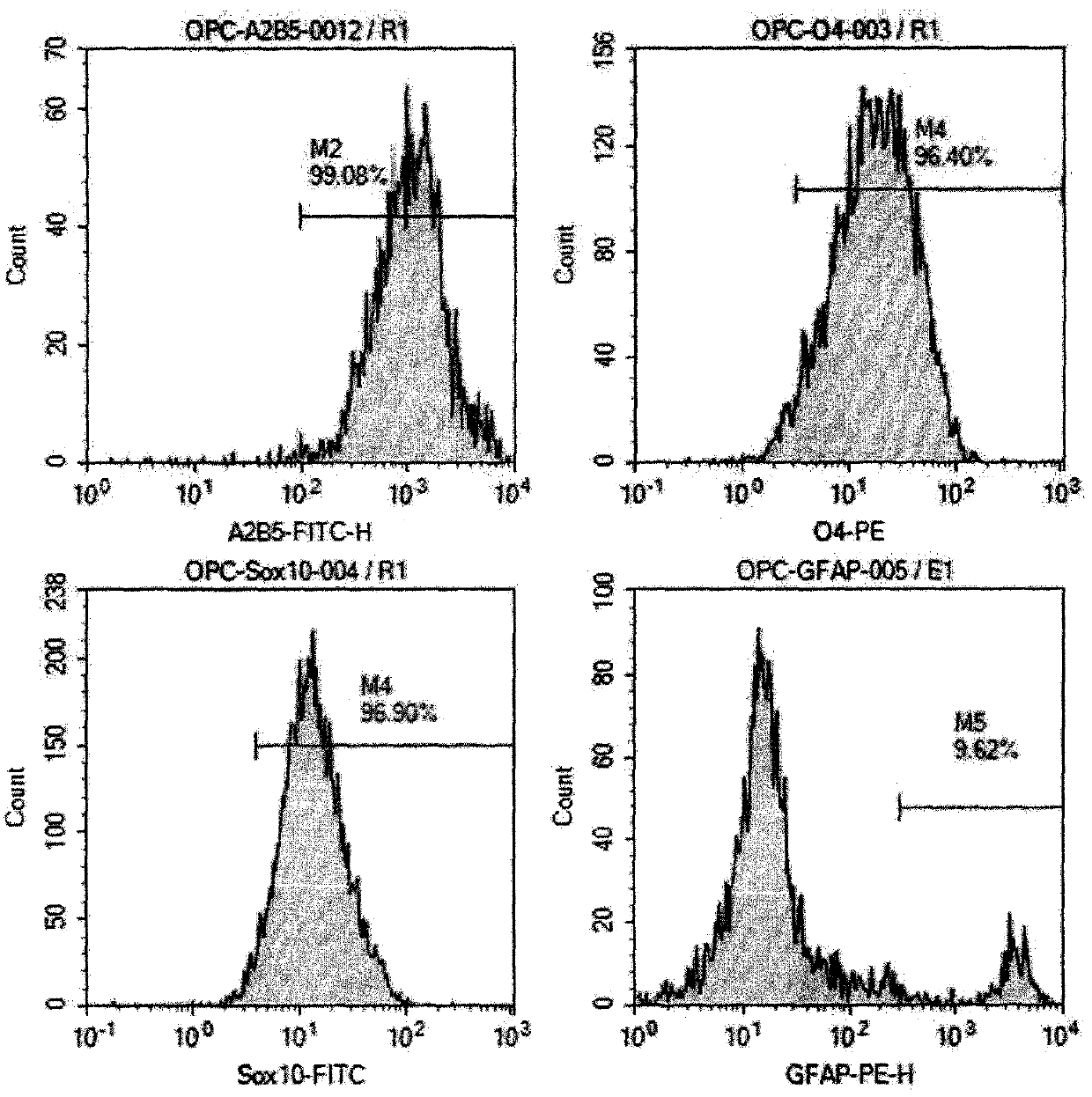
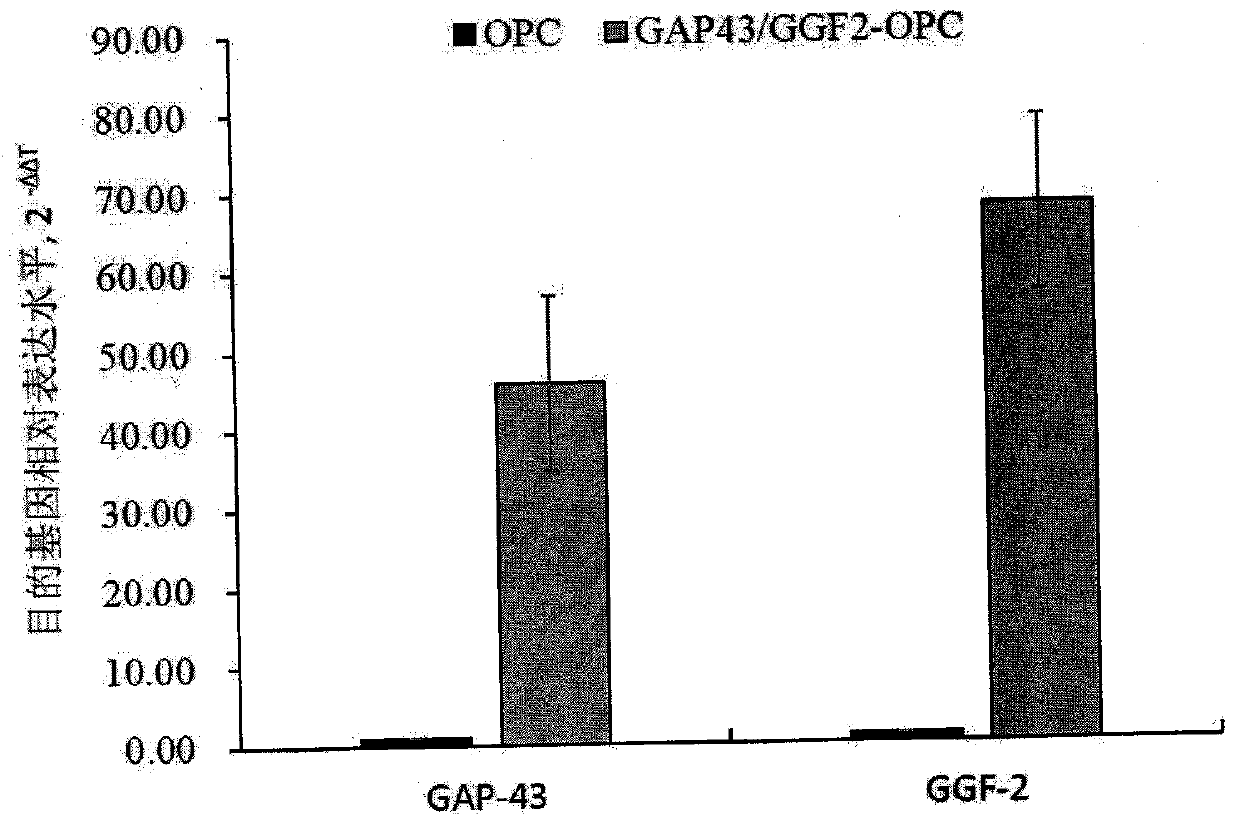
ActiveCN106011173AInhibition of secondary damageProtect physiological functionNervous disorderNeuregulinsVirus typeGlial Growth Factor
The invention provides a preparation method of a human oligodendrocyte precursor cell inhibiting nerve secondary. Specifically, the method includes: constructing a virus expression vector of two glial growth factors, conducting cotransfection of a human oligodendrocyte precursor cell to prepare the human oligodendrocyte precursor cell, which can achieve high expression of the two glial growth factors, i.e. a growth related factor 43 and a glial growth factor 2, give play to the combined action of the two to promote oligodendrocyte repair, and inhibit nerve secondary injury caused by proliferative response and glial scar formation after oligodendrocyte injury. Also, the proliferation and differentiation ability of oligodendrocyte precursor cell itself is also utilized to participate in nerve function repair, and no tumorigenesis exists. The invention also provides a kit for preparation of the human oligodendrocyte precursor cell inhibiting nerve secondary. The human oligodendrocyte precursor cell provided by the invention has very good therapeutic effect on nervous system diseases.
Owner:江西美奥生物技术有限公司
Protease-activated receptor 3 and uses thereof
Disclosed are cDNAs and genomic DNAs encoding protease-activated receptor 3 (PAR3) from mouse and human, and the recombinant polypeptides expressed from such cDNAs. The recombinant receptor polypeptides, receptor fragments and analogs expressed on the surface of cells are used in methods of screening candidate compounds for their ability to act as agonists or antagonists to the effects of interaction between thrombin and PAR3. Agonists are used as therapeutics to treat wounds, thrombosis, atherosclerosis, restenosis, inflammation, and other thrombin-activated disorders. Antagonists are used as therapeutics to control blood coagulation and thereby treating heart attack and stroke. Antagonists mediate inflammatory and proliferative responses to injury as occur in normal wound healing and variety of diseases including atherosclerosis, restenosis, pulmonary inflammation (ARDS) and glomerulosclerosis. Antibodies specific for a protease-activated receptor 3 (or receptor fragment or analog) and their use as a therapeutic are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Adjuvant therapy
InactiveUS7214380B1Decrease IgE productionReduce productionBacterial antigen ingredientsPeptide/protein ingredientsAdjuvantTh2 cytokines
Methods are provided for the treatment of allergic and other immune disorders associated with overproduction of Th2 type cytokines by antigen specific T cells. Immunotherapy with adjuvants, as provided in the present invention, greatly inhibits the development of airway hyperreactivity and airway inflammation. Such immunotherapy is shown to reverse ongoing airway disease, and convert allergic inflammatory responses into protective immune responses. Conditions of particular interest include allergic conditions associated with production of Th2 cytokines and / or IgE antibodies, asthma, allergic rhinitis, and anaphylactic reactions. The addition of adjuvant induces a Th1-type immune response and can redirect an established Th2-type response to a Th1-type response for the selected antigen. Preferably, antigen-specific IgE production is reduced without altering the intensity of the antigen-specific proliferative response. One particularly preferred adjuvant for use in accordance with the present invention is a Listeria adjuvant.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Inhibition of graft versus host disease
InactiveUS20030108536A1Reduce development riskReduce productionBiocideElectrotherapyHost diseaseT cell
The development of graft versus host disease in a mammalian patient undergoing cell transplantation therapy for treatment of a bone marrow mediated disease, is prevented or alleviated by subjecting at lest the T-cells of the allogeneic cell transplantation composition, in admixture with red blood cells of the donor, extracorporeally, to oxidative stress, in appropriate dosage amounts, such as bubbling a gaseous mixture of ozone and oxygen through a suspension of the T-cells. The process may also include irradiation of the cells with UV light, simultaneously with the application of the oxidative stress. The oxidative stress induces reduced inflammatory cytokine production and a reduced proliferative response in the T-cells.
Owner:VASOGEN IRELAND LTD
Composition for improving proliferative response during adapation of gastrointestinal tract and use in short bowel syndrome
InactiveCN1411347ADigestive systemPharmaceutical non-active ingredientsDocosahexaenoic acidProliferative response
Methods for the treatment of short bowel syndrome in patients in need thereof are provided, comprising the administration to the patients of an effective amount of a formulation comprising arachidonic acid and docosahexanoic acid.
Owner:WYETH LLC
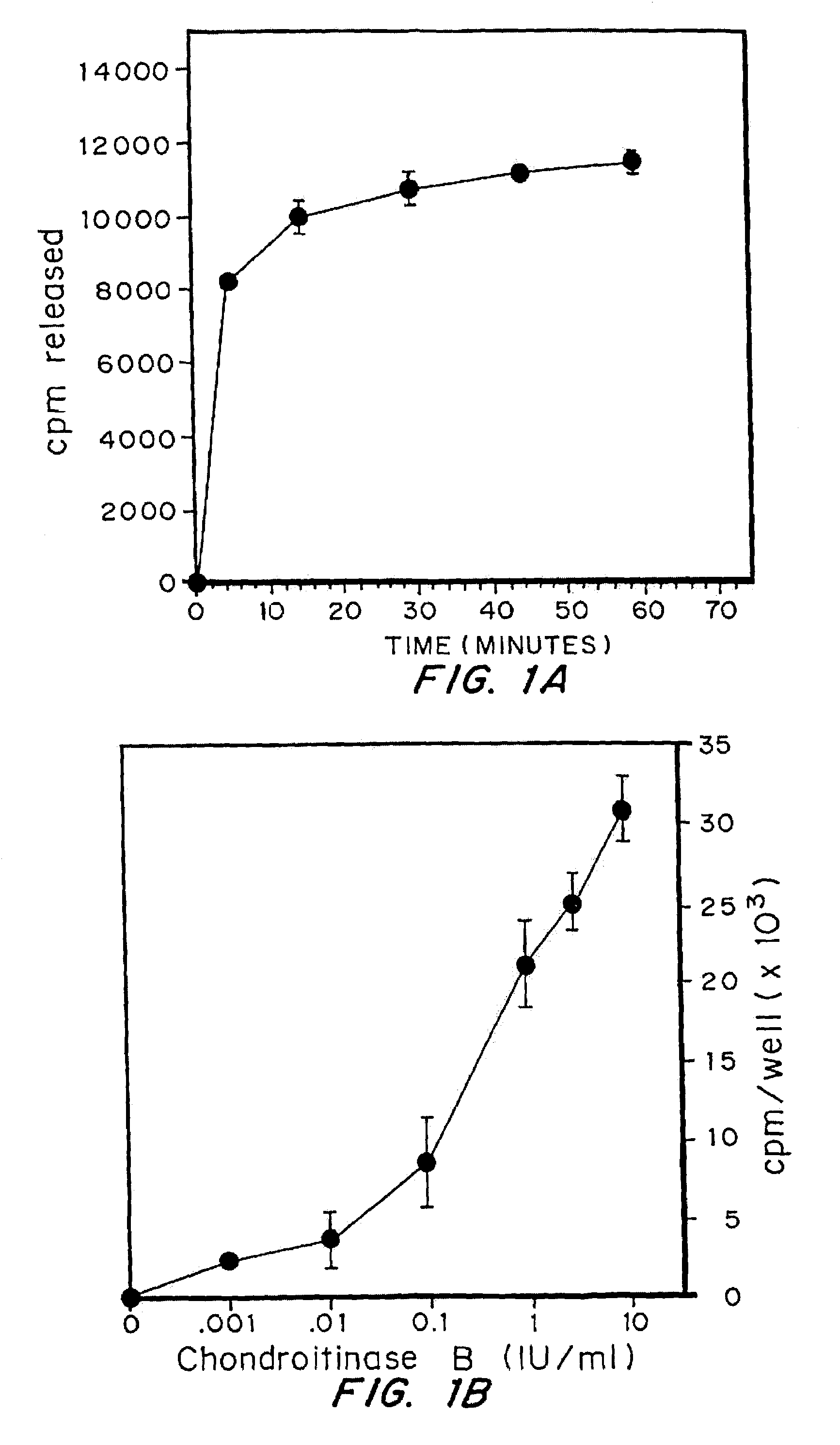
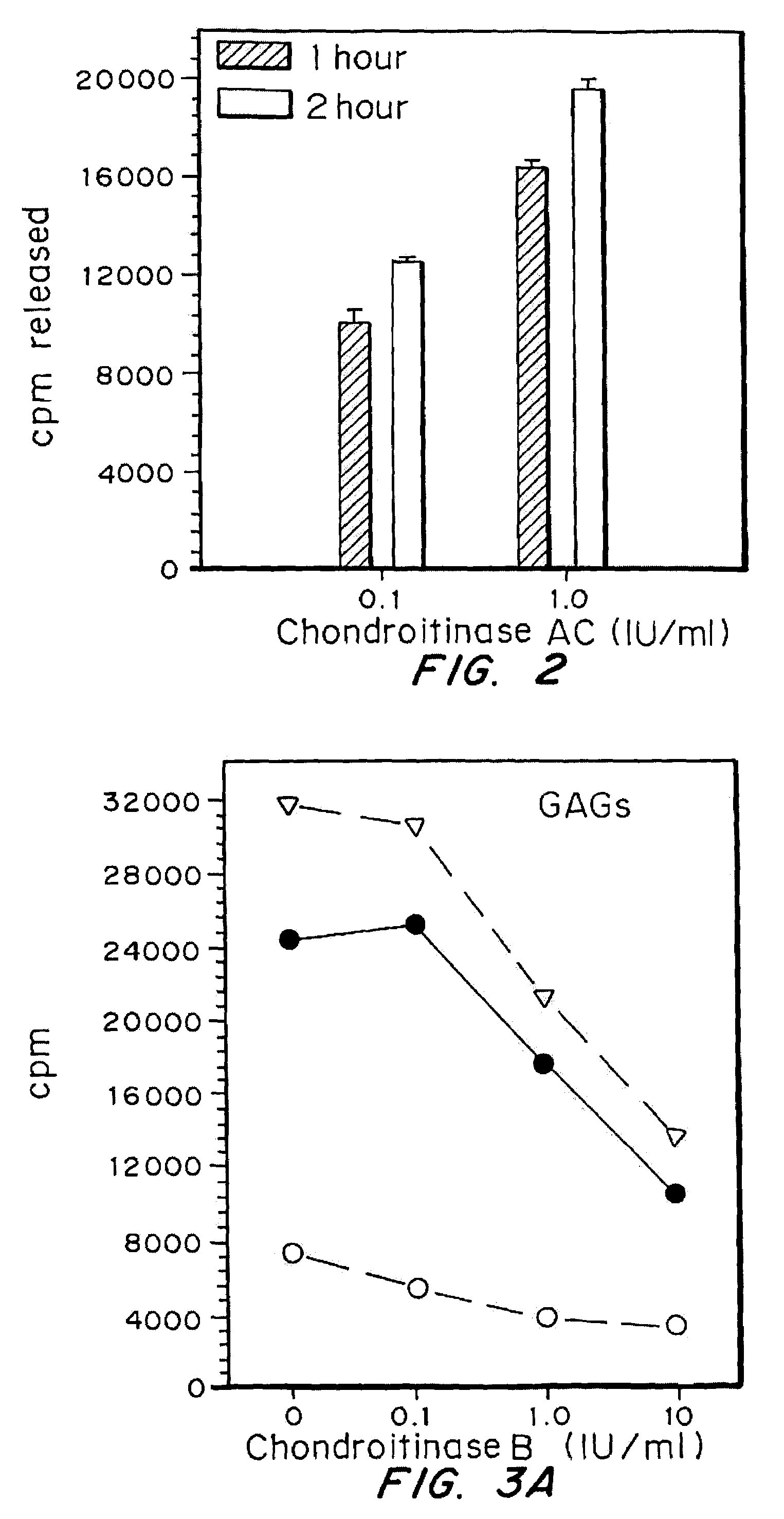
Attenuation of fibroblast proliferation
InactiveUS7056711B2Promote growthReduce secretionPeptide/protein ingredientsSkeletal disorderDiseaseCell-Extracellular Matrix
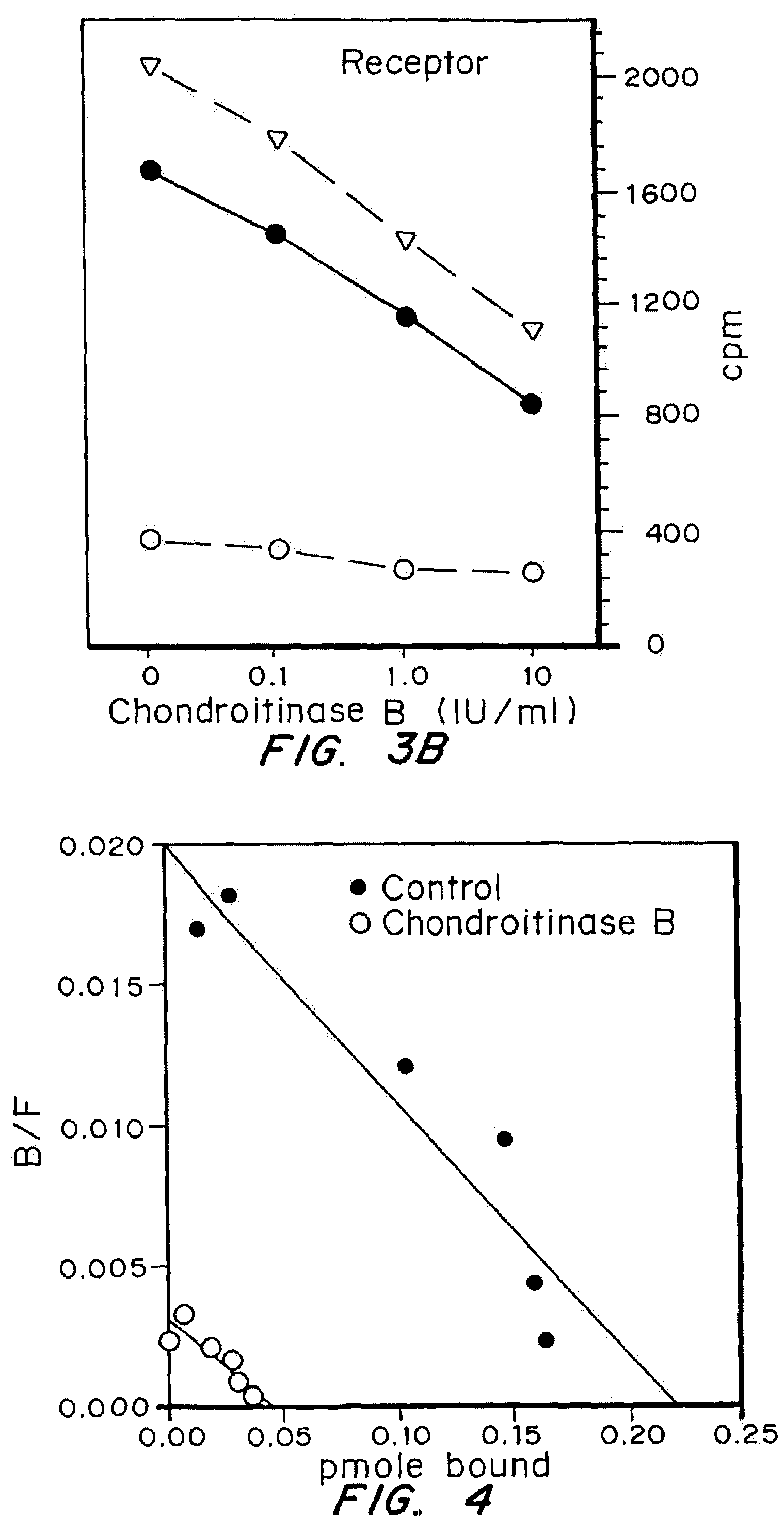
Highly purified and specific glycosaminoglycan degrading enzymes, chondroitinase B and chondroitinase AC, are used to treat fibroproliferative diseases. The enzymatic removal of chondroitin sulfate B(dermatan sulfate), and to a lesser extent, chondroitin sulfate A or C, from cell surfaces effectively decreases growth factor receptors on the cells and thereby decreases the cell proliferative response to such growth factors. In addition, removal of chondroitin sulfates reduces secretion of collagen, one of the major extracellular matrix components. Through the combined inhibition of fibroblast proliferation and collagen synthesis, treatment with chondroitinase B or chondroitinase AC decreases the size of fibrous tissue found in psoriasis, scleroderma, keloids, pulmonary fibrosis and surgical adhesions.
Owner:BIOMARIN PHARMA INC
Kit and applications of beta-glucan extract product in immunological enhancement of poultry animals
PendingCN110433288AEnhanced proliferative responseQuick responseSsRNA viruses negative-senseOrganic active ingredientsMortality rateCell wall
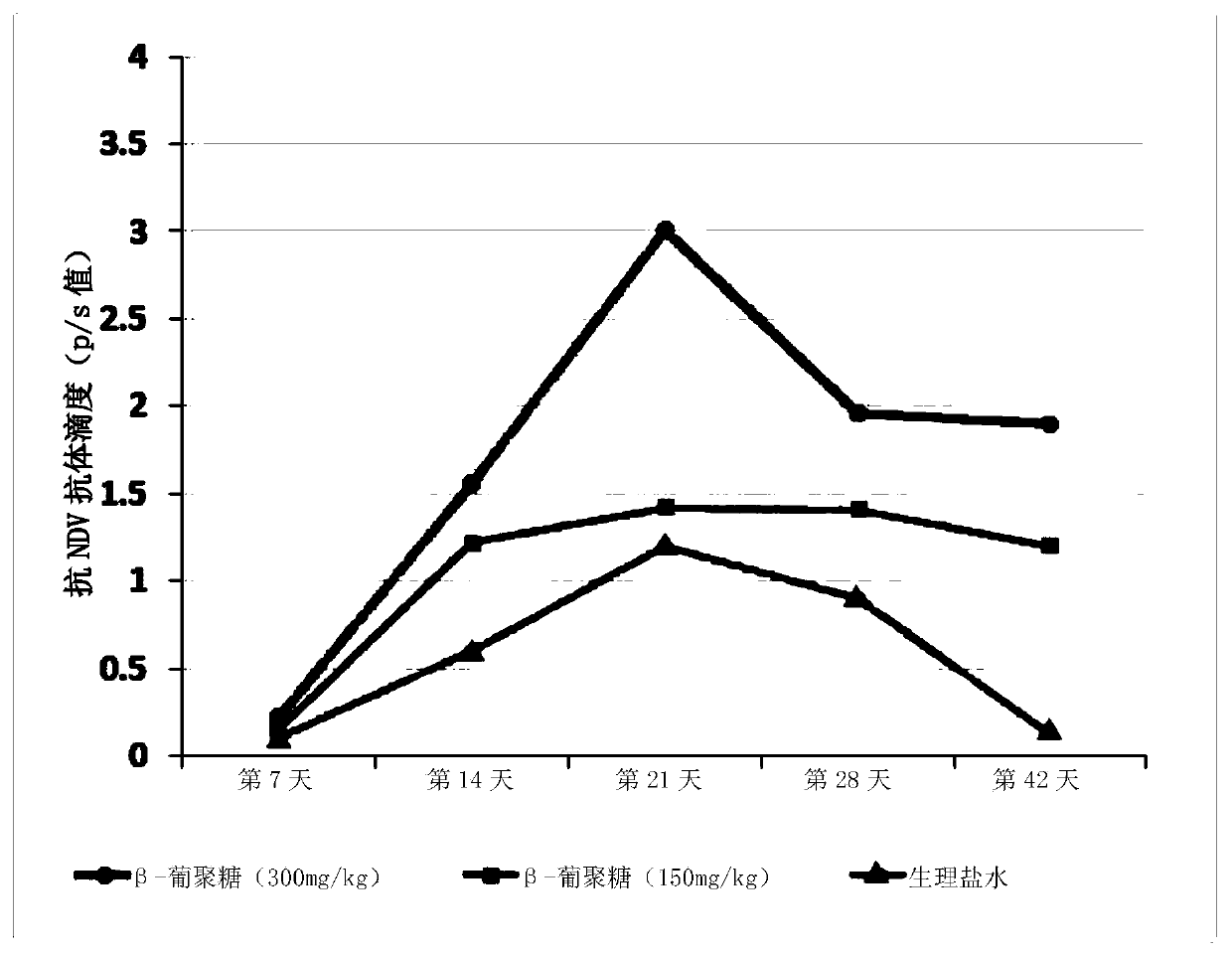
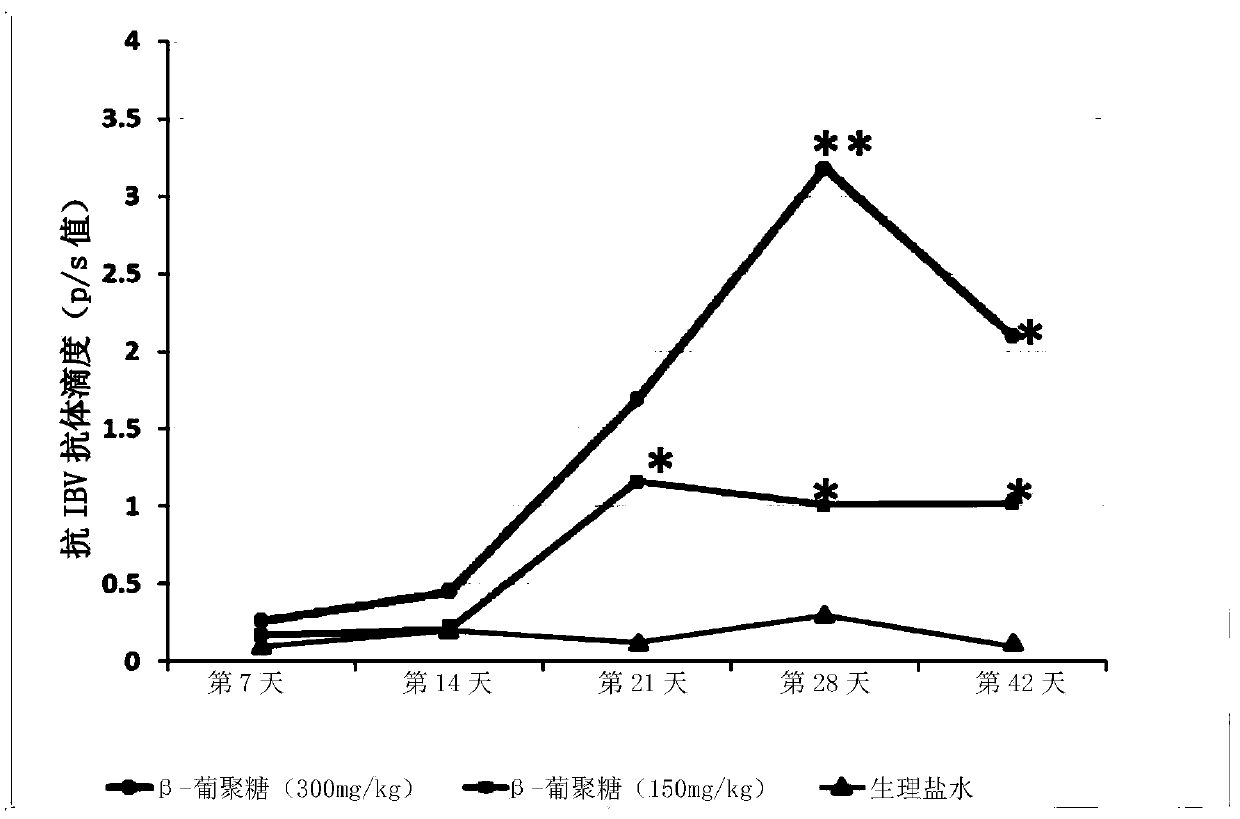
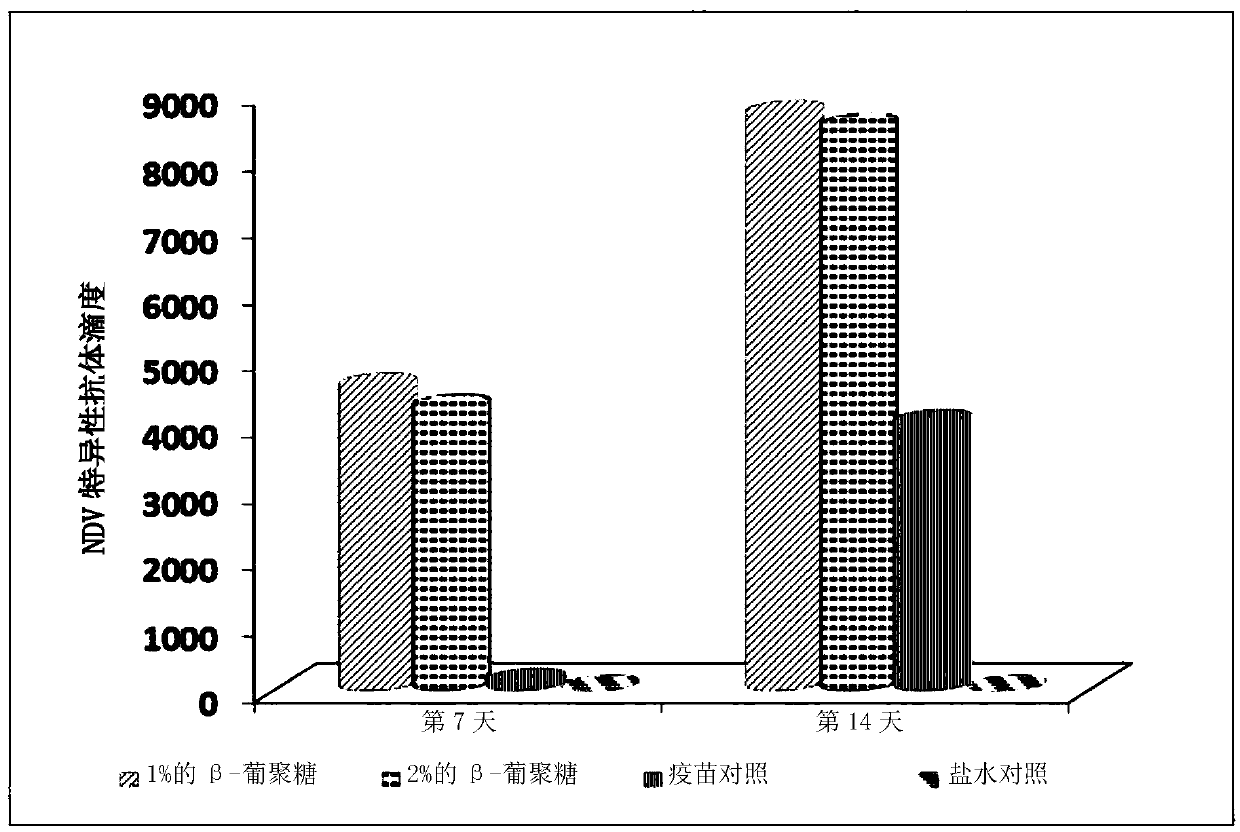
The invention relates to applications of a beta-glucan extract product in the immunological enhancement of poultry animals, and specifically provides a kit. The kit includes as follows: (1) an immune-enhancer used for enhancing the immunity of poultry animals and includes a beta-glucan extract product extracted from saccharomyces cerevisiae cell walls; and (2) a virus vaccine. Applications of thebeta-glucan extract product extracted from the saccharomyces cerevisiae cell walls in the preparation of the kit and immune-enhancer for enhancing the immunity of the poultry animals are also provided. Immunological enhancement can be performed on the poultry animals by using the beta-glucan extracted from the saccharomyces cerevisiae cell walls, so that lymphocyte proliferative response and T cell response of the poultry animals inoculated with the vaccine can be enhanced; viral load can be reduced; pathogenic rates or mortality can be lowered; antibodies can be generated in advance; antibodyproduction can be enhanced; and / or the diffusion of viruses can be restricted.
Owner:LESAFFRE & CIE
A preparation method, kit and application of human oligodendrocyte progenitor cells that inhibit secondary nerve injury
ActiveCN106011173BInhibition of secondary damageProtect physiological functionNervous disorderNeuregulinsGlial Growth FactorTherapeutic effect
The invention provides a preparation method of a human oligodendrocyte precursor cell inhibiting nerve secondary. Specifically, the method includes: constructing a virus expression vector of two glial growth factors, conducting cotransfection of a human oligodendrocyte precursor cell to prepare the human oligodendrocyte precursor cell, which can achieve high expression of the two glial growth factors, i.e. a growth related factor 43 and a glial growth factor 2, give play to the combined action of the two to promote oligodendrocyte repair, and inhibit nerve secondary injury caused by proliferative response and glial scar formation after oligodendrocyte injury. Also, the proliferation and differentiation ability of oligodendrocyte precursor cell itself is also utilized to participate in nerve function repair, and no tumorigenesis exists. The invention also provides a kit for preparation of the human oligodendrocyte precursor cell inhibiting nerve secondary. The human oligodendrocyte precursor cell provided by the invention has very good therapeutic effect on nervous system diseases.
Owner:江西美奥生物技术有限公司
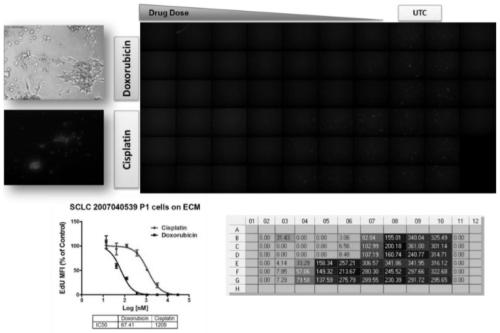
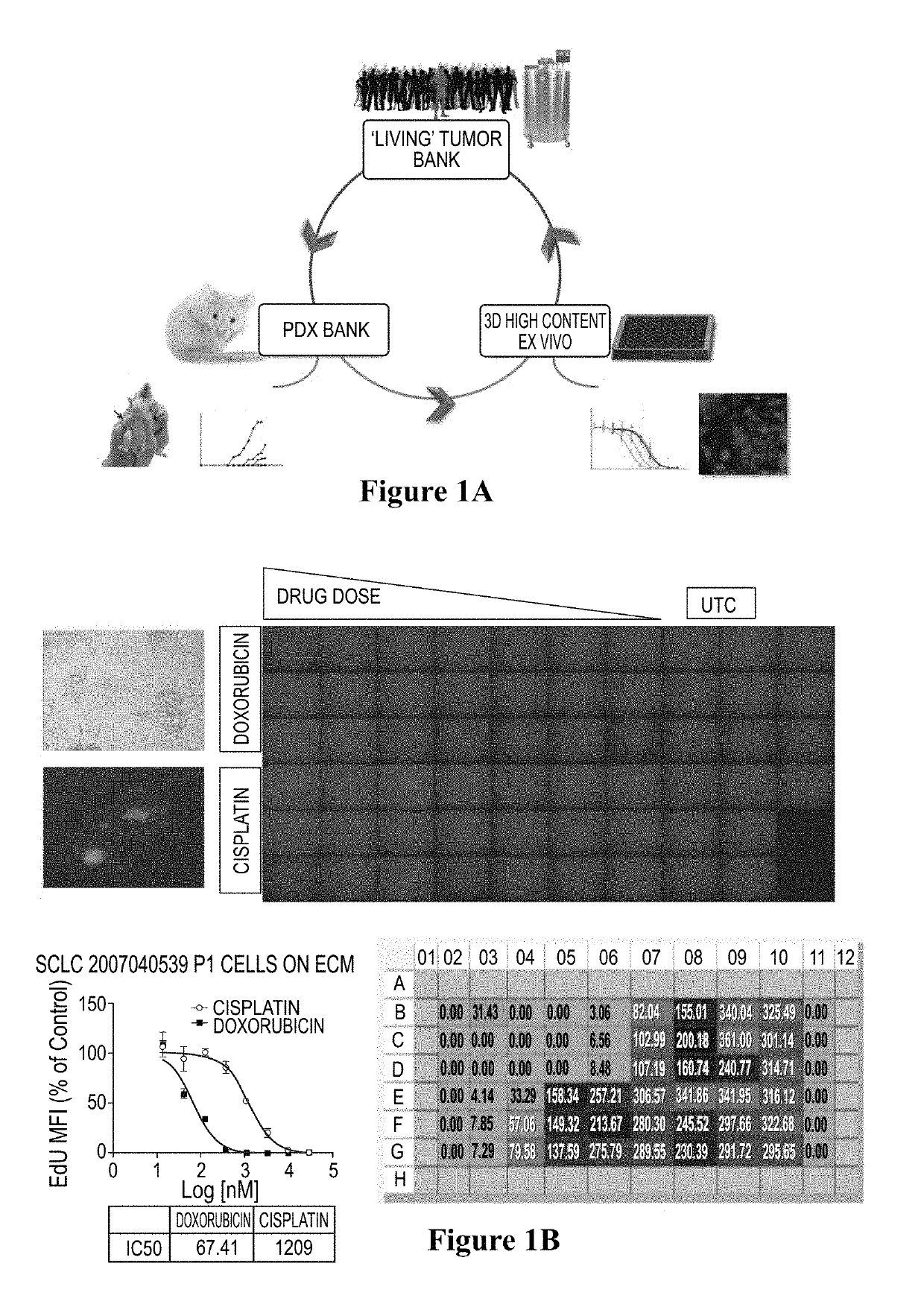
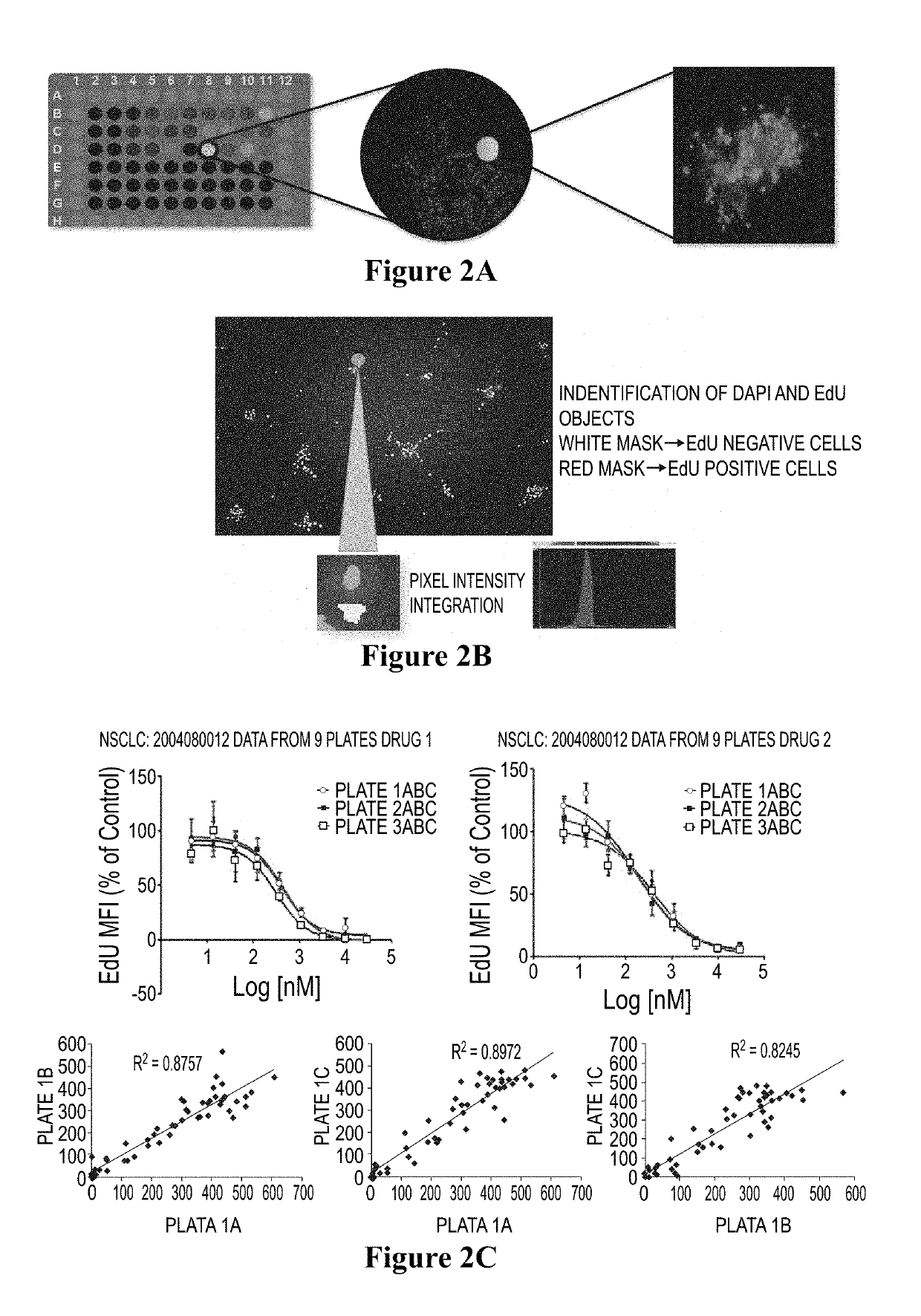
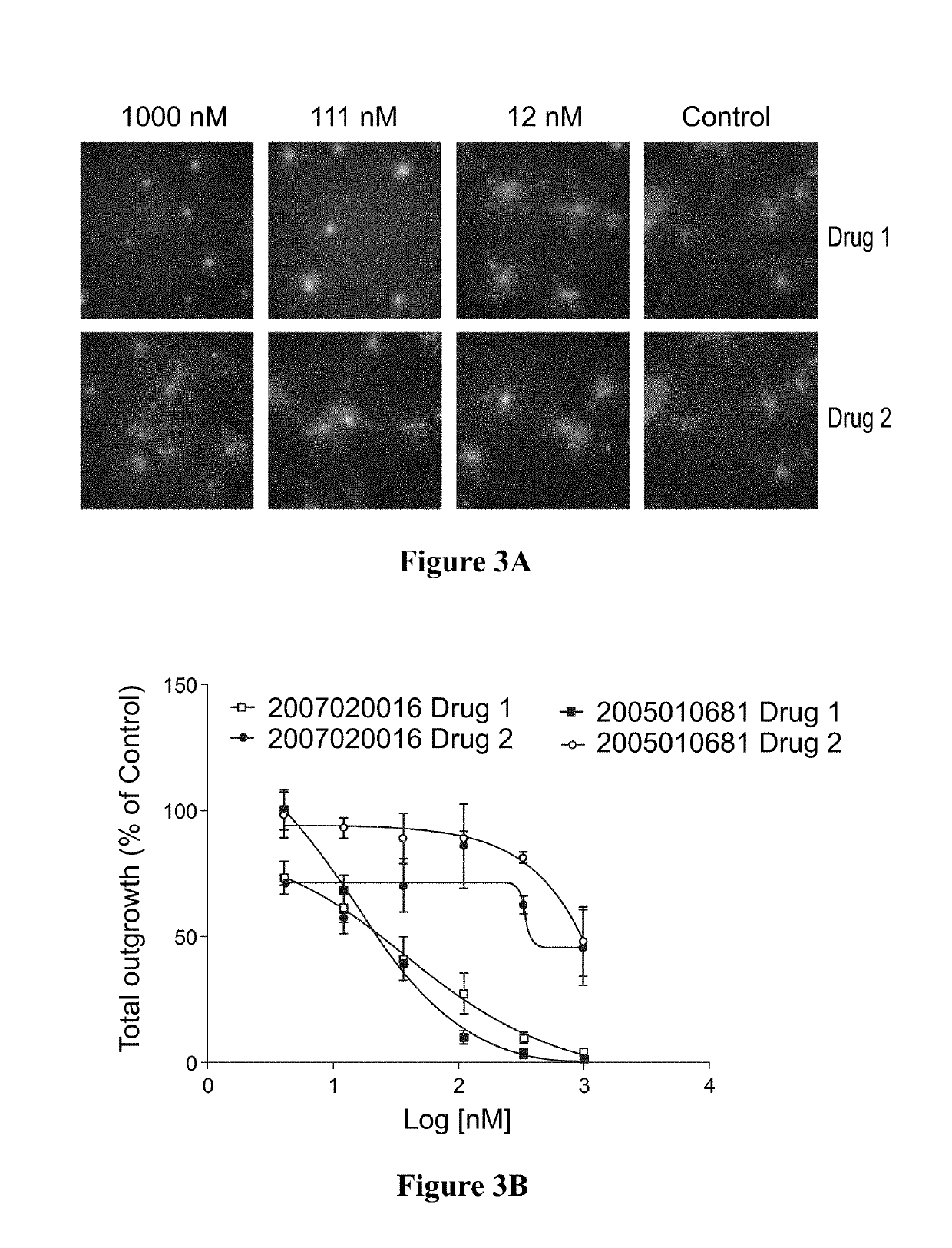
Method for testing proliferative response of drug to tumor cell derived from patient
PendingCN110029141AReliable simulationPredicted responseCompound screeningApoptosis detectionDrug testing methodsCell-Extracellular Matrix
The invention relates to use of a 3D cell culture technology in an in-vitro drug test method, and more particularly to a method for testing a proliferative response of a drug to tumor cell derived from a patient, which can reliably screen the candidate drugs by predictive proliferation assay for efficacy and / or mechanism of action. The method comprises the steps of: (1) obtaining cells from a biopsy or tumor resection material; (2) culturing the obtained cells on a 3D extracellular matrix; (3) treating the tumor cells cultured on the 3D extracellular matrix with drug treatment; (4) performinghigh-content imaging on the processed tumor cells; and (5) evaluating the high-content imaging of the processed tumor cells, and testing the proliferative response of the drug to the tumor cells derived from the patient.
Owner:SHANGHAI BIODURO BIOLOGICS
3D cell culture and ex vivo drug testing methods
Provided herein are methods for testing proliferative responses of a drug on patient-derived tumor cells; the method comprising obtaining cells from biopsy or tumor resection material; culturing the cells on a 3D extracellular matrix (ECM); treating the cells in ECM with a drug; subjecting the treated cells to high-content (HC) imaging; and evaluating the HC imaging of the treated cells; thereby testing the proliferative responses of the drug on the patient-derived tumor cells. In some embodiments, the methods disclosed herein comprise obtaining cells from biopsy or tumor resection material; xenografting the cells into an animal model (patient-derived xenograft; PDX) for tumor formation; and obtaining tumor cells from the animal.
Owner:MOLECULAR RESPONSE
DC cell preparation method for improving antigen presenting T cell and improving T cell killing efficiency and application thereof
ActiveCN114032212AHigh killing efficiencyHigh antigen presentation efficiencyMammal material medical ingredientsBlood/immune system cellsT lymphocyteTumor antigen
The invention provides a DC cell preparation method for improving antigen presenting T cells and improving T cell killing efficiency and application thereof. A tumor antigen modified by 4-phenyl isothiocyanate alpha-D-mannopyranoside glycosylation is loaded on a DC cell, SDF-1 and IL-8 chemotactic factors are added, the DC cell is chemotactic, more mature DCs are obtained, interaction between the DC and T cell is enhanced, T cell activation is further promoted, a gene expression silencing agent shRNA is introduced into the DC cell, an IDO gene is silenced, the surface antigen of the DC is obviously increased, and the proliferation reaction of T lymphocytes is enhanced and stimulated; after the DC cells and the T cells are subjected to mixed culture, the pH value is adjusted, and the DC cells and the T cells are cultured in a weak acid environment, so that the antigen presentation efficiency of the DC cells and the T cells is improved. The result shows that the antigen presentation efficiency between the DC cells and the T cells is higher, and the T cells with higher antigen specificity and higher killing efficiency can be obtained through stimulation.
Owner:北京荟科柘生物科技有限公司
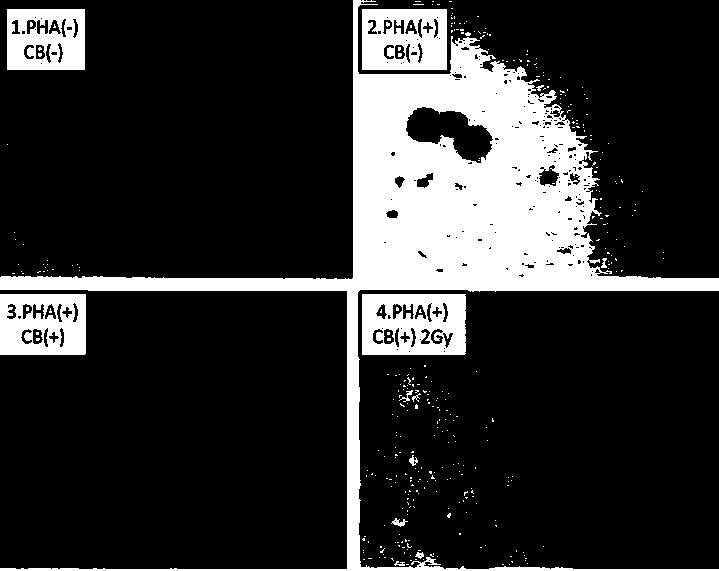
Method for detecting lymphopoiesis by utilizing medicament cytochalasin B
InactiveCN104017876AMorphological observation is intuitive and objectiveReduce experiment costMicrobiological testing/measurementMaterial analysisBiophysicsBinucleated cells
The invention belongs to the technical field of biological detection, and particularly relate to a method for detecting lymphopoiesis by utilizing a medicament cytochalasin B. The principle of the invention is that the medicament cytochalasin B can retard cell division and cannot retard nuclear division, so that cells which are subjected to breeder reaction are treated by the cytochalasin B to form visual binucleated cells; peripheral blood lymphocyte can enable corresponding lymphocyte to be cloned and multiplicated under the stimulation of phytohemagglutinin, and ionizing radiation can inhibit multiplication of lymphocyte; human peripheral blood can be irradiated by a 2Gygamma ray, and the cytochalasin B is added to treat after the stimulation by PHA (phytohemagglutinin), and difference of binuclear rate of radiated and non-radiated lymphocyte is detected; the lymphocyte stimulated by PHA forms binucleated cells, and the binuclear rate of radiated lymphocyte decreases obviously, which shows that the multiplication level of lymphocyte can be reflected well by detecting the binuclear rate of lymphocyte by utilizing the cytochalasin B. The method provided by the invention is visual in detecting indexes, is simple, rapid and economical in operation, and is accurate in result.
Owner:FUDAN UNIV
Uses of bispecific antibody coated dendritic cells pulsed with antigens and GM-CSF in immune regulation
GM-CSF administered before immunization exerted a sustained suppressive effect against the induction of myasthenia gravis (MG). This suppression was associated with lowered serum autoantibody levels, reduced T cell proliferative responses to AChR, and an expansion in the population of FoxP3+ regulatory T cells. Manipulating DCs to expand regulatory T cells is useful for the control of autoimmune diseases such as myasthenia gravis MG.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Saturated fatty chain alcohol His-Gly-AA tripeptide ester, synthetic method and application thereof
InactiveCN101899090BExcellent immunosuppressive effectTripeptide ingredientsPeptide preparation methodsAlcoholProliferative response
The invention relates to 12 saturated fatty chain alcohol His-Gly-AA tripeptide ester conjugates with immunosuppressive activity in a general formula His-Gly-AA-O-CH2-(CH2)nCH3 I, wherein AA stands for Glu or Lys. In saturated fatty chain alcohol, n is equal to 6, 8, 10, 12, 14 or 16. the invention also relates to a preparation method and application of the conjugates as an immunosuppressant. An experimental result of the saturated fatty chain alcohol His-Gly-AA tripeptide ester which has the inhibition effects of splenic lymphocyte mitogen breeder reaction and macrophage phagocytosis activity indicates that the conjugates of the invention have excellent immunosuppressive action and can be clinically used as an immunosuppressant.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Nitrogenated trans-stilbene analogs, method for the obtention and medical applications thereof
This invention is related to new nitrogenated trans-stilbene compounds, more specifically, imine, pyrrole and indole derivatives, with procedures for the preparation and use thereof as pharmaceutical compositions for the treatment and / or chemoprevention of those mammalian diseases such as cancer, fibrosclerosis and acute / chronic inflammation, graft-versus-host reaction, ischemic-reperfusion tissue injury in stroke and heart attack, neurodegeneration, and during organ transplantation, whose pathogenic and pathophysiological mechanisms depend on or are significantly contributed by undesirable oxidative stress, angiogenic and proliferative responses.
Owner:DOMINION PHARMAKINE +1
A kind of porcine circovirus type 2 immune protection polypeptide and vaccine
ActiveCN104098657BHigh titerRaise antibody levelsViral antigen ingredientsVirus peptidesFactor iiPeptide vaccine
The invention relates to the technical field of porcine circovirus, in particular to a porcine circovirus 2 (PCV2) immune protection polypeptide, and a vaccine containing the PCV2 immune protection polypeptide. The amino acid sequence of the PCV2 immune protection polypeptide is shown in the sequence table 1-4. According to the vaccine, tests show that after 42-63 days of first immune of a vaccine of P5013 and a vaccine of P1300, the antibody titer and the neutralizing antibody titer of an ELISA antibody are remarkably increased, the lymphocyte proliferative response and IL-4 and IFN-Gamma cytokines are significantly higher than those of a control group, and the antibody level and the cellular immunity level of the vaccine group of P5013 are the highest; mice tests show that PCV2 immune response induced by the synthetic peptide vaccine of P5013 is the highest and the strongest; pig tests find that the synthetic polypeptide vaccine of P5013 can induce pigs to generate a PCV2-specific immune response, can reduce viremia caused by PCV2 virulent virus attack, alleviate the clinical symptoms and pathological lesions of viremia, and has immune protection effect on PCV2.
Owner:NANJING AGRICULTURAL UNIVERSITY
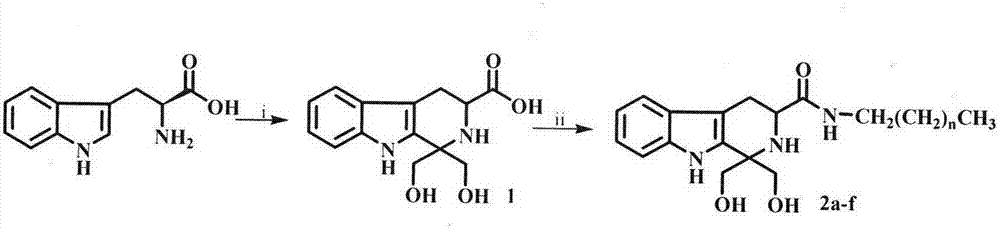
Tetrahydro-beta-carbolinyl-3-formyl aliphatic chain amines, and preparation, nano structure, immunosuppression action and application thereof
The invention relates to 6 aliphatic chain amine modified carbolinyl carboxylic acid analogs disclosed as general formula I, wherein n is equal to 6, 8, 10, 12, 14 or 16. The invention also relates to a preparation method of the 6 aliphatic chain amine modified carbolinyl carboxylic acid analogs disclosed as general formula I. The invention further relates to a nano structure of the 6 aliphatic chain amine modified carbolinyl carboxylic acid analogs disclosed as general formula I. By researching the inhibiting action of the saturated fatty chain amine modified carbolinyl carboxylic acid analogs on the spleen lymphocyte mitogen breeder reaction and the survival time after transplanting the mouse postotic cardiac muscle, the invention further relates to excellent immunosuppression action of the 6 aliphatic chain amine modified carbolinyl carboxylic acid analogs disclosed as general formula I. The 6 aliphatic chain amine modified carbolinyl carboxylic acid analogs with immunosuppressive activity disclosed as general formula I have wide application prospects in preparing immunosuppressive drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com