Method for testing proliferative response of drug to tumor cell derived from patient
A tumor cell, proliferation response technology, applied in the field of tumor cell proliferation response, can solve problems such as low usability, poor reproducibility and stability, complex amplification or reduction of components, and insufficiently characterized samples, etc., to achieve predictive The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In Vitro Proliferation Assay
[0034] Thaw patient-derived tumor cells in a 37°C water bath until the frozen cell suspension is fully thawed to form a slurry solution, slowly transfer the cell suspension to a 50ml conical tube through a cell strainer, and pour into the conical tube Fill up with conditioned medium warmed to 37°C; spin the conical tube at 800 RPM for 5 min at 4°C to pellet the cells; remove the supernatant carefully without disturbing the cell pellet by aspirating the supernatant, Resuspend the cell pellet in 1-2 ml of warmed conditioned medium, count the cells using TC10, and finally remove 10 μl of the cell suspension and transfer to a tube for trypan blue exclusion assay to assess cell density and vial Overall health of cells, counting live-dead cells.
[0035] use (ECM from Trevigen) to coat a 6-well plate, dilute the ECM to 1:20 with ice-cold conditioned medium without growth factors and supplements, add 1 ml of diluted Cultrex solution to each we...
Embodiment 2
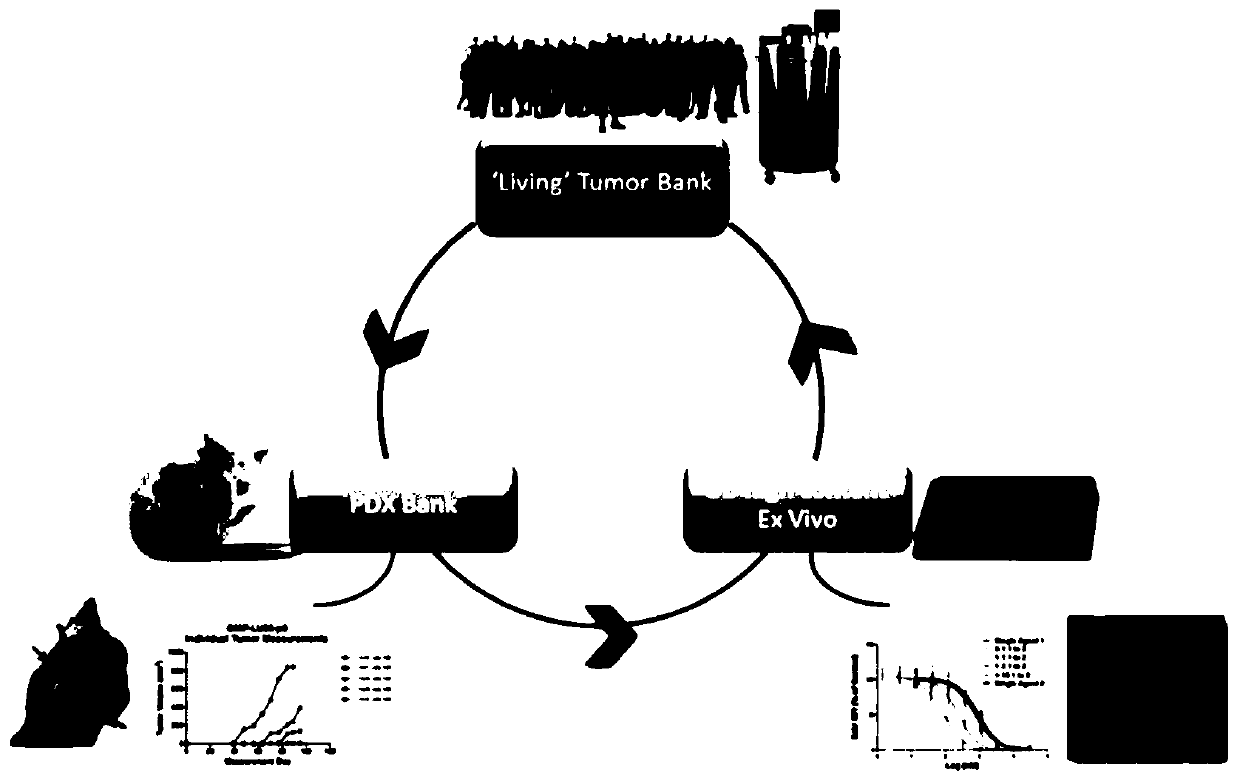
[0048] Use of Patient-Derived Xenografts (PDX)
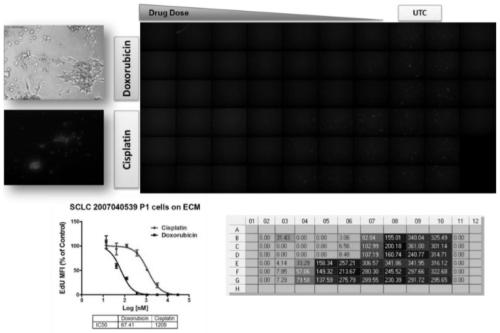
[0049] Fresh or banked patient-derived tumor cells were injected into immunocompromised mice, cells were harvested and plated to form spheroids, and spheroids were processed ex vivo with chemotherapeutic candidates, cisplatin, and doxorubicin Drug treatment, cell proliferation measured by HC imaging of EdU incorporation assay. ( Figure 1A -B)
[0050] Cell images are captured and processed using a variety of algorithms corresponding to the experiment. Select DAPI on channel 1 to identify valid objects and EdU on channel 2 to identify DNA-incorporated cell populations, record and integrate the intensity of each pixel for each channel to obtain the total intensity, divide the total intensity In total pixels, the mean fluorescence intensity (MFI) of the channel was obtained. The integrated mean fluorescence intensity of multiple cells was divided by the total number of cells in each well to obtain the mean value of MFI. To norma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com