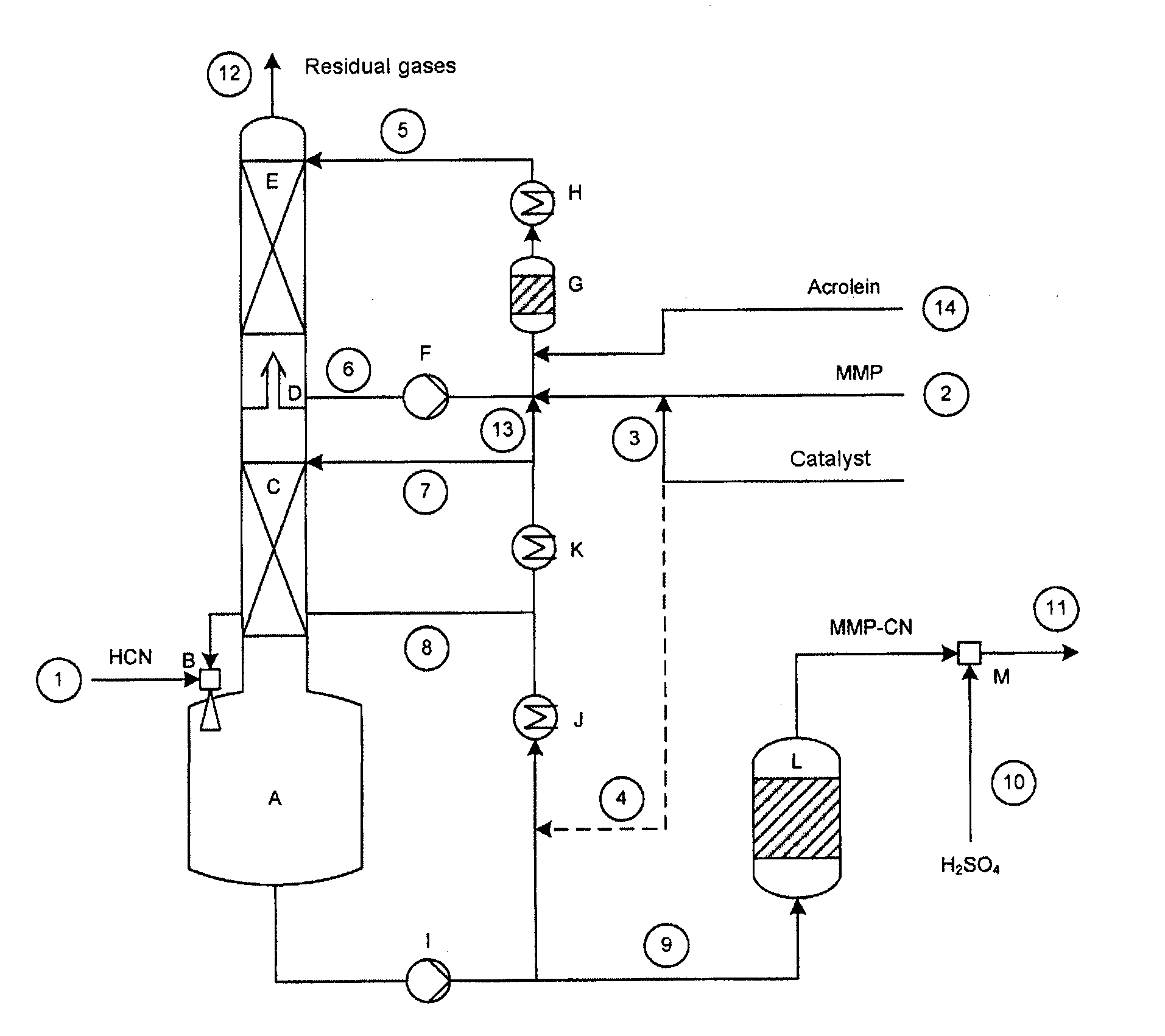
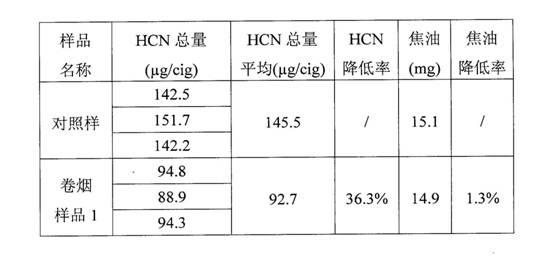
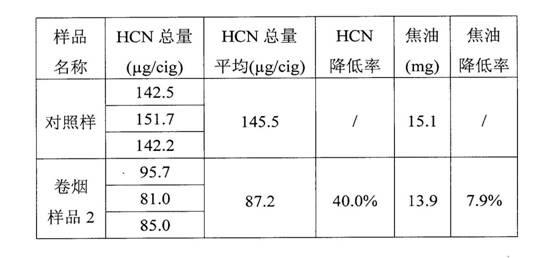
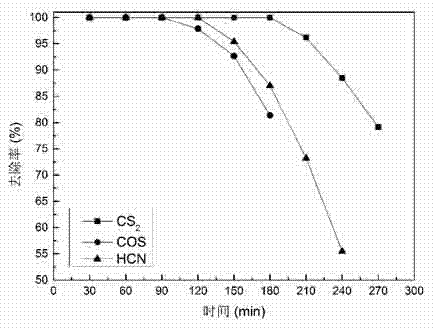
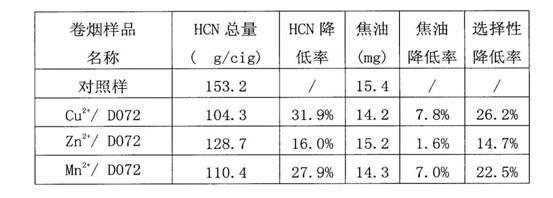
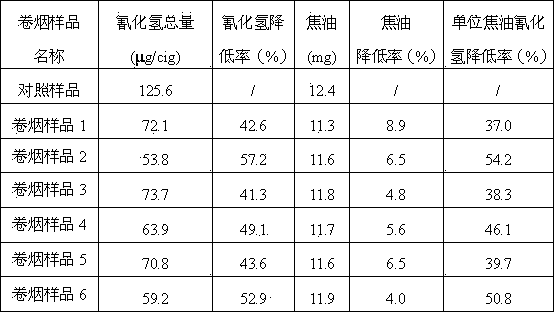
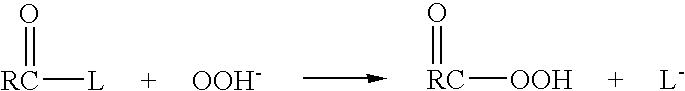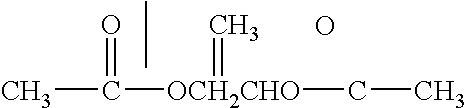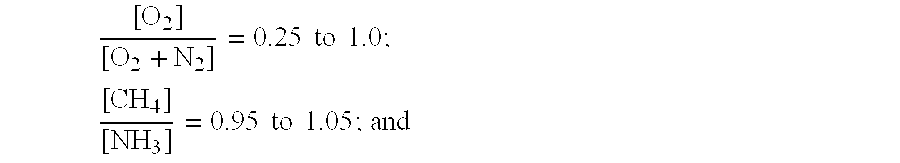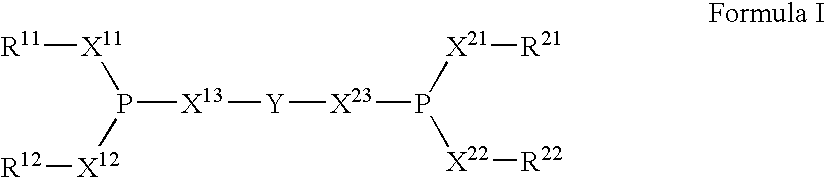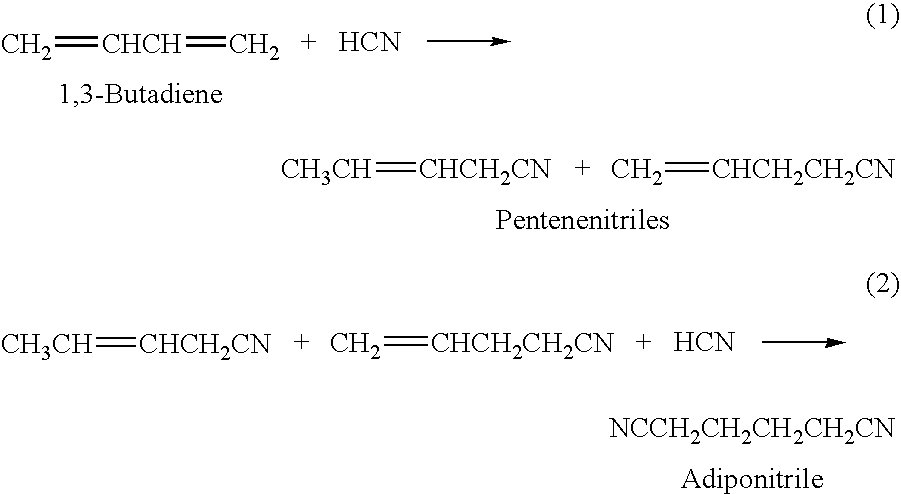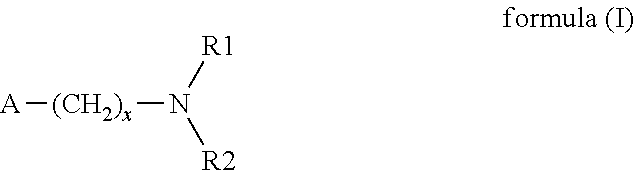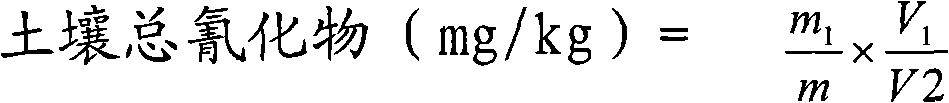Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
430 results about "Hydrogen cyanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrogen cyanide (HCN), sometimes called prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valuable precursor to many chemical compounds ranging from polymers to pharmaceuticals.
Electrical smoking system and method
InactiveCN1633247AReduce gaseous componentsIncandescent ignitionCigar manufactureIntegratorAcrylonitrile
An electric smoking system includes a cigarette including a cylindrical tobacco web partially filled with tobacco material to define a filled tobacco rod portion and an unfilled tobacco rod portion, and an electric lighter. The wrapper includes a filler of ammonium-containing compounds effective to reduce the gaseous constituents of the smoke produced during smoking. The system includes a pilot burner including at least one heating vane and a controller adapted to control heating of the heating vane. The lighter is configured to at least partially contain the cigarette such that the heater blade heats the heating region of the cigarette. Manipulating the controller to limit the heating of the heater blades to a predetermined temperature range which allows the delivery of the smoke generated when the portion of the tobacco rod is heated while at least reducing the amount of smoke present in the smoke as compared to smoking a cigarette having only calcium carbonate as filler. A gaseous component. The gaseous components that can be reduced include carbon monoxide, 1,3 butadiene, isoprene, acrolein, acrylonitrile, hydrogen cyanide, 0-toluidine, 2-naphthylamine, nitrogen oxide, benzene, NNN, Phenol, catechol, benzanthracene and benzopyrene.
Owner:PHILIP MORRIS PROD SA
Reactive formulations for a neutralization of toxic industrial chemicals
InactiveUS7125497B1Efficiently neutralizedHydrogen peroxideLiquid degasificationBoron trichlorideMalathion
Decontamination formulations for neutralization of toxic industrial chemicals, and methods of making and using same. The formulations are effective for neutralizing malathion, hydrogen cyanide, sodium cyanide, butyl isocyanate, carbon disulfide, phosgene gas, capsaicin in commercial pepper spray, chlorine gas, anhydrous ammonia gas; and may be effective at neutralizing hydrogen sulfide, sulfur dioxide, formaldehyde, ethylene oxide, methyl bromide, boron trichloride, fluorine, tetraethyl pyrophosphate, phosphorous trichloride, arsine, and tungsten hexafluoride.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Grapheme type fiber cigarette filter and preparation method thereof
InactiveCN103005712ALarge surface atomic ratioUnique adsorptionTobacco smoke filtersFiberHazardous substance
The invention discloses a grapheme type fiber cigarette filter and a preparation method thereof. The filter comprises a fiber carrier, wherein the fiber carrier is provided with a grapheme type material for absorbing harmful components in smoke. The filter loads the grapheme type material on a carbon fiber or acetate fiber carrier; and the carrier is added in the filter by adopting the binary filter production mode in the existing industries to produce a compound filter for absorbing harmful substances in the smoke which is generated when cigarettes are smoked. The filter applies unique phi absorption characteristics and higher surface active atom ratio of novel two-dimensional nanometer materials to efficiently absorb benzo [a] pyrene, volatile aldehyde ketone type cancer promoting molecules, phenol and HCN (Hydrogen Cyanide) under the condition of smaller adding quantity, so that the filter is environment-friendly and has no additional poison; as the amount is small and the effect is high, the harm of the cigarettes to human bodies is reduced under the condition of not influencing the inherent sensory quality of the cigarettes. And at the same time, the method has the advantages of simple technique, large-scale production and low cost, and has wide application foreground in the cigarette field of harm reduction and tar reduction.
Method of producing hydrogen cyanide
InactiveUS6096173AReduce the temperatureCurrent densityEnergy based chemical/physical/physico-chemical processesHydrogen cyanide preparation/purification/separationGas phaseCorona discharge
A method of producing hydrogen cyanide by the gas-phase reaction of methane with ammonia at elevated temperature and an ammonia / methane ratio of 1.001 to 1.1. The conducting of the gaseous reaction mixture through a corona discharge causes the reaction to start at temperatures below 1000 DEG C. without the action of a catalyst. This results in considerable savings of the necessary investment expenses as well as of the running costs for energy.
Owner:DEGUSSA HULS AG
Hydrogen cyanide synthesis process
InactiveUS6979432B2Improve productivityOperational securityHydrogen cyanide preparation/purification/separationPlatinumNitrogen
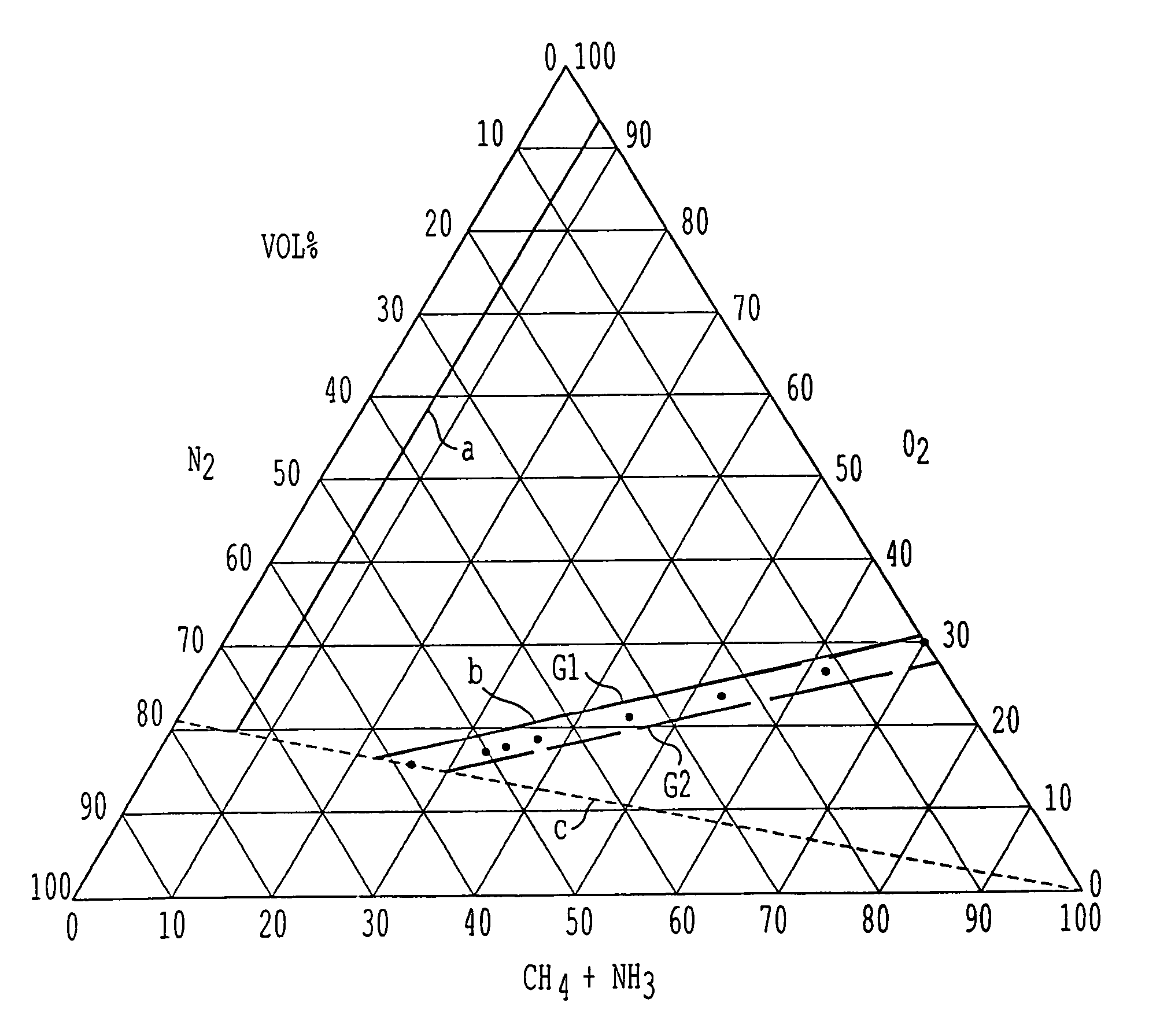
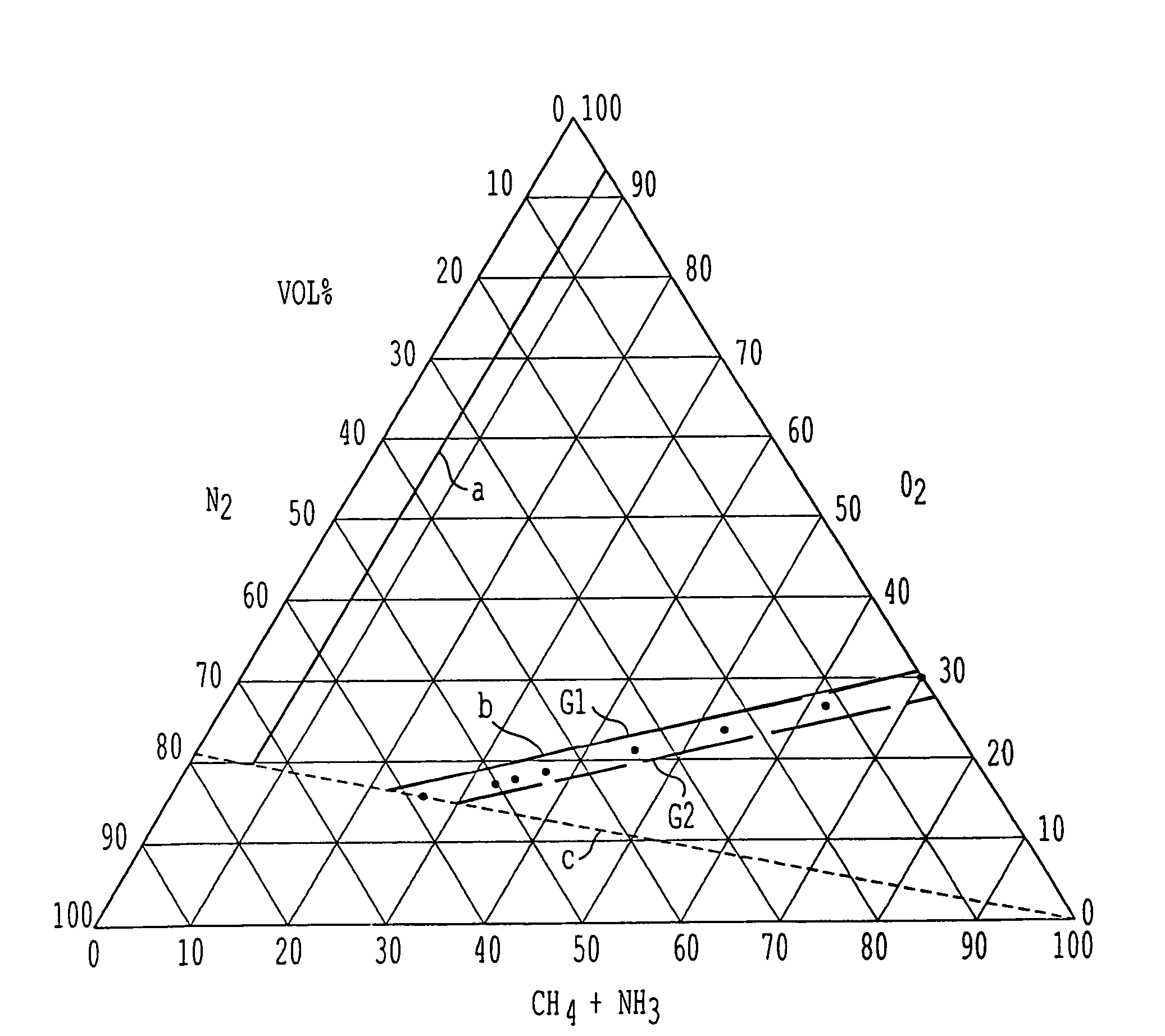
A process for the production of hydrogen cyanide is provided, wherein hydrogen cyanide is synthesized by reacting methane or methane-containing natural gas, ammonia and oxygen-enriched air or oxygen in the presence of a catalyst comprising platinum or a platinum alloy; wherein the reactants are present in the following molar ratios [O2][O2+N2]=0.25to1.0;[CH4][NH3]=0.95to1.05;andwhere a molar ratio of ammonia to the sum of oxygen and nitrogen obeys the following relationship:Y=m·X−a,wherein Y=[NH3][O2+N2]X=[O2][O2+N2]m=1.25 to 1.40; and a=0.05 to 0.14.
Owner:EVONIK OPERATIONS GMBH
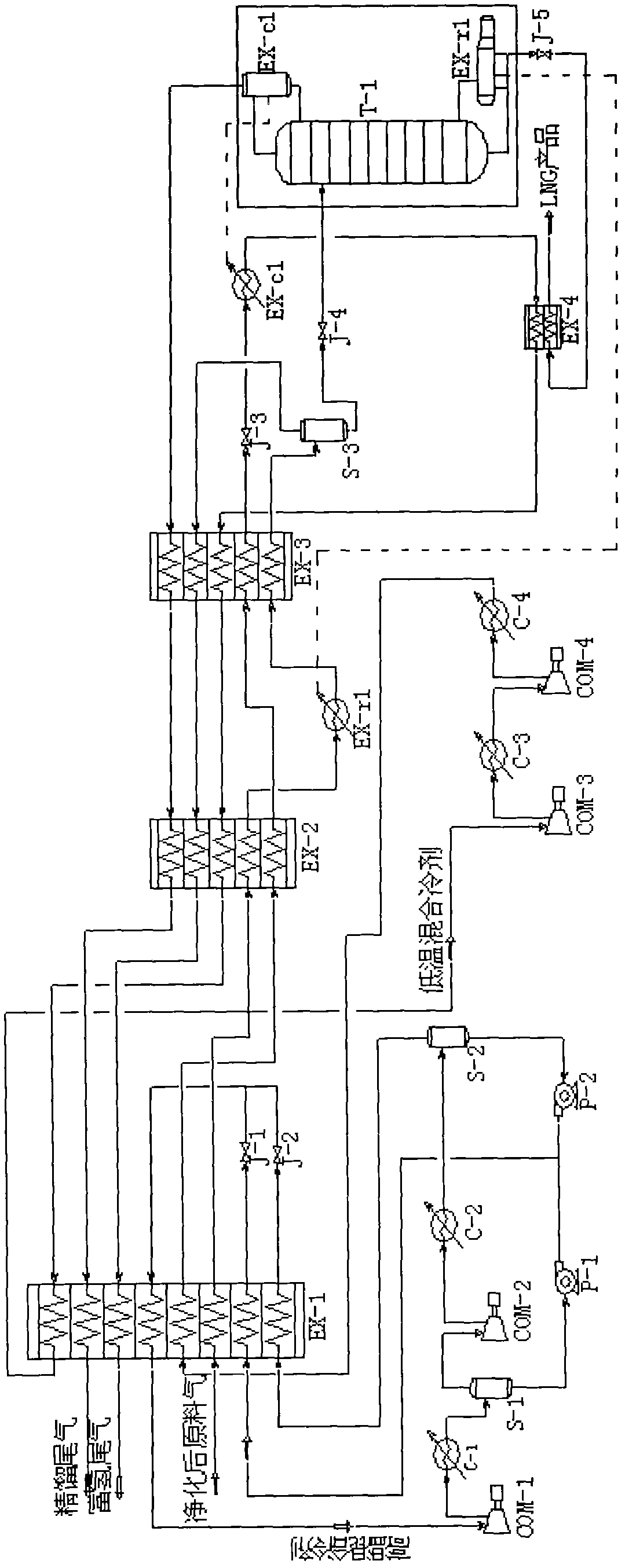
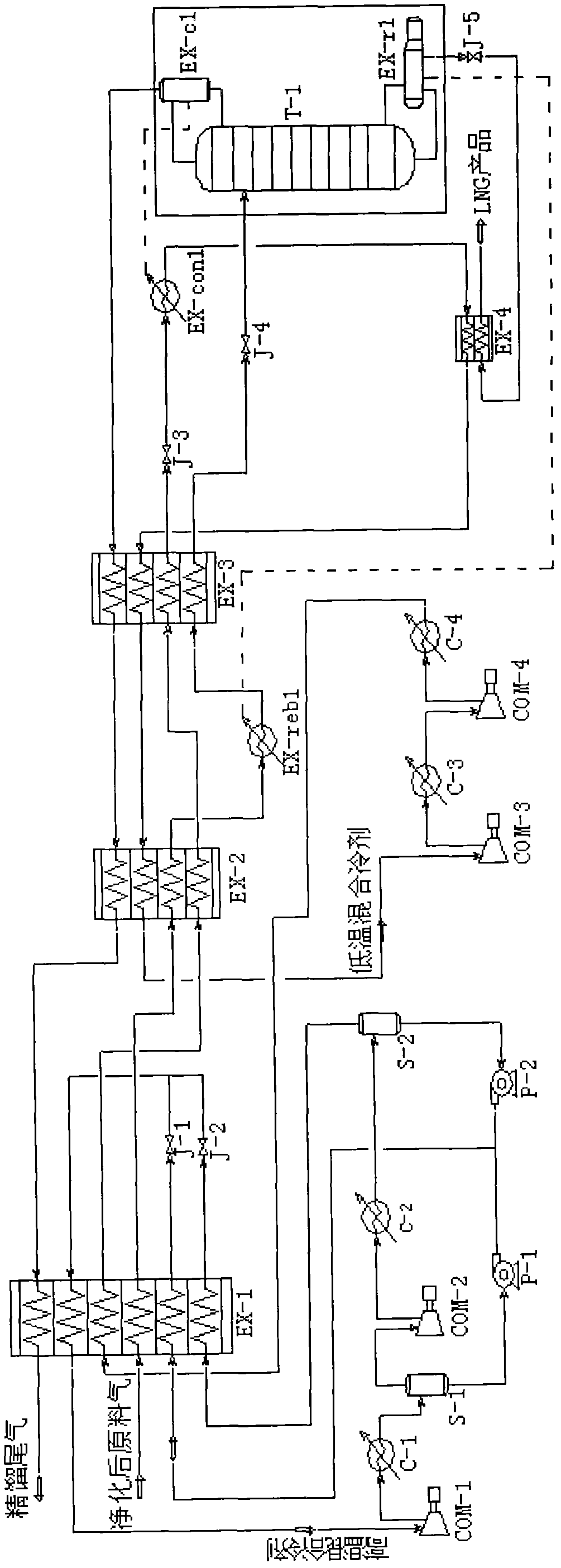
Method for producing liquefied natural gas by using coke oven gas
The invention provides a method for producing liquefied natural gas by using coke oven gas. The method comprises the following steps: (1) the coke oven gas is pretreated to remove benzene, naphthalene, tar and HCN (hydrogen cyanide); (2) sulfur is removed; (3) carbon is removed and the coke oven gas is dried; (4) the coke oven gas is led to a first heat exchanger and pre-cooled; (5) the pre-cooled coke oven gas passes through a second heat exchanger, a reboiler at the bottom of a low-temperature rectifying tower and a third heat exchanger in sequence, so that the temperature is lowered step by step; (6) gaseous liquid coming out of the third heat exchanger is led to a gas-liquid separator for gas-liquid separation, then the liquid coming out of the gas-liquid separator enters the low-temperature rectifying tower, and a liquefied natural gas product is acquired at the bottom of the low-temperature rectifying tower; and otherwise, the gaseous liquid coming out of the third heat exchanger is directly led to the low-temperature rectifying tower, and the liquefied natural gas product is acquired at the bottom of the low-temperature rectifying tower. The method for producing the liquefied natural gas by using the coke oven gas as raw material has the advantages of high output as well as low energy consumption and cost, and is easy to operate.
Owner:BEIJING ZHONGKE RUIAO ENERGY TECH CO LTD
Hydrocyanation process with reduced yield losses
ActiveUS20080015382A1Investment exemptionSelective and efficient and stableOrganic compound preparationPreparation by hydrogen cyanide additionHydrogenPhosphite ester
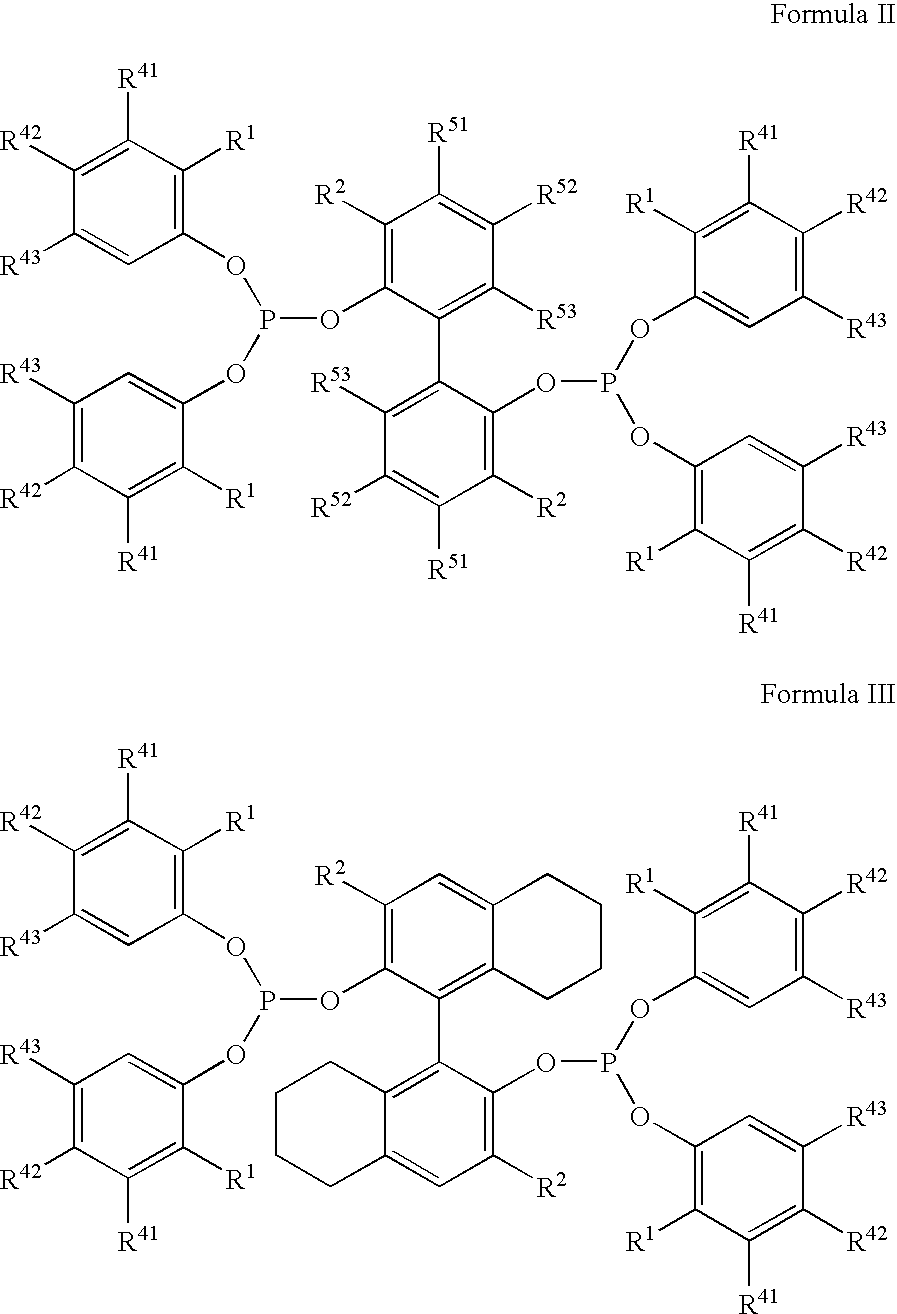
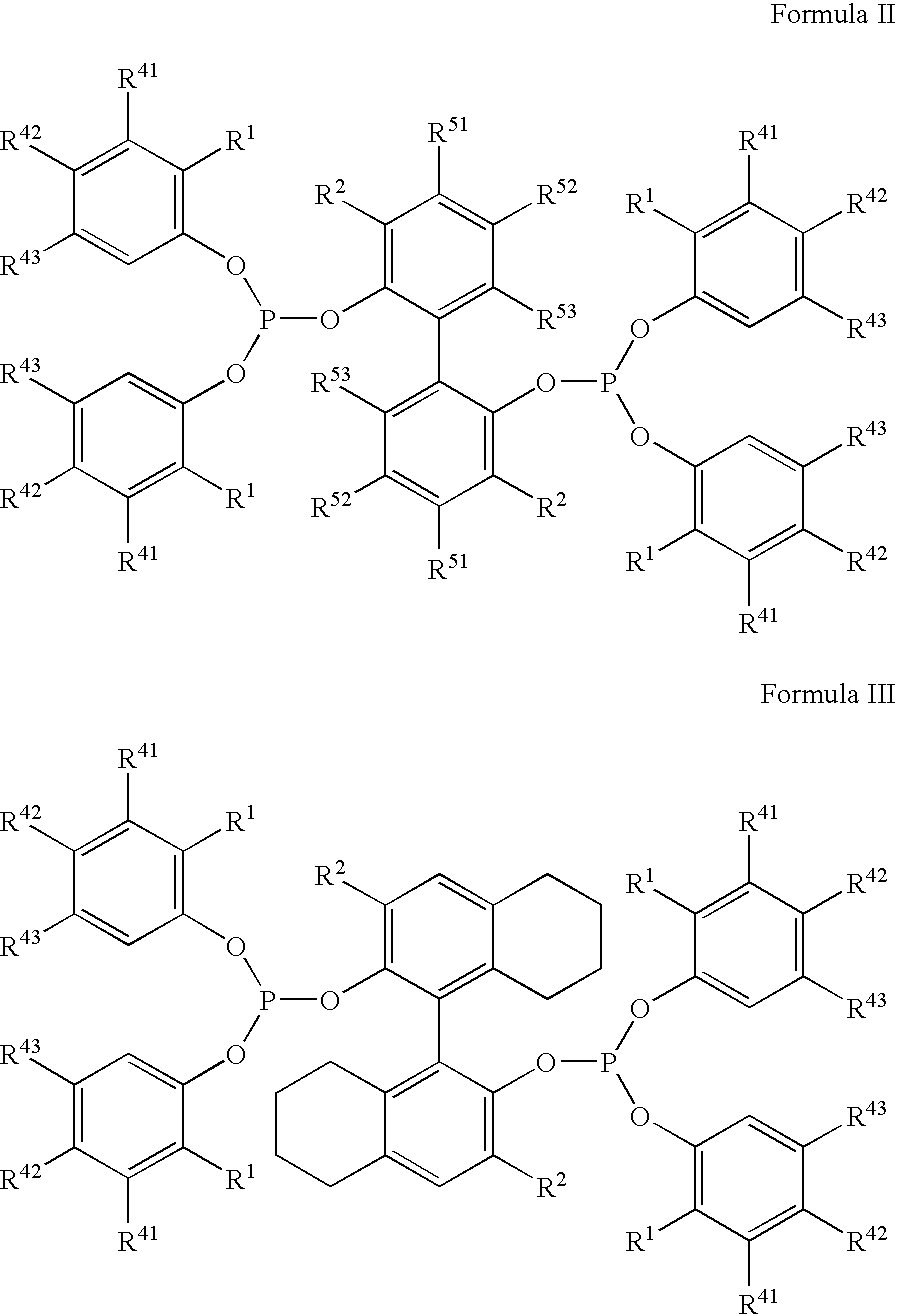
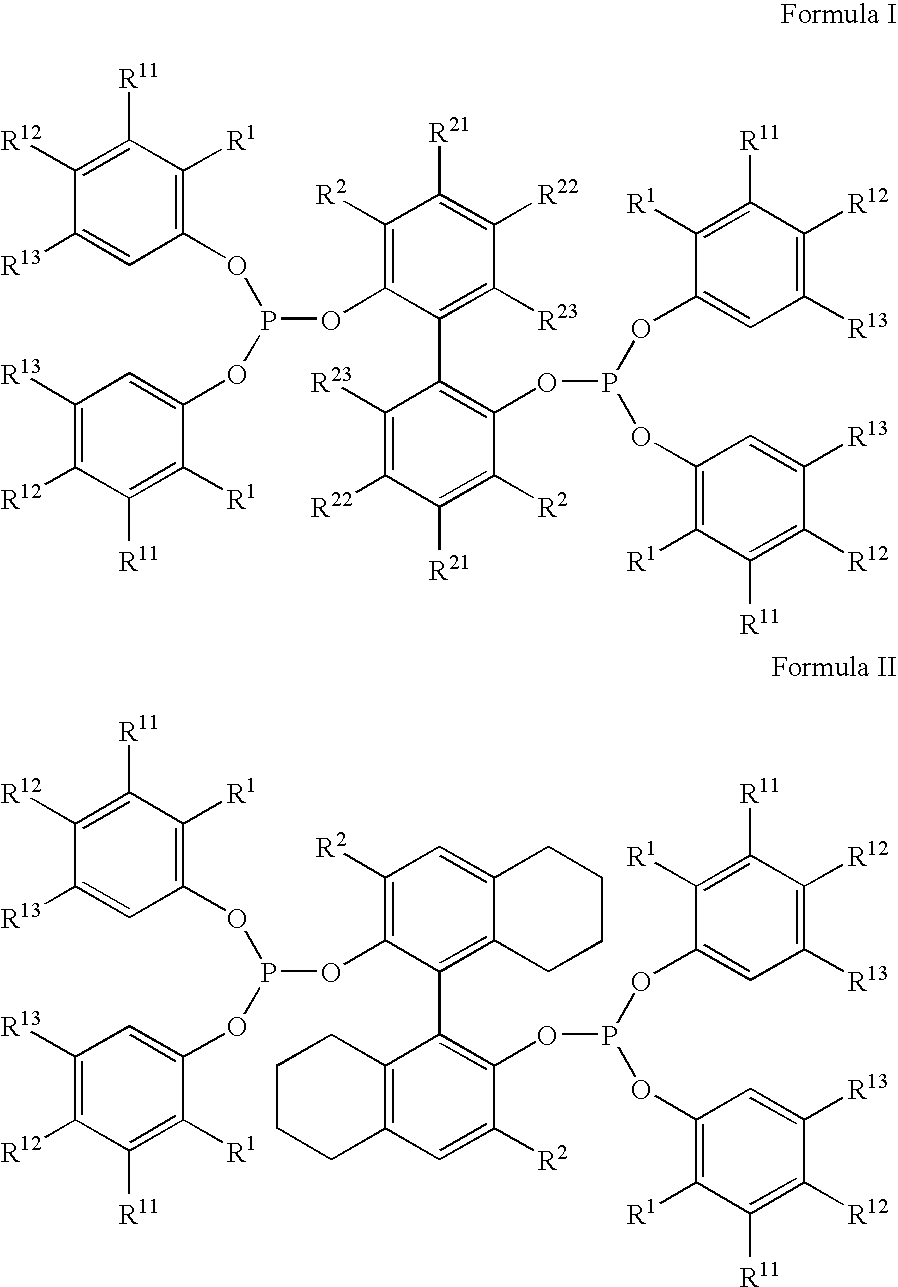
The invention provides a hydrocyanation process for the production of adiponitrile and other dinitriles having six carbon atoms, the process comprising:a) forming a reaction mixture in the presence of at least one Lewis acid, said reaction mixture comprising ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition, by continuously feeding ethylenically unsaturated nitriles, hydrogen cyanide, and a catalyst precursor composition;b) controlling X and Z, wherein X is the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and Z is the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles, by selecting a value for X in the range from about 0.001 to about 0.5, and a value for Z in the range from about 0.5 / 1 to about 0.99 / 1, such that the value of quotient Q, whereinQ=X[(moles3PN+4PNinthefeed) / (molesallunsaturatednitrilesinthefeed)]-Zis in the range from about 0.2 to about 10, wherein 3PN is 3-pentenenitriles and 4PN is 4-pentenenitrile; andc) withdrawing a reaction product mixture comprising adiponitrile;wherein the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles in the reaction mixture is from about 0.2 / 1 to about 10 / 1;wherein the catalyst precursor composition comprises a zero-valent nickel and at least one multidentate phosphorus-containing ligand;wherein the multidentate phosphorus-containing ligand is selected from the group consisting of a phosphite, a phosphonite, a phosphinite, a phosphine, and a mixed phosphorus-containing ligand or a combination of such members; andwherein the multidentate phosphorus-containing ligand gives acceptable results according to at least one protocol of the 2-Pentenenitrile Hydrocyanation Test Method.
Owner:INV NYLON CHEM AMERICAS LLC
Hydrocyanation process with reduced yield losses
ActiveUS7659422B2Investment exemptionSelective and efficient and stableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenHydrogen cyanide
A hydrocyanation process produces adiponitrile and other dinitriles having six carbon atoms. The process involves forming a reaction mixture in the presence of at least one Lewis acid. The reaction mixture includes ethylenically unsaturated nitriles having five carbon atoms, hydrogen cyanide, and a catalyst precursor composition. The reaction mixture is continuously fed while controlling the overall feed molar ratio of 2-pentenenitriles to all unsaturated nitriles and the overall feed molar ratio of hydrogen cyanide to all unsaturated nitriles. In the reaction product mixture, including adiponitrile, the ratio of the concentration of 2-pentenenitriles to the concentration of 3-pentenenitriles is from about 0.2 / 1 to about 10 / 1. Included in the catalyst precursor composition is a zero-valent nickel and at least one bidentate phosphite ligand.
Owner:INV NYLON CHEM AMERICAS LLC
Method for the production of 2-hydroxy-4-(methylthio)butyronitrile from 3-(methylthio)propanal and hydrogen cyanide
ActiveUS20120215022A1Increase response rateLow byproduct formationOrganic compound preparationOrganic chemistry methodsCyanidePropynal
A method for the production of 2-hydroxy-4-(methylthio)butyronitrile having good storage stability in a multi-zone reactor, is provided. 3-methylmercaptopropionaldehyde is reacted with hydrogen cyanide in the presence of a base as catalyst in a main reaction zone of the multizone reactor to form a reaction mixture comprising the 2-hydroxy-4-(methylthio)butyronitrile, unreacted 3-methylmercaptopropionaldehyde, the catalyst and residual amounts of gaseous hydrogen cyanide. The residual gaseous hydrogen cyanide is removed from the main reaction zone to an absorption and post-reaction zone of the reactor which comprises a mixture of 3-methylmercaptopropionaldehyde and the catalyst; and the gaseous hydrogen cyanide is further reacted with the 3-methylmercaptopropionaldehyde in the absorption and post reaction zone. A molar ratio of hydrogen cyanide to 3-(methylthio)propanal in the main reaction zone is from 0.98 to 1.03.
Owner:EVONIK OPERATIONS GMBH
Water retaining material, preparation method thereof and application of water retaining material in filter rod
ActiveCN102029146AReduce hydrophilicityImprove hydrophilic abilityOther chemical processesTobacco smoke filtersAcid releaseEnvironmental engineering
The invention relates to a water retaining material, a preparation method thereof and an application of the water retaining material in a filter rod. The invention is characterized in that water-retaining substances and alkaline components are introduced into a porous material with larger specific surface area simultaneously, and the water-retaining material for selectively reducing hydrogen cyanide (HCN) in mainstream cigarette smoke is obtained. The material has certain content of water due to water-retaining substances in the material, simultaneously the alkaline components can generate acid-alkaline reaction with the hydrophilic HCN in the mainstream cigarette smoke, and the selective harm reduction performance of the material to the HCN in the mainstream cigarette smoke is further enhanced. 10-40mg of the material is added in per piece of the filter rod of the cigarette, therefore, the release amount of the hydrogen cyanide in the mainstream cigarette smoke can be reduced by more than 30 percent, while the release amount of tar is basically not changed or reduced slightly, and simultaneously no bad influence is caused on the smoking quality of cigarettes, and the purpose of realizing selective harm reduction is further achieved.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Process for making nitriles
ActiveUS20130211126A1Reduce nickel contentOrganic compound preparationChemical recyclingMethyl groupHydrogen cyanide
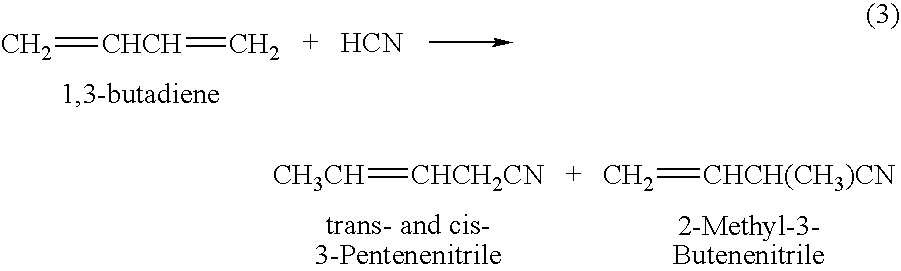
Adiponitrile is made by reacting 3-pentenenitrile with hydrogen cyanide. The 3-pentenenitrile is made by reacting 1,3-butadiene with hydrogen cyanide and by isomerizing 2-methyl-3-butenenitrile. The reaction of 1,3-butadiene with hydrogen cyanide to produce 3-pentenenitrile also produces small amounts of dinitrile compounds, including adiponitrile (ADN) and methylglutaronitrile (MGN). Methylglutaronitrile is removed to provide an adiponitrile-enriched stream, which is used in a catalyst purification step.
Owner:INV NYLON CHEM AMERICAS LLC
HCN (hydrogen cyanide) electrochemical sensor
ActiveCN103336041AEliminate Cross InterferenceAccurately measure concentrationMaterial electrochemical variablesElectrochemical gas sensorNitrogen monooxide
The invention relates to the field of electrochemical sensors, and in particular relates to an HCN (hydrogen cyanide) four-electrode electrochemical sensor resistant to nitrogen monoxide and nitrogen dioxide interference. The HCN electrochemical sensor provided by the invention comprises a first working electrode, a second working electrode, a reference electrode and a counter electrode which form ion conduction via electrolyte, wherein the first working electrode is a carbon element electrode, and the second working electrode an Au-containing electrode. The HCN electrochemical sensor provided by the invention is capable of effectively eliminating the cross interference between NO and NO2 and accurately measuring the concentration of an HCN gas via the design of a four-electrode sensor system comprising the reference electrode, the counter electrode and the two working electrodes.
Owner:RAE SYST SHANGHAI
Preparation method of modified bio-charcoal based catalyst
InactiveCN104772146AImprove removal effectFully removedDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsPtru catalystCarbonyl sulfide
Owner:KUNMING UNIV OF SCI & TECH
Metal complexing material and preparation method thereof, and application of metal complexing material to cigarettes
The invention relates to a metal complexing material and a preparation method thereof, and application of the metal complexing material to cigarettes. The invention is characterized in that: the complexing material is obtained by complexing a porous carrier with large specific surface area, such as ion exchange resin, ion exchange fibers, macroporous adsorption resin and the like, and different metal ions, and is applied to the cigarettes in a form of a binary composite filter stick; 10 to 40mg of complexing material is added into each cigarette, so that hydrogen cyanide (HCN) in mainstream smoke can be selectively reduced by over 25 percent; meanwhile, the complexing material does not have negative effects on the sensory quality of the cigarettes. The porous carrier in the material has large specific surface area, so smoke can be effectively contacted with the functional group of the material when passing through the material; meanwhile, the metal ions on the surface can be subjectedto complexing reaction with the HCN, so that the HCN in the smoke of the cigarettes is selectively removed.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Catalytic oxidation purification method for hydrogen cyanide in industrial waste gas
InactiveCN101269297AHigh catalytic activityImprove stabilityDispersed particle separationPurification methodsPhosphoric acid
The invention relates to a method of the catalytic oxidation and purification of tail gas containing hydrocyanic acid, in particular to a method for purifying the tail gas containing hydrocyanic acid by using a chemical method. The tail gas containing hydrocyanic acid is enabled to react with a prepared mixed catalyst solution containing palladium and copper under the temperature of 4 to 100 DEG C in a absorption tower, and the tail gas containing hydrocyanic acid is blown into the absorption tower in a gas-liquid negative-direction contact way, part of the hydrocyanic acid contacts and reacts with the palladium ions to form insoluble palladium simple substance, and part of the hydrocyanic acid reacts with the copper ions to form insoluble phosphor copper, part of the hydrocyanic acid is oxidized into phosphoric acid; all the generated substances enter into the liquid phase, and the mixed catalyst solution which absorbs the hydrocyanic acid and the purified and qualified tail gas not containing the hydrocyanic acid are acquired, the mixed hydrocyanic acid catalyst solution is oxidized into a by-product of the phosphoric acid to be used as resources again, the problems of hydrocyanic acid pollution is effectively eliminated, and the method provided by the invention provides a practical purifying method for the calcium carbide furnace gas and yellow phosphorus exhaust gas used as monocarbide chemical raw materials.
Owner:KUNMING UNIV OF SCI & TECH
Modified natural plant filter tip additive material capable of selectively reducing burst size of hydrogen cyanide in main stream smoke of cigarettes and preparation method of modified natural plant filter tip additive material
ActiveCN103393219AImprove securitySimple preparation processTobacco smoke filtersEnvironmental engineeringHydrogen cyanide
The invention discloses a modified natural plant filter tip additive material capable of selectively reducing burst size of hydrogen cyanide in main stream smoke of cigarettes and a preparation method of the modified natural plant filter tip additive material. The modified natural plant filter tip additive material takes the natural plant material as a carrier and is prepared through carboxylation and loading of transition metal ions sequentially. According to the invention, the filter tip additive material prepared by taking the natural plant material as the carrier has the porous adsorption property and is rich in transition metal ions; as CN- has strong complexation to the transition metal ions, the filter tip additive material can perform effective adsorption and complexation on hydrogen cyanide, and the purpose of selectively reducing burst size of hydrogen cyanide in main stream smoke of cigarettes is realized. According to the modified natural plant filter tip additive material and the preparation method, the source of the raw material is extensive, green and environmentally friendly; the prepared filter tip additive material is high in safety, simple in preparation technology, low in cost, and easy to produce on a large scale; when applied to cigarette filter tips, the modified natural plant filter tip additive material can selectively reduce burst size of hydrogen cyanide in main stream smoke of cigarettes by more than 35% with no negative effect on the sensory quality of cigarettes.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Environmental-friendly new method for preparing N-phosphonomethyl iminodiacetic acid by utilizing acrylonitrile byproduct hydrocyanic acid
InactiveCN101747369AQuality improvementHigh yieldGroup 5/15 element organic compoundsIminodiacetic acidControl system
The invention relates to an environmental-friendly new method for preparing N-phosphonomethyl iminodiacetic acid by utilizing acrylonitrile byproduct hydrocyanic acid, belonging to the comprehensive utilization of acrylonitrile device byproduct hydrocyanic acid in industrial scale. The method includes that: step first, acrylonitrile byproduct hydrocyanic acid is used for preparing hydroxyl acetonitrile, and then iminodiacetonitrile is prepared; step two, the obtained iminodiacetonitrile is used for preparing iminodiacetic acid by acid hydrolysis method; and step three, the obtained iminodiacetic acid is used for preparing N-phosphonomethyl iminodiacetic acid. No report of preparing PMIDA by utilizing acrylonitrile byproduct hydrocyanic acid in industrial scale is seen, and the inventor uses local materials, utilizes the advantages of being adjacent to Qilu petrochemical and having resource of hydrocyanic acid (4000 ton / year) transmitted by pipeline and initially provides the method for preparing PMIDA by utilizing acrylonitrile byproduct hydrocyanic acid in industrial scale. The invention solves the problem of region restriction of product production caused by inconvenient transportation of hydrocyanic acid. A gas and liquor mixer is applied to iminodiacetonitrile reaction, and by virtue of DCS control system, quality and yield of iminodiacetonitrile are improved.
Owner:YINGKOU YINGXIN CHEM TECH CO LTD
Selective Filtration of Cigarette Smoke using Chitosan Derivatives
InactiveUS20070295345A1Improve performanceEffectively adsorbing and absorbing undesirable smoke ingredientsTobacco treatmentTobacco smoke filtersCross-linkMass ratio
A smoking article filter having a porous resin with a high surface area to mass ratio comprised of a chitosan derivative. Preferred embodiments include chitosan cross-linked with glutaraldehyde and chitosan cross-linked with glyoxal. The chitosan derivative provides for the selective filtration of cigarette smoke, particularly for the removal of aldehydes, hydrogen cyanide, heavy metals and carbonyls.
Owner:R J REYNOLDS TOBACCO COMPANY
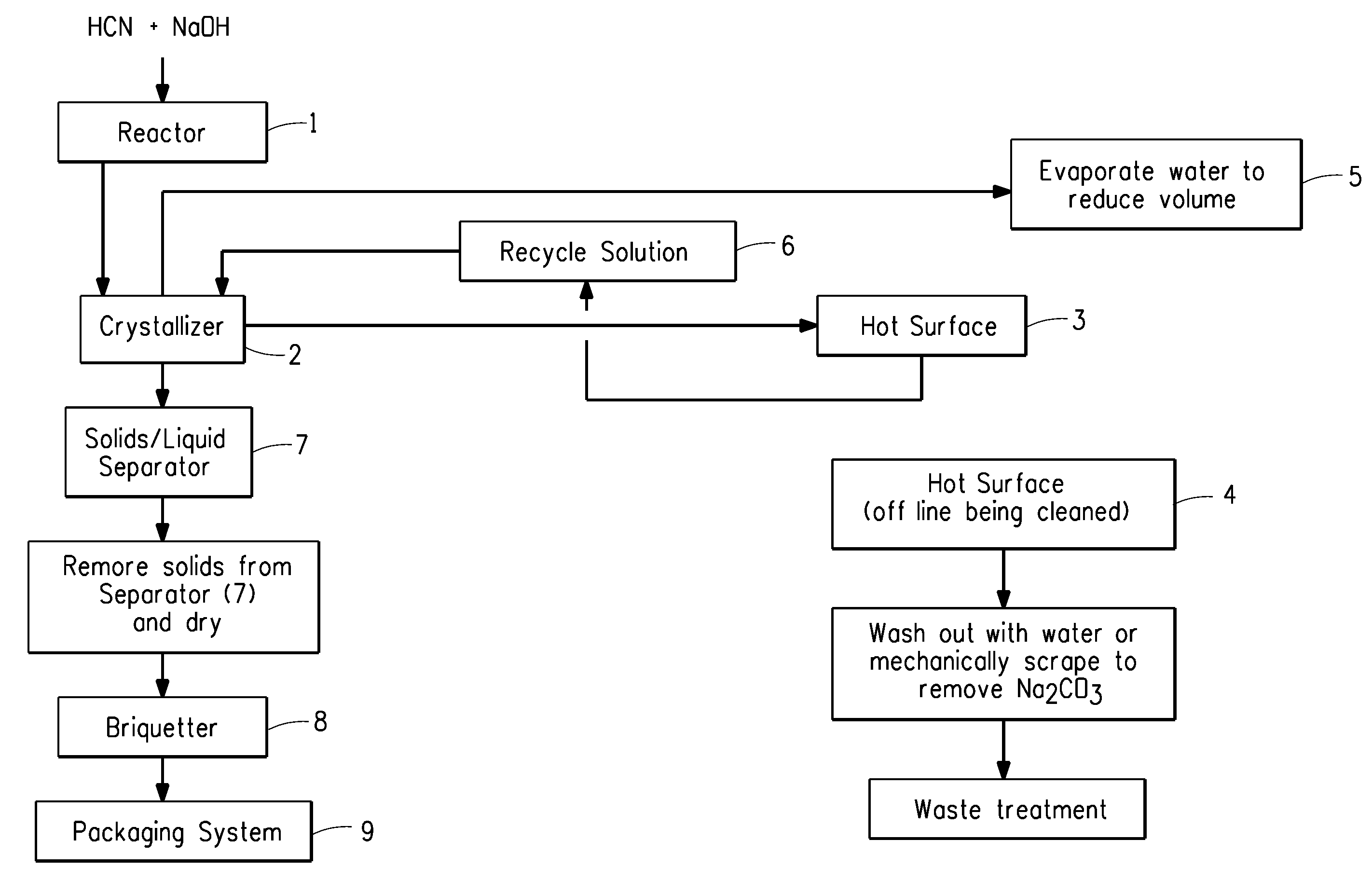
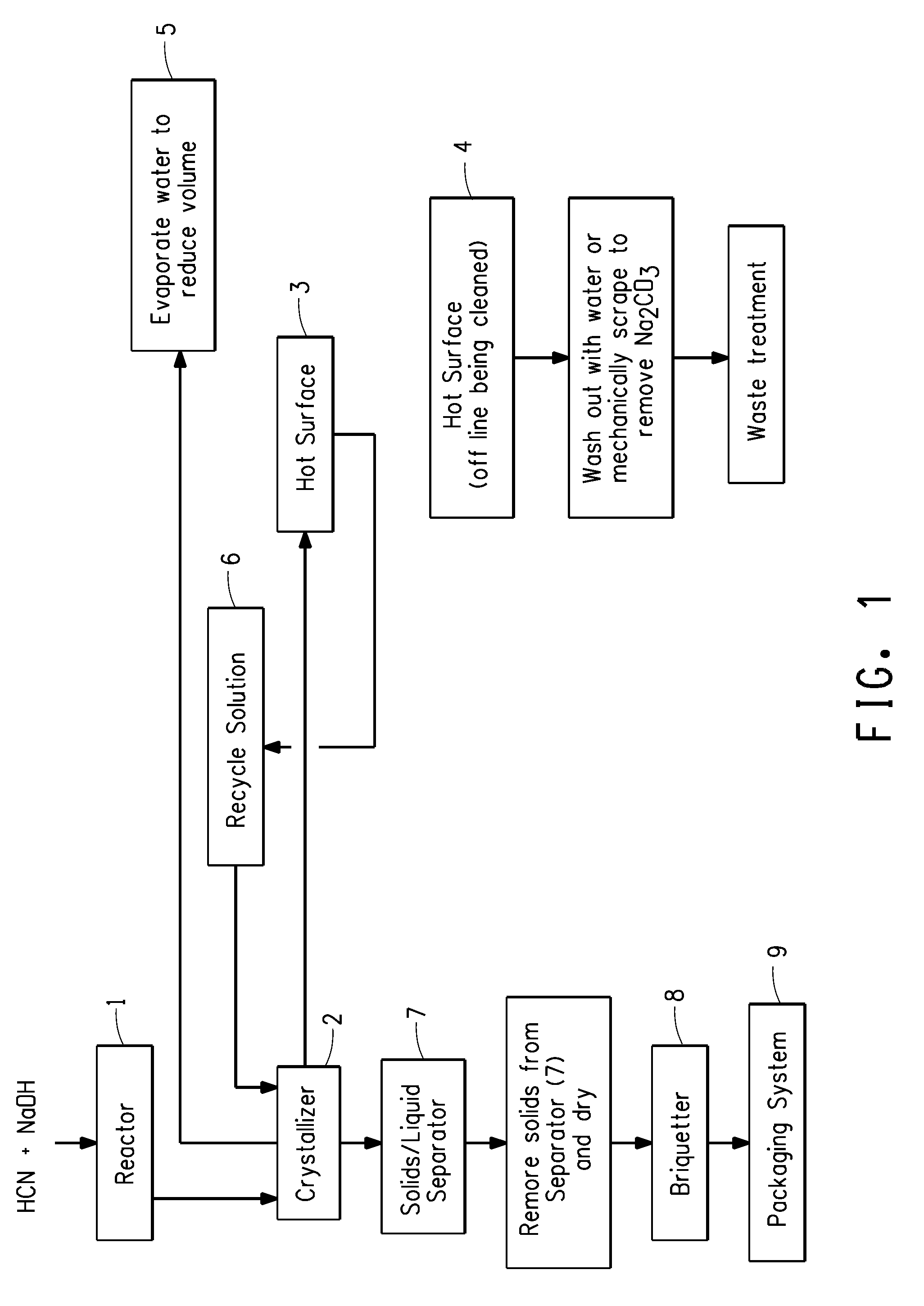
Sodium cyanide process
A process for the production of sodium cyanide crystals comprising;(a) contacting impure hydrogen cyanide and sodium hydroxide in a reactor with mixing for a maximum contact time of about 5 seconds;(b) feeding the resulting mixture to a continuous evaporative crystallizer to produce a slurry of sodium cyanide crystals;(c) passing the slurry of sodium cyanide crystals from the crystallizer over a hot surface to precipitate onto the surface and remove sodium carbonate, and passing said slurry back to the crystallizer; and(d) separating the sodium cyanide crystals from the slurry.
Owner:THE CHEMOURS CO FC LLC
Method for determining soil total cyanide--isonicotinic acid--barbituric acid spectrophotometric method
InactiveCN101256138AMeet analysisMeet the jobMaterial analysis by observing effect on chemical indicatorSpecial data processing applicationsCyanideRepeatability
The invention provides a test method, which includes the following steps: distilling soil in pH<2 acidic medium, hydrogen cyanide is formed, absorbing with the sodium hydrate solution, and analyzing total cyanide in soil with isonicotinic acid-barbituric acid spectrophotometry. The invention has the advantages of good sensitivity and good repeatability, which provides a convenient and reliable method for analyzing total cyanide in soil, and meets the needs of soil pollutant analysis as well as research work.
Owner:谱尼测试集团股份有限公司
Selective filtration of cigarette smoke using chitosan derivatives
A smoking article filter having a porous resin with a high surface area to mass ratio comprised of a chitosan derivative. PreferedPreferred embodiments include chitosan cross-linked with glutaraldehyde and chitosan cross-linked with glyoxal. The chitosan derivative provides for the selective filtration of cigarette smoke, particularly for the removal of aldehydes, hydrogen cyanide, heavy metals and carbonyls.
Owner:BROWN & WILLIAMSON HLDG INC
Hydrocyanation method for ethylenically unsaturated organic compounds
The invention concerns a hydrocyanation method for ethylenically unsaturated organic compounds in particular to obtain nitriles, and more particularly hydrocyanation of substituted diolefins or of olefins for producing dinitriles, and / or the isomerization of nitriles obtained by hydrocyanation. The invention more particularly concerns hydrocyanation catalysed by a nickel-based compound. Thus the catalyst used in said production method is treated in output with hydrogen cyanide to recover and re-dissolve the nickel precipitated in the form of nickel hydroxide. The method enables to regenerate and prolong the life span of a catalyst charge. Moreover, it enables to reduce pollution in the installations using said method.
Owner:RHODIA POLYAMIDE INTERMEDIATES
Electrical smoking system and method
InactiveCN1287699CReduce gaseous componentsIncandescent ignitionCigar manufactureAcrylonitrileEngineering
An electrical smoking system comprising a cigarette and an electric lighter, wherein the cigarette comprises a wrapper surrounding a tubular tobacco mat partially filled with material tobacco so as to define a filled tobacco rod portion and an unfilled tobacco rod portion. The wrapper includes an ammonium containing compound filler therein effective to reduce gaseous components of the tobacco smoke produced during smoking of the cigarette. The system includes a lighter comprising at least one heating blade and a controller adapted to control heating of the heater blade, the lighter arranged to at least partially receive the cigarette such that the heater blade heats a heating zone of the cigarette. The controller is operable to limit heating of the heater blade to a predetermined temperature range which allows delivery of tobacco smoke generated by heating the tobacco rod portion while reducing the content of at least one gaseous component in the tobacco smoke compared to smoking a cigarette having only calcium carbonate as the wrapper filler. The gaseous components which can be reduced include carbon monoxide, 1,3-butadiene, isoprene, acrolein, acrylonitrile, hydrogen cyanide, o-toluidine, 2-naphtylamine, nitrogen oxide, benzene, NNN, phenol, catechol, benz(a)anthracene, and benzo(a)pyrene.
Owner:PHILIP MORRIS PROD SA
Processing method of nitrile contained organic sewage difficult to degrade
InactiveCN1907881AWater/sewage treatment by flocculation/precipitationWater/sewage treatment by reductionElectrolysisDecomposition
This invention concerns a treatment method for the organic wastewater containing hydroxyacetonitrile, phenylaminoacetonitrile, aniline, diazo value, etc. nitrile-bearing refractory organism. Said method comprises carrying out microelectrolysis under acidic condition, and then adding ferrous sulfate for redox reaction and flocculation process. The mechanism is to make nitriles and CN-NH3-N into small molecules or produce insoluble precipitate through synergetic effect of a series reactions like electrolysis, redox and aeration oxidation, and further decomposition, oxidation and reduction, and remove them through flocculation. The nitriles and hydrogen cyanide like hydroxyacetonitrile and phenylaminoacetonitrile in the water can be removed completely. Aniline can be degraded and removed. CODcr removal percentage is 35-45%.
Owner:CHONGQING UNISPLENDOUR CHEM
Measuring method for cyanides in cyanide-containing wastewater of gold mine
InactiveCN104833674AAccurate measurementGood effectMaterial analysis by observing effect on chemical indicatorPhosphoric acidWastewater
The invention discloses a measuring method for cyanides in cyanide-containing wastewater of a gold mine. In order to solve the problem that a great number of foams are produced by heating and distilling under an acidic condition, a defoaming agent GC-1 is added into a cyanide-containing wastewater sample to inhibit activities of a surfactant in wastewater, so that the water sample does not produce foams in a distilling process; phosphoric acid and Na2-EDTA are added into the water sample, and heated and distilled in a medium with pH lower than 2, so that cyanide ions are separated from complex cyanide by utilizing the characteristic that the complexing capacity between the metal ions and the EDTA is higher than that between the metal ions and the cyanide ions, the cyanide ions are distilled in the form of hydrogen cyanide, and the content of the cyanide ions is titrated by silver nitrate after the cyanide ions are absorbed by a sodium hydroxide solution. Under an alkaline condition, the cyanide ions act with the silver nitrate to form soluble sliver-cyanogen complex irons (Ag(CN)2<->), and the excessive sliver ions react with 5-(4-dimethylaminobenzylidene)rhodanine indicator liquor, so that the solution is changed into orange red from yellow, thereby ending the measurement. The method has the advantages of obvious effect and accurate measured result, and is simple to operate and easy to grasp.
Owner:CHANGCHUN GOLD RES INST
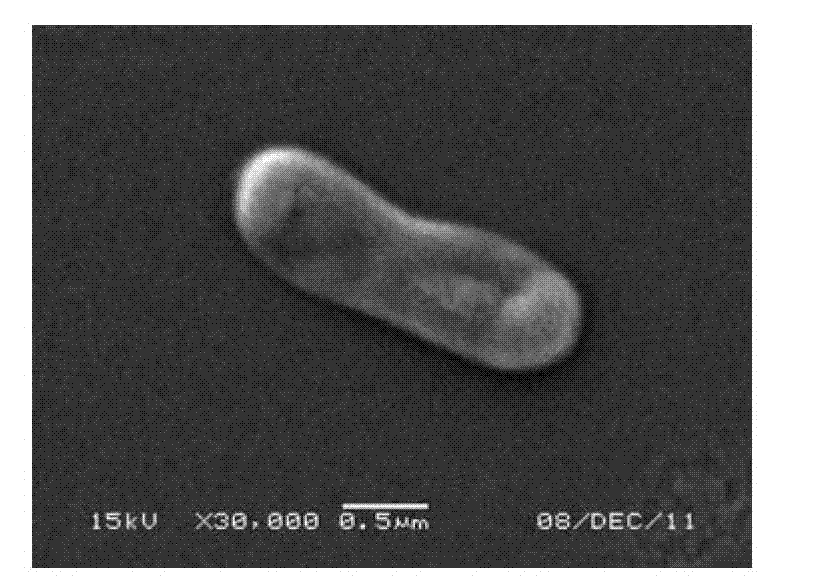
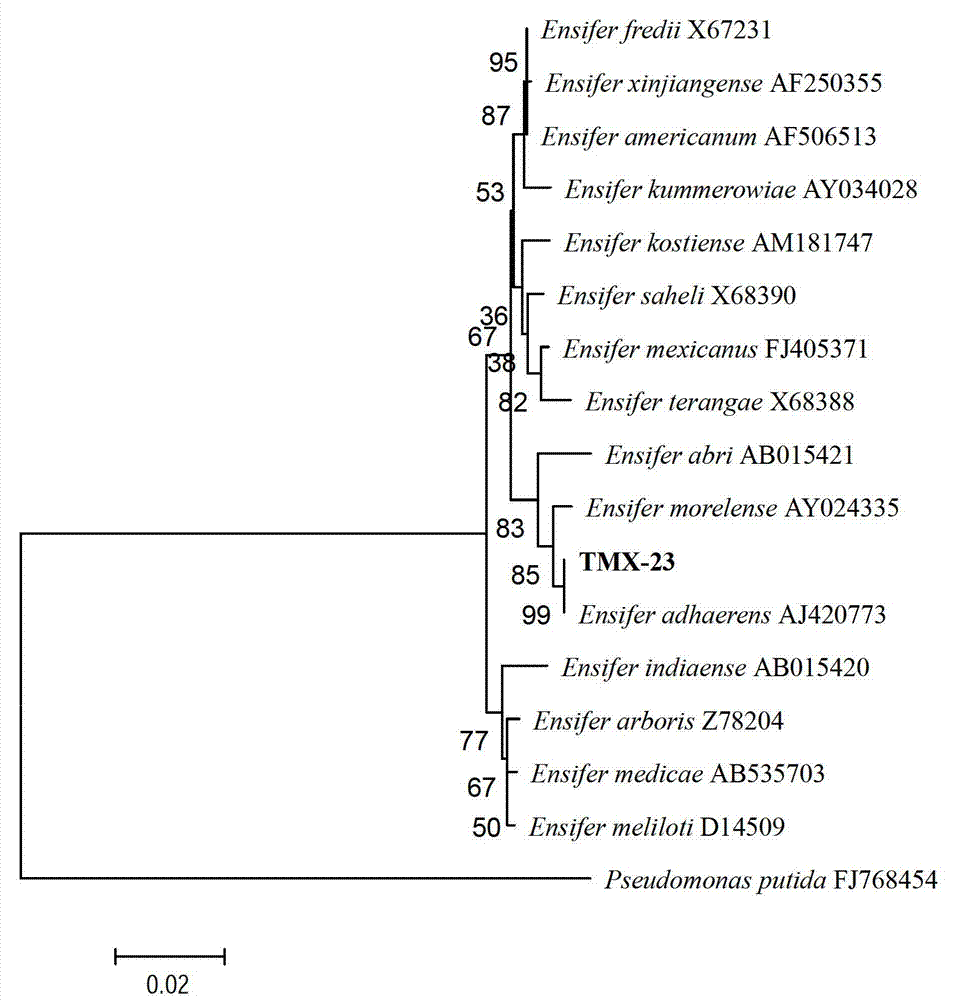
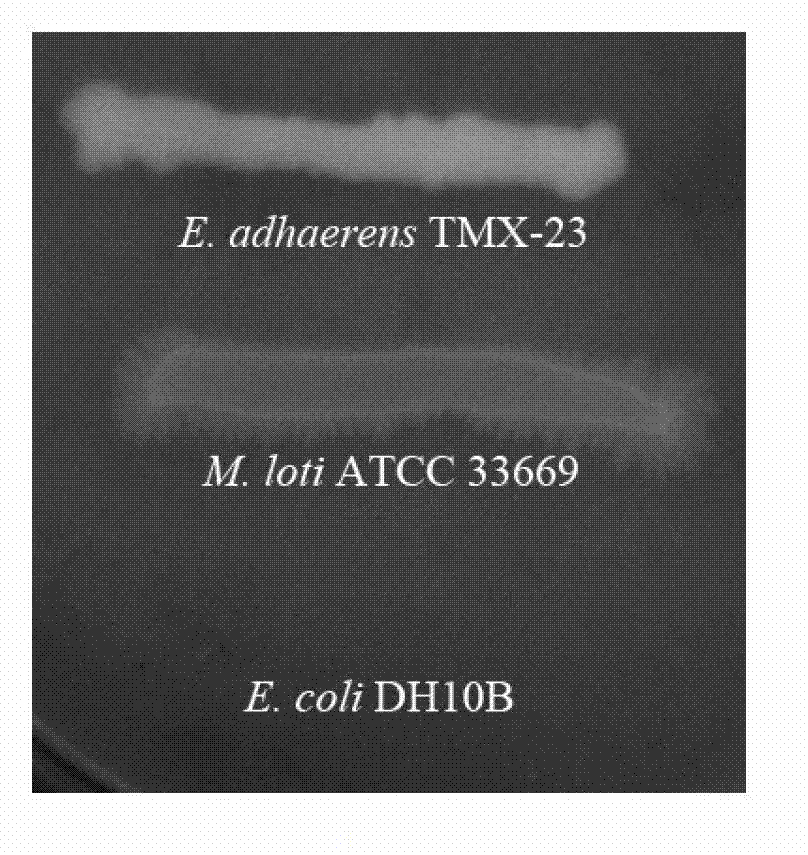
Ensiferadhaerens and application thereof
InactiveCN102851237AHas the ability to fix nitrogenHas PGPR activityBacteriaMicroorganism based processesBiotechnologyBenzoic acid
The invention discloses Ensiferadhaerens CGMCC 6315 which is a bacterium having a nitrogen-fixing capability. The strain is also a plant growth promoting rhizobacterium (PGPR), and can produce indoleacetic acid (IAA), extracellular polysaccharide (EPS), siderophore, salicylic acid (SA), 2,3-dihydroxybenzoic acid (DHBA), hydrogen cyanide (HCN) and ammonia. Under the stress condition of a salt containing sodium chloride, soybeans inoculated with the E.adhaerens CGMCC 6315 can have a higher germination rate in comparison with a control group which is not inoculated with the strain.
Owner:NANJING NORMAL UNIVERSITY
Processes for the anaerobic bioconversion of syngas to oxygenated organic compound with in situ protection from hydrogen cyanide
Owner:SYNATA BIO INC
Process for producing glycolic acid from formaldehyde and hydrogen cyanide
A process is provided for producing glycolic acid from formaldehyde and hydrogen cyanide. More specifically, heat-treated formaldehyde and hydrogen cyanide are reacted to produce glycolonitrile having low concentrations of impurities. The glycolonitrile is subsequently converted to an aqueous solution of ammonium glycolate using an enzyme catalyst having nitrilase activity derived from Acidovorax facilis 72W (ATCC 57746). Glycolic acid is recovered in the form of the acid or salt from the aqueous ammonium glycolate solution using a variety of methods described herein.
Owner:THE CHEMOURS CO FC LLC
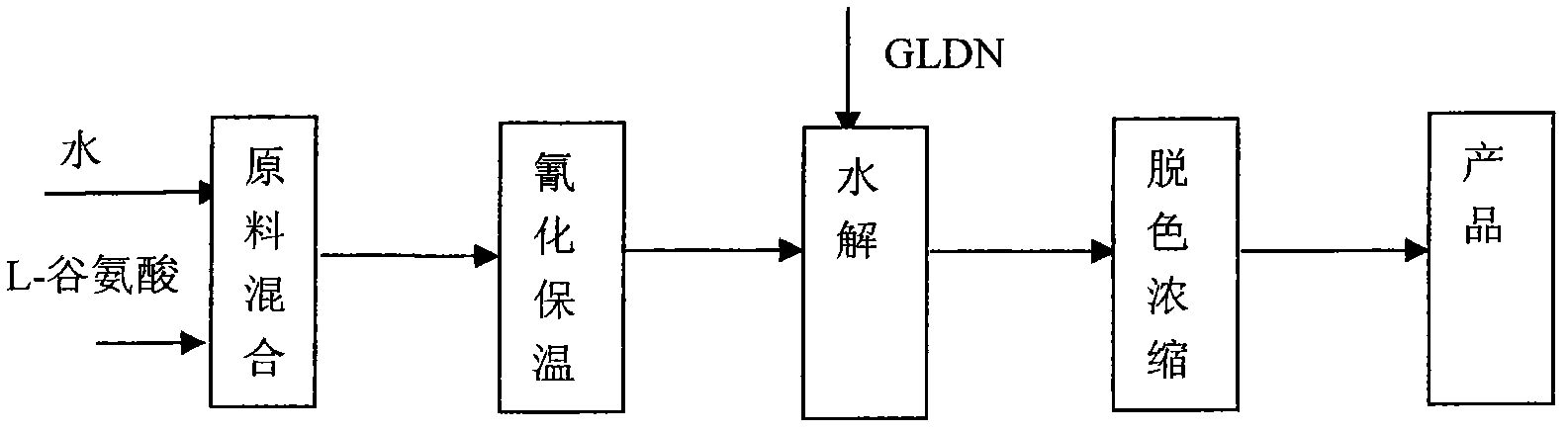
Preparation of novel green chelating agent glutamic diacetate tetracetic acid metal salt
ActiveCN102827016AReduce usageEasy to handleOrganic compound preparationAmino-carboxyl compound preparationToxic materialEthyl Chloride
The invention relates to a novel preparation of a green chelating agent glutamic diacetate tetracetic acid metal salt. The preparation comprises the steps of synthesizing GLDA-4Na tetrasodium glutamate diacetate (GLDA-4M) through L-glutamic acid, 2-chloroacetonitrile organic alkali, zinc chloride and sodium hydroxide; carrying out cyanation and hydrolysis of L-glutamic acid to obtain the aqueous solution of the GLDA-4Na; and then carrying out decolorizing and concentrating to obtain the product. According to the preparation, the 2-chloroacetonitrile organic alkali is adopted to replace the common HCN (hydrogen cyanide) in the prior art, so that the use of the extremely toxic substance is reduced, and the produced wastewater can be easily processed; and green chelating agent glutamic diacetate tetracetic acid metal salt has high yield, high purity, and low cost, and is simple in technology, and easy to realize post-treatment to the wastewater, and achieve industrial production.
Owner:石家庄杰克化工有限公司
Gas analyzer
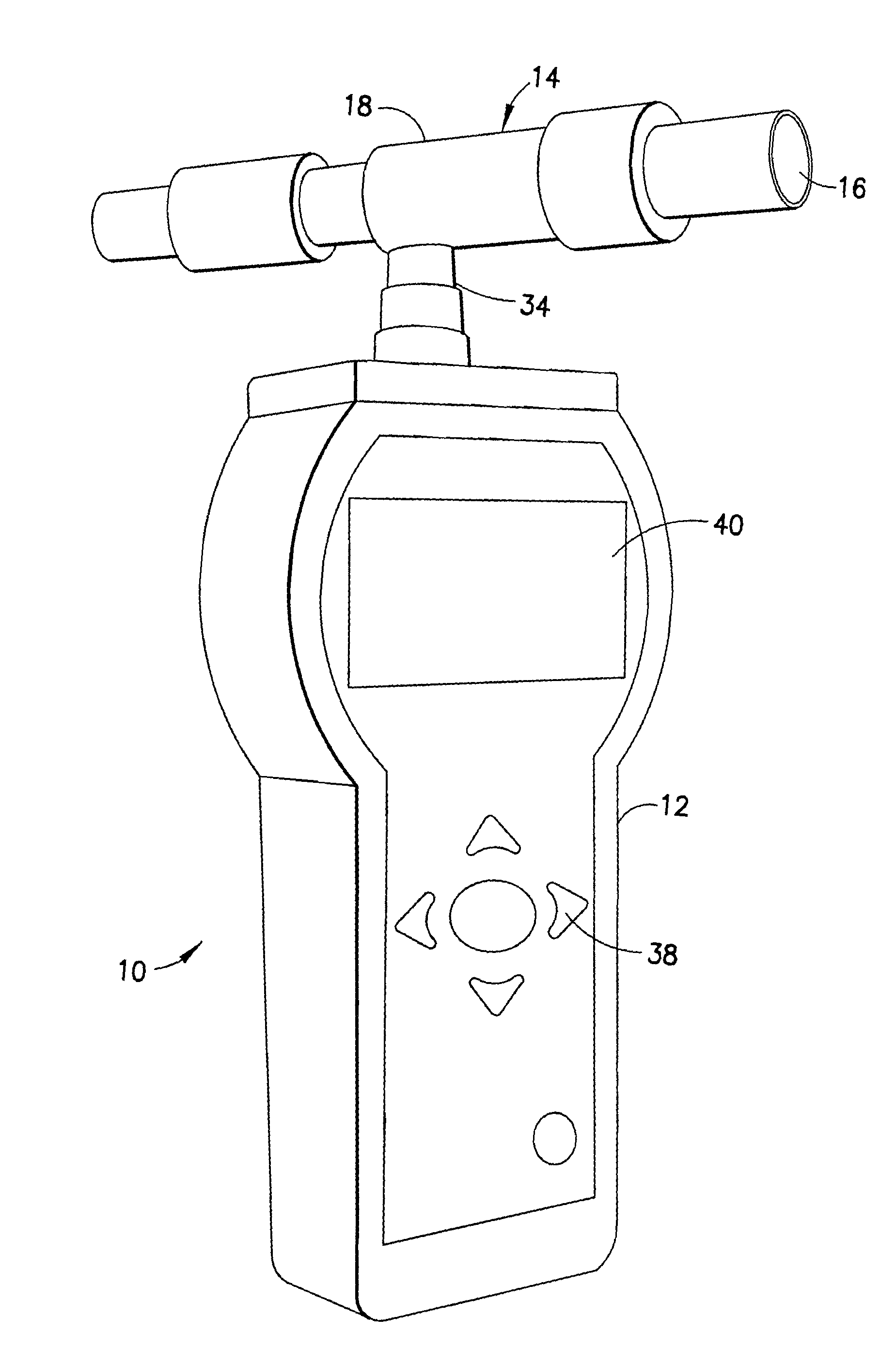
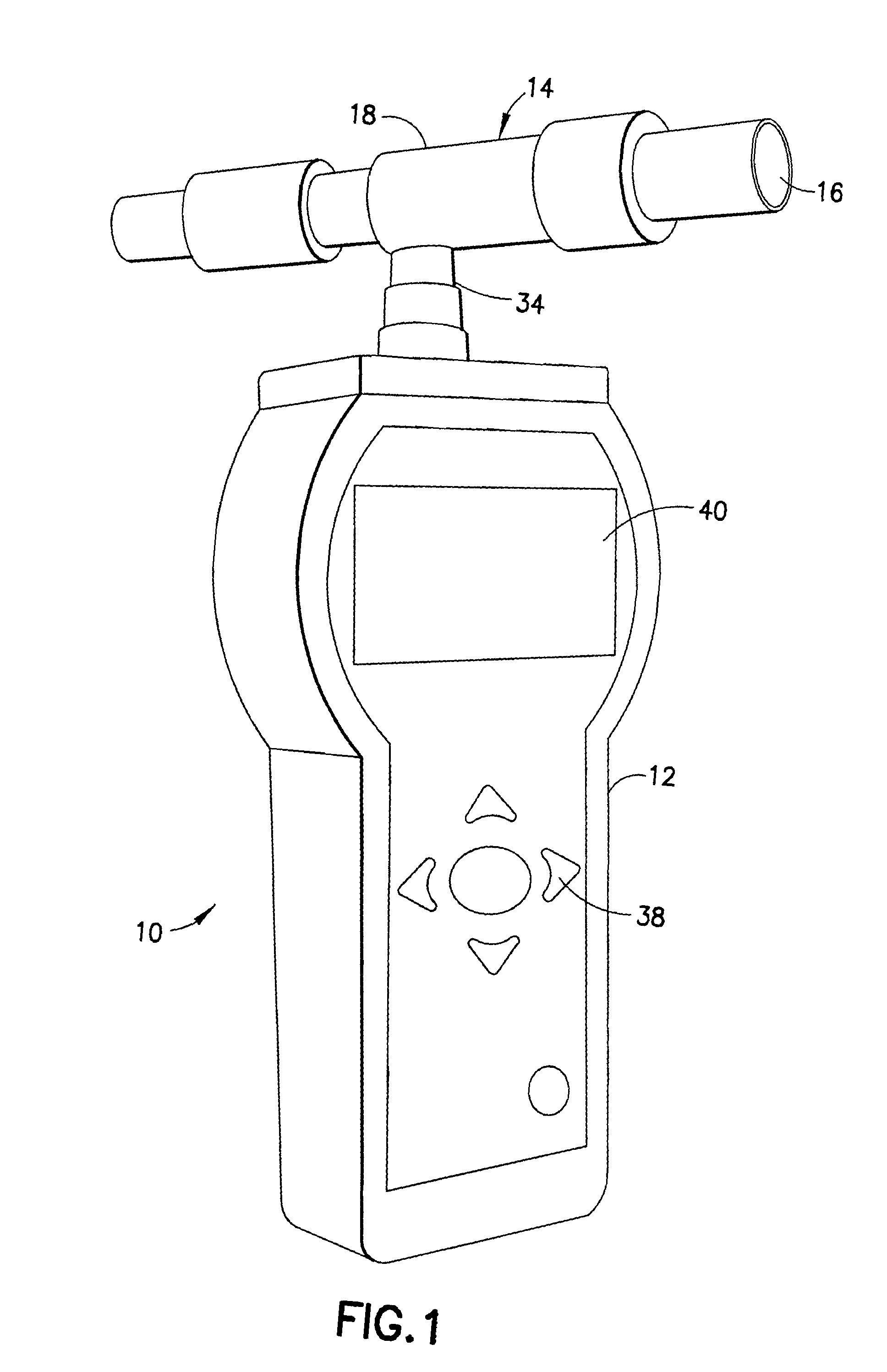
InactiveUS20110001625A1Quickly determineQuick levelingWithdrawing sample devicesDiagnostic recording/measuringToxic gasHydrogen
The subject invention is directed to a breath analyzer which is capable of detecting toxic gas levels from breath analysis. The subject invention includes a mouthpiece which is in communication with a plurality of discrete chambers, such as first and second discrete chambers, each being provided with a separate probe for breath analysis. The probes are connected to analyzers for determining detected levels of gas. In a first embodiment, a first probe may be provided for carbon monoxide detection with a second probe being provided for hydrogen cyanide detection. Advantageously, with this arrangement, breath analysis may be conducted on-site, for example at the site of a fire, to quickly and simultaneously determine carbon monoxide and hydrogen cyanide levels in a person's blood stream. In a second embodiment, a first probe may be provided for detection of carbon monoxide and a second probe may be provided for detection of hydrogen. With this arrangement, a calibrated correction of measured carbon monoxide data can be made to correct for improperly detected hydrogen. As such, a highly accurate on-site measurement for carbon monoxide can be achieved.
Owner:FSP INSTR
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com