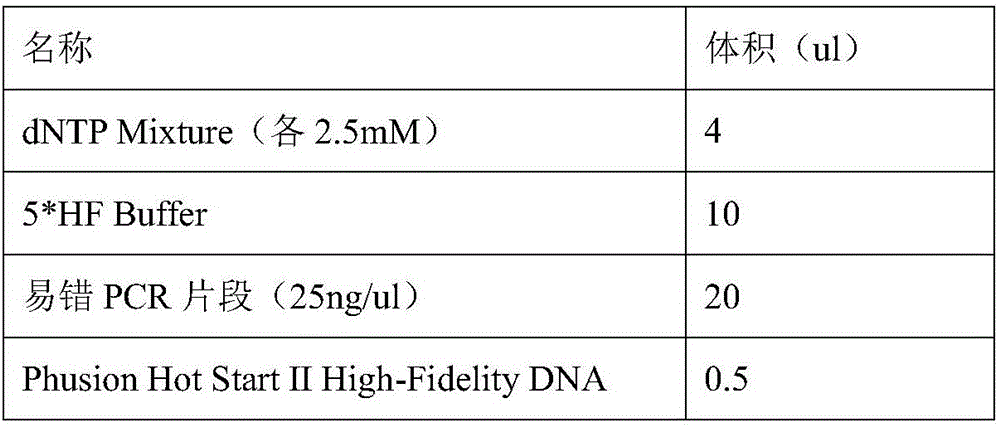
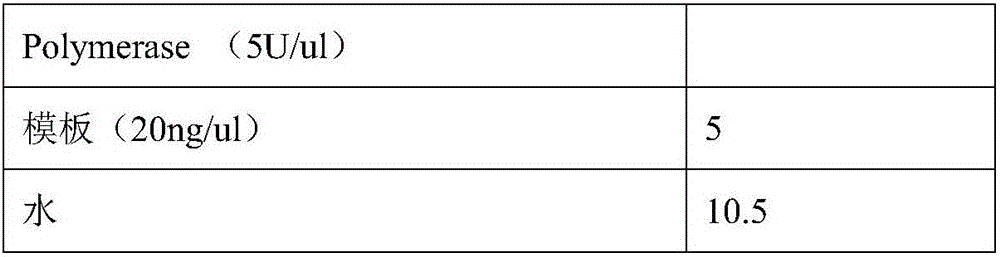
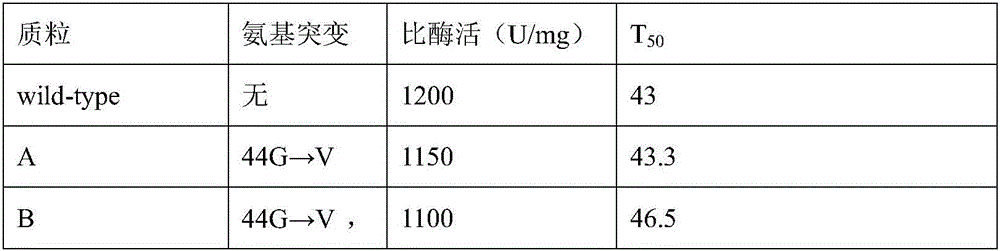
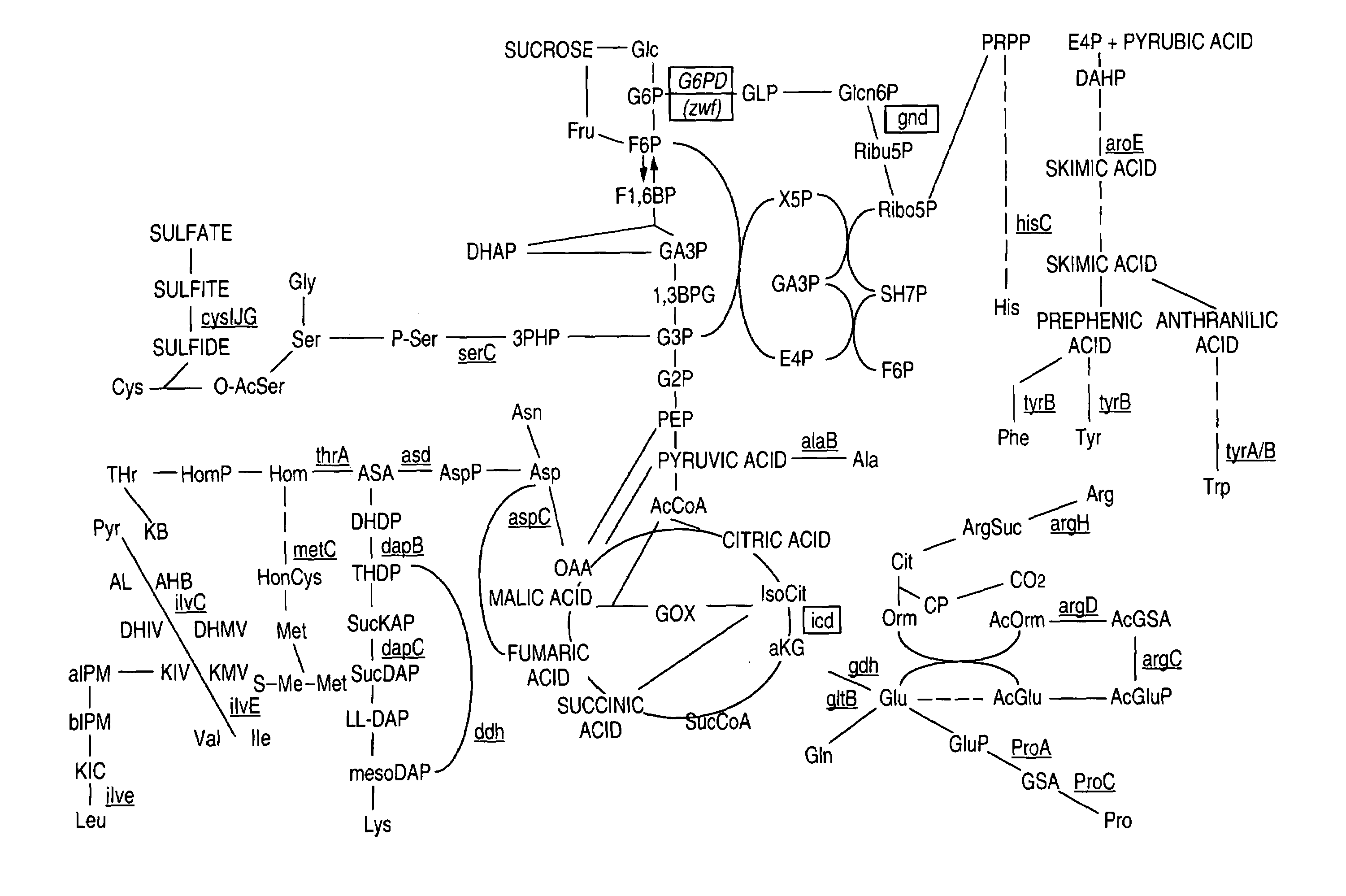
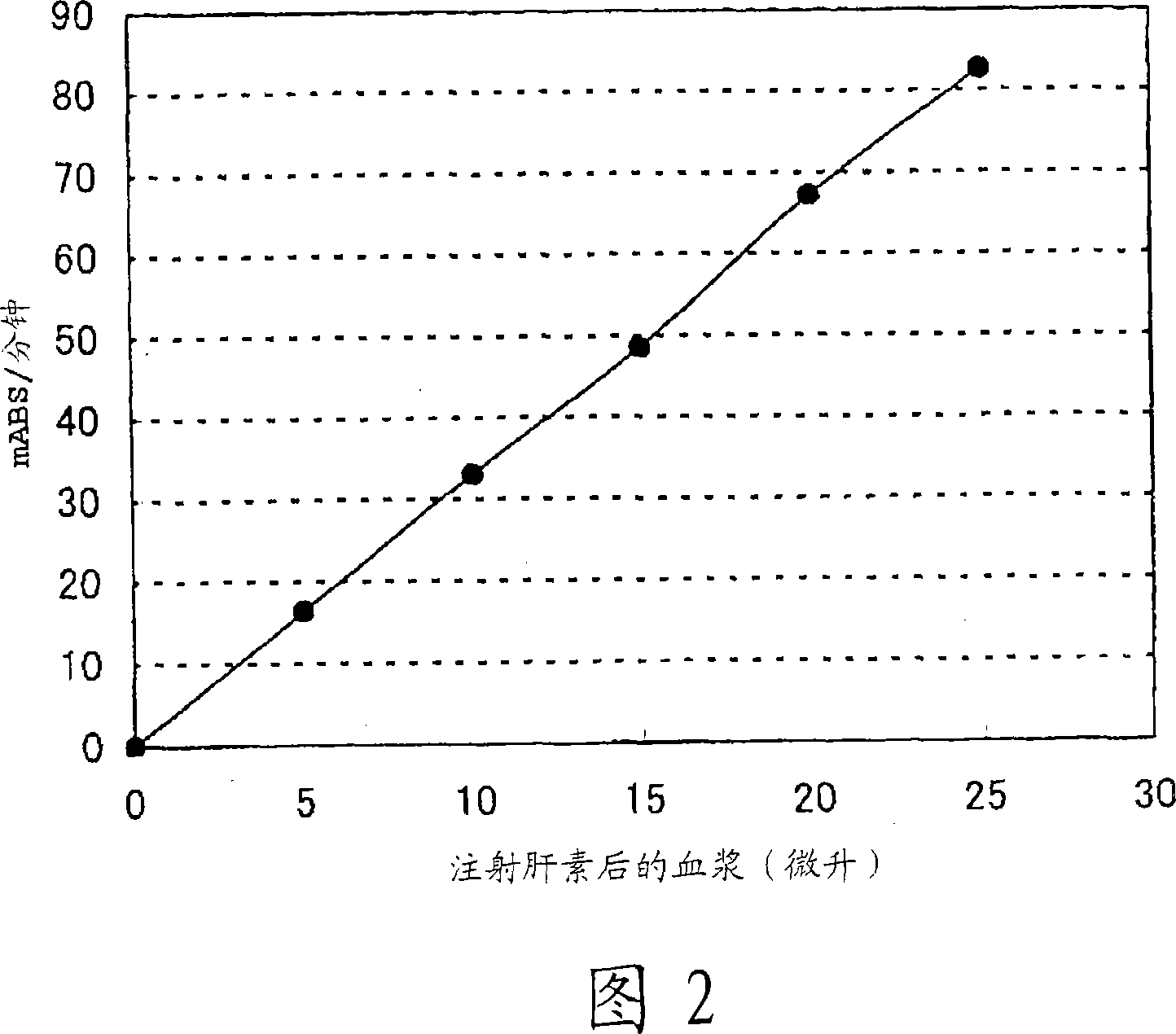
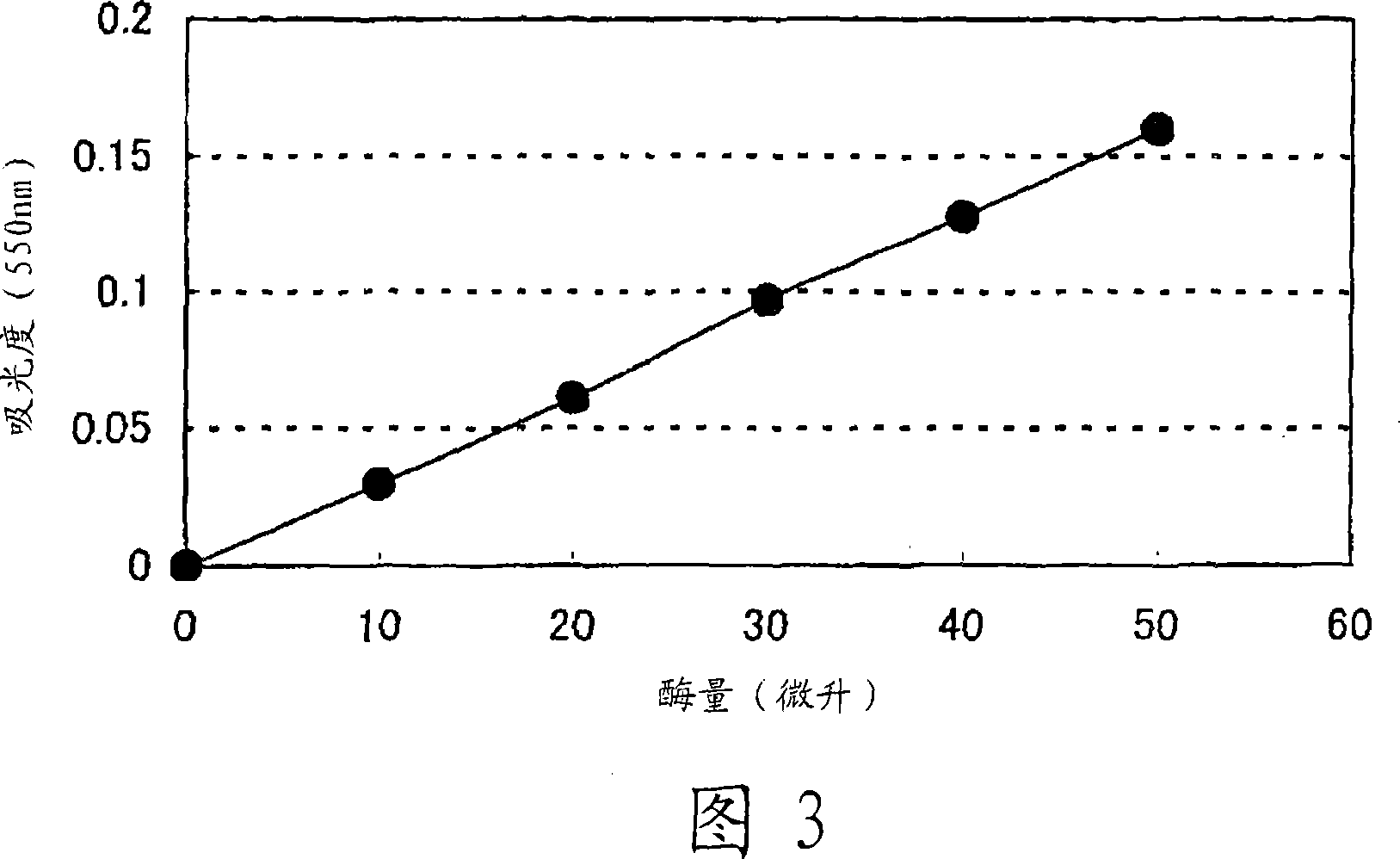
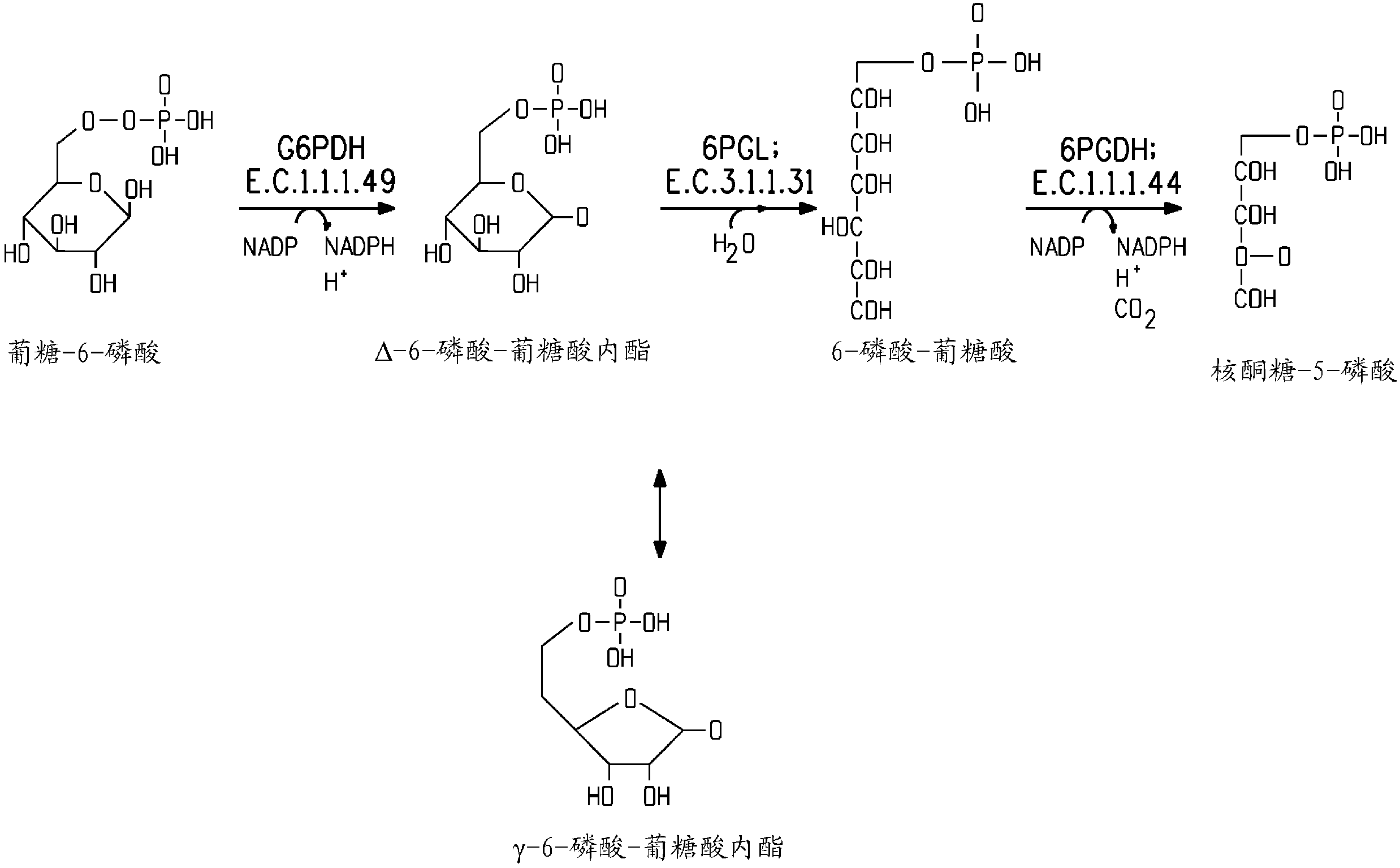
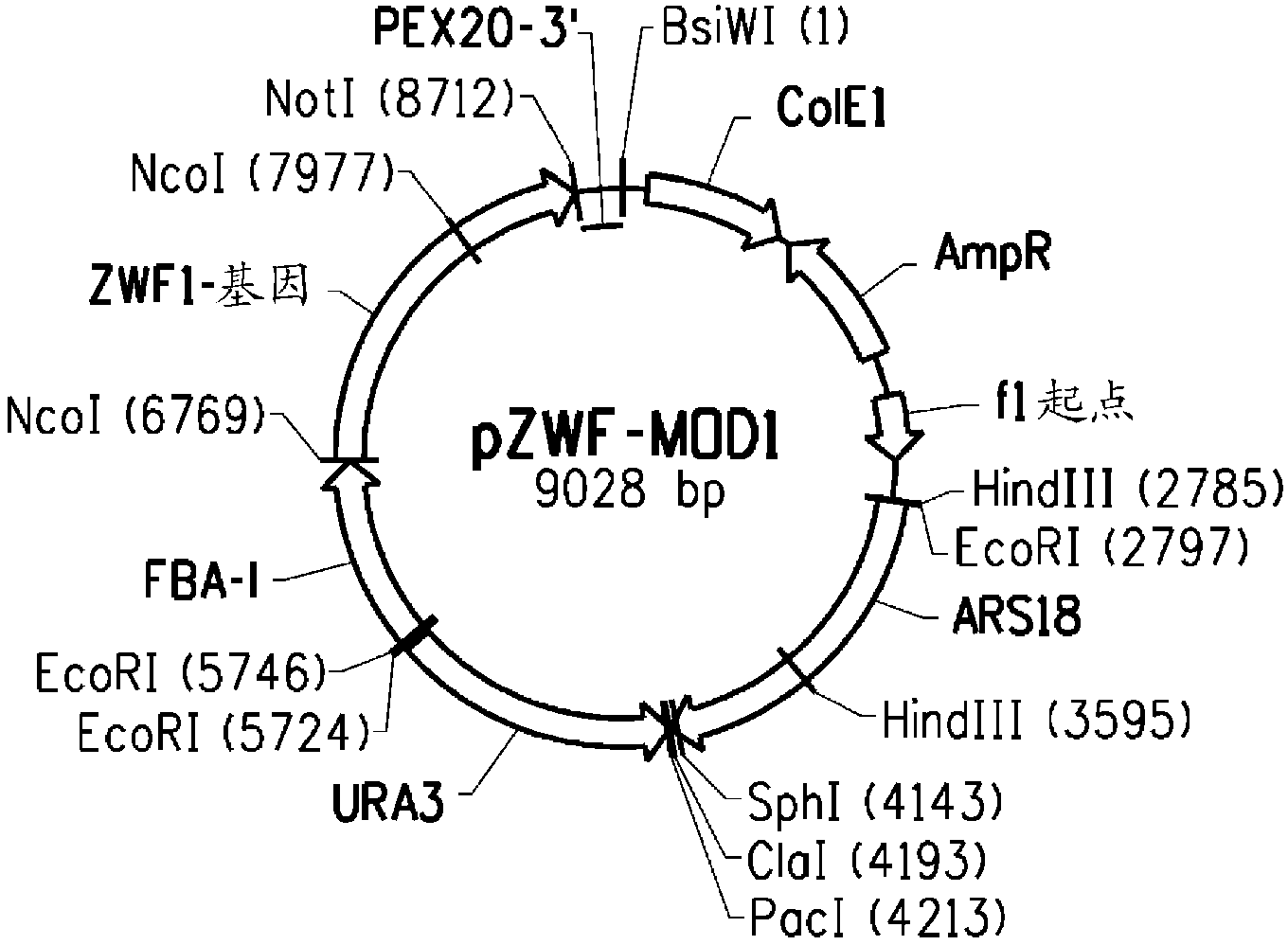
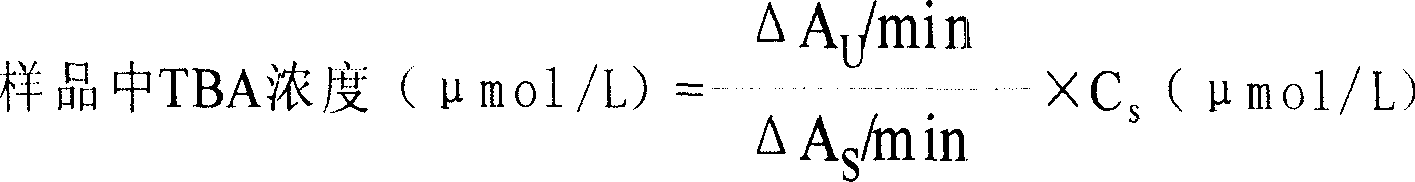Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Glucose-6-phosphate dehydrogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
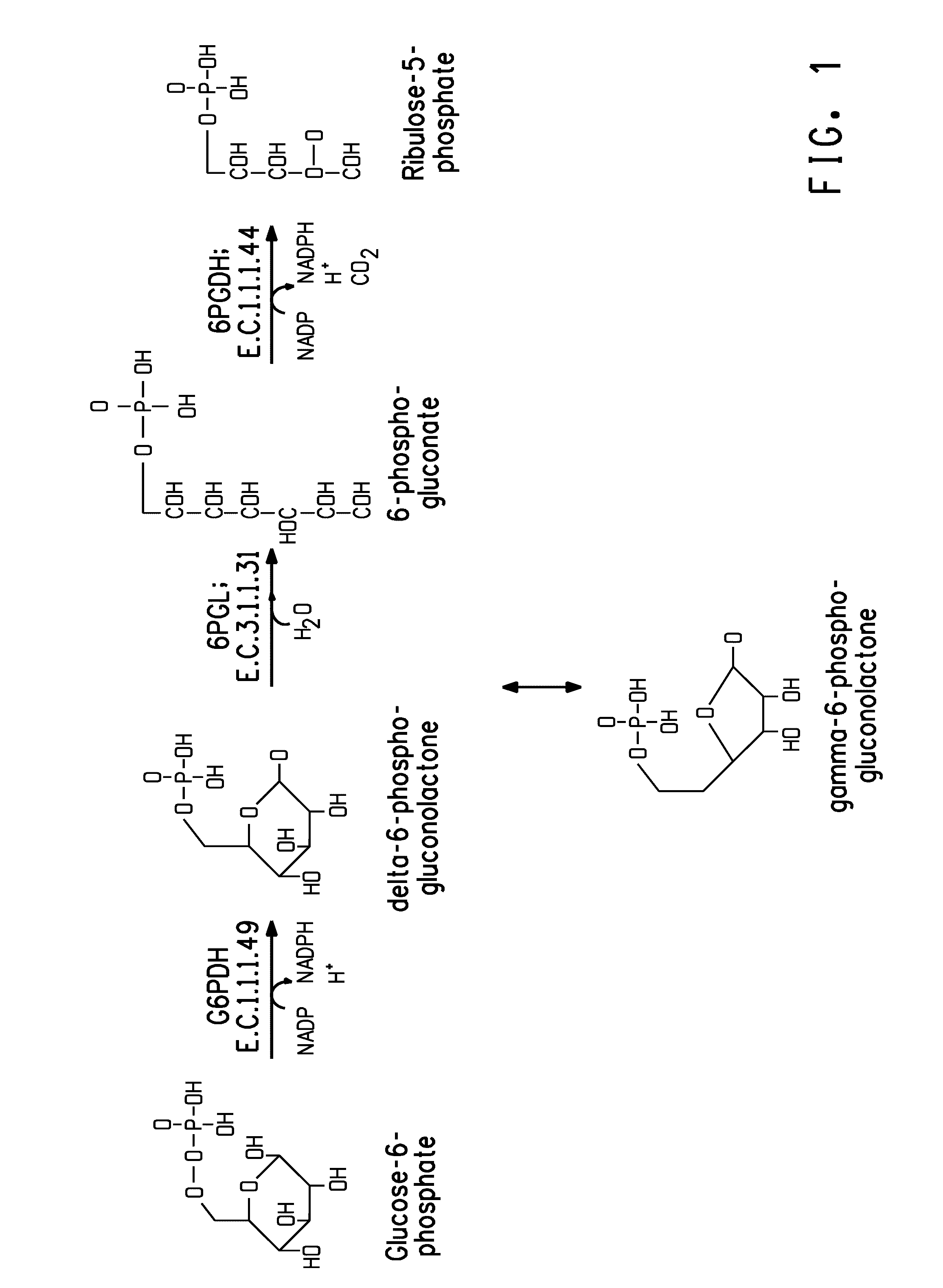
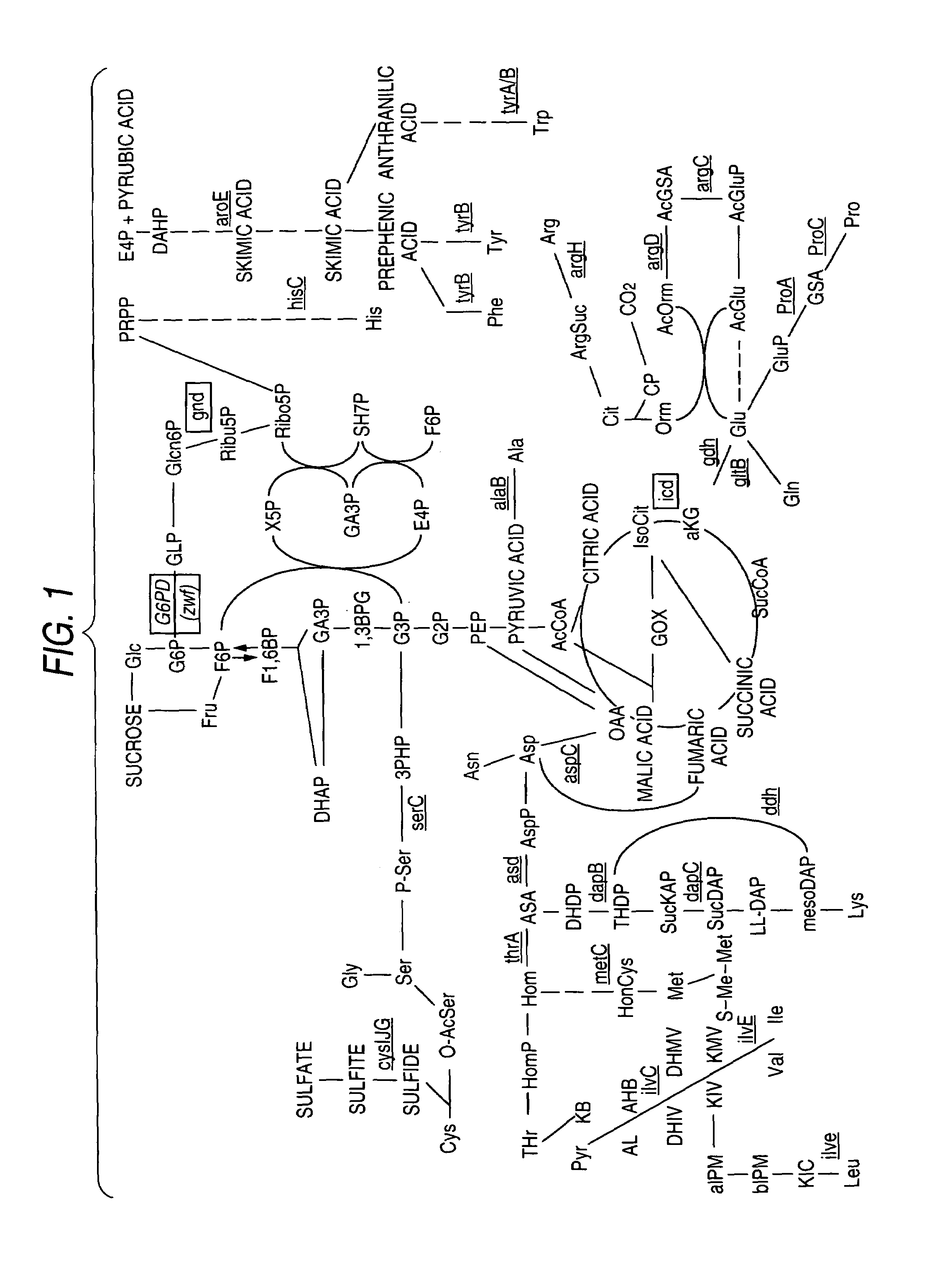
Glucose-6-phosphate dehydrogenase (G6PD or G6PDH) (EC 1.1.1.49) is a cytosolic enzyme that catalyzes the chemical reaction...
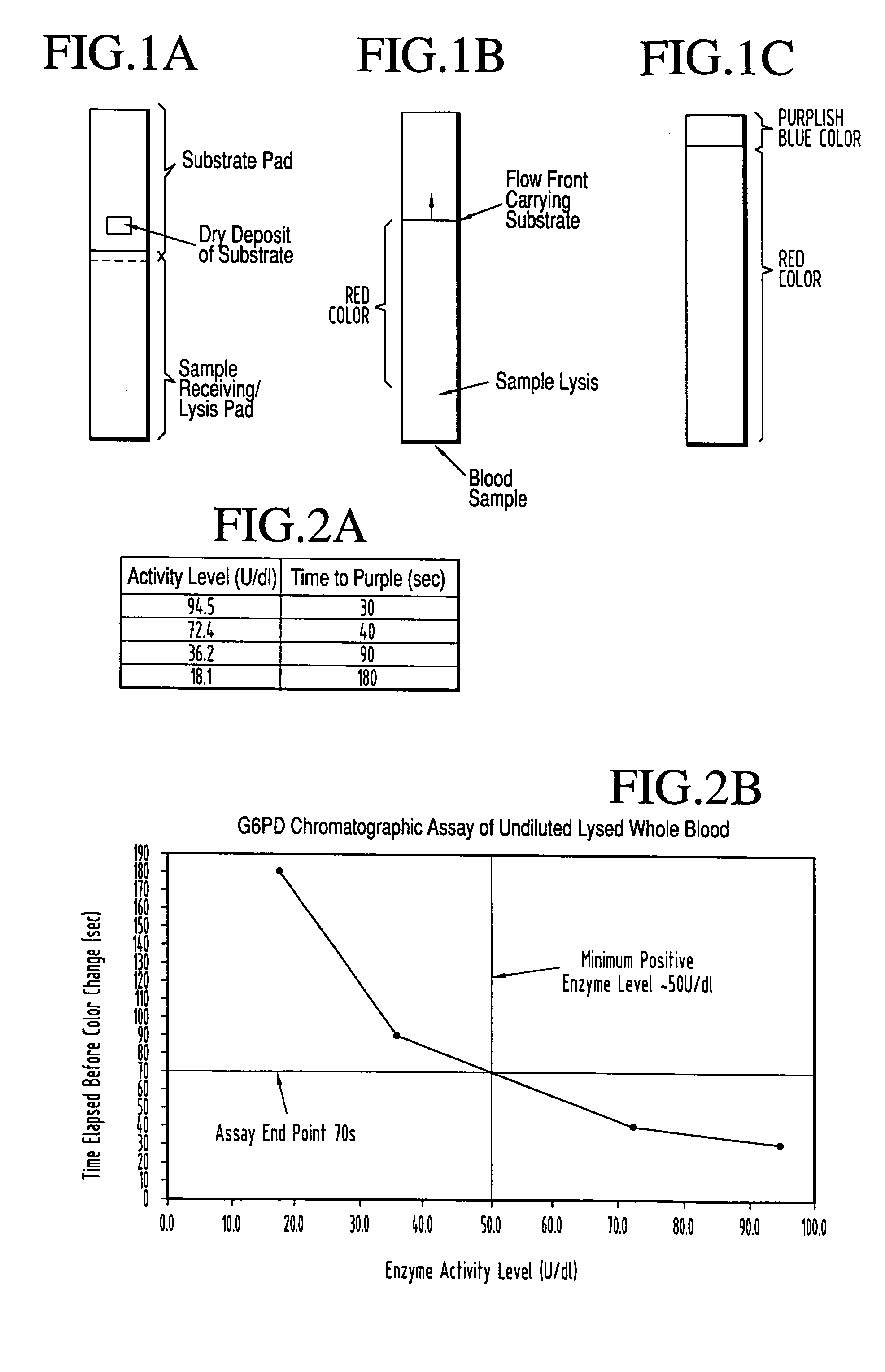
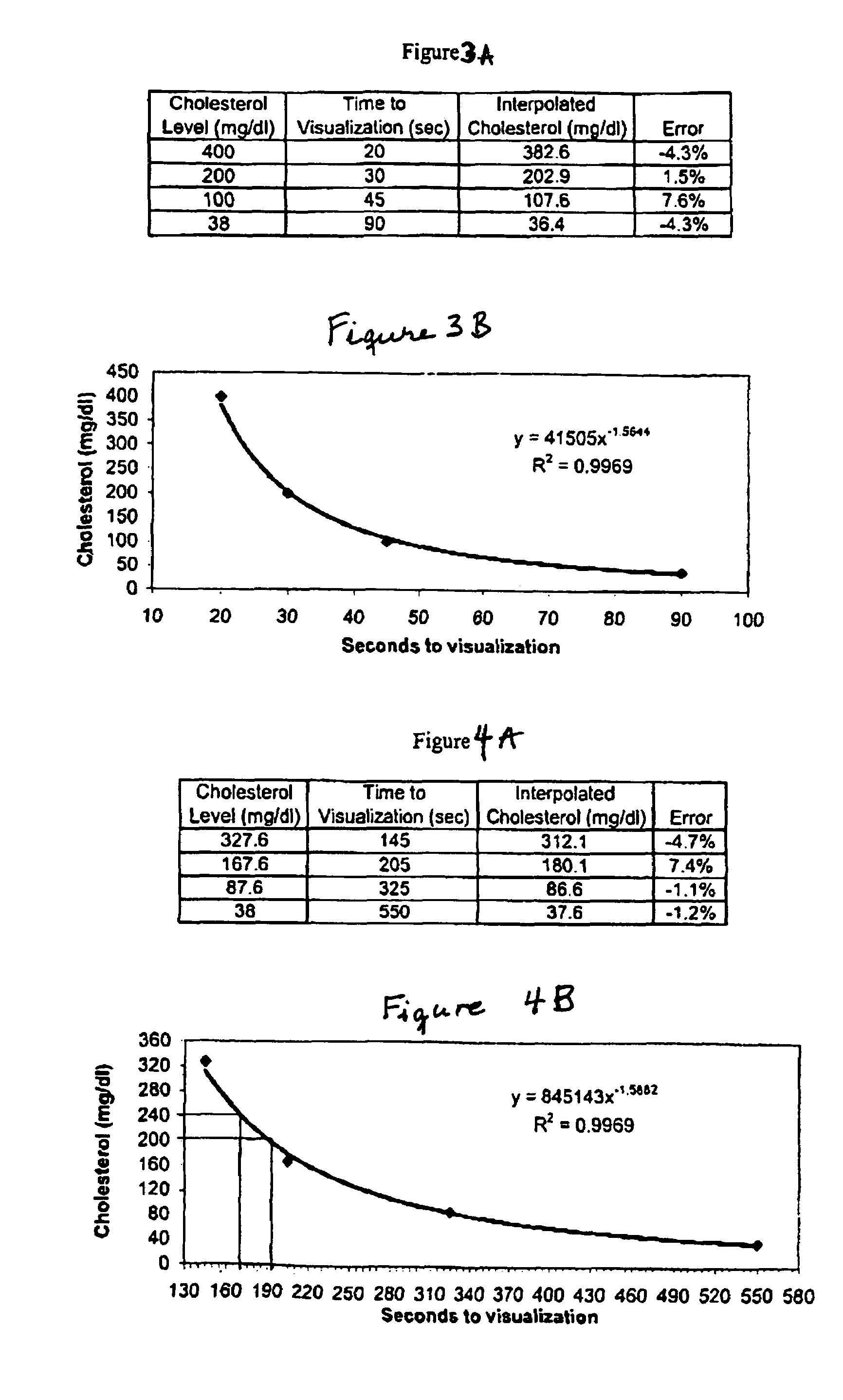
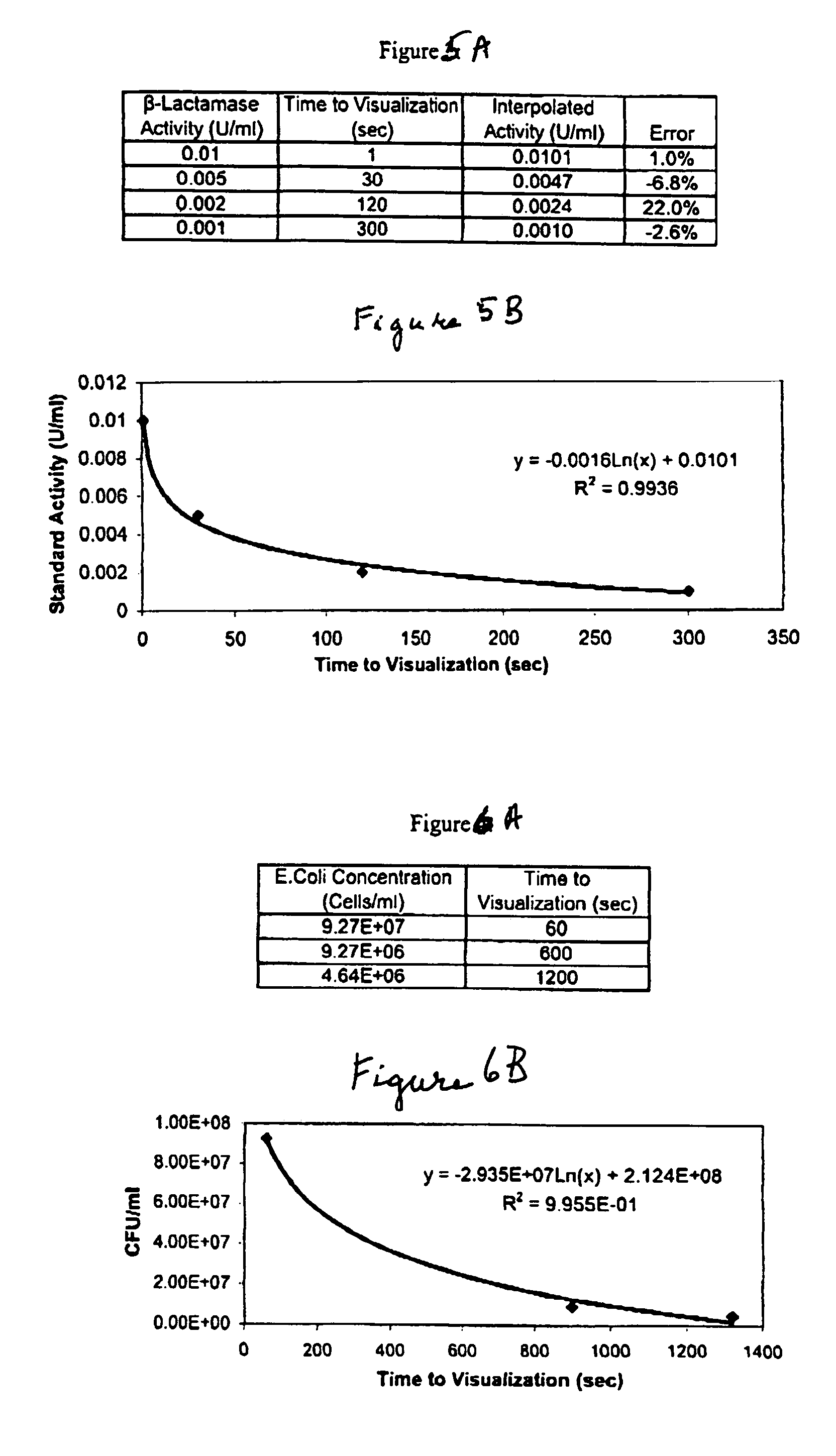
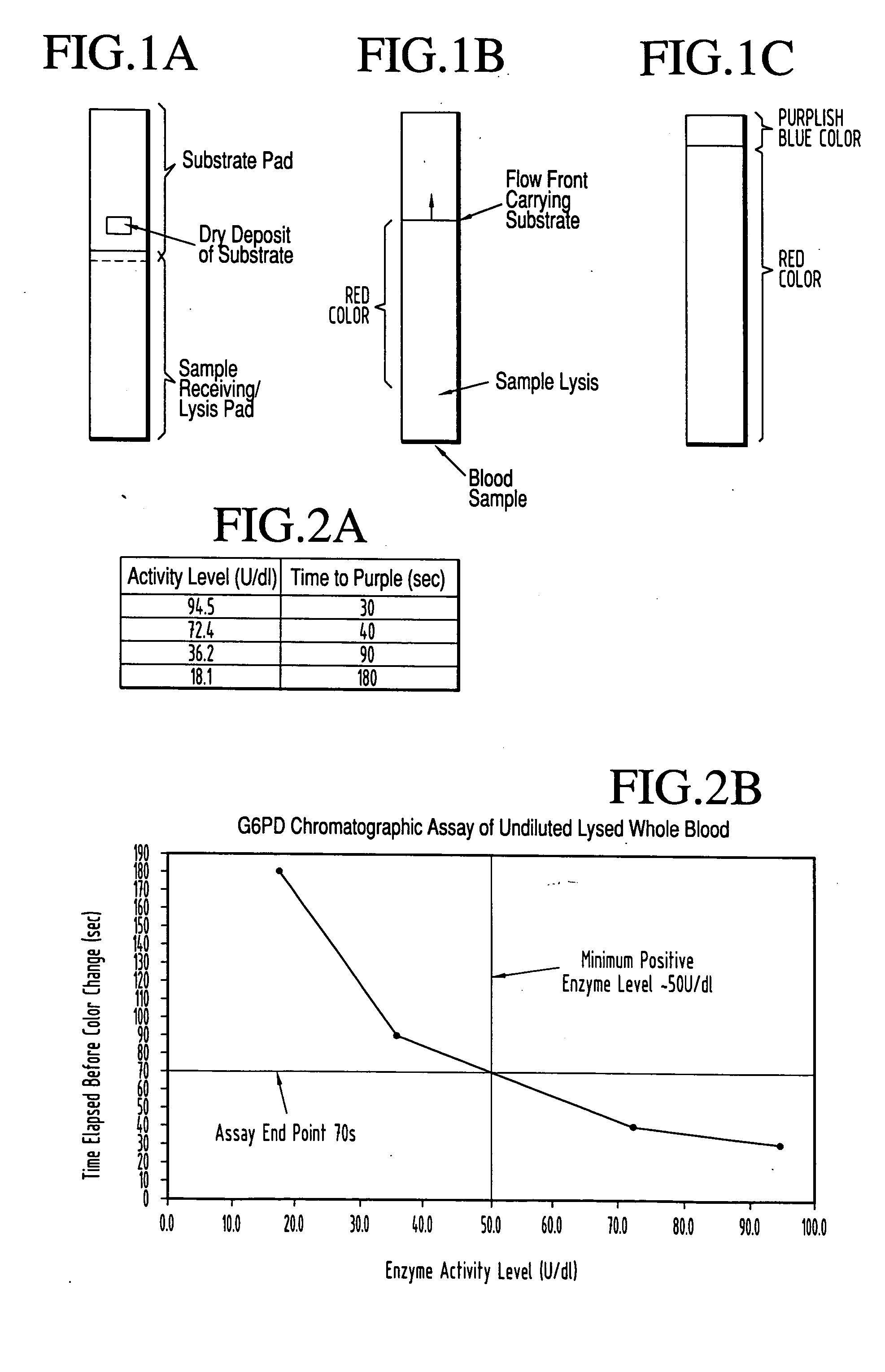
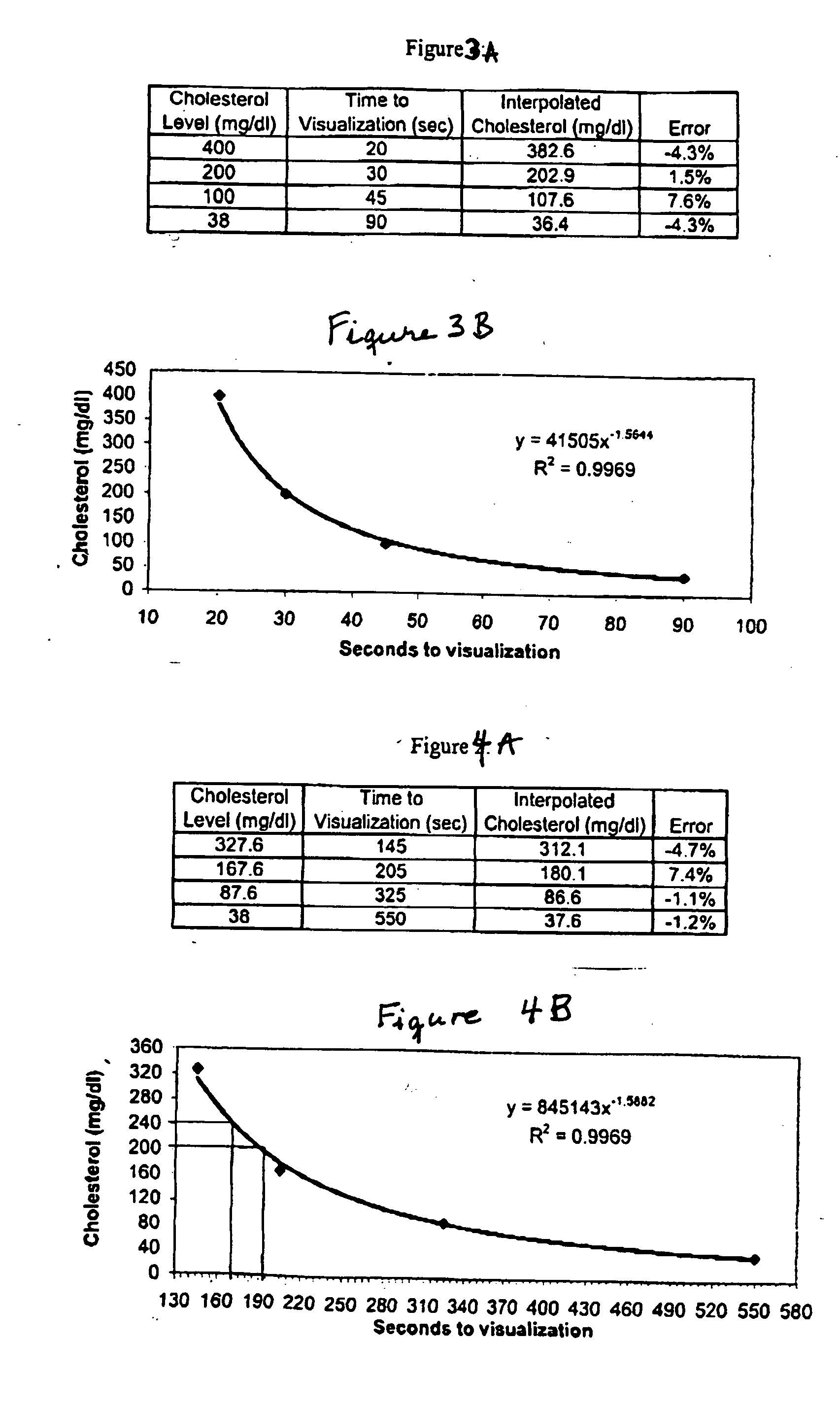
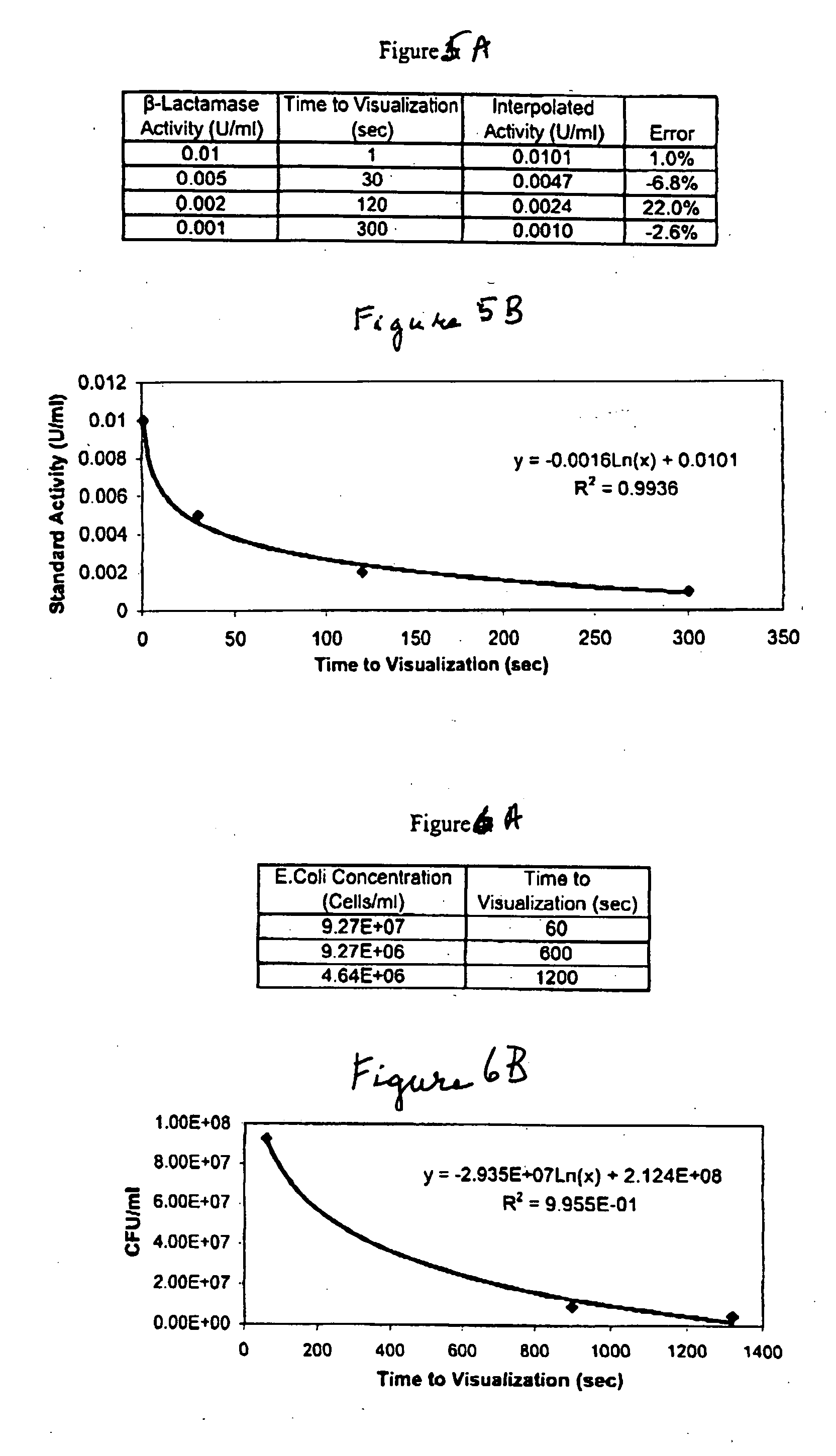
Dry chemistry, lateral flow-reconstituted chromatographic enzyme-driven assays
InactiveUS7425302B2Easy to observeHigh detection sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsAdditive ingredientPeroxidase
A lateral flow chromatographic assay format for the performance of rapid enzyme-driven assays is described. A combination of components necessary to elicit a specific enzyme reaction, which are either absent from the intended sample or insufficiently present therein to permit completion of the desired reaction, are predeposited as substrate in dry form together with ingredients necessary to produce a desired color upon occurrence of the desired reaction. The strip is equipped with a sample pad placed ahead of the substrate deposit in the flowstream, to which liquid sample is applied. The sample flows from the sample pad into the substrate zone where it immediately reconstitutes the dried ingredients while also intimately mixing with them and reacting with them at the fluid front. The fluid front moves rapidly into the final “read zone” wherein the color developed is read against predetermined color standards for the desired reaction. Pretreatment pads for the sample, as needed, (e.g. a lysing pad for lysing red blood cells in whole blood) are placed in front of the sample pad in the flow path as appropriate. The assay in the format of the invention is faster and easier to perform than analogous wet chemistry assays.Specific assays for glucose-6-phosphate dehydrogenase (“G-6PD”), total serum cholesterol, β-lactamase activity and peroxidase activity are disclosed.
Owner:ABBOTT DIAGNOSTICS SCARBOROUGH INC
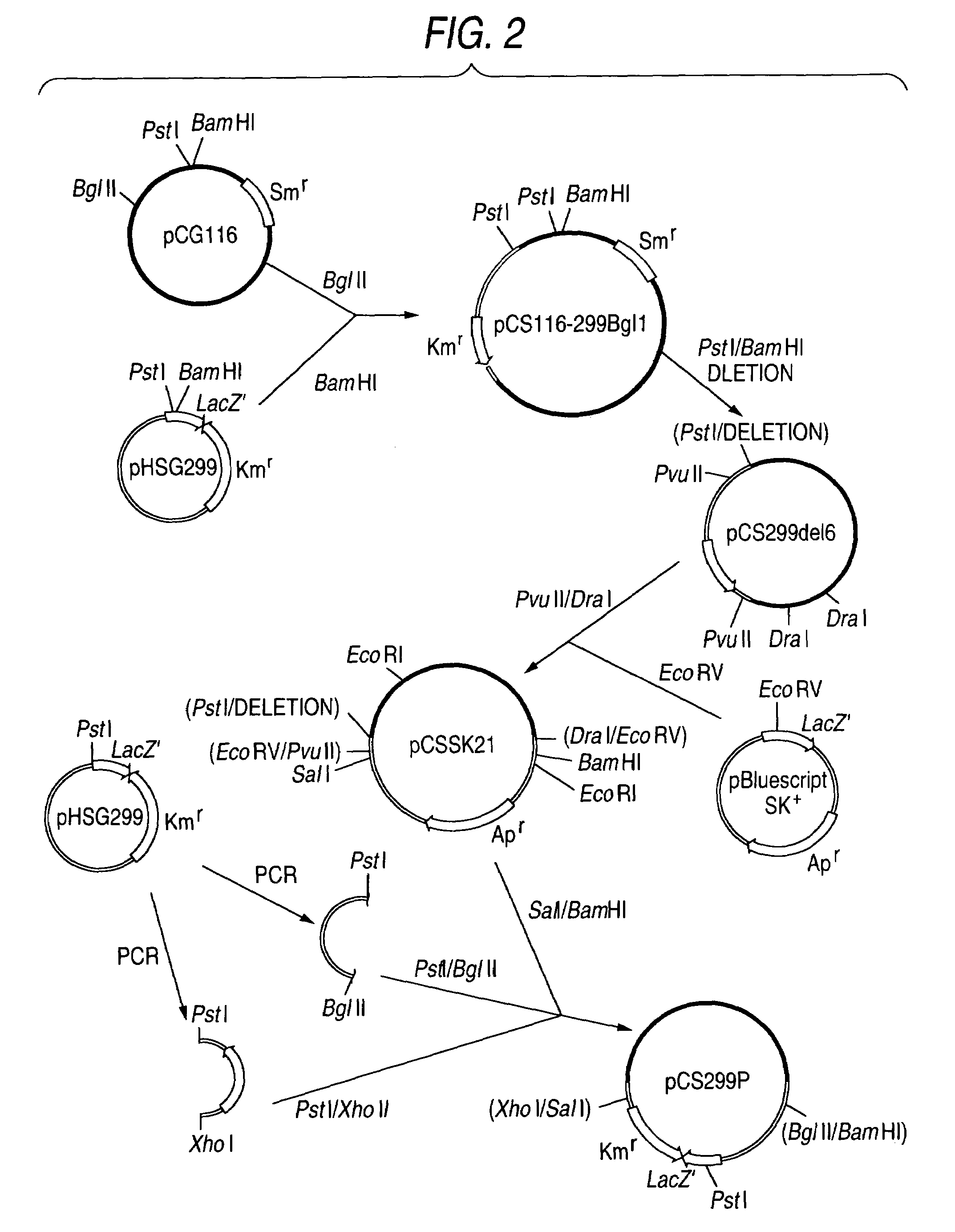
Alleles of the zwf gene from coryneform bacteria
The invention relates to mutants and alleles of the zwf gene of coryneform bacteria, which encode variants of the Zwf subunit of glucose 6-phosphate dehydrogenase (EC: 1.1.1.49), and to processes for preparing amino acids, in particular L-lysine and L-tryptophan, by using bacteria which harbor said alleles.
Owner:EVONIK OPERATIONS GMBH
6-glucose-6-phosphate dehydrogenase mutant and application thereof in preparing detection reagent
InactiveCN110174363AColor/spectral properties measurementsBulk chemical productionHigh fluxWild type
The application relates to a 6-glucose-6-phosphate dehydrogenase mutant and an application thereof in preparing detection reagent. Specifically, the 6-glucose-6-phosphate dehydrogenase mutant comprises one of the following mutants or combination thereof: D306C, D375C, and G426C in comparison with the wild type 6-glucose-6-phosphate dehydrogenase. The detection kit prepared from the 6-glucose-6-phosphate dehydrogenase mutant has the advantages of being strong in specificity, high in sensitivity, convenient to operate, short in detection time, accurate in quantification, and suitable for high-flux detection.
Owner:BEIJING STRONG BIOTECH INC
Homogeneous enzyme immunoassay for oral fluid
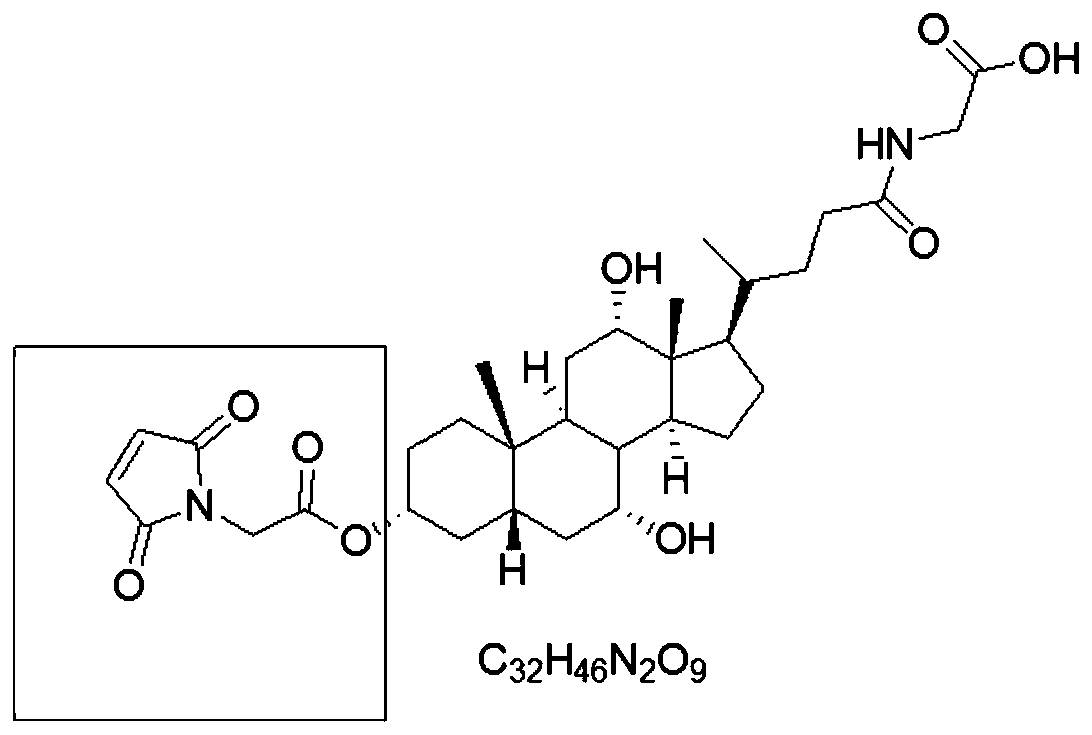
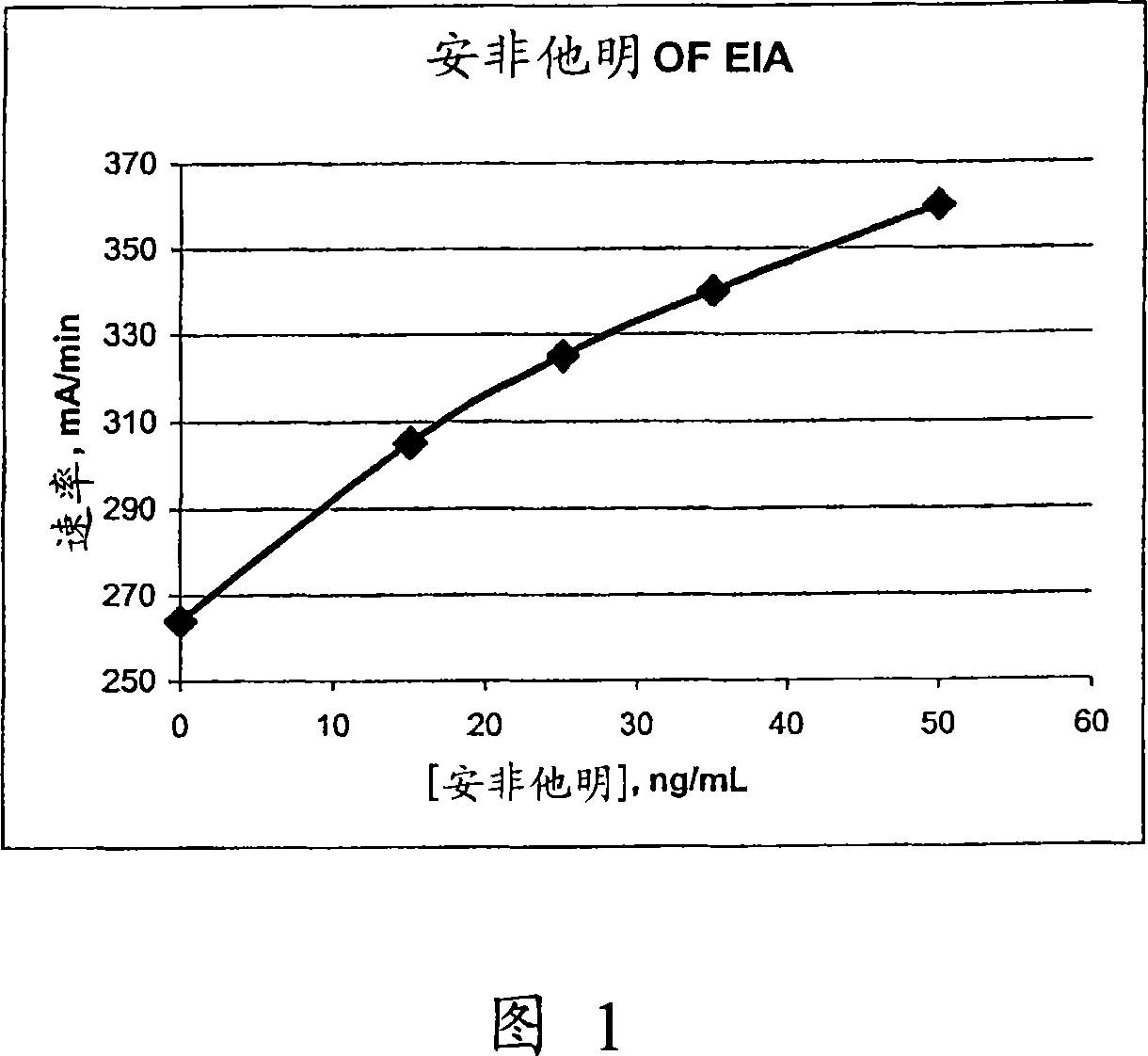
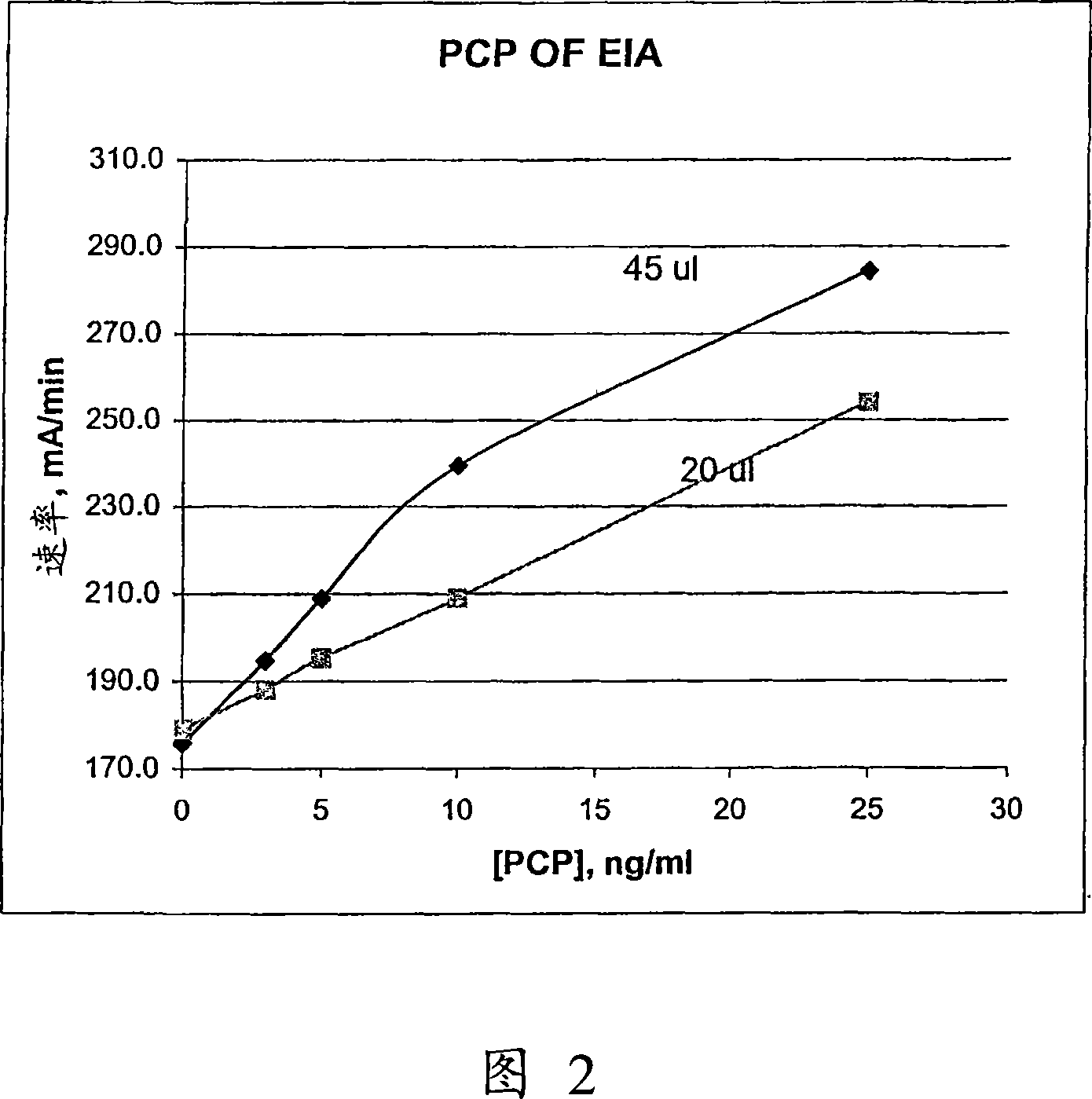
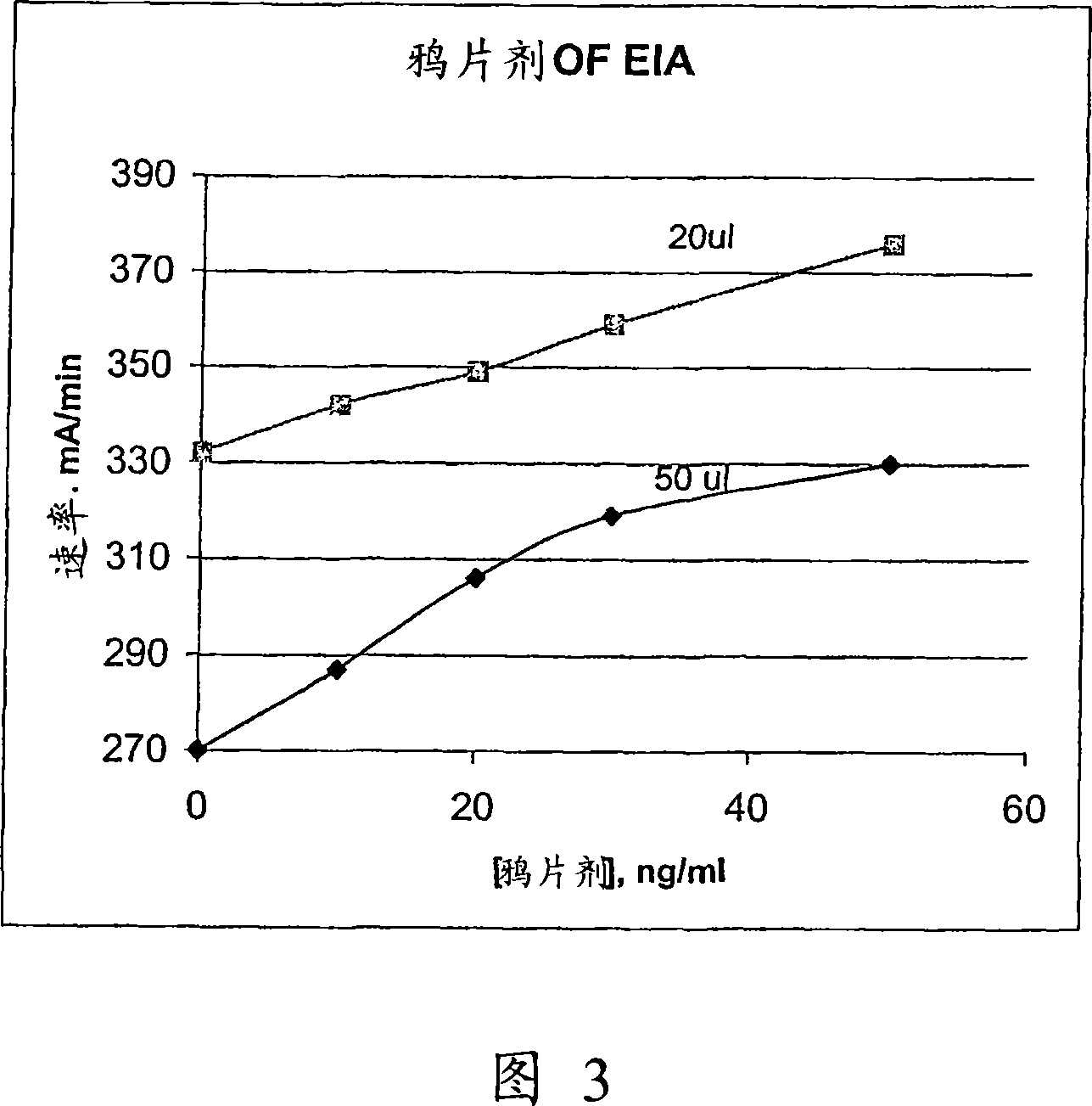
The present invention discloses homogeneous enzyme immunoassay systems, methods and kits useful for the qualitatively and quantitatively determination of analytes in oral fluid samples. The system involves a competitive enzyme immunoassay employing a conjugate comprising glucose-6-phosphate dehydrogenase (G6PDH) and an analyte. The methods and kits are particularly useful in the detection of recent drug use and for fast determination of analytes using auto-analyzers.
Owner:LIN ZHI INT
Dry chemistry, lateral flow-reconstituted chromatographic enzyme-driven assays
ActiveUS20040203086A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPeroxidaseAdditive ingredient
A lateral flow chromatographic assay format for the performance of rapid enzyme-driven assays is described. A combination of components necessary to elicit a specific enzyme reaction, which are either absent from the intended sample or insufficiently present therein to permit completion of the desired reaction, are predeposited as substrate in dry form together with ingredients necessary to produce a desired color upon occurrence of the desired reaction. The strip is equipped with a sample pad placed ahead of the substrate deposit in the flowstream, to which liquid sample is applied. The sample flows from the sample pad into the substrate zone where it immediately reconstitutes the dried ingredients while also intimately mixing with them and reacting with them at the fluid front. The fluid front moves rapidly into the final "read zone" wherein the color developed is read against predetermined color standards for the desired reaction. Pretreatment pads for the sample, as needed, (e.g. a lysing pad for lysing red blood cells in whole blood) are placed in front of the sample pad in the flow path as appropriate. The assay in the format of the invention is faster and easier to perform than analogous wet chemistry assays. Specific assays for glucose-6-phosphate dehydrogenase ("G-6PD"), total serum cholesterol, beta-lactamase activity and peroxidase activity are disclosed.
Owner:ABBOTT DIAGNOSTICS SCARBOROUGH INC
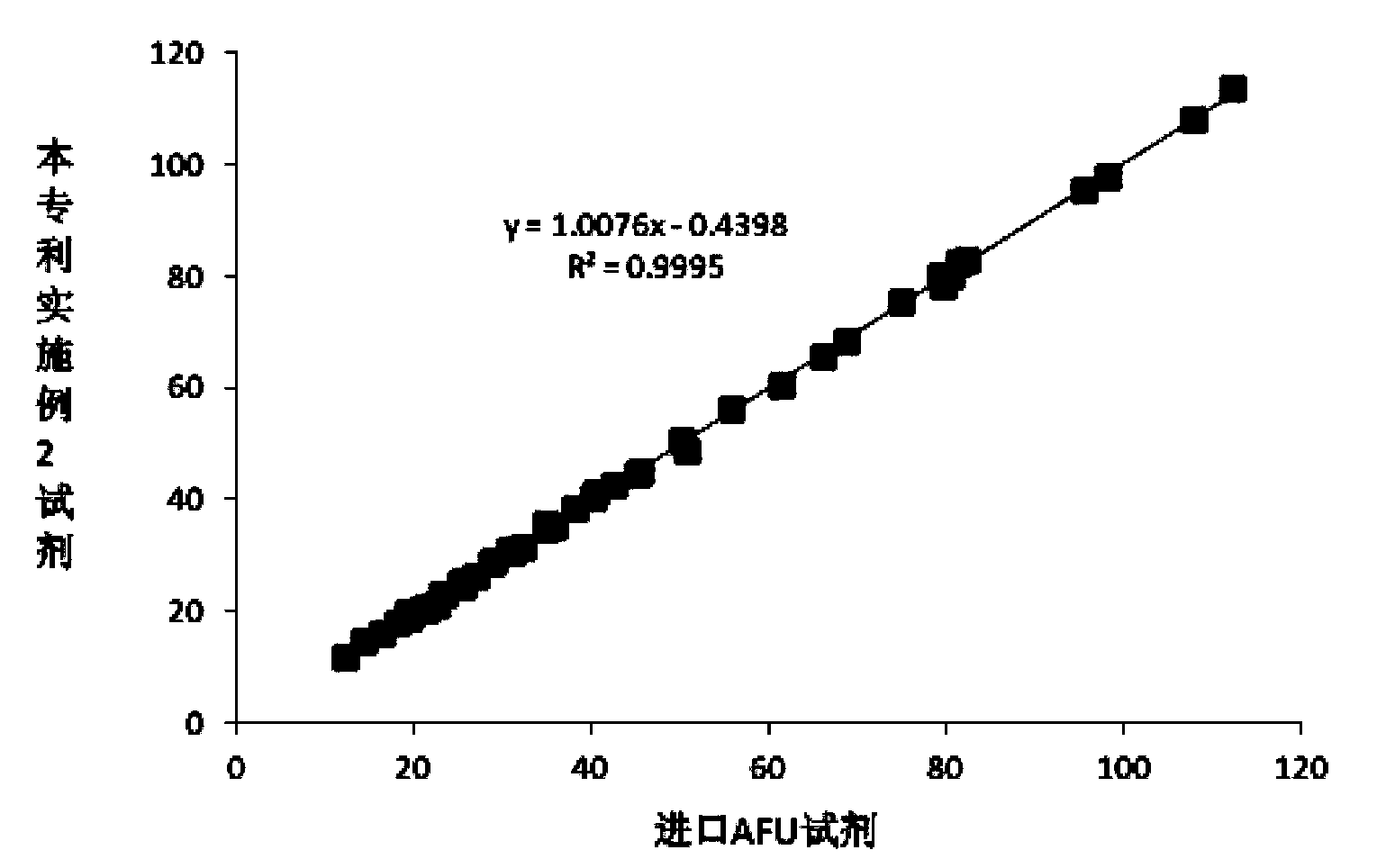
Serum AFU detection kit
ActiveCN104267178ASolve instabilityImprove protectionMicrobiological testing/measurementBiological testingPreservativeHexokinase
The invention relates to a serum AFU detection kit. The kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises 50-500 mmol / L of a buffer solution (pH 4.0-4.5), 1-50 g / L of a surfactant, 1-20 g / L of an anti-interference agent and 0.1-100 g / L of a preservative; the reagent 2 comprises 50-500 mmol / L of a buffer solution (pH 7.0-7.5), 2-60 g / L of glucose, 1-20 KU / L of hexokinase, 1-20 KU / L of glucose-6-phosphate dehydrogenase, 0.3-5 g / L of magnesium sulfate, 0.2-5 g / L of a substrate, 1-100 mmol / L of a stabilizer, 0.1-40 g / L of a protective agent and 0.1-100 g / L of a preservative. With the adoption of the kit, problems of the poor substrate stability, the poor anti-interference capacity and the lower sensitivity which exist universally are solved.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Kit for diagnosing diseases in system of liver and gall
InactiveCN101003831AExtended storage timeUndisturbedMicrobiological testing/measurementBiological testingDisease causePolymer
This invention relates to a test kit for diagnosing hepatic and biliary diseases. The test kit has such advantages as high stability, high accuracy and high sensitivity. The test kit comprises two reagents. Reagent 1 comprises oxidized thio coenzyme, buffer solution, preservative, and anti-interference agent. Reagent 2 comprises 3alpha-HSD, reduced coenzyme, buffer solution, preservative, complexing agent, polymer accelerator, stabilizer, and one of formate dehydrogenase-formic acid-NAD, glutamate dehydrogenase-glutamic acid-oxidized nicotinamide coenzyme, glucose-6-phosphate dehydrogenase-glucose-oxidized nicotinamide coenzyme, glucose dehydrogenase-xylose-oxidized nicotinamide coenzyme, lactate dehydrogenase-alpha-ketoglutarate-oxidized nicotinamide coenzyme, and glycerol dehydrogenase-ethylene glycol-oxidized nicotinamide coenzyme.
Owner:王贤理
Bacillus licheniformis engineered bacterium capable of high production of poly-Gamma-glutamic acid
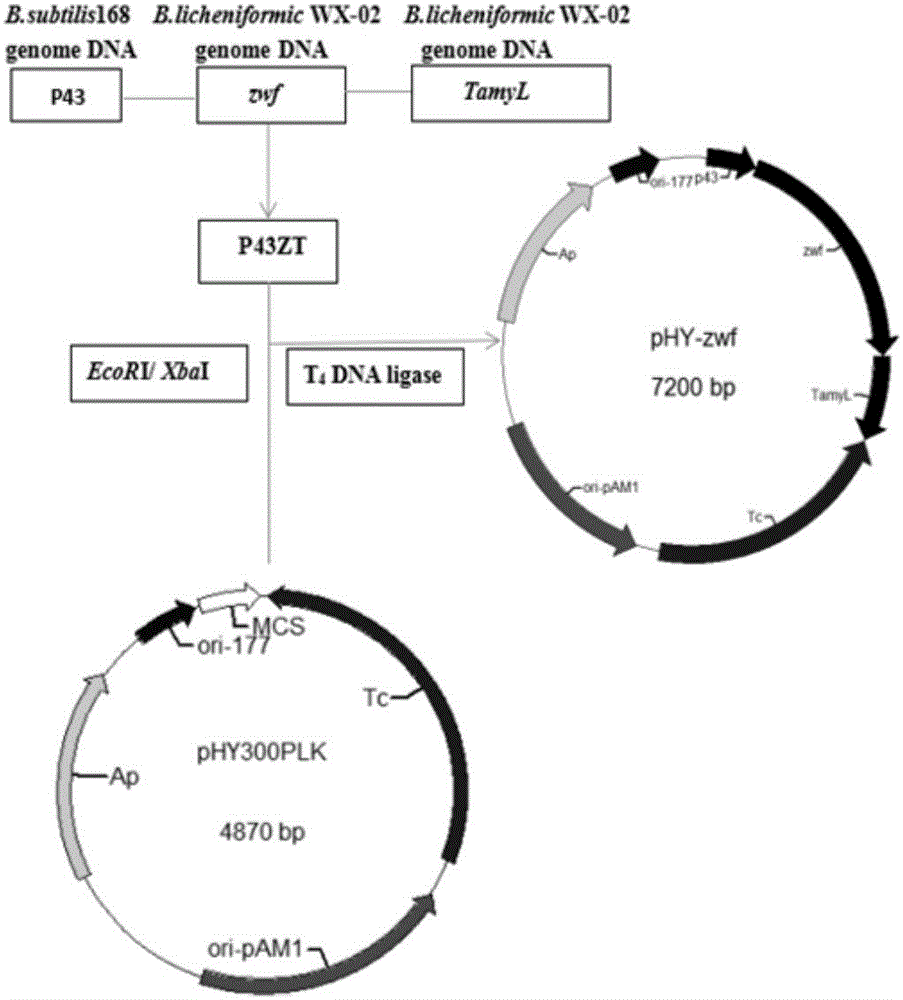
ActiveCN106497857AIncrease synthesis levelIncrease productionBacteriaMicroorganism based processesBacillus licheniformisGlucose-6-phosphate dehydrogenase
The invention provides a Bacillus licheniformis engineered bacterium capable of high production of poly-Gamma-glutamic acid, collected under CCTCC No: M2016439 and named as Bacillus licheniformis (WX-02) / pHY-zwf, and further provides a construction method of the Bacillus licheniformis (WX-02) / pHY-zwf and a method for high production of poly-Gamma-glutamic acid. The synthetic level of intracellular NADPH is increased through genetic engineering modification, and the yield of Gamma-PGA. By constructing glucose-6-phosphate dehydrogenase overexpression support pHY-zwf and converting to Gamma-PGA-producing Bacillus licheniformis WX-02 pre-stored in a lab, the Bacillus licheniformis (WX-02) / pHY-zwf capable of producing Gamma-PGA is re-constructed. The bacterium may provide significantly synthesis of Gamma-PGA under optimized media and culture conditions. The yield of Gamma-PGA is up to 51.13 g / L which is 49% significantly higher than the Gamma-PGA yield of an original bacterium.
Owner:HUBEI UNIV
Pentose phosphate pathway upregulation to increase production of non-native products of interest in transgenic microorganisms
Coordinately regulated over-expression of the genes encoding glucose 6-phosphate dehydrogenase [“G6PDH”] and 6-phospho-gluconolactonase [“6PGL”] in transgenic strains of the oleaginous yeast, Yarrowia lipolytica, comprising a functional polyunsaturated fatty acid [“PUFA”] biosynthetic pathway, resulted in increased production of PUFAs and increased total lipid content in the Yarrowia cells. This is achieved by increased cellular availability of the reduced form of nicotinamide adenine dinucleotide phosphate [“NADPH”], an important reducing equivalent for reductive biosynthetic reactions, within the transgenic microorganism.
Owner:EI DU PONT DE NEMOURS & CO
Engineering strain for generating allulose, construction method and application thereof
InactiveCN110079488AHigh yieldSuitable for large-scale productionBacteriaHydrolasesO-Phosphoric AcidPhosphate
The invention discloses a construction method of an engineering strain for generating allulose and an application thereof. The construction method comprises the following steps: increasing content ofintracellular fructose 6-phosphoric acid by reducing enzymatic activity of fructose 6-phosphokinase and glucose 6-phosphate dehydrogenase in corynebacterium glutamicum and enhancing enzymatic activityof glucokinase and glucose 6-phophate isomerase by regulating glucose intracellular metabolism; constructing a synthetic route of allulose composed of 6-aloxone phosphate 3-epimerase and 6-aloxone phosphate phosphorylase; constructing a metabolic pathway of fructose composed of fructose transmittase and fructokinase; constructing a metabolic pathway of glycerinum composed of glycerol transmittase, glycerol dehydrogenase and dihydroxyacetone kinase, thereby acquiring a corynebacterium glutamicum recombinant strain. The strain is capable of metabolizing glycerinum, glucose, fructose or saccharose for synthesizing allulose. Compared with the present reported method for compounding allulose through bioconversion of fructose, the construction method provided by the invention has the advantagesof high conversion rate, low production cost, and the like, and is suitable for large-scale production of allulose.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Glucose detection reagent being strong in stability and low in cost and adopting hexokinase
ActiveCN106868096AEasy to storeImprove protectionMicrobiological testing/measurementPolyethylene glycolHexokinase
The invention relates to the technical field of detection for GLU in the blood by adopting hexokinase, and in particular relates to a detection reagent for GLU in the blood, which is strong in stability and low in cost and adopts liquid hexokinase. The detection reagent adopts liquid double reagents, wherein the reagent I mainly contains a buffer solution, Mg<2+>, an ion balancing agent, a preservative, NAD (oxidized coenzyme I), a protective agent and a surfactant, and the reagent II contains a buffer solution, a protective agent, a surfactant, preservative, hexokinase (HK), glucose-6-phosphate dehydrogenase (G6P-DH), ATP.NA2, and the like. In order to guarantee the stability of enzymes in the reagent, various protective agents including polyethylene glycol-20000, tween-80, FAD and the like are added in the reagent in a pertinence manner, in order to reduce the cost, especially the use of enzymes, various surfactants are added in the reagent, so that the emulsifying for enzymes is enhanced, the service efficiency for enzymes is improved, thus the cost of the reagent is greatly reduced, and the detection reagent is particularly suitable for being used and popularized in clinic.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Glucose 6 phosphate dehydrogenase mutant
ActiveCN106190996AIncreased specific enzyme activityImprove thermal stabilityBiological material analysisOxidoreductasesMutantSorbitol-6-phosphate dehydrogenase
The invention relates to a glucose 6 phosphate dehydrogenase mutant. The mutant is obtained by mutating 44th-site glycine into valine and 319th-site glycine into valine in a glucose 6 phosphate dehydrogenase of which the amino acid sequence is disclosed as SEQ ID No.1; and compared with the glucose 6 phosphate dehydrogenase of which the amino acid sequence is disclosed as SEQ ID No.1 before mutation, the mutant has higher heat stability. The glucose 6 phosphate dehydrogenase mutant has the advantages of high heat stability and high inhibition rate; and the mutant enzyme can be used as a raw material for multiple small molecule detection kits, so that the obtained kit has higher stability and sensitivity.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Glucose-6-phosphate dehydrogenase
The present invention relates to a novel glucose-6-phosphate dehydrogenase (hereinafter referred to as “G6PD”) derived from a bacterium belonging to the genus Corynebacterium, a DNA encoding the enzyme, a recombinant DNA comprising the DNA, a transformant comprising the recombinant DNA, a transformant comprising the DNA on its chromosome, and a process for producing L-amino acid or G6PD which comprises culturing the transformant.According to the present invention, a modified G6PD and a DNA encoding the G6PD are obtained, and the productivity of L-amino acid by a microorganism can be improved by using the modified G6PD.
Owner:KYOWA HAKKO BIO CO LTD +1
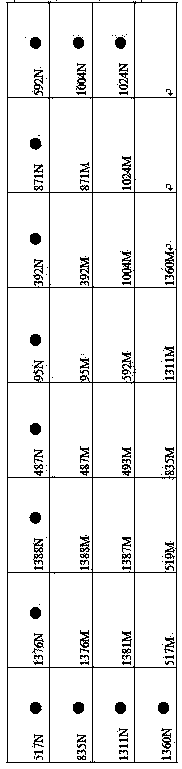
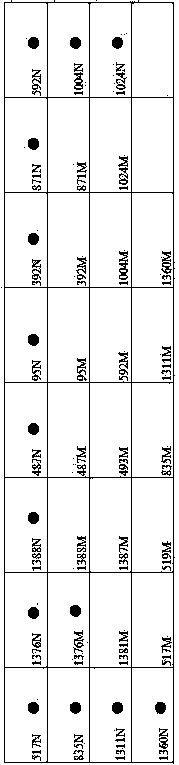
G6PD (Glucose-6-Phosphate Dehydrogenase) deficiency gene detection membrane strip and PCR (Polymerase Chain Reaction) primer
InactiveCN103695554AImprove accuracyReduce the possibility of missed detectionMicrobiological testing/measurementDNA/RNA fragmentationGlucose-6-phosphate dehydrogenaseFalse positives and false negatives
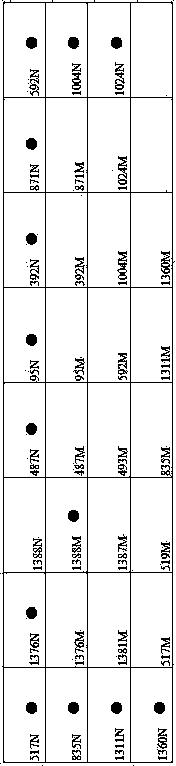
The invention relates to a gene detection membrane strip and a related primer for G6PD (Glucose-6-Phosphate Dehydrogenase) deficiency, in particular to a G6PD deficiency gene detection membrane strip and a PCR (Polymerase Chain Reaction) primer. The membrane strip and the primer detect 17 mutation sites and 20 mutation types, wherein the 20 mutation types are respectively A95G, G392T, G487A, A493G, T517C, C519T, C519G, C592T, A835G, A835T, G871A, C1004T, C1004A, C1024T, C1311T, C1360T, G1376T, G1381A, C1387T and G1388A; the PCR primer comprises 7 pairs of 14 primers; the 14 primers are simplified in the forms of G1F, G1R, G2F, G2R, G3F, G3R, G4F, G4R, G5F, G5R, G6F, G6R, G7F and G7R. The G6PD deficiency gene detection membrane strip and the PCR primer largely reduce the detection leaking frequency of patients, and protect the life and health of the patients; the detection membrane strip detects quickly and accurately, and little appears false positive and false negative.
Owner:东莞市石龙博爱医院
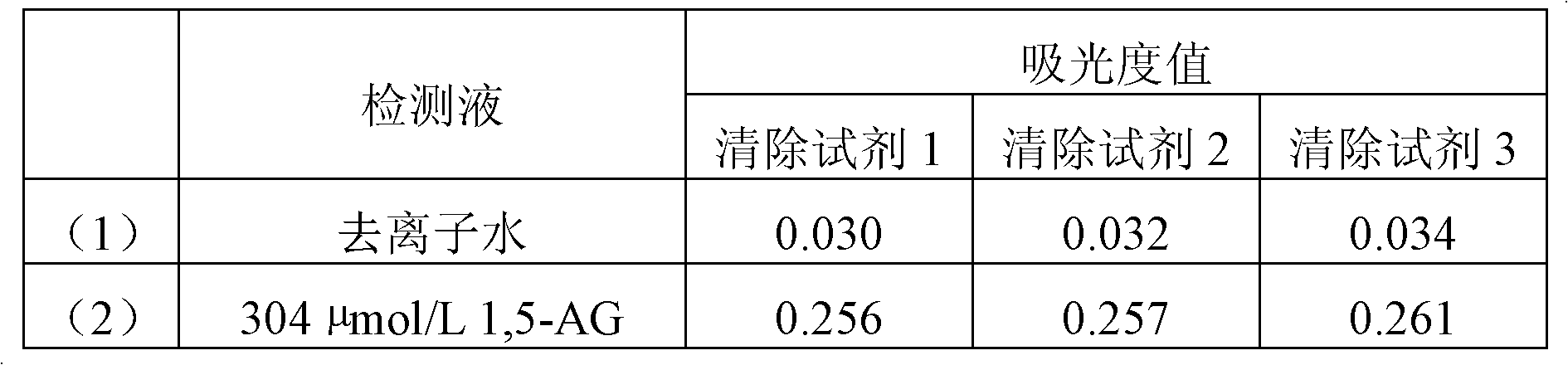
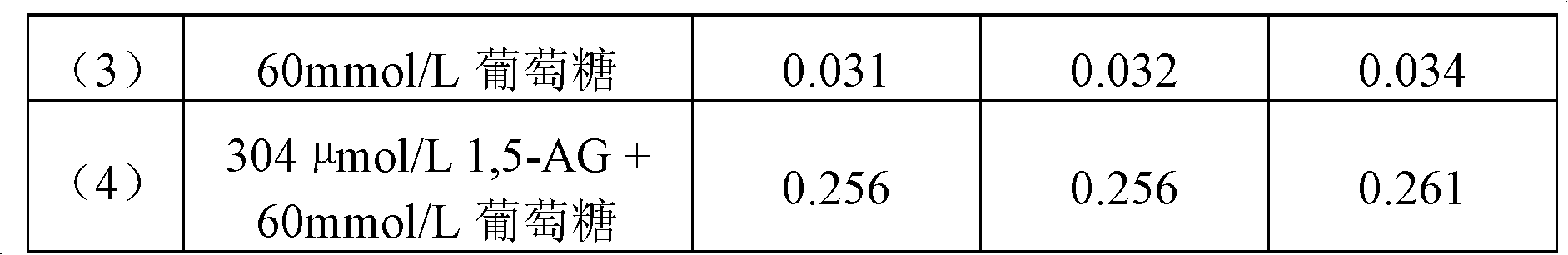
High-concentration glucose removal method and kit for determining serum 1,5 anhydro-D-glucitol based on pyranose oxidase method
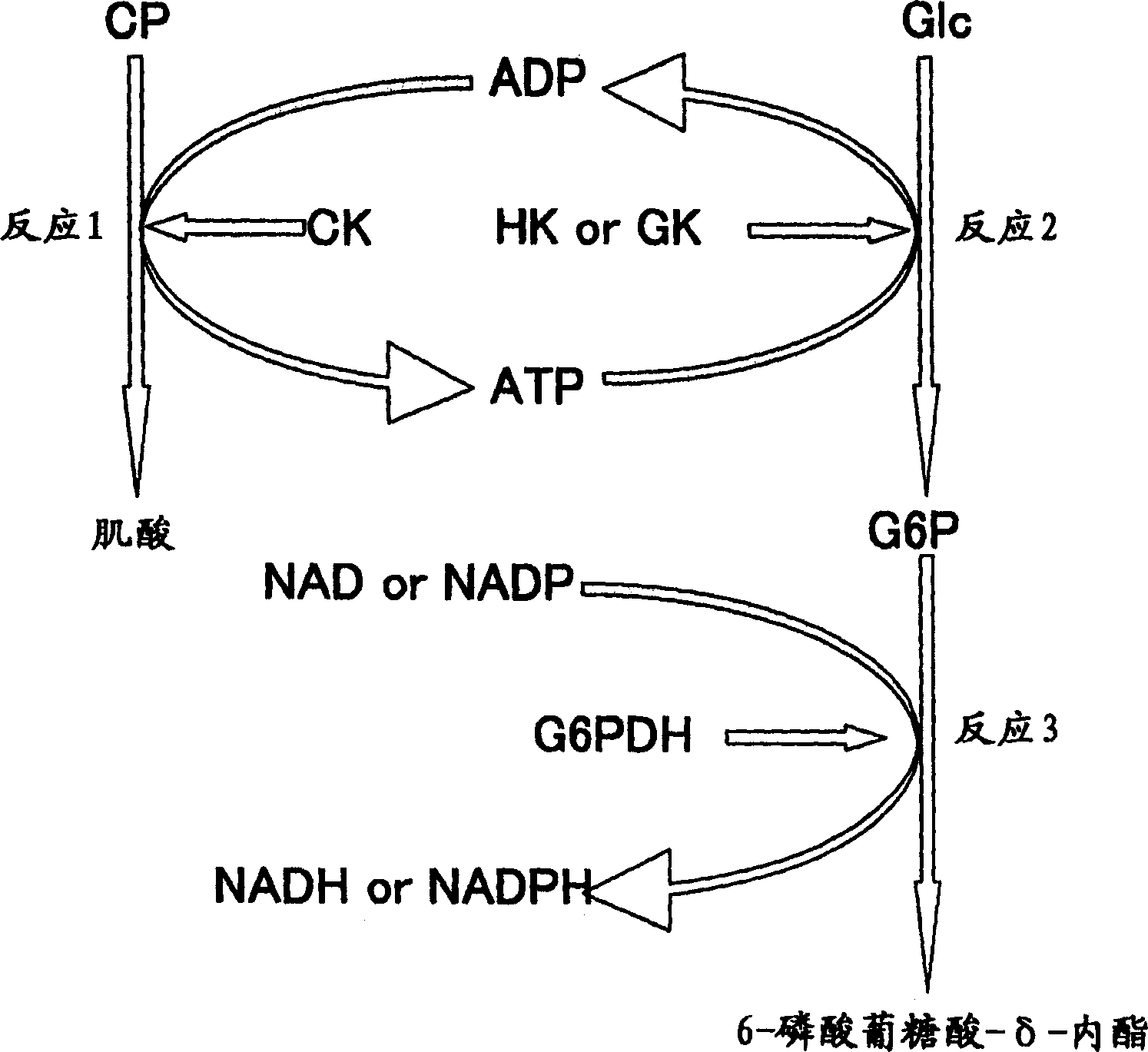
The invention discloses a high-concentration glucose removal method for determining serum 1,5 anhydro-D-glucitol based on a pyranose oxidase method, which comprises the following steps: preparing a 2-morpholinoethanesulfonic acid (MES) buffer solution having a pH value of 6.0-9.0, wherein the MES buffer solution contains a certain amount of glucokinase (GK), glucose-6-phosphate dehydrogenase (G6PD), nicotinamide adenine dinucleotide phosphate (NADP), pyruvate kinase (PK), phosphoenolpyruvate (PEP) and adenosine triphosphate (ATP); and adding into a serum sample containing glucose (Glu), and reacting at 20-50 DEG C for 3-5 minutes, wherein the GK converts the Glu into glucose-6-phosphoric acid (G6P) in the presence of the ATP, and adenosine diphosphate (ADP) is generated at the same time; the G6P is further converted into glucose-6-phosphate lactone (G6PL) by the G6PD in the presence of the NADP+; and the ADP generated in the reaction is converted into ATP again under the action of thePK in the presence of the PEP. The glucose removal agent prepared by the method can be stably stored at 2-8 DEG C for 14 months, and can be used for a clinical biochemical autoanalyzer extensively used at present.
Owner:浙江夸克生物科技有限公司
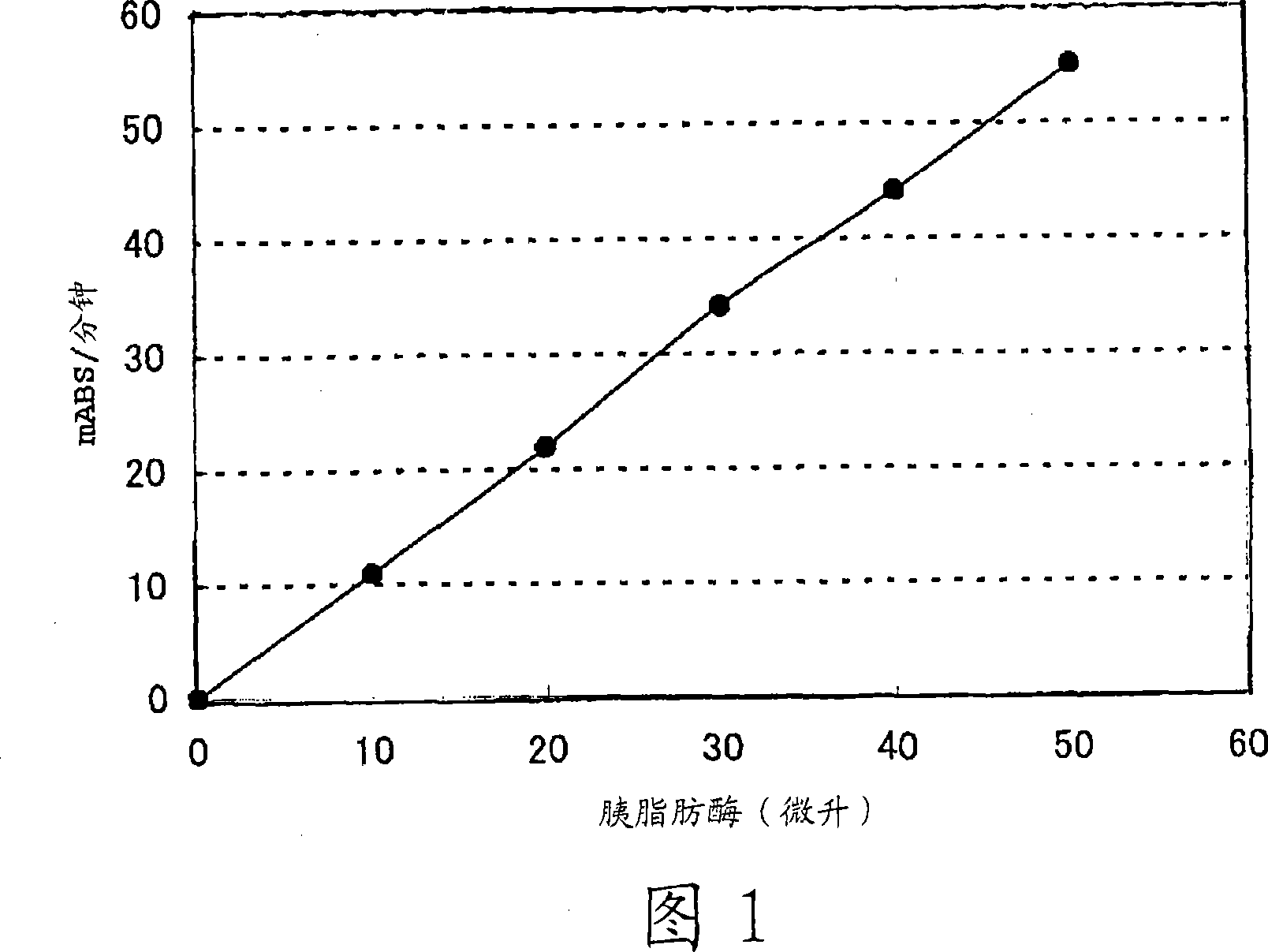
Compositions for lipase activity determination and method of determining activity
InactiveCN101061234AEasy to useExcellent long-term storage stabilityMicrobiological testing/measurementLactate dehydrogenaseMonoglyceride
To provide lipase activity determination reagents which function by the enzymatic method. The reagents are easy to use in an ordinary clinical examination, have excellent handleability, and are excellent in accuracy and reproducibility. Lipase activity is determined with any of reagents which comprise a low-concentration buffer and a diglyceride dissolved therein. The diglyceride is used as a substrate for lipase, whereby the liquid reagents can have long-term storage stability. One of the reagents converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, pyruvate kinase, lactate dehydrogenase, reduced NAD, ATP, and phosphoenol pyruvate. Another reagent converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, glucose, ADP-dependent hexokinase, glucose-6-phosphate dehydrogenase, oxidized NAD or oxidized NADP, and ATP. A further reagent converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, glycero-3-phosphate oxidase, peroxidase, and a dye which colors in the presence of hydrogen peroxide.
Owner:ASAHI KASEI PHARMA
Pentose phosphate pathway upregulation to increase production of non-native products of interest in transgenic microorganisms
Coordinately regulated over-expression of the genes encoding glucose 6-phosphate dehydrogenase ["G6PDH"] and 6-phospho-gluconolactonase ["6PGL"] in transgenic strains of the oleaginous yeast, Yarrowia lipolytica, comprising a functional polyunsaturated fatty acid ["PUFA"] biosynthetic pathway, resulted in increased production of PUFAs and increased total lipid content in the Yarrowia cells. This is achieved by increased cellular availability of the reduced form of nicotinamide adenine dinucleotide phosphate ["NADPH"], an important reducing equivalent for reductive biosynthetic reactions, within the transgenic microorganism.
Owner:EI DU PONT DE NEMOURS & CO
Recombinant strain producing l-amino acids, constructing method therefor and method for producing l-amino acids
The present application discloses recombinant bacteria producing L-amino acid(s) its construction method and the method of producing L-amino acid(s). The recombinant bacteria producing L-amino acid(s) according to the present invention has reduced expression of the glucose-6-phosphate isomerase Pgi and improved expression of the glucose-6-phosphate dehydrogenase Zwf-OpcA than the starting bacteria, wherein: said starting bacterium is a bacterial strain which can accumulate target amino acid(s). During fermenting and culturing the recombinant bacteria according to the present invention, it is observed that the effect of improving yield can be additive and the yield of L-amino acid(s) is improved obviously. The strategy of combinational modification according to the present invention develops a new method of improving the yield of L-amino acid(s) and hence it can be applied to produce L-amino acid(s) through bacterialfermentation.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
In-vitro diagnostic kit for gene mutation of glucose-6-phosphate dehydrogenase (G6PD) deficiency
ActiveCN104017862AThe instrument is simpleEasy to operateMicrobiological testing/measurementGlucose-6-phosphate dehydrogenaseMolecular diagnostics
The invention provides an in-vitro diagnostic kit for gene mutation of glucose-6-phosphate dehydrogenase (G6PD) deficiency. The kit comprises SEQ IDNO.1-24. Aiming at six high frequency mutation sites of a G6PD gene, the invention provides a quick in-vitro diagnostic kit for gene mutation of G6PD deficiency, wherein the kit is accurate, good in specificity, high in detection rate, low in cost and good in generalization performance and can differentiate heterozygous and homozygous mutations, thereby providing a quick and reliable molecular diagnostic basis for immediately diagnosing and preventing the G6PD deficiency in gene level. The kit can make a definite diagnosis to the patient within the shortest time, treat immediately suiting the case and avoid further deterioration of the pathogenetic condition, so that the kit not only has important social and economic significance in reducing harm of the G6PD deficiency and reducing the economic and spiritual burden of families, but also has high clinical popularization and application value and wide market prospect.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV +1
Detection kit for fluorescent PCR (Polymerase Chain Reaction) melting curve of gene mutation of glucose-6-phosphate dehydrogenase
The invention discloses a detection kit for a fluorescent PCR (Polymerase Chain Reaction) melting curve of gene mutation of glucose-6-phosphate dehydrogenase. The kit provided by the invention can detect 16 mutations at 16 sites in a two-tube PCR system, and the genotype of a sample can be known through a primary fluorescent PCR melting curve analysis after PCR amplification. The whole operation is completed within 2-3 hours, so that the operating step is less, the time consumed is short, the flux is high, the detection sites are more and the mutation coverage is high. In addition, the kit provided by the invention uses homogeneous detection in a closed tube, so that the probability of PCR product pollution is reduced, the detection specificity is high and the result is easy to judge.
Owner:XIAMEN UNIV +1
Application of G6PDH gene for improving steroid C11 alpha-hydroxylation ability of rhizopus nigricans and strain
ActiveCN103627721AIncrease profitGrow fastFungiMicroorganism based processesRhizopusTransformation efficiency
The invention provides an application of G6PDH gene for improving steroid C11 alpha-hydroxylation ability of rhizopus nigricans, a method for constructing glucose-6-phosphate dehydrogenase genetically engineered bacteria and a strain screened out after construction. G6PDH is cloned into rhizopus nigricans through a molecular biological method to transform the strain, and the G6PDH catalyzes NADPH regeneration in the transformed rhizopus nigricans cells to provide coenzyme necessary for a catalytic reaction of a cytochrome P450 enzyme system so as to improve the steroid C11 alpha-hydroxylation ability of the rhizopus nigricans. The application provided by the invention has the beneficial effects that the genetically engineered bacteria created by the method provided by the invention are used for converting Wolfowitz oxide C11 alpha-hydroxylation to produce mold oxide; compared with a starting strain, the strain grows faster and is high in raw material utilization rate and high in conversion rate and conversion efficiency, so that the strain has far-reaching theoretical significance and high application value in development and application process of steroid medicine.
Owner:ZHEJIANG UNIV OF TECH
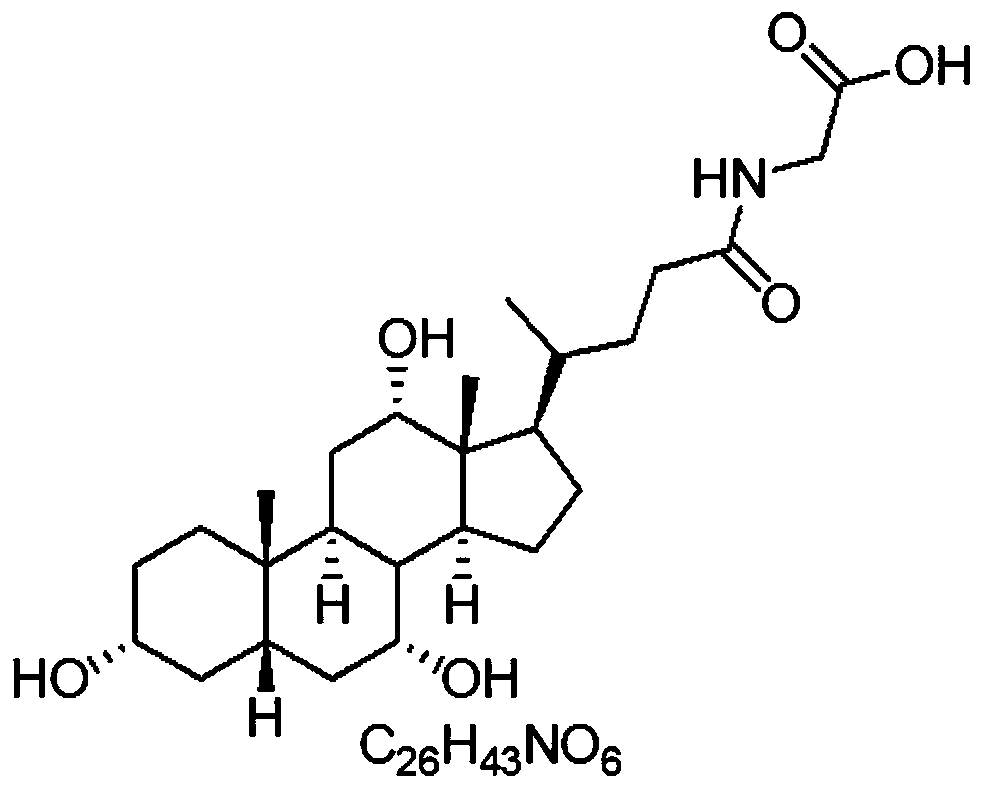
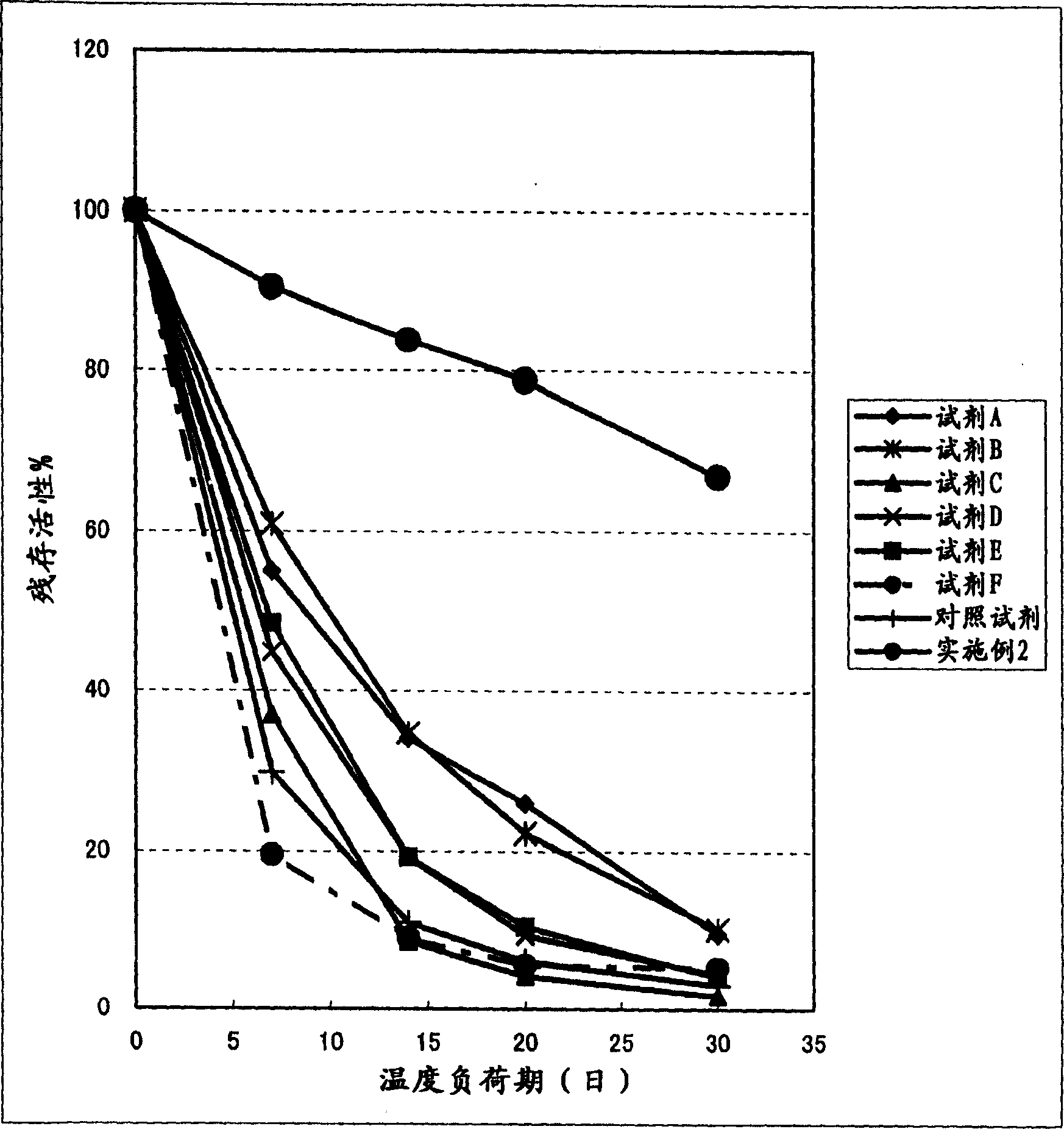
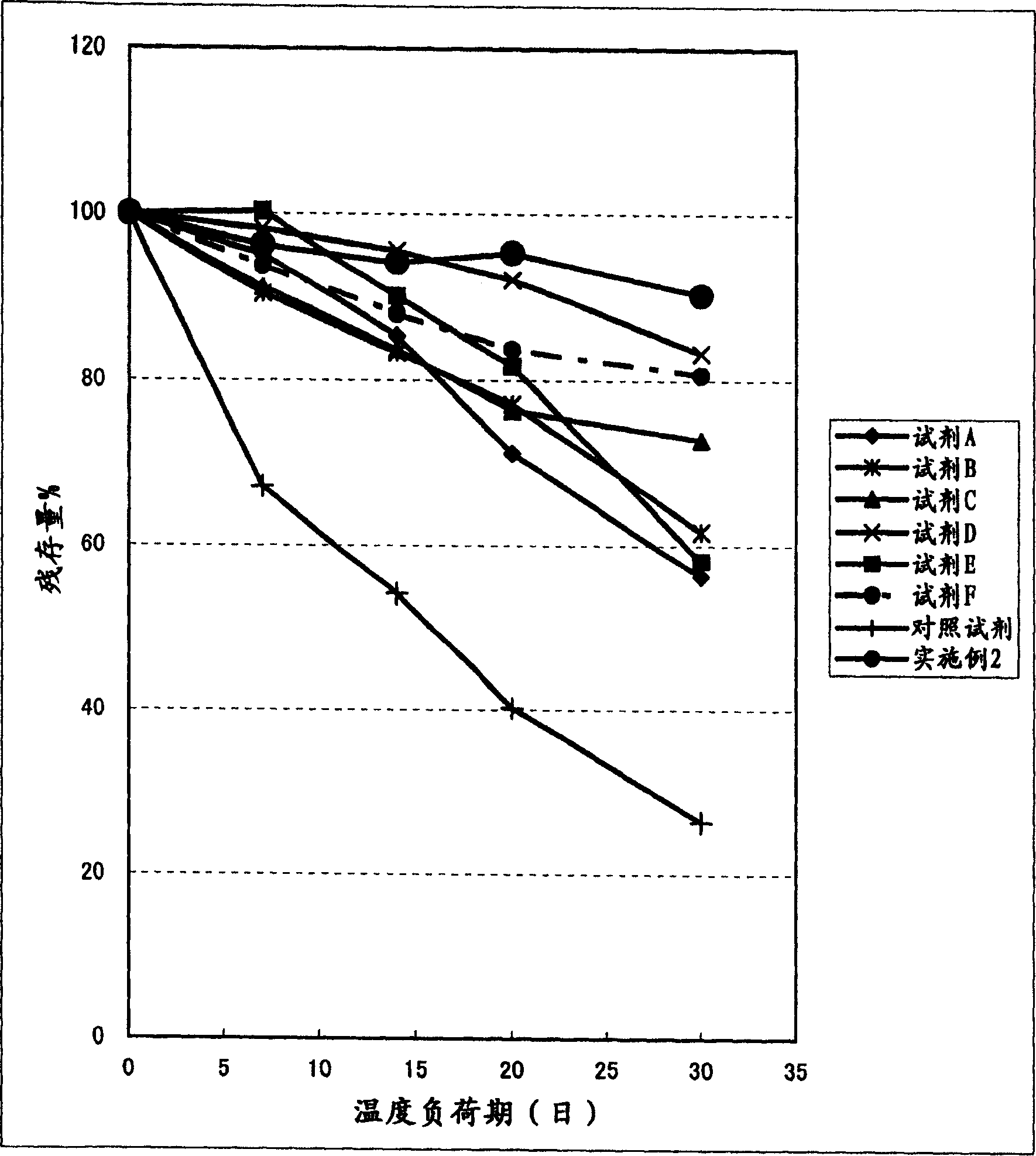
Clinical diagnostic reagent comprising glucose 6-phosphate dehydrogenase (G6PDH), method for stabilizing G6PDH, and use of a stabilizer for g6pdh
A clinical diagnostic reagent is described. The reagent comprises glucose 6-phosphate dehydrogenase (G6PDH) and a stabilizer for the G6PDH represented by formula (I): wherein R1 represents a hydrogen, a halogen, a hydroxyl group, a substituted or unsubstituted lower alkyl group, a cycloalkyl group, a lower alkoxy group, a lower alkenyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted heterocyclic group, a lower alkanoyl group, a substituted or unsubstituted aroyl group, a pyridylcarbonyl group, a lower alkoxycarbonyl group, or a substituted or unsubstituted amino group.
Owner:SYSMEX CORP
Lyophilized whole blood controls for G6PD (glucose-6-phosphate dehydrogenase) and preparation method of lyophilized whole blood controls
ActiveCN105758700AImprove uniformityImprove stabilityPreparing sample for investigationMedicineNormal level
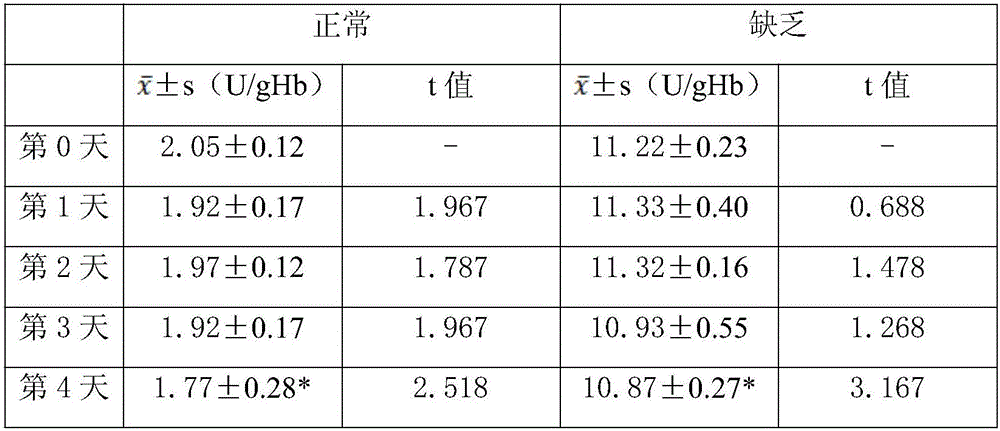
The invention discloses lyophilized whole blood controls for G6PD (glucose-6-phosphate dehydrogenase) and a preparation method of the lyophilized whole blood controls. The controls are lyophilized products prepared from CPD whole blood matrixes and G6PD. The preparation method of the lyophilized whole blood controls comprises steps as follows: red blood cells and plasma of the CPD whole blood matrixes are separated centrifugally; red blood cell hemolysates are prepared with a physical method and are mixed with hemolysates in different ratios, and the CPD whole blood matrixes are obtained; different quantities of G6PD are added to the whole blood matrixes, the control containing G6PD in a normal level and the control containing G6PD in a deficient level are prepared, the two controls are subpackaged and subjected to lyophilization and vacuum capping, and the lyophilized whole blood controls for G6PD are obtained. The controls have good product homogeneity, good long-term sample storage stability and good stability after redissolving, are not influenced by factors such as transportation, temperature and the like, are wide in applicable range, can be suitable for different brands of quantitative detection kits, can meet the clinical quality control requirement for G6PD detection, and can improve the accuracy of a clinical sample detection result.
Owner:THE PEOPLES HOSPITAL OF GUANGXI ZHUANG AUTONOMOUS REGION
Gene coding for glucose-6-phosphate-dehydrogenase proteins
Owner:DAESANG CORP
Creatine kinase isoenzyme detection reagent kit
InactiveCN109837324AImprove stabilityHigh precisionMicrobiological testing/measurementCreatine kinaseCreatine kinase isoenzyme
The invention is suitable for the technical field of medical inspection, and provides a creatine kinase isoenzyme detection reagent kit. The reagent kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises the following components of 10-20g / L of biologic buffering agents, 1-3g / L of magnesium acetate, 5-10g / L of N-broncholysin, 0.5-2KU / L of hexokinase, 0.5-2.5g / L of a surfactant and 0.8-2.5mL / L of creatine kinase isoenzyme antibodies, and the reagent R2 comprises the following components of 5-20g / L of creatine phosphate, 1.5-5g / L of adenosine diphosphate and 8-18KU / L of glucose-6-phosphate dehydrogenase. The reagent kit disclosed by the invention can be applied to detection of the activity of the creatine kinase isoenzymes in serum of human bodies, the reagent stabilityis high, and the detection result is high in precision and accuracy.
Owner:曾宪亮
Kit for detecting glucose-6-phosphate isomerase and use method of kit
InactiveCN109593825AShorten detection timeMicrobiological testing/measurementSolventFructose 6-phosphate
The invention provides a kit for detecting glucose-6-phosphate isomerase and a use method of the kit, and belongs to the technical field of enzyme detection. The kit comprises a first reagent and a second reagent, wherein the first reagent uses Tris buffer as a solvent and comprises adenosine triphosphate, beta-nicotinamide adenine dinucleotide trihydrate, a protective agent and a preservative; the second reagent uses the Tris buffer as the solvent and comprises magnesium chloride, fructose-6-phosphate, glucose-6-phosphate dehydrogenase, a stabilizer and the preservative; and the concentrationof the Tris buffer is 50-500 mmol / L. The kit provided by the invention can obtain a result of detecting glucose-6-phosphate isomerase at 6 min 20 s to 9 min 40 s, thereby shortening detection time.
Owner:浙江达美生物技术有限公司
Coenzyme II NADP+ or NADPH content testing kit and its method
InactiveCN107782684AEfficient determinationEffective contentColor/spectral properties measurementsHydrogenDistilled water
The invention discloses a coenzyme II NADP+ or NADPH content testing kit and its method. The kit comprises acid extractive fluid, alkali extractive fluid, mixed solution of HEPES and EDTA, glucose 6-phosphate, PMS, MTT, glucose 6-phosphate dehydrogenase solution, NaCl solution and mixed solution of ethanol and double distilled water. The method includes steps of extracting NADP+ and NADPH in a sample by acid and alkali extracting fluids on the basis of a spectrophotometric method; performing PMS hydrogen delivering action on NADPH, and reducing MTT to be formazan; detecting light absorption value under 570 nm, and testing the NADPH content; reducing the NADP+ to be NADPH by glucose 6-phosphate dehydrogenase, thereby detecting the NADP+ content. The kit and its testing method can effectively test the content of the coenzyme II NADP+ or NADPH, and are featured by high specifity, wide application range, high stability, and others.
Owner:SUZHOU COMIN BIOTECH
Gluconobacter oxydans engineering bacteria and preparation method and application thereof in preparing xylitol
InactiveCN102978148ASolve the problem of low conversion rate and low concentration of xylitolSimple and fast operationBacteriaMicroorganism based processesD-arabitolGluconobacter oxydans
The invention discloses a preparation method of gluconobacter oxydans engineering bacteria, which performs over-expression of the key restriction enzyme glucose-6-phosphate dehydrogenase (G6PDH) for controlling the phosphopentose path to increase the metabolic flux of the path so as to realize cyclic regeneration of coenzyme NADH. The invention also discloses a fermentation technology of the engineering bacteria and application thereof in converting and preparing xylitol. According to the invention, the effect of the engineering bacteria in converting D-arabitol to generate xylitol is obviously superior to that of an original strain; and the conversion rate of xylitol against D-arabitol can reach 60-90%. The technology solves the problems of low conversion rate and low xylitol concentration of a bioconversion method for preparing xylitol to a certain degree, and has the advantages of economy and practicability, simplicity and convenience in operation and energy conservation and environmental protection.
Owner:NANJING UNIV OF TECH
Alleles of the zwf gene from coryneform bacteria
The invention relates to mutants and alleles of the zwf gene of coryneform bacteria, which encode variants of the Zwf subunit of glucose 6-phosphate dehydrogenase (EC: 1.1.1.49), and to processes for preparing amino acids, in particular L-lysine and L-tryptophan, by using bacteria which harbor said alleles.
Owner:DEGUSSA AG
CK, CKMB, LDH and AST combined detection reagent
ActiveCN107988315APlay a role in de-interferenceMicrobiological testing/measurementBiological material analysisVitamin CL-Aspartate
The invention discloses a CK, CKMB, LDH and AST combined detection reagent which comprises a diluent 1, a diluent 2 and 4 freeze-dried balls. The diluent 1 comprises trismetyl aminomethane, a surfactant, a dehydrobilirubin interference agent, vitamin C oxidase and a preservative; the diluent 2 comprises a buffer solution ad an inhibitory CK-M antibody; the freeze-dried ball 1 comprises a buffer solution, alpha-ketoglutaric acid, a reduced coenzyme I, malic dehydrogenase, L-aspartate and a freeze-drying protective additive; the freeze-dried ball 2 comprises a buffer solution, L-lithium lactate,an oxidized coenzyme I and a freeze-drying protective additive; the freeze-dried balls 3 and 4 comprise a buffer solution, adenosine diphosphate, glucose-6-phosphate dehydrogenase, hexokinase, D-glucose, phosphocreatine, an enzyme activator and a freeze-drying protective additive. The combined detection reagent is applicable to a multifunctional full-spectrum POCT (Point-of-care Testing) biochemical analyzer, has good correlation to detection results of clinically common liquid reagents on a large biochemical analyzer, and has the advantages of being simple and convenient to operate, convenient in reagent preservation and transport, low in detection cost and the like.
Owner:NINGBO MEIKANG BAOSHENG BIOMEDICAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com