Lyophilized whole blood controls for G6PD (glucose-6-phosphate dehydrogenase) and preparation method of lyophilized whole blood controls
A technology of phosphate dehydrogenase and glucose, which is applied in the field of medical testing to achieve the effect of low cost, good stability and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
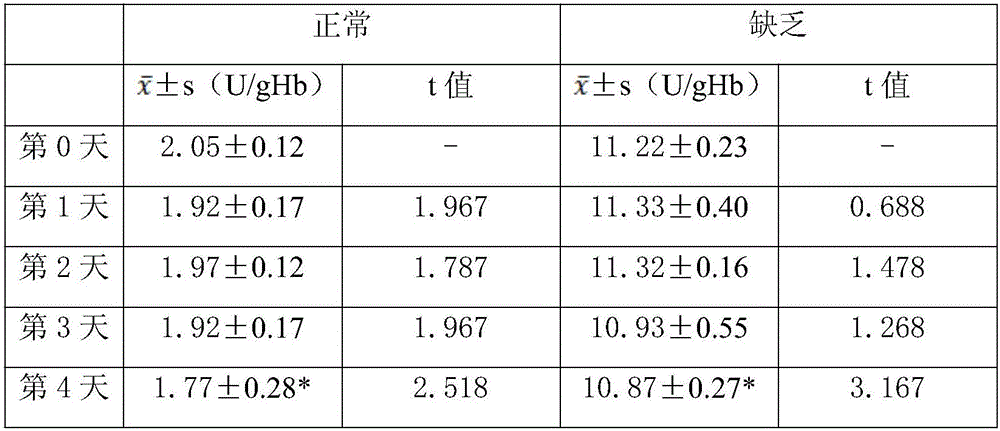
[0037] The G6PD freeze-dried whole blood quality control substance in this embodiment takes the G6PD normal and lack of quality control substances as examples. Both normal and lacking controls were lyophilized from CPD whole blood matrix and C6PD. After lyophilization, it is a red cake-like solid, and the two colors are the same. The CPD whole blood matrix is composed of blood and CPD preservation solution.
[0038] The preparation method of CPD preservation solution: Weigh 2.6g of trisodium citrate, 0.32g of citrate, 2.5g of glucose and 0.2g of sodium dihydrogen phosphate, then add 1000mL of distilled water and fully dissolve to obtain CPD preservation solution.
[0039] 1. Preparation of G6PD freeze-dried whole blood quality control products
[0040] The preparation method of glucose-6-phosphate dehydrogenase freeze-dried whole blood quality control product comprises the following steps:
[0041] 1. Select healthy adults who are negative for infectious markers such as H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com