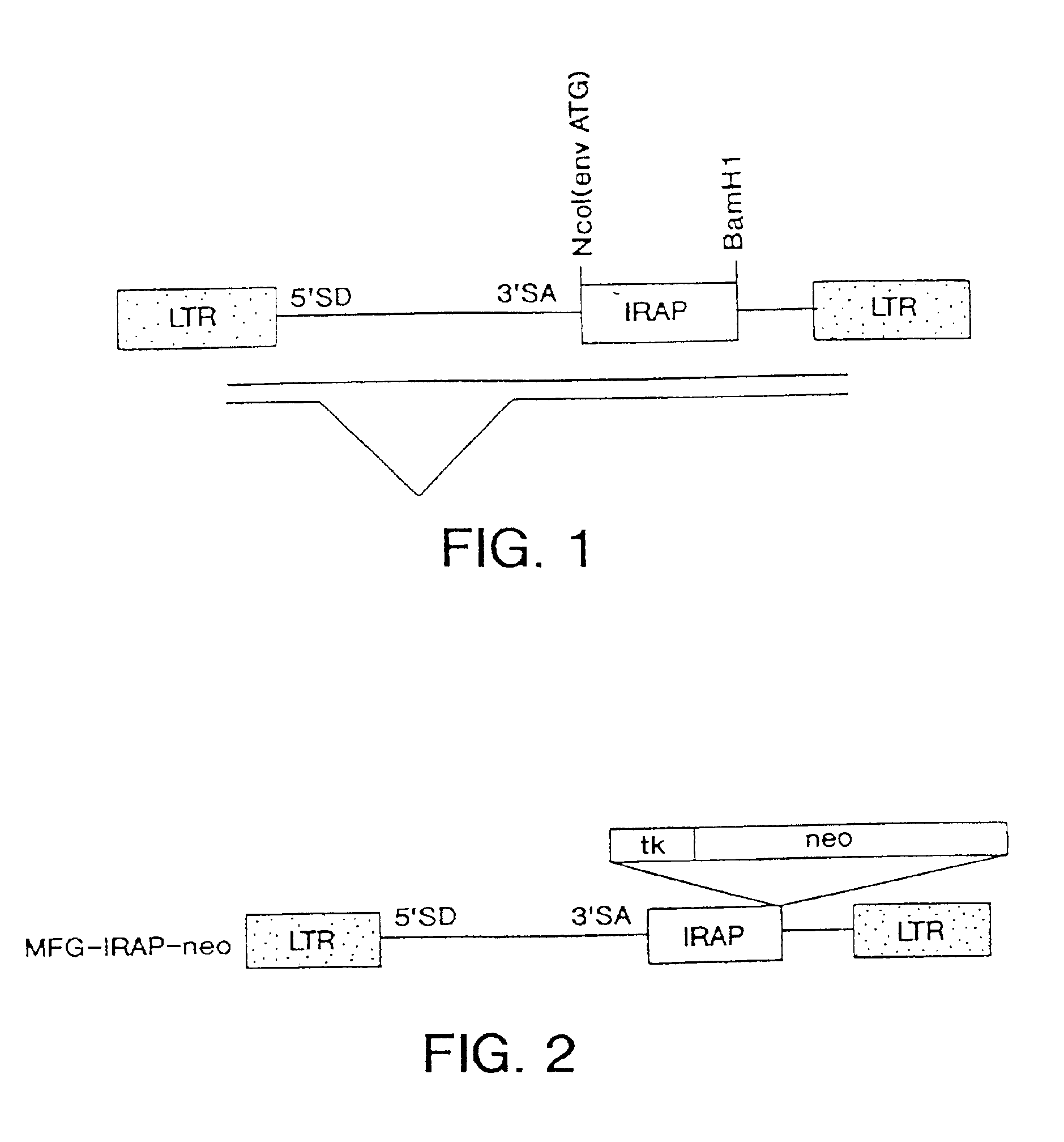
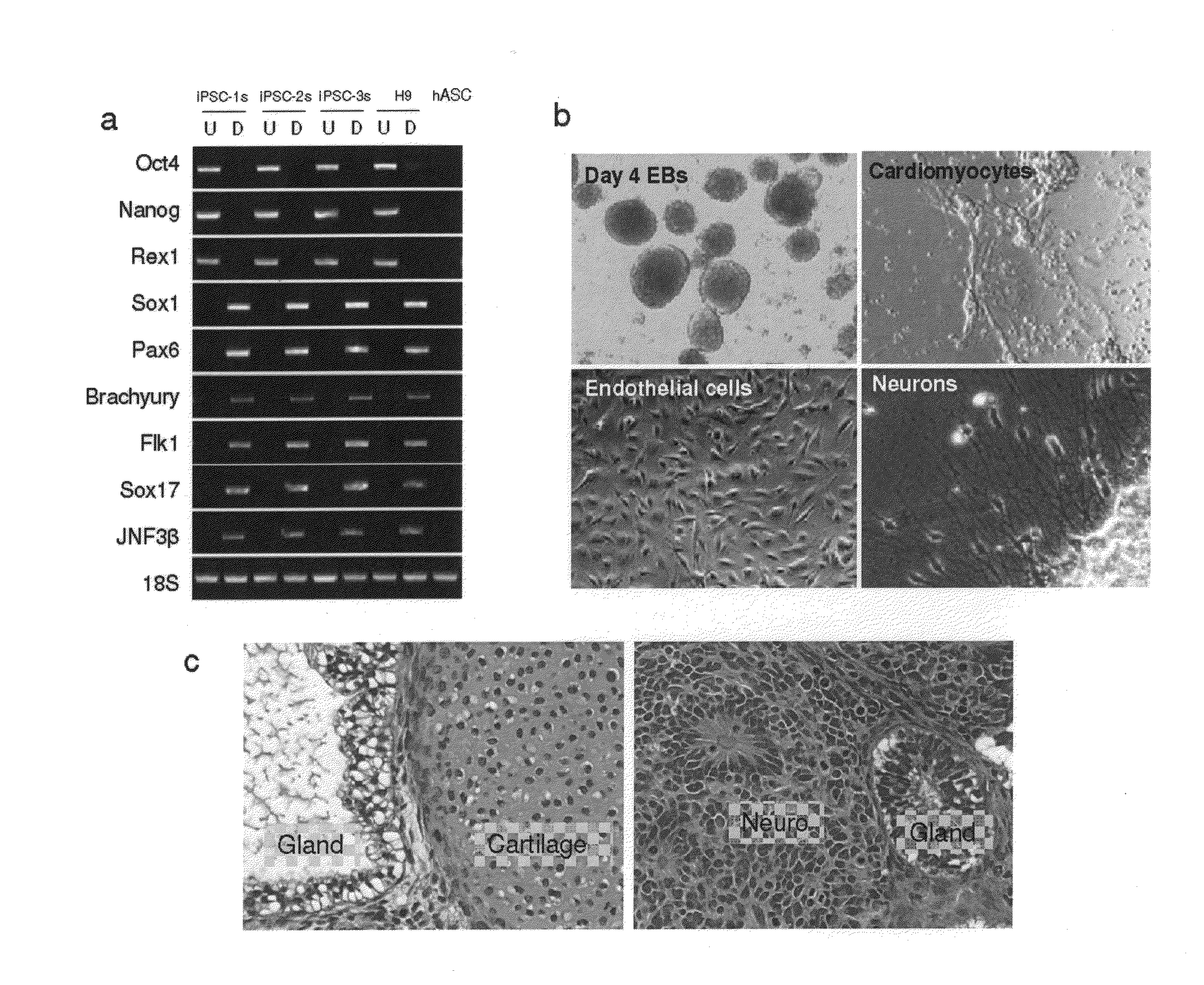
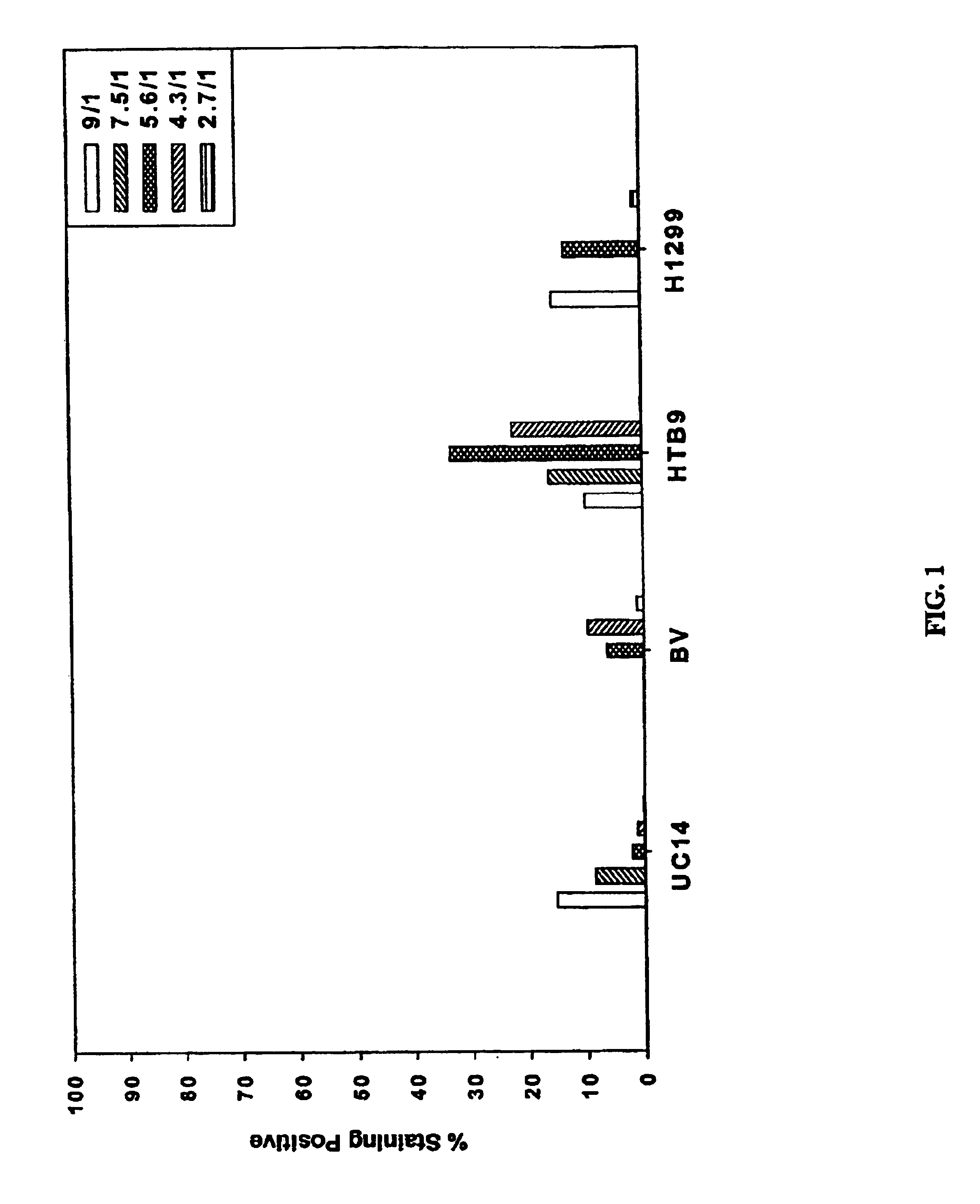
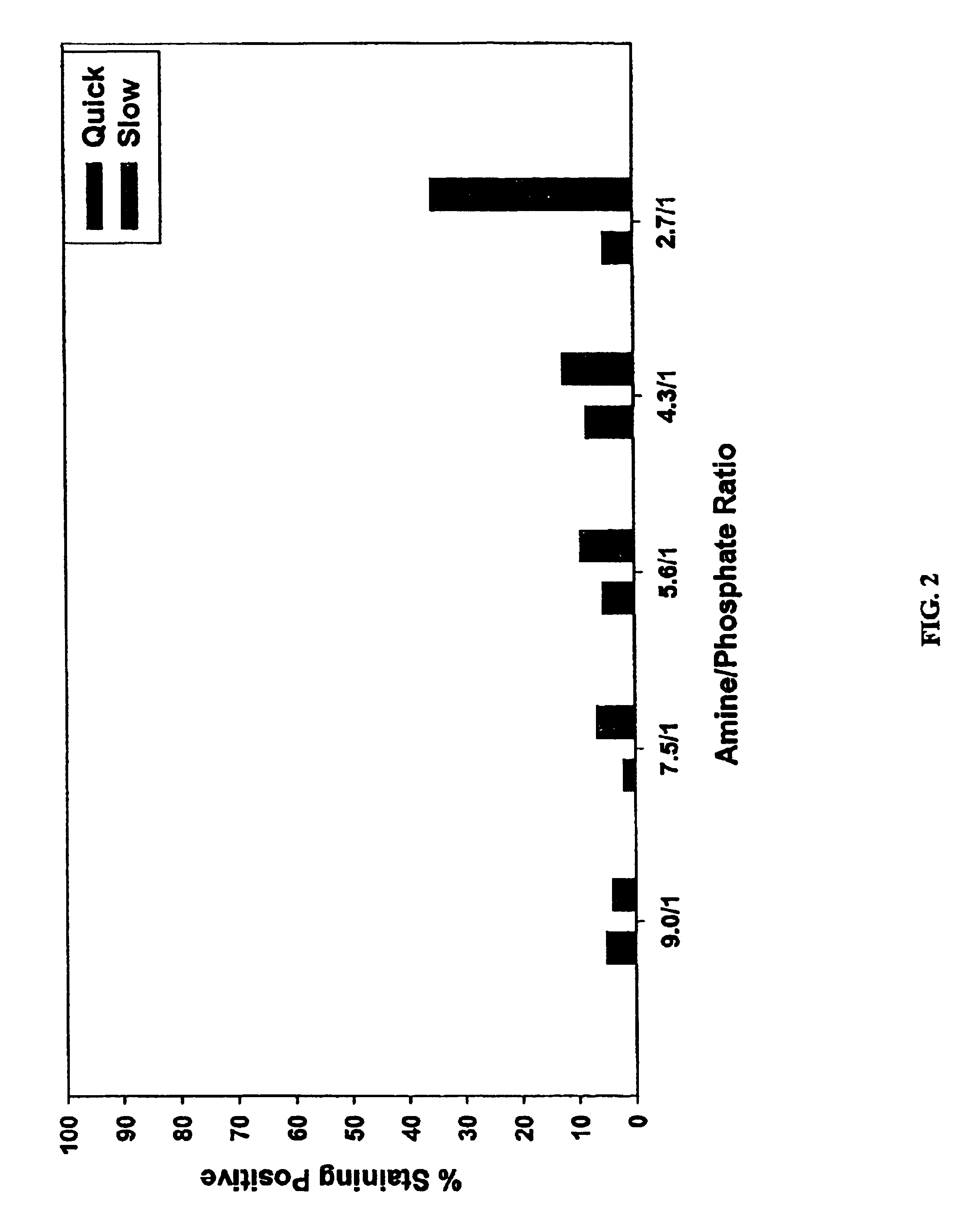
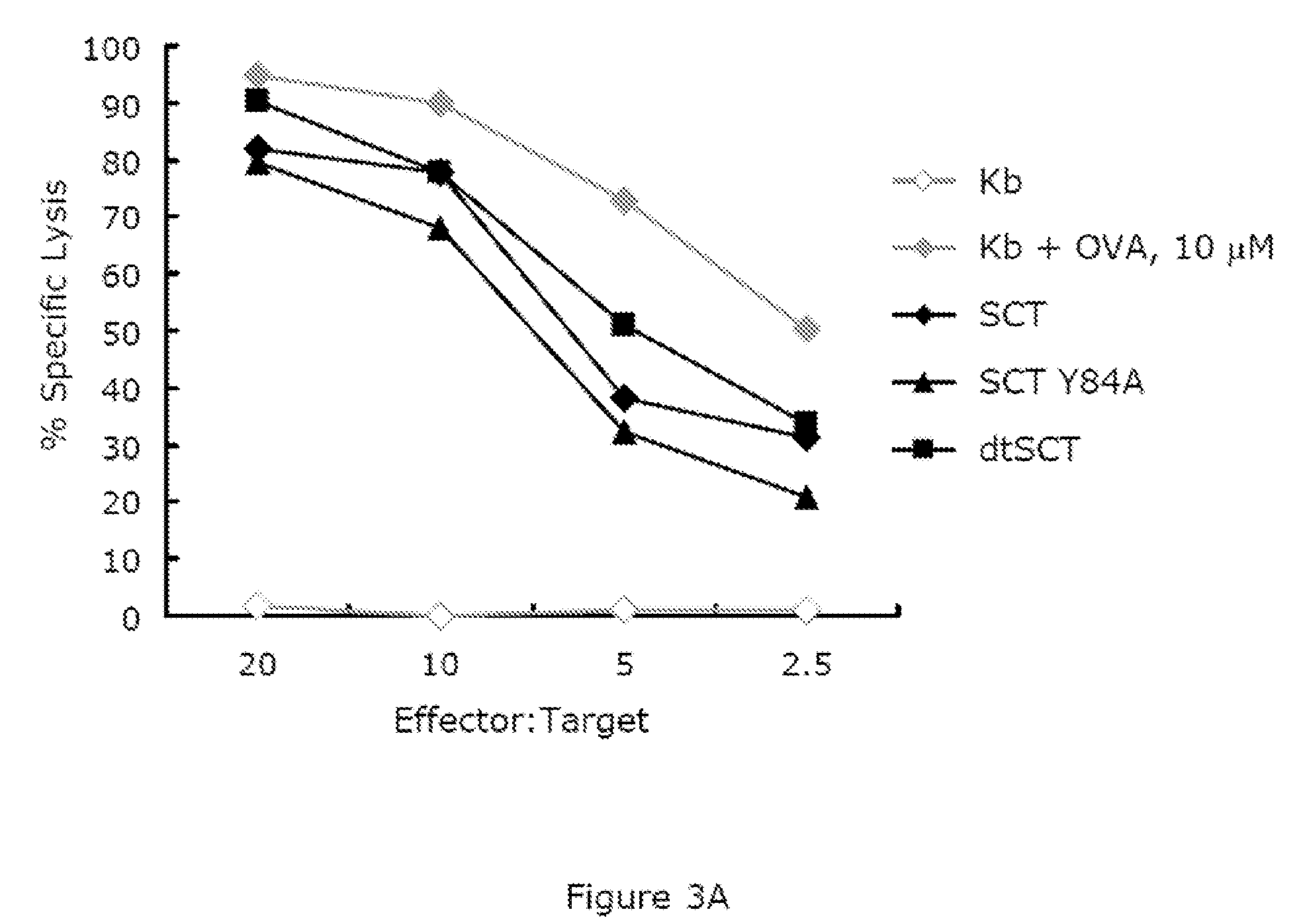
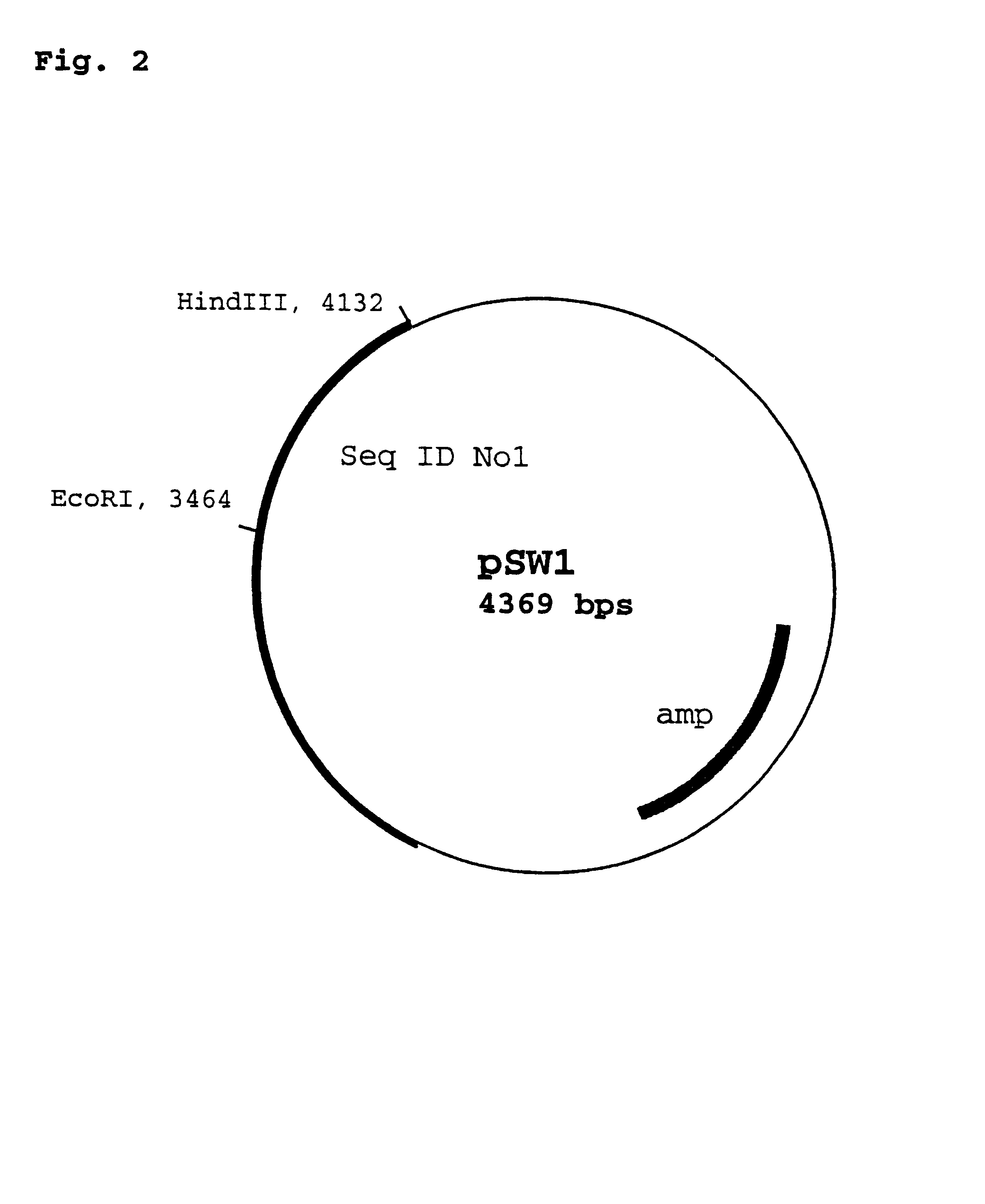
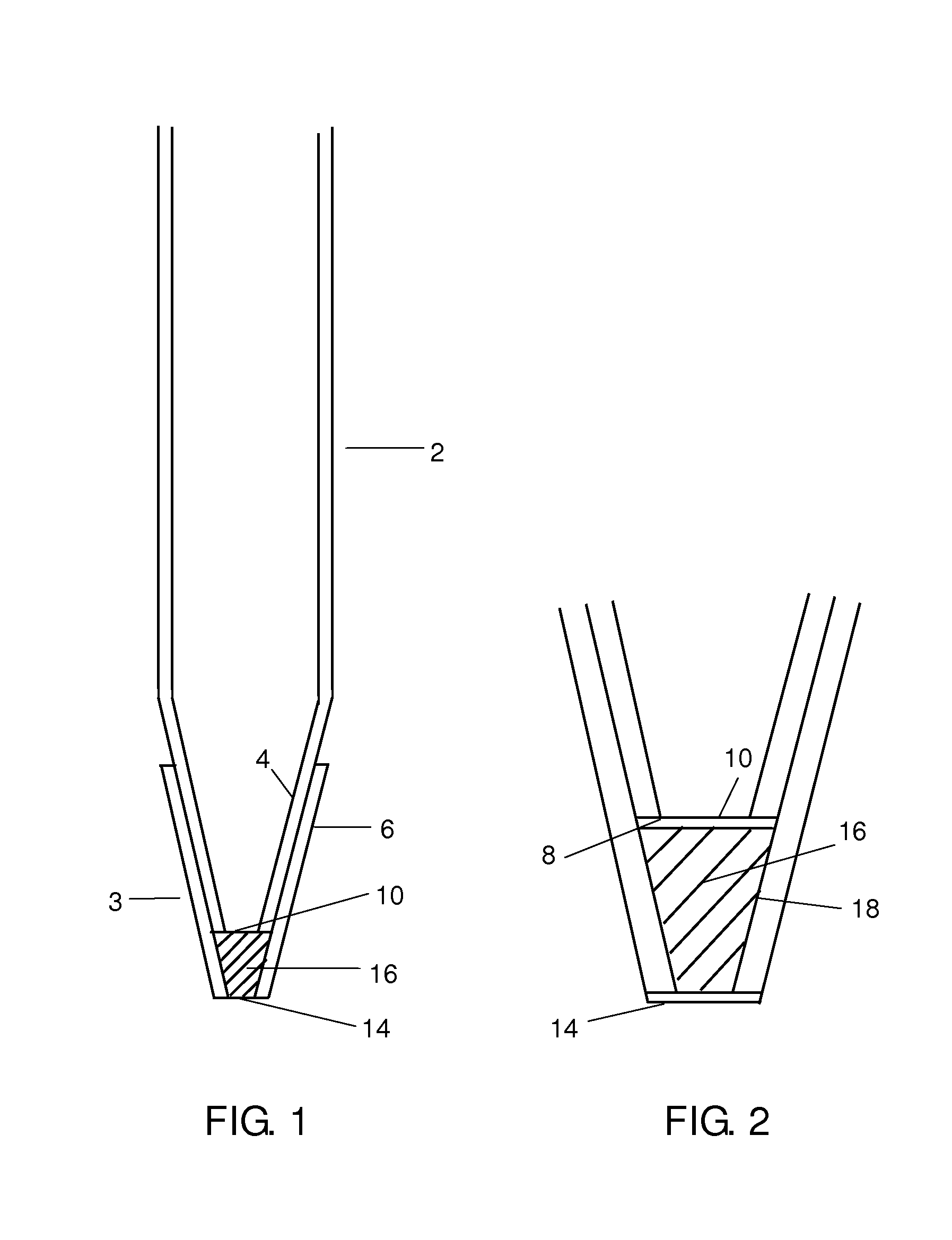
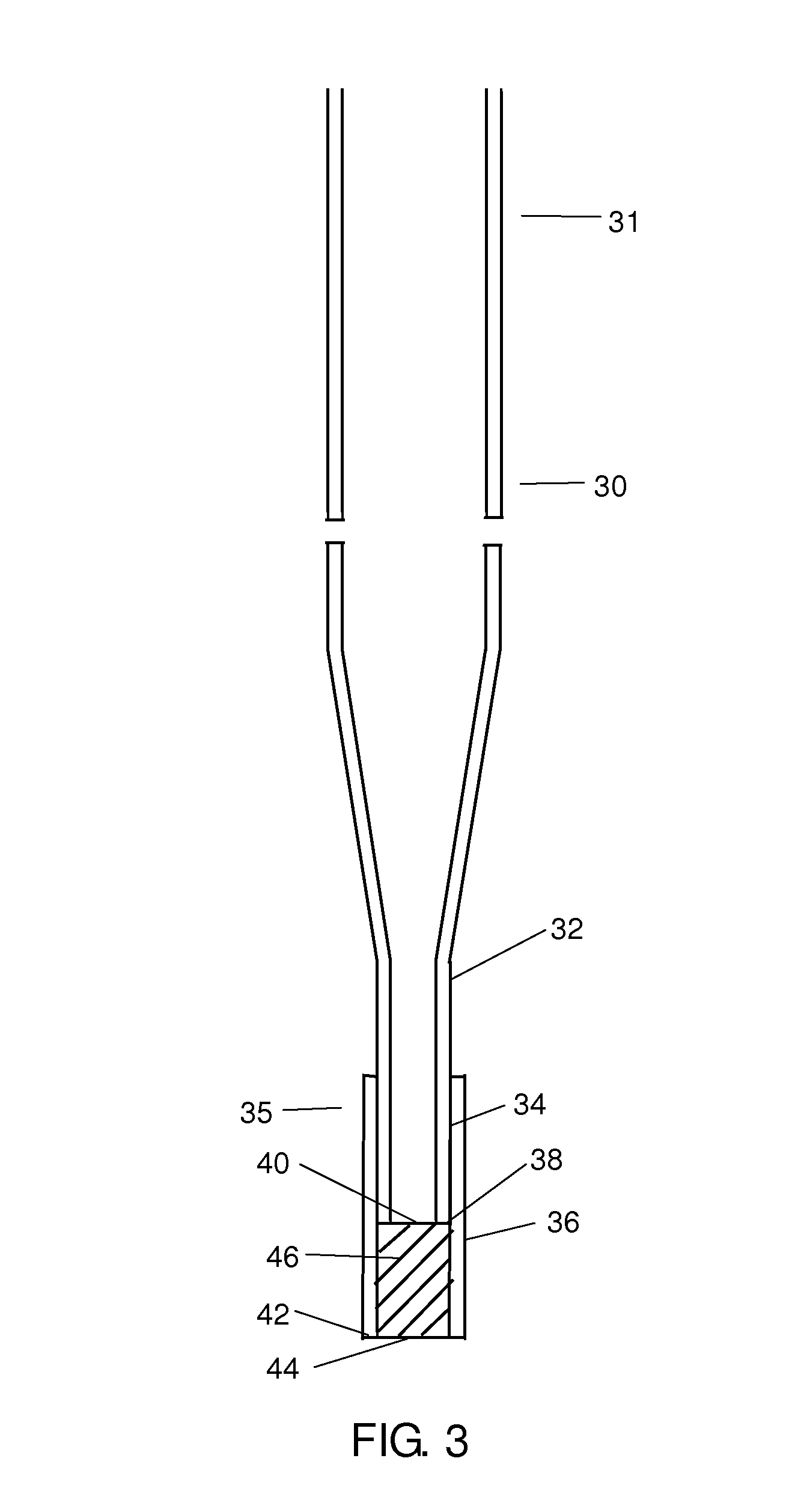
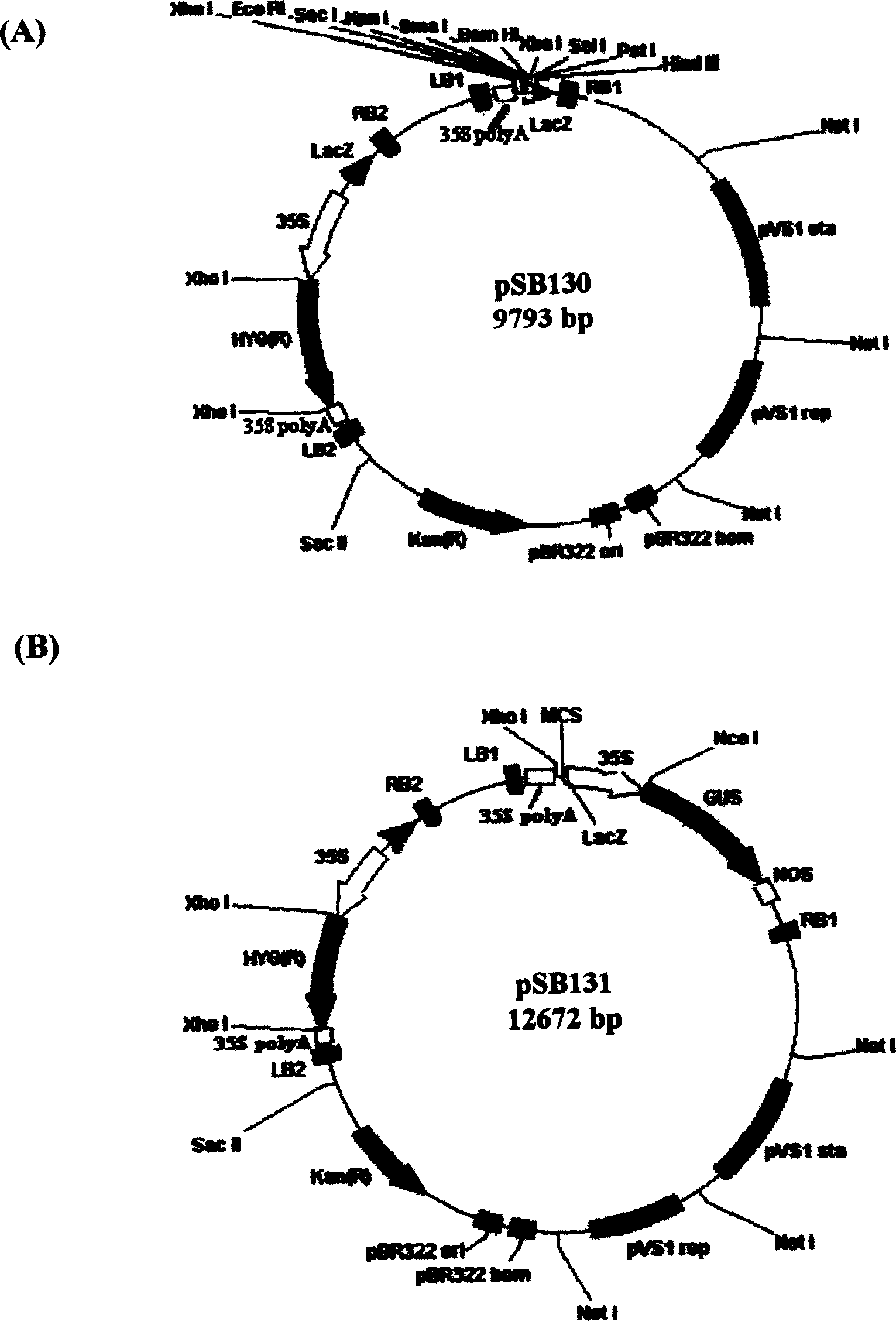
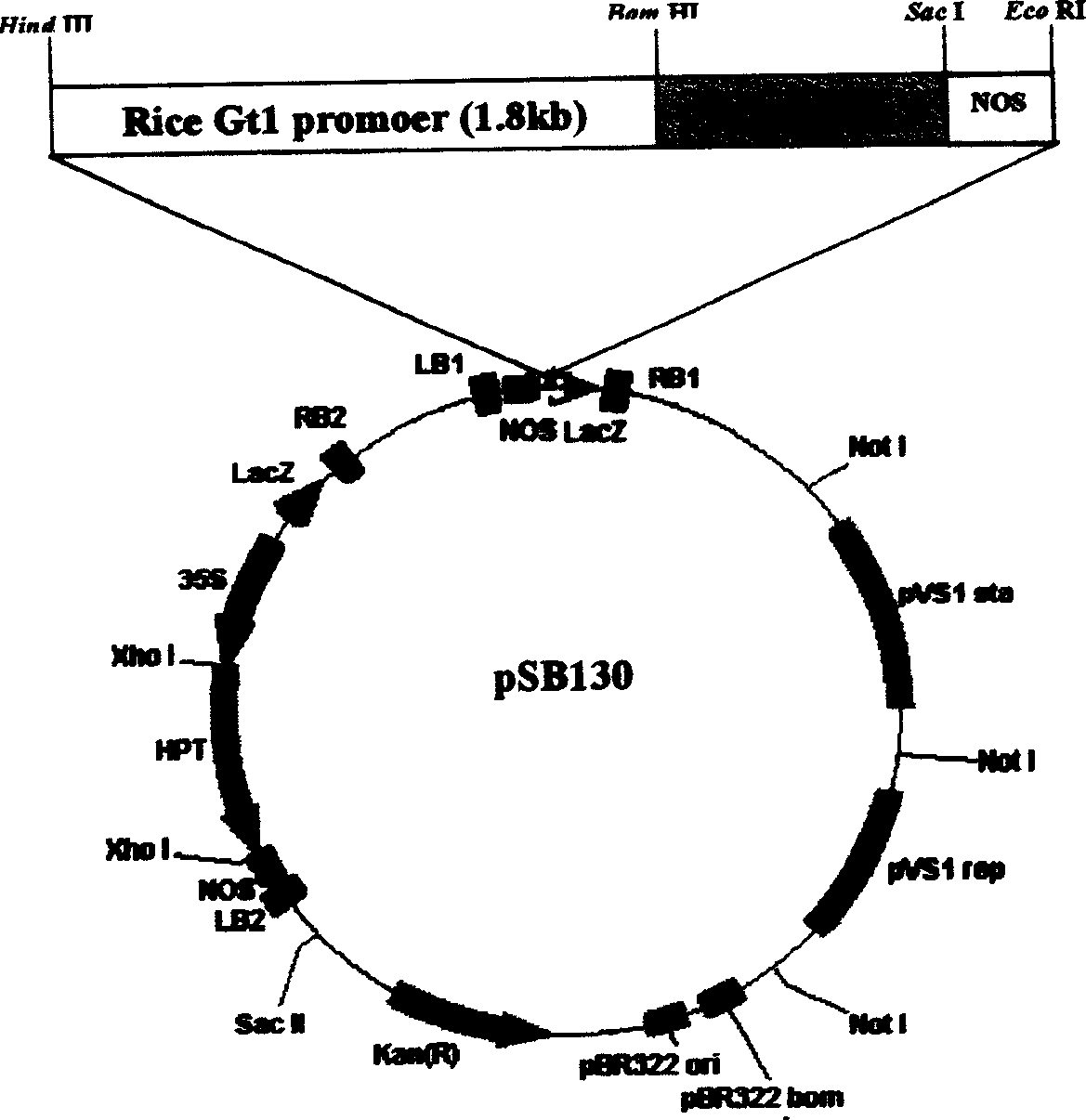
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
143 results about "Dna carrier" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carrier DNA Insight® is a comprehensive preconception and prenatal carrier screening test. Carrier DNA Insight® follows the American College of Obstetricians and Gynecologists (ACOG) recommendations, and screen patients for more than 120 recessive genetic diseases.
Methods of modifying eukaryotic cells
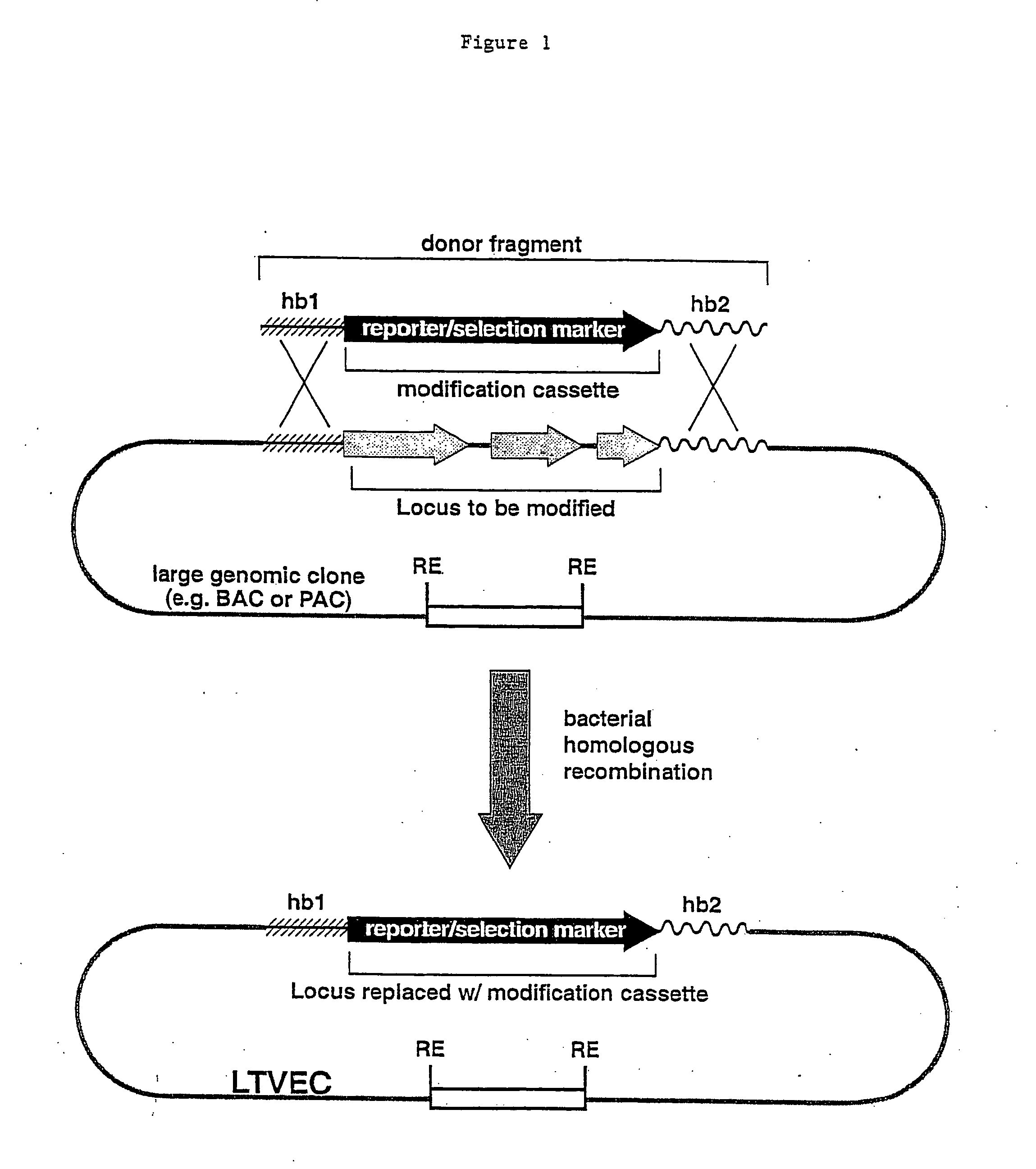
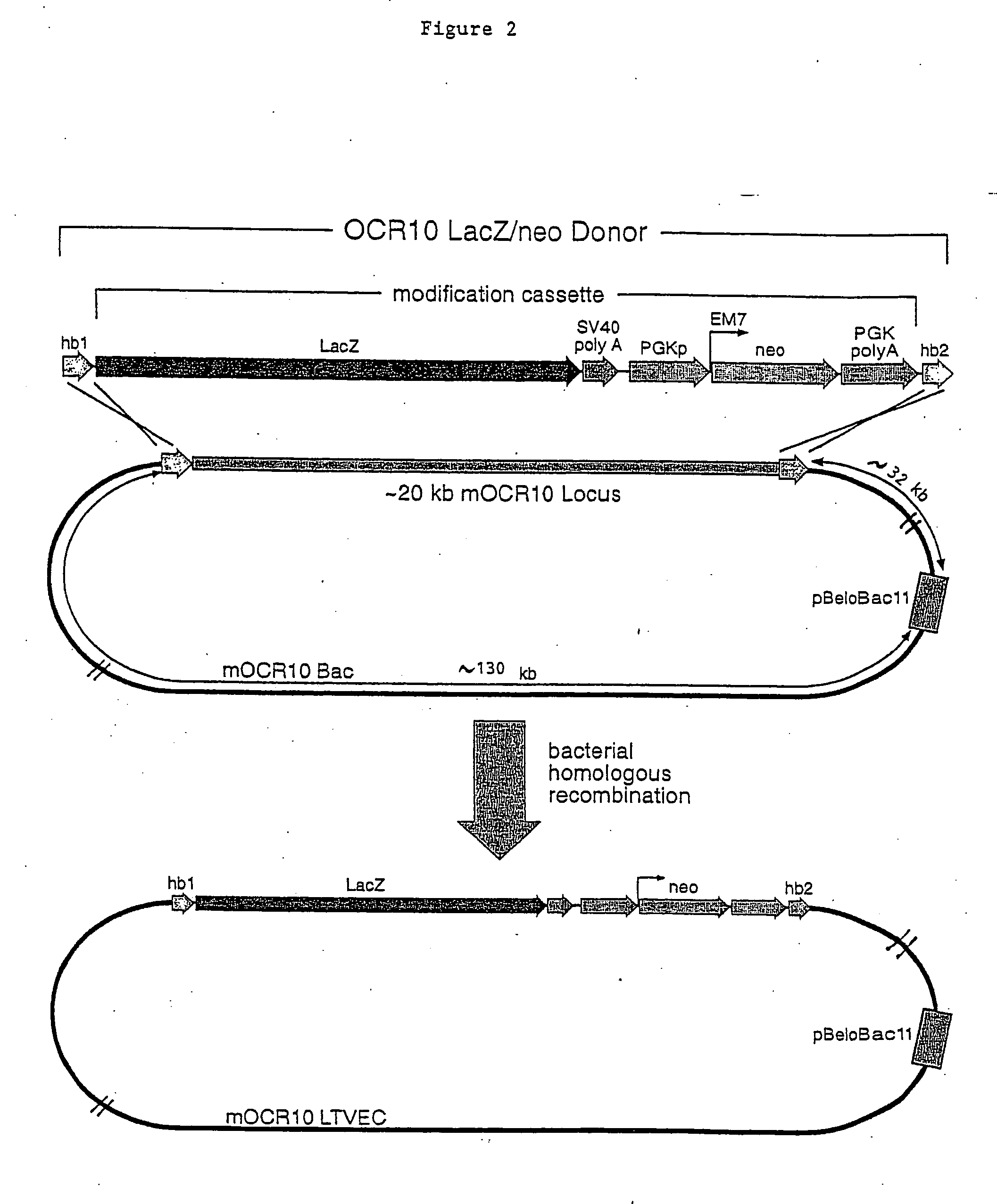
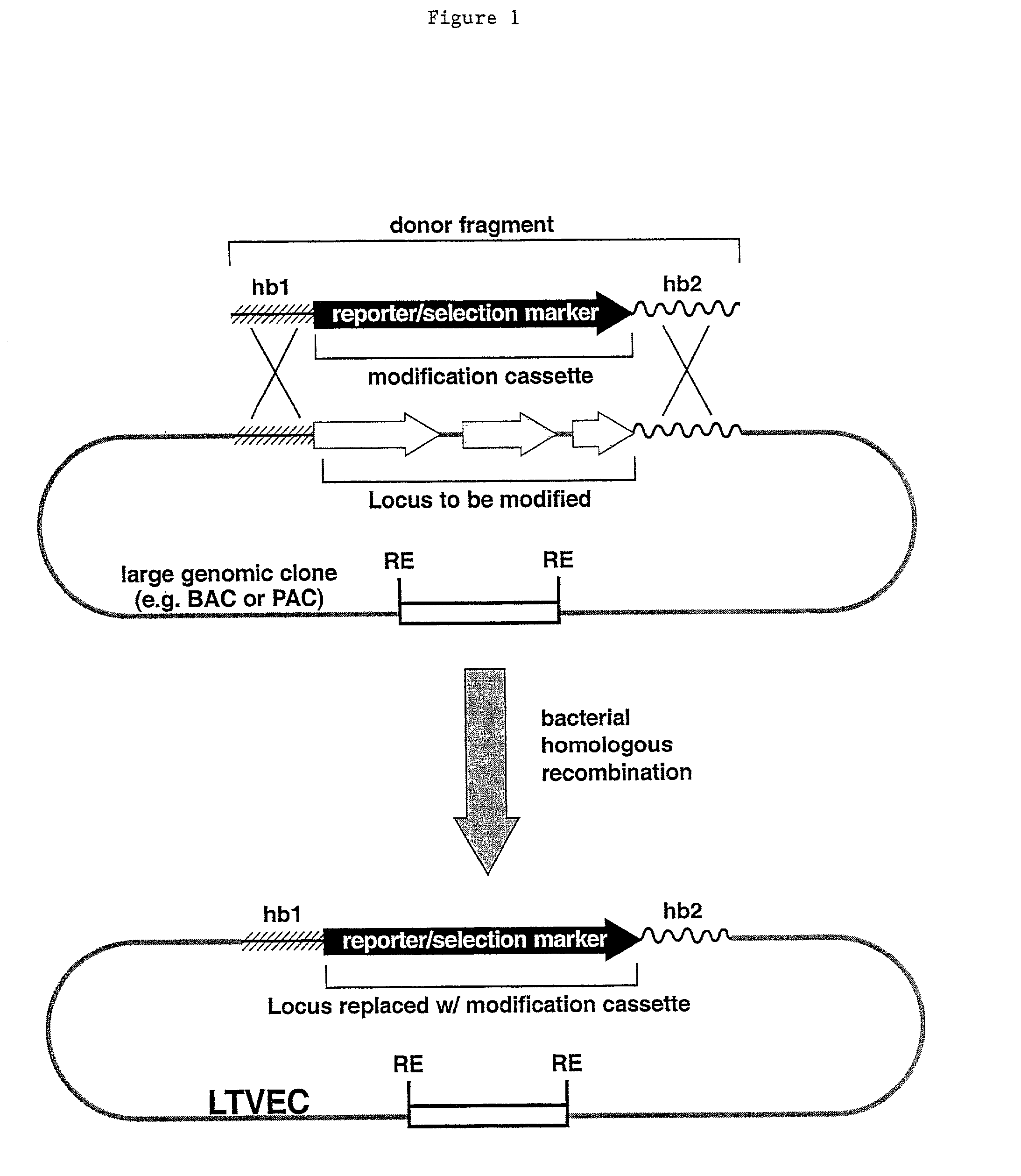
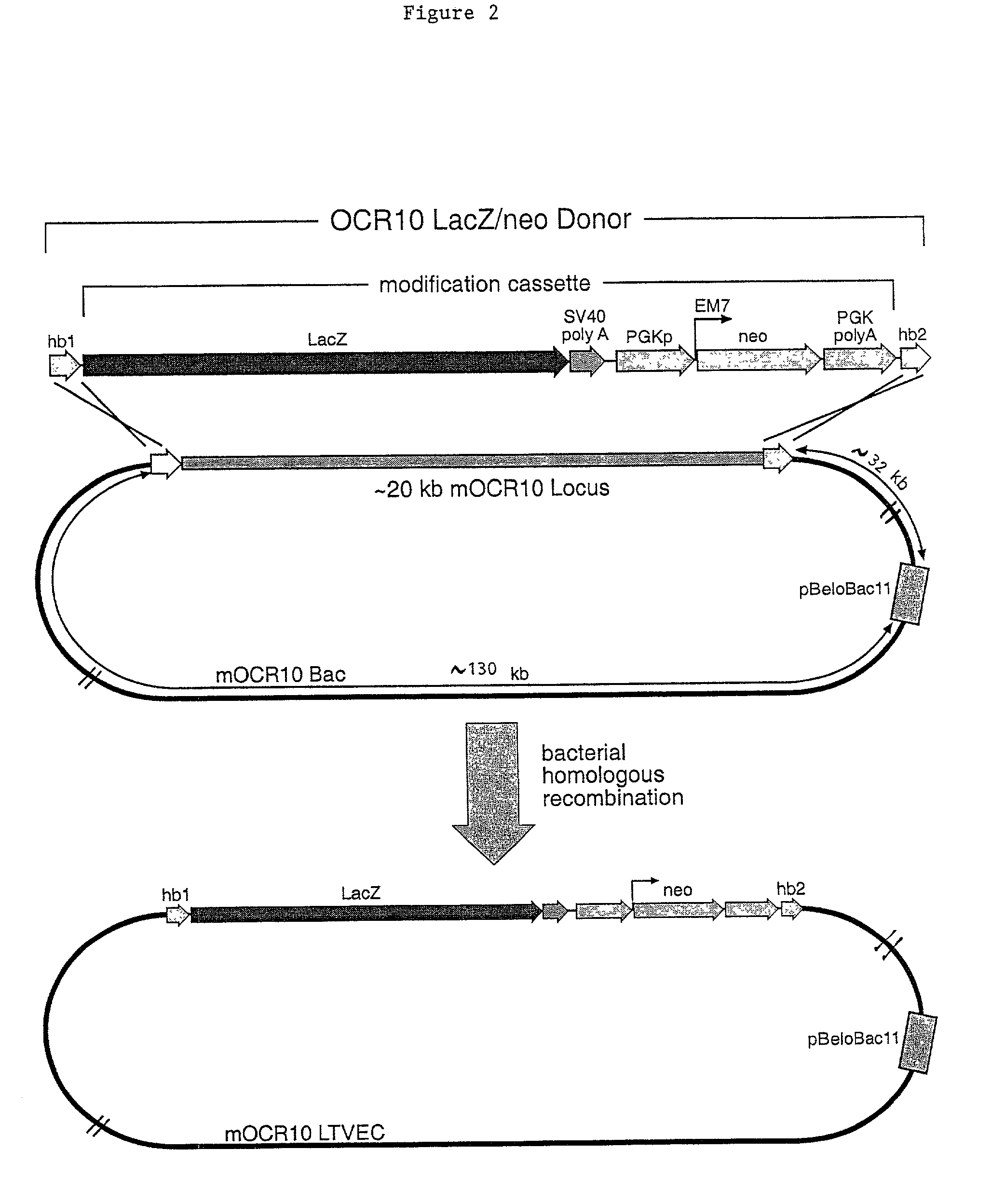
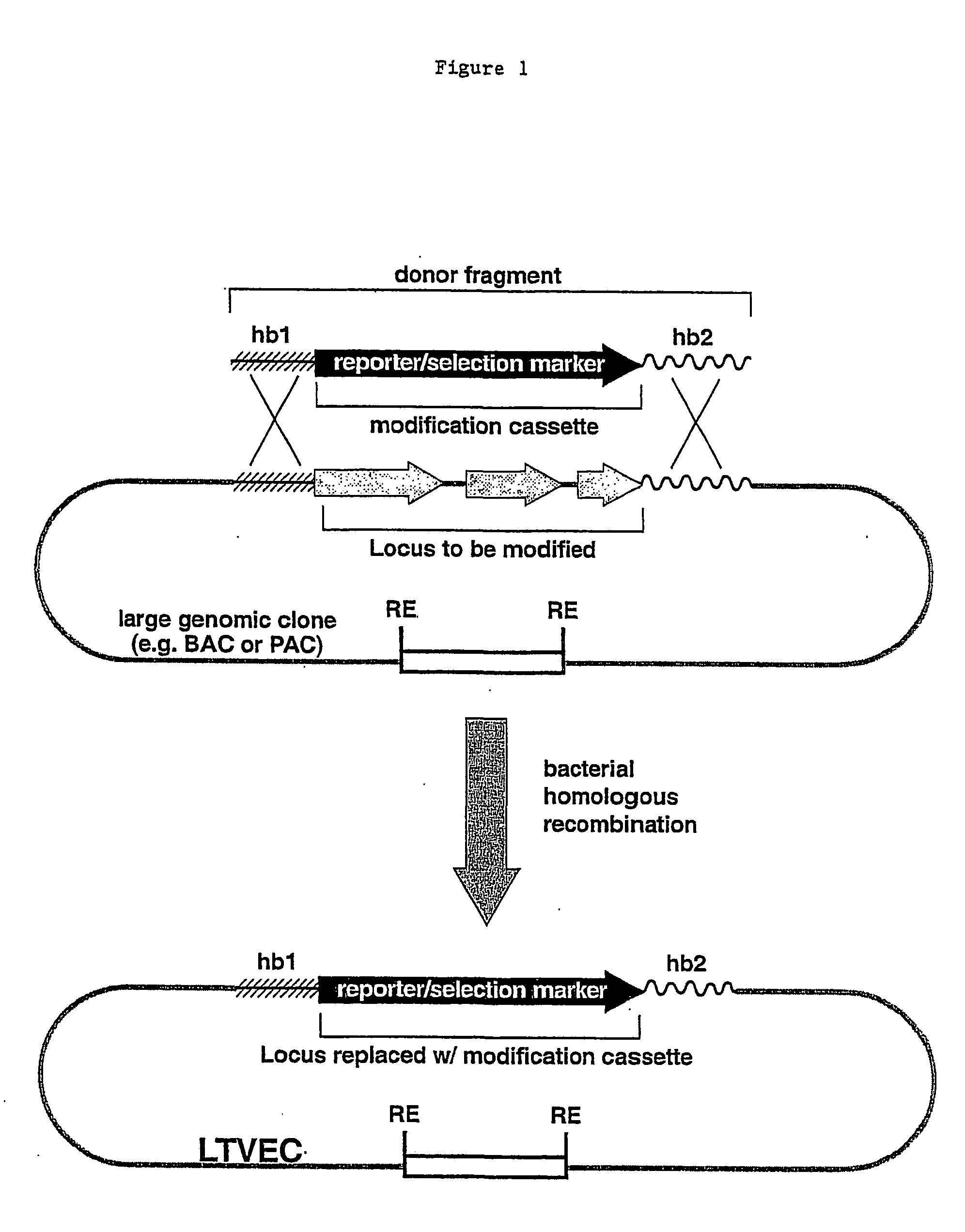
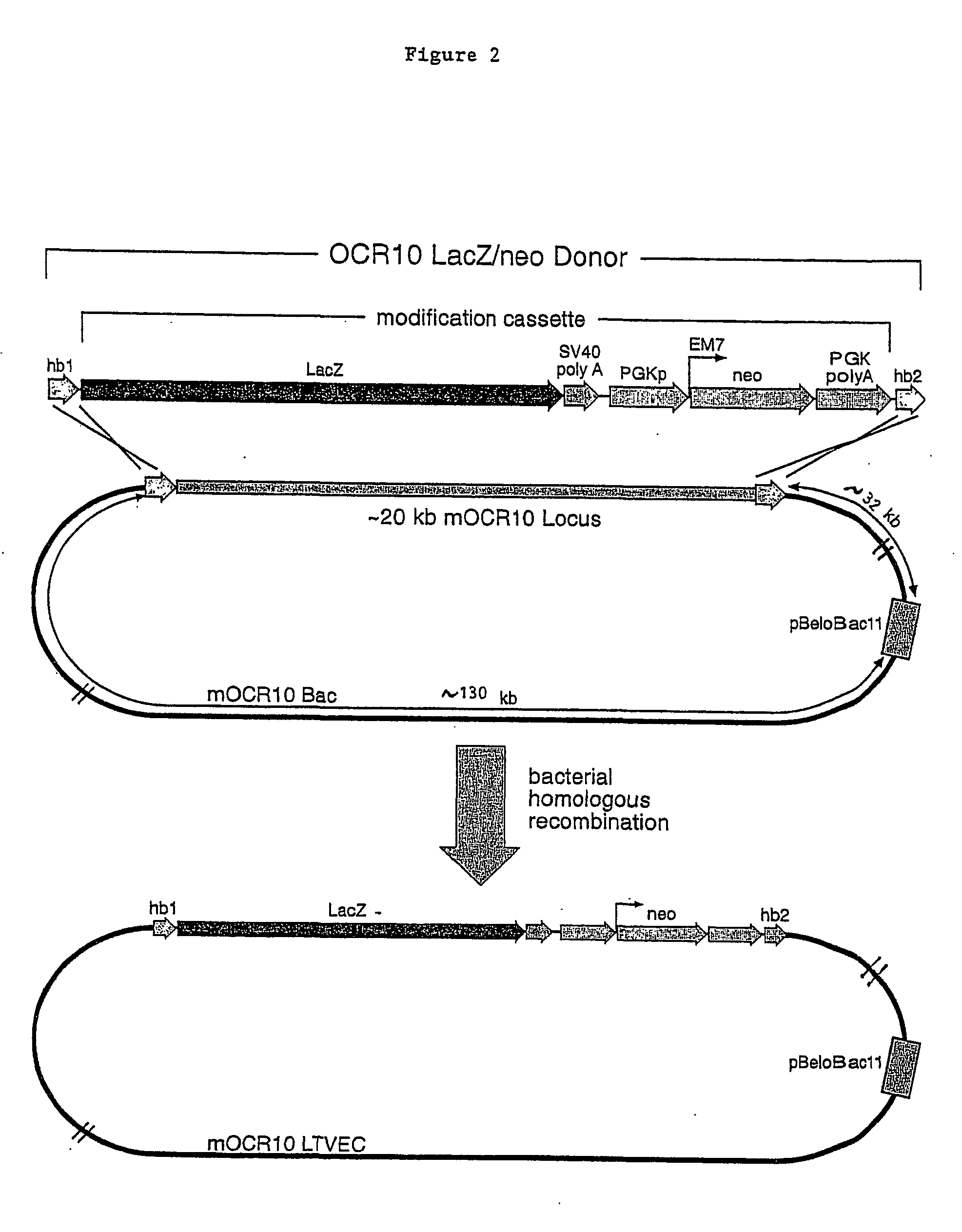
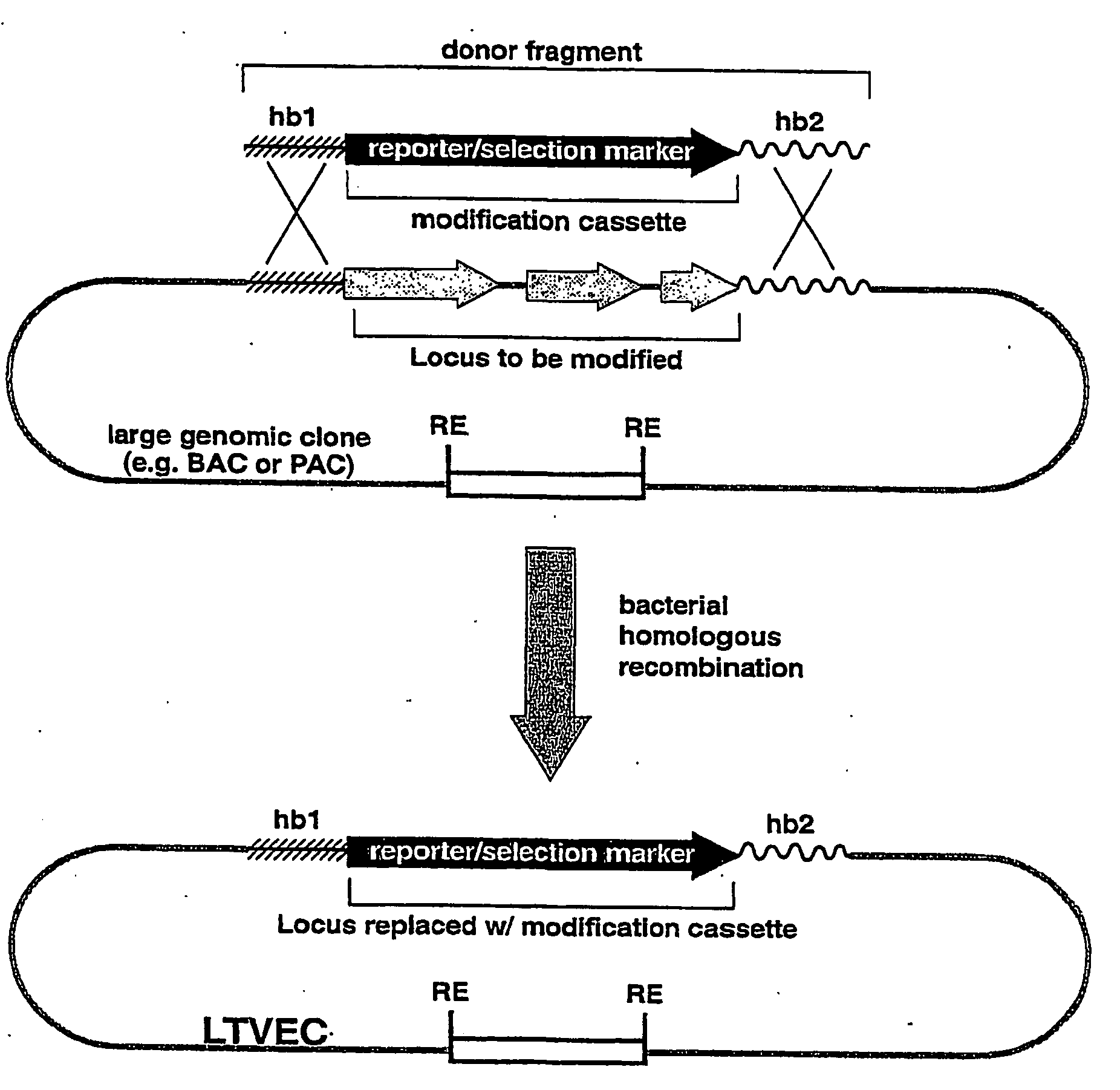
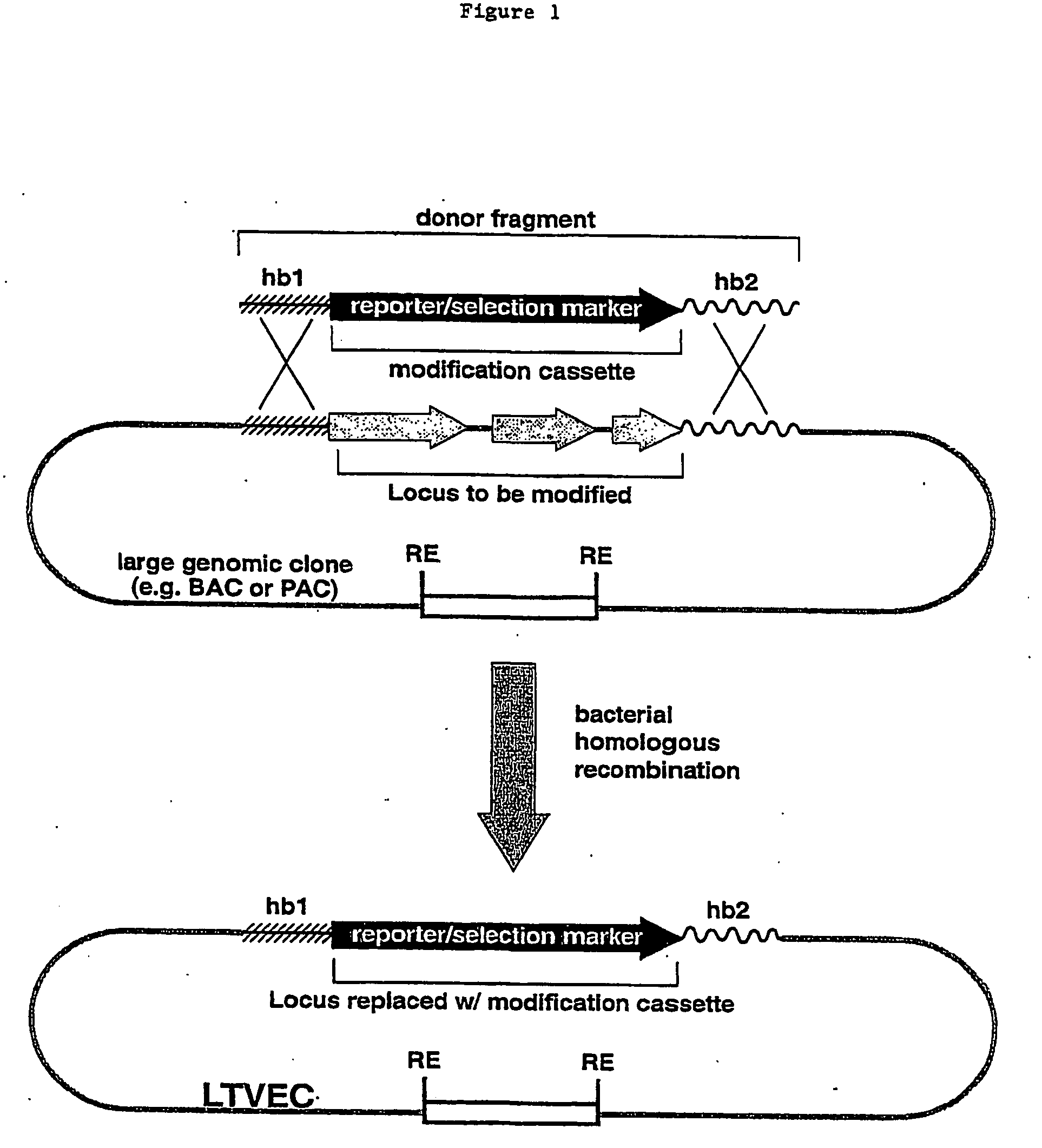
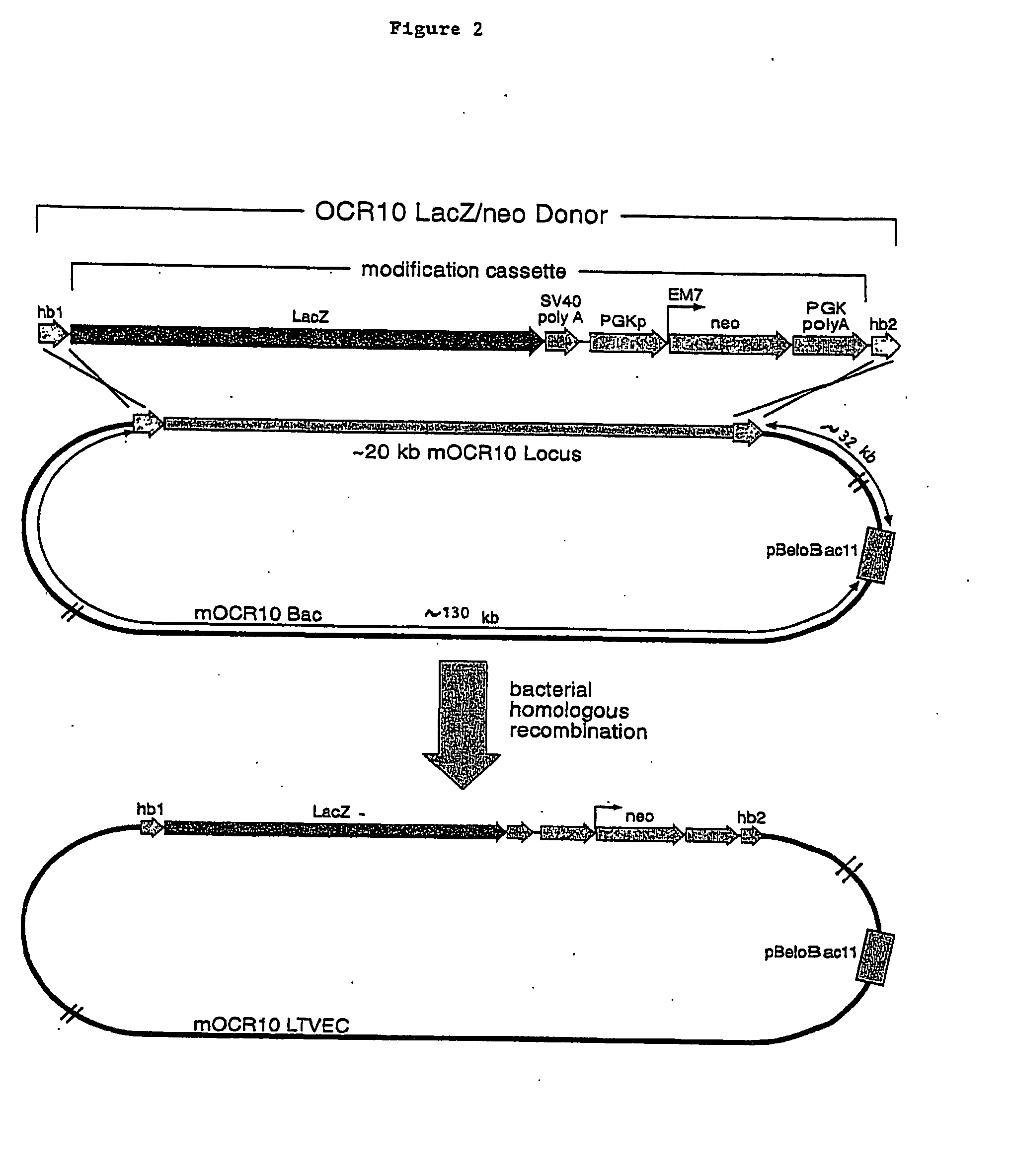
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous gene(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
Methods of modifying eukaryotic cells
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous gene(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
Methods of modifying eukaryotic cells
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous genes(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
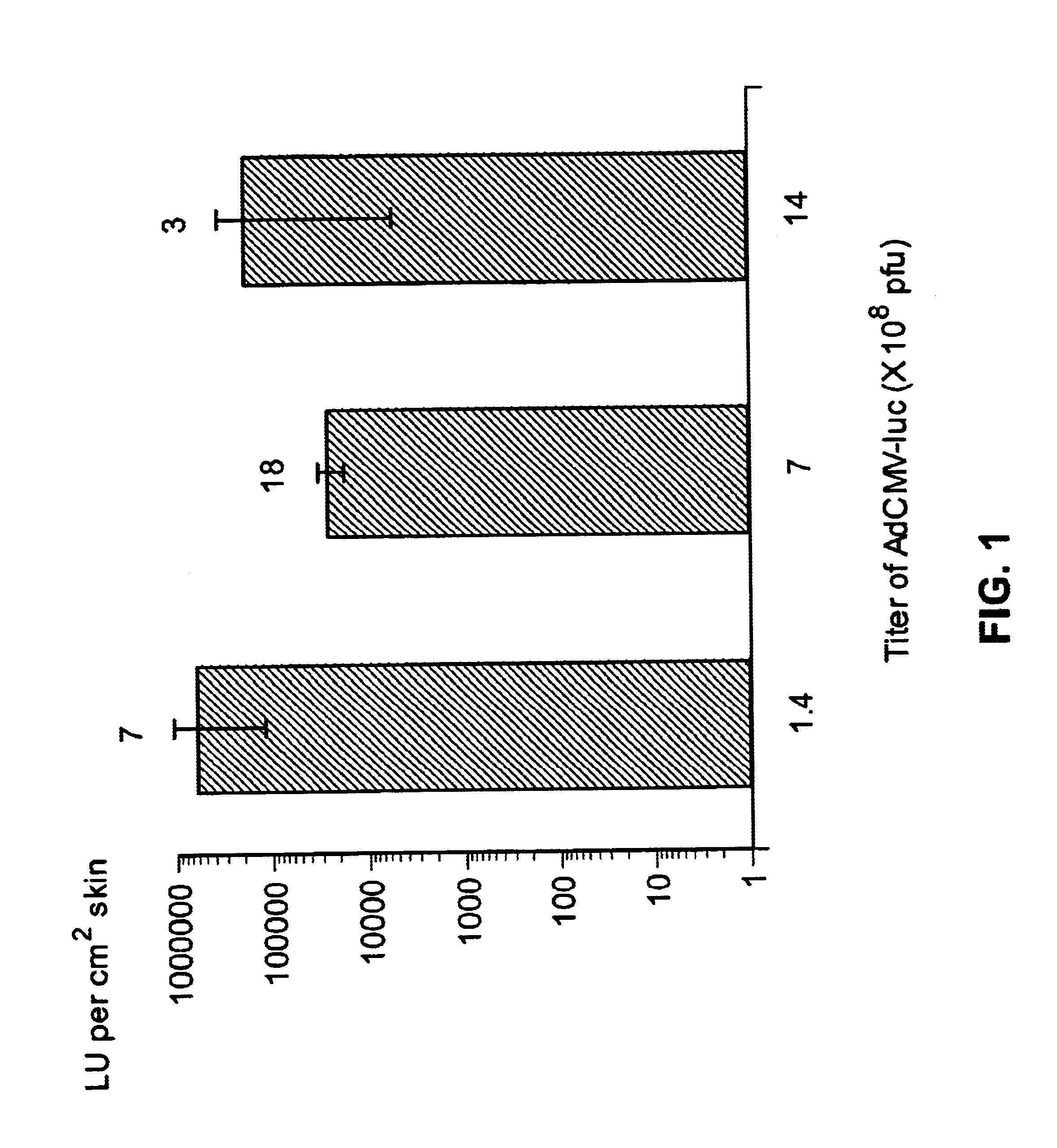
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
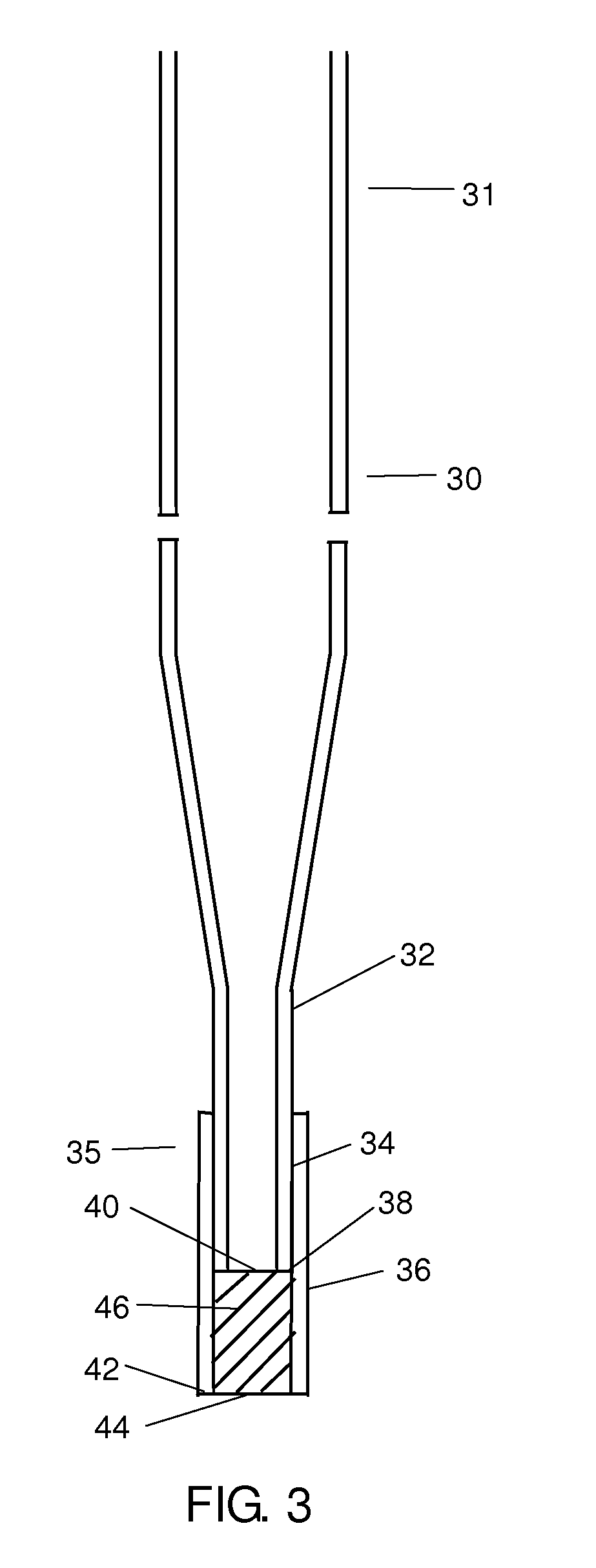
Circular DNA vectors for synthesis of RNA and DNA
InactiveUS20080227160A1Low-cost and efficientEasy to identifySugar derivativesPeptide/protein ingredientsRibonucleotide synthesisRibonucleotide
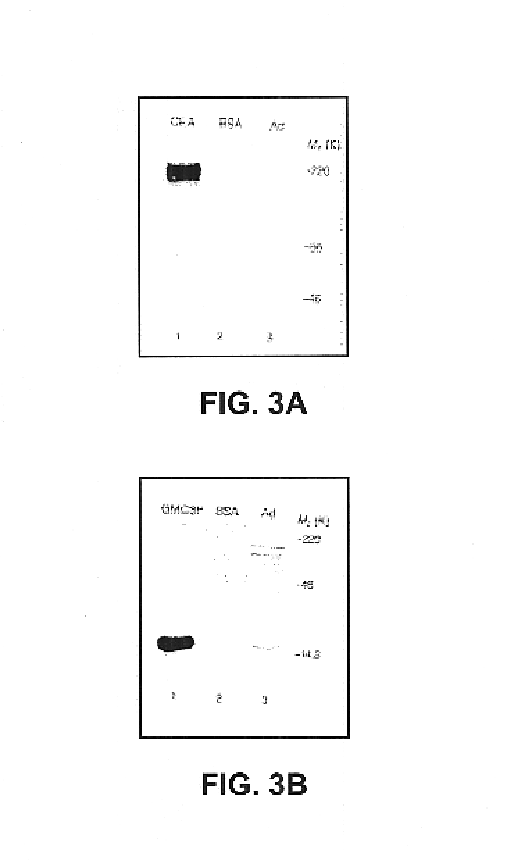
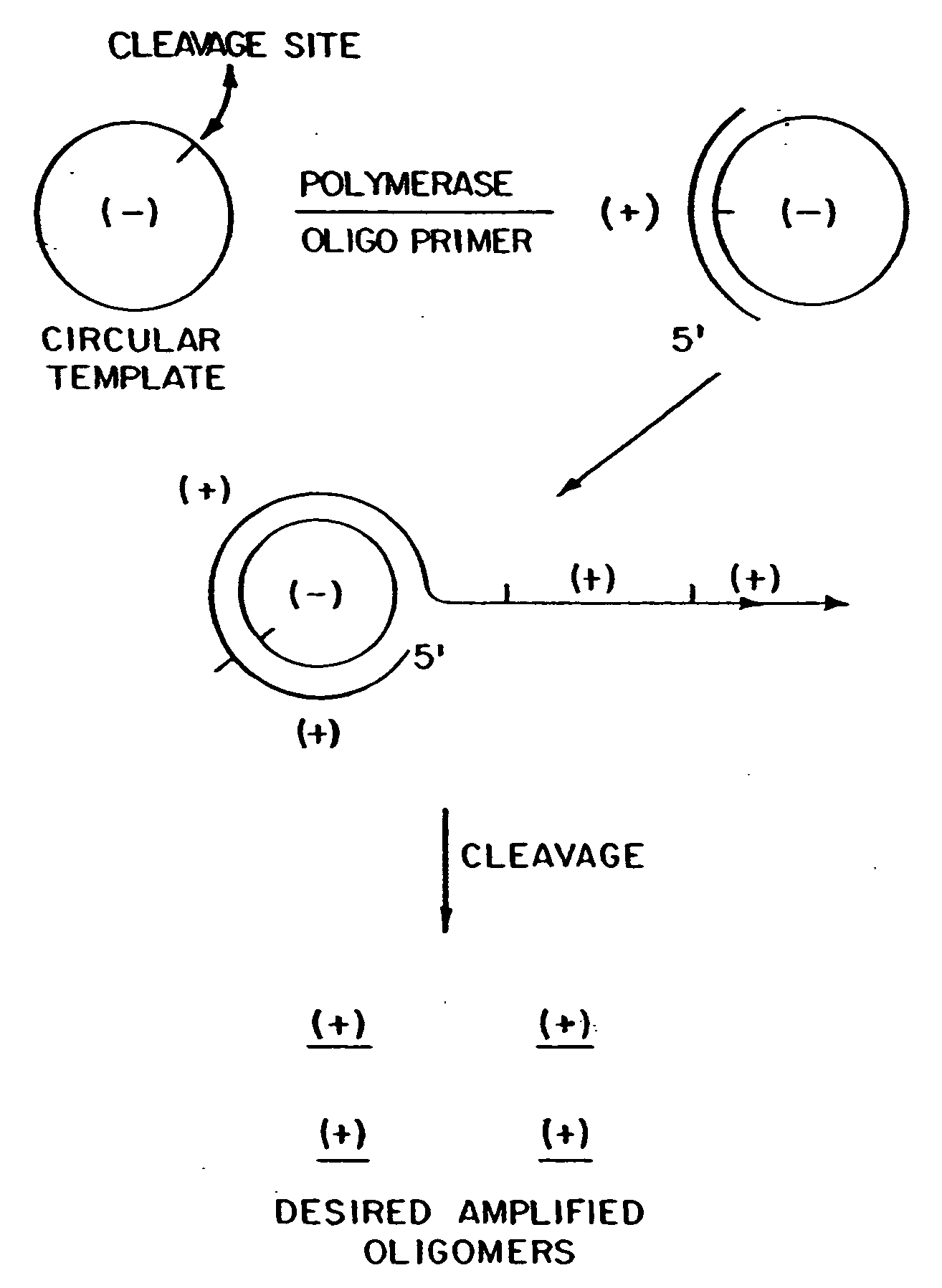
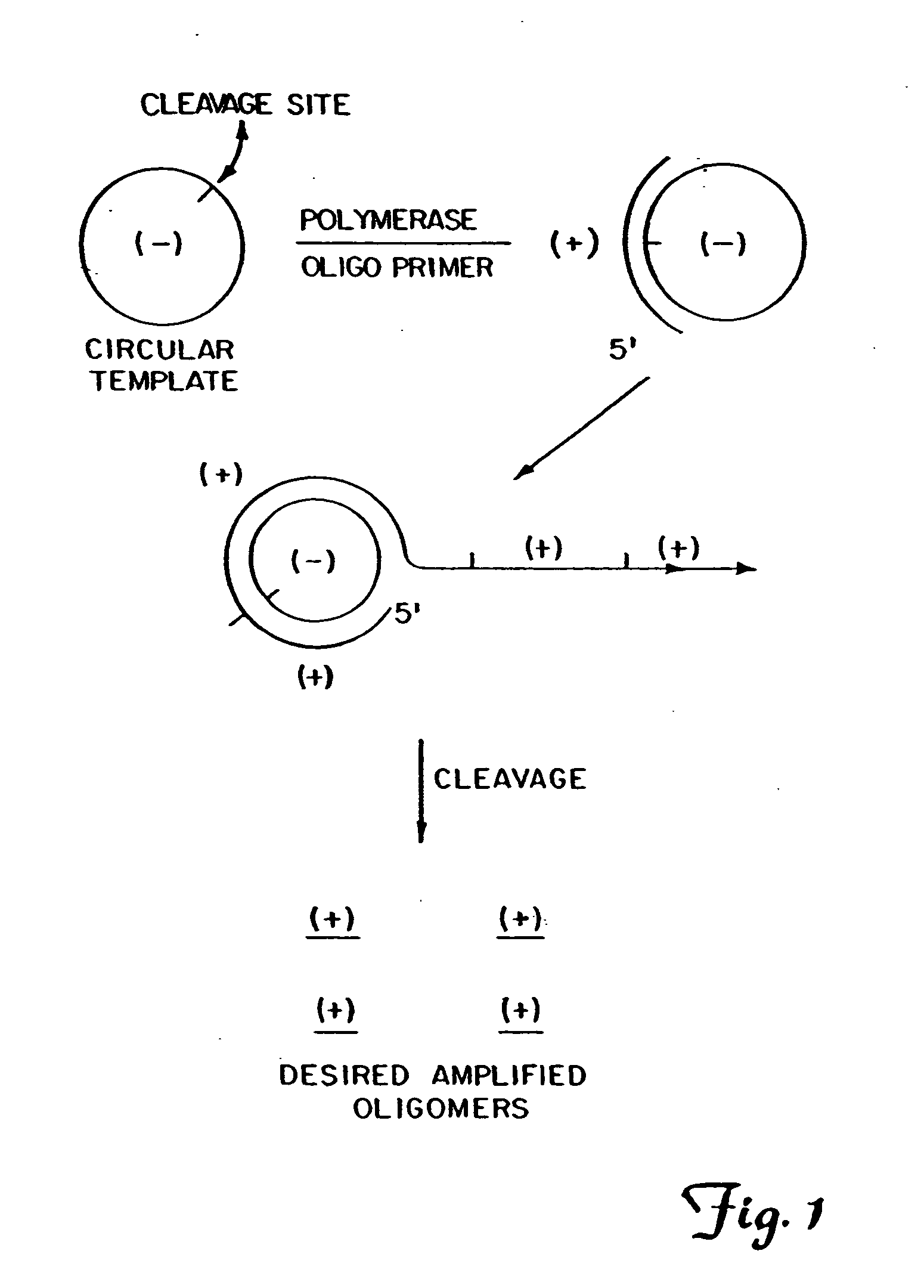
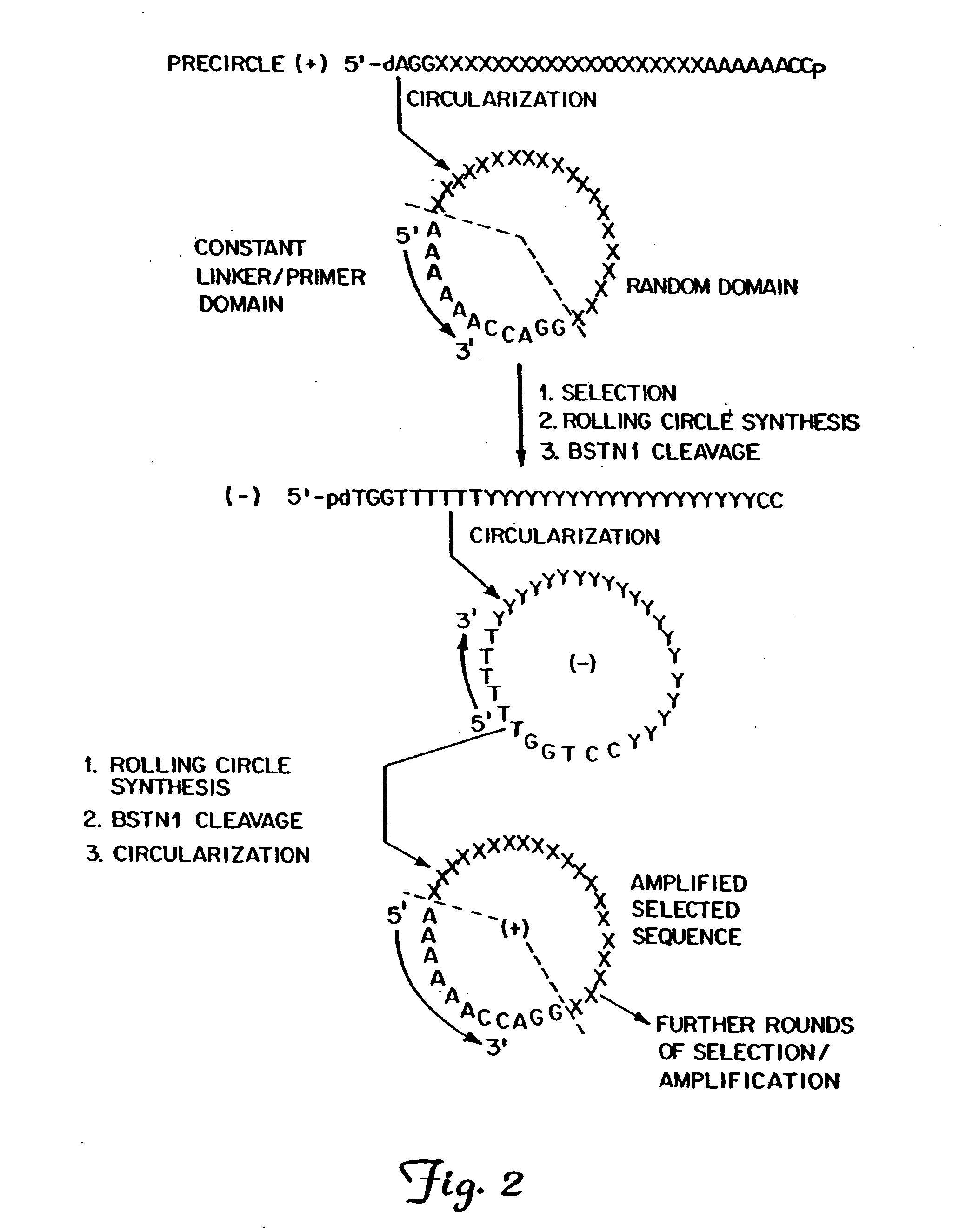
The present invention provides methods for synthesis and therapeutic use of DNA and RNA oligonucleotides and analogs. RNA oligonucleotides are synthesized using a small, circular DNA template which lacks an RNA polymerase promoter sequence. The RNA synthesis is performed by combining a circular single-stranded oligonucleotide template with an effective RNA polymerase and at least two types of ribonucleotide triphosphate to form an RNA oligonucleotide multimer comprising multiple copies of the desired RNA oligonucleotide sequence. Preferably, the RNA oligonucleotide multimer is cleaved to produce RNA oligonucleotides having well-defined ends. Preferred RNA oligonucleotide multimers contain ribozymes capable of both cis (autolytic) and trans cleavage.
Owner:UNIVERSITY OF ROCHESTER
Gene transfer for studying and treating a connective tissue of a mammalian host
InactiveUS7037492B2Reducing at least one deleterious joint pathologyCompounds screening/testingBiocideConnective tissue fiberMammal
Methods for introducing at least one gene encoding a product into at least one target cell of a mammalian host for use in treating the mammalian host are disclosed. These methods include employing recombinant techniques to produce a vector molecule that contains the gene encoding for the product, and infecting the target cells of the mammalian host using the DNA vector molecule. A method to produce an animal model for the study of connective tissue pathology is also disclosed.
Owner:UNIVERSITY OF PITTSBURGH
Nanoparticles from chitosan
InactiveUS20050226938A1Material nanotechnologyPeptide/protein ingredientsControlled releaseBiopolymer
Methods are disclosed for preparing crosslinked core and core-shell nanoparticle polymers from chitosan. The final products of the present invention may be used as detergents and as additives for pharmaceutical composition and for drug delivery, and DNA carrier system. The nanoparticles made from biopolymers of the present invention may also be used in controlled release, superabsorbent materials and biomaterials like enzyme immobilization.
Owner:UNIVERSITY OF DEBRECEN
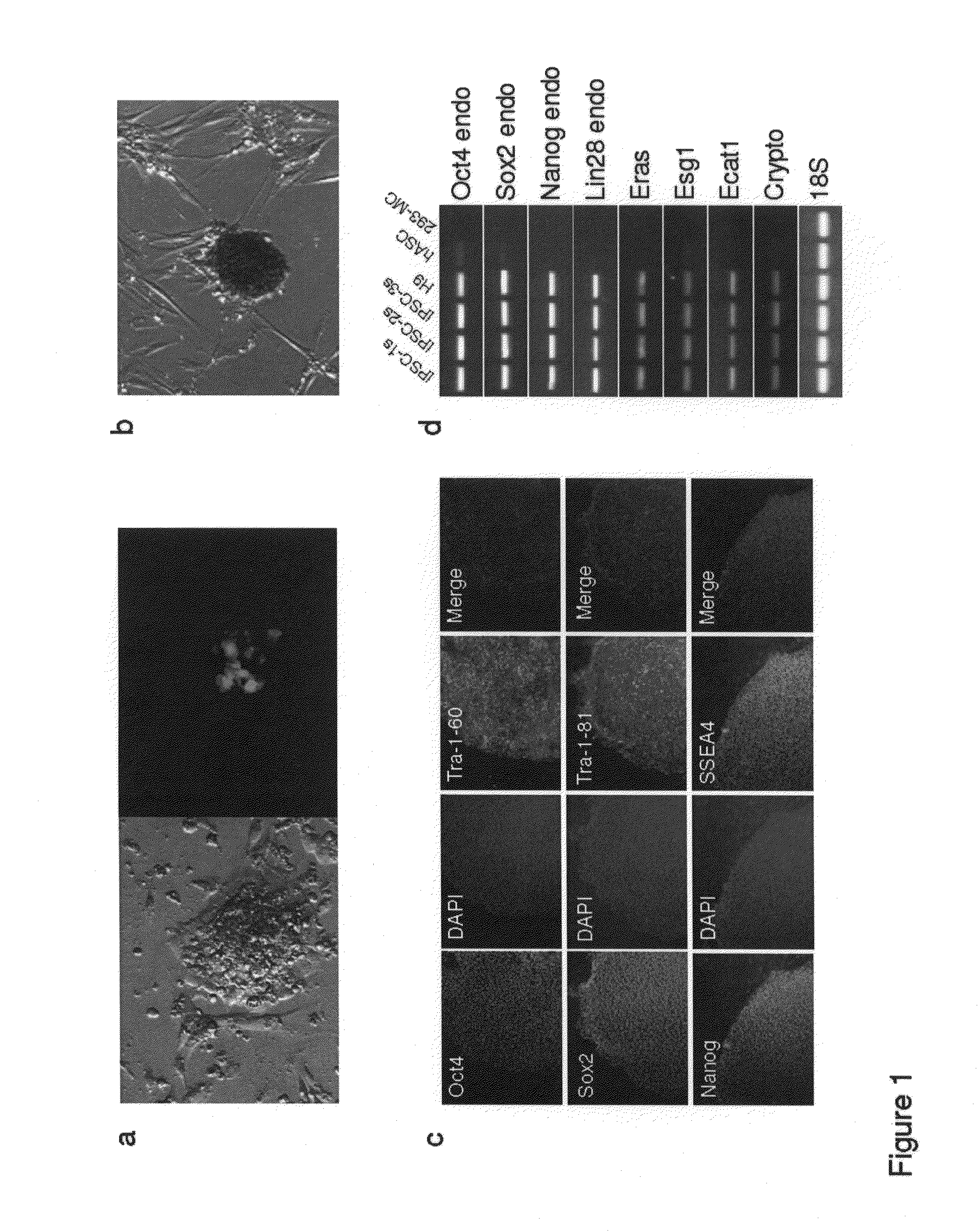
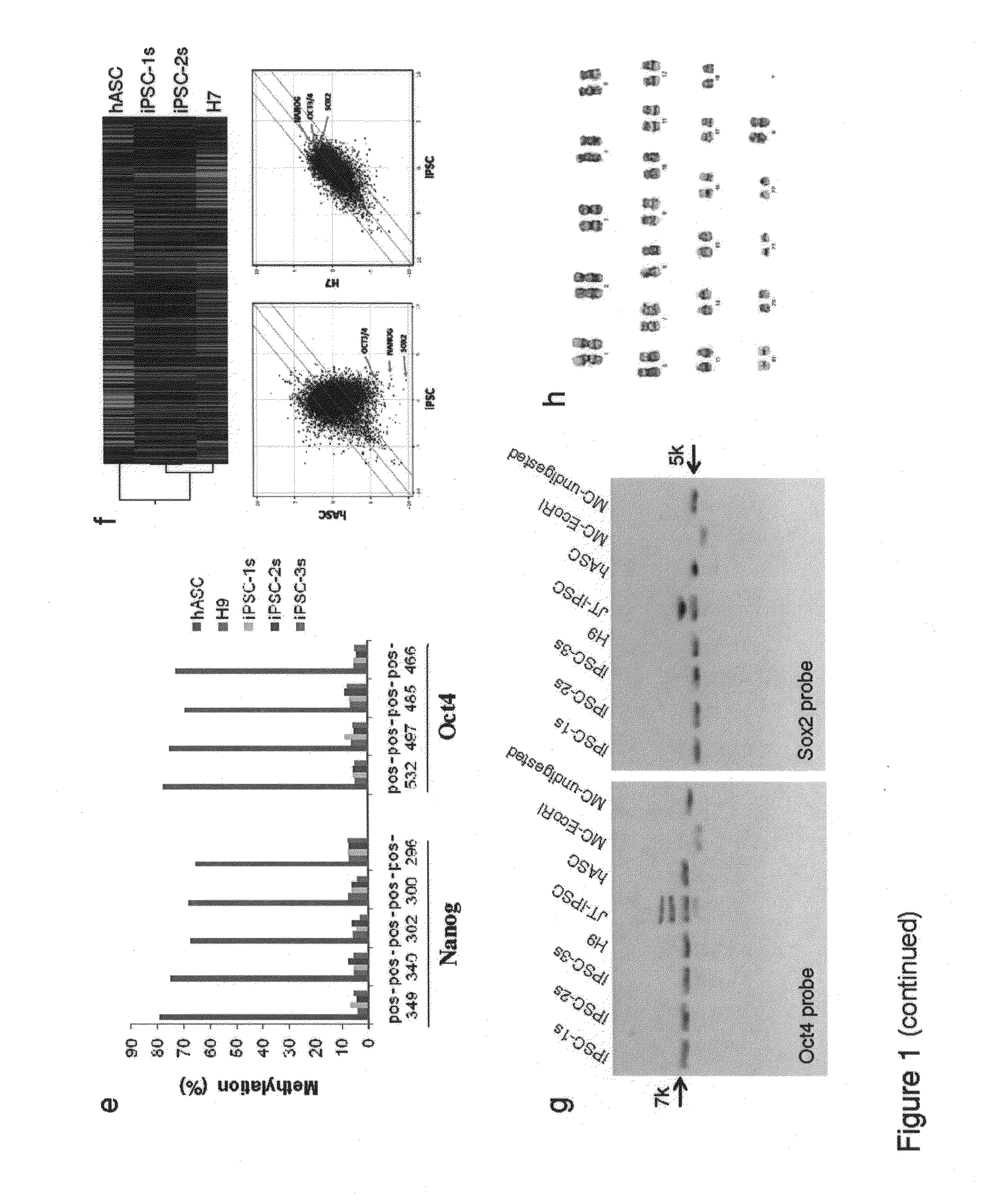
Enhanced efficiency of induced pluripotent stem cell generation
Human somatic cells are reprogrammed to become induced pluripotent stem cells (iPS cells) by the introduction of a minicircle DNA vector. Cells of interest include adipose stem cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Vectors for plant transformation and methods of use
InactiveUS20060041956A1Easy to eliminateImprove efficiencyOther foreign material introduction processesFermentationNormal appearanceRead through
The present invention is directed to a vector for identifying read-through of non-T-DNA in a T-DNA vector. In one embodiment, the vector provides a visually detectable change in the normal appearance of transformants wherein read-through has occurred. In another embodiment, the vector also provides for expression of a readily detectable fluorescent protein that allows for the early detection and elimination of transformants wherein read-through has occurred. In a further aspect, the present invention is directed to a method for detecting read-through of non-T-DNA in plants transformed with a T-DNA vector. In another aspect, the present invention is directed to a method for producing a transgenic plant containing a polynucleotide of interest but being substantially free of non-T-DNA.
Owner:PIONEER HI BRED INT INC
PEI: DNA vector formulations for in vitro and in vivo gene delivery
InactiveUS6846809B2Easy constructionGood suitPowder deliveryGenetic material ingredientsGene deliveryIn vivo
The present invention relates generally to the fields of nucleic acid transfection. More particularly, it concerns novel polycation:nucleic acid compositions, methods of preparation of such compositions and methods of transfecting cells with such compositions.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions and methods for improved plant transformation
The present invention provides methods for eliminating plants containing non-T-DNA sequences derived from a T-DNA vector. More specifically, the present invention provides a method for killing plant cells that receive non-T-DNA sequences based on incorporation of a lethal polynucleotide sequence into the non-T-DNA portion of the vector.
Owner:MONSANTO CO (MONSANTO CY)
Disulfide trap MHC class I molecules and uses therefor
ActiveUS8992937B2Extended cell surface half-lifeLow efficiencyBiocideAntibody mimetics/scaffoldsAntigenMHC class I
Owner:WASHINGTON UNIV IN SAINT LOUIS
Vector for integration of heterologous sequences into poxviral genomes
The present invention provides a DNA vector comprising a nucleic acid sequence useful for inserting heterologous sequences into the genome of poxviruses by homologous recombination. The present invention relates also, inter alia, to recombinant poxvirses carrying heterologous coding sequences transferred by the vector according to the present invention.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
Methods for nucleic acid mapping and identification of fine-structural-variations in nucleic acids
ActiveUS20090325239A1Quick buildEfficient sortingSugar derivativesMicrobiological testing/measurementNucleic acid mappingA-DNA
A method of juxtaposing sequence tags (GVTs) that are unique positional markers along the length of a population of target nucleic acid molecules is provided, the method comprising: fragmenting the target nucleic acid molecule to form target DNA insert; ligating the target DNA insert to a DNA vector or backbone to create a circular molecule; digesting the target DNA insert endonuclease to cleave the target DNA insert at a distance from each end of the target DNA insert yielding two GVTs comprising terminal sequences of the target DNA insert attached to an undigested linear backbone; recircularizing the linear backbone with the attached GVTs to obtain a circular DNA containing a GVT-pair having two juxtaposed GVTs; and recovering the GVT-pair DNA by nucleic acid amplification or digestion with endonuclease having sites flanking the GVT-pair. Cosmid vectors are provided for creating GVT-pairs of ˜45- to 50-kb separation sequencable by next-generation DNA sequencers.
Owner:VERSITECH LTD
Method and device for sample preparation
InactiveUS20100200509A1Easily plug columnAvoid purificationMaterial nanotechnologyComponent separationPipetteFrit
The invention provides pipette tip extraction columns for the purification of a DNA vector from un-clarified cell lysate containing cell debris as well as methods for making and using such columns. The columns typically include a bed of extraction media positioned in the pipette tip column, above a bottom frit and with an optional top frit.
Owner:PHYNEXUS
Double T-DNA carrier and its application in cultivating of non selecting sign transgene rice
InactiveCN1597969ASmall molecular weightHigh co-transformation efficiencyFermentationVector-based foreign material introductionGenetically modified ricePlant nodule
The invention provides a simple and convenient Agrobacterium Tumefacies dual carrier containing double T-DNA structural regions, and a method of using the carrier system to cultivate transgenic rice without resistance selection label. With the help of the principle of co-conversion mediated by root nodule Agrobacterium Tumefacies, the system contains two separate T-DNA structural sections, where the first T-DNA region contains antibiotic resistance selection label gene and the second one contains a universal polyclonal site able to be arbitrarily inserted with destination gene. The double-T-DNA carrier has small molecular weight, easy to clone and after the destination gene and necessary regulation and control series are cloned in the T-DNA region containing the polyclonal site, it realizes rice co-conversion; by selfing, it selects transgenic individual with destination gene but without selectin label gene from the self-bred progeny, thus eliminating the negative effect on transgenic plant commercialized production, etc, possibly caused by selection label gene.
Owner:YANGZHOU UNIV
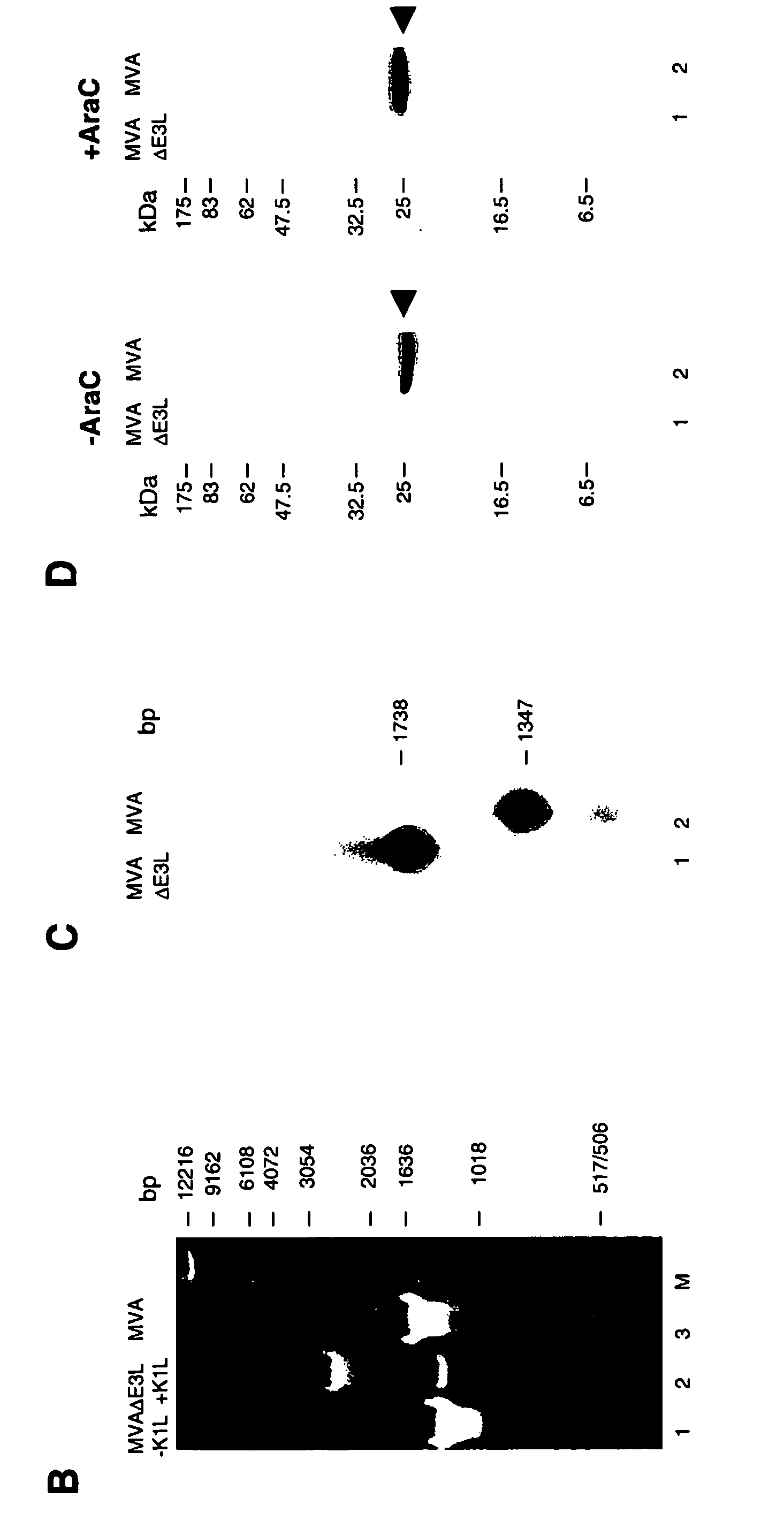
Vaccinia virus MVA-E3L-knockout-mutants and use thereof
InactiveUS7049145B2Quickly and efficiently produceIncrease choiceInactivation/attenuationVertebrate cellsMutantVaccinia viruses
The present invention relates to mutant MVA vaccinia viruses, which are used for the generation of recombinant MVA viruses, as well as host cells, which have been infected with these mutant MVA viruses. The present invention further relates to DNA-vector constructs, and a method for the generation of recombinant MVA by using the mutant MVA viruses and the DNA-vector constructs. The mutant MVA vaccinia viruses of the present invention are characterized in that the MVA ORF 050L gene or a functional part thereof has been inactivated in the viral genome.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
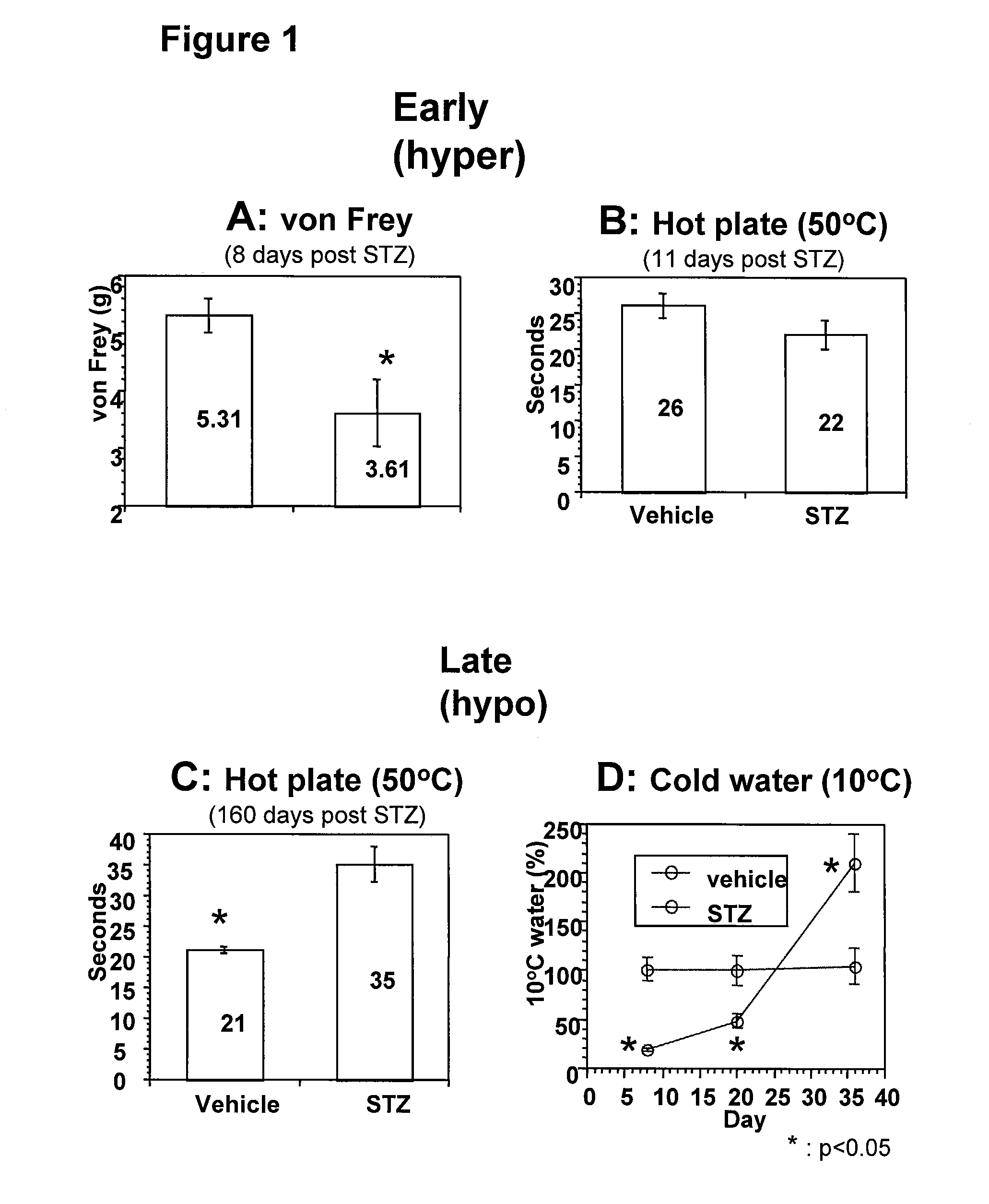
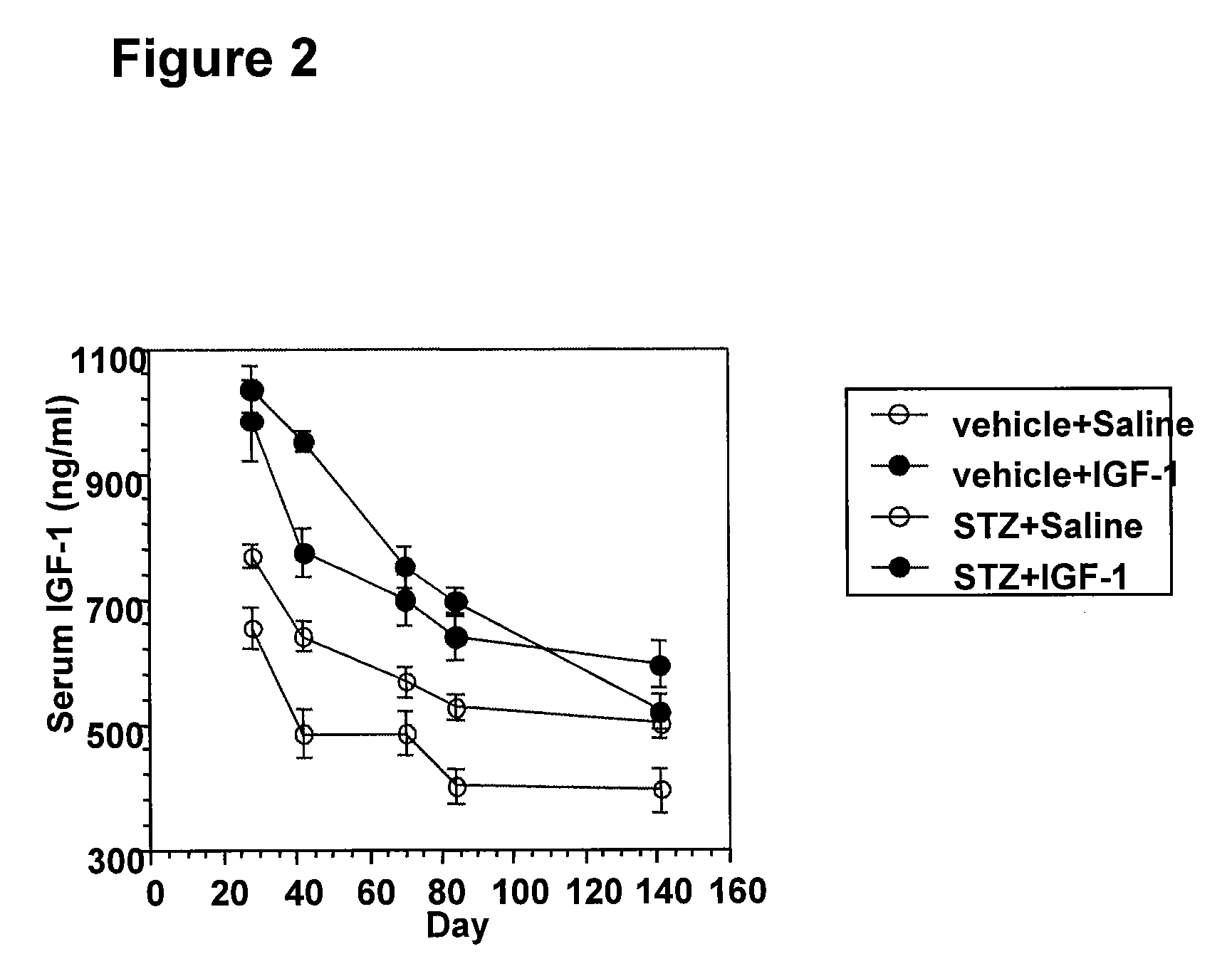
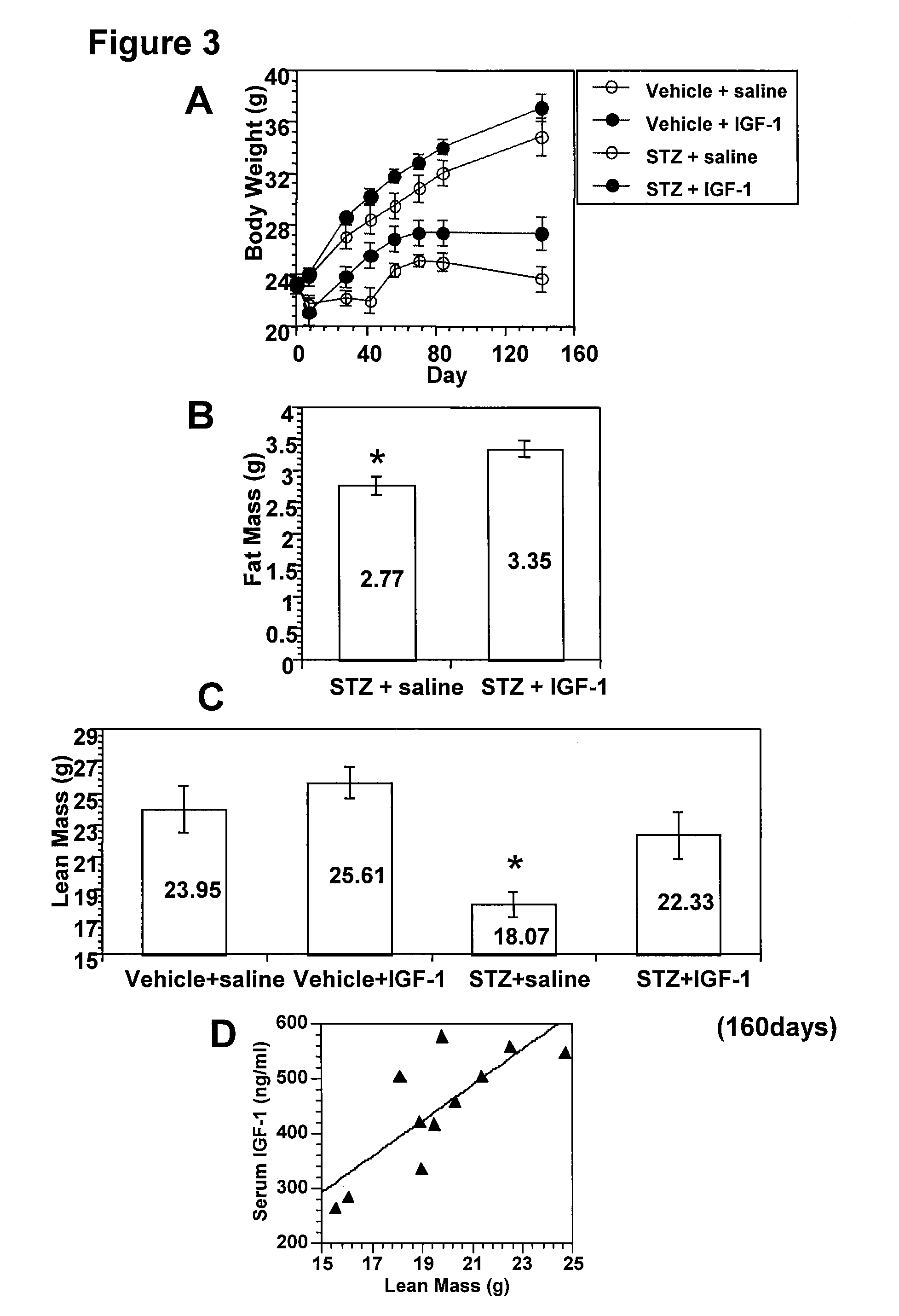
Systemic insulin-like growth factor-1 therapy reduces diabetic peripheral neuropathy and improves renal function in diabetic nephropathy
InactiveUS20100216709A1Prevents subsequent hyposensitivityEasy maintenanceOrganic active ingredientsNervous disorderInsulin-like growth factorHyperglycemic disorder
The present invention provides methods of treatment of patients suffering from the complications of blood sugar disorders: diabetic peripheral neuropathy and diabetic nephropathy by administration of IGF-1 via protein therapy or gene therapy. It relates to methods of treating an individual having a diabetic disorder or a hyperglycemic disorder, comprising administering to the individual an effective amount of a DNA vector expressing IGF-1Eb or IGF-1Ec in vivo or an effective amount of at the IGF-1Eb or IGF-1Ec protein in the early hyperalgesia stage or in patients that have advanced to the hyposensitivity stage. Treatment at the early hyperalgesia stage prevents subsequent hyposensitivity with increases or maintenance of sensory nerve function. IGF-1Eb or IGF-1Ec treatment also increases muscle mass and improves overall mobility, which indicates a treatment-related improvement in motor function. Treatment with IGF-1Eb or IGF-1Ec at the hyposensitivity stage reverses hyposensitivity and improves muscle mass and overall health. Systemic IGF-1 provides a therapeutic modality for treating hyposensitivity associated with DPN. In addition, IGF-1Eb or IGF-1Ec provides a therapeutic modality for treating diabetic nephropathy. IGF-1Eb or IGF-1Ec improves renal function as evidenced by a modulation in serum albumin concentration and a reduction in urine volume and protein levels. IGF-1Eb or IGF-1Ec also reduces diabetic glomerulosclerosis.
Owner:GENZYME CORP
Methods of modifying eukaryotic cells
A method for engineering and utilizing large DNA vectors to target, via homologous recombination, and modify, in any desirable fashion, endogenous genes and chromosomal loci in eukaryotic cells. These large DNA targeting vectors for eukaryotic cells, termed LTVECs, are derived from fragments of cloned genomic DNA larger than those typically used by other approaches intended to perform homologous targeting in eukaryotic cells. Also provided is a rapid and convenient method of detecting eukaryotic cells in which the LTVEC has correctly targeted and modified the desired endogenous gene(s) or chromosomal locus (loci) as well as the use of these cells to generate organisms bearing the genetic modification.
Owner:REGENERON PHARM INC
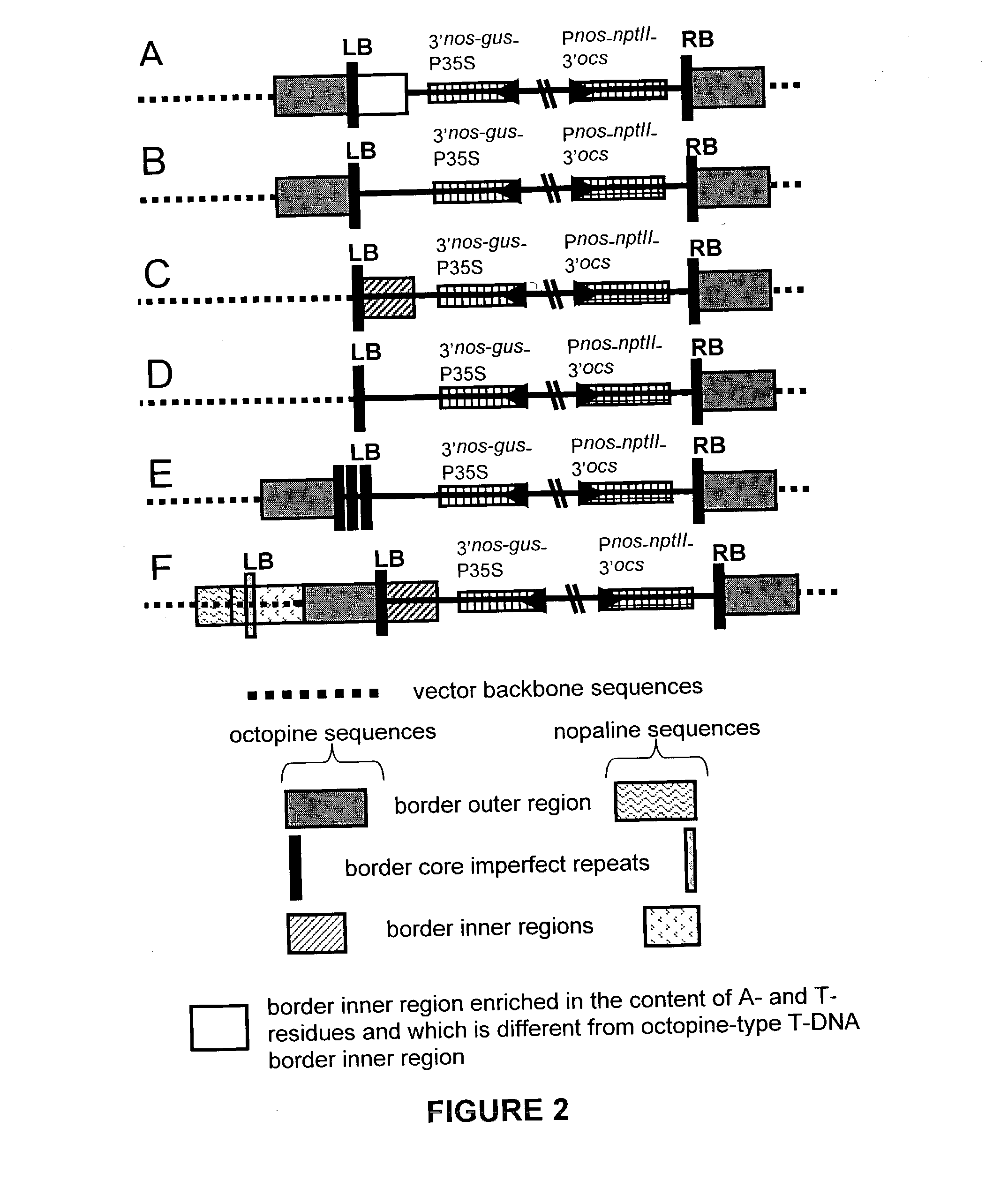
Optimized t-dna transfer and vectors therefor
The present invention relates to T-DNA vectors and methods for obtaining transgenic eukaryotes using said vectors. The transgenic eukaryotes are characterized in that they contain the T-DNA but not the illegitimately transferred vector backbone sequence. This is achieved by modifying the T-DNA borders such that they are more efficiently nicked or such that they allow elimination of illegitimately transferred vector backbone sequences by means of recombination.
Owner:CROPDESIGN NV
Method for detecting nucleic acid or cells based on enzymatic cycle amplification and nano-particle reinforced SPR (surface plasmon resonance)
InactiveCN105648070ARealize highly sensitive detectionIncrease loadMicrobiological testing/measurementBiological testingMagnetic beadBarcode
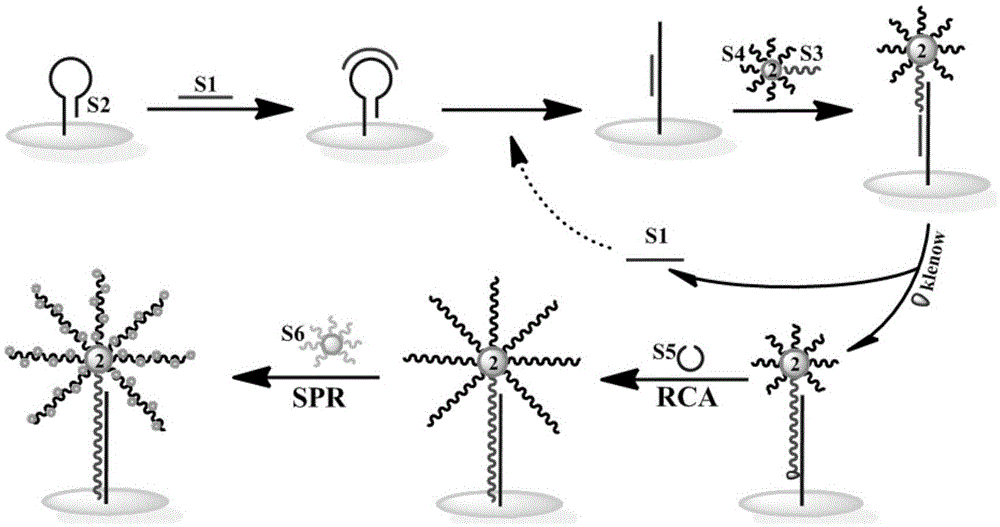
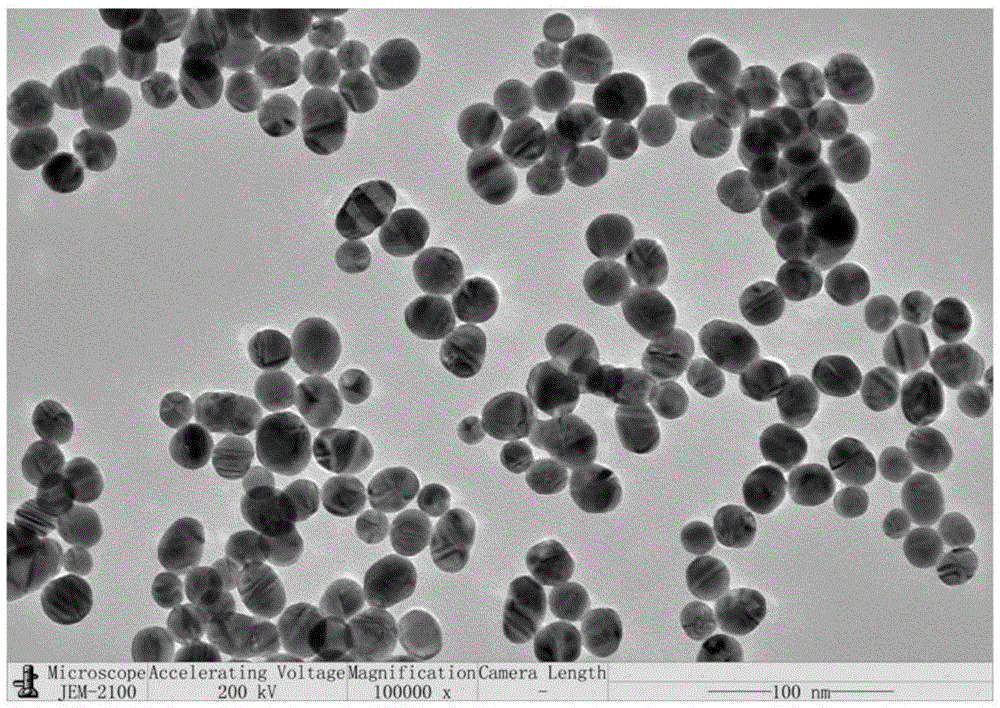
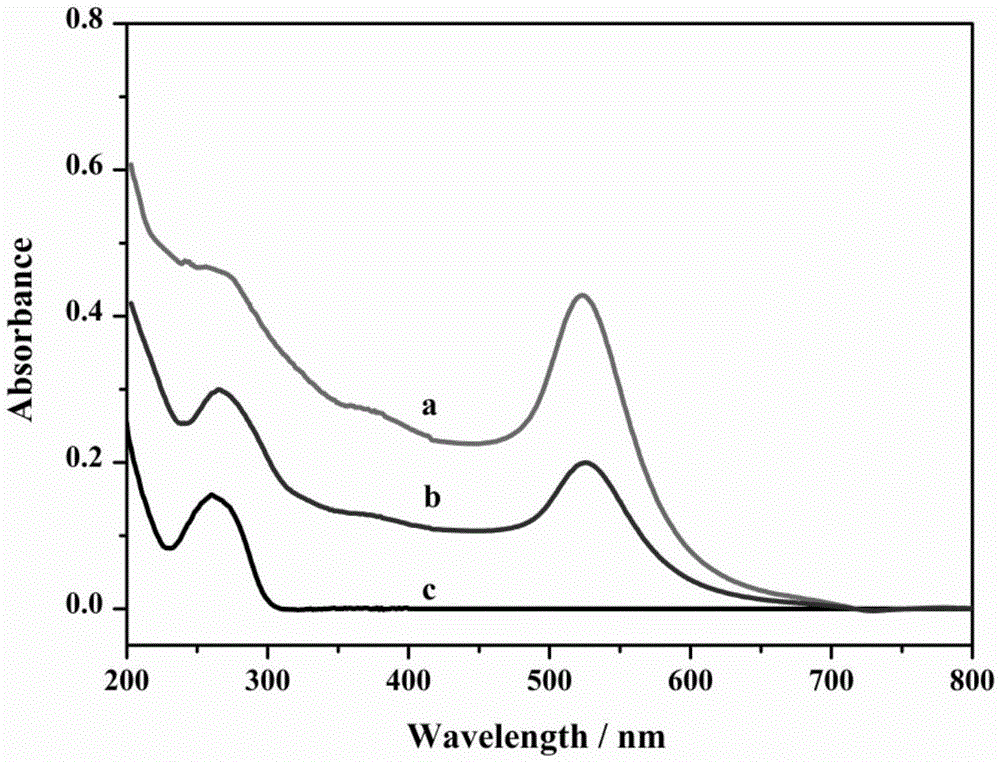
The invention discloses a method for detecting nucleic acid or cells based on enzymatic cycle amplification and nano-particle reinforced SPR (surface plasmon resonance). Strand displacement cycle amplification and rolling circle replication amplification are triggered by a target DNA (deoxyribonucleic acid), a double-signal amplification reaction is formed, a magnetic bead is taken as a DNA carrier and serves as a transfer station for signal amplification, a magnetic bead bio-barcode probe has a strand displacement cycle amplification reaction as a reaction unit and also increases the load capacity of a rolling circle replication product as a signal amplification carrier, a multi-stage signal amplification system is formed, high-sensitivity detection of the target DNA is realized with gold nano-particles as signal probes with an SPR technique, and the limit of detection can reach 1fM.
Owner:QINGDAO UNIV OF SCI & TECH
Methods for treating blood coagulation disorders
InactiveUS7615537B2Optimally promote proper disulfide bondingPrecise BondingBiocideVirusesFactor VIIaA-DNA
The present invention relates to a method of treating an individual having a blood coagulation defect (e.g., hemophilia A, hemophilia B), comprising administering to the individual an effective amount of a DNA vector encoding modified Factor VII (FVII), wherein the modified Factor VII leads to generation of Factor VIIa in vivo. In a particular embodiment, the invention pertains to a method of treating an individual having a blood coagulation defect comprising administering to the individual an effective amount of a nucleic acid encoding a modified FVII wherein the modified FVII comprises a signal which codes for precursor cleavage by furin at the activation cleavage site of the modified FVII. The invention also relates to a method of treating an individual having a blood coagulation disorder comprising administering to the individual an effective amount of a nucleic acid encoding the light chain of human FVII and a nucleic acid encoding the heavy chain of human FVII operably linked to a leader sequence. Compositions, expression vectors and host cells comprising nucleic acid which encodes a modified Factor VII, wherein the modified Factor VII leads to generation of Factor VIIa in vivo is also encompassed by the present invention.
Owner:GENZYME CORP
One-step preparation method of multicolor fluorescent functionalized graphene quantum dots
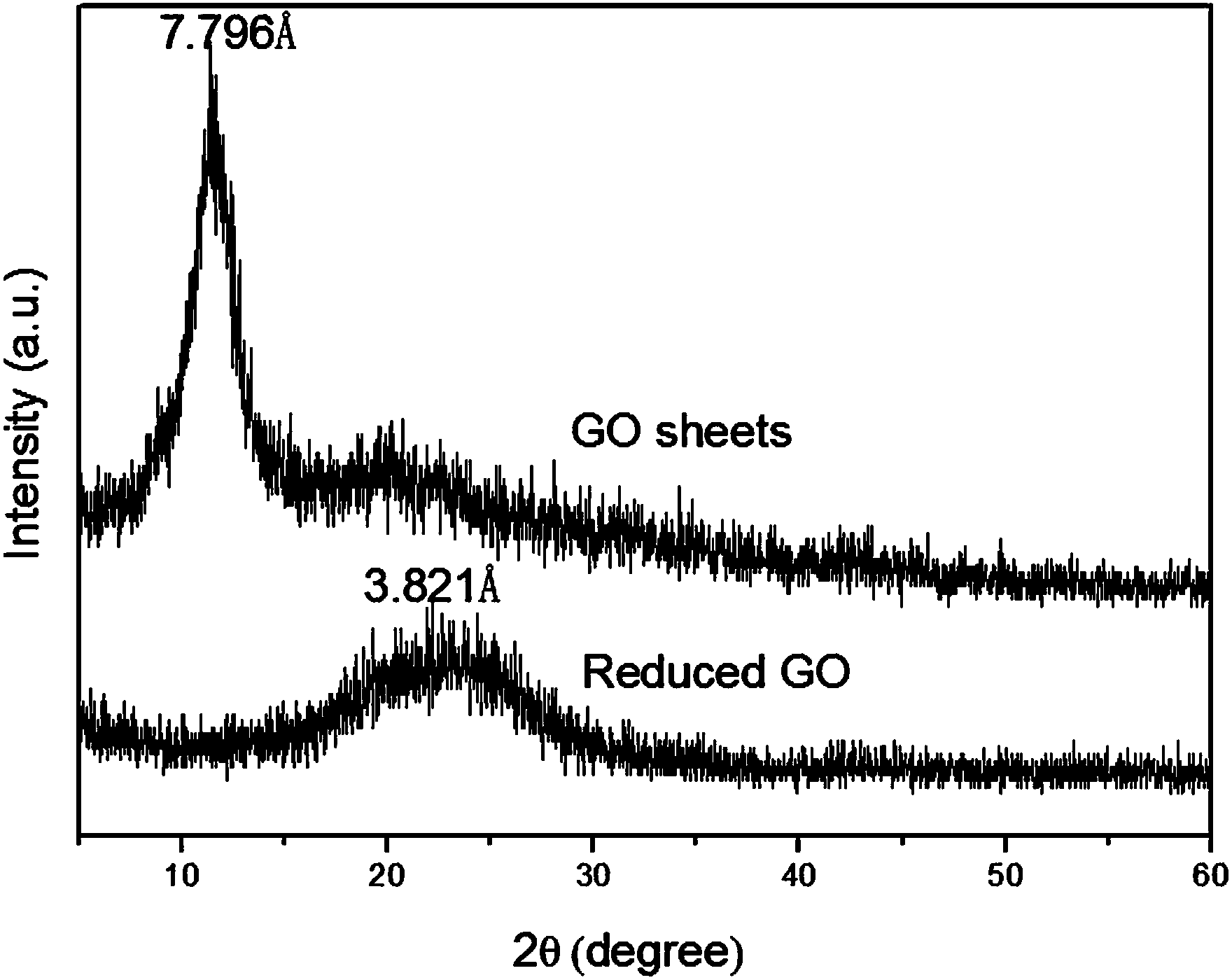
ActiveCN103980893AGood biocompatibilityHigh fluorescence intensityLuminescent compositionsQuantum yieldSolvent
The invention discloses a one-step preparation method of multicolor fluorescent functionalized graphene quantum dots, belonging to the technical field of nanomaterial preparation. The one-step preparation method comprises the following steps of with graphene oxide sheets as raw materials, dispersing the graphene oxide sheets into different solvents such as ethanol and the like; adding polyethyleneimine, and regulating the pH value to 12 by using ammonia water; after sufficiently stirring, carrying out heat treatment in a reaction kettle at the temperature of 100-200 DEG C for 1-24h; naturally cooling, and collecting filtrate after filtering; after drying, purifying through column chromatography and carrying out size separation to obtain functionalized graphene quantum dots with different fluorescence colors. The method provided by the invention is simple and convenient in synthesis process and high in efficiency; the obtained fluorescent functionalized graphene quantum dots are high in purity, moderate in monodispersity and particle size, water-soluble, strong in fluorescence property (the quantum yield is up to 18.1%) and expected to be applied to LED (Light Emitting Diode) membrane materials, fluorescence labeling, DNA (Deoxyribonucleic Acid) carriers, targeted drug carrying particles and the like.
Owner:TAIYUAN UNIV OF TECH
Method and device for sample preparation
InactiveUS8377715B2Easily plug columnsAvoid purificationMaterial nanotechnologyComponent separationFritPipette
The invention provides pipette tip extraction columns for the purification of a DNA vector from un-clarified cell lysate containing cell debris as well as methods for making and using such columns. The columns typically include a bed of extraction media positioned in the pipette tip column, above a bottom frit and with an optional top frit.
Owner:PHYNEXUS
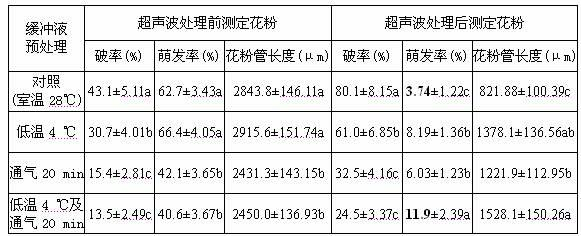
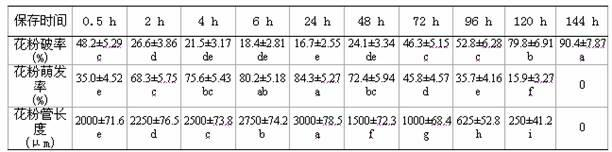
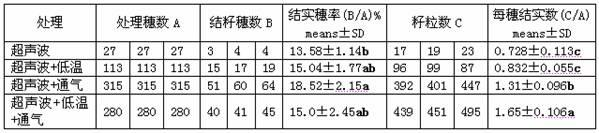
Ultrasonic-assisted pollen mediated plant genetic transformation method
InactiveCN102127567AImprove seed setting rateAvoid the cultivation processVector-based foreign material introductionEscherichia coliSaccharum
The invention relates to an improved ultrasonic-assisted pollen mediated plant genetic transformation method, and aims to obviously increase the setting percentage of the plant fertilized by pollen subjected to ultrasonic treatment, thereby increasing the number of transformants obtained for each treatment. The method comprises the following steps: by using an agrobacterium Ti plasmid carrying an exogenous gene segment, colibacillus plasmid or any other DNA vector as a genetic donor and using plant pollen as a receptor, mixing the pollen and exogenous DNA in a 5-50% sucrose solution subjected to aeration and low-temperature treatment, and transferring the exogenous gene into the receptor pollen under the assisting action of ultrasonic; fertilizing the treated pollen onto the stigma of the plant, and harvesting when the grains become ripe; and in the subsequent growth season, sowing the harvested seeds which are obtained after the fertilization of the transformed pollen, screening the germinated seeds and seedlings, carrying out PCR (Polymerase Chain Reaction) amplification and Southern hybridization on the DNA of a seedling sample, and further determining the transformant. The method provided by the invention does not need tissue culture, does not have species or genotype dependency, and can shorten the genetic transformation breeding time and save the manpower and material resources.
Owner:AGRI BIOTECH RES CENT OF SHANXI PROVINCE
Methods for nucleic acid mapping and identification of fine-structural-variations in nucleic acids
ActiveUS8329400B2Quick buildEfficient sortingSugar derivativesMicrobiological testing/measurementA-DNADigestion
A method of juxtaposing sequence tags (GVTs) that are unique positional markers along the length of a population of target nucleic acid molecules is provided, the method comprising: fragmenting the target nucleic acid molecule to form target DNA insert; ligating the target DNA insert to a DNA vector or backbone to create a circular molecule; digesting the target DNA insert endonuclease to cleave the target DNA insert at a distance from each end of the target DNA insert yielding two GVTs comprising terminal sequences of the target DNA insert attached to an undigested linear backbone; recircularizing the linear backbone with the attached GVTs to obtain a circular DNA containing a GVT-pair having two juxtaposed GVTs; and recovering the GVT-pair DNA by nucleic acid amplification or digestion with endonuclease having sites flanking the GVT-pair. Cosmid vectors are provided for creating GVT-pairs of ˜45- to 50-kb separation sequencable by next-generation DNA sequencers.
Owner:VERSITECH LTD
Small interfering RNA and pharmaceutical composition for treatment of hepatitis b comprising the same
InactiveUS20100063132A1Inhibit expressionOrganic active ingredientsSugar derivativesA-DNATherapeutic treatment
The present invention relates to RNA interference mediated inhibition of Hepatitis B virus (HBV) by short interfering RNA (siRNA) molecules. Specially, siRNAs of the present invention which are double-stranded RNAs concern directing the sequence-specific degradation of viral RNA in mammalian cells. Disclosed is a DNA vector encoding the RNA molecules and synthesized siRNA molecules as well as method of therapeutic treatment for inhibition of HBV gene expression and viral replication by the administration of RNA molecules of the present invention.
Owner:MOGAM BIOTECH RES INST
Hyaluronic acid-based cross-linked nanoparticles
InactiveUS7879818B2Reaction is slowReduce crosslink densityPowder deliveryOrganic active ingredientsCross-linkBiopolymer
Methods are disclosed for preparing novel biodegradable cross-linked nanoparticles based on covalently cross-linking modifications of hyaluronic acid. The final products of the present invention are stable in aqueous media, and may be used as detergents and as additives for pharmaceutical compositions for drug delivery, DNA carrier system and other applications. The nanoparticles made from the biopolymers of the present invention may also be used in controlled release applications, super-absorbent materials as well as biomaterials like enzyme immobilization.
Owner:BORBELY JANOS +3
Method for isolating DNA
Method for isolating DNA contained in a biological sample. The method includes combining in a solution a DNA-containing biological sample, a salt, a cationic surfactant, and a DNA-binding carrier, the solution having a salt concentration higher than the DNA precipitation inhibition-initiating concentration, to lyse the DNA-containing biological sample and to bind DNA to the DNA-binding carrier while in the solution to form a bound DNA-carrier. The method also includes separating the DNA-bound carrier from other components. The method further includes dissociating the bound DNA from the DNA-binding carrier. The method still further includes recovering dissociated DNA.
Owner:RIKEN
Method of introducing a plurality of DNA fragments simultaneously into DNA vector
InactiveCN101016551AImprove recombination efficiencyVector-based foreign material introductionCompetent cellDNA fragmentation
The invention discloses a method to introducing multiple DNA fragment in DNA carrier, which comprises the following steps: augmenting two or more goal DNA fragment with oligonucleotide isogeneic limb through polyose chain-reaction; transforming to permissive state cell with recombinant enzyme vector and armed reforming carrier; getting the reforming carrier with one step through consangnuinity reconstruction of oligonucleotide; making the first fragment 5' end of goal DNA fragment possesses the same sequence with armed replacing position left side of target carrier; setting the last 3' end possesses the same sequence with armed replacing position left side of target carrier; making each fragment 5' end possesses the reversing complementary sequence with the last fragment 3' end from the second fragment. The method of this invention is simple, which can be a general method to reform DNA carrier.
Owner:北京华诺奥美基因生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com