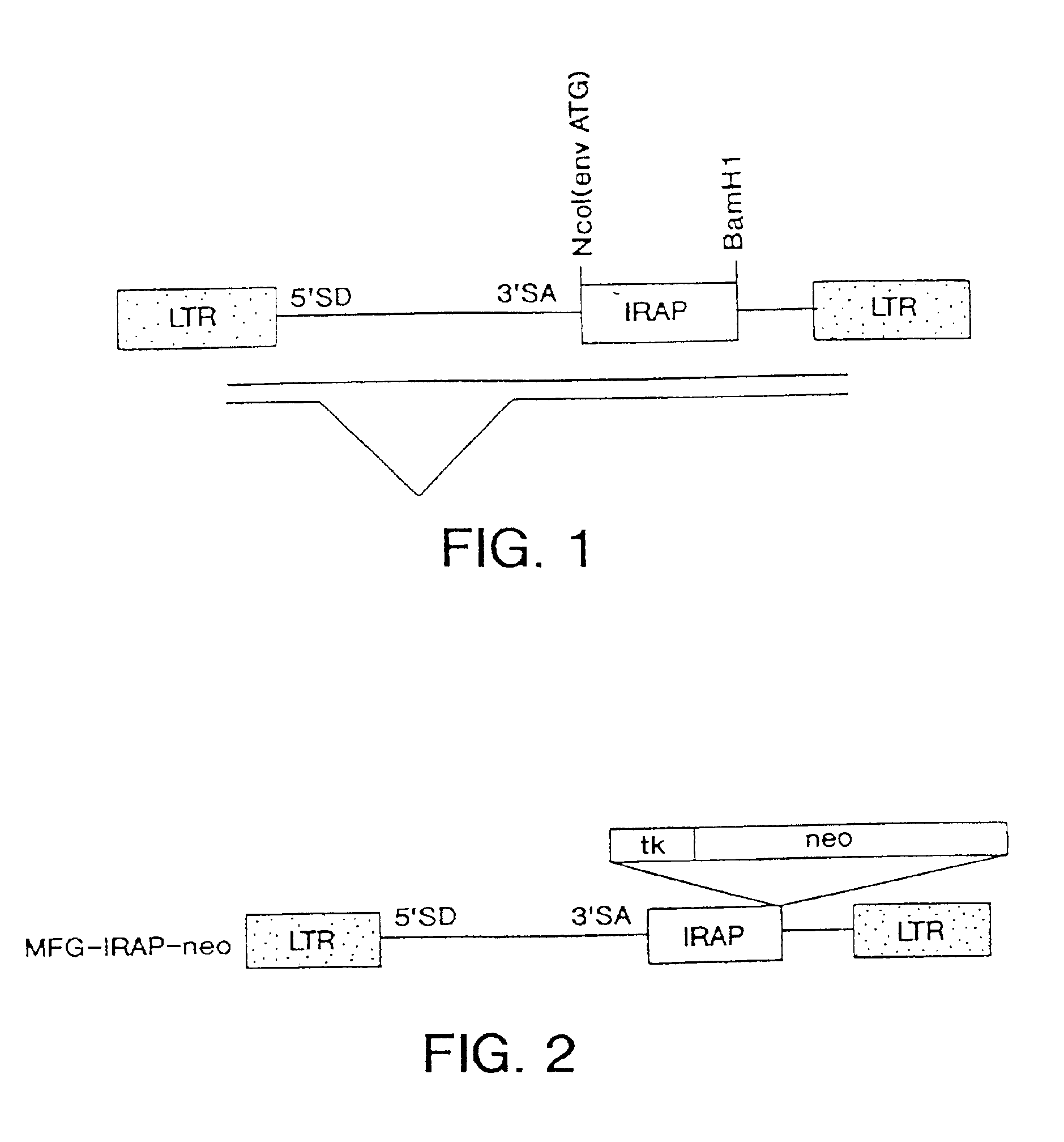
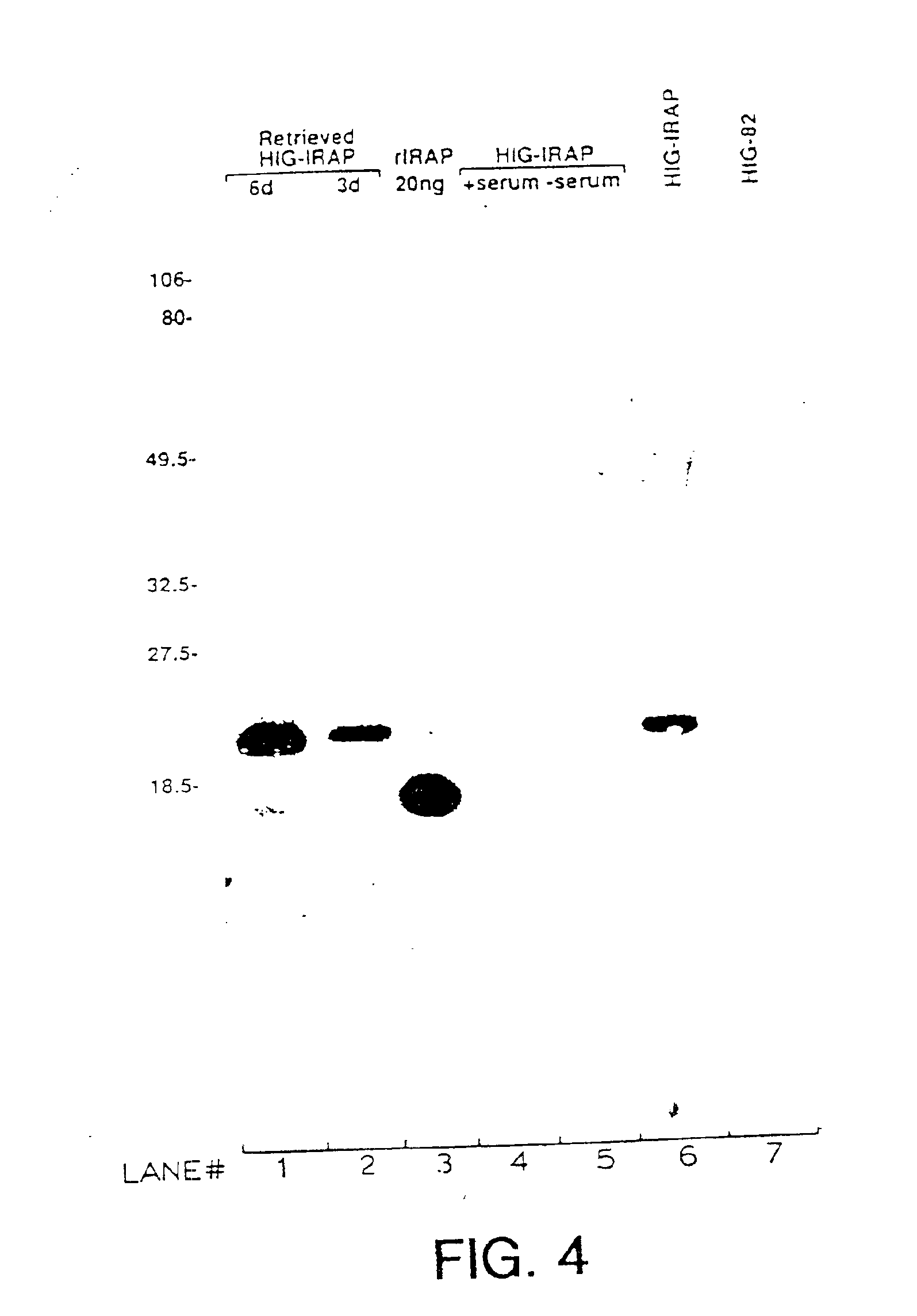
Gene transfer for studying and treating a connective tissue of a mammalian host
a technology of connective tissue and gene transfer, which is applied in the field of gene transfer for studying and treating the connective tissue of a mammalian host, can solve the problems of less efficient diffusion with the increase of the target molecule size, affecting the therapeutic intervention of arthritis, and affecting the survival rate of the host, so as to prevent arthritis, resist the degradation of cartilage, and improve the survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0151]The following examples are intended to illustrate the present invention, and should not be construed as limiting the invention in any way.
example i
Packaging of AAV
[0152]The only cis-acting sequences required for replication and packaging of recombinant adeno-associated virus (AAV) vector are the AAV terminal repeats. Up to 4 kb of DNA can be inserted between the terminal repeats without effecting viral replication or packaging. The virus rep proteins and viral capsid proteins are required in trans for virus replication as is an adeno-associated virus helper. To package a recombinant AAV vector, the plasmid containing the terminal repeats and the therapeutic gene is co-transfected into cells with a plasmid that expresses the rep and capsid proteins. The transfected cells are then infected with adeno-associated virus and virus isolated from the cells about 48-72 hours post-transfection. The supernatants are heated to about 56° Centigrade to inactivate the adeno-associated virus, leaving an active virus stock of recombinant AAV.
example ii
[0153]The connective tissue cells to be electroporated are placed into Herpes buffer saline (HBS) at a concentration of about 107 cells per ml. The DNA to be electroporated is added at a concentration of about 5-20 ug / ml of HBS. The mixture is placed into a cuvette and inserted into the cuvette holder that accompanies the Bio-RAD electroporation device (1414 Harbour Way South, Richmond, Calif. 94804). A range between about 250 and 300 volts at a capacitance of about 960 ufarads is required for introduction of DNA into most eukaryotic cell types. Once the DNA and the cells are inserted into the Bio-RAD holder, a button is pushed and the set voltage is delivered to the cell-DNA solution. The cells are removed from the cuvette and replated on plastic dishes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com