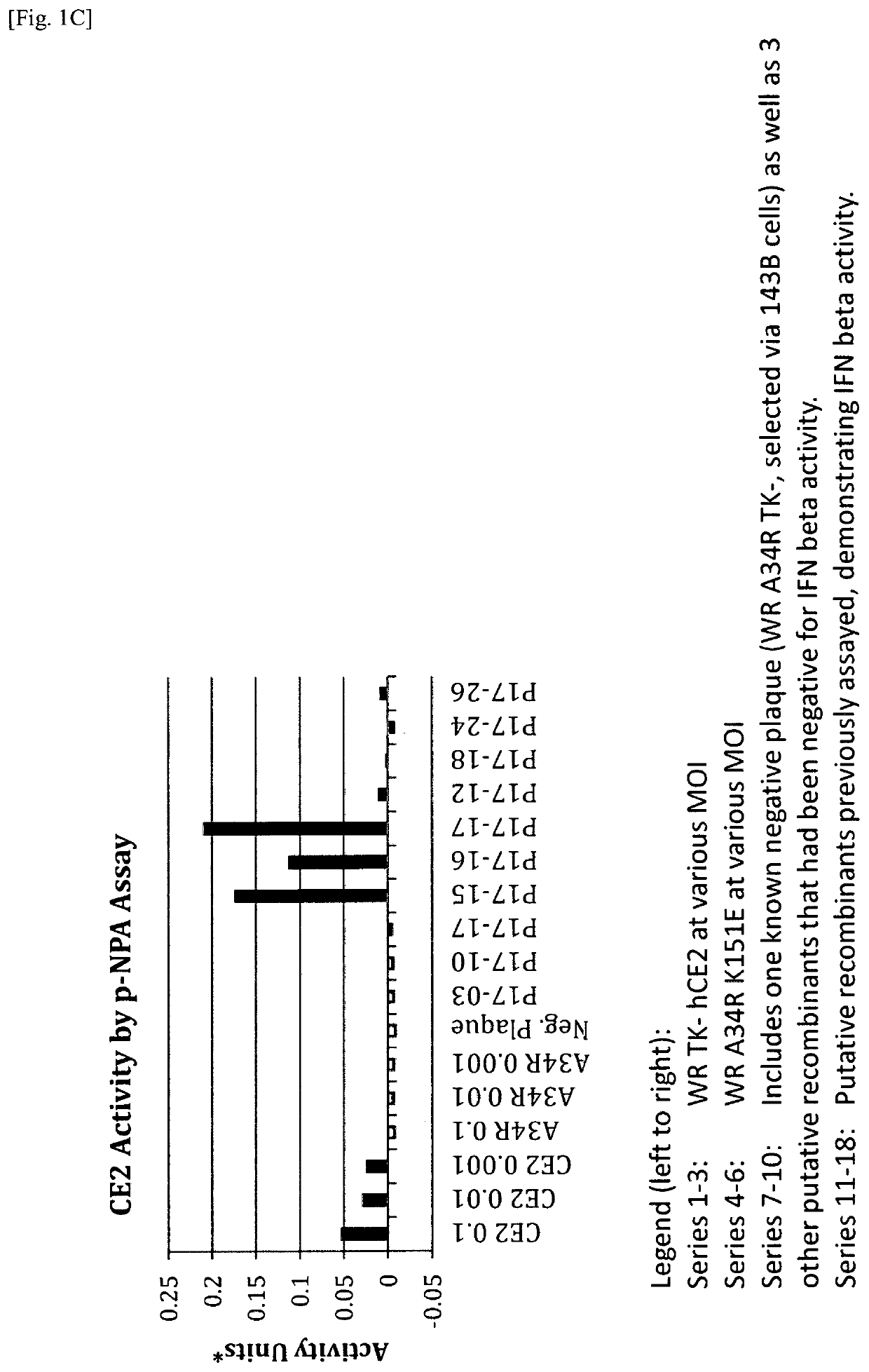
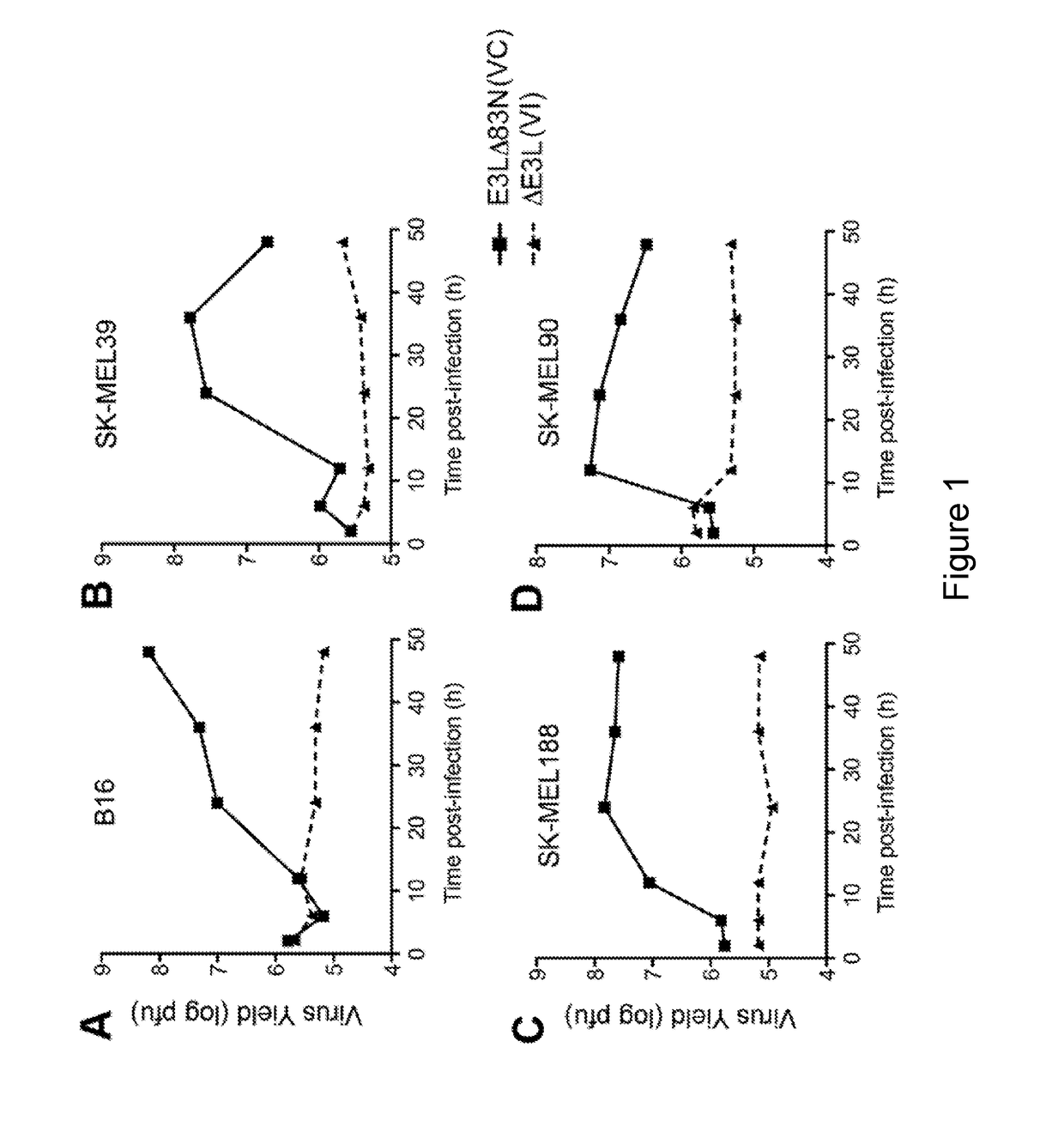
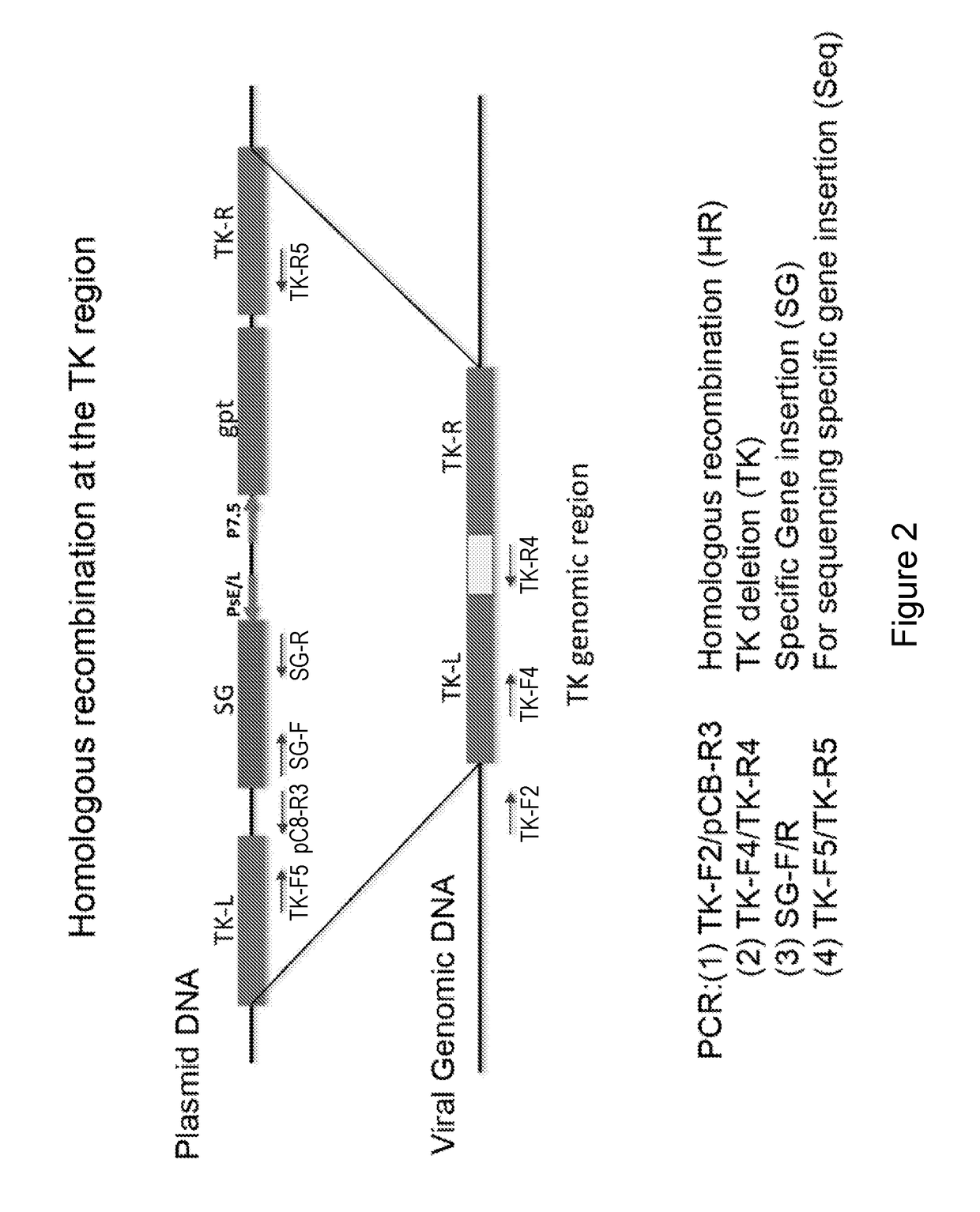
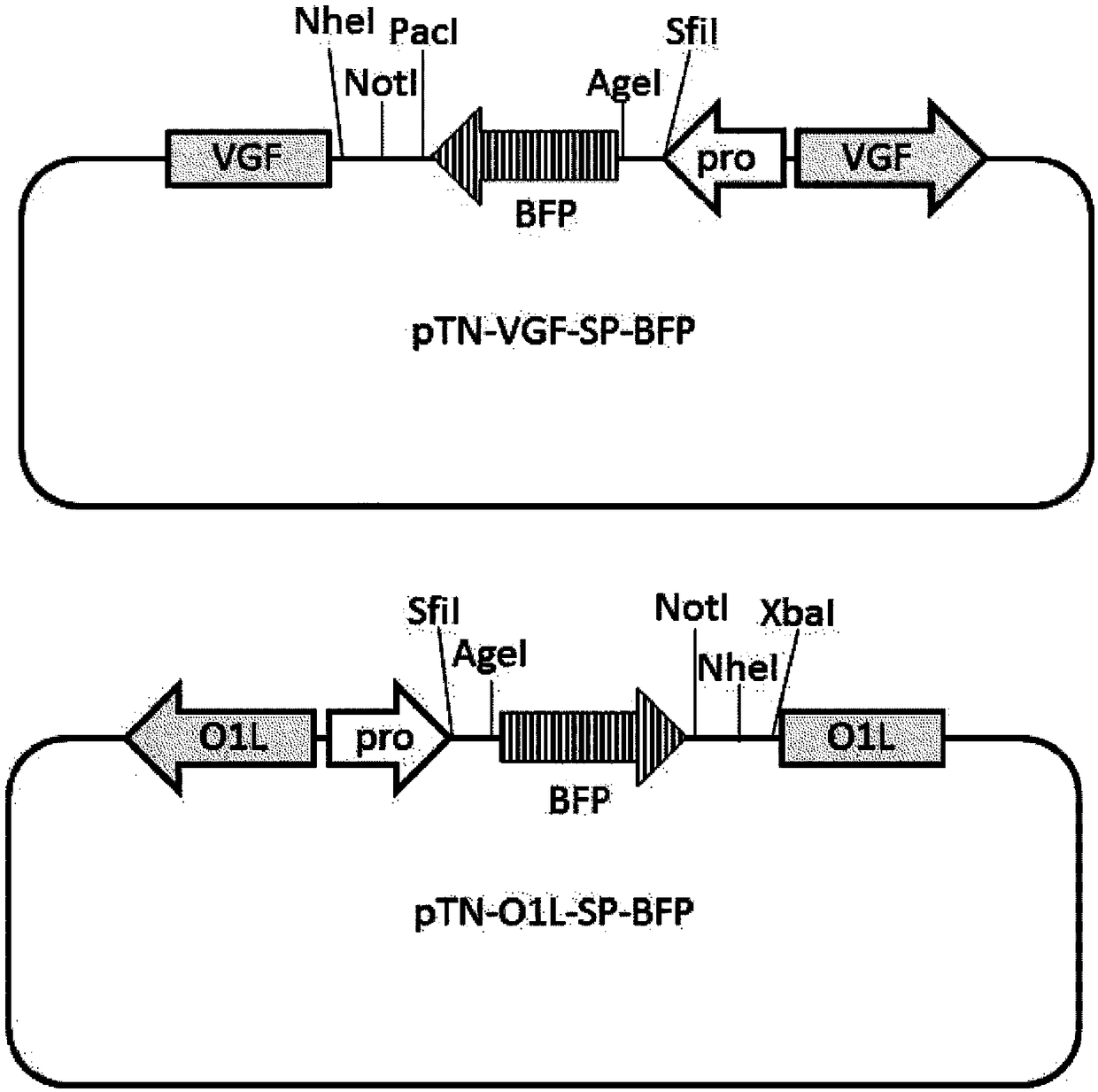
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Vaccinia viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clonal strains of attenuated vaccinia viruses and methods of use thereof
Clonal strains of vaccinia viruses are provided. Also provided are methods of identifying and isolating attenuated and oncolytic clonal strains from virus preparations, Modified recombinant forms of the clonal strains also are provided. The clonal strains and virus preparations can be used for diagnostic and therapeutic methods, in particular for therapy and diagnosis or monitoring treatment of proliferative disorders, including neoplastic diseases, such as, but are not limited to, solid tumors and blood cancers.
Owner:GENELUX
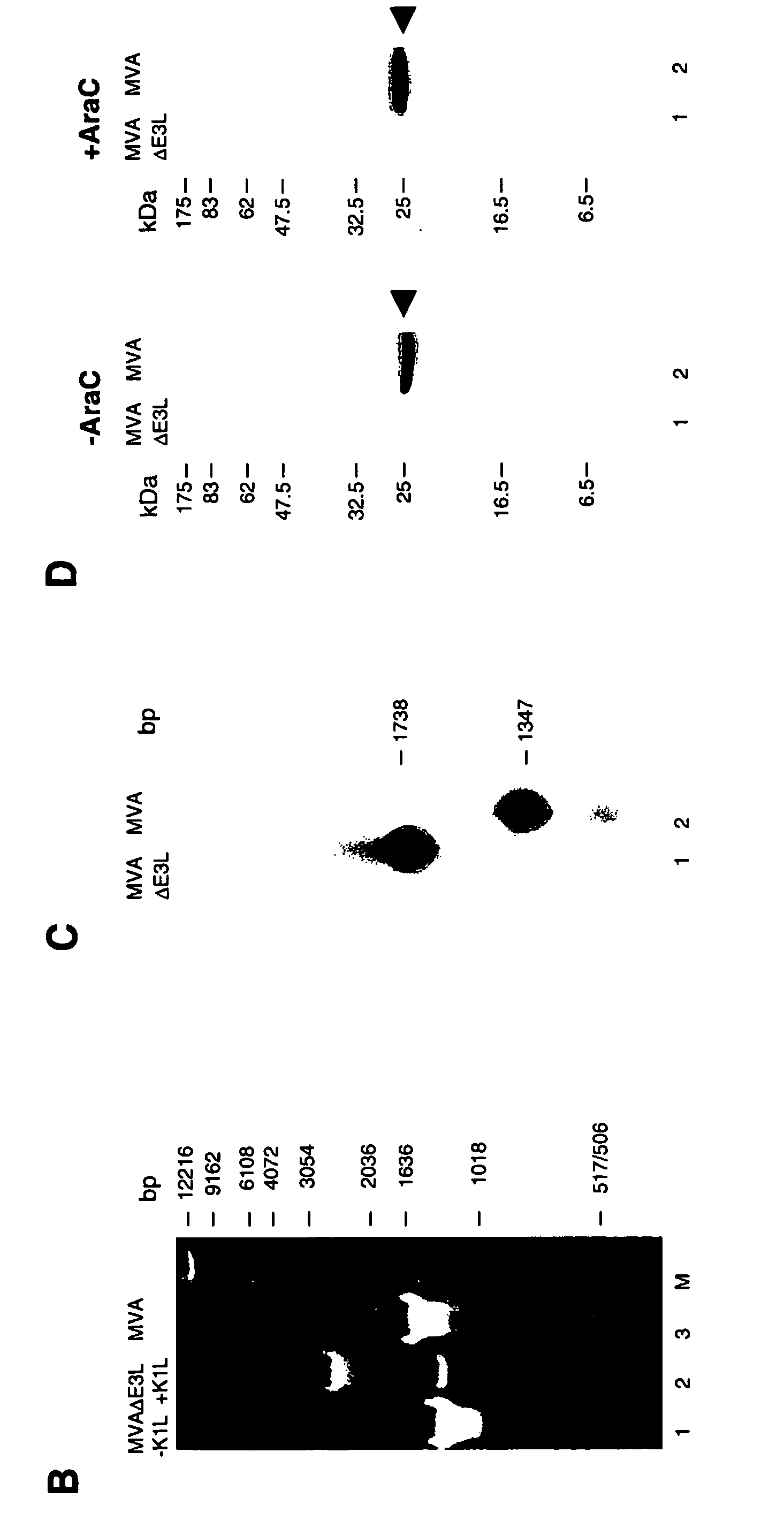
Vaccinia virus MVA-E3L-knockout-mutants and use thereof
InactiveUS7049145B2Quickly and efficiently produceIncrease choiceInactivation/attenuationVertebrate cellsMutantVaccinia viruses
The present invention relates to mutant MVA vaccinia viruses, which are used for the generation of recombinant MVA viruses, as well as host cells, which have been infected with these mutant MVA viruses. The present invention further relates to DNA-vector constructs, and a method for the generation of recombinant MVA by using the mutant MVA viruses and the DNA-vector constructs. The mutant MVA vaccinia viruses of the present invention are characterized in that the MVA ORF 050L gene or a functional part thereof has been inactivated in the viral genome.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
Immuno-Oncolytic Therapies
ActiveUS20160235793A1Reduced responseImprove immunityViral antigen ingredientsUnknown materialsAbnormal tissue growthInterleukin-18 binding protein
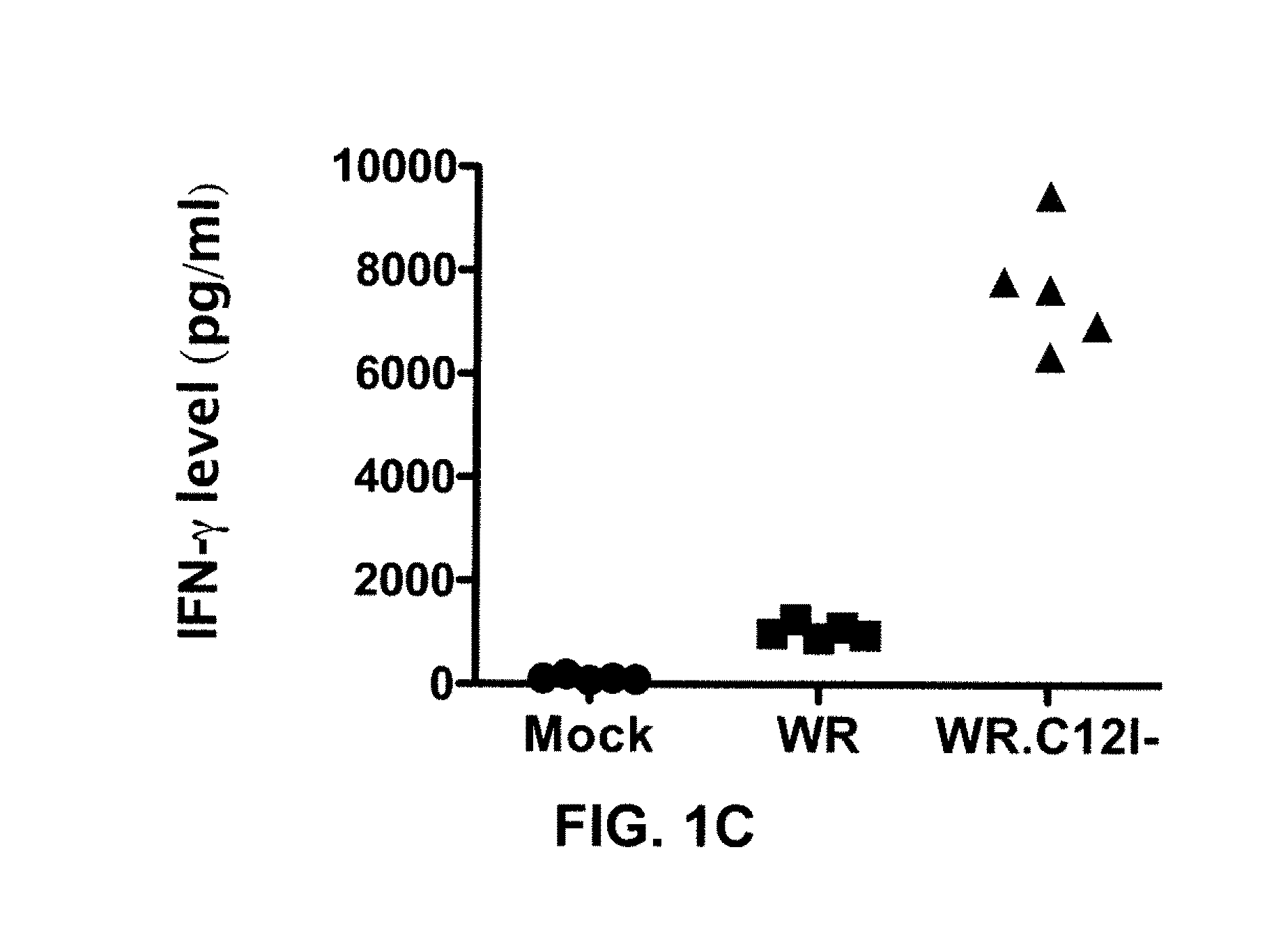
The present invention relates to oncolytic vaccinia viruses which have been modified to promote anti-tumor immunity and / or reduce host immunity and / or antibody response against the virus. It is based, at least in part, on the discovery that oncolytic vaccinia virus (i) bearing a genome deletion of a gene that reduces T cell immunity (interleukin-18 binding protein); (ii) treated with a sialidase enzyme which is believed to reduce TLR2 activation and therefore the antibody response; (iii) carrying a gene that enhances cytotoxic T lymphocyte induction (e.g., TRIF) and / or (iv) reduces tumor myeloid-derived suppressor cells by reducing prostaglandin E2 reduces tumor growth. Accordingly, the present invention provides for immunooncolytic vaccinia viruses and methods of using them in the treatment of cancers.
Owner:UNIVERSITY OF PITTSBURGH
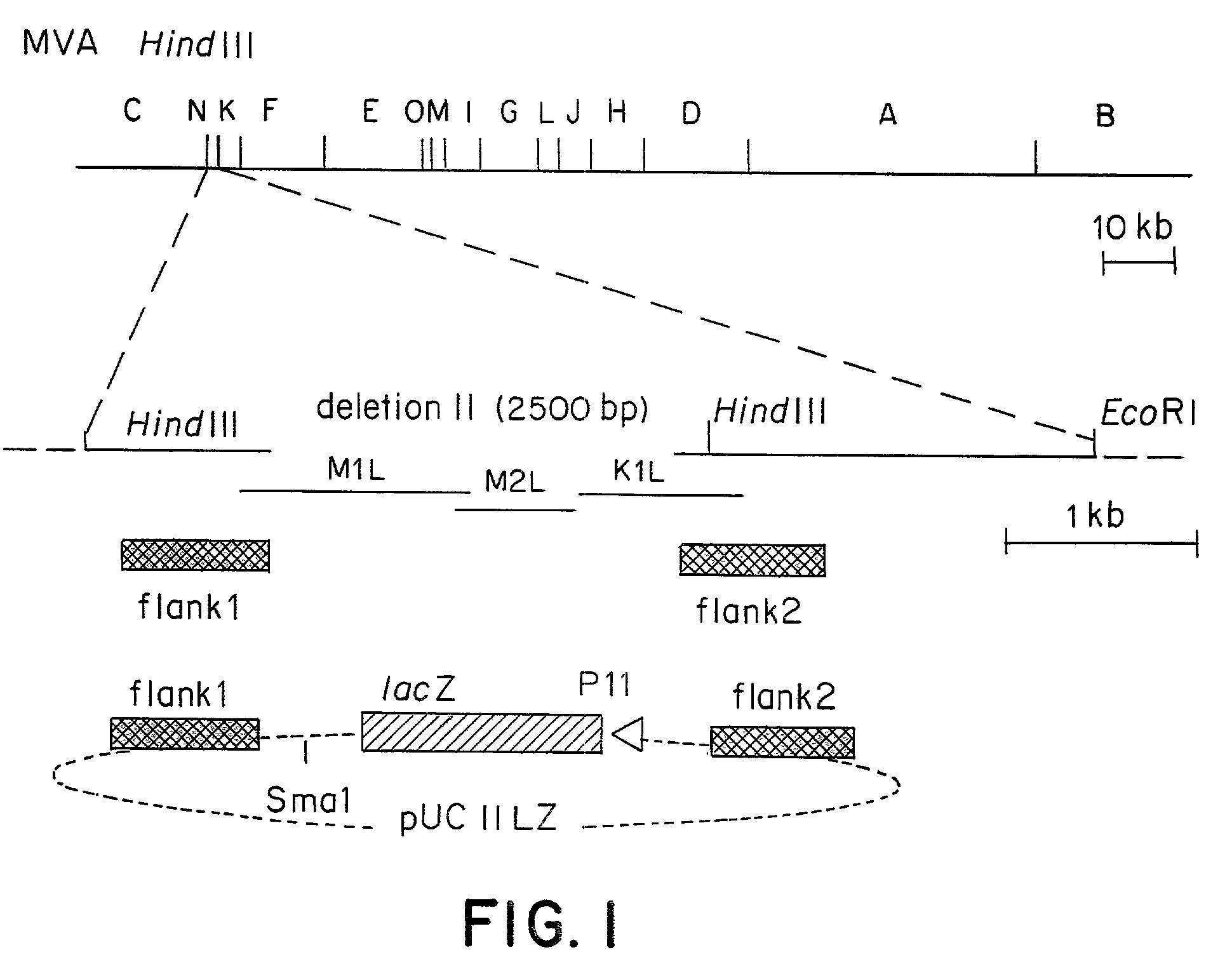
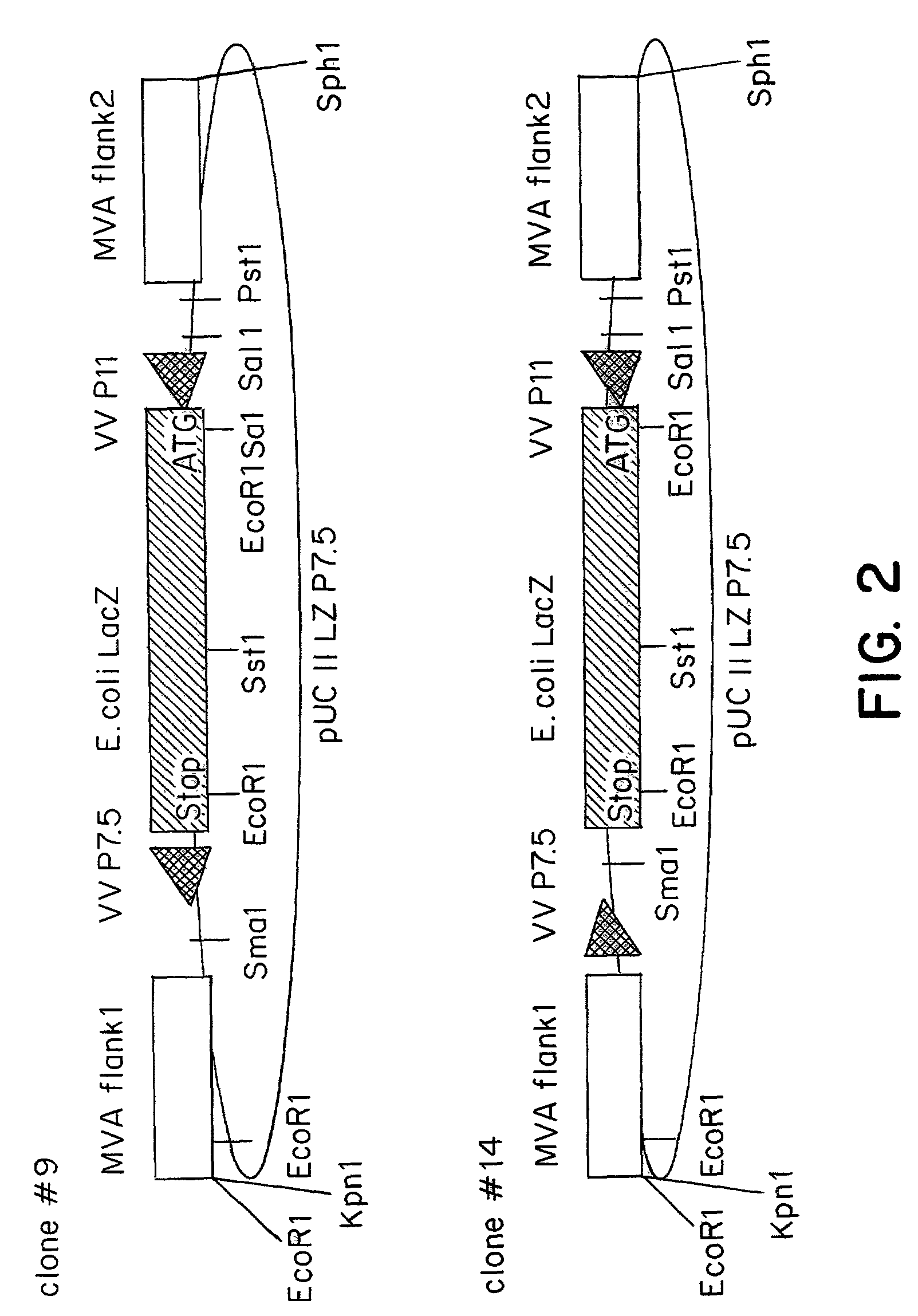
Recombinant MVA virus, and the use thereof
InactiveUS7198934B2Improve efficiencyReduce the amount of solutionOrganic active ingredientsVirusesAntigenViral vector
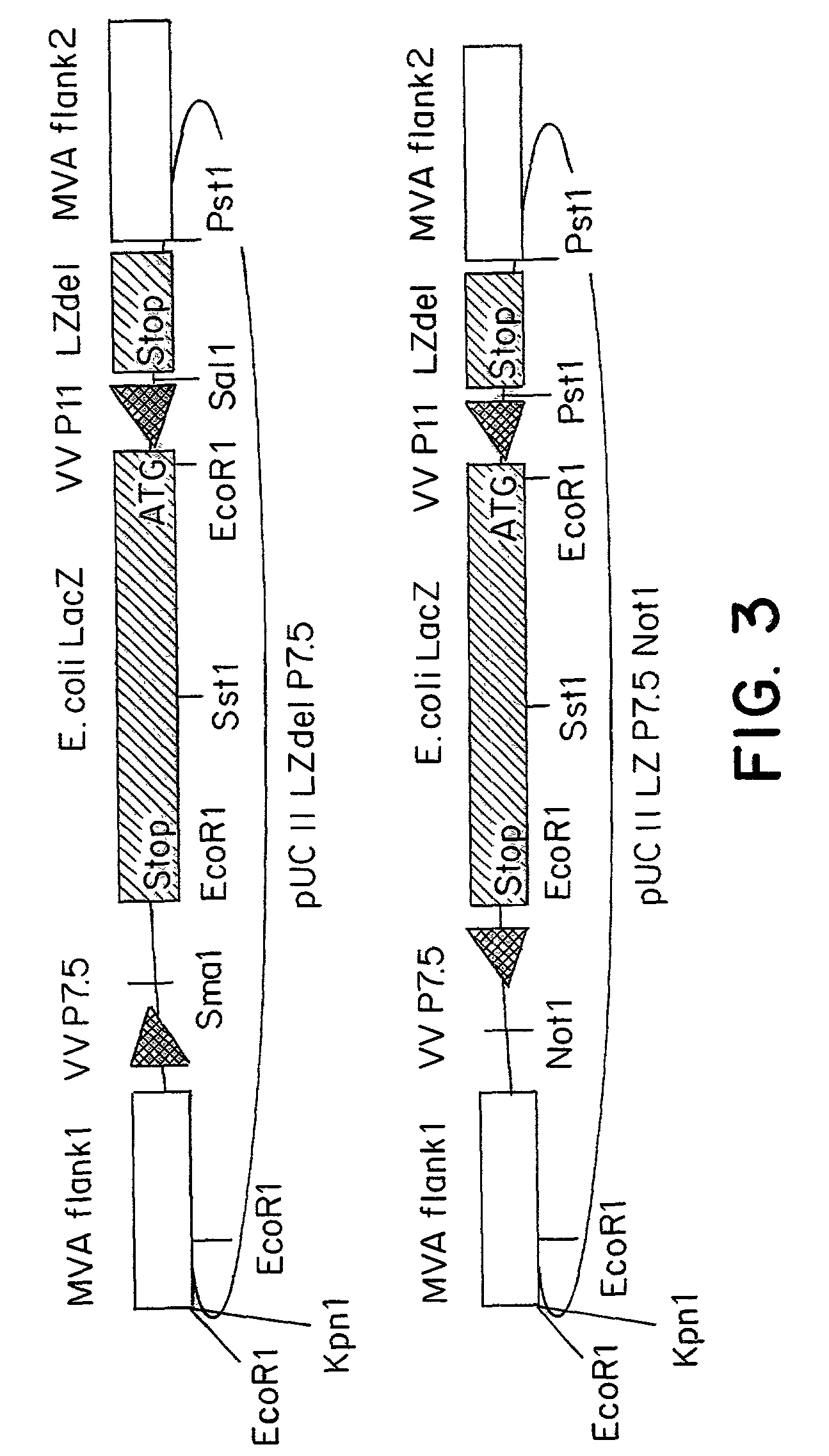
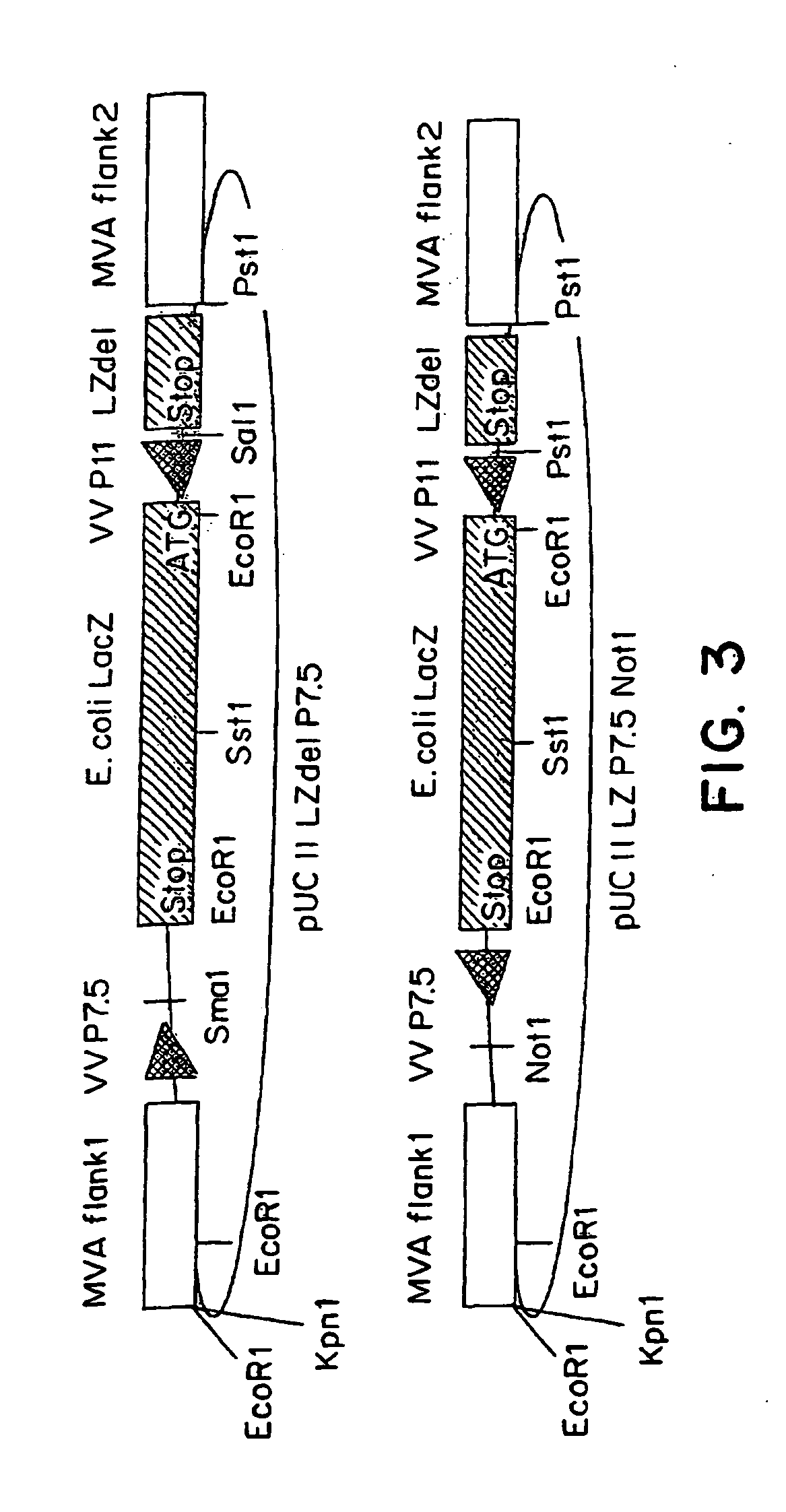
The present invention relates to recombinant vaccinia viruses derived from the modified vaccinia virus Ankara (MVA) and containing and capable of expressing foreign genes which are inserted at the site of a naturally occurring deletion in the MVA genome, and the use of such recombinant MVA viruses for the production of polypeptides, e.g. antigens or therapeutic agents, or viral vectors for gene therapy, and the use of such recombinant MVA viruses encoding antigens as vaccines.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
Recombinant viral-based malaria vaccines
Owner:JANSSEN VACCINES & PREVENTION BV
Recombinant MVA virus, and the use thereof
InactiveUS20070071769A1Improve efficiencyReduce the amount of solutionOrganic active ingredientsVirusesAntigenViral vector
The present invention relates to recombinant vaccinia viruses derived from the modified vaccinia virus Ankara (MVA) and containing and capable of expressing foreign genes which are inserted at the site of a naturally occurring deletion in the MVA genome, and the use of such recombinant MVA viruses for the production of polypeptides, e.g. antigens or therapeutic agents, or viral vectors for gene therapy, and the use of such recombinant MVA viruses encoding antigens as vaccines.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
T-cell vaccination with viral vectors via mechanical epidermal disruption
ActiveUS8691502B2Strong immune responseProtected growthAntibacterial agentsAntimycoticsAdjuvantCuticle
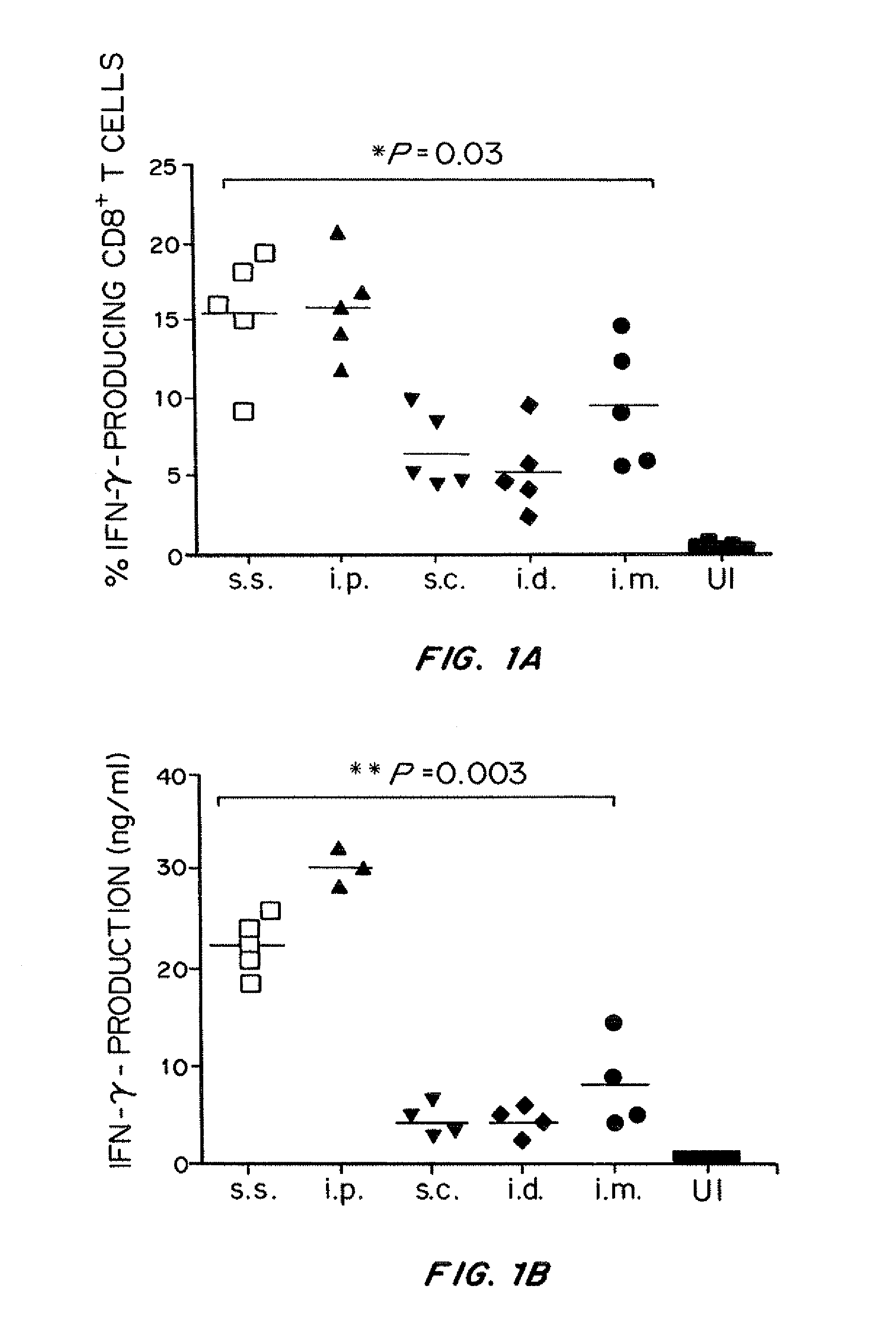
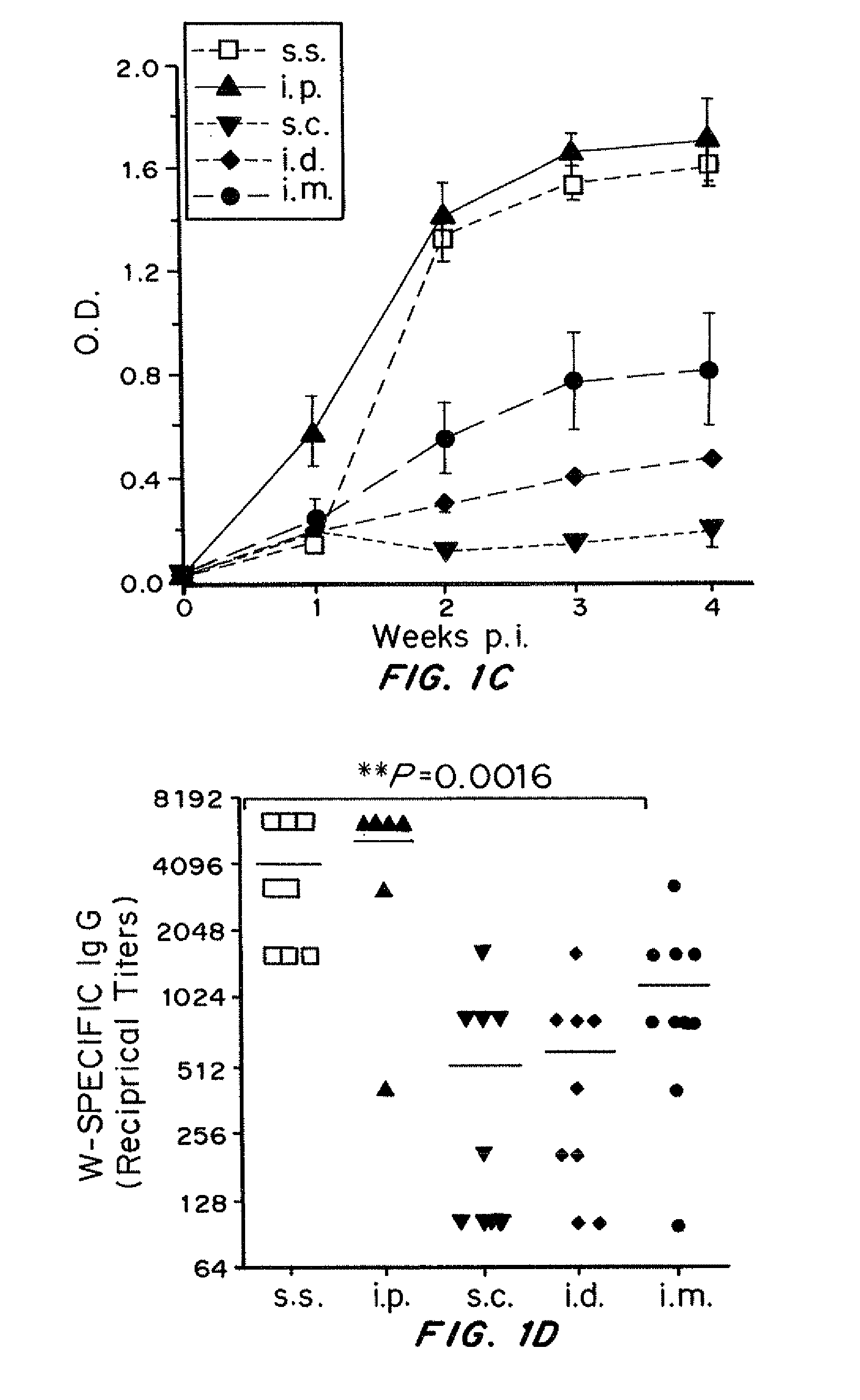
Attenuated, replication-deficient viruses such as vaccinia viruses are used to deliver an exogenous viral, bacterial, parasitic or tumor antigen to an epidermal tissue such as the skin, lungs or gastrointestinal tract, which has been mechanically disrupted, in an amount effective to elicit or stimulate a cell mediated immune response. The epidermal tissue may be mechanically disrupted by a device such as a scarification needle or an abrader device. The epidermis may be mechanically disrupted prior to, at the same time, or immediately after the administration of the vaccine. The vaccine can be used to induce immunity against a pathogen, such as a virus, bacteria, or parasite, or against a cancer in a subject that has or is at risk of developing cancer. In some embodiments a co-stimulatory molecule, a growth factor, an adjuvant and / or a cytokine is administered before, with or after the viral vaccine. In some embodiments, the co-stimulatory molecule is co-expressed with the antigen by the virus.
Owner:TREMRX
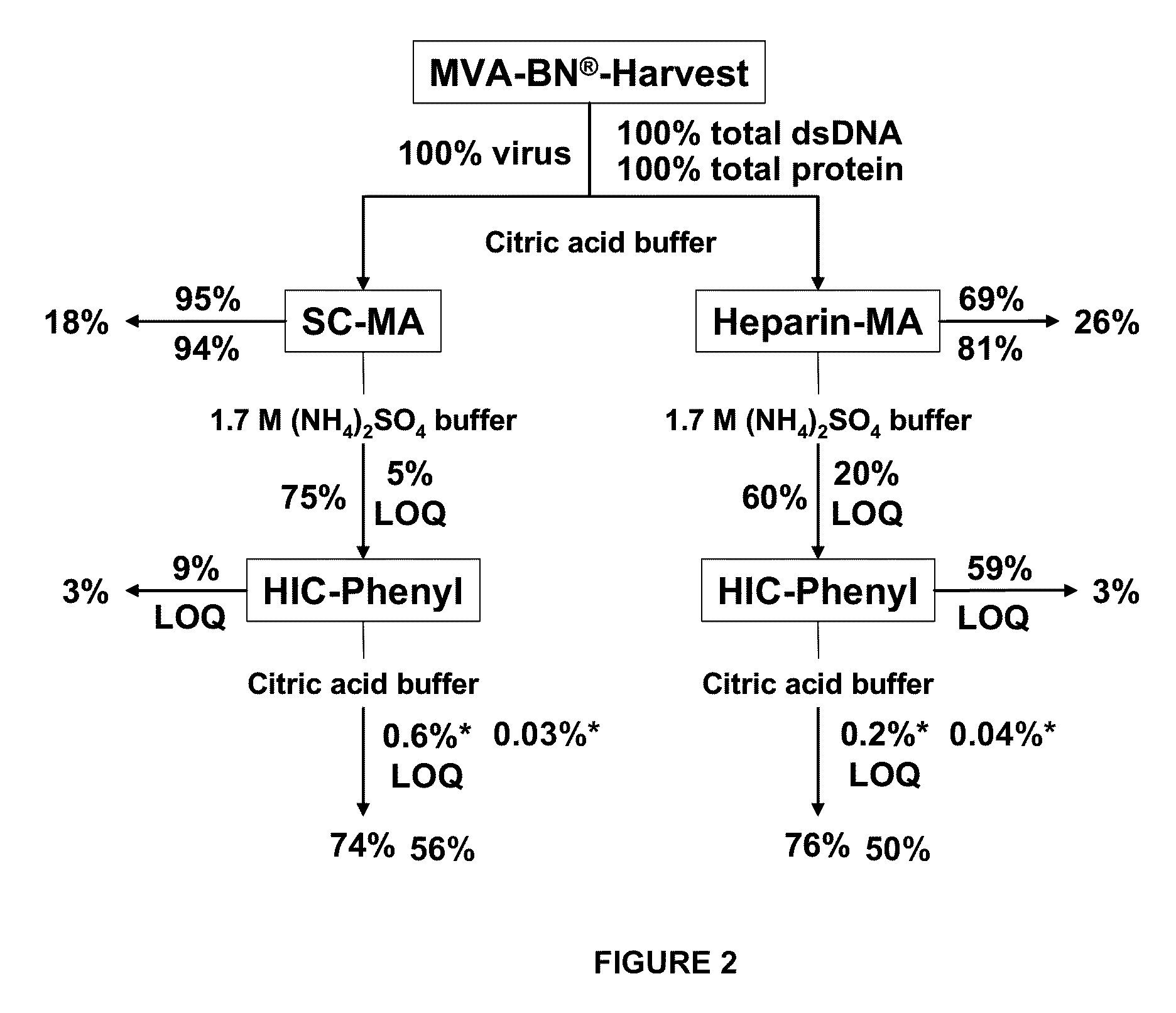
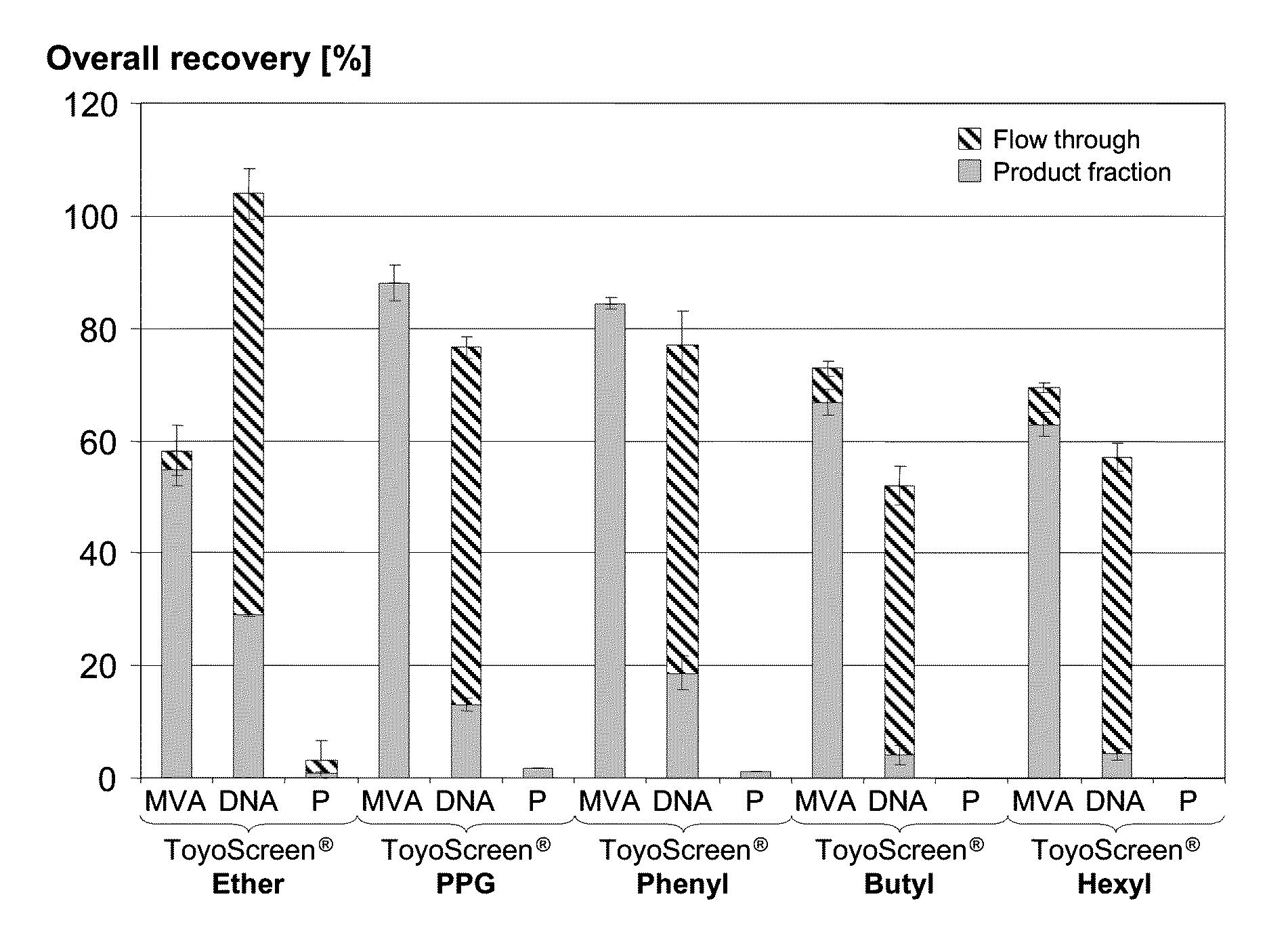
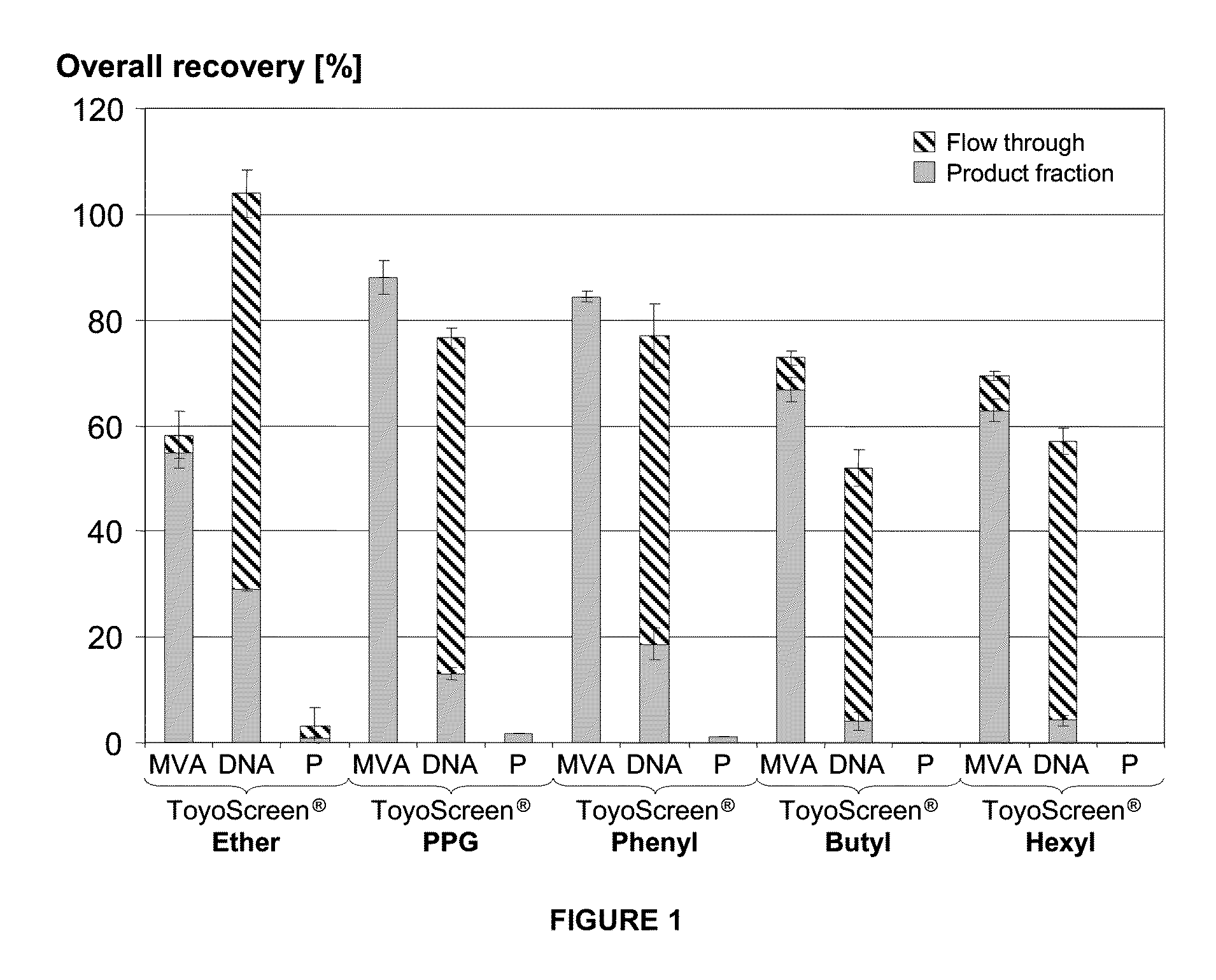
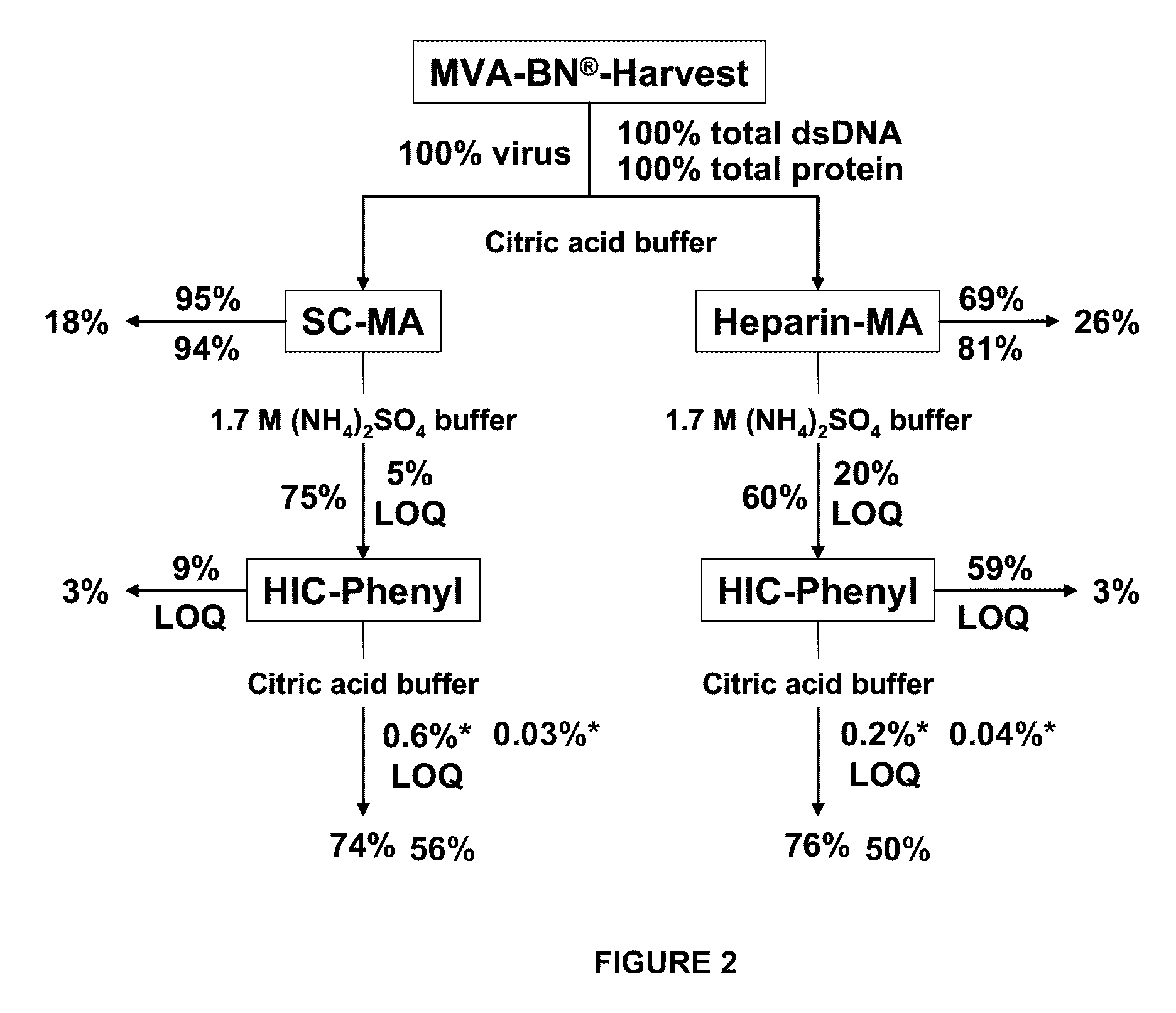
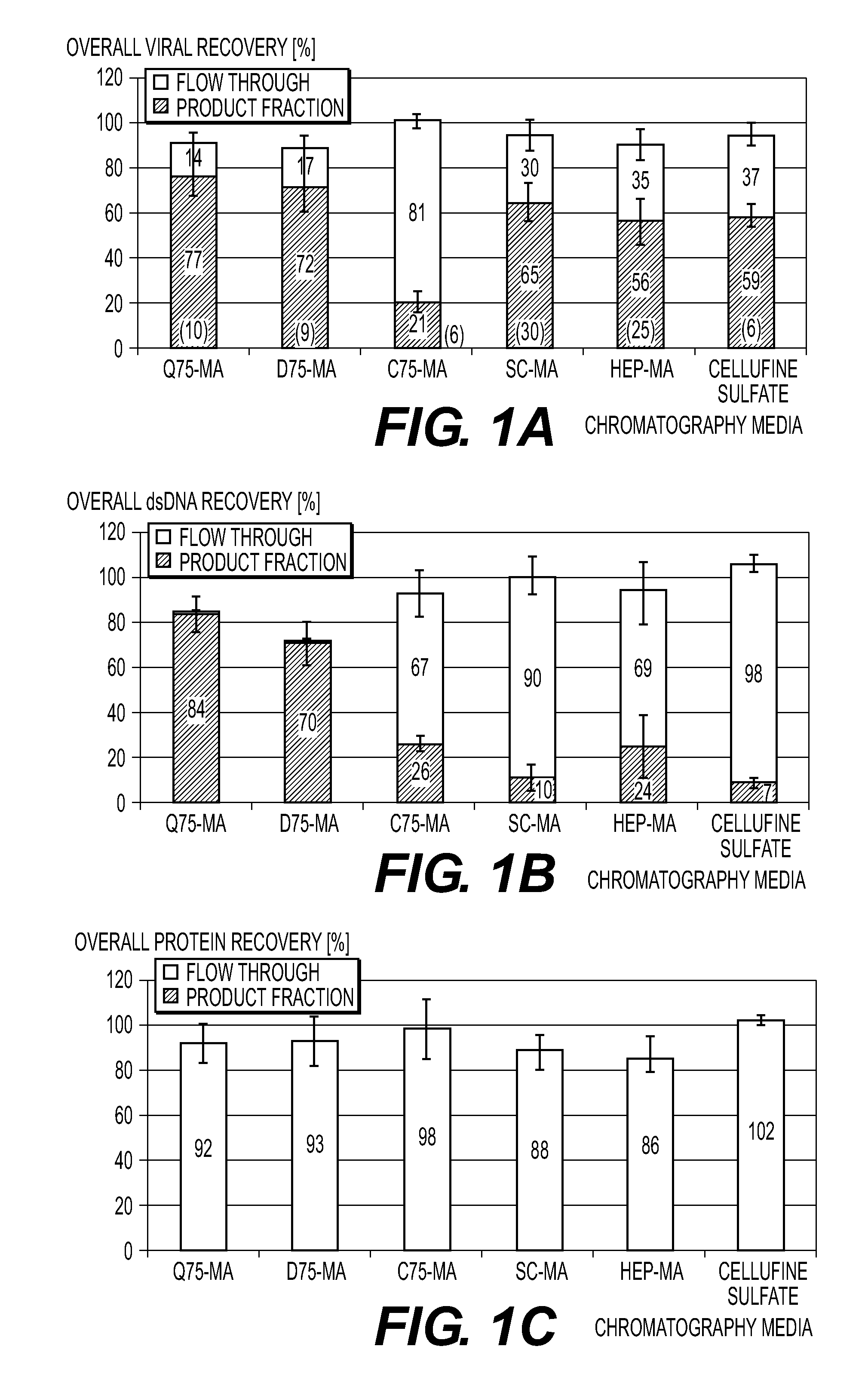
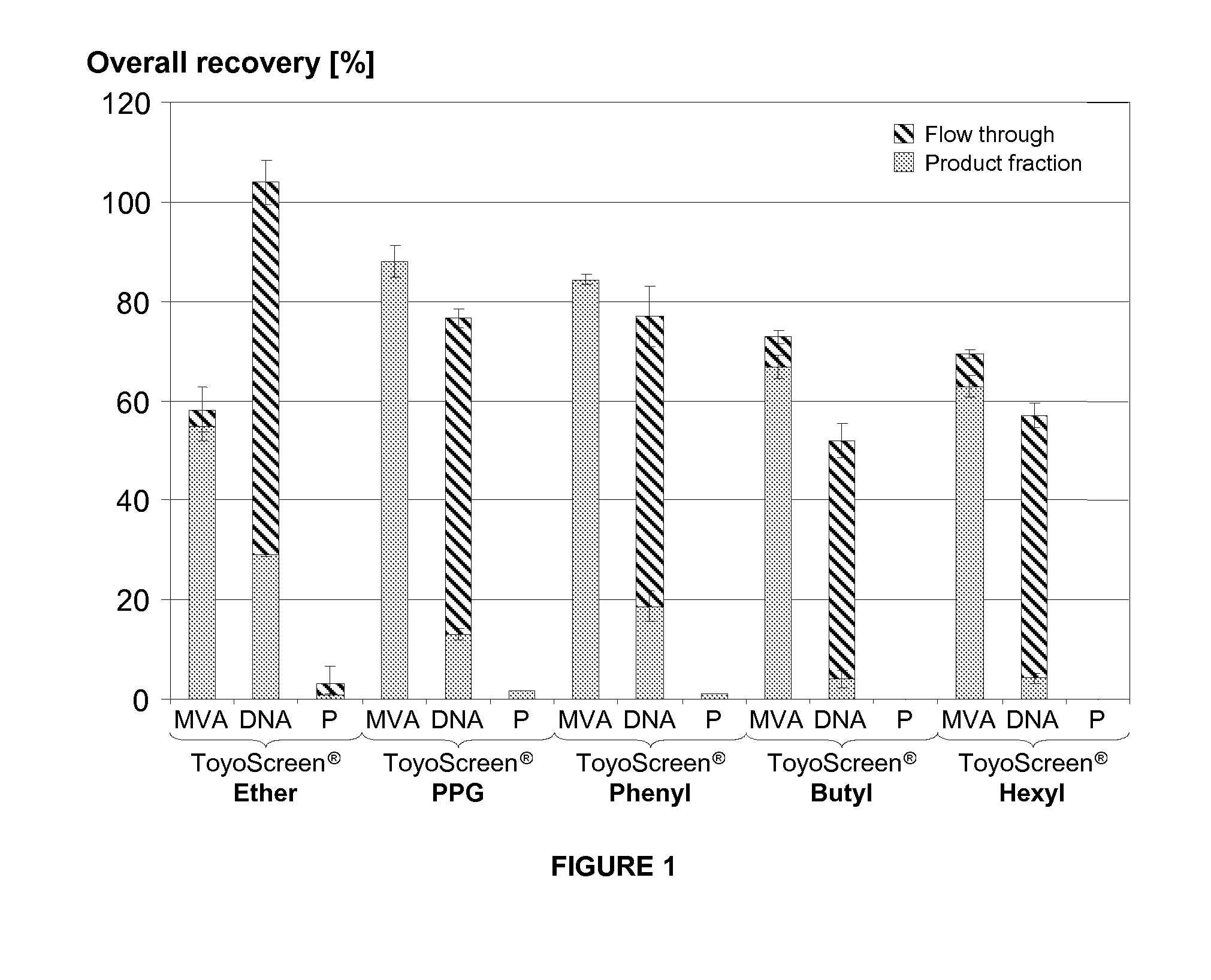
Purification of vaccinia viruses using hydrophobic interaction chromatography
ActiveUS8003364B2Reduce the amount requiredViral antigen ingredientsAntiviralsOrganismDrug biological activity
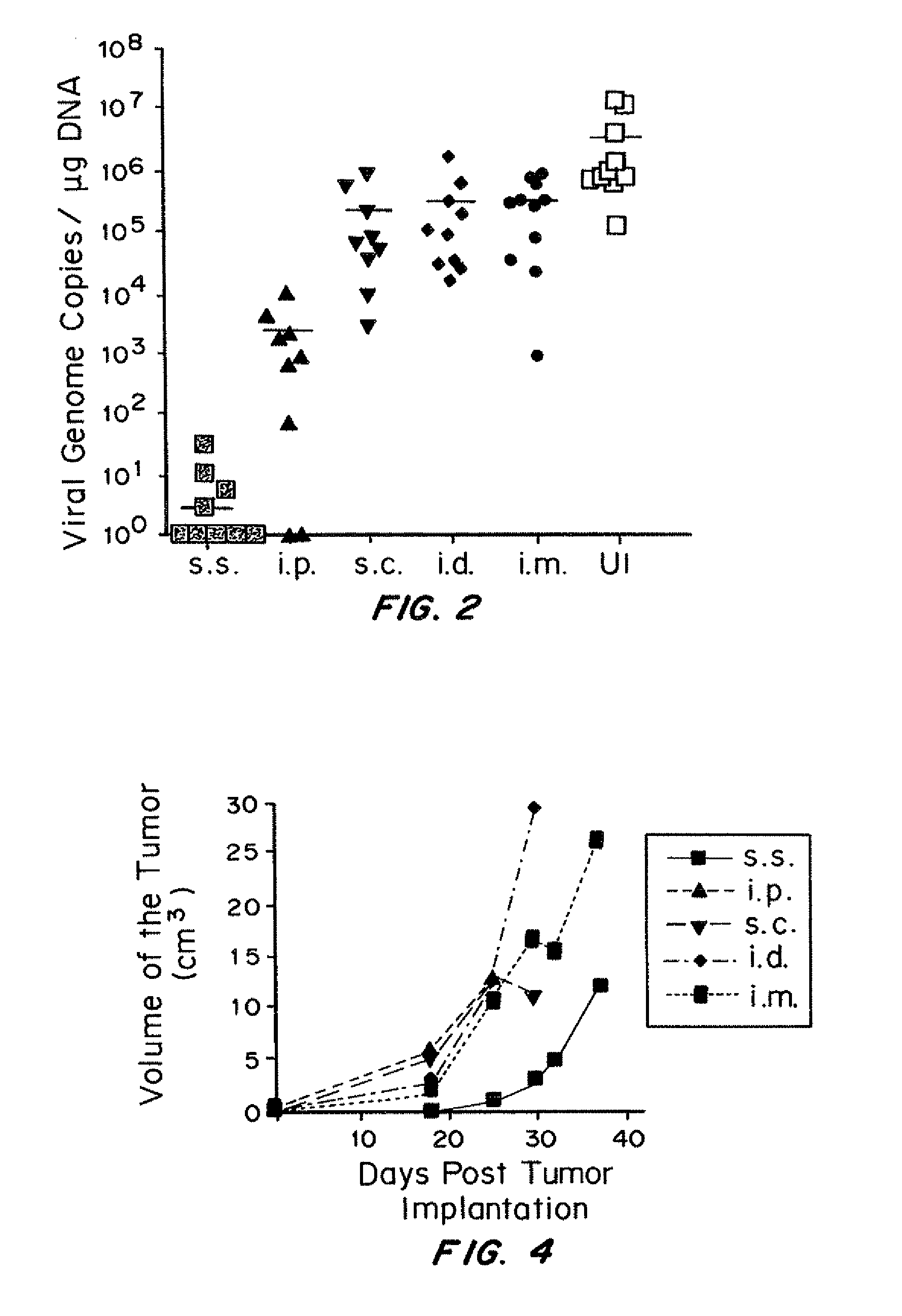
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:OTTO VON GUERICKE UNIV MAGDEBURG +2
T-cell vaccination with viral vectors via mechanical epidermal disruption
ActiveUS20110274649A1Strong immune responseReduce doseAntibacterial agentsAntimycoticsAdjuvantCuticle
Attenuated, replication-deficient viruses such as vaccinia viruses are used to deliver an exogenous viral, bacterial, parasitic or tumor antigen to an epidermal tissue such as the skin, lungs or gastrointestinal tract, which has been mechanically disrupted, in an amount effective to elicit or stimulate a cell mediated immune response. The epidermal tissue may be mechanically disrupted by a device such as a scarification needle or an abrader device. The epidermis may be mechanically disrupted prior to, at the same time, or immediately after the administration of the vaccine. The vaccine can be used to induce immunity against a pathogen, such as a virus, bacteria, or parasite, or against a cancer in a subject that has or is at risk of developing cancer. In some embodiments a co-stimulatory molecule, a growth factor, an adjuvant and / or a cytokine is administered before, with or after the viral vaccine. In some embodiments, the co-stimulatory molecule is co-expressed with the antigen by the virus.
Owner:TREMRX
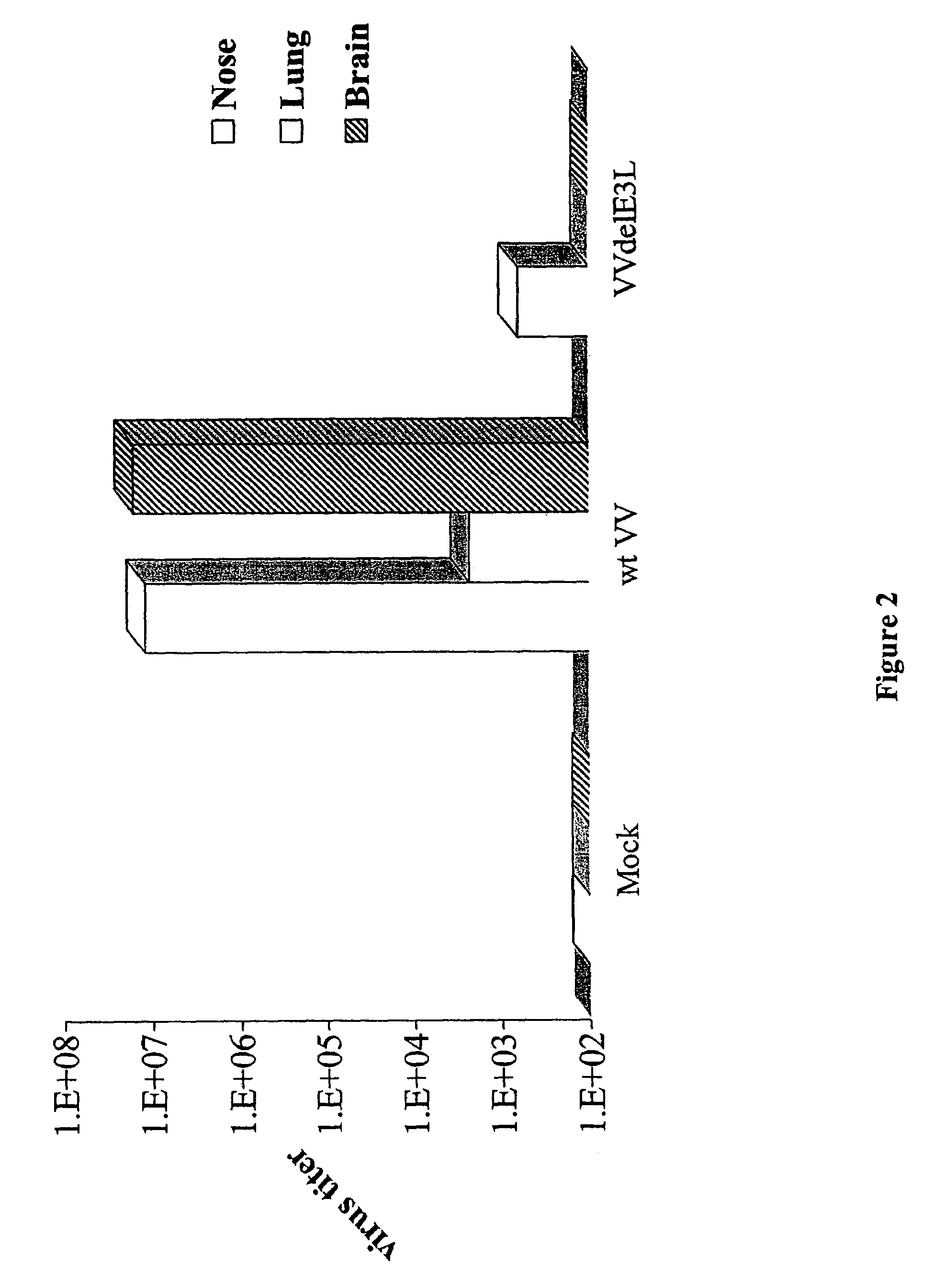
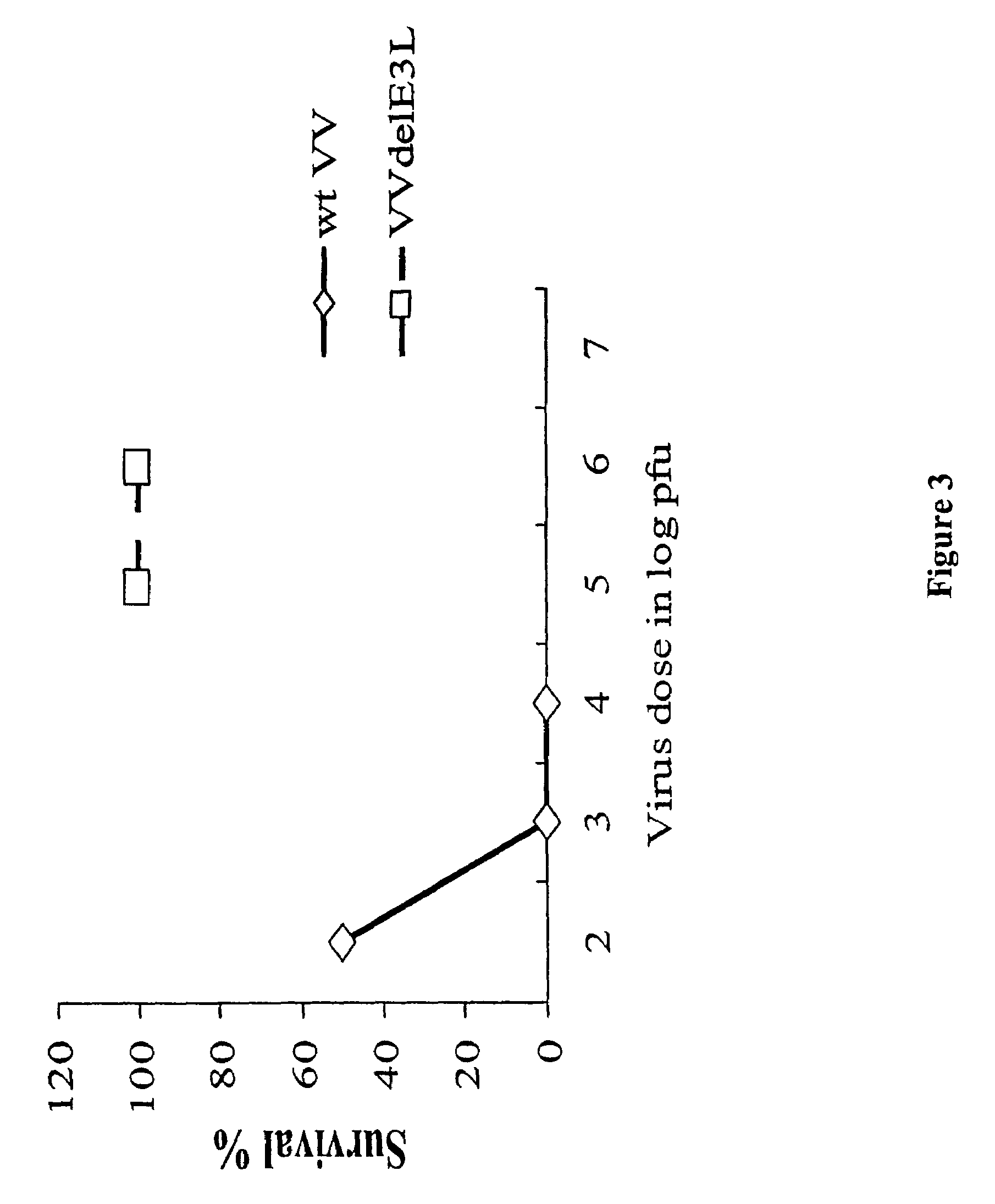
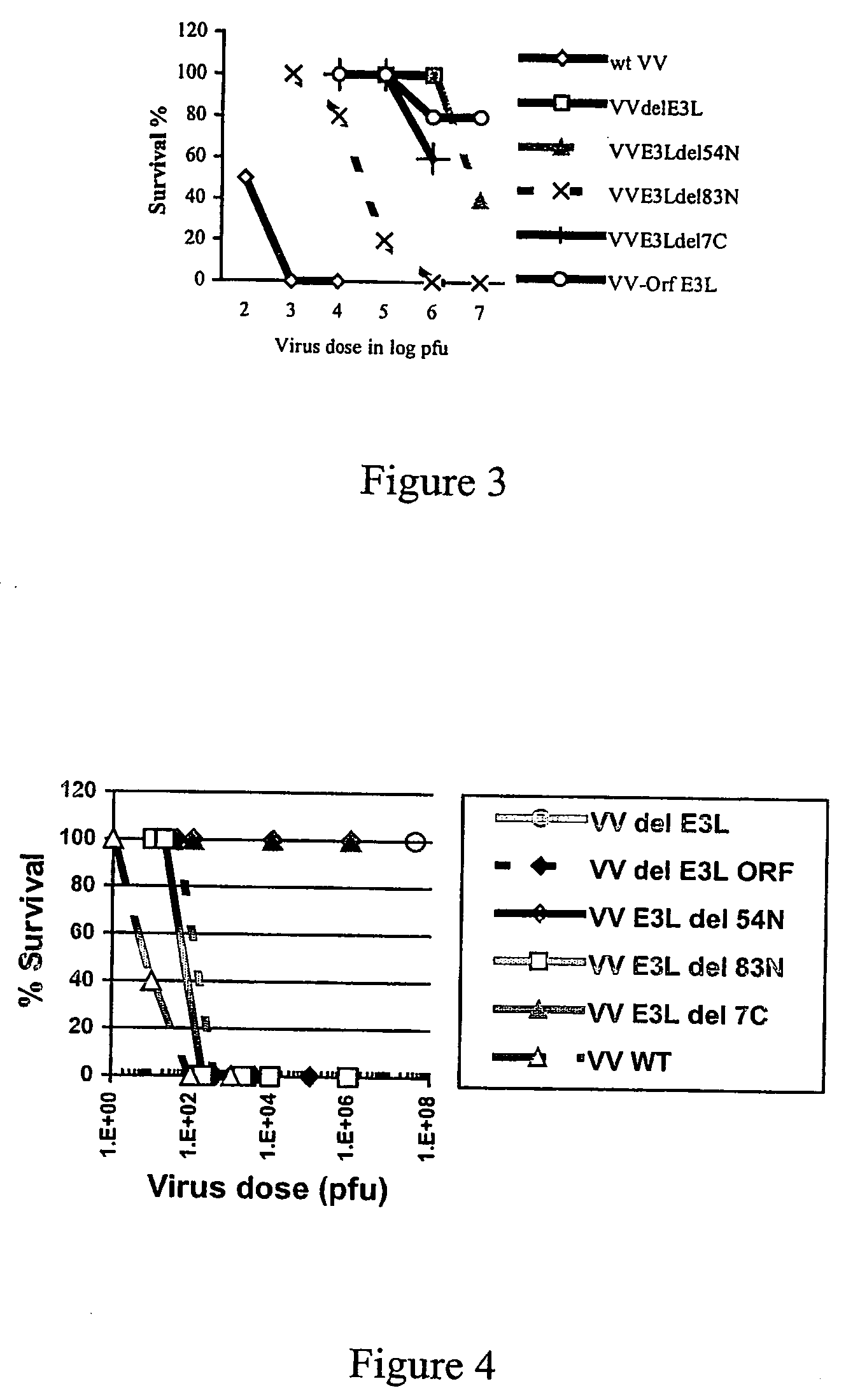
Use of vaccinia virus deleted for the E3L gene as a vaccine vector
The present invention relates to vaccines having an increased level of safety comprising recombinant vaccinia viruses containing an inactivated E3L region. The invention also relates to methods for stimulating a protective immune response in an immunized host using the vaccines of the invention. The invention is based on the discovery that vaccinia virus mutants having deletions in the E3L region exhibit dramatically reduced pathogenesis while remaining highly immunogenic. In addition, the invention relates to modified recombinant vaccinia viruses engineered to express heterologous polypeptides and the use of such viruses in vaccines designed to stimulate a protective immune response against such polypeptides in a host. The invention further relates to an interferon-sensitive recombinant vaccinia virus with broad host range wherein a salamander eIF2α is inserted into the viral genome in place of at least a portion of the E3L gene.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
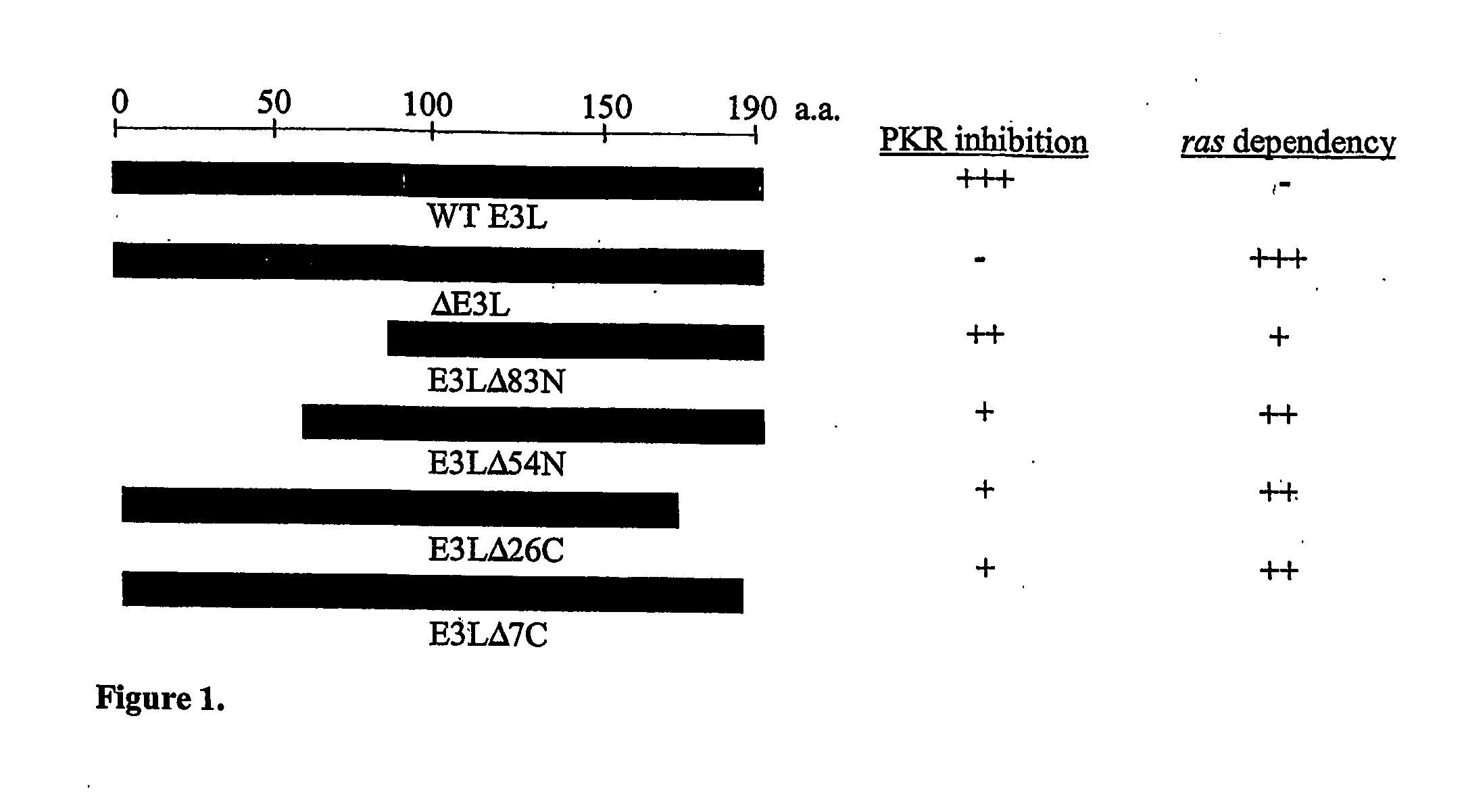
Mutants of vaccinia virus as oncolytic agents
InactiveUS20070036758A1Wide base of knowledgeImprove efficacyBiocideGenetic material ingredientsCancer cellLysis
The present invention relates to mutant oncolytic vaccinia viruses and their use for selective destruction of cancer cells. The mutant vaccinia viruses of the invention include those having a reduced ability to inhibit the antiviral dsR-NA dependent protein kinase (PKR) and increased sensitivity to interferon. Such mutants include, for example, vaccinia viruses having mutations in the E3L and / or K3L regions. The invention is based on the discovery that vaccinia viruses having mutations in the E3L region are capable of replication in oncogenic cells resulting in cell lysis. The invention further provides methods for treating proliferative disorders, such as neoplasms, in a host comprising administration of mutant vaccinia virus under conditions which result in substantial lysis of the proliferating cancer cells.
Owner:JACOBS BERSTREETCAR +2
Purification of vaccinia viruses using hydrophobic interaction chromatography
ActiveUS20100119552A1Reduce the amount requiredViral antigen ingredientsAntiviralsHigh effectivenessDrug biological activity
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:OTTO VON GUERICKE UNIV MAGDEBURG +2
Mutants of replication competent vaccinia virus
The present invention relates to vaccines having an increased level of safety comprising recombinant vaccinia viruses. The invention also relates to methods for stimulating a protective immune response in an immunized host using the vaccines of the invention. The vaccines and recombinant vaccinia viruses of the invention comprise a first nucleic acid comprising an expression control sequence and a second nucleic acid comprising an exogenous nucleic acid encoding a conditional replication gene product, wherein the expression control sequence is operably linked to the exogenous nucleic acid. The exogenous nucleic acid may, by its expression or non-expression, confer upon the recombinant vaccinia virus either sensitivity or dependence upon an exogenous molecule (e.g. a drug) or a condition. Importantly, to allow the recombinant vaccinia viruses of the invention to replicate normally under permissive conditions, the exogenous nucleic acid is inserted into a non-essential locus, e.g., the E2L / E3L inter-genic locus, K1L / K2L inter-genic locus, the superoxide dismutate locus, and the 7.5 K locus.
Owner:ARIZONA STATE UNIVERSITY
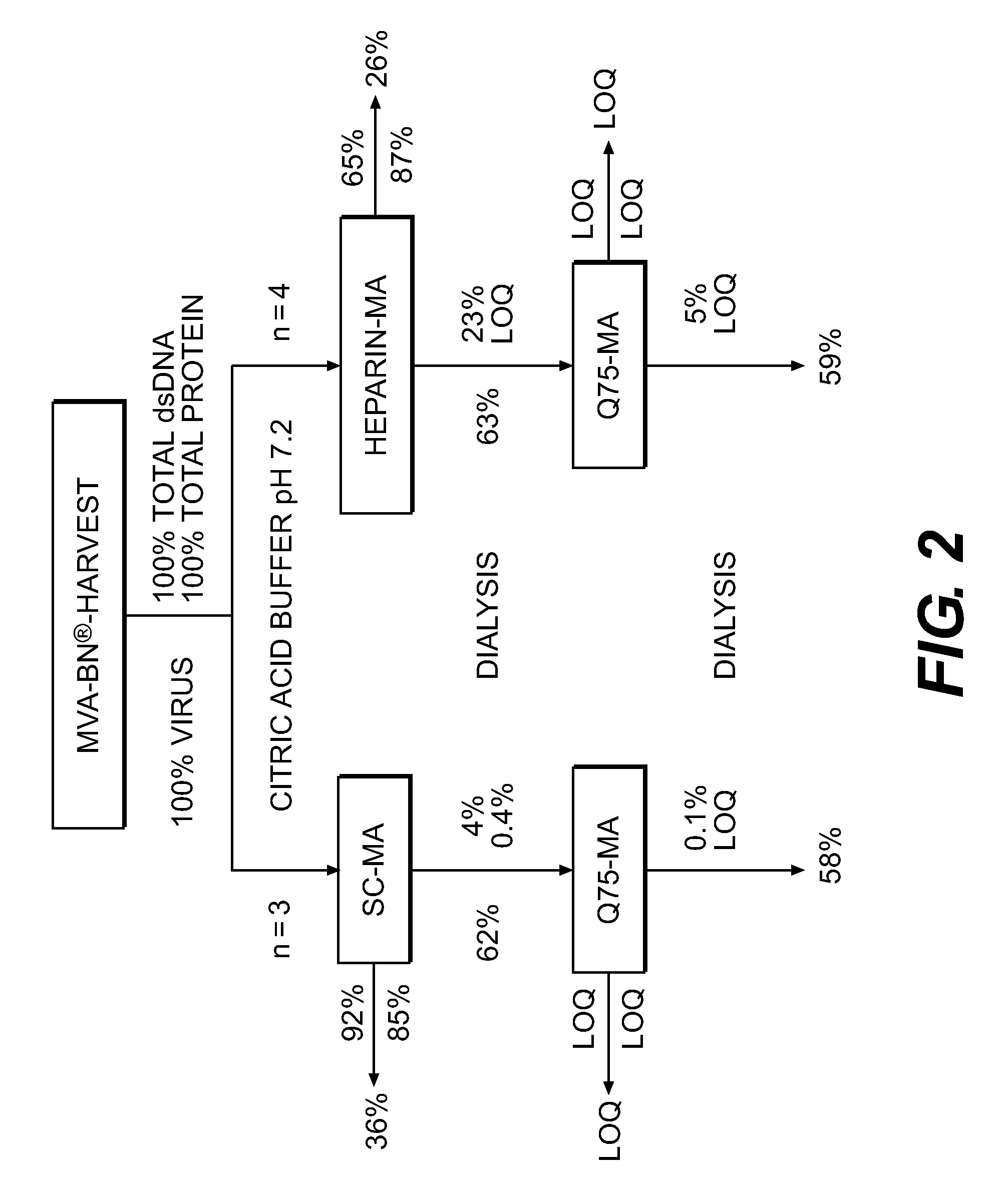
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS8003363B2Reduce the amount requiredViral antigen ingredientsRecovery/purificationOrganismVaccinia viruses
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
Purification of vaccinia viruses using hydrophobic interaction chromatography
ActiveUS20110306114A1Reduce the amount requiredViral antigen ingredientsAntiviralsCowpox virusVaccinia viruses
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS +2
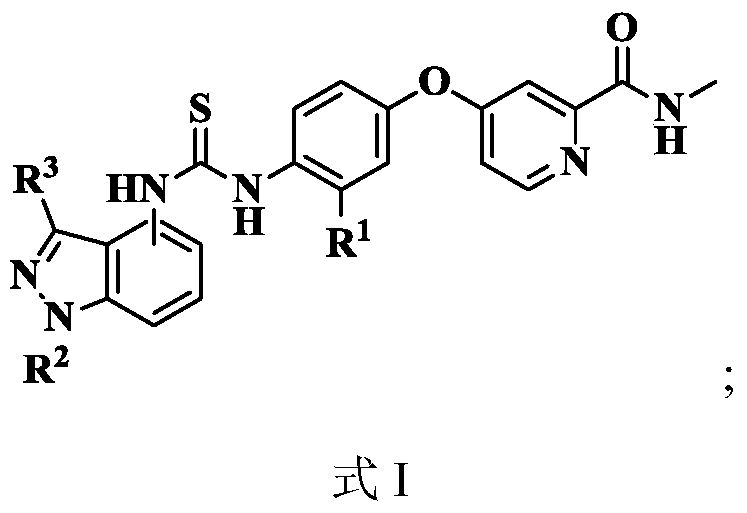
N-indazole substituted thiourea derivatives and preparation method and application thereof
InactiveCN105753841AImproves antioxidant activityImprove clearanceOrganic active ingredientsOrganic chemistryDPPHThiourea
The invention discloses N-indazole substituted thiourea derivatives and a preparation method and application thereof and belongs to the technical field of chemical medicine.The thiourea derivatives are a series of compounds simultaneously containing the 1H-indazole ring structure and the asymmetrical thiourea structure, and the compounds are not reported in the literature.The bioactivity test result analysis of the thiourea derivatives show that the compounds are good in antioxidant activity, the average scavenging rate is above 80%, the scavenging rate of the compound 12b, the compound 12c, and the compound 12d and the compound 12h is higher than 90%, the scavenging activity IC50 of the compound 12h on DPPH is 0.14mg / mL; part of the target compounds has certain inhibition activity on herpes viruses, vaccinia viruses, reoviruses, Coxsackie viruses, Feline coronary herpes viruses, HIV viruses and the like, and the compound 12c and the compound 12n are high in antivirus activity; the synthesized compounds hopefully have new bioactivity which is not expounded, and a certain material basis is provided for the development of new medicine.
Owner:XI AN JIAOTONG UNIV
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS20100112001A1Reduce the amount requiredViral antigen ingredientsRecovery/purificationViral VaccineOrganism
The present invention relates to methods for purification of Vaccinia viruses (VV) and / or Vaccinia virus (VV) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS8012738B2High yieldMore cost-effectiveBiocideViral antigen ingredientsViral VaccineVaccinia viruses
The present invention relates to methods for purification of Vaccinia viruses (W) and / or Vaccinia virus (W) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
Purification of vaccinia virus- and recombinant vaccinia virus-based vaccines
ActiveUS20100129326A1Improve purification effectImprove efficiencyBiocideViral antigen ingredientsViral VaccineDrug biological activity
The present invention relates to methods for purification of Vaccinia viruses (W) and / or Vaccinia virus (W) particles, which can lead to highly pure and stable virus preparations of predominantly biologically active viruses. The invention encompasses purifying a virus preparation in a sterilized way with high efficiency and desirable yield in terms of purity, biological activity and stability, aspects advantageous for industrial production.
Owner:BAVARIAN NORDIC AS
Mutant vaccinia viruses and use thereof
The present invention discloses recombinant vaccinia virus (VV) virions that are resistant to antiviral defenses and have enhanced anti-tumor activities. In one embodiment, the recombinant VV comprise one or more variant VV proteins that have mutations at one or more neutralizing antibody epitopes, thereby conferring viral escape from the neutralizing antibodies. In another embodiment, the recombinant VV is resistant to complement-mediated neutralization due to the expression of a regulator of complement activation (e.g. CD55). In another embodiment, the recombinant VV has enhanced anti-tumor activities due to the expression of bi-specific antibodies co-targeting cancer cells and immune effector cells, or the expression of a polypeptide blocking the PD-1 pathway. The recombinant vaccinia virus virions can be used to treat cancer in a subject.
Owner:ICELLKEALEX THERAPEUTICS LLC
Use of vaccinia virus deleted for the E3L gene as a vaccine vector
InactiveUS20060008470A1Reduce pathogenicityMaintain immunogenicityViral antigen ingredientsVirus peptidesHeterologousProtection sex
The present invention relates to vaccines having an increased level of safety comprising recombinant vaccinia viruses containing an inactivated E3L region. The invention also relates to methods for stimulating a protective immune response in an immunized host using the vaccines of the invention. The invention is based on the discovery that vaccinia virus mutants having deletions in the E3L region exhibit dramatically reduced pathogenesis while remaining highly immunogenic. In addition, the invention relates to modified recombinant vaccinia viruses engineered to express heterologous polypeptides and the use of such viruses in vaccines designed to stimulate a protective immune response against such polypeptides in a host. The invention further relates to an interferon-sensitive recombinant vaccinia virus with broad host range wherein a salamander eIF2α is inserted into the viral genome in place of at least a portion of the E3L gene.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
T-Cell Vaccination With Viral Vectors Via Mechanical Epidermal Disruption
ActiveUS20140186298A1Strong immune responseProtected growthAntibacterial agentsAntimycoticsCuticleTumor antigen
Attenuated, replication-deficient viruses such as vaccinia viruses are used to deliver an exogenous viral, bacterial, parastic or tumor antigen to an epidermal tissue such as the skin, lungs or gastrointestinal tract, which has been mechanically disrupted, in an amount effective to elicit or stimulate a cell mediated immune response. The epidermis may be mechanically disrupted prior to, at the same time, or immediately after the administration of the vaccine. The vaccine can be used to induce immunity against a pathogen, such as a virus, bacteria, or parasite, or against a cancer in a subject that has or is at risk of developing cancer.
Owner:TREMRX
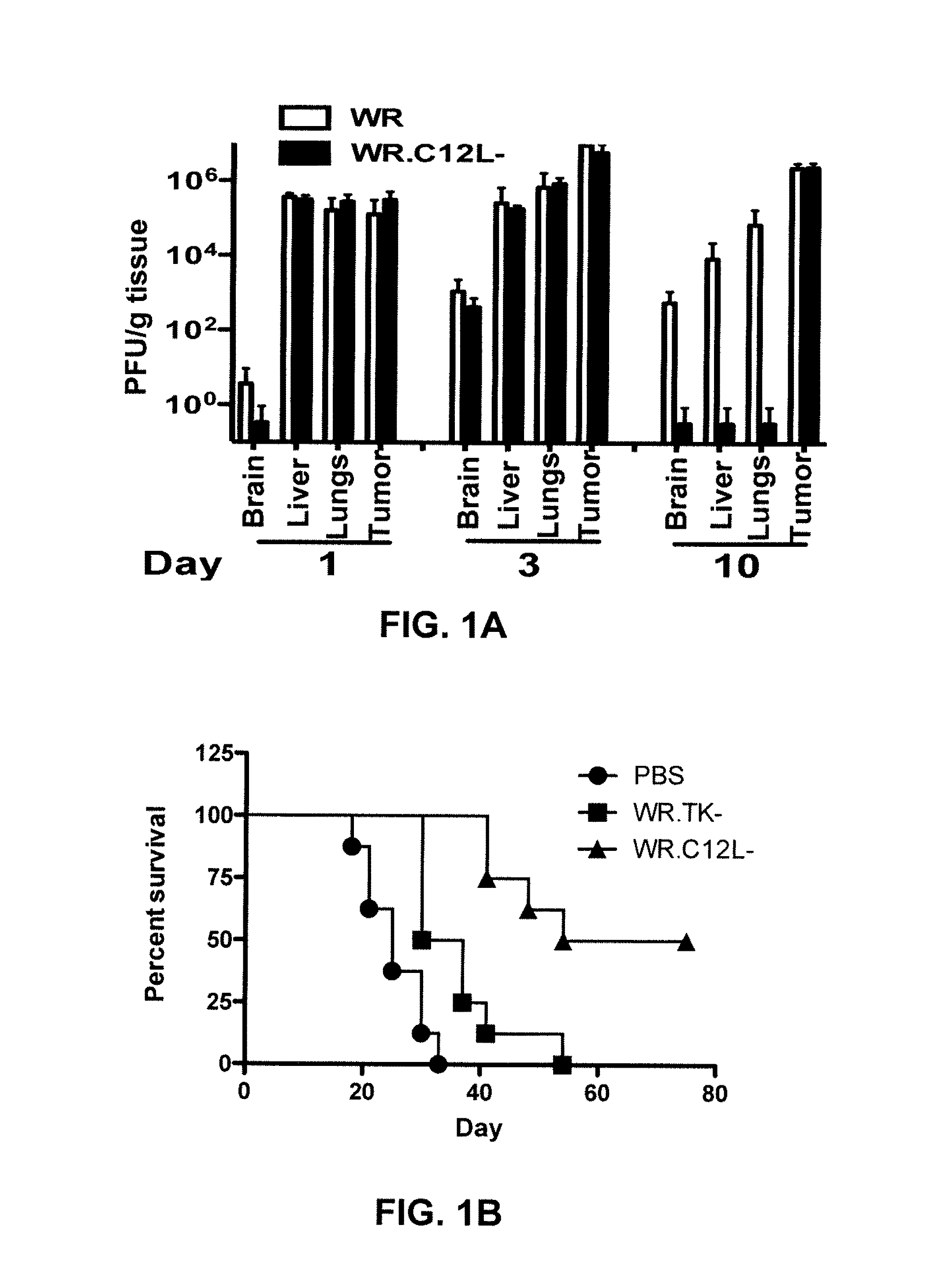
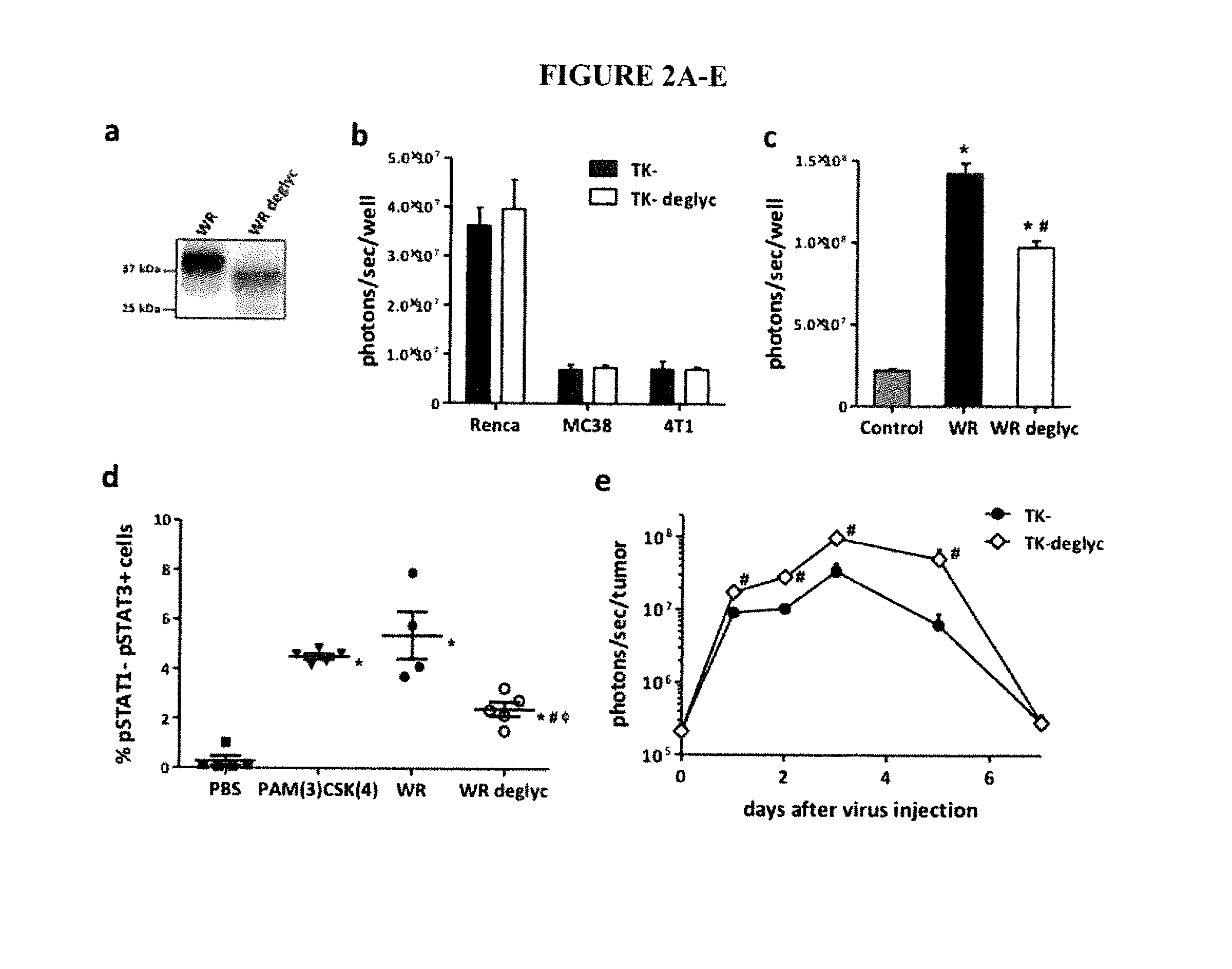
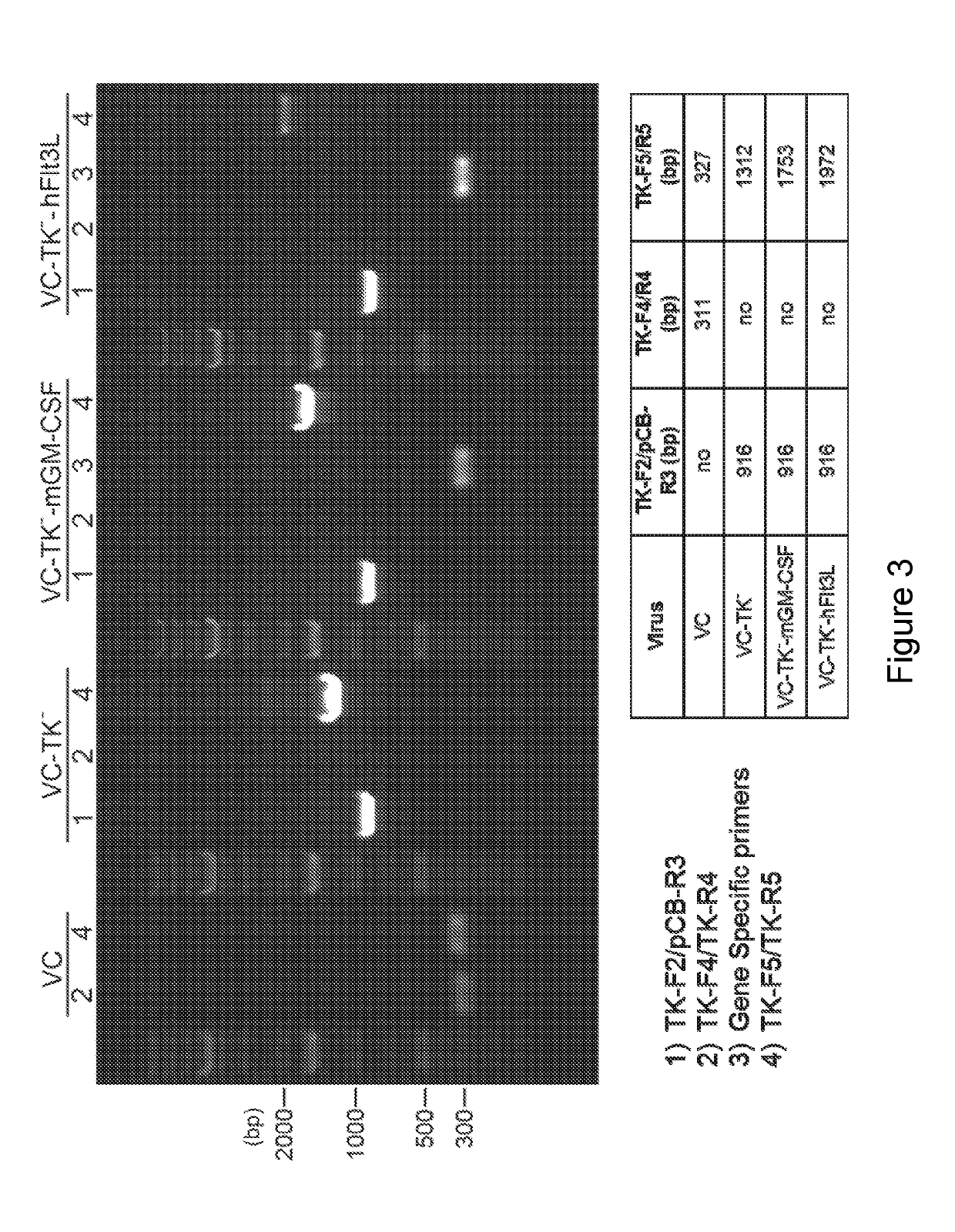
Replication competent attenuated vaccinia viruses with deletion of thymidine kinase with and without the expression of human Flt3L or GM-CSF for cancer immunotherapy
ActiveUS10512662B2Facilitate recruitment and differentiation and functionPromote resultsChemokinesPeptide/protein ingredientsVaccinia virusesPoxvirus officinale
The present invention relates generally to the fields of oncology, virology and immunotherapy. More particularly, it concerns the use of poxviruses, specifically the replication competent attenuated vaccinia virus with deletion of thymidine kinase (VC-TK−) with and without the expression of human Flt3L or GM-CSF as oncolytic and immunotherapy. The foregoing poxviruses can also be used in combination with immune checkpoint blocking agents. The foregoing poxviruses can also be inactivated via Heat or UV-treatment and the inactivated virus can be used as immunotherapy either alone or in combination with immune checkpoint blocking agents.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Mutant vaccinia viruses and use thereof
PendingUS20210388388A1High killing efficiencyHeightened tumor specificityVirus peptidesUnknown materialsCancer cellCowpox virus
The present invention discloses recombinant vaccinia virus (VV) virions that are resistant to antiviral defenses and have enhanced anti-tumor activities. In one embodiment, the recombinant VV comprise one or more variant VV proteins that have mutations at one or more neutralizing antibody epitopes, thereby conferring viral escape from the neutralizing antibodies. In another embodiment, the recombinant VV is resistant to complement-mediated neutralization due to the expression of a regulator of complement activation (e.g. CD55). In another embodiment, the recombinant VV has enhanced anti-tumor activities due to the expression of bi-specific antibodies co-targeting cancer cells and immune effector cells, or the expression of a polypeptide blocking the PD-1 pathway. The recombinant vaccinia virus virions can be used to treat cancer in a subject.
Owner:ICELLKEALEX THERAPEUTICS LLC
Modified oncolytic vaccinia viruses expressing a cytokine and a car-boxylesterase and methods of use thereof
ActiveUS10646524B2High concentrationReduce the possibilityPeptide/protein ingredientsHydrolasesPharmaceutical drugCarboxylic acid
The present disclosure pertains to a compositions and combinations for simultaneous, separate or sequential use which comprises (a) an oncolytic vaccinia virus that expresses a cytokine and a carboxylesterase enzyme, and, preferably, (b) a cancer co-drug, and to their uses for the treatment of cancer.
Owner:SILLAJEN
Replication competent attenuated vaccinia viruses with deletion of thymidine kinase with and without the expression of human flt3l or gm-csf for cancer immunotherapy
ActiveUS20190054131A1Facilitate recruitmentFacilitate differentiationChemokinesPeptide/protein ingredientsPox virusesUv treatment
The present invention relates generally to the fields of oncology, virology and immunotherapy. More particularly, it concerns the use of poxviruses, specifically the replication competent attenuated vaccinia virus with deletion of thymidine kinase (VC-TK−) with and without the expression of human Flt3L or GM-CSF as oncolytic and immunotherapy. The foregoing poxviruses can also be used in combination with immune checkpoint blocking agents. The foregoing poxviruses can also be inactivated via Heat or UV-treatment and the inactivated virus can be used as immunotherapy either alone or in combination with immune checkpoint blocking agents.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
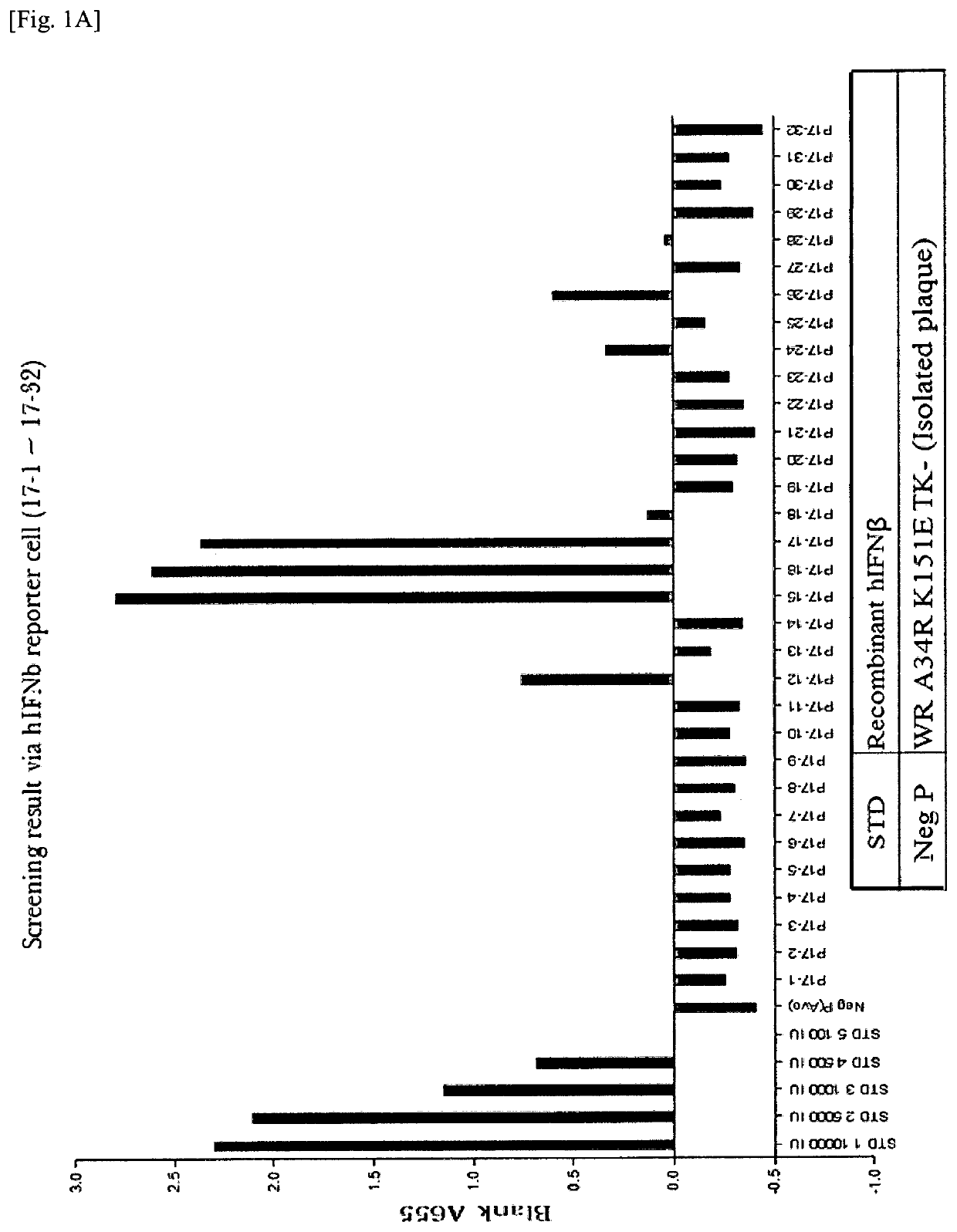
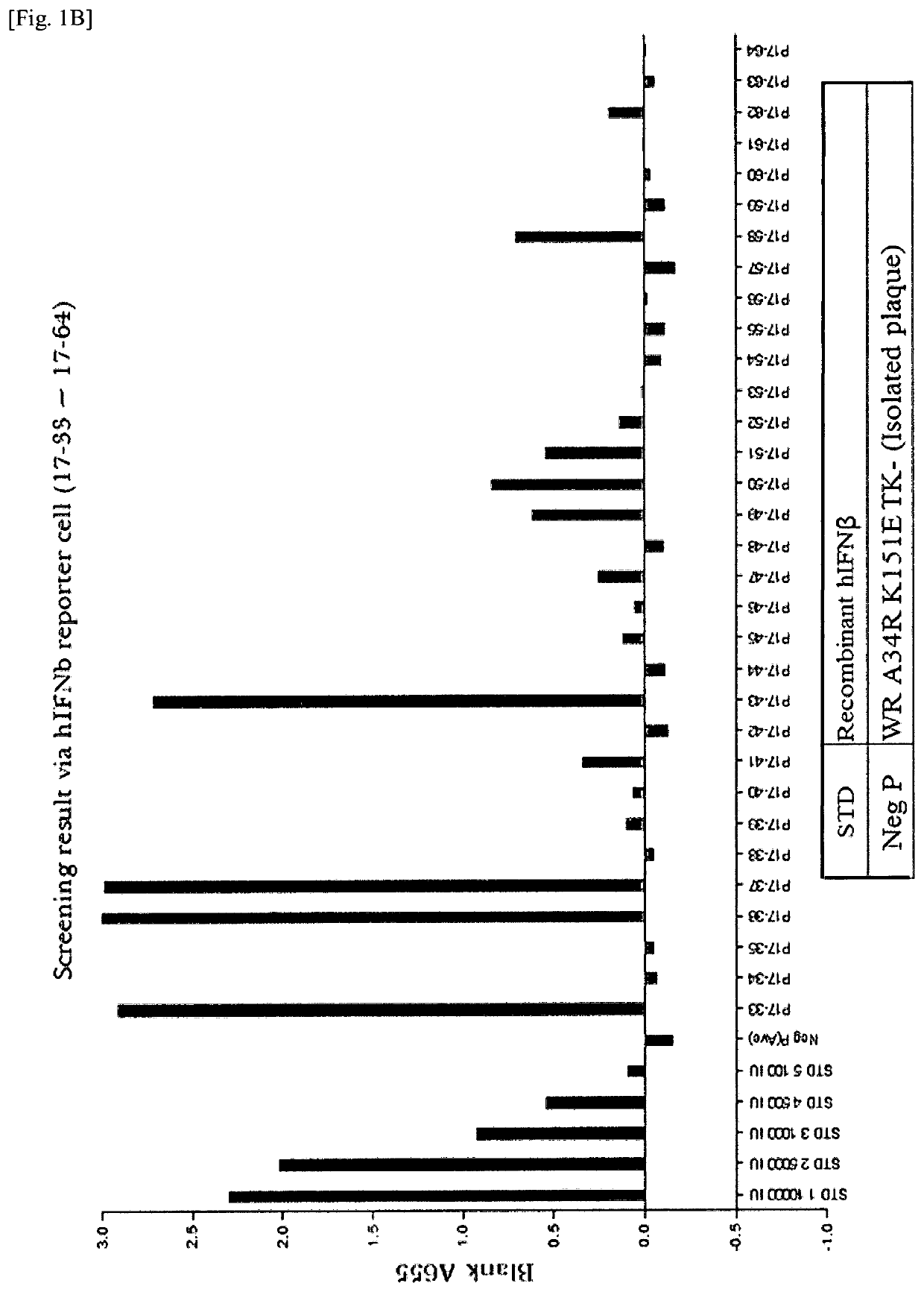
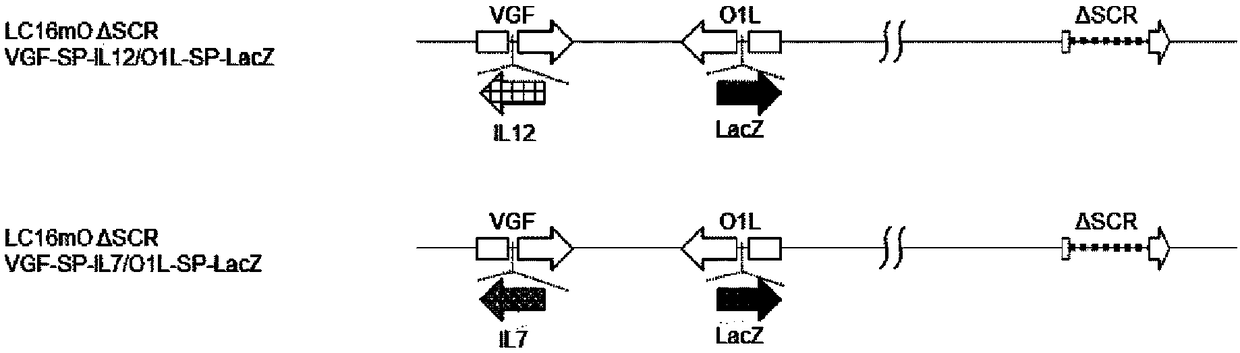
New genetically-modified vaccinia virus
The invention provides a genetically-modified vaccinia virus that is effective for the prevention or treatment of cancer. Specifically, the invention provides: a vaccinia virus containing two polynucleotides which are a polynucleotide that codes for IL-7 and a polynucleotide that codes for IL-12; a combination kit including two vaccinia viruses which are a vaccinia virus containing a polynucleotide that codes for IL-7 and a vaccinia virus containing a polynucleotide that codes for IL-12; and a combination use of the two vaccinia viruses.
Owner:ASTELLAS PHARMA INC +1
Synthetic chimeric vaccinia virus
The invention relates in various aspects to a synthetic chimeric vaccinia virus or compositions comprising such viruses, and the development and use of systems and methods for producing such synthetic chimeric vaccinia viruses. The synthetic chimeric vaccinia viruses are well suited, among others, as virus vaccines or to generate an oncolytic response and pharmaceutical formulations.
Owner:TONIX PHARMA LTD
Modified oncolytic vaccinia viruses expressing a cytokine and a car- boxylesterase and methods of use thereof
ActiveUS20180271921A1Prevents and delays build-upGood effectOrganic active ingredientsPeptide/protein ingredientsCytokineVaccinia viruses
The present disclosure pertains to a compositions and combinations for simultaneous, separate or sequential use which comprises (a) an oncolytic vaccinia virus that expresses a cytokine and a carboxylesterase enzyme, and, preferably, (b) a cancer co-drug, and to their uses for the treatment of cancer.
Owner:SILLAJEN
Immuno-oncolytic therapies
ActiveUS11478518B2Reduced responseImprove immunityViral antigen ingredientsUnknown materialsTumor reductionAntiendomysial antibodies
The present invention relates to oncolytic vaccinia viruses which have been modified to promote anti-tumor immunity and / or reduce host immunity and / or antibody response against the virus. It is based, at least in part, on the discovery that oncolytic vaccinia virus (i) bearing a genome deletion of a gene that reduces T cell immunity (interleukin-18 binding protein); (ii) treated with a sialidase enzyme which is believed to reduce TLR2 activation and therefore the antibody response; (iii) carrying a gene that enhances cytotoxic T lymphocyte induction (e.g., TRIF) and / or (iv) reduces tumor myeloid-derived suppressor cells by reducing prostaglandin E2 reduces tumor growth. Accordingly, the present invention provides for immunooncolytic vaccinia viruses and methods of using them in the treatment of cancers.
Owner:UNIVERSITY OF PITTSBURGH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com