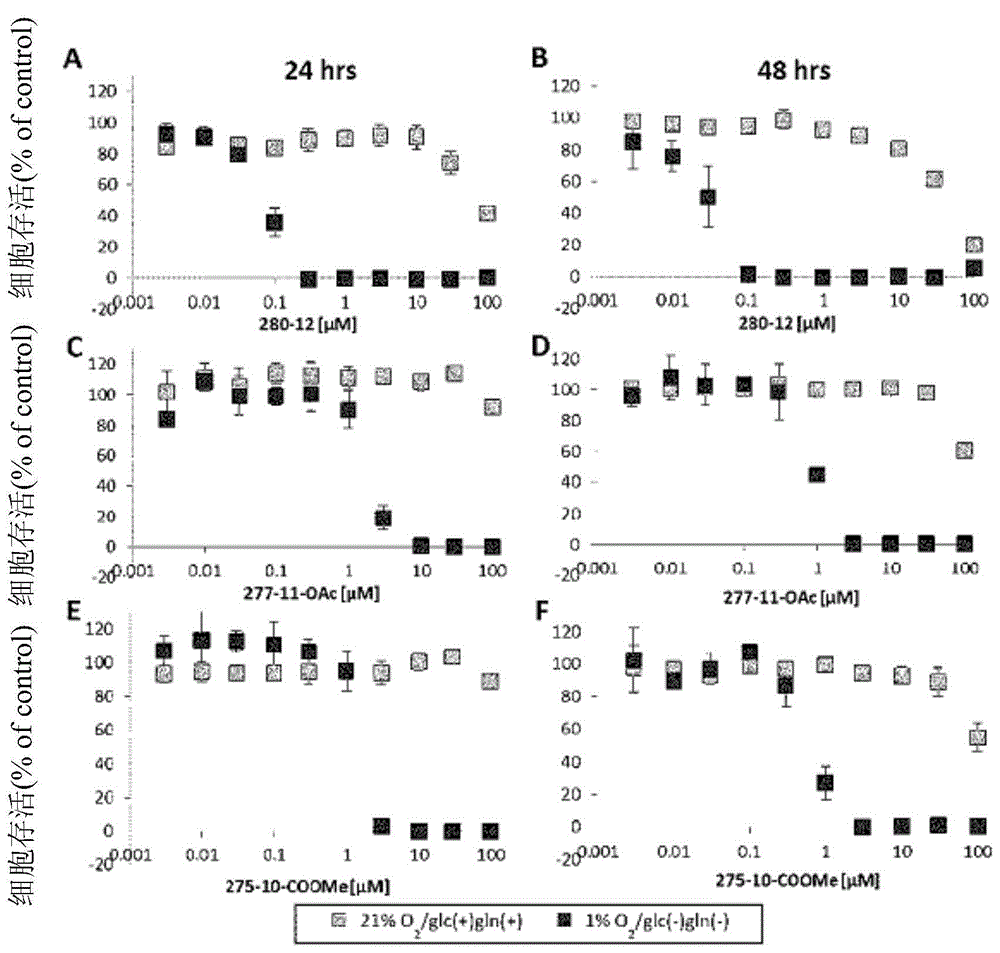
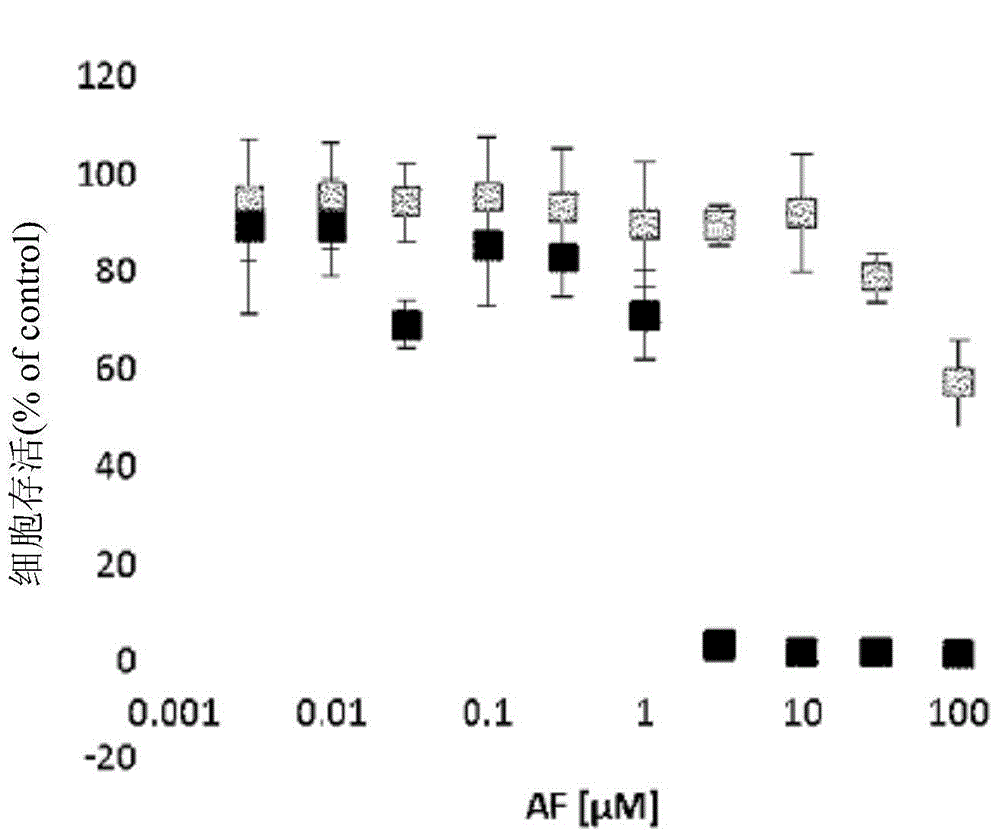
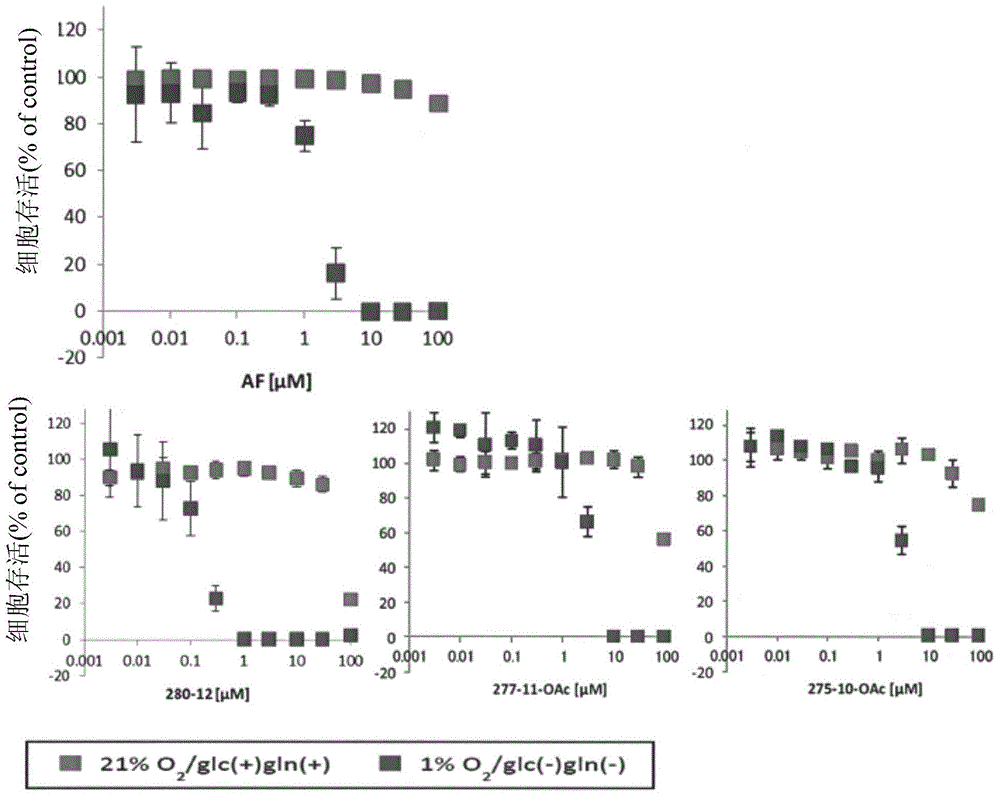
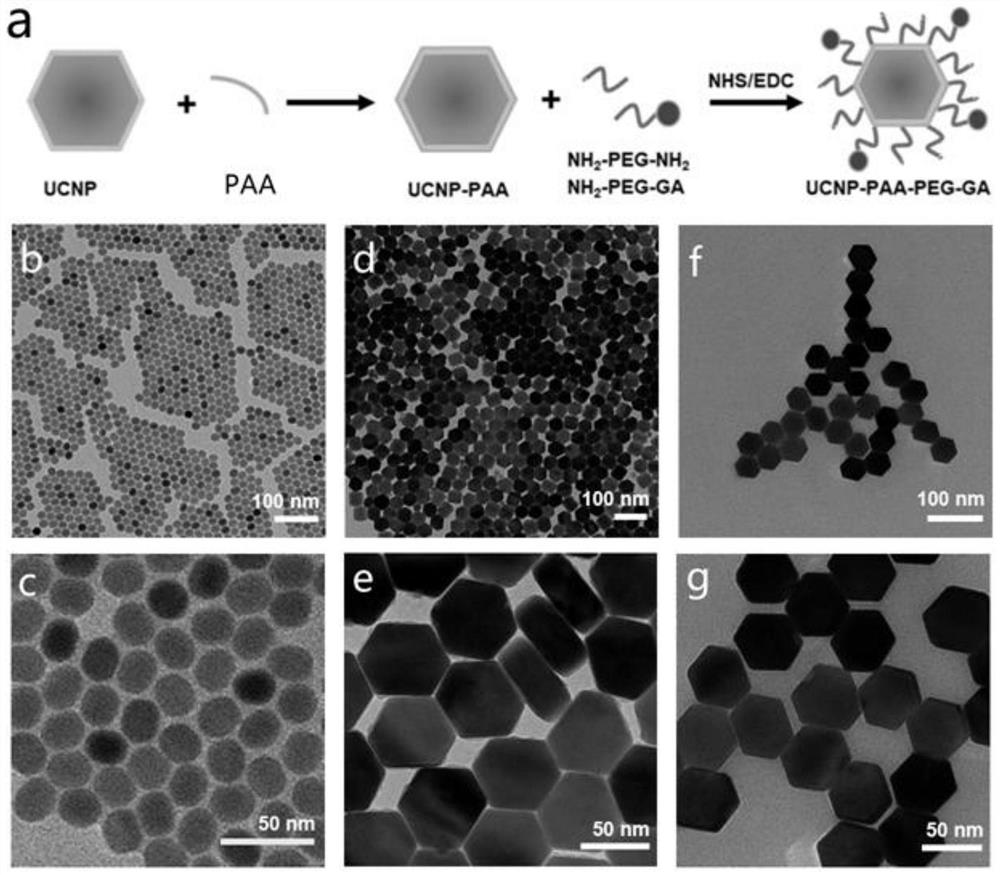
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Diabetes mellitus therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
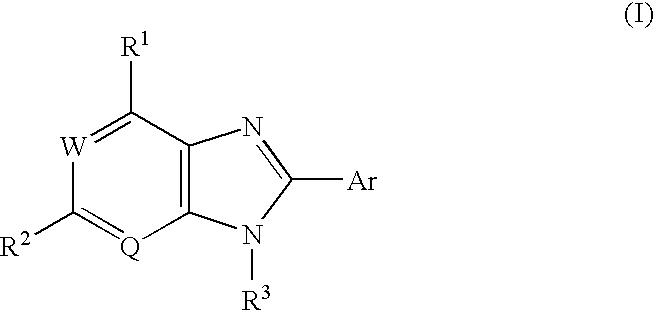
Condensed imidazole compounds and a therapeutic agent for diabetes mellitus
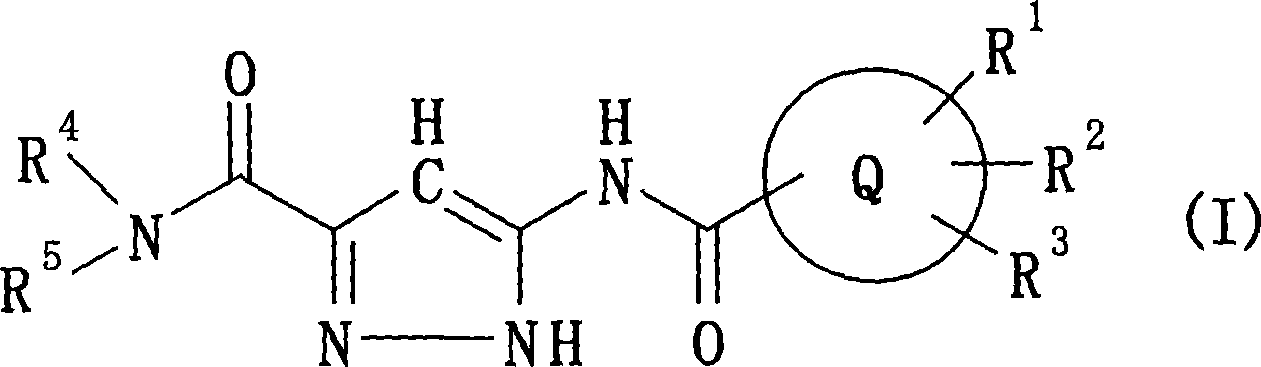
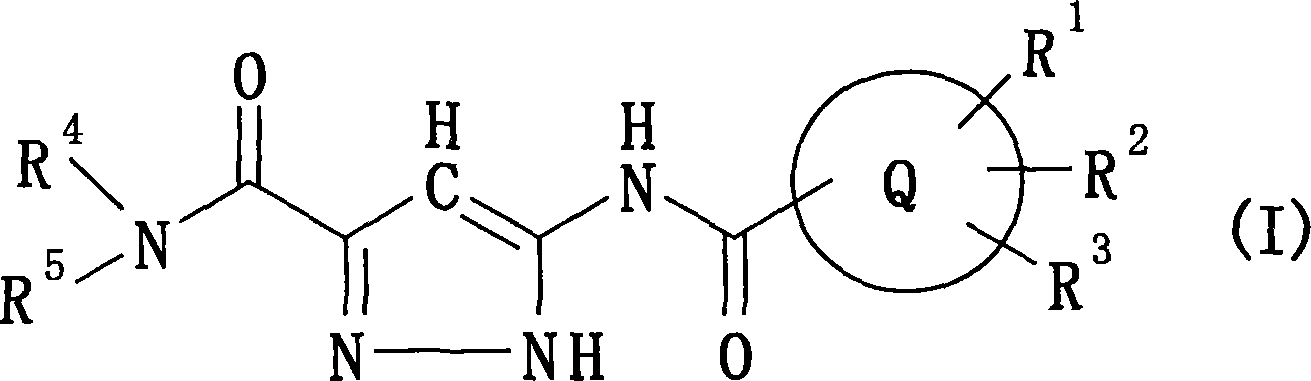
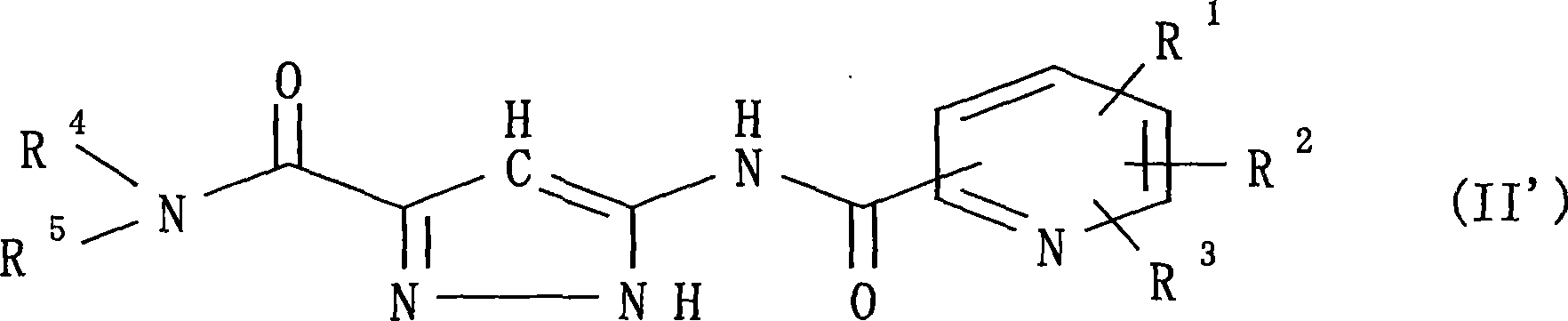
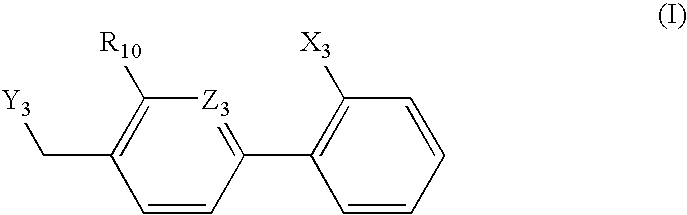
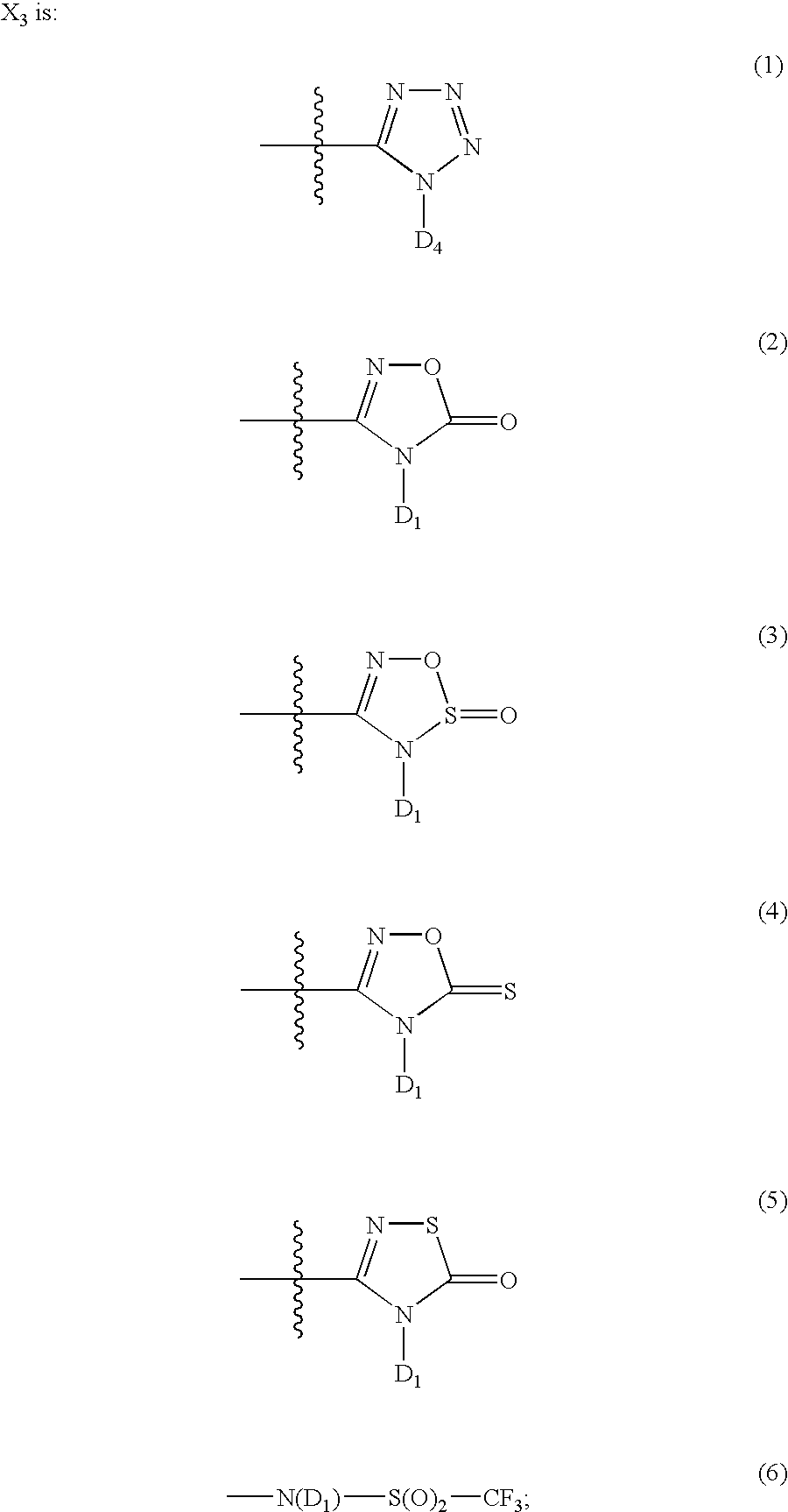
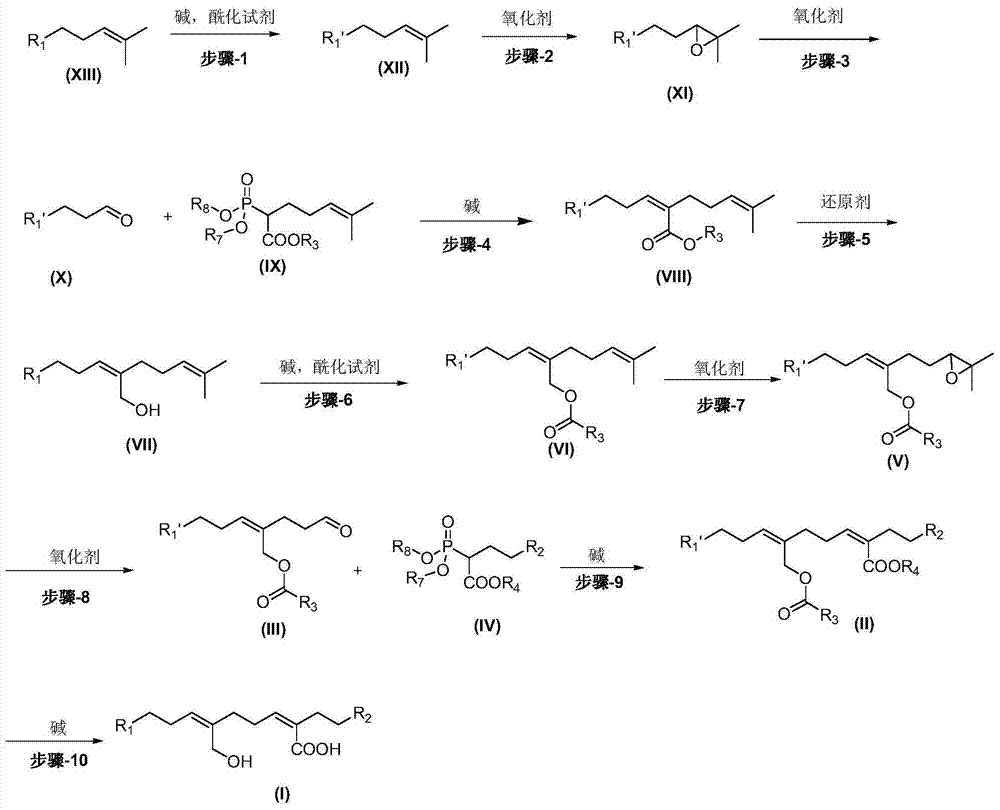
The present invention provides a preventive or therapeutic agent for diabetes mellitus and diabetic complications, which is a new type based on an adenosine A2 receptor antagonist action.That is, it provides a novel condensed imidazole compound which has an adenosine A2 receptor antagonist action, is effective for preventing or treating diabetes mellitus and diabetic complications, and is represented by the formula (I); (wherein R1 represents e.g. an amino group which may be substituted with an alkyl group; R2 represents e.g. hydrogen atom, an alkyl group, a cycloalkyl group or an alkyl group, alkenyl group or alkynyl group which may be substituted with hydrox etc.; R3 represents e.g. an optionally substituted alkyl group, alkenyl group, alkynyl group, aryl group, heteroaryl group, pyridinone group, pyrimidinone group or piperadinone group; Ar represents e.g. an optionally substituted aryl or heteroaryl group; and Q and W are the same as or different from each other and each represents N or CH), a pharmacologically acceptable salt or hydrates thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Organic nitric oxide enhancing salts of angiotensin ii antagonists, compositions and methods of use
The invention describes compositions and kits comprising at least one organic nitric oxide enhancing salt of an angiotensin π antagonist, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The organic nitric oxide enhancing compounds that form salts with the angiotensin II antagonists are organic nitrates, organic nitrites, nitrosothiols, thionitrites, thionitrates, NONOates, heterocyclic nitric oxide donors and / or nitroxides. The heterocyclic nitric oxide donors are furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines.
Owner:NICOX SA
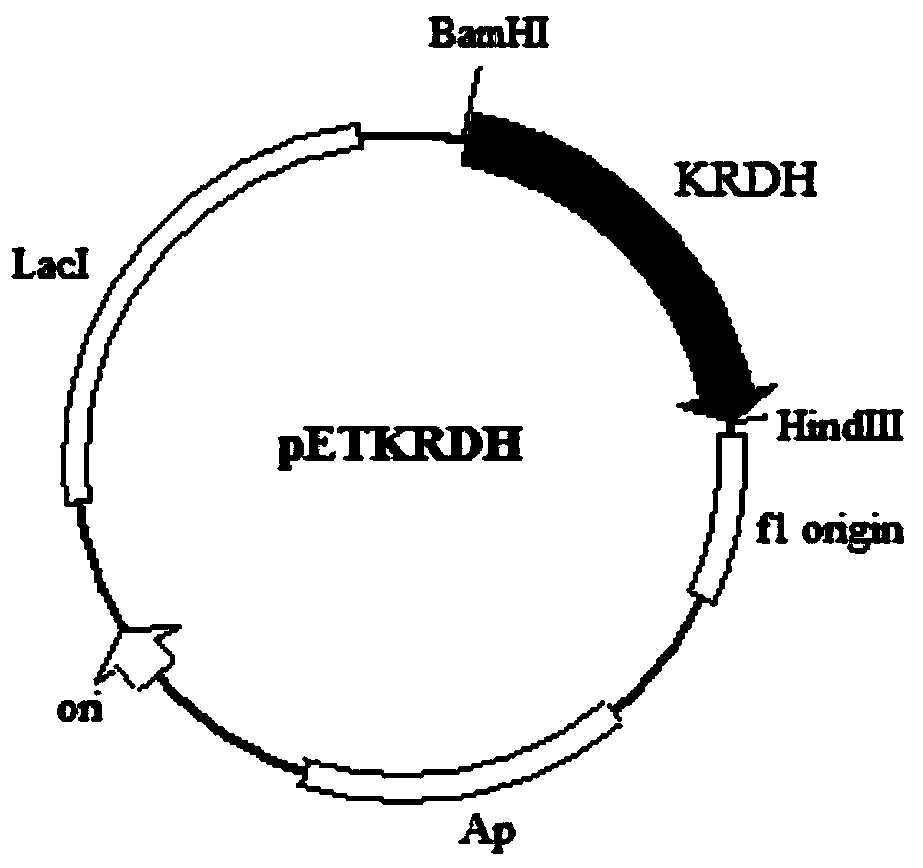
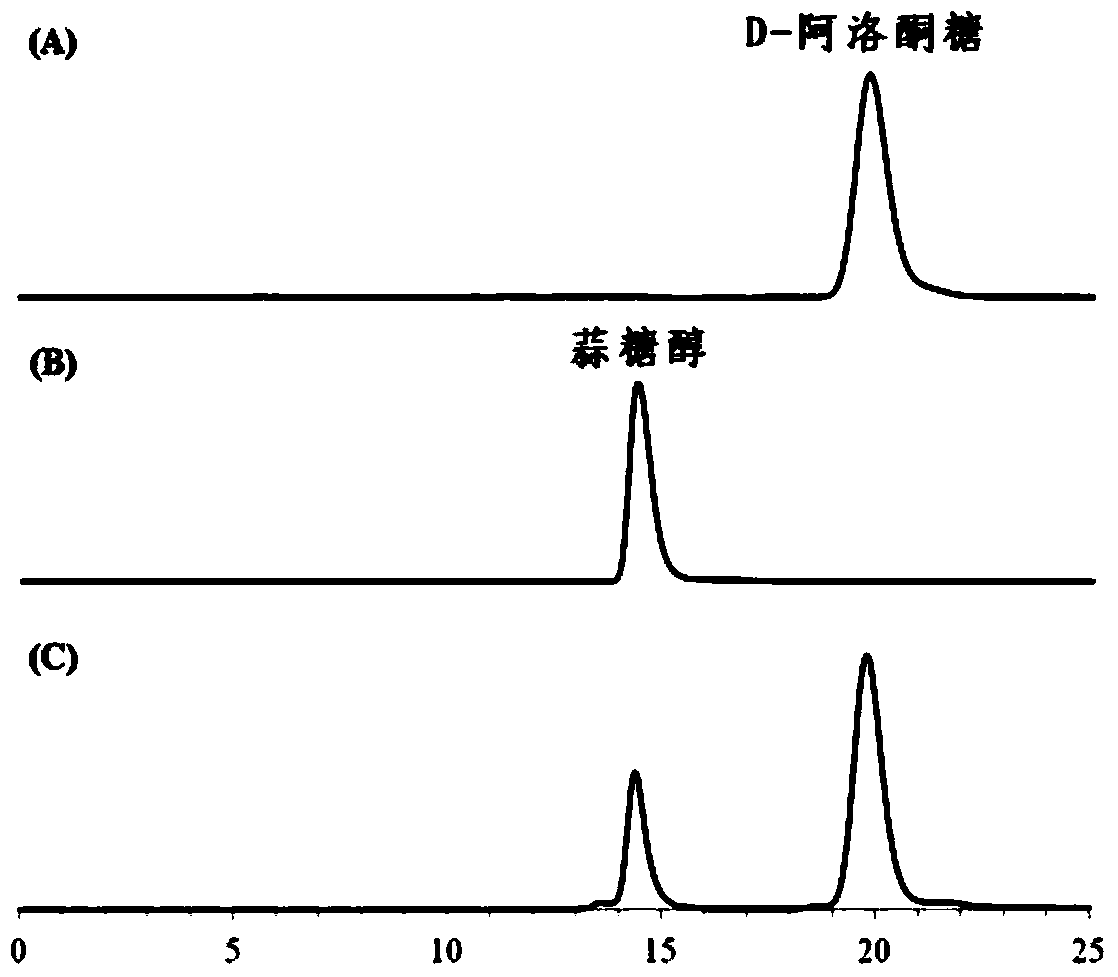
Ribitol dehydrogenase (RDH) derived from Klebsiella oxytoca, and coding gene and application thereof
InactiveCN103397006AReduce dosageImprove conversion rateBacteriaMicroorganism based processesEscherichia coliKlebsiella oxytoca
The invention discloses a ribitol dehydrogenase (RDH) derived from Klebsiella oxytoca G4A4CGMCC No.7662, and realizes expression thereof in Escherichia coli. The experiment proves that the enzyme can realize the biotransformation production of allitol, and D-allulose can be converted into the functional rare sugar alcohol (allitol) with high conversion rate (96% or above at most) and less pollution; and an NADH (reduced nicotinamide adenine dinucleotide) regeneration system is added in the conversion process, and the NAD (nicotinamide adenine dinucleotide) consumption is low, thereby greatly lowering the cost. The allitol can be used in multiple fields such as preparation of pharmaceuticals (such as diabetes treatment medicaments and the like), health products and other raw materials of rare sugar and the like, and has wide application prospects.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Cardiovascular Compounds Comprising Nitric Oxide Enhancing Groups, Compositions and Methods of Use
The invention describes compositions and kits comprising at least one cardiovascular compound comprising at least one nitric oxide enhancing group, or pharmaceutically acceptable salts thereof, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; Q) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The cardiovascular compounds are angiotensin II antagonists, aldosterone antagonists, endothelin antagonists, hydralazine compounds, neutral endopeptidase inhibitors and renin inhibitors. The nitric oxide enhancing groups are nitroxides and / or heterocyclic nitric oxide donors.
Owner:NICOX SA
Chiral CB1 (cannabinoid) receptor inhibitor, and preparation method and medical application thereof
ActiveCN102603713AOvercome Toxicity and Side EffectsLow toxicityNervous disorderOrganic chemistryDrugIsrapafant
The invention relates to a chiral CB1 (cannabinoid) receptor inhibitor which is showed as a general formula A1 and a general formula A2 and has optical asymetery performance, and slat or solvate which is physiologically acceptable, in particular relates to a diaryl-replaced pyrazole ramification CB1 receptor inhibitor which is showed as the general formula A and the general formula B, has optical asymetery performance and is hypotoxic, and the method also discloses a preparation method and an application used for preparing medicaments for detoxification, weight losing, diabetes mellitus treatment or cardiovascular disease preventing, and all application on medical science related with the CB1 receptor inhibitor.
Owner:范如霖 +1
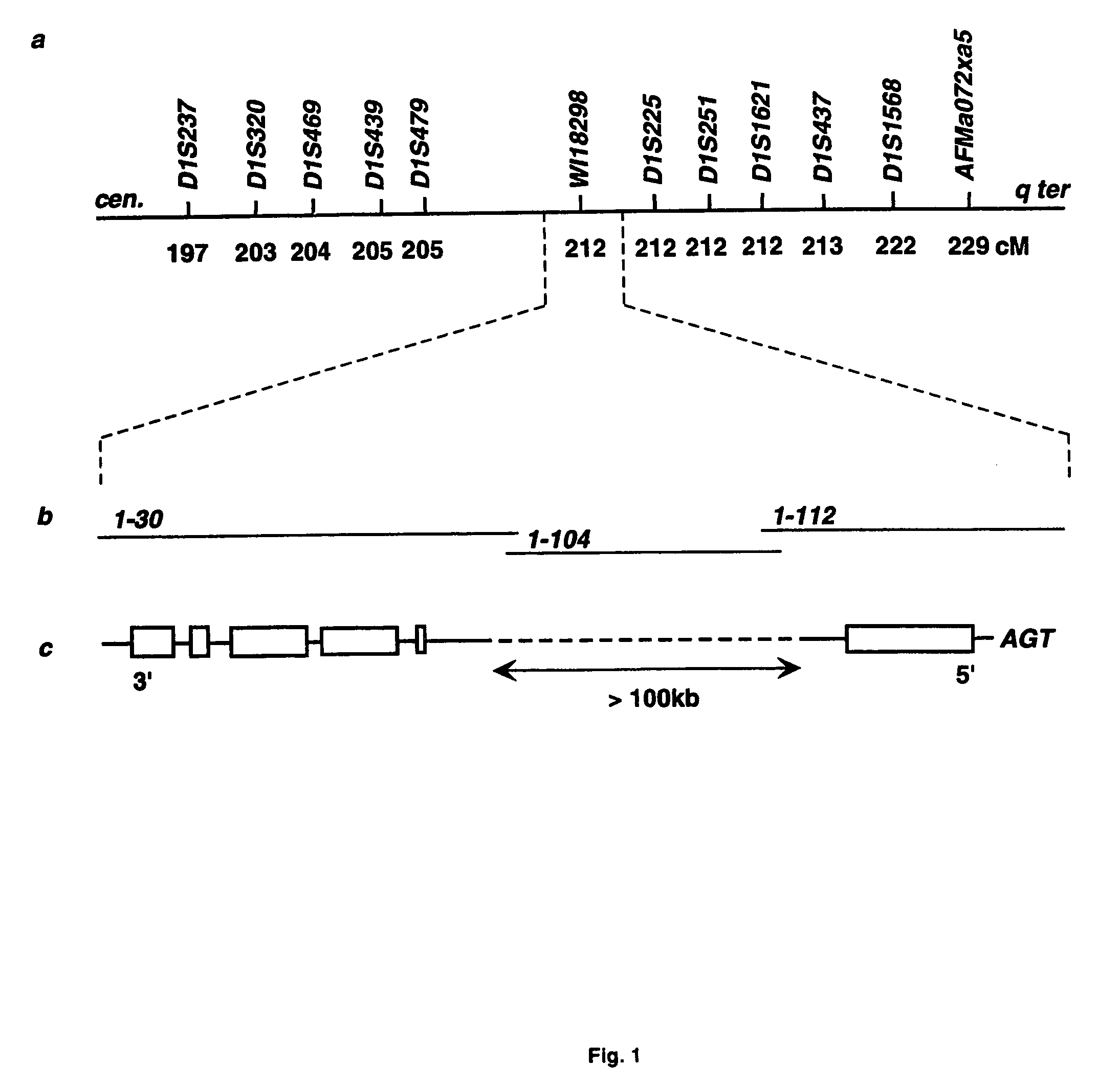
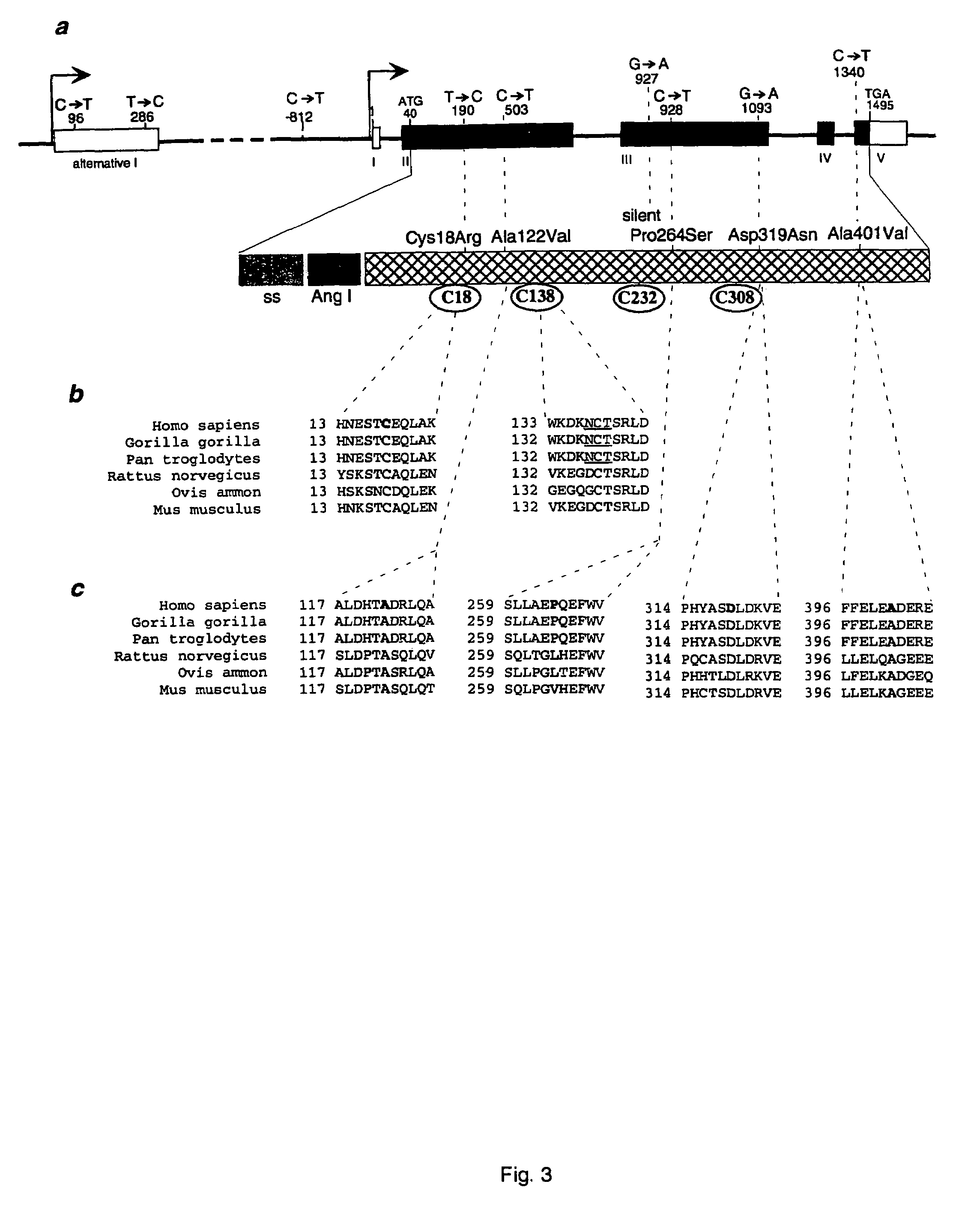
Diabetes gene
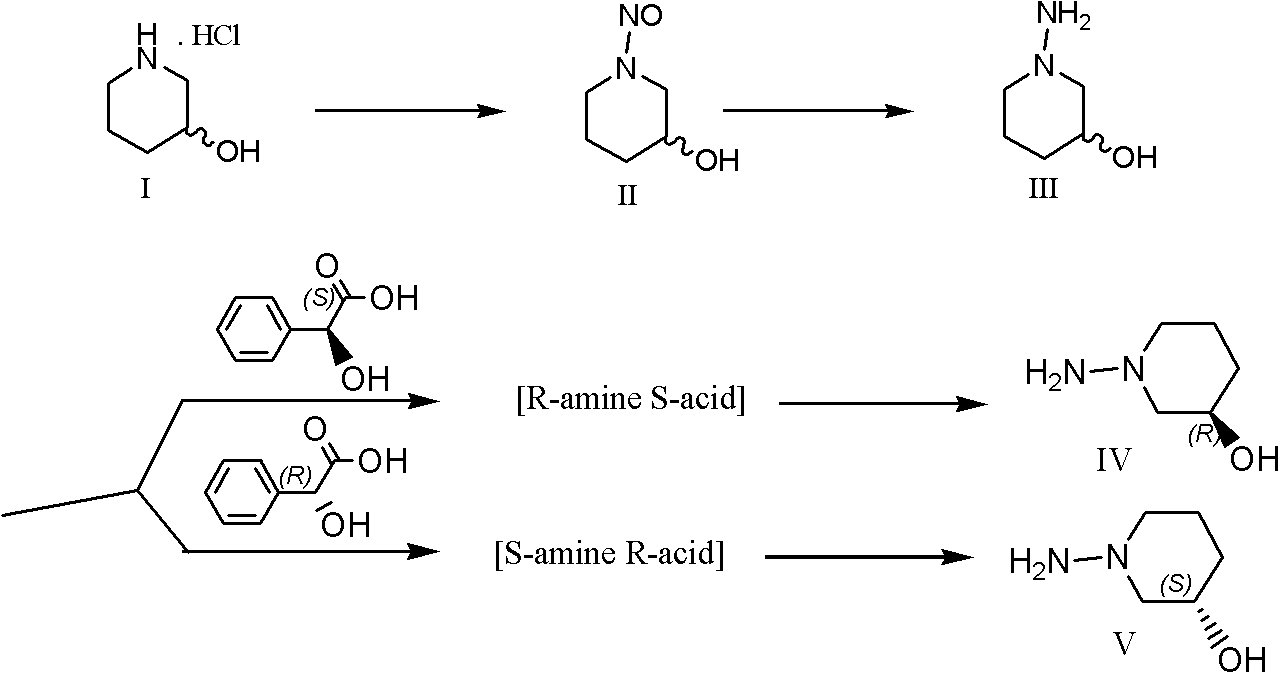
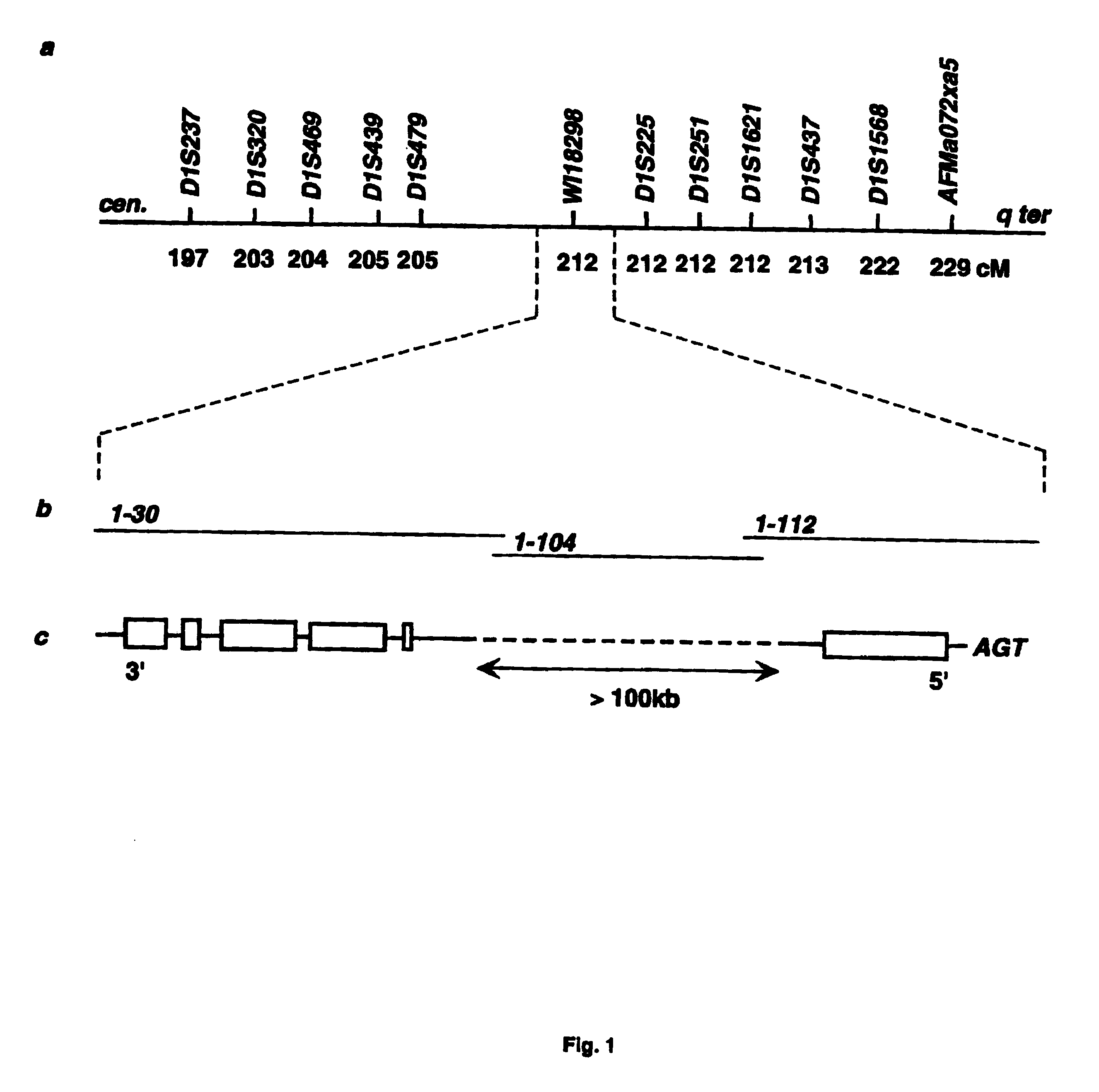
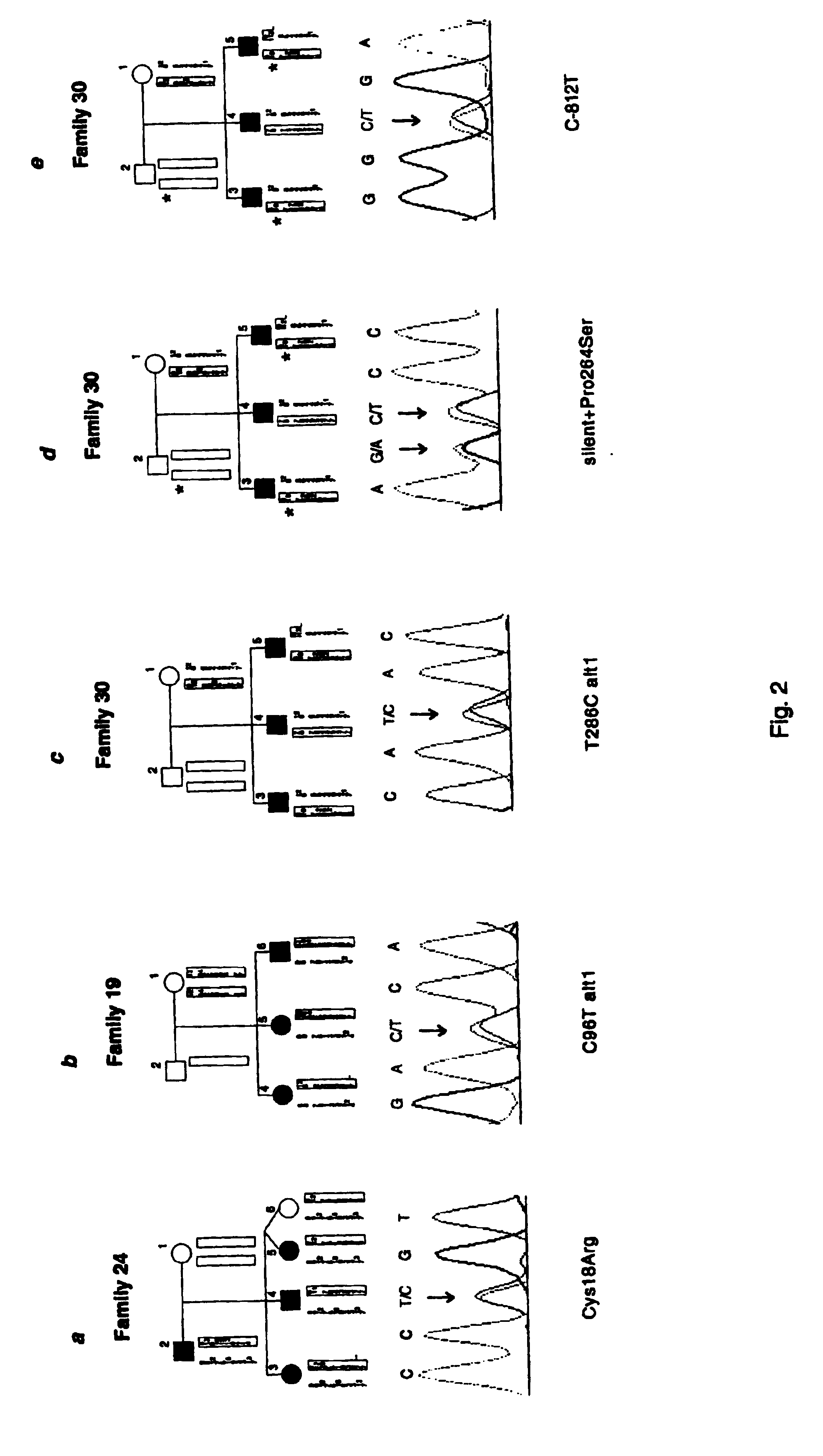
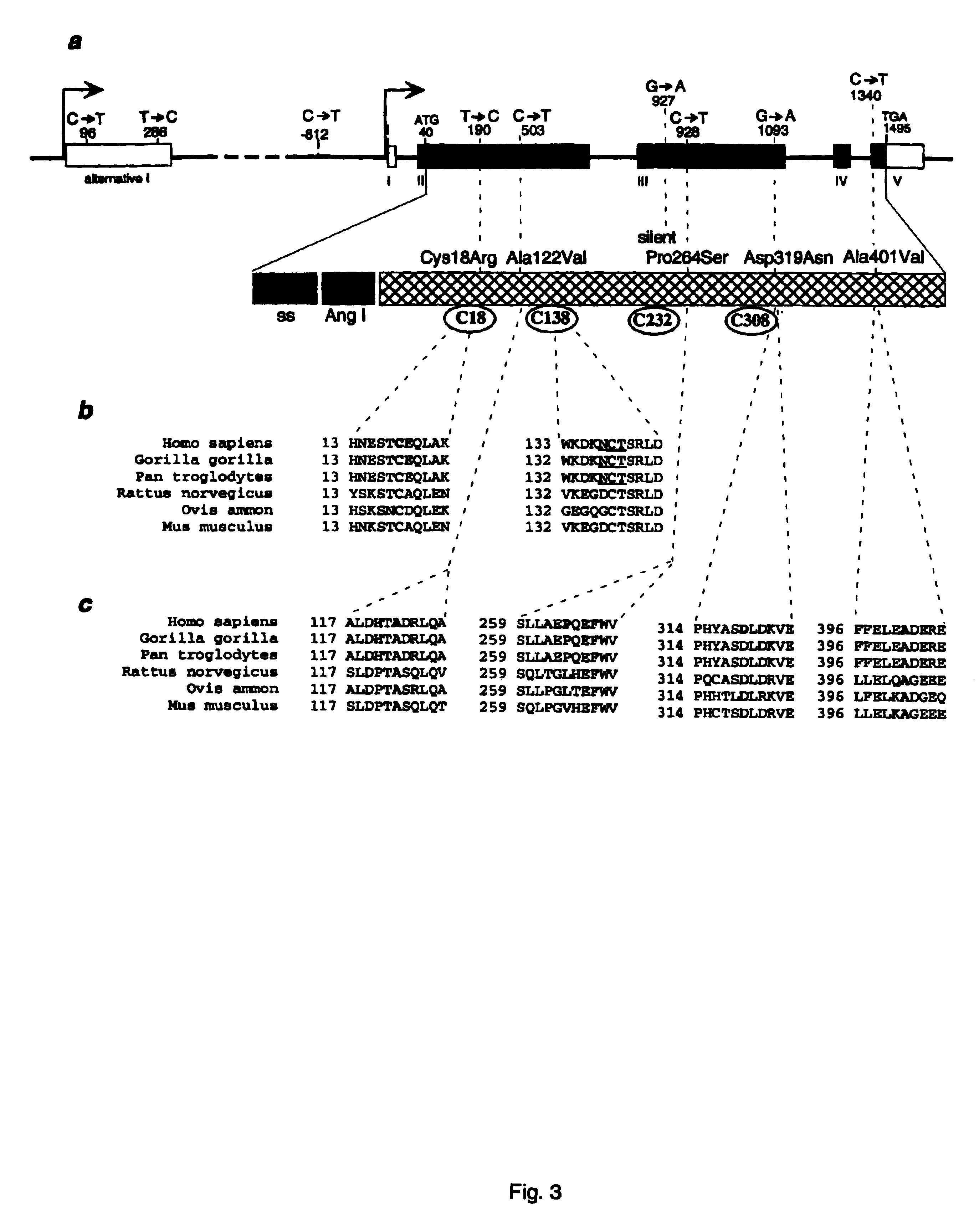
InactiveUS6902888B1Preventing and treating diabetesReduce and prevent gene expressionSugar derivativesPeptide/protein ingredientsInsulin dependent diabetesDiabetes Therapy
The present invention relates generally to the field of human genetics. Specifically, the present invention relates to methods and materials used to isolate and detect human diabetes mellitus predisposing gene, specifically the angiotensinogen (AGT) gene, some mutant alleles of which cause susceptibility to insulin-dependent diabetes mellitus (IDDM). More specifically, the invention relates to gernline mutations in the AGT gene and their use in the diagnosis of predisposition to diabetes. The invention also relates to the prophylaxis and / or therapy of diabetes associated with a mutation in the AGT gene. The invention further relates to the screening of drugs for diabetes therapy. Finally, the invention relates to the screening of the AGT gene for mutations, which are useful for diagnosing the predisposition to diabetes.
Owner:MYRIAD GENETICS
Pyrazole compounds and antidiabetes agents containing the same
InactiveCN101208306AHigh activityNo side effectsOrganic chemistryHeterocyclic compound active ingredientsAzideDiabetes mellitus
A pyrazole compound represented by the general formula (I) or a salt thereof which has a hepatic glycogen phosphorylase inhibitory activity and therefore is useful as a therapeutic or prophylactic agent for diabetes or a pharmacologically salt thereof : wherein the ring Q represents an aryl group or an aromatic heterocyclic group; R<1> represents a hydrogen atom, a halogen atom, a C1-6 alkyl group or a C1-6 alkoxy group; R<2> represents a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group or an azide group; R<3> represents a halogen atom, a hydroxyl group, a C1-6 alkyl group, a halo-C1-6 alkyl group, a C1-6 alkoxy group, an azide group, an amino group, an acylamino group or a C1-6 alkylsulfonylamino group; R<4> and R<5> independently represent a hydrogen atom, a C1-6 alkyl group which may be substituted, a a C3-8 cycloalkyl group, a saturated heterocyclic group which may be substituted, an aryl group which may be substituted, a C7-14 aralkyl group, an aromatic heterocyclic group or the like.
Owner:JAPAN TOBACCO INC
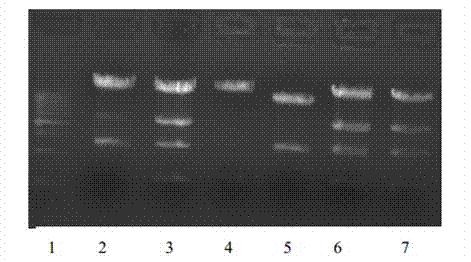
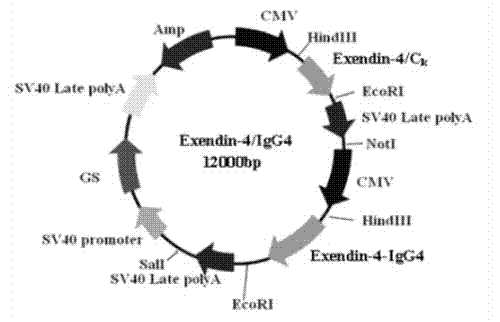
Long-acting immune fusion protein for treating diabetes mellitus
ActiveCN103204944AImprove stabilityExtended half-lifePeptide/protein ingredientsMetabolism disorderApoptosisPancreatic hormone
The invention provides a long-acting immune fusion protein for treating diabetes mellitus. The immune protein is an Exendin-4-IgG4 immune fusion protein. The long-acting immune fusion protein has the advantages that the Exendin-4-IgG4 immune fusion protein has functions of promoting insulin secretion, suppressing programmed apoptosis of beta-cells, stimulating multiplication of the beta-cells, reducing the glucagon level, retarding gastric emptying, reducing ingestion appetite and the like, in-vivo blood glucose is reduced in multiple ways without causing hypoglycemia, the effective phase of the Exendin-4-IgG4 immune fusion protein is longer than that of Exendin-4 immune fusion protein, a certain weight loss effect can be realized after Exendin-4-IgG4 immune fusion proteins are used for a long time, and the long-acting immune fusion protein can be used for treating type-II diabetes mellitus.
Owner:JIANGSU JIANDE BIOLOGICAL PHARMA
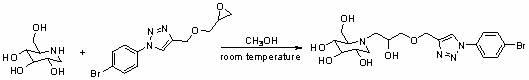
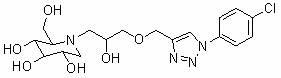
Use of derivatives of 1-deoxynojiri mycin containing 1,2,3-triazoles as alpha-glucosidase inhibitors
The invention belongs to the field of chemical medicaments, particularly relates to use of derivatives of 1-deoxynojiri mycin containing 1,2,3-triazoles as alpha-glucosidase inhibitors. The derivatives of 1-deoxynojiri mycin containing 1,2,3-triazoles have the activity of inhibiting alpha-glucosidase. Therefore, the e derivatives can be used for treating diseases such as diabetes and have a quite wide application prospect.
Owner:FUDAN UNIV
Dihydroorotic acid dehydrogenase inhibitor
InactiveCN104582694AGood DHOD inhibitory activityAntibacterial agentsOrganic chemistryDiseaseRheumatism
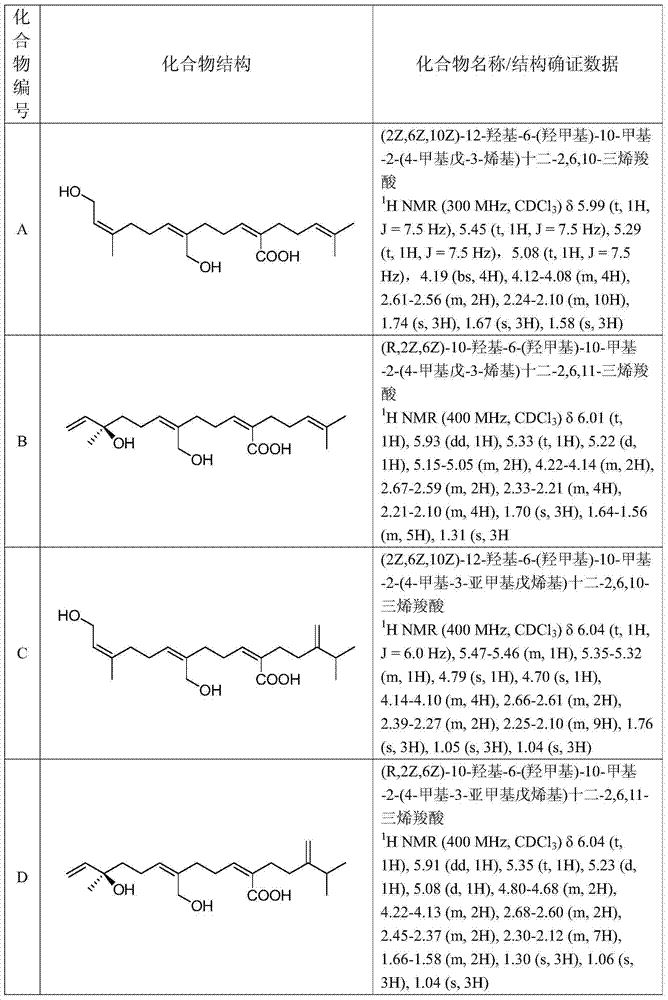
The present invention provides a novel dihydroorotic acid dehydrogenase inhibitor which is applicable to various diseases. When used as an active ingredient, a compound represented by formula (I): (wherein X represents a halogen atom, R 1 represents a hydrogen atom, R 2 represents an alkyl group containing 1 to 7 carbon atoms, R 3 represents -CHO, and R 4 represents -CH 2 -CH=C(CH 3 )-R 0 (wherein R 0 represents an alkyl group containing 1 to 12 carbon atoms which may have a substituent on the terminal carbon and / or on a non-terminal carbon, etc.)), an optical isomer thereof or a pharmaceutically acceptable salt thereof has a high inhibitory effect on dihydroorotic acid dehydrogenase and can be used as an immunosuppressive agent, a therapeutic agent for rheumatism, an anticancer agent, a therapeutic agent for graft rejection, an antiviral agent, an anti- H . pylori agent, a therapeutic agent for diabetes or the like.
Owner:INST OF MITOCHONDRIA SCI INC
Non-invasive near-infrared light-controlled nano material for treating diabetes mellitus
ActiveCN111840551AAvoid takingGet rid of the bondagePowder deliveryMaterial nanotechnologyFluorescenceGlycan metabolism
The invention relates to a non-invasive near-infrared light-controlled nano material for treating diabetes mellitus, and claims to protect the application of an up-conversion fluorescence nano material in preparation of a tool for treating diabetes mellitus. The up-conversion fluorescence nano material comprises a rare earth element doped inorganic nano material, a hepatocyte targeting molecule and a water-soluble polymer. The invention discloses a new application of the non-invasive up-conversion fluorescent nano material. An invasive optical fiber does not need to be implanted into an animalthrough an operation during diabetes treatment, near-infrared light with high tissue penetrability is used for exciting an up-conversion nano material in a living body, and light in the near-infraredband is converted into visible light through the up-conversion material so that photosensitive protein is activated, and glucose metabolism related signal pathways in cells are remotely regulated andcontrolled without relying on insulin under the condition of high time-space resolution, glycogen synthesis is promoted, glycogenesis is inhibited, and the blood glucose level is reduced.
Owner:SUZHOU UNIV
Insulin-loaded nanoparticles and application thereof
ActiveCN108721605AUniform sizeImprove thermal stabilityPeptide/protein ingredientsMetabolism disorderNanoparticleGlucose lowering
The invention discloses insulin-loaded nanoparticles. The insulin-loaded nanoparticles are obtained by carrying out reversible crosslinking reaction on glucomannan, concanavalin A and a crosslinking agent. The invention develops glucose induction type insulin oral nanoparticles prepared from a natural polymer; a glucose induction type insulin oral transportation system can be constructed by utilizing the nanoparticles; the system has basic properties of a transportation system which has glucose induction performance, can control blood glucose and has low toxin; the insulin-loaded nanoparticleshave a relatively good effect in bodies and have a slow glucose lowering effect for relatively long time; the blood glucose control curative effect of the glucose induction type insulin oral transportation system is improved, and the insulin-loaded nanoparticles have relatively great application potential in the fields of development of diabetes mellitus treatment, natural polymer medical functions and the like.
Owner:SOUTH CHINA NORMAL UNIVERSITY
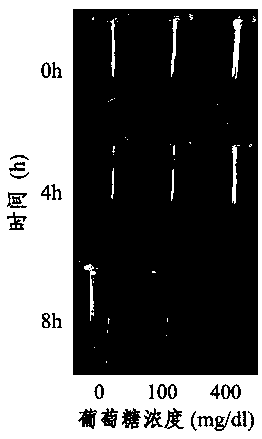
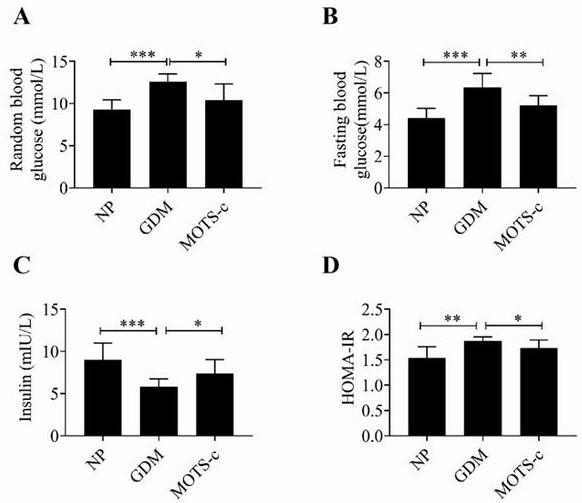
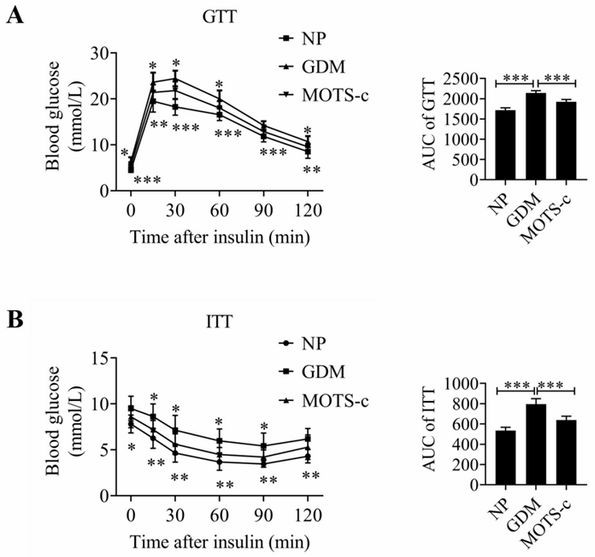
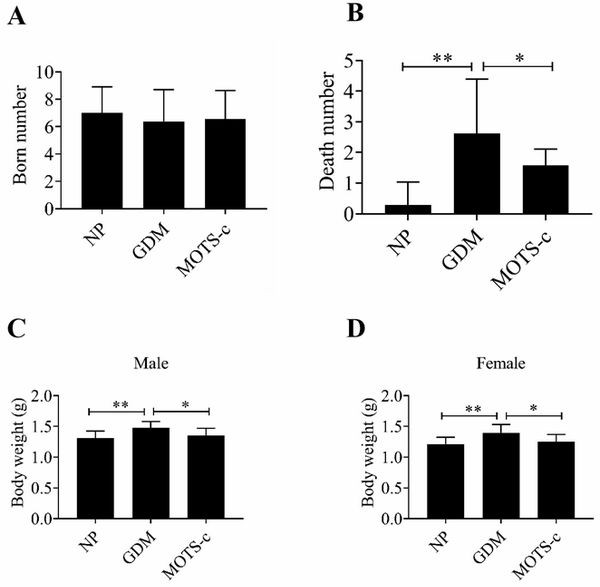
Application of MOTS-c peptide and derivative thereof in preparation of medicine for treating gestational diabetes mellitus
PendingCN113440599ALower blood sugar levelsReduce resistancePeptide/protein ingredientsMetabolism disorderPregnancyPancreatic hormone
The invention provides application of mitochondria-derived polypeptide MOTS-c and a derivative thereof in preparation of a medicine for treating gestational diabetes mellitus (GDM), and belongs to the technical field of biomedicine. The MOTS-c is highly conserved in humans, rodents and various mammals, and it is found that the plasma MOTS-c content of a GDM pregnant woman is obviously reduced; the MOTS-c treatment can reduce pregnancy blood glucose of GDM model mice, improve insulin resistance, improve glucose tolerance, reduce offspring birth weight, protect pancreas islet functions and maintain insulin secretion; and therefore, the mitochondria-derived polypeptide MOTS-c has the potential of preparing medicines for preventing and treating GDM and complications thereof.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
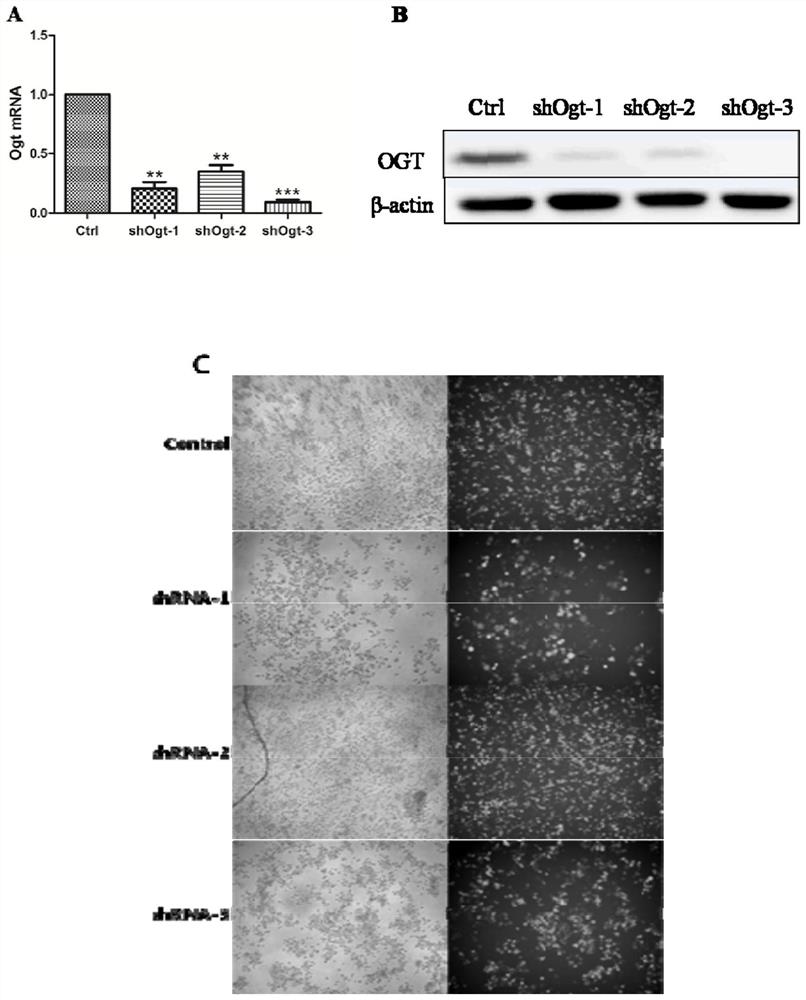
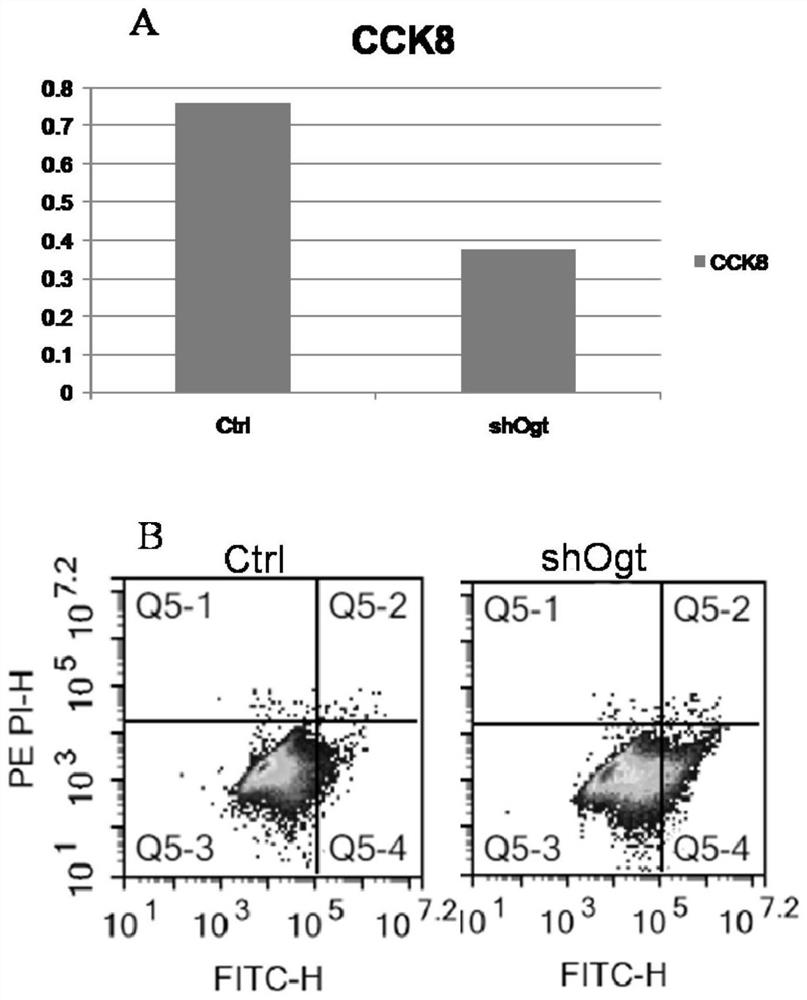
Application of OGT as target in preparation of medicine for treating abnormal secretion of glucagon in diabetes
InactiveCN112891540AReduce secretionPromote witheringMetabolism disorderMicrobiological testing/measurementAntiendomysial antibodiesIslet cells
The invention discloses application of OGT as a target in preparation of a medicine for treating abnormal secretion of glucagon in diabetes. The medicine is a medicine for inhibiting expression of O-GlcNAc glycosyltransferase OGT, wherein the functional component can be at least one of shRNA, siRNA, dsRNA, miRNA, cDNA, antisense RNA / DNA, a low molecular compound, peptide and an antibody. Studies find that the secretion amount of glucagon can be reduced by knocking out or inhibiting O-GlcNAc glycosyltransferase OGT, meanwhile, withering of islet alpha cells can be promoted, proliferation of the islet alpha cells is inhibited, and O-GlcNAc glycosyltransferase OGT can be used as a target for treating diabetes.
Owner:BINZHOU MEDICAL COLLEGE
Polypeptide nanoparticle for treating diabetes mellitus, polypeptide nanoparticle microneedle and preparation method of polypeptide nanoparticle microneedle
InactiveCN111053891AImprove stabilityImprove mechanical propertiesPowder deliveryPeptide/protein ingredientsTransdermal patchChitosan nanoparticles
The invention belongs to the technical field of pharmaceutical preparations and preparation methods thereof, and particularly relates to a polypeptide nanoparticle for treating diabetes, a polypeptidenanoparticle microneedle and a preparation method of the polypeptide nanoparticle microneedle. The method comprises the following steps: 1) preparing chitosan nanoparticles entrapped with exenatide;2) preparing a polymer composite microneedle with strong hardness, high solubility and good biocompatibility by a physical crosslinking technology; and 3) freeze-drying the nanoparticles into a powder, and loading the powder into the polymer microneedle to prepare the dry powder drug-loaded microneedle. The exenatide nanoparticle freeze-dried powder is loaded by utilizing holes naturally formed inthe preparation process of the polymer microneedle to prepare the exenatide dry powder microneedle for treating type 2 diabetes. A exenatide chitosan nanoparticle microneedle transdermal patch disclosed by the invention can effectively reduce the risks of strong inflammation and immune system reaction caused by the medicine, so the painless, needleless, transdermal and slow blood glucose-releasing purposes are achieved.
Owner:ZHEJIANG UNIV OF TECH
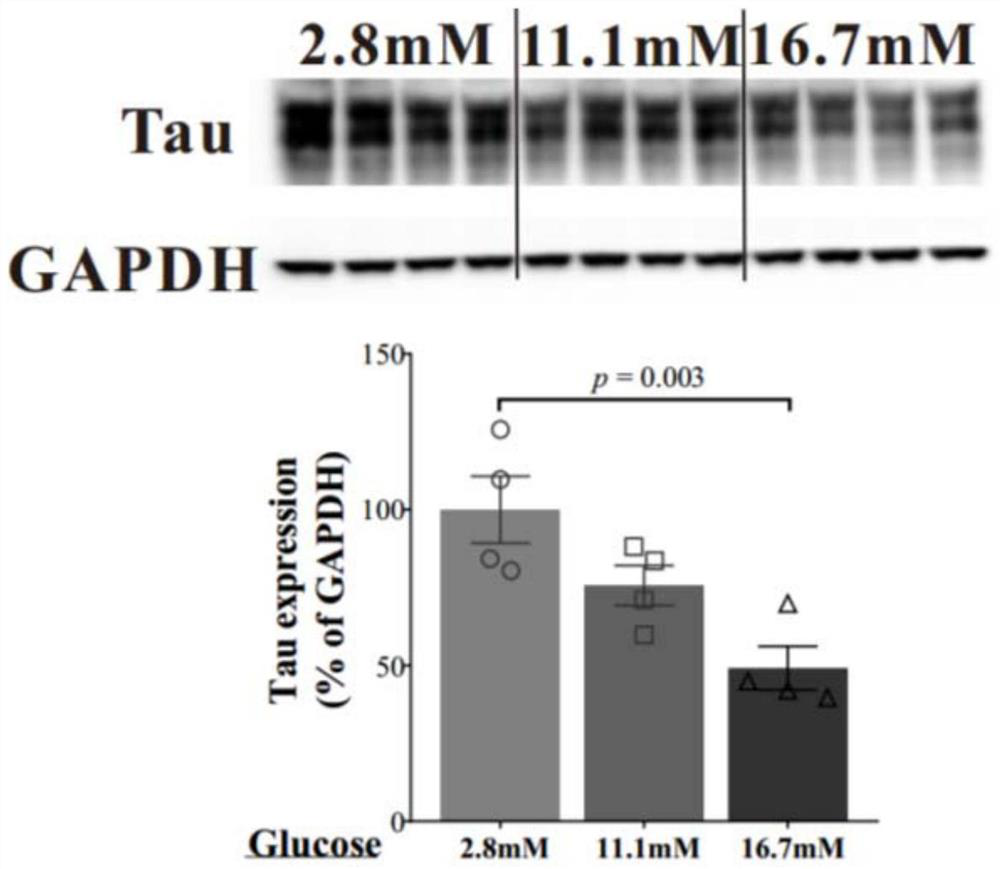
Application of tau protein and genes thereof as drug targets in preparation of drugs for treating diabetes mellitus
ActiveCN112569354AInhibitory activityPromote secretionMetabolism disorderPharmaceutical active ingredientsPharmaceutical drugPancreatic hormone
The invention discloses tau protein and application of a gene of the tau protein as a drug target in preparation of a drug for treating diabetes, and relates to the technical field of diabetes treatment. The invention discloses an application of a reagent taking tau protein or tau gene as a target point in preparation of a medicine for preventing or treating diabetes mellitus. Embodiment of the invention creatively shows that tau protein has an important regulation and control effect in insulin secretion, and diabetes mellitus can be treated by targeting the tau protein or the tau gene. The invention provides a new thought and strategy for diabetes treatment and drug research and development or screening.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Diabetes gene
InactiveUS7374884B2Preventing and treating diabetesImprove disease symptomsSugar derivativesPeptide/protein ingredientsInsulin dependent diabetesDiabetes Therapy
The present invention relates generally to the field of human genetics. Specifically, the present invention relates to methods and materials used to isolate and detect human diabetes mellitus predisposing gene, specifically the angiotensinogen (AGT) gene, some mutant alleles of which cause susceptibility to insulin-dependent diabetes mellitus (IDDM). More specifically, the invention relates to germline mutations in the AGT gene and their use in the diagnosis of predisposition to diabetes. The invention also relates to the prophylaxis and / or therapy of diabetes associated with a mutation in the AGT gene. The invention further relates to the screening of drugs for diabetes therapy. Finally, the invention relates to the screening of the AGT gene for mutations, which are useful for diagnosing the predisposition to diabetes.
Owner:MYRIAD GENETICS
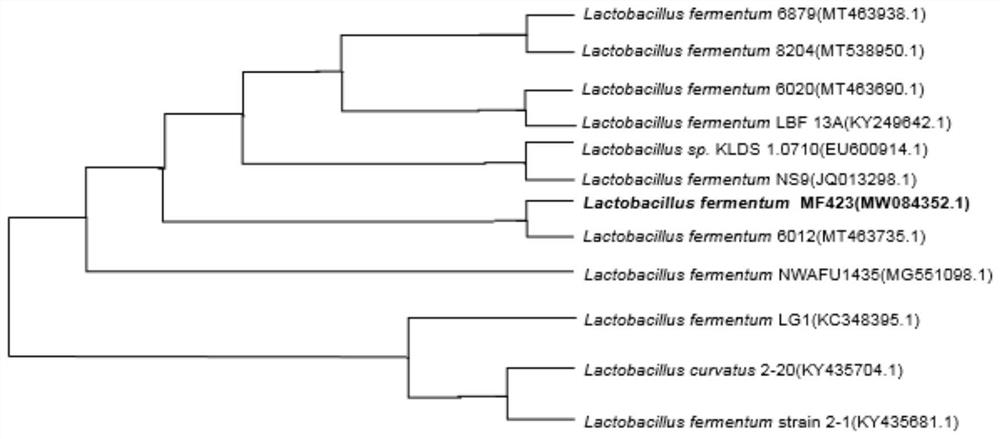
Lactobacillus fermentum MF423, fermented rice bran extract thereof and application of lactobacillus fermentum MF423 and fermented rice bran extract
ActiveCN112940967AImproves antioxidant activityIncrease glucose consumptionBacteriaMicroorganism based processesLactobacillus fermentumPancreatic hormone
The invention relates to the technical field of microorganisms, in particular to lactobacillus fermentum MF423 from rice flour fermentation wastewater and application of the lactobacillus fermentum MF423. The lactobacillus fermentum MF423 fermented rice bran extract disclosed by the invention has an effect of improving insulin resistance in vivo and in vitro. Through determination, on the cell and animal level, the rice bran extract fermented by the bacterium has relatively high antioxidant activity, and can be used for remarkably increasing the consumption of glucose in a culture medium by cells, reducing the blood sugar of mice, remarkably eliminating lipid accumulation of the mice and inhibiting the occurrence of low-grade inflammation, and has important value in subsequent efficient utilization of low-value resource rice bran and application of the rice bran in clinical treatment of the type 2 diabetes mellitus.
Owner:CENT SOUTH UNIV
Nitrosated And Nitrosylated Cardiovascular Compounds, Compositions And Methods Of Use
InactiveUS20070238740A1Improve propertiesBiocideSenses disorderRenovascular diseaseAngiotensin ii antagonist
The invention describes novel nitrosated and / or nitrosylated cardiovascular compounds or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitrosated and / or nitrosylated cardiovascular compound, and, optionally, at least one nitric oxide donor and / or at least one therapeutic agent. The invention also provides novel compositions and kits comprising at least one cardiovascular compound of the invention, that is optionally nitrosated and / or nitrosylated, and, optionally, at least one nitric oxide donor compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; and (k) treating nephropathy. The nitrosated and / or nitrosylated cardiovascular compounds are preferably nitrosated and / or nitrosylated aldosterone antagonists, nitrosated and / or nitrosylated angiotensin II antagonists, nitrosated and / or nitrosylated calcium channel blockers, nitrosated and / or nitrosylated endothelin antagonists, nitrosated and / or nitrosylated hydralazine compounds, nitrosated and / or nitrosylated neutral endopeptidase inhibitors and nitrosated and / or nitrosylated renin inhibitors.
Owner:NICOX SA
DcR3 (Decoy Receptor 3) and GAD65 (Glutamic Acid Decarboxylase 65) double gene co-expression recombinant adenovirus as well as preparation method and application thereof
InactiveCN101875920ASuppression of immune toleranceProlong lifeMicroorganism based processesViruses/bacteriophagesGene terminatorPancreatic islet transplantation
Owner:ARMY MEDICAL UNIV
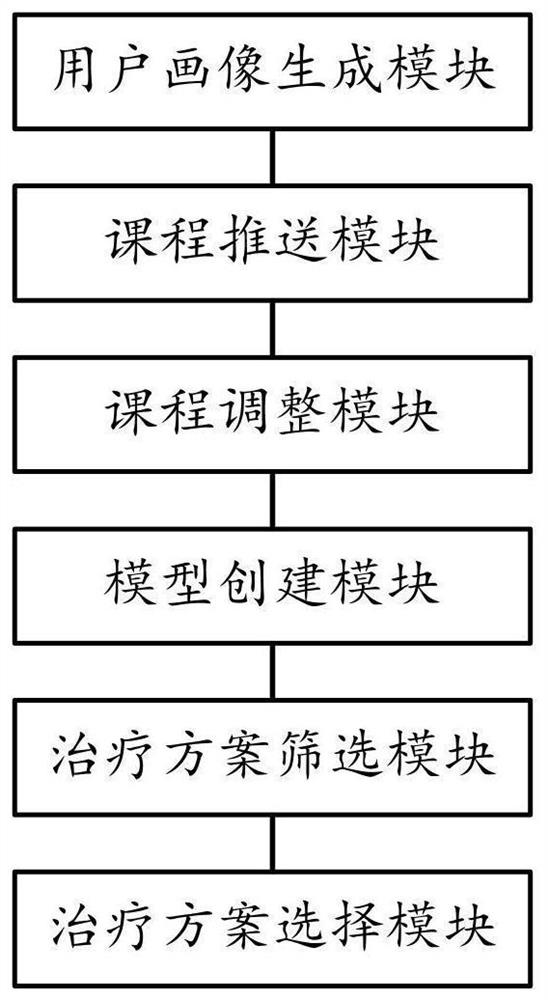
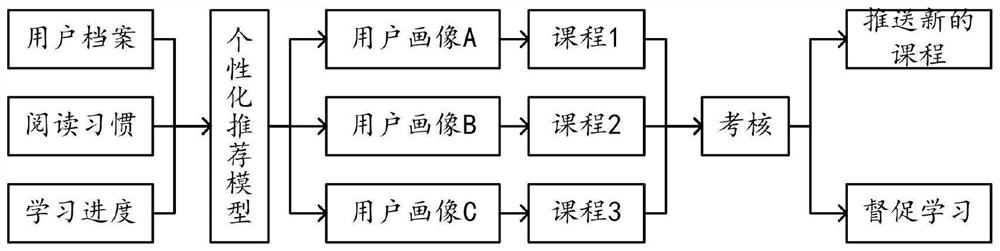
Diabetic patient education and treatment scheme adjustment method and system
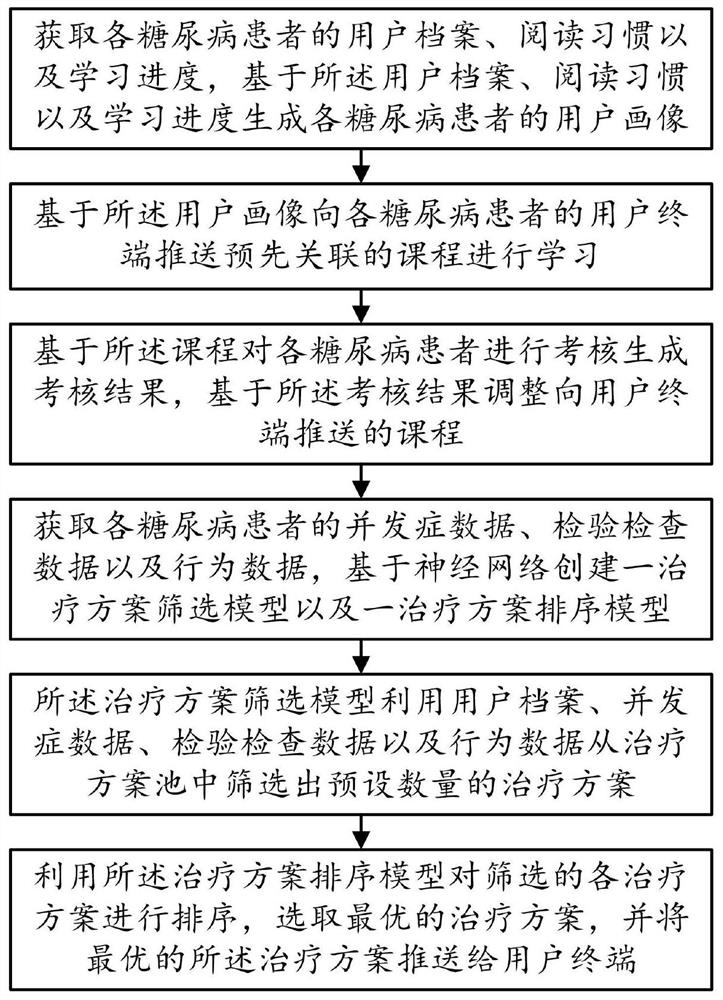
PendingCN114171152AGood treatment effectSteady and steadyDigital data information retrievalTherapiesDiabetes managementBehavioral data
The invention provides a diabetic patient education and treatment scheme adjustment method and system in the technical field of diabetes management. The method comprises the following steps: step S10, obtaining a user file, a reading habit and a learning progress to generate a user portrait of a diabetic patient; step S20, pushing courses based on the user portrait; s30, examining the diabetic patient based on the course, and adjusting the pushed course; step S40, acquiring complication data, examination data and behavior data, and creating a treatment scheme screening model and a treatment scheme sorting model; s50, the treatment scheme screening model screens a treatment scheme by using the user file, the complication data, the inspection data and the behavior data; and step S60, sorting the treatment schemes by using the treatment scheme sorting model, selecting an optimal treatment scheme, and pushing the optimal treatment scheme to the user terminal. The system and the method have the advantages that the education of the diabetic patient and the dynamic adjustment of the treatment scheme are realized, so that the diabetes treatment effect is greatly improved.
Owner:福州康为网络技术有限公司
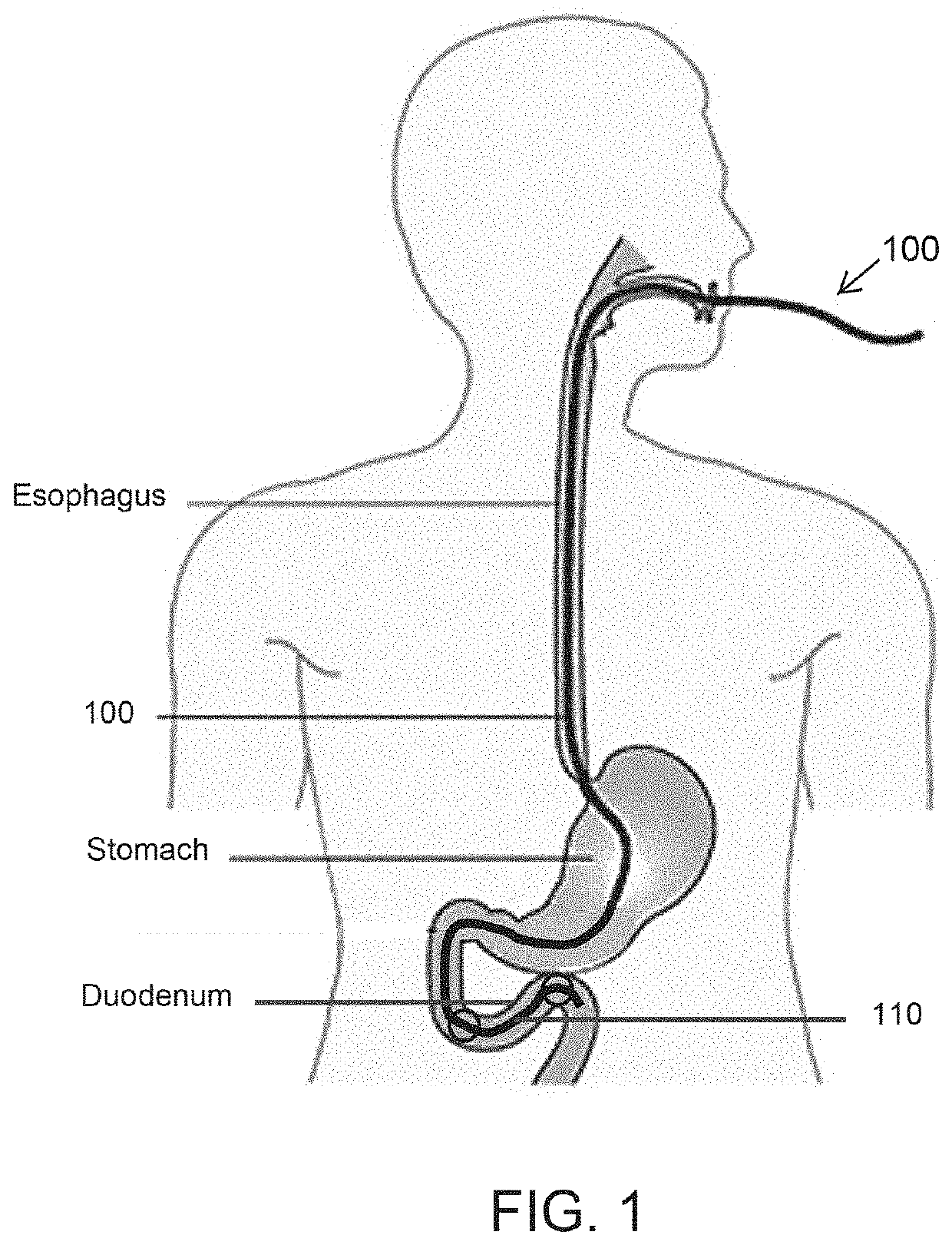
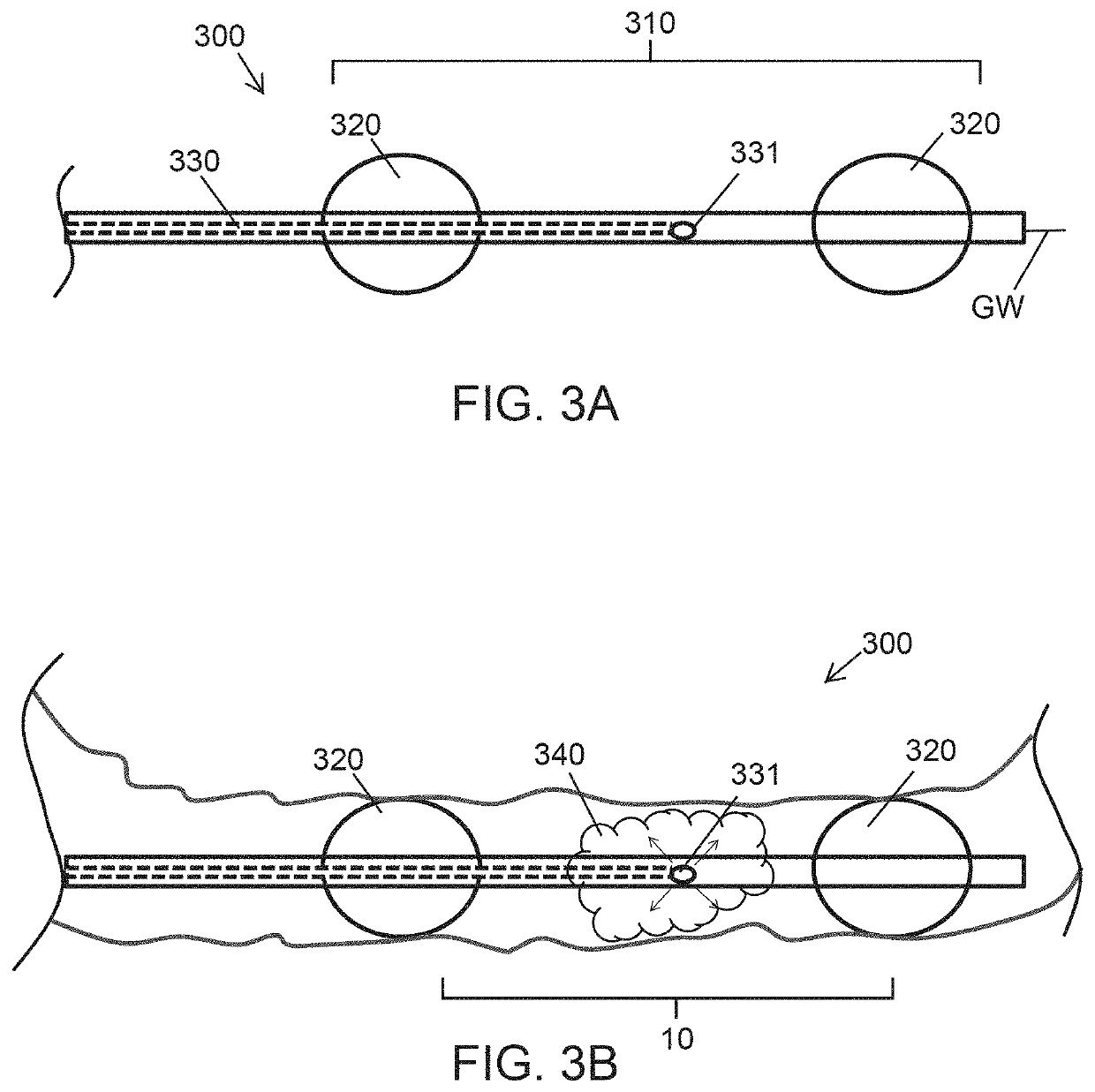
Diabetes Treatment Methods and Devices
Endoluminal devices and methods that facilitate treatment of a desired treatment region of the gastrointestinal tract, in particular the duodenum, are provided herein. Such devices include a catheter having a treatment delivery portion disposed between proximal and distal balloons. The treatment can include thermal ablation of the treatment region by delivering a treatment fluid to the treatment region between the inflated balloons. In one aspect, the treatment fluid is delivered so as to fill the entire treatment region between the balloons without regard to the inflated balloon pressure. To ensure filling, the treatment fluid can be delivered into the treatment region until a pressure increase is observed or until a pre-determined volume is delivered. Other treatment devices and methods include means of uniformly distributing a treatment gas for plasma ablation, electrical ablation energy or a chemical or drug eluting stent.
Owner:METABOSCOPY MEDICAL INC
Traditional Chinese medicine composition for treating type II diabetes and application
ActiveCN113521134AImprove clinical symptomsHigh fasting blood sugarMetabolism disorderPlant ingredientsDyslipidemiaLiver functions
The invention discloses a traditional Chinese medicine composition for treating type II diabetes and application. The traditional Chinese medicine composition comprises the following components: 10-25 parts of cocculus orbiculatus, 15-50 parts of cassia twig, 15-40 parts of coptis chinensis, 20-80 parts of poria cocos, 20-50 parts of radix glehniae and 20-50 parts of trichosanthes kirilowii maxim (kernel). The traditional Chinese medicine composition can be used for preparing a preparation for treating type II diabetes. The traditional Chinese medicine composition has a good curative effect on high fasting blood glucose, postprandial blood glucose rise, dyslipidemia and particularly insulin resistance caused by type 2 diabetes, and can remarkably improve clinical symptoms of type 2 diabetes patients; the composition has no obvious damage to the liver function, and has a protective effect, so that the safety is relatively high, and the compound has a wide application prospect in the field of treatment of type II diabetes mellitus.
Owner:北京精医和生医药科技有限公司
Preparation method of smallanthus sonchifolitus diterpene acid compound and compound obtained by using preparation method
ActiveCN103880653AStrong inhibitory activityOrganic active ingredientsOrganic compound preparationAlgluceraseCompound a
The invention discloses a preparation method of a smallanthus sonchifolitus diterpene acid compound and a compound obtained by using the preparation method. The smallanthus sonchifolitus diterpene acid is prepared by reacting a compound as shown in the specification with an acidifying agent, and by performing a series of oxidation reaction, reduction reaction and hydrolysis reaction. The smallanthus sonchifolitus diterpene acid compound and a midbody compound thereof can be prepared by using the method disclosed by the invention, in-vitro experience shows that a compound A has good inhibition activity for alpha-glucosidase, the activity degree is equivalent to that of a diabetes therapeutic medicine glucobay, and the compound can be applied to preparation of anti-diabetic medicines.
Owner:珍奥集团股份有限公司
Method For Detecting And Purifying Pancreatic Beta Cells
InactiveUS20120070847A1Improve treatmentEasy diagnosisAntibody mimetics/scaffoldsPancreatic cellsPancreatic isletsMammalian cell
The invention is based, in part, on the discovery that a polypeptide, referred to herein as Betacam, is selectively expressed on the surface of pancreatic islet cells, Thus, in one aspect, the invention is directed to compositions comprising Betacam or that can be used to detect Betacam. In another aspect, the invention provides methods of detecting (e.g., non-invasively) pancreatic beta cells from a mammalian cell source. Another aspect of the invention is directed to cellular purification of pancreatic beta cells from a heterogeneous cell source of multiple kinds. In another aspect, the invention provides methods of identifying agents that modulate activity of Betacam-In yet another aspect, the invention provides for improved treatment and diagnosis of diabetes.
Owner:THE CLEVELAND CLINIC FOUND +1
Method for acquisition of sugar-reducing medicine and sugar-reducing medicine
InactiveCN107034264AReduce R&D costsGeneral adaptabilityCompounds screening/testingOrganic active ingredientsPancreatic structureInsulin resistance
The invention discloses a method for acquisition of a sugar-reducing medicine and the sugar-reducing medicine, and relates to the technical field of the medical treatment and public health. The method includes steps of through inhibiting inflammation, improving the insulin secretion capacity and the insulin resistance capacity of an early-stage INS-1 pancreas islet Beta cell line and a rodent experimental animal, and realizing the reduction of blood sugar, specifically using a pharmaceutical containing a p38 MAPK inhibitor ingredient SB203580 to realize. The provided sugar-reducing medicine is capable of preparing a pharmaceutical for nursing, delaying and / or treating diabetes or future application in a health care product. The p38 MAPK inhibitor SB203580 is capable of remarkably improving the insulin secretion capacity and the insulin resistance capacity of diabetes patient pancreas islet Beta cells, reducing the blood sugar level of the diabetes patient, and delaying and improving the development of the diabetes through inhibiting the inflammation, and has extensive application prospect in the treatment and nursing of the diabetes.
Owner:PEKING UNIV FIRST HOSPITAL
Modified pig islets for diabetes treatment
InactiveUS20130336940A1Increase insulin productionCAMP level increasedBiocidePancreatic cellsBlood sugarPancreatic islets
The present invention relates to a modified pig islet capable of producing higher levels of glucagon than a native pig islet or capable of producing a glucagon analog, and methods for obtaining thereof. The invention also relates to a method for treating Diabetes Mellitus, and / or for regulating blood glucose levels in a subject in need thereof, comprising the administration of the modified pig islets of the invention.
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN
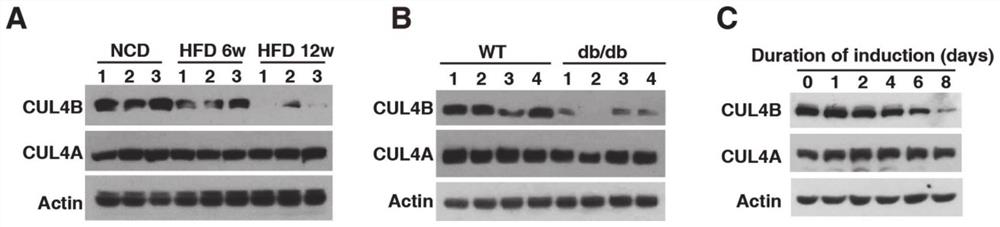
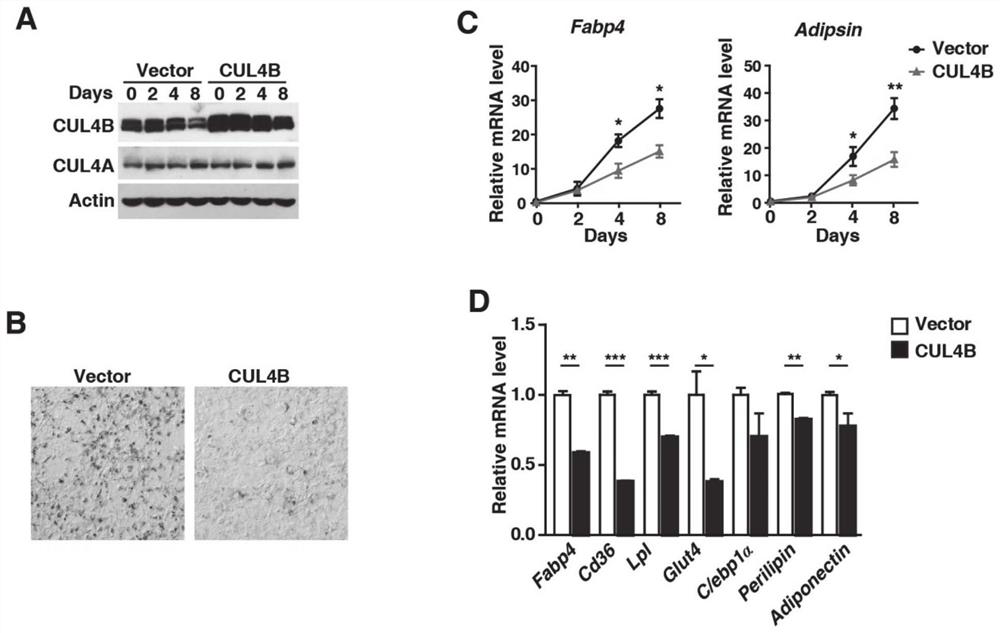
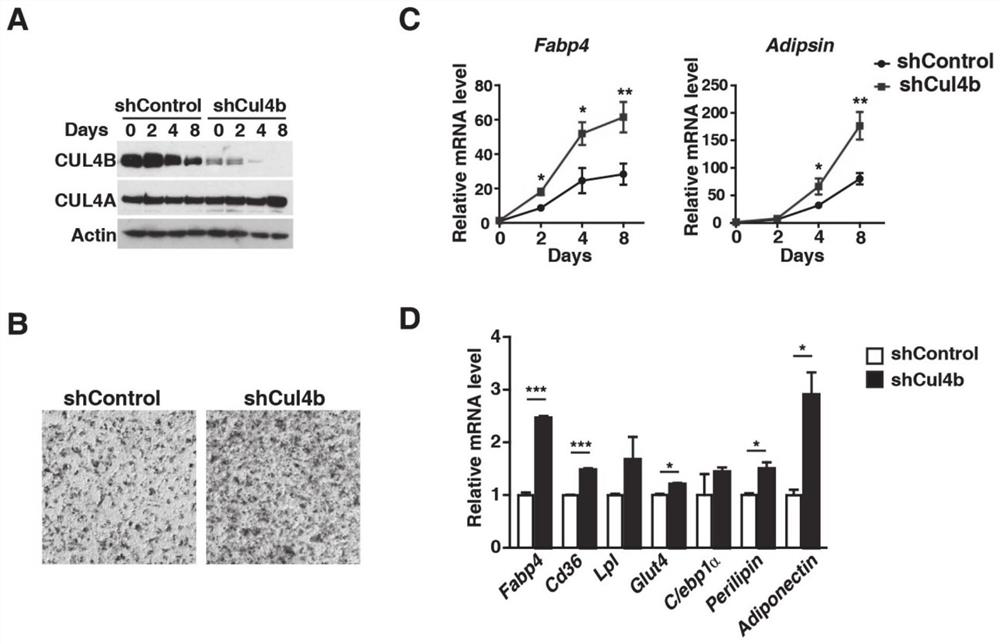
Application of CUL4B in treatment of type 2 diabetes mellitus
InactiveCN112516318AArrestin activityPromote absorptionMetabolism disorderEndocrine system disorderCUL4BKnockout animal
The invention provides an application of CUL4B in treatment of type 2 diabetes mellitus. Research finds that the CUL4B can negatively regulate adipose differentiation, and further research proves thatCul4b knockout mice can be improved in insulin resistance and type 2 diabetes mellitus symptom. In terms of mechanism, insulin signaling pathways in AKO mice are found to be more sensitive, and adipocytes are found to absorb glucose more easily. Meanwhile, the infiltration of macrophages in adipose tissues of the AKO mice is obviously reduced. Therefore, the CUL4B can be used as a potential drugtarget, the protein activity of the CUL4B can be inhibited to reduce the blood sugar and improve the symptom of the type 2 diabetes mellitus, and the CUL4B has good practical application value.
Owner:SHANDONG UNIV
8-(C-beta-D-glycopyranyl)-7,3',4'-trihydroxyflavone, its separating process, medical composition and used for medicine for diabets
InactiveCN1453286AHas antidiabetic activitySimple methodOrganic active ingredientsSaccharide with heterocyclic radicalsPharmaceutical drugTraditional medicine
Owner:COUNCIL OF SCI & IND RES
Preparation process of medicine for treating diabetes
InactiveCN114522217ANo cloggingFully brokenMetabolism disorderInanimate material medical ingredientsMedicinal herbsAngelica Sinensis Root
The invention discloses a preparation process of a medicine for treating diabetes mellitus, which comprises the following specific steps: weighing various medicinal materials; the invention relates to the technical field of medicines for treating diabetes mellitus, and relates to the technical field of medicines for treating diabetes mellitus. The medicines comprise 10-35g of codonopsis pilosula, 5-30g of raw bighead atractylodes rhizome, 10-35g of poria cocos, 2-10g of coptis chinensis, 2-10g of dried ginger, 8-22g of cassia twig, 32-50g of angelica sinensis, 3-15g of medicated leaven, 4-15g of fructus amomi, 5-20g of white paeony root, 15-40g of radix astragali preparata, 5-30g of red ginseng, 23-35g of golden cypress, 17-35g of pericarpium citri reticulatae, 4-20g of pseudo-ginseng, 4-20g of amber, 4-20g of crude dragon bone, 3-20g of raw oyster shell and the like. According to the preparation technology of the medicine for treating the diabetes, crushed medicinal materials enter the discharging barrel, gravity sensors are arranged on the top of an opening and closing plate and in the opening and closing plate, and according to the total weight of the added medicinal materials, when the gravity sensors detect that the weight of medicine powder is close to the total weight of the added medicinal materials, the opening and closing plate is controlled to be opened; and the medicine powder can fall into the collecting box, so that the device automatically detects the crushing process and timely removes the internal medicine powder.
Owner:范伟彪
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com