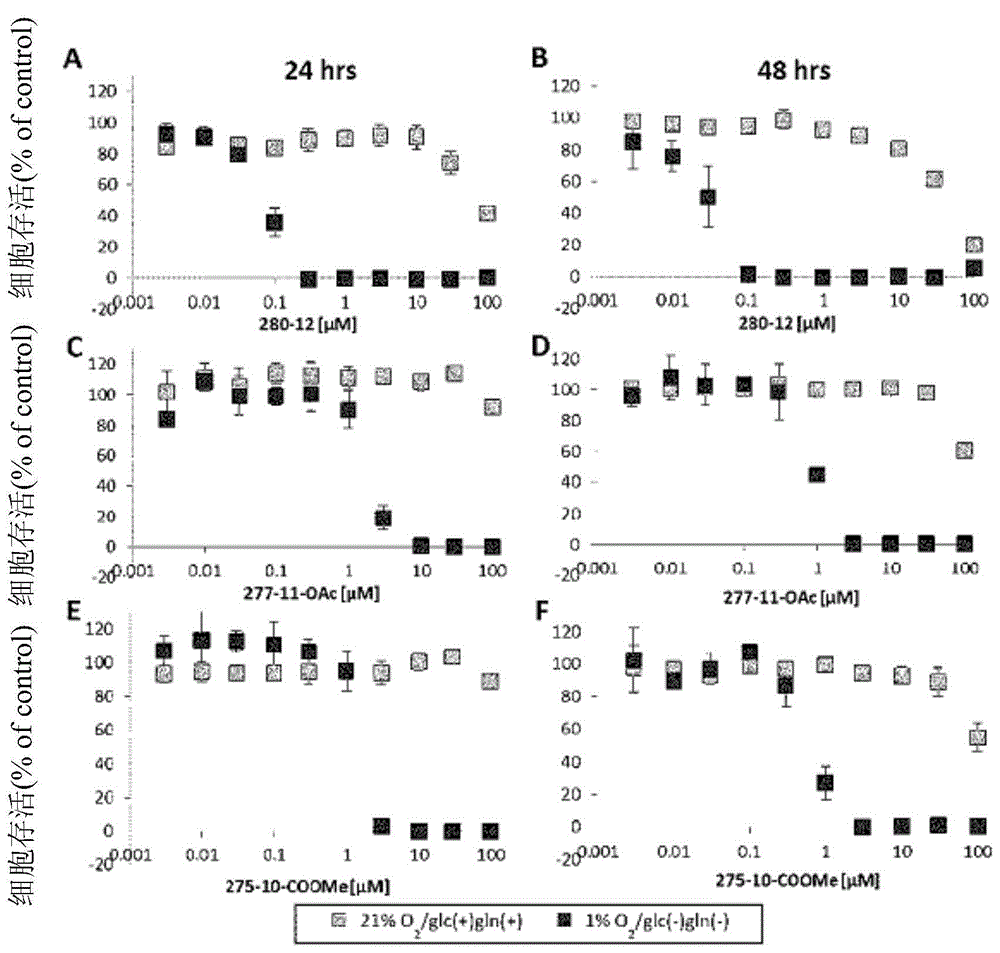
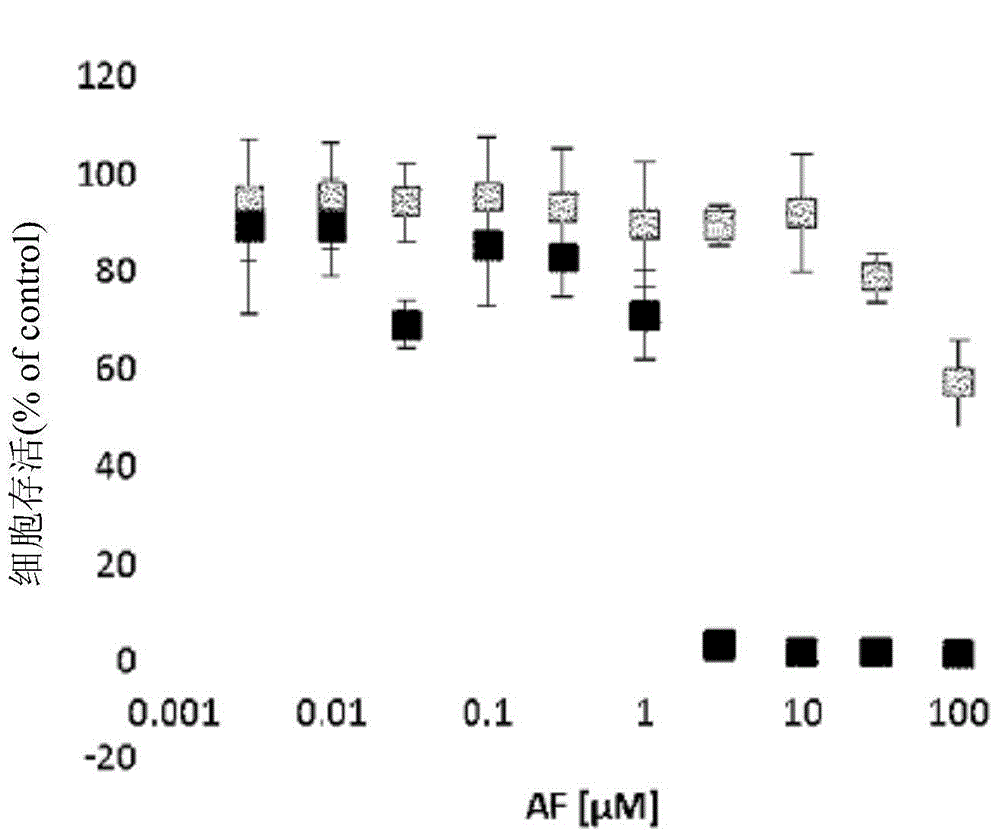
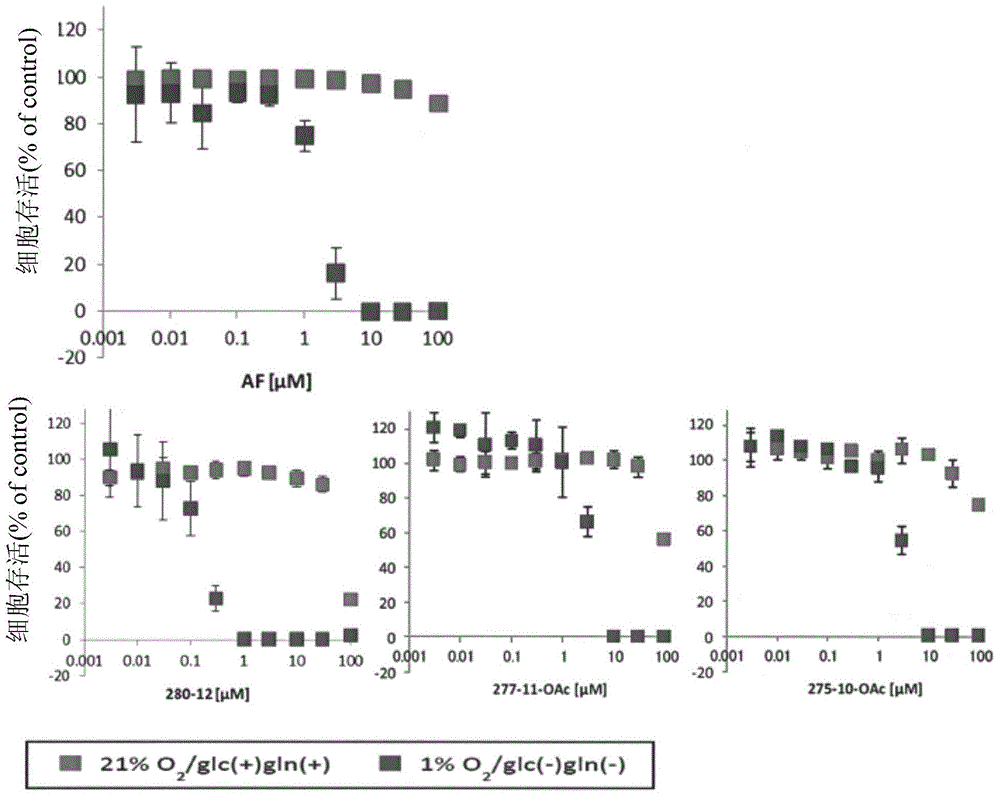
Dihydroorotic acid dehydrogenase inhibitor
A technology of dihydroorotic acid and dehydrogenase, which is applied in anti-inflammatory agents, pill delivery, antiviral agents, etc., and can solve problems such as hyperplasia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
[0126] The following examples illustrate the compounds used in the present invention in detail. Again, these compounds do not limit the scope of the present invention.
[0127] 1. Derivatives 215-15-COOEt, 215-15-COOIPr, 215-13-COOH
[0128] [chemical 1]
[0129]
[0130] Ethyl 12-(3-chloro-5-formyl-2,6-dihydroxy-4-methylphenyl)dodecanate (215-15-COOEt).
[0131] CHCl in acetic anhydride (12.5ml, 132mmol) 3 (16ml) solution, 30% aqueous hydrogen peroxide (10ml, 98mmol) was added at 0°C, and stirred at the same temperature for 1 hour. Next, maleic anhydride (10.0 g, 102 mmol) was added while maintaining its individuality, and the mixture was stirred for 2 hours while gradually returning to room temperature. After confirming that the reaction solution was exothermic, cyclododecanone (compound 1, 2.52 g, 13.8 mmol) was added while maintaining its individuality, and stirred at 35° C. for 16 hours. After returning the reaction solution to room temperature, it was further...
Embodiment 1
[0686] [Example 1] DHOD inhibitor assay
[0687] Using the compounds of the present invention, the DHOD inhibitor activity was determined at concentrations of 200 nM and 1000 nM.
[0688] Add 190 μl of Assay Buffer (100 mM HEPES pH8.0, 150 mM NaCl, 5% Glycerol, 0.05% Triton X-100, 200 μM dihydroorotate, 120 μM DCIP, 11 μM Decylubiquinone) and 5 μl of DMSO solution of each inhibitor, and add 5 μl of 8 μg / ml human DHOD solution , start the enzyme reaction (human DHOD with an end concentration of 0.2μg / ml (4nM)), measure the reduction of DCIP at 600nm for 20 minutes, and control it with End Point Assay () according to the change of the absorbance value at 600nm after 0 minutes and 20 minutes The value when only DMSO solution was added, and the human DHOD inhibition rate of each compound at concentrations of 200 nM and 1000 nM were determined. Also, the concentration (nM) that inhibits 50% of the activity of human DHOD was defined as IC50 (50% inhibitory concentration). The resu...
Embodiment 2
[0691] [Example 2] Anticancer effect
[0692] Conventional anticancer agents act directly on the process of cell division, but have low specificity to tumor cells and strong damage to normal cells, so serious side effects appear, which is a serious problem. Afterwards, molecular targeted drugs appeared, targeting molecules related to tumor cell proliferation, invasion, and metastasis to inhibit tumor cell deterioration, not only inhibit tumor cells, but also inhibit tumor metastasis.
[0693] Therefore, screening was carried out by using a cancer cell growth inhibition test using a panel of 39 kinds of human cancer cell lines (JFCR39) and using Cancer Cell Informatics (Kong D., T. . Yamori; Bioorganic & Med. Chem., 20, 1947-51, 2012). The concentration (μM) that inhibits the growth of human cancer cells by 50% was defined as GI50 (50% inhibitory concentration).
[0694] The results are shown in Table 2.
[0695] Inhibitory effect on various human cancer cells.
[0696] [Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com