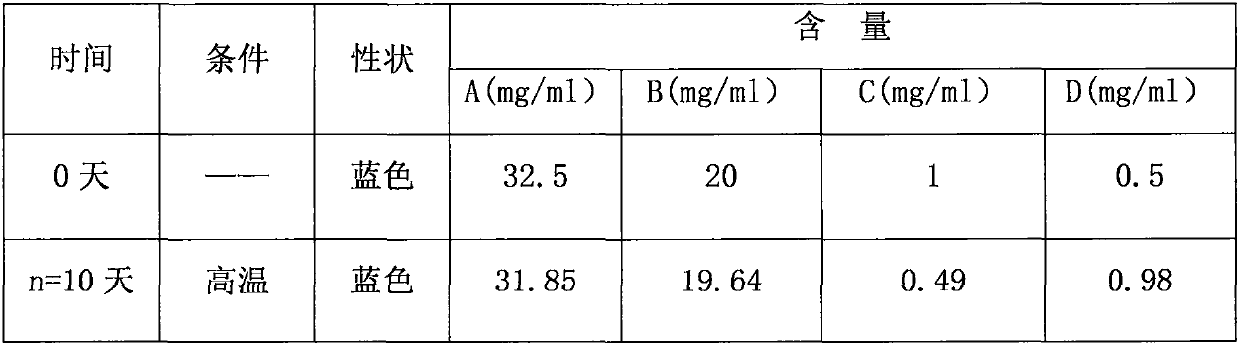
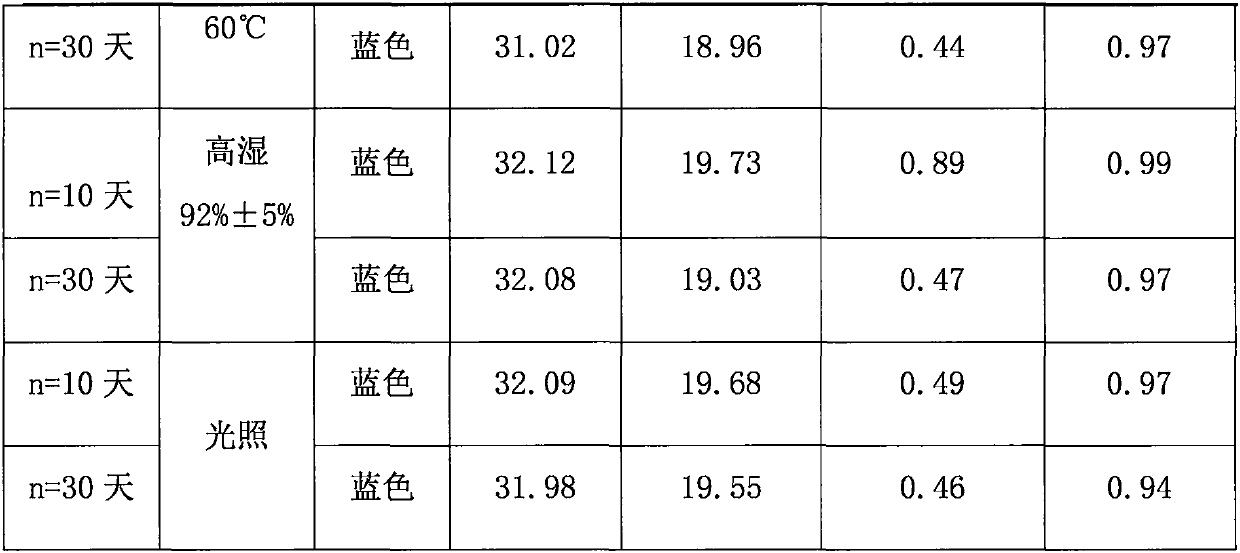
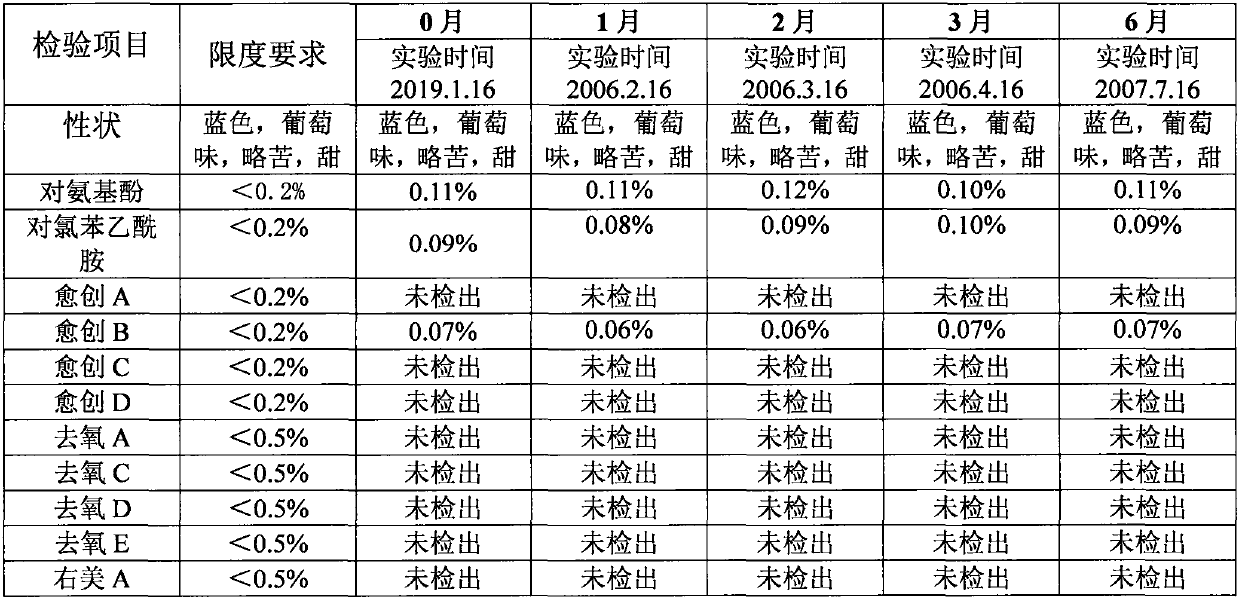
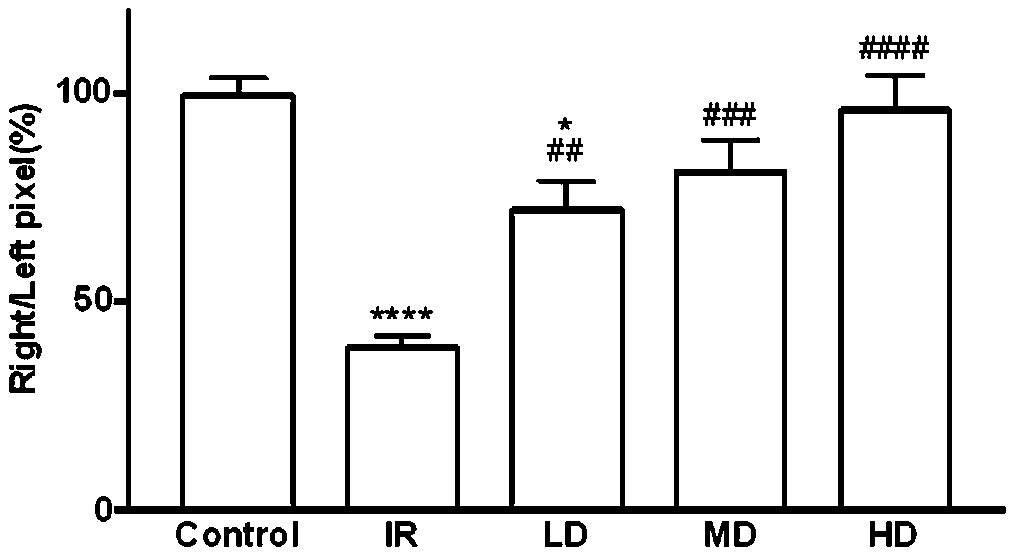
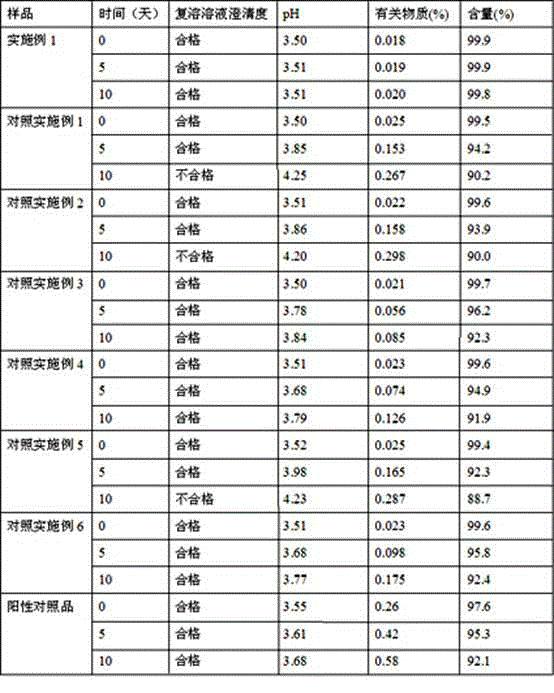
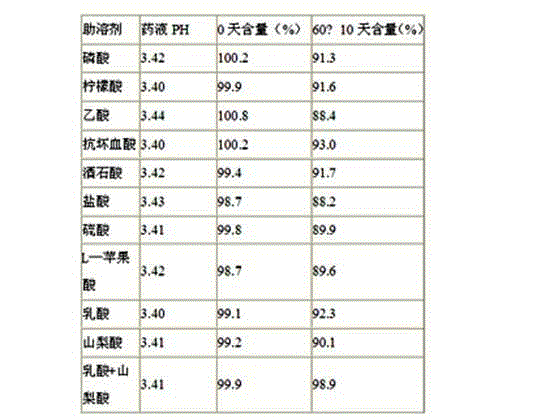
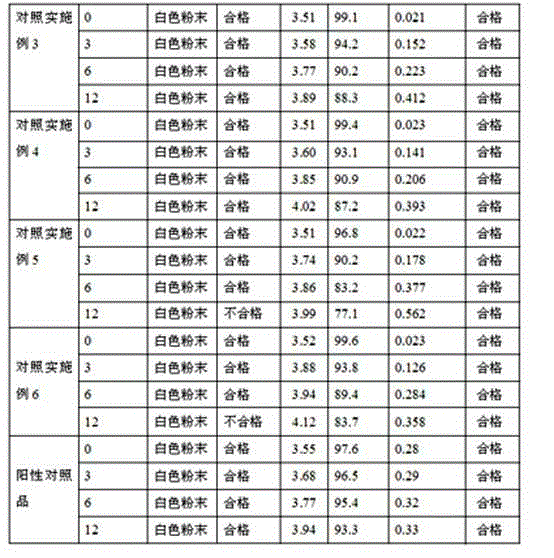
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Dextromethorphan hbr" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
How to use Dextromethorphan Hbr Syrup. Take this medication by mouth, usually every 4 to 12 hours as needed or as directed by your doctor.If stomach upset occurs, take with food or milk. Use a ...
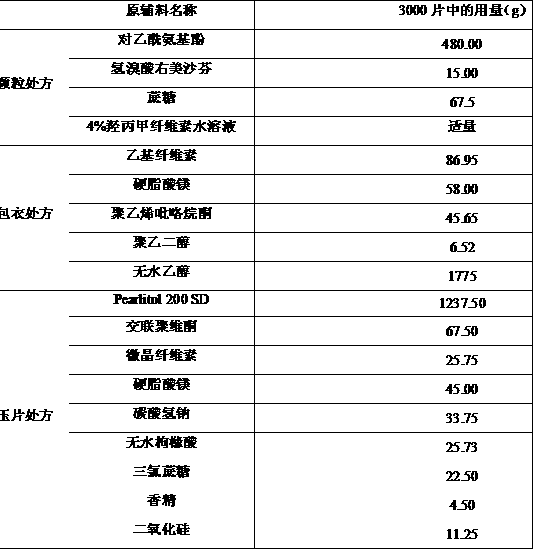
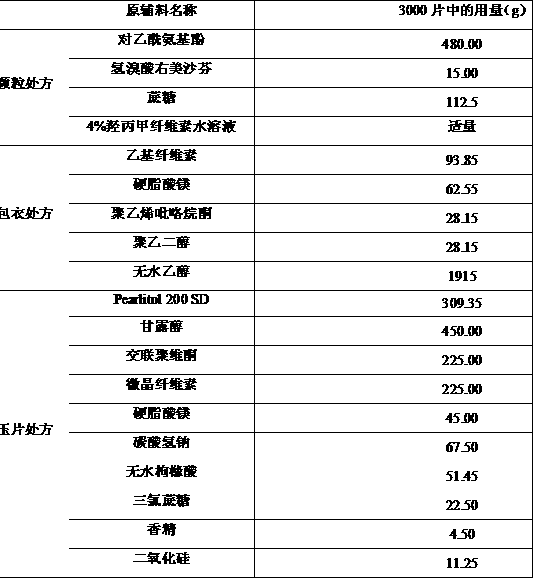
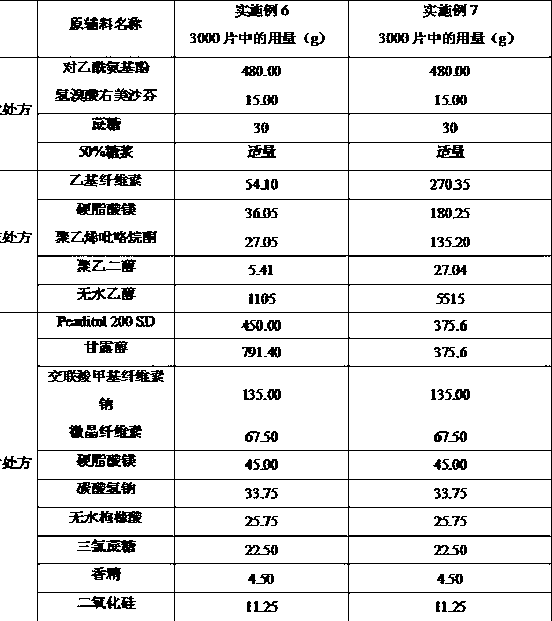
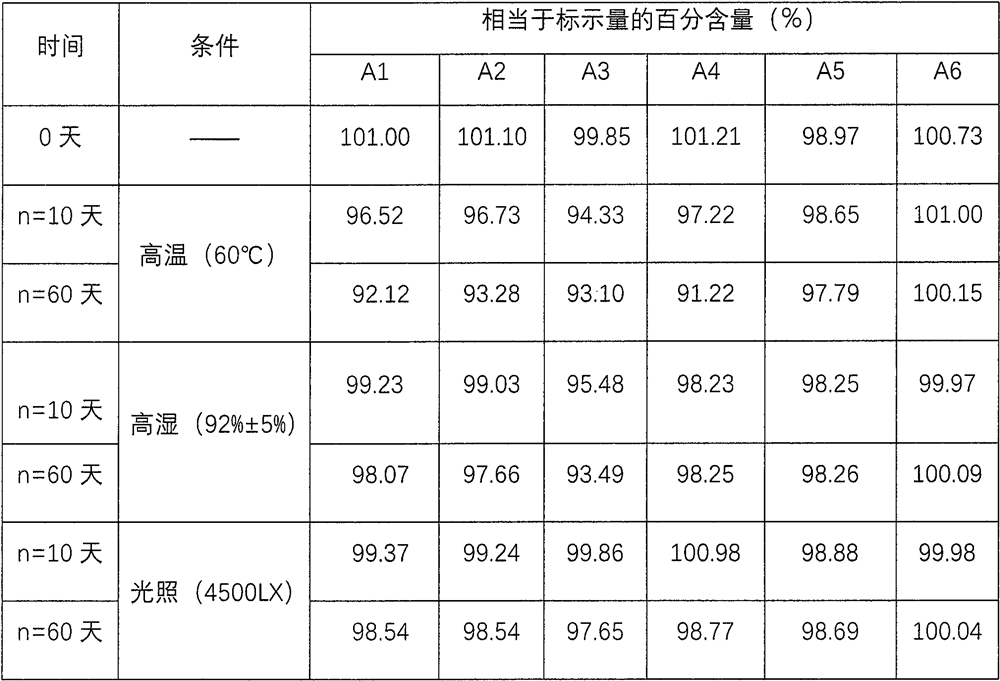
Oral disintegrant of compound paracetamol
InactiveCN1679525ASimple preparation processDisintegration has little adverse effectOrganic active ingredientsAntipyreticChlorphenamine maleateOrally disintegrating tablet
An oral disintegrating tablet of compound paracetamol is prepared from paracetamol, pseudoephedrine hydrochloride, dextromethorphan HBr, chlorphenamine maleate, filler, disintegrant, adhesive or moistening agent, lubricant, flavouring and pigment through direct tabletting.
Owner:FUDAN UNIV
Compound dextromethorphan oral solution, preparation method thereof and use thereof
InactiveCN110327339AEasy dischargeTreat and relieve minor painDispersion deliveryAntipyreticSolventBronchial inflammation
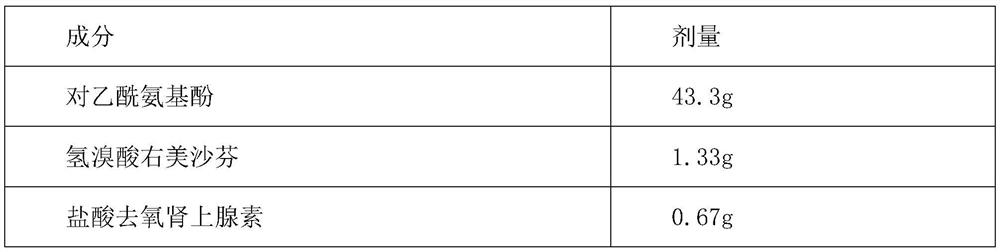
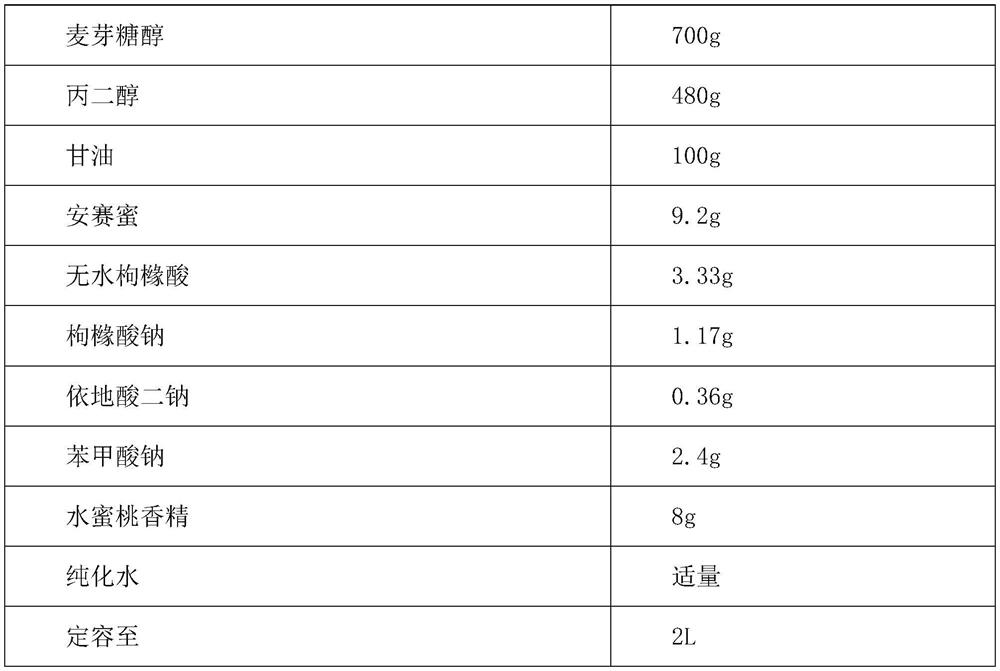
The present invention provides a compound dextromethorphan oral solution preparation, a preparation method thereof and a use thereof. The compound dextromethorphan oral solution preparation is prepared by using acetaminophen, guaiacol glyceryl ether, phenylephrine hydrochloride and dextromethorphan hydrobromide as active drugs, and combining a certain amount of stabilizers, solubilizers, thickeners, suspending agents, emulsifiers, pH adjusters, preservatives, corrigents suitable for children to accept and other accessory materials, and processed by a scientific method. The compound dextromethorphan oral solution is suitable for consumption of children aged 6-12 years old. The compound dextromethorphan oral solution can treat and alleviate symptoms of common colds and flu, such as nasal congestion, cough and intensity of impulsivity caused by mild throat and bronchial inflammation and cough, can treat and alleviate mild pains and headache, and sore throat, and temporarily reduces fever.
Owner:北京博智绿洲医药科技有限公司
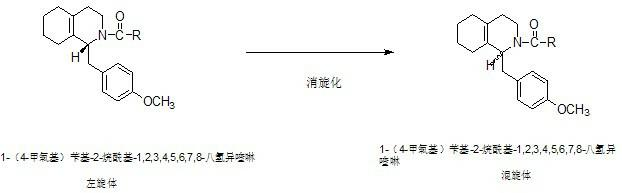
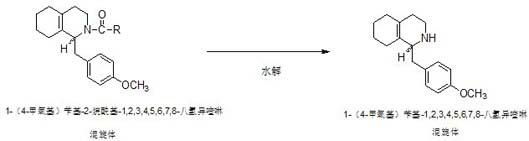
Preparation process for key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as cough relieving medicine
ActiveCN102219737ALow costSimple and safe operationOrganic chemistryIsoquinolineDextromethorphan Hydrobromide
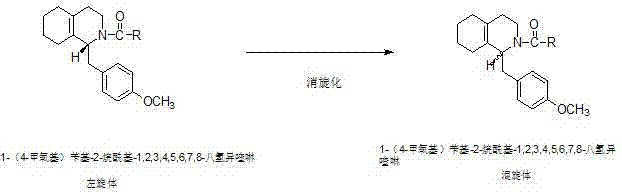
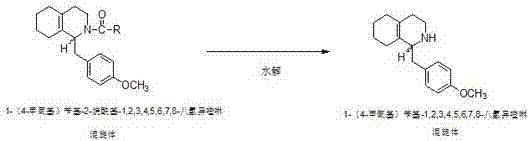
The invention discloses a preparation process for a key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as a cough relieving medicine, which comprises the following steps of: performing acylation reaction to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (laevo isomer); and racemizing under the alkaline condition to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer); and hydrolyzing under the alkaline condition to obtain the 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer). The process aims to overcome the defect of the synthetic process and reduce cost, so that the process is simply and safely operated, the industrial production is qualified, and the yield of products is between 55 and 69 percent.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Anti-cold drug suitable for children aged 4-11 years old and preparation method of drug
InactiveCN110302149AImprove complianceRelieve nasal congestionOrganic active ingredientsDispersion deliveryCommon coldDextromethorphan Hydrobromide
The invention relates to an anti-cold drug suitable for children aged 4-11 years old and a preparation method of the drug. Dextromethorphan hydrobromide and phenylephrine hydrochloride are taken as active ingredients, a pH regulator is added to regulate the pH value to 3.8 + / - 2, and auxiliary ingredients such as a cosolvent, a stabilize, a thickening agent, a bacteriostat and the like are added,so that the defects in the prior art are effectively solved, and the stability of the drug is increased. At the same time, a certain amount of flavoring agent and aromatic agent are added to improve the compliance of children. The drug is mainly used for relieving nasal obstruction and cough caused by common cold of children aged 4-11 years old.
Owner:北京博智绿洲医药科技有限公司
Dextromethorphan hydrobromide chewable tablet and preparation method thereof
The invention belongs to the technical field of medicinal preparations, and discloses dextromethorphan chewing gum tablets and an industrially applicable preparation method thereof. The preparation is delivered through a chewing process, so that the defects of the marketed products at present can be overcome, and the tablets have the advantages of no need of water for taking, convenience in taking, good mouthfeel, less adverse effects, quick response, capability of eliminating thirst and the like. The dextromethorphan chewing gum tablets prepared by the invention are mainly used for dry coughand are suitable for cold, acute or chronic bronchitis, bronchial asthma, faucitis, phthisis and cough during other upper respiratory tract infections. The dextromethorphan chewing gum tablets provided by the invention have the advantages of simple preparation process, low cost, easiness in control and easiness for industrial production.
Owner:CHONGQING MEDICAL UNIVERSITY
Compound oral disintegrating tablet containing acetaminophen and dextromethorphan
ActiveCN103169706ASimple preparation processGreat tasteOrganic active ingredientsNervous disorderPyrrolidinonesMannitol
The invention relates to the field of pharmaceutical preparation, and in particular relates to a compound oral disintegrating tablet containing acetaminophen and dextromethorphan hydrobromide. The compound oral disintegrating tablet contains the following components of: a. acetaminophen and dextromethorphan hydrobromide coating particles, wherein the particle diameter of the coating particles is 60-100 meshes, the coating particles contain particles including acetaminophen and dextromethorphan hydrobromide, a coating material coated on the particle surface, and the coating material comprises ethylcellulose and at least one pore-foaming agent; b. a filler which is directly compressible mannitol, mannitol, microcrystalline cellulose or a mixture thereof; c. a disintegrating agent selected from crospolyvinylpyrrolidone or croscarmellose sodium; d. a lubricant which is talcum powder or magnesium stearate; and e. an effervescing agent.
Owner:CHONGQING PHARMA RES INST
Phenylephrine hydrochloride containing liquid composition
InactiveCN112076183AInorganic non-active ingredientsPharmaceutical delivery mechanismSinusitisDisease
The invention provides six liquid compositions containing phenylephrine hydrochloride. The six liquid compositions contain the following active substances: a composition 1: phenylephrine hydrochloride, brompheniramine maleate and dextromethorphan hydrobromide; a composition 2: phenylephrine hydrochloride, acetaminophen, guaiacol glyceryl ether and dextromethorphan hydrobromide; a composition 3: phenylephrine hydrochloride, chlorpheniramine maleate, acetaminophen and dextromethorphan hydrobromide; a composition 4: phenylephrine hydrochloride, acetaminophen and dextromethorphan hydrobromide; a composition 5: phenylephrine hydrochloride, acetaminophen and diphenhydramine hydrochloride; and a composition 6: phenylephrine hydrochloride, acetaminophen and guaiacol glyceryl ether. The inventor finds that the stability of the six liquid compositions is related to the pH value through research. The liquid composition can be used for treating respiratory diseases caused by cold, including virusinfection, such as influenza and cold, as well as allergy, sinusitis, rhinitis and the like.
Owner:北京博智绿洲医药科技有限公司
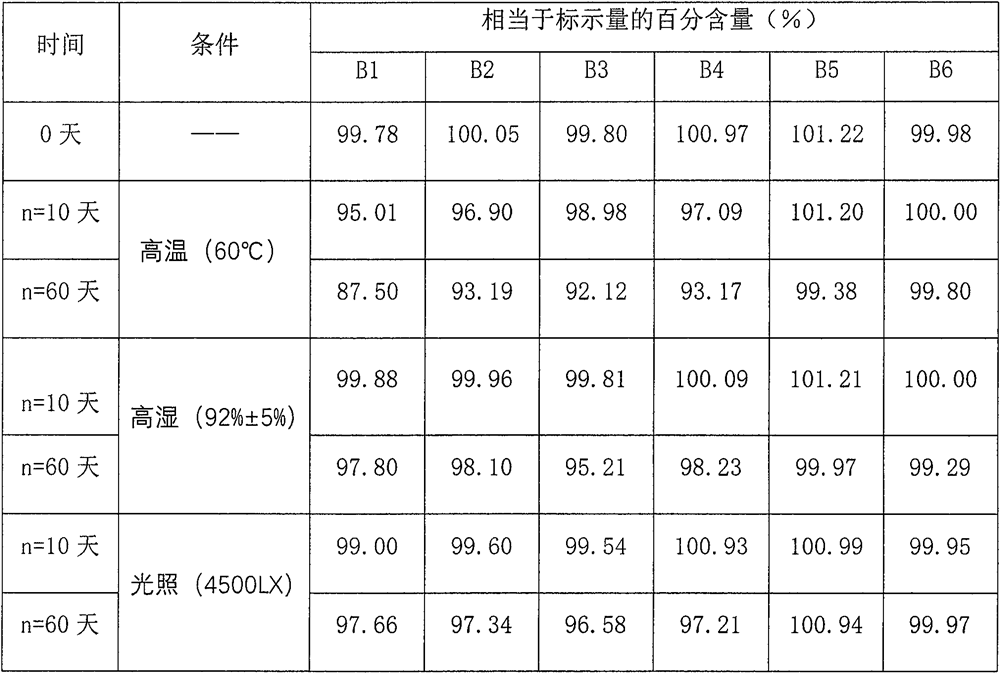
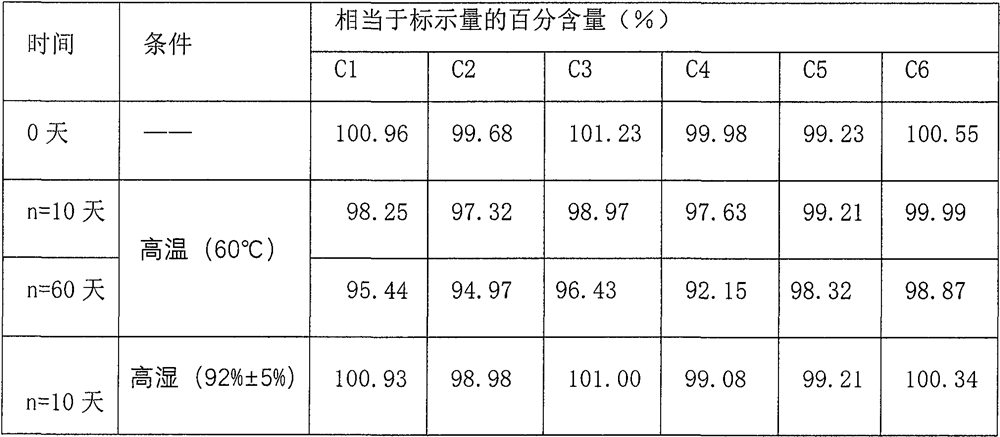
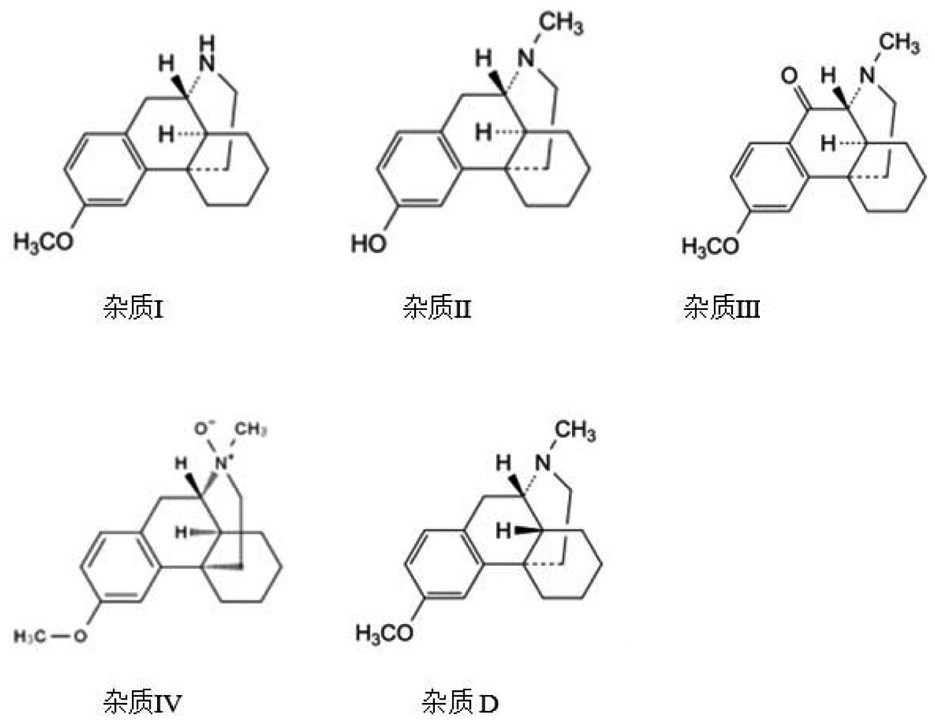
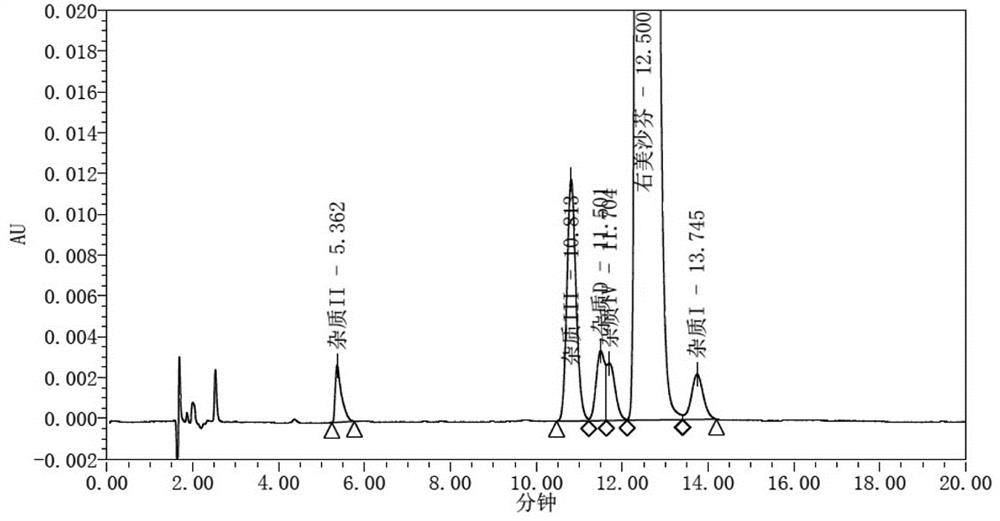
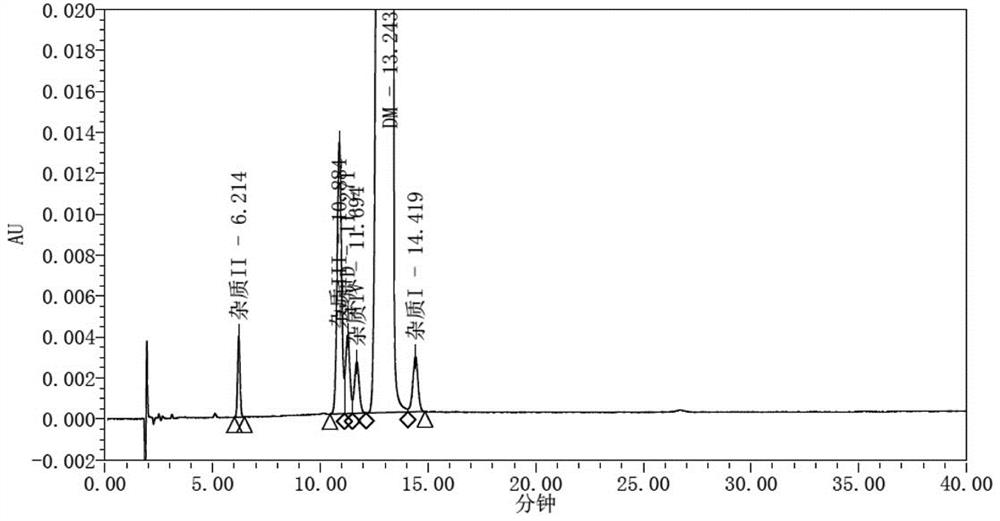
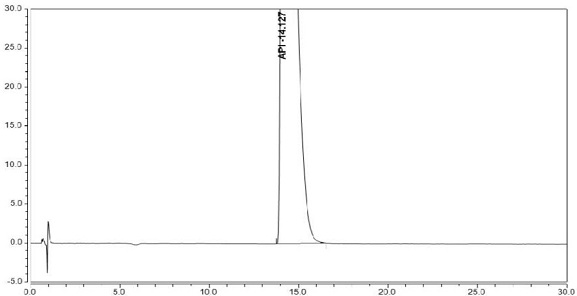
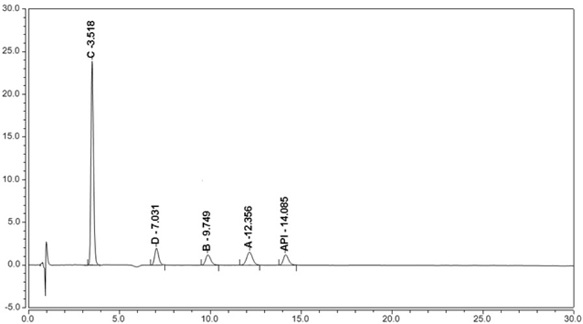
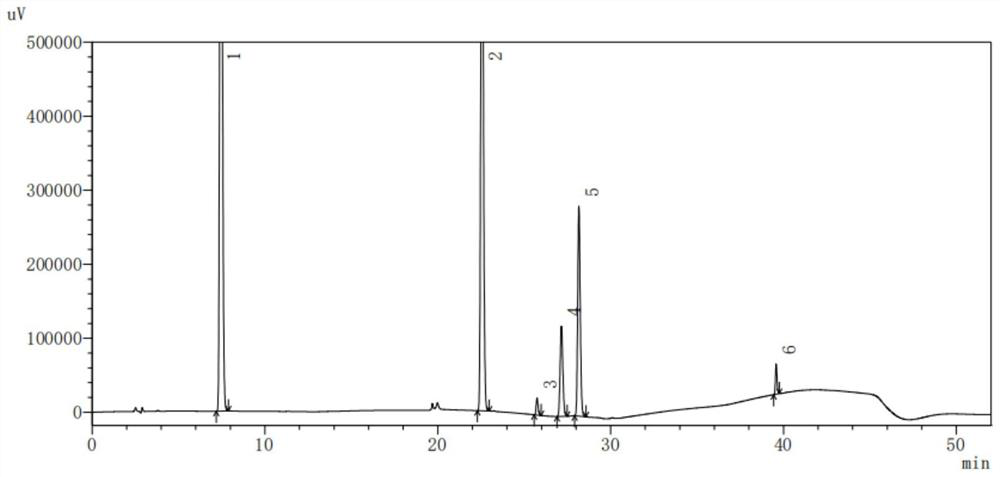
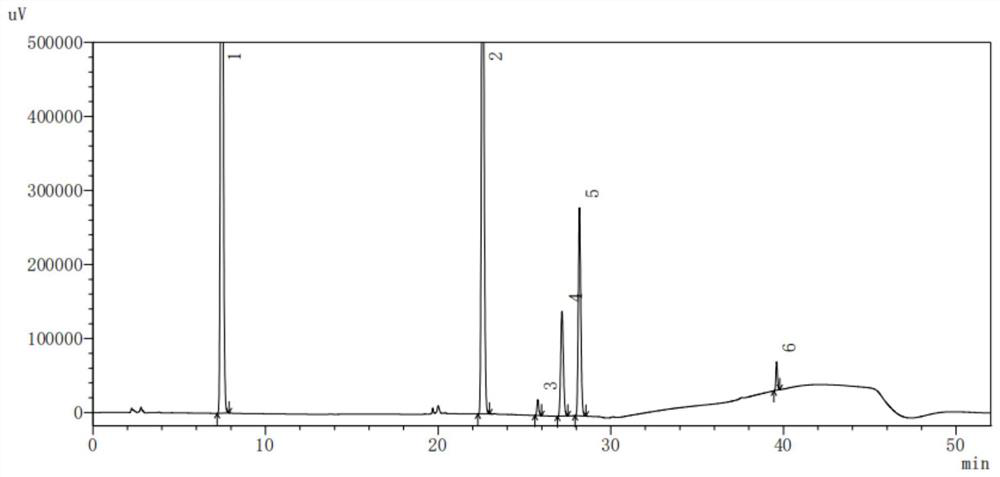
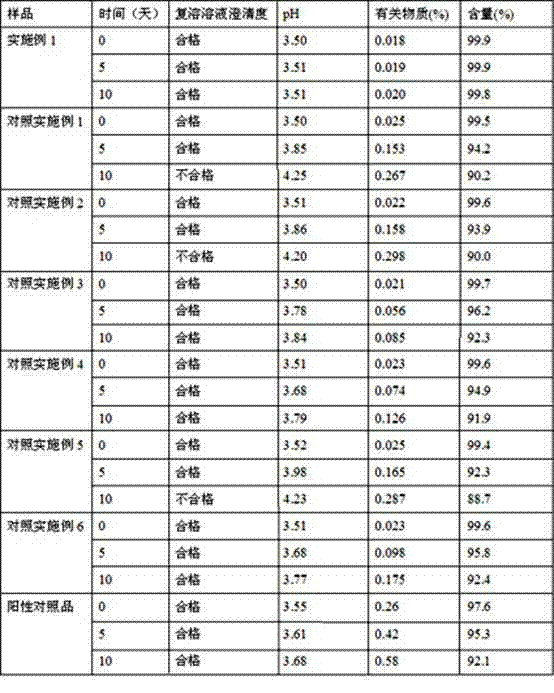
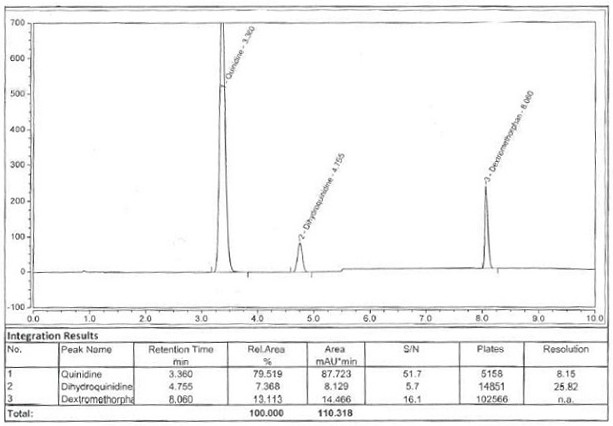
Dextromethorphan hydrobromide related substance detection method
PendingCN114544828AEfficient separationEfficient detectionComponent separationDextromethorphan HydrobromidePhotochemistry
The invention relates to a dextromethorphan hydrobromide related substance detection method, which can be used for simultaneously detecting five impurities and accurately determining the relative retention time and correction factor of each known impurity. The method is short in separation time, good in separation degree, low in quantification limit and accurate in result, and related substances in dextromethorphan hydrobromide can be effectively detected.
Owner:SHIJIAZHUANG YILING PHARMA
Liquid composition containing phenylephrine hydrochloride as well as preparation and application thereof
PendingCN111588694AImprove stabilityReduce solubilitySalicyclic acid active ingredientsAntipyreticSalicylic acidSuccinic acid
The invention relates to a liquid composition containing phenylephrine hydrochloride as well as a preparation and application thereof. The composition comprises three compositions: the first is a combination of the phenylephrine hydrochloride, choline salicylic acid and guaifenesin; the second is a combination of the phenylephrine hydrochloride, acetaminophen and pheniramine maleate; and the thirdis a combination of the phenylephrine hydrochloride, the acetaminophen, dextromethorphan hydrobromide and doxylamine succinate. The composition disclosed by the invention can be prepared into a liquid preparation, and a preferable dosage form is suspension.
Owner:北京博智绿洲医药科技有限公司
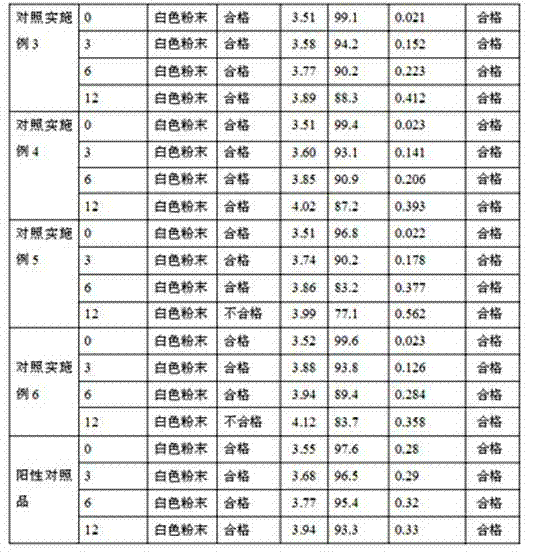
Determination method of dextromethorphan hydrobromide related substances
ActiveCN114324642AResolve detectionImprove quality controlComponent separationDrug utilisationDextromethorphan Hydrobromide
The invention belongs to the technical field of pharmaceutical analysis, particularly relates to a determination method of dextromethorphan hydrobromide related substances, and provides a convenient, efficient and accurate detection method for solving the problem of detection of the dextromethorphan hydrobromide related substances, and the method can detect the dextromethorphan hydrobromide related substances, so that the medication safety is effectively guaranteed, and the detection cost is reduced. The method is convenient, efficient and accurate, completely conforms to the guidance principle verified by methods in Chinese pharmacopoeia in the aspects of system applicability, repeatability, specificity and accuracy, and can be used for quality control of dextromethorphan hydrobromide.
Owner:珠海润都制药股份有限公司
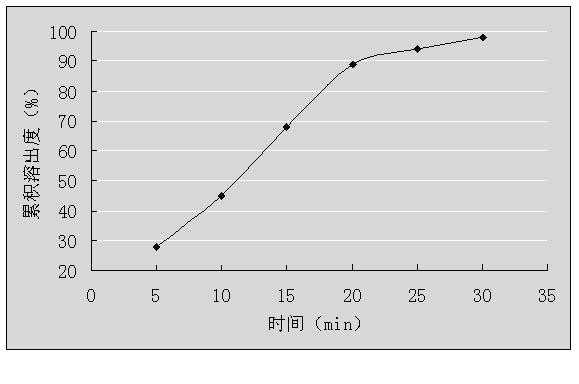
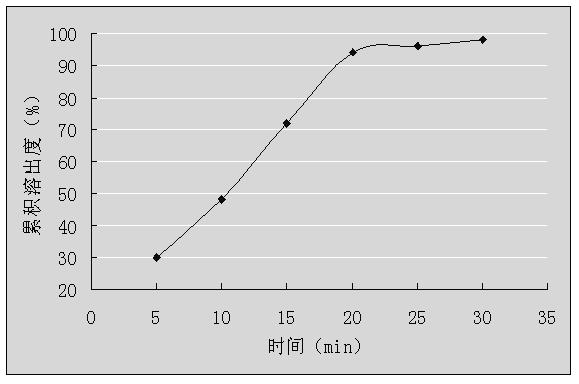
Granules containing dextromethorphan hydrobromide and guaiacol glycerin and preparation method thereof
InactiveCN102283840AQuick releaseSmooth releaseEther/acetal active ingredientsGranular deliverySide effectGuaiacol
The invention relates to a granule containing dextromethorphan hydrobromide and guaiacol glyceryl ether and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The granules are composed of dextromethorphan hydrobromide cyclodextrin inclusion compound, guaiacol glycerin ether, diluent and binder, wherein the dextromethorphan hydrobromide cyclodextrin inclusion compound The molar ratio of the dosage of dextromethorphan hydrobromide to the cyclodextrin is 1:1-1:3, and the cyclodextrin is hydroxypropyl-β-cyclodextrin. The granule containing dextromethorphan hydrobromide and guaiacol glyceryl ether of the present invention has good stability and good taste, and at the same time solves the problem that the blood concentration of dextromethorphan hydrobromide fluctuates greatly in patients after taking the medicine, and is effective Reduced drug side effects.
Owner:NANJING CHENGONG PHARM CO LTD
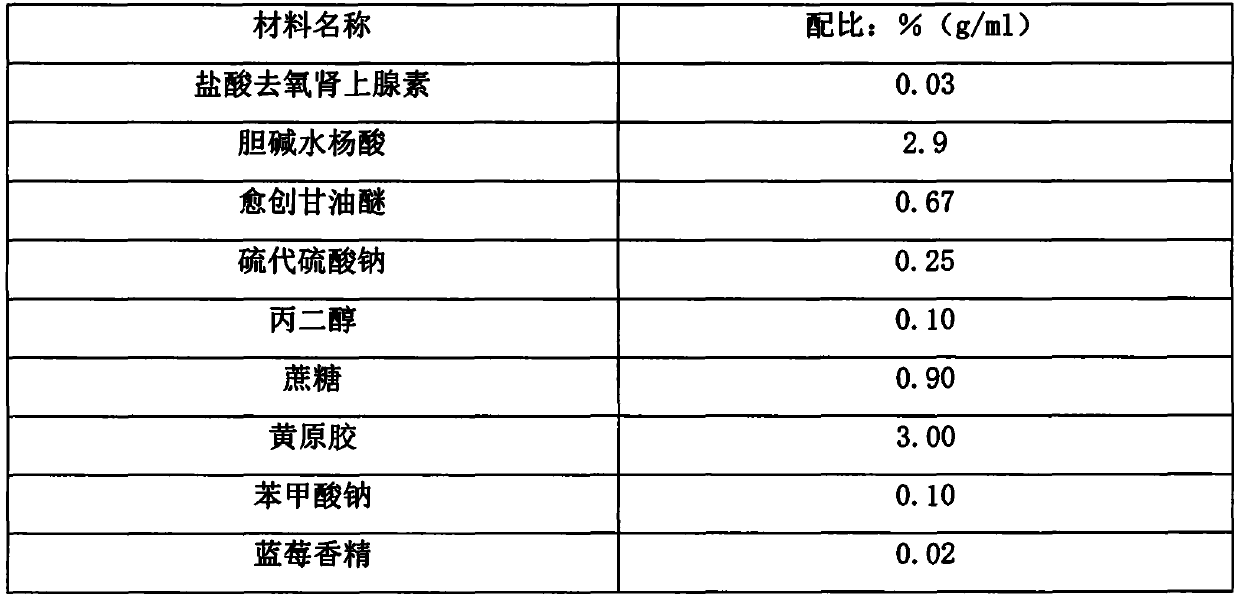
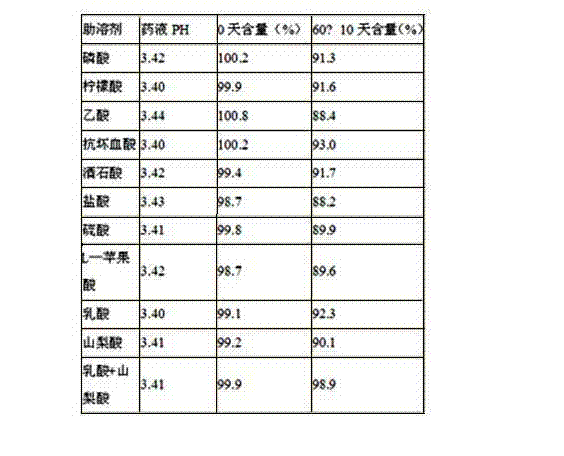
Stable dextromethorphan hydrobromide, chlorphenamine maleate and ammonium chloride oral solution and preparation method thereof
ActiveCN111437250AImprove stabilityImprove securityOrganic active ingredientsDispersion deliverySolubilityChlorobenzene
The invention provides a stable dextromethorphan hydrobromide, chlorphenamine maleate and ammonium chloride oral solution and a preparation method thereof. The dextromethorphan hydrobromide, chlorphenamine maleate and ammonium chloride oral solution is prepared from 0.03-0.3% (w / v) of dextromethorphan hydrobromide, 0.01-0.2% (w / v) of chlorpheniramine maleate, 0.1-3.0% (w / v) of ammonium chloride, 0.02-5% (w / v) of a pH regulator, 0.01-2% (w / v) of an aromatizer, 0.008-0.1% (w / v) of a colorant, water and 10%-25% (w / v) of propylene glycol. The stable dextromethorphan hydrobromide, chlorphenamine maleate and ammonium chloride oral solution can take effect quickly; the prescription does not contain sucrose, so that incompatibility of sucrose with ammonium chloride is avoided; preservatives are not contained, and the safety of the solution is improved; the solubility of dextromethorphan hydrobromide is increased by using the propylene glycol; and the content of the propylene glycol is 10%-25%,the antibacterial effect is obvious, and the preservatives do not need to be added.
Owner:HEFEI IND PHARMA INST
Dextromethorphan hydrobromide injection and preparation method thereof
ActiveCN103536527AOrganic active ingredientsPharmaceutical delivery mechanismDextromethorphan HydrobromidePolyethylene glycol
The invention relates to a dextromethorphan hydrobromide injection prepared from the following raw materials: 15g of dextromethorphan hydrobromide, 13g of lactic acid, 7g of sorbic acid, 42g of polyethylene glycol 2000 and 2000ml of water for injection through adjusting the pH value to 3.4-3.5.
Owner:南通丝乡丝绸有限公司
Synthesis method of 2-(1-cyclohexenyl) ethylamine
ActiveCN111807968ARaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationHydroxy compound preparationCyclohexanoneMeth-
The invention belongs to the technical field of organic chemistry, and particularly relates to a synthesis method of a compound 2-(1-cyclohexenyl) ethylamine (I). Cyclohexanone (II) and a Grignard reagent are subjected to a Grignard reaction in an organic solvent to be converted into 1-vinyl cyclohexanol (III), the 1-vinyl cyclohexanol (III) and a chlorination reagent are subjected to a chlorination / rearrangement one-pot reaction in an organic solvent in the presence of organic alkali to prepare (2-chloroethylene methylene) cyclohexane (IV), the (2-chloroethylene methylene) cyclohexane (IV) and urotropine are subjected to quaternization in an organic solvent to form N-cyclohexylidene ethyl urotropine hydrochloride (V), and finally, hydrolysis rearrangement is carried out in a solvent in the presence of inorganic mineral acid to obtain the 2-(1-cyclohexenyl) ethylamine (I). The compound (I) has important industrial application value as an intermediate for synthesizing the antitussive drug dextromethorphan hydrobromide. The method has the advantages of cheap and accessible raw materials, mild reaction conditions, high yield and high product purity, is simple to operate, and is convenient for industrial production.
Owner:FUDAN UNIV
Preparation method of dextromethorphan hydrobromide N-oxide impurity
PendingCN110143921AHigh yieldHigh purityOrganic chemistryOrganic solventDextromethorphan Hydrobromide
The invention discloses a preparation method of a compound of dextromethorphan hydrobromide N-oxide impurity as shown in the formula I. The invention provides a preparation method of a dextromethorphan hydrobromide N-oxide impurity as shown in the formula I, comprising the following steps: dissolving dextromethorphan hydrobromide in water, dissociating by using alkali, extracting by using an organic solvent, and reacting under the action of an oxidizing agent. The preparation method of the invention is convenient to operate, the reaction condition is mild and controllable, reaction stability is high, yield of the reaction product is high, and purity is also high.
Owner:上海葆隆生物科技有限公司
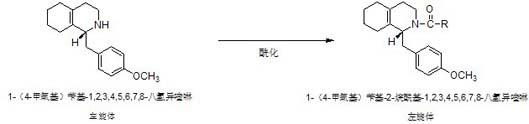
Preparation process for key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as cough relieving medicine
ActiveCN102219737BLow costSimple and safe operationOrganic chemistryIsoquinolineDextromethorphan Hydrobromide
The invention discloses a preparation process for a key intermediate 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer) of dextromethorphan hydrobromide serving as a cough relieving medicine, which comprises the following steps of: performing acylation reaction to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (laevo isomer); and racemizing under the alkaline condition to obtain 1-(4-methoxyl)benzyl-2-alkylacyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer); and hydrolyzing under the alkaline condition to obtain the 1-(4-methoxyl)benzyl-1,2,3,4,5,6,7,8-octahydro isoquinoline (mixed isomer). The process aims to overcome the defect of the synthetic process and reduce cost, so that the process is simply and safely operated, the industrial production is qualified, and the yield of products is between 55 and 69 percent.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Dextromethorphan hydrobromide transdermal patch
PendingCN112370438AReduce total intakeMeet needsOrganic active ingredientsOrganic non-active ingredientsTransdermal patchDextromethorphan Hydrobromide
A dextromethorphan hydrobromide transdermal patch comprises a medicine carrying layer, a backing layer and an anti-sticking layer, and the medicine carrying layer is composed of dextromethorphan hydrobromide, a penetration enhancer and a pressure-sensitive adhesive matrix, and the weight percentage content of dextromethorphan hydrobromide in the medicine carrying layer is 2%-4%. The weight percentage of the penetration enhancer in the medicine carrying layer is 1%-3%, and the penetration enhancer is propylene glycol, myristic acid and menthone in the weight ratio of 1: (0.2-0.4): (0.1-0.2), wherein the pressure-sensitive adhesive matrix is a polysiloxane pressure-sensitive adhesive matrix.
Owner:北京茗泽中和药物研究有限公司
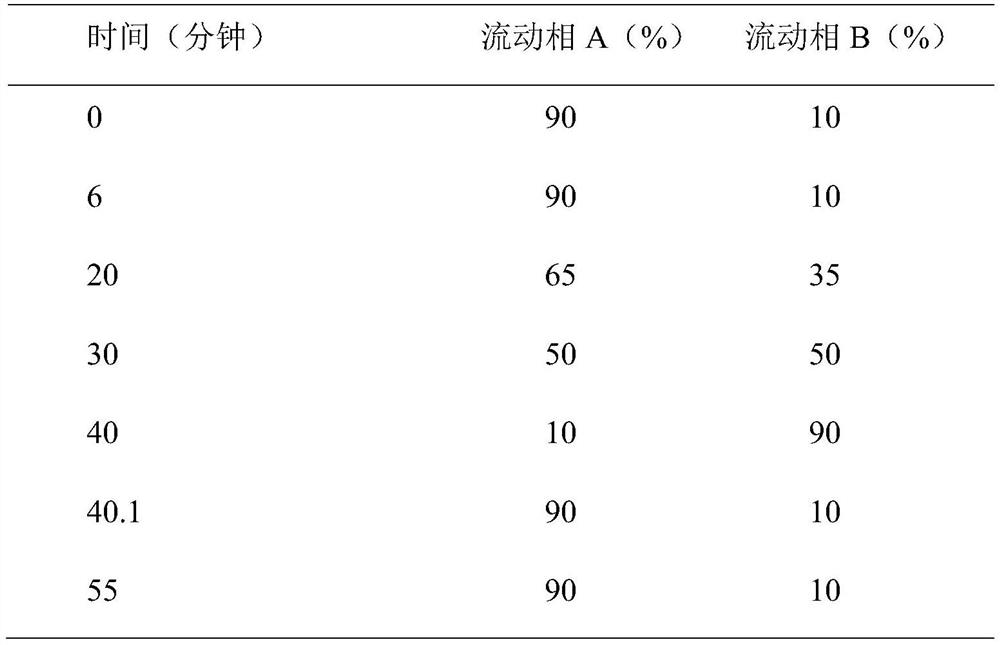
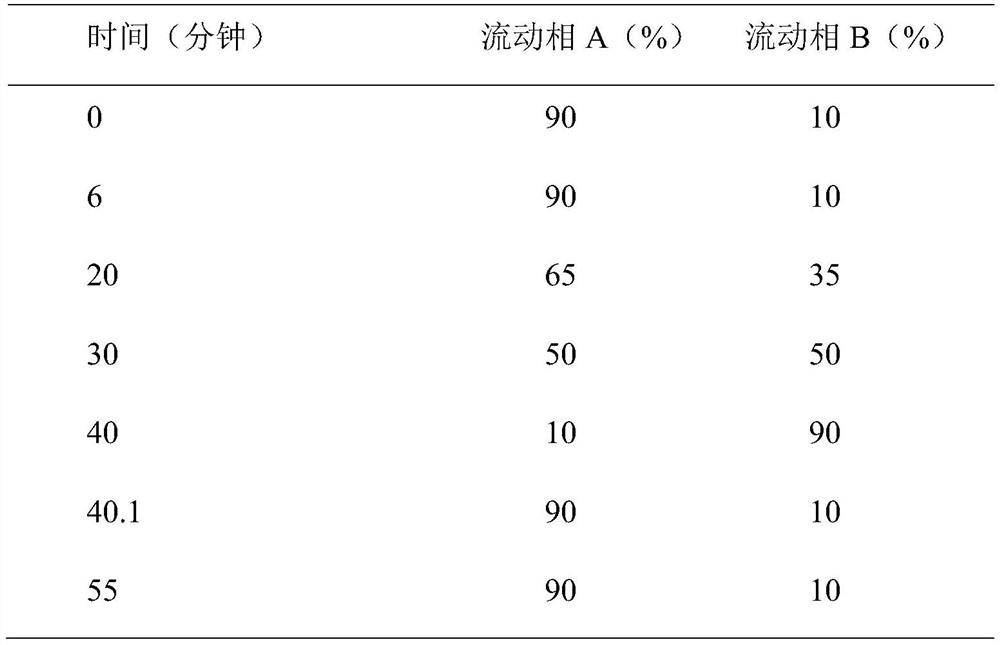
Method for detecting content of multiple components in amimex oral solution by using HPLC method
The invention discloses a method for detecting the content of multiple components in an amimex oral solution by using an HPLC method, and the chromatographic conditions of the HPLC method are as follows: octadecylsilane bonded silica is used as filler, a mixed solution of a phosphate buffer solution and methanol is used as a mobile phase A, and a mixed solution of the phosphate buffer solution andacetonitrile is used as a mobile phase B for gradient elution, the flow rate is 0.5-1.5ml / min, the column temperature is 25-40DEG C, and the detection wavelength is 200-254nm. The method can be usedfor simultaneously detecting acetaminophen, dextromethorphan hydrobromide, guaiacol glyceryl ether, phenylephrine hydrochloride, sodium benzoate and propyl gallate in the amimex oral solution, and identifying components of common peaks, and has the advantages of good reproducibility and high accuracy, and is suitable for the quality control of the active ingredient content of the amimex oral solution.
Owner:SICHUAN FENGCHUN PHARMA
Dextromethorphan hydrobromide oral dispersible film agent and preparation method thereof
ActiveCN103705493BGreat tasteImprove complianceOrganic active ingredientsPharmaceutical non-active ingredientsPolacrilinBiology
Owner:山西皇城相府药业股份有限公司
Dextromethorphan hydrobromide lyophilized powder and preparation method thereof
ActiveCN103536539APowder deliveryOrganic active ingredientsDextromethorphan HydrobromidePolyethylene glycol
The invention relates to dextromethorphan hydrobromide lyophilized powder. The dextromethorphan hydrobromide lyophilized powder is prepared from the following raw materials: 16g of dextromethorphan hydrobromide, 11g of lactic acid, 8g of sorbic acid, 45g of polyethylene glycol 2000 and 2000ml of water for injection; and the pH of adjusted to 3.3-3.6.
Owner:南通丝乡丝绸有限公司
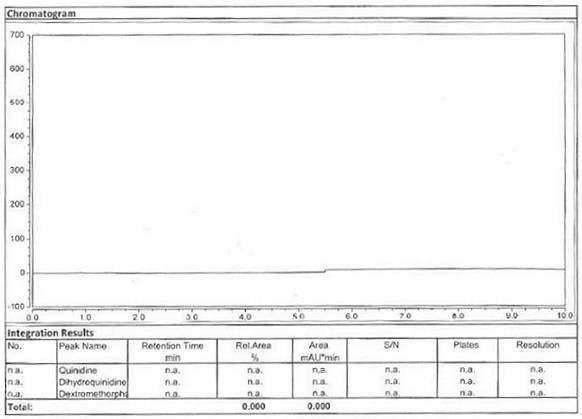
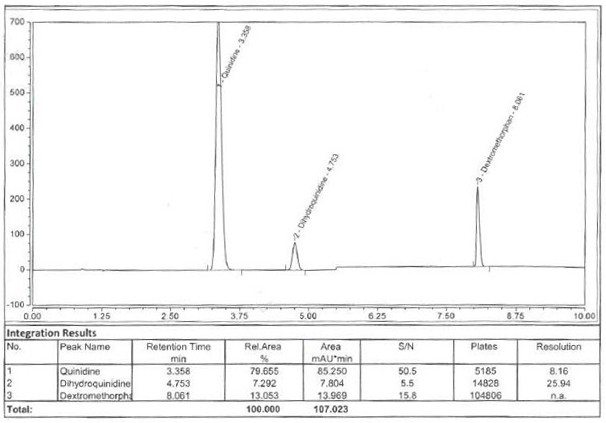
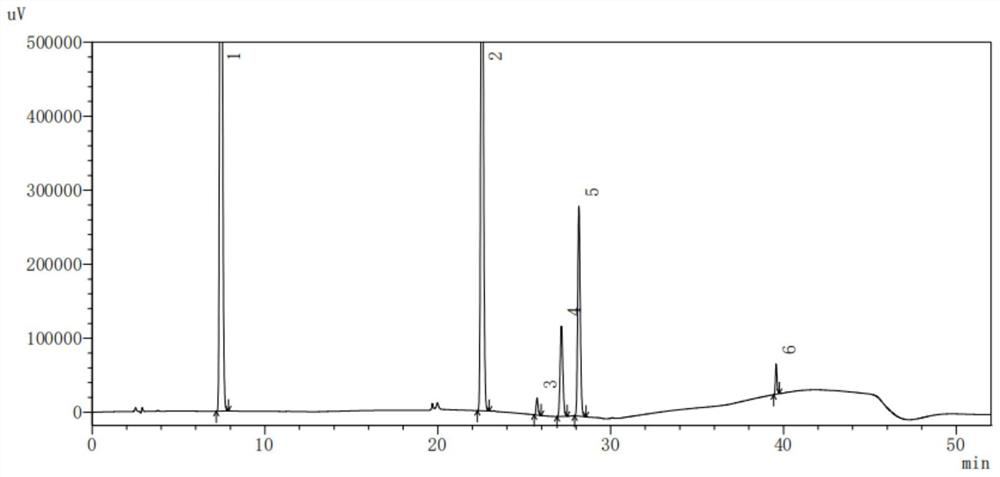
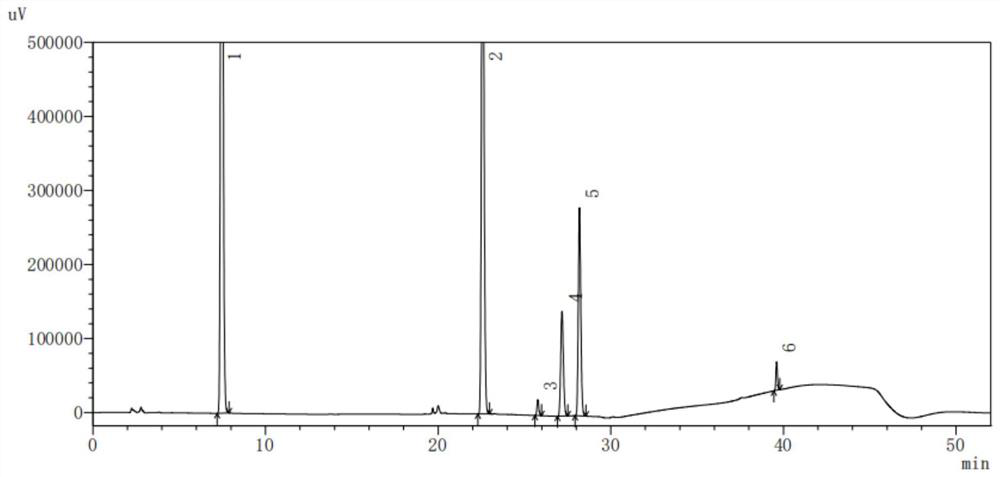
Method for detecting content of dextromethorphan hydrobromide quinidine sulfate capsules
ActiveCN113720944AThe detection method is convenient and fastEfficient detection methodComponent separationDextromethorphan HydrobromidePharmaceutical drug
The invention belongs to the technical field of pharmaceutical analysis, particularly relates to a method for detecting the content of dextromethorphan hydrobromide and quinidine sulfate capsules, and provides a convenient, efficient and accurate detection method for solving the detection problem of a method for detecting the content of dextromethorphan hydrobromide and quinidine sulfate capsules. The method can be used for detecting the content of the dextromethorphan hydrobromide and quinidine sulfate capsules, so that the medication safety is effectively guaranteed, the quality control of the dextromethorphan hydrobromide and quinidine sulfate capsules is facilitated, the method is convenient, efficient and accurate, the system applicability, repeatability, specificity and accuracy completely accord with the guidance principle verified by a Chinese pharmacopoeia method, and the method can be used for quality control of the dextromethorphan hydrobromide quinidine sulfate capsules.
Owner:珠海润都制药股份有限公司
A method for detecting the content of various components in Ammeguasu oral solution by HPLC method
The invention discloses a method for detecting the content of various components in the oral solution of Ammeguasu by HPLC. The chromatographic conditions of the HPLC method are as follows: octadecylsilane bonded silica gel is used as filler, and phosphate The mixture of buffer solution and methanol is mobile phase A, the mixture of phosphate buffer solution and acetonitrile is mobile phase B for gradient elution, the flow rate is 0.5-1.5ml per minute, the column temperature is 25-40°C, and the detection wavelength 200‑254nm. The present invention can not only detect acetaminophen, dextromethorphan hydrobromide, guaiacol glyceryl ether, phenylephrine hydrochloride, sodium benzoate and peproterate in the oral solution of Ammegualine simultaneously, but also have a common peak among them All components are identified, have good reproducibility and high accuracy, and are suitable for the quality control of the active component content of the ammeguabu oral solution of the present invention.
Owner:SICHUAN FENGCHUN PHARMA
Dextromethorphan chewing gum tablets and preparation method thereof
ActiveCN102258489BOrganic active ingredientsPill deliveryBronchial epitheliumObstructive chronic bronchitis
Owner:CHONGQING MEDICAL UNIVERSITY
A kind of stable oral solution of mepheniramine ammonium and preparation method thereof
ActiveCN111437250BImprove stabilityImprove applicabilityOrganic active ingredientsDispersion deliveryChlorobenzeneSucrose
The invention provides a stable oral solution of mepheniramine ammonium and a preparation method thereof. Mepheniramine ammonium oral solution of the present invention comprises dextromethorphan hydrobromide 0.03~0.3% (w / v), chlorpheniramine maleate 0.01~0.2% (w / v), ammonium chloride 0.1~3.0% % (w / v), pH regulator 0.02-5% (w / v), fragrance 0.01-2% (w / v), colorant 0.008-0.1% (w / v) and water, the oral solution It also contains 10% to 25% (w / v) of propylene glycol. The mephenamine ammonium oral solution of the present invention can take effect quickly; the prescription does not contain sucrose, which avoids incompatibility with ammonium chloride; does not contain preservatives, which improves the safety of the solution; The propylene glycol increases the solubility of dextromethorphan hydrobromide; and the propylene glycol content of 10% to 25% has obvious bacteriostasis effect, and there is no need to add preservatives.
Owner:HEFEI IND PHARMA INST CO LTD
Dextromethorphan hydrobromide oral solution
ActiveCN114159387AOrganic active ingredientsDispersion deliveryDextromethorphan HydrobromideAntioxidant
The invention relates to a dextromethorphan hydrobromide oral solution. Specifically, the invention provides the pharmaceutical composition, and the pharmaceutical composition comprises dextromethorphan hydrobromide, a flavoring agent, a stabilizer, a complexing agent, an antioxidant and a solvent. The dextromethorphan hydrobromide oral solution disclosed by the invention has excellent mouth feel and stability.
Owner:JIANGSU HANCHEN PHARMA
Compound preparation for treating cold and preparation method thereof
PendingCN113893249AGreat tasteInhibit growthOrganic active ingredientsDispersion deliveryAntiseptic AgentOral solutions
The invention discloses a compound preparation for treating cold. The compound preparation is a liquid preparation, comprises a solution consisting of purified water, dextromethorphan hydrobromide, acetaminophen and phenylephrine hydrochloride, and further comprises a pH regulator, a preservative, an antioxidant, a sweetening agent and a cosolvent. According to the compound oral solution containing the acetaminophen, the dextromethorphan hydrobromide and the phenylephrine hydrochloride, a flavoring agent is added, so that the compound oral solution tastes good and is particularly suitable for children; a pH regulator buffer system is adopted, so that the pH value of the product is stable; a bacteriostatic agent is added, so that microbial growth can be prevented, and chemical degradation is remarkably reduced; the compound oral solution consisting of the acetaminophen, the dextromethorphan hydrobromide and the phenylephrine hydrochloride is high in stability; the antioxidant is added, so that the chemical degradation is remarkably reduced; and the preparation process is simple, the quality is controllable, the production cost is low, and the compound preparation is suitable for large-scale production.
Owner:合肥远志医药科技开发有限公司
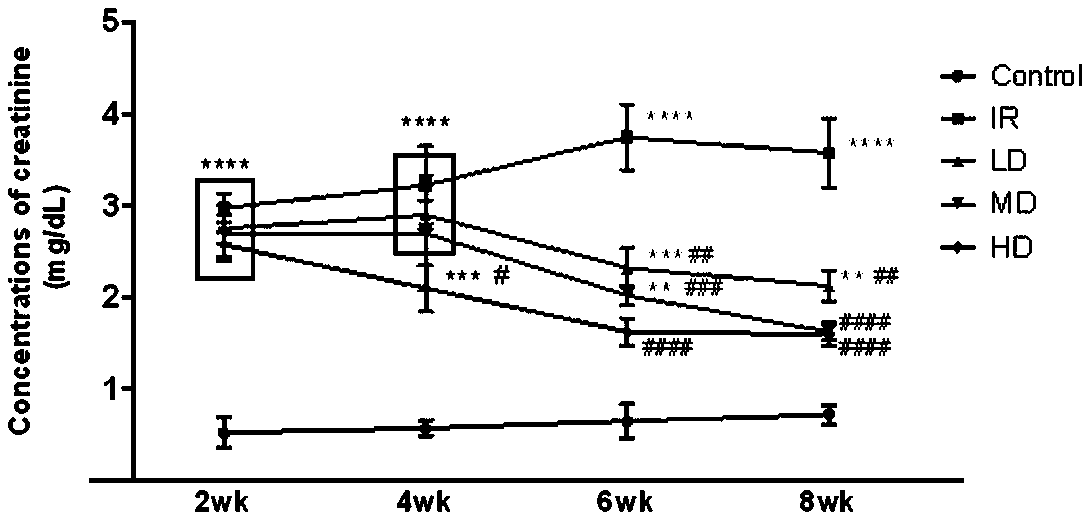
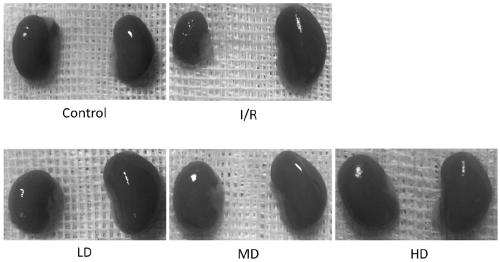
Application of dextromethorphan hydrobromide in the treatment of acute and chronic renal fibrosis
ActiveCN107260739BPrevent and inhibit renal fibrosisOrganic active ingredientsInorganic non-active ingredientsDextromethorphan HydrobromideAcute Renal Injury
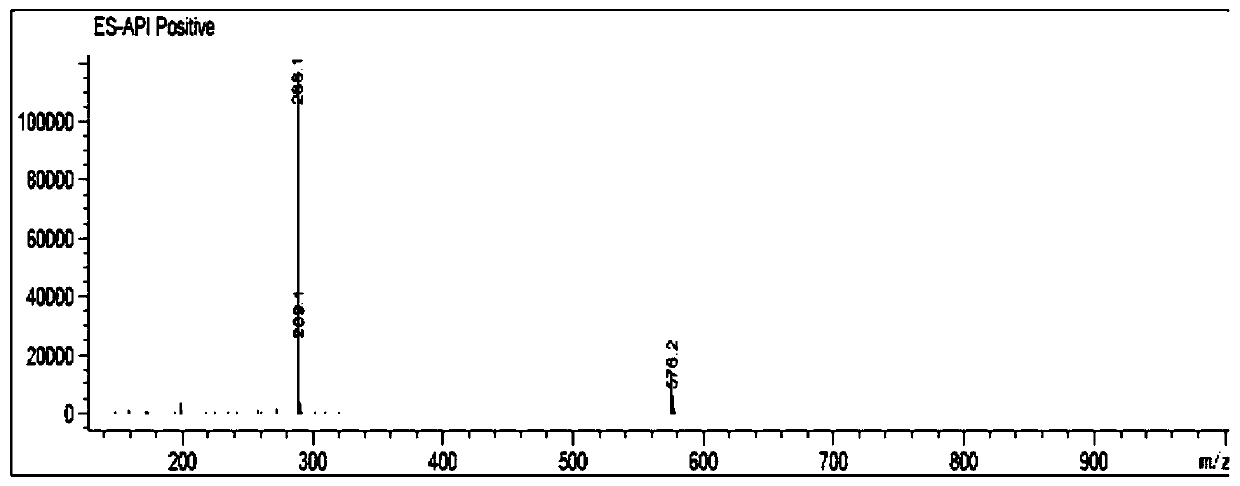
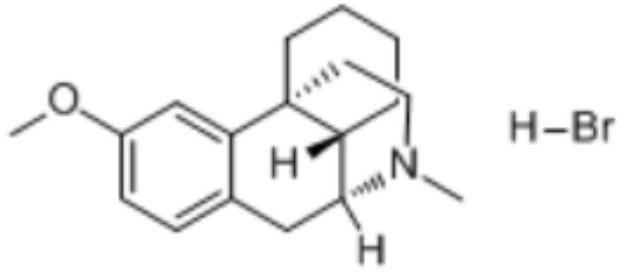
The invention provides application of dextromethorphan hydrobromide to treatment of acute and chronic renal fibrosis. The molecular formula of dextromethorphan hydrobromide is C18H25NO HBr H2O. The dextromethorphan hydrobromide has the shape of white powder and the dosage form of injection; the injection is obtained after diluting mother liquid prepared from dextromethorphan hydrobromide powder and physiological saline. The invention provides the application of the existing medicine (dextromethorphan hydrobromide) to the treatment of the acute and chronic renal fibrosis. The technical scheme and the technical effect can prove that the dextromethorphan hydrobromide can be used for effectively preventing and inhibiting the long-period prognostic renal function weakening and renal fibrosis due to renal ischaemia; the ingredients can be used for medium-long-period renal function protection after the acute kidney injury; the novel scheme is provided for the acute chronic renal fibrosis; the novel direction is provided for the medicine study of the dextromethorphan hydrobromide.
Owner:ZHEJIANG UNIV
Dextromethorphan hydrobromide lyophilized powder and preparation method thereof
ActiveCN103536539BOrganic active ingredientsPowder deliveryDextromethorphan HydrobromidePolyethylene glycol
The invention relates to dextromethorphan hydrobromide freeze-dried powder injection, which is made of the following raw materials: 16g dextromethorphan hydrobromide, 11g? Lactic acid, 8g sorbic acid, 45g polyethylene glycol 2000, 2000ml water for injection; adjust the pH to 3.3-3.6.
Owner:南通丝乡丝绸有限公司
Hydrobromic acid dextro methaphen oral cavity disintegretion tablet and its preparation method
An oral disintegrating tablet of dextromethorphan hydrobromide contains dexromethorphan hydrobromide and auxiliaries including sorbitol, methylacrylic copolymer resin (Eudragit L30D), citric acid, sodium dicarbonate, magnesium stearate, SiO2 and lemon perfume.
Owner:范敏华
Compound dextromethorphan hydrobromide dropping pills and preparation method thereof
InactiveCN110721168APromote oral absorptionDetoxificationAntibacterial agentsOrganic active ingredientsFritillaria cirrhosaPolyethylene glycol
The invention discloses compound dextromethorphan hydrobromide dropping pills and a preparation method thereof. The product comprises 1-20 parts of dextromethorphan hydrobromide; 5-30 parts of andrographolide; 5-30 parts of herba ephedrae, 5-30 parts of long-noded pit viper; 5-30 parts of saxifraga stolonifera; 5-30 parts of firmiana root; 5-30 parts of semen armeniacae amarae; 5-30 parts of honey-fried licorice root; 5-30 parts of platycodon grandiflorum, 5-30 parts of polygala tenuifolia; 5-30 parts of flos farfarae, 5-30 parts of liquorice, 5-30 parts of aster, 5-30 parts of bulbus fritillariae cirrhosae, 5-30 parts of folium eriobotryae, 5-30 parts of radix stemonae, 5-30 parts of radix peucedani, 5-30 parts of cortex mori radicis, 5-30 parts of mint, 5-30 parts of folium eriobotryae,1-20 parts of polyethylene glycol A, 1-20 parts of glycerin, and 1-5 parts of tween. According to the compound dextromethorphan hydrobromide dropping pill, dextromethorphan hydrobromide and traditional Chinese medicine components are taken in a combined mode, the effect is more beneficial to treatment of dry cough and pharyngitis pain, and the product stability is good.
Owner:山东禹王制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com