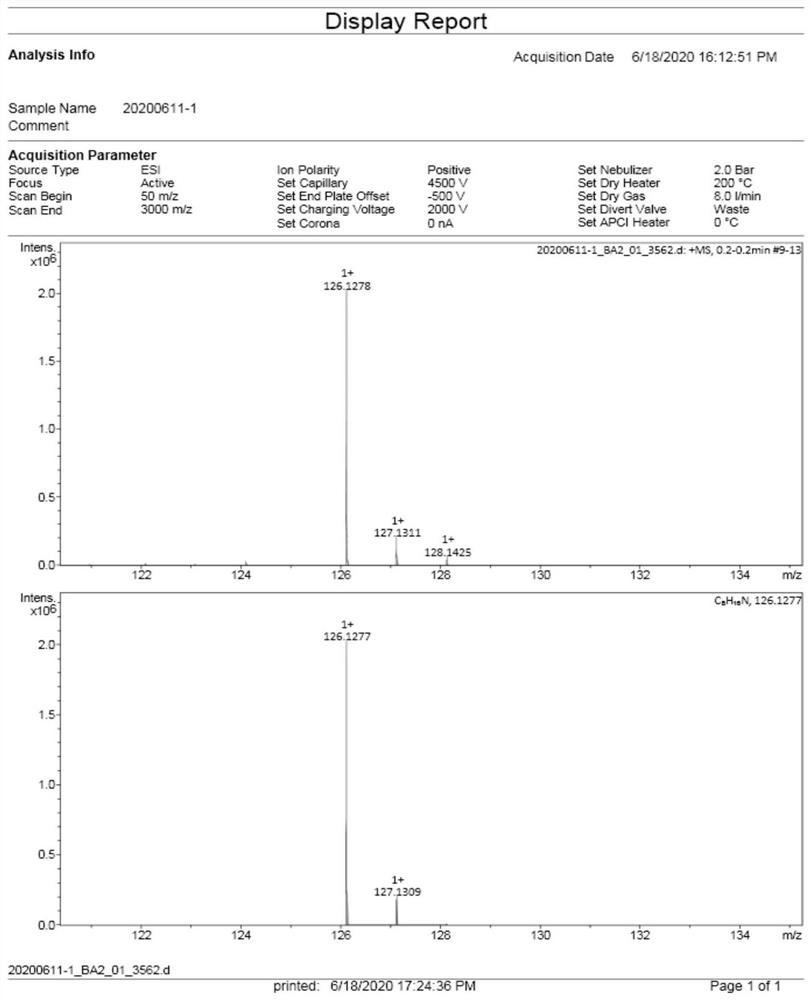
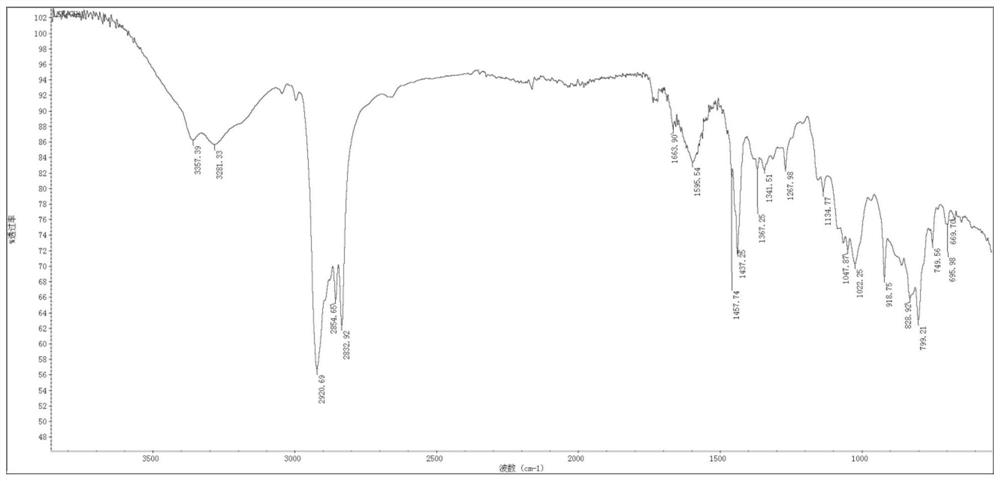
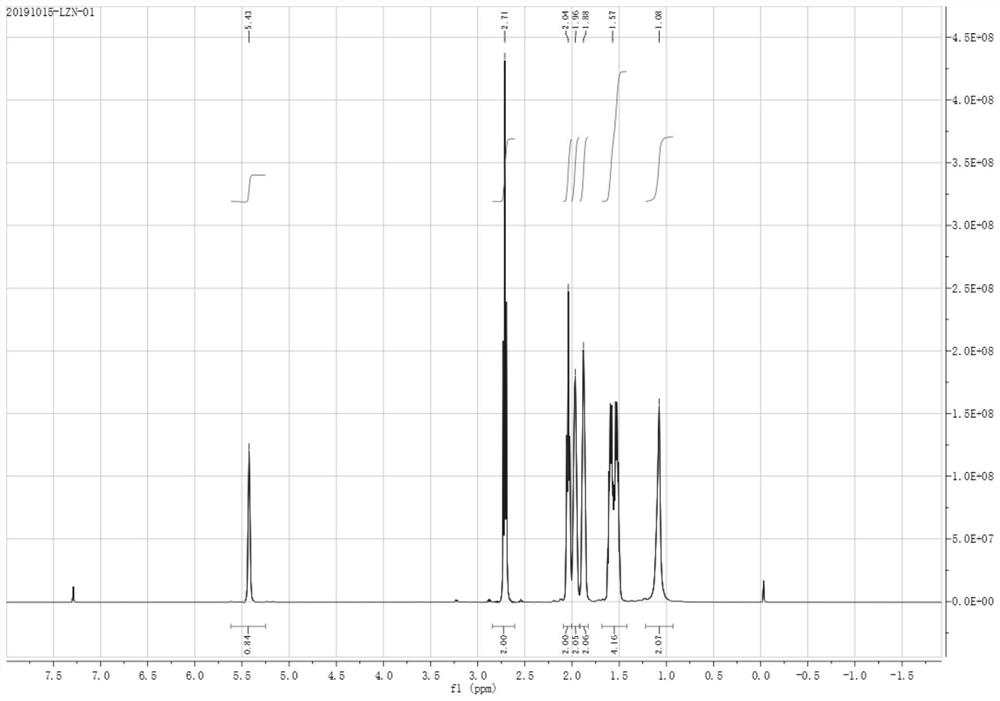
Synthesis method of 2-(1-cyclohexenyl) ethylamine
A technology of cyclohexenyl and synthesis method, applied in the field of synthesis of compound 2-ethylamine, can solve the problems of difficult industrial implementation, harsh reaction conditions, long process route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: The compound cyclohexanone (II, 9.8g, 0.1mol) was mixed with 200mL tetrahydrofuran, and after cooling to 0°C in an ice bath, a tetrahydrofuran solution of vinylmagnesium chloride (1.6M, 100mL) was added, and stirred at room temperature for 10 hours After quenching with a saturated ammonium chloride solution, the organic phase was separated, the aqueous phase was extracted twice with ethyl acetate, and the organic phases were combined and concentrated to obtain 12.4 g of crude product of 1-vinylcyclohexanol (III).
Embodiment 2
[0043] Example 2: The compound cyclohexanone (II, 9.8g, 0.1mol) was mixed with 200mL tetrahydrofuran, and after cooling to 0°C in an ice bath, a tetrahydrofuran solution (1M, 150mL) of vinylmagnesium bromide was added, and stirred at room temperature for 6 Quenched with ammonium chloride saturated solution after 1 hour, separated the organic phase, extracted the aqueous phase twice with ethyl acetate, combined the organic phases, and concentrated it to obtain 12.6 g of the crude product of 1-vinylcyclohexanol (III) .
[0044] Two, the preparation of (2-chloroethylmethylene) cyclohexane (IV)
[0045] Example 1: 12.6g of 1-vinylcyclohexanol (III, 12.6g, 0.1mol) prepared in the previous step was mixed with 150mL of tetrahydrofuran, and after ice bathing to 0°C, pyridine (14.22g, 0.18mol) and Thionyl chloride (18.88g, 0.16mol), after stirring the reaction for 45min, 3 The saturated solution was quenched, the organic phase was separated, the aqueous phase was extracted twice with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com