Dextromethorphan hydrobromide lyophilized powder and preparation method thereof
A technology of dextromethorphan hydrobromide and freeze-dried powder injection, which can be applied in freeze-dried delivery, powder delivery, pharmaceutical formulation and other directions, can solve the problems of high product safety risk, increase product cost, etc., and achieve high safety for clinical use, The effect of less excipients and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
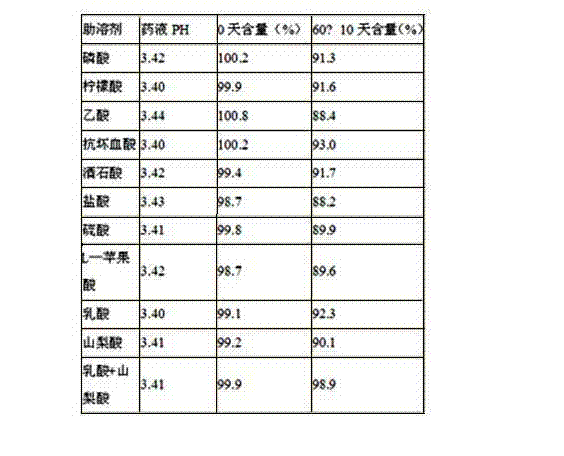
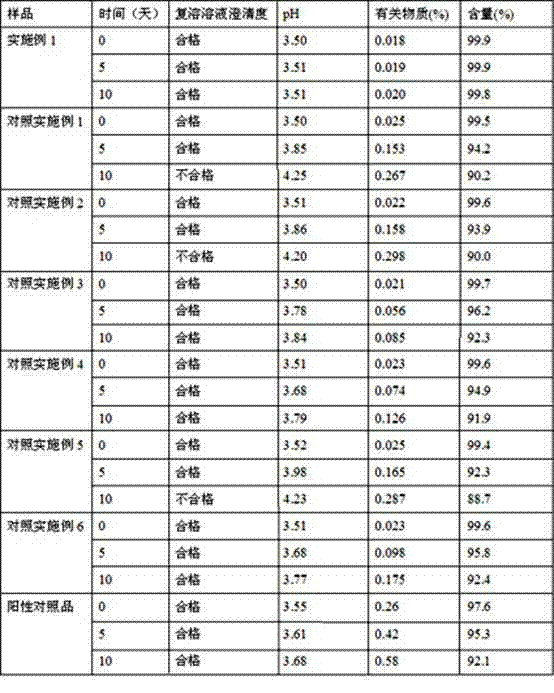
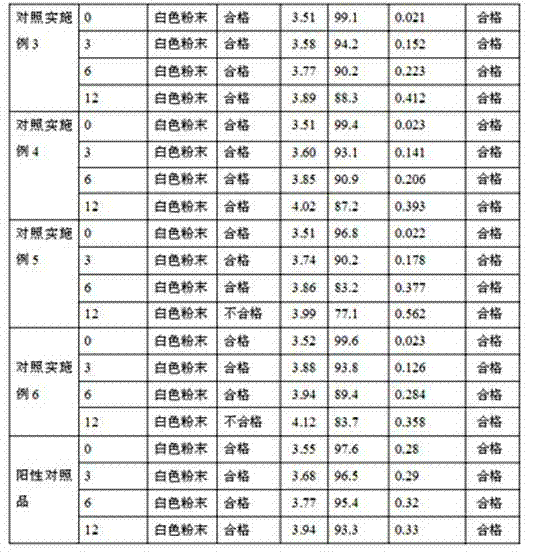
Image
Examples
Embodiment 1
[0020] Dextromethorphan hydrobromide: 16g
[0021] Lactic acid and sorbic acid: 11g lactic acid + 8g sorbic acid
[0022] Polyethylene glycol 2000: 45g
[0023] PH regulator: appropriate amount
[0024] Water for injection: 2000ml
[0025] Process:
[0026] 1. Take prescription amount of lactic acid and sorbic acid, add 50% water for injection, heat to 50~55℃, stir to dissolve, add prescription amount of dextromethorphan hydrobromide to the solution, stir to dissolve and continue stirring for 15 minutes .
[0027] 2. Take the prescription amount of polyethylene glycol 2000, add 40% water for injection, stir for 15 minutes, adjust the pH to 3.0-3.5 with hydrochloric acid.
[0028] 3. Combine 1 and 2 solutions, adjust the pH to 3.3-3.6 with a pH regulator (sodium hydroxide solution), add water for injection to the full amount, add 0.15% activated carbon for needles, stir for 25 minutes, filter and decarburize, intermediate After passing the inspection, filter and sterilize with a 0.22μm fi...
Embodiment 1
[0030] Dextromethorphan hydrobromide: 16g
[0031] Lactic acid and sorbic acid: 11g lactic acid + 8g sorbic acid
[0032] PH regulator: appropriate amount
[0033] Water for injection: 2000ml
[0034] Process:
[0035] 1. Take prescription amount of lactic acid and sorbic acid, add 80% water for injection, heat to 50~55℃, stir to dissolve, add prescription amount of dextromethorphan hydrobromide to the solution, stir to dissolve and continue stirring for 15 minutes .
[0036] 2. Adjust the pH to 3.3~3.6 with pH regulator (sodium hydroxide solution), add water for injection to the full amount, add 0.15% activated carbon for needles, stir for 25 minutes, filter and decarburize, check the intermediate, use 0.22μm after passing Sterilize through filtration membrane, pour the filtrate into a vial and freeze-dry to obtain a freeze-dried powder injection, which is packaged after passing the inspection.
Embodiment 2
[0038] Dextromethorphan hydrobromide: 16g
[0039] Polyethylene glycol 2000: 45g
[0040] pH regulator: appropriate amount
[0041] Water for injection: 2000ml
[0042] Process:
[0043] 1. Take the prescription amount of polyethylene glycol 2000, add 80% water for injection, stir for 15 minutes, adjust the pH to 3.0-3.5 with hydrochloric acid. Add the prescribed amount of dextromethorphan hydrobromide to the solution, stir to dissolve and continue stirring for 15 minutes.
[0044] 2. Adjust the pH to 3.3~3.6 with pH regulator (sodium hydroxide solution), add water for injection to the full amount, add 0.15% activated carbon for needles, stir for 25 minutes, filter and decarburize, check the intermediate, use 0.22μm after passing Sterilize through filtration membrane, pour the filtrate into a vial and freeze-dry to obtain a freeze-dried powder injection, which is packaged after passing the inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com