Method for detecting content of dextromethorphan hydrobromide quinidine sulfate capsules
A technology for dextromethorphan hydrobromide and quinidine sulfate, which is applied in the field of detection of the content of dextromethorphan hydrobromide and quinidine sulfate capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Prepare the solution:
[0056] Blank solution: mobile phase A.
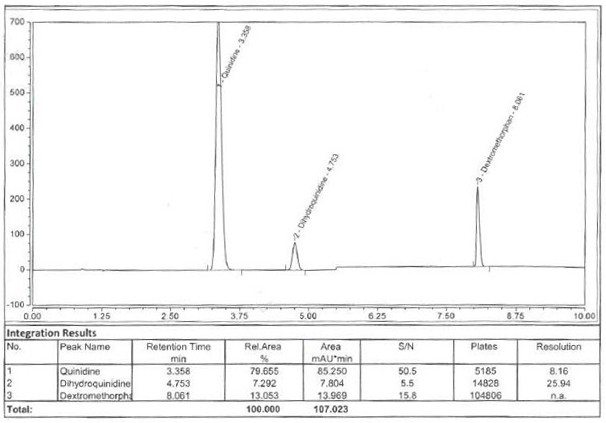
[0057] Reference substance solution: Take about 20 mg of dextromethorphan hydrobromide reference substance and about 10 mg of quinidine sulfate reference substance respectively, accurately weigh them, put them in the same 100ml measuring bottle, add mobile phase A and sonicate for 2 minutes to dissolve and dilute to the mark , shake well. (Concentration: Dextromethorphan Hydrobromide 0.2mg / ml, Quinidine Sulfate 0.1mg / ml)
[0058] Test solution 1: Take 1 capsule of this product, place it in a 100ml measuring bottle, add an appropriate amount of mobile phase A, soak for 30 minutes, ultrasonicate for 5 minutes to dissolve, let cool to room temperature, dilute to the mark with mobile phase A, shake well, filter.
[0059] Test solution 2: Take 5 capsules of this product, place them in a 100ml measuring bottle, add an appropriate amount of mobile phase A, soak for 30 minutes, ultrasonicate for 5 minutes t...
Embodiment 2
[0096] Example 2: System Suitability
[0097] The system applicability is checked by repeating the 5-point reference solution, and the RSD of the peak area of the 5-point reference solution is required to be ≤ 2.0%, and the RSD of 6 needles (or more than 6 needles) is ≤ 3.0%; the recovery rate of the reference solution should be 98.0% ~102.0%; the number of theoretical plates of dextromethorphan hydrobromide peak and quinidine sulfate peak is not less than 2000. After the system is stabilized, inject 1 injection of blank solution and 5 injections of reference substance solution.
[0098]
[0099]
Embodiment 3
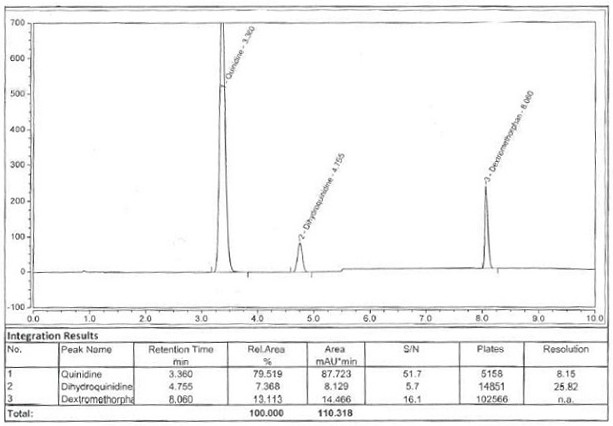
[0100] Example 3: Specificity
[0101] Specificity requires that the blank solution and blank excipients (including capsule shell) have no interference to the detection, and the blank solution and blank excipients (including capsule shell) have no interference on the detection. Dextromethorphan hydrobromide and quinidine sulfate in the test solution The retention time of the test solution is consistent with that of the reference substance solution. If there is an unknown peak in the test solution, the separation degree of the main peak and the adjacent peak is not less than 1.5, and the number of theoretical plates of the dextromethorphan hydrobromide peak and the quinidine sulfate peak is not less than 2000. The purity matching value of dextromethorphan hydrobromide peak and quinidine sulfate peak in the test solution and reference solution is not less than 980. After the system is stabilized, blank solution, blank excipient (including capsule shell) solution, reference soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com