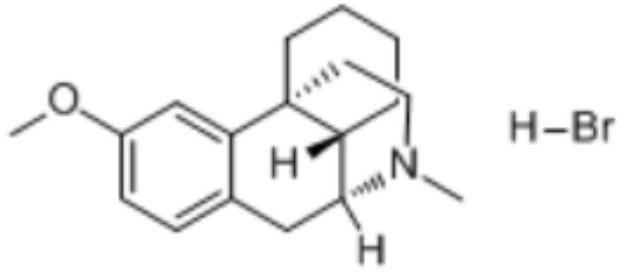
Dextromethorphan hydrobromide oral solution
A technology of dextromethorphan hydrobromide and sodium bisulfite, which is applied in the directions of dispersion liquid delivery, inorganic inactive ingredients, and drug combination, etc., can solve the problems of easy precipitation and crystallization, poor oral compliance of patients, and poisoning of essences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
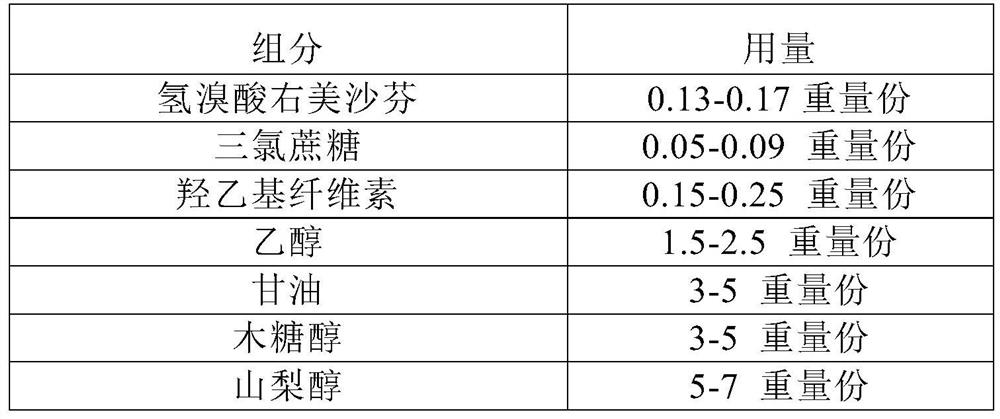
[0164] Present embodiment 1 provides a kind of dextromethorphan hydrobromide oral solution, and the prescription of dextromethorphan hydrobromide oral solution is as shown in table 1 below:
[0165] The prescription composition of table 1 dextromethorphan hydrobromide oral solution (specification 1.5mg / ml)
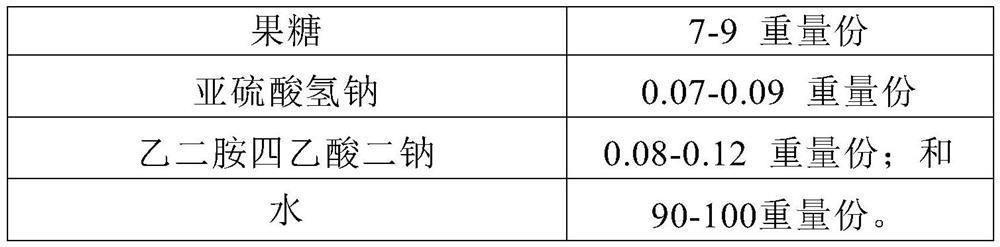
[0166] components Dosage Dextromethorphan Hydrobromide 0.15g Sucralose 0.07g Hydroxyethyl cellulose 0.2g ethanol 2.0g glycerin 4.0g Xylitol 4.0g Sorbitol 6.0g fructose 8.0g sodium bisulfite 0.08g Disodium edetate 0.1g purified water up to 100ml
[0167] The preparation method of the dextromethorphan hydrobromide oral solution of present embodiment 1 is as follows:
[0168] (1) Boil the prescribed amount of purified water, keep it for 10 minutes, and set aside.
[0169] (2) After mixing 45% of the purified water of the prescribed amount, the glycerol of the prescribed amount and the ethanol o...
Embodiment 2
[0173] Present embodiment 2 provides a kind of dextromethorphan hydrobromide oral solution, and the prescription of dextromethorphan hydrobromide oral solution is as shown in table 2 below:
[0174] The prescription composition of table 2 dextromethorphan hydrobromide oral solution (specification 1.5mg / ml)
[0175] components Dosage Dextromethorphan Hydrobromide 0.15g Sucralose 0.07g ethanol 2.0g Xylitol 4.0g Sorbitol 6.0g fructose 8.0g sodium bisulfite 0.08g Disodium edetate 0.1g purified water up to 100ml
[0176] The preparation method of the dextromethorphan hydrobromide oral solution of present embodiment 2 is as follows:
[0177] (1) Boil the prescribed amount of purified water, keep it for 10 minutes, and set aside.
[0178] (2) After mixing 45% of the purified water of the prescribed amount and the ethanol of the prescribed amount, a mixed solvent is obtained, and the temperature of the mixed solven...
Embodiment 3
[0182] Present embodiment 3 provides a kind of dextromethorphan hydrobromide oral solution, and the prescription of dextromethorphan hydrobromide oral solution is as shown in table 3 below:
[0183] The prescription composition of table 3 dextromethorphan hydrobromide oral solution (specification 1.5mg / ml)
[0184] components Dosage Dextromethorphan Hydrobromide 0.15g Sucralose 0.07g Hydroxyethyl cellulose 0.2g ethanol 2.0g glycerin 4.0g Sorbitol 6.0g fructose 8.0g sodium bisulfite 0.08g Disodium edetate 0.1g purified water up to 100ml
[0185] The preparation method of the dextromethorphan hydrobromide oral solution of present embodiment 3 is as follows:
[0186] (1) Boil the prescribed amount of purified water, keep it for 10 minutes, and set aside.
[0187] (2) After mixing 45% of the purified water of the prescribed amount, the glycerol of the prescribed amount and the ethanol of the prescribed am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com