Preparation method of dextromethorphan hydrobromide N-oxide impurity
A technology of dextromethorphan hydrobromide and sodium hydroxide, applied in the direction of organic chemistry and the like, can solve the problem of not finding the preparation documents of nitrogen oxides, etc., and achieve the effects of high stability, mild and controllable reaction conditions, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
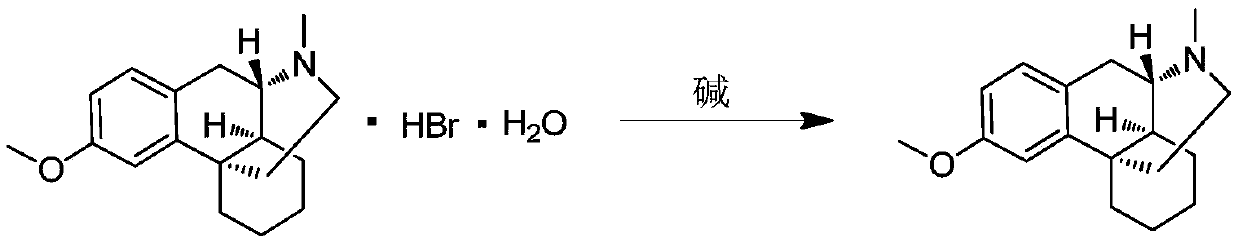
[0033] Take 3.7g (1.0eq) of dextromethorphan hydrobromide, dissolve it in 150ml of water, add 5ml of 5N aqueous sodium hydroxide solution, stir for 3-5 minutes, add 100ml of dichloromethane for extraction, and dry the organic phase with anhydrous sodium sulfate , and concentrated the solvent under reduced pressure to obtain dextromethorphan (free state). Add 50ml of absolute ethanol to dextromethorphan, then add dropwise 1.7g (1.5eq) of 30% hydrogen peroxide, and keep stirring at 10-20°C for 16h after the addition is complete. Post-processing: the reaction solution was concentrated under reduced pressure, purified and separated by silica gel column chromatography, the eluent was DCM / MeOH=20 / 1-10 / 1, and concentrated to obtain 2.5 g of the product with a yield of 87%.
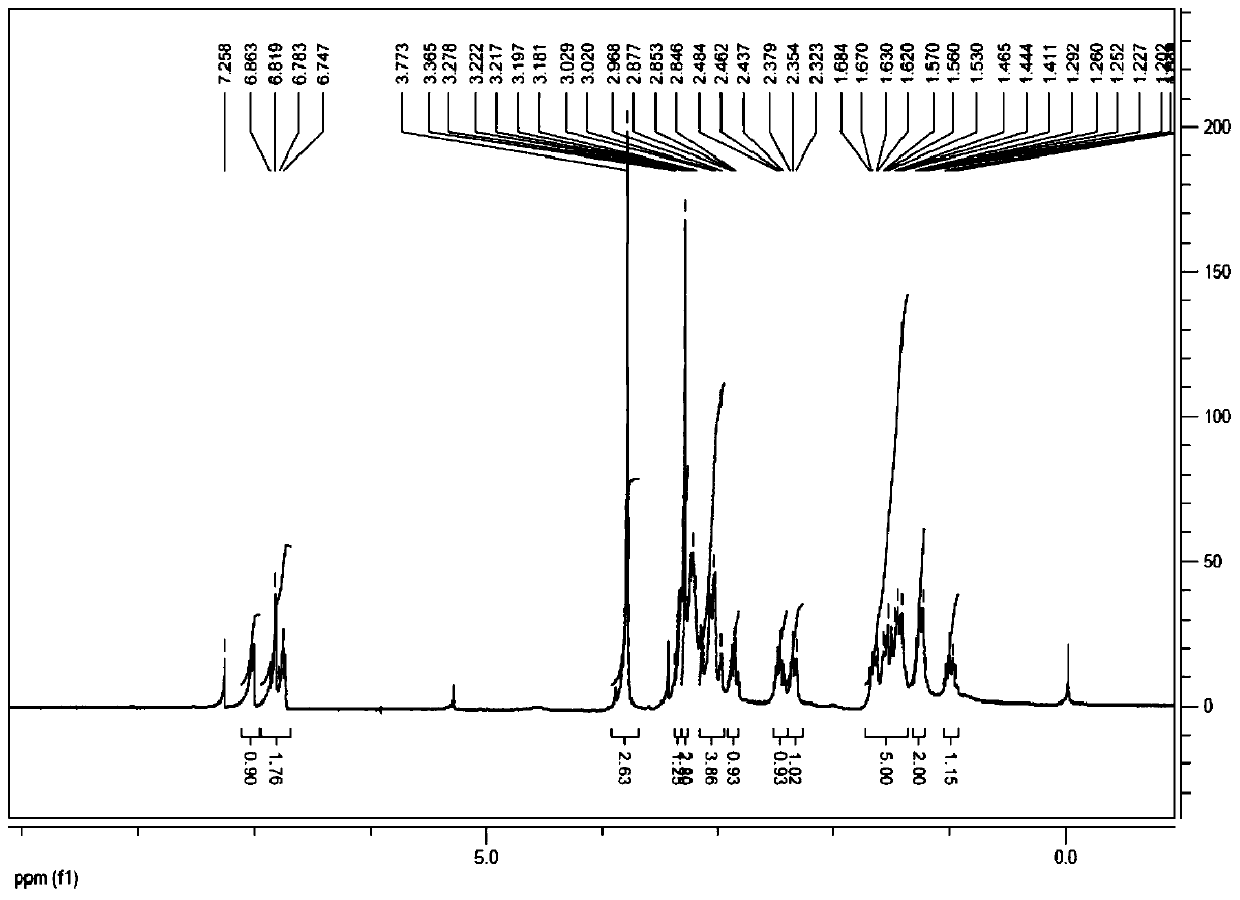
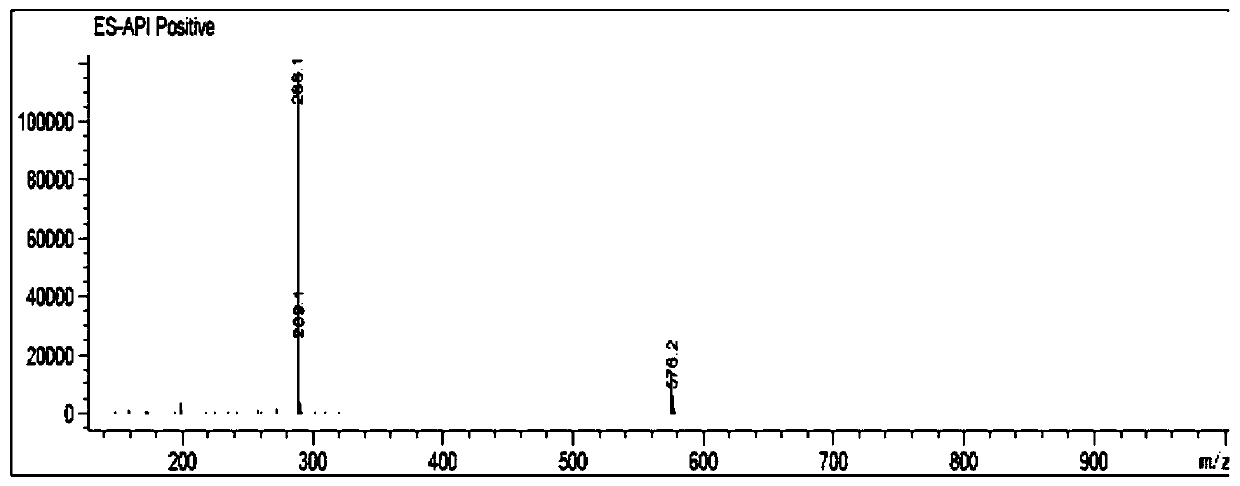
[0034] MS(m / z):288.1[M+H] + , 576.2[2M+H] + .
[0035] 1 HNMR (400MHz, CDCl 3 )δ:0.97-0.98(m,1H),1.22-1.26(m,2H),1.42-1.64(m,5H),2.32(d,1H),2.49(d,1H),2.84(d,1H) , 2.98-3.03 (m, 4H), 3.06 (s, 3H...
Embodiment 2
[0037]
[0038] Take 1.85g (1.0eq) of dextromethorphan hydrobromide, dissolve it in 75ml of water, add 3ml of 5N aqueous sodium hydroxide solution, stir for 3-5 minutes, add 50ml of dichloromethane for extraction, and dry the organic phase with anhydrous sodium sulfate , and concentrated the solvent under reduced pressure to obtain dextromethorphan (free state). Add 25ml of methanol to dextromethorphan, then dropwise add 0.85g (1.5eq) of 30% hydrogen peroxide, and keep stirring at 20-30°C for 16h after the addition is complete. Post-processing: the reaction solution was concentrated under reduced pressure, purified and separated by silica gel column chromatography, the eluent was DCM / MeOH=20 / 1-10 / 1, and concentrated to obtain 1.09 g of the product, with a yield of 76%.
Embodiment 3
[0040]
[0041] Take 7.4g (1.0eq) of dextromethorphan hydrobromide, dissolve it in 300ml of water, add 10ml of 5N aqueous sodium hydroxide solution, stir for 3-5 minutes, add 150ml of dichloromethane for extraction, and dry the organic phase with anhydrous sodium sulfate , and concentrated the solvent under reduced pressure to obtain dextromethorphan (free state). Add 100ml of absolute ethanol to dextromethorphan, then add 8.12g (2.0eq) of 85% m-chloroperoxybenzoic acid, and keep stirring at 0-10°C for 16h after the addition is complete. Post-processing: the reaction solution was concentrated under reduced pressure, purified and separated by silica gel column chromatography, the eluent was DCM / MeOH=20 / 1-10 / 1, and concentrated to obtain 4.6 g of the product with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com