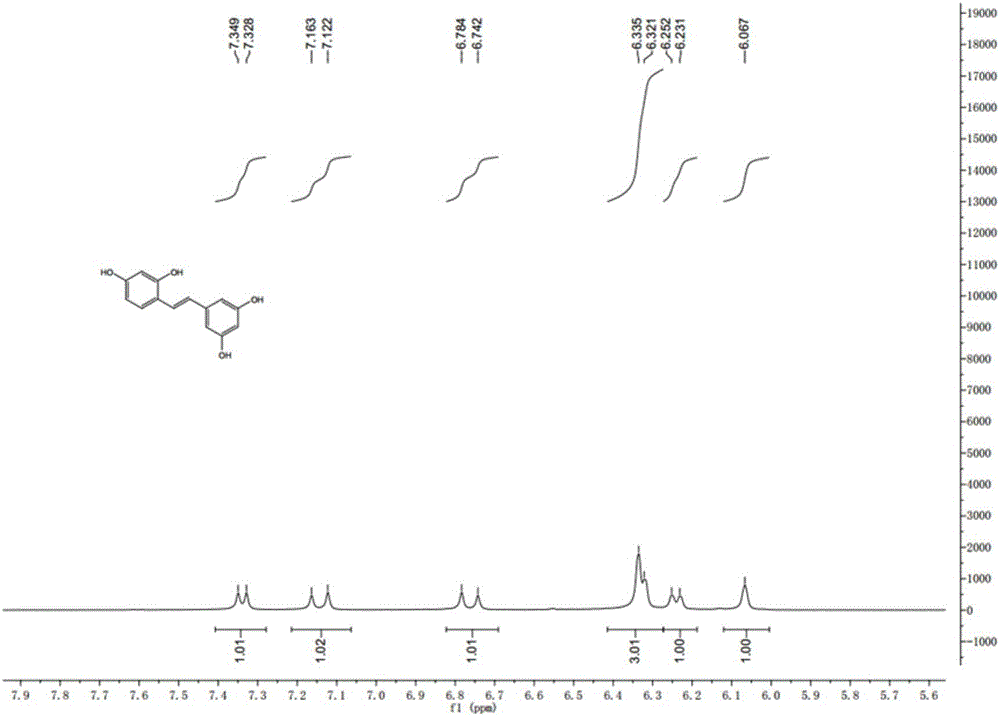
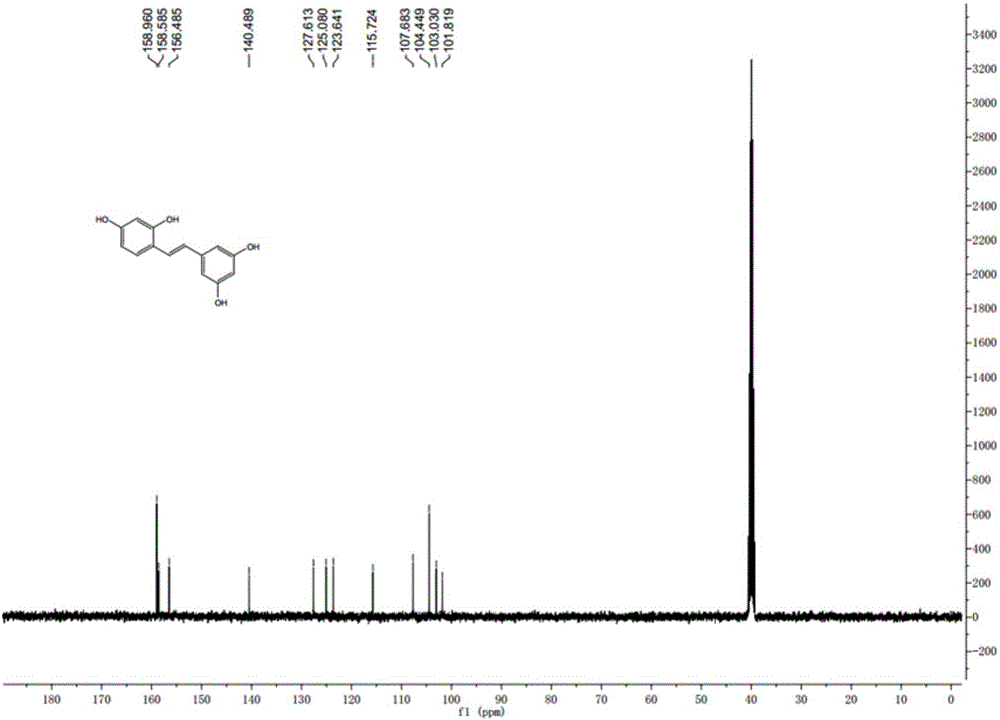
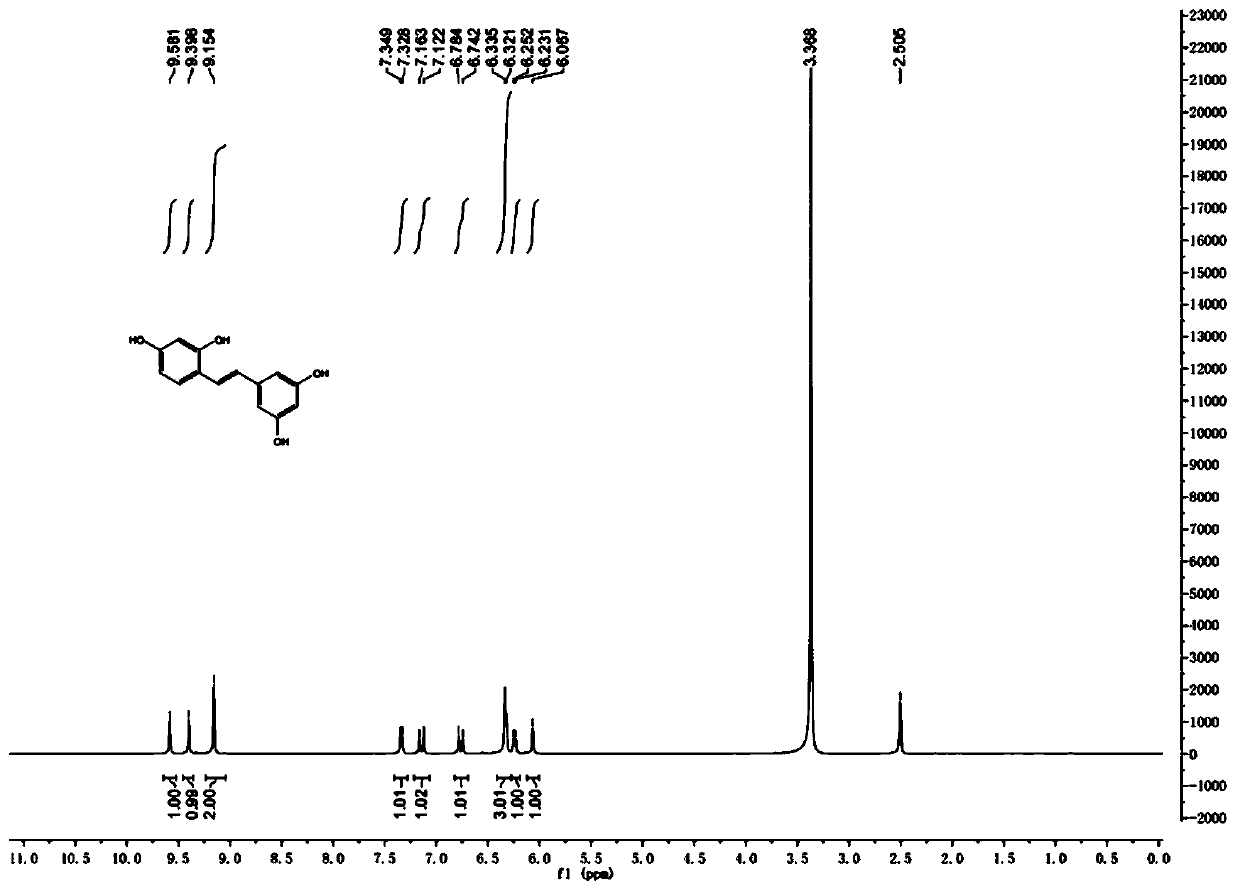
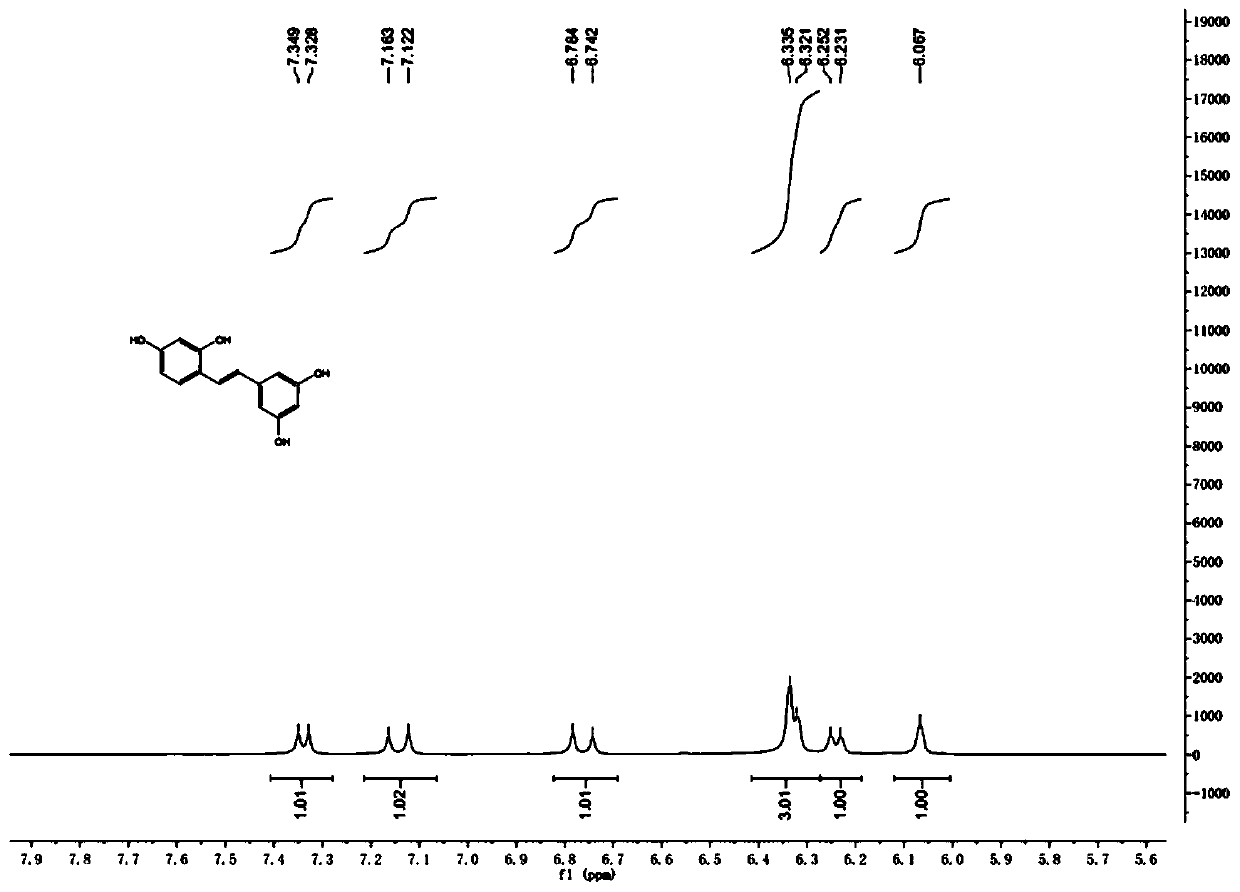
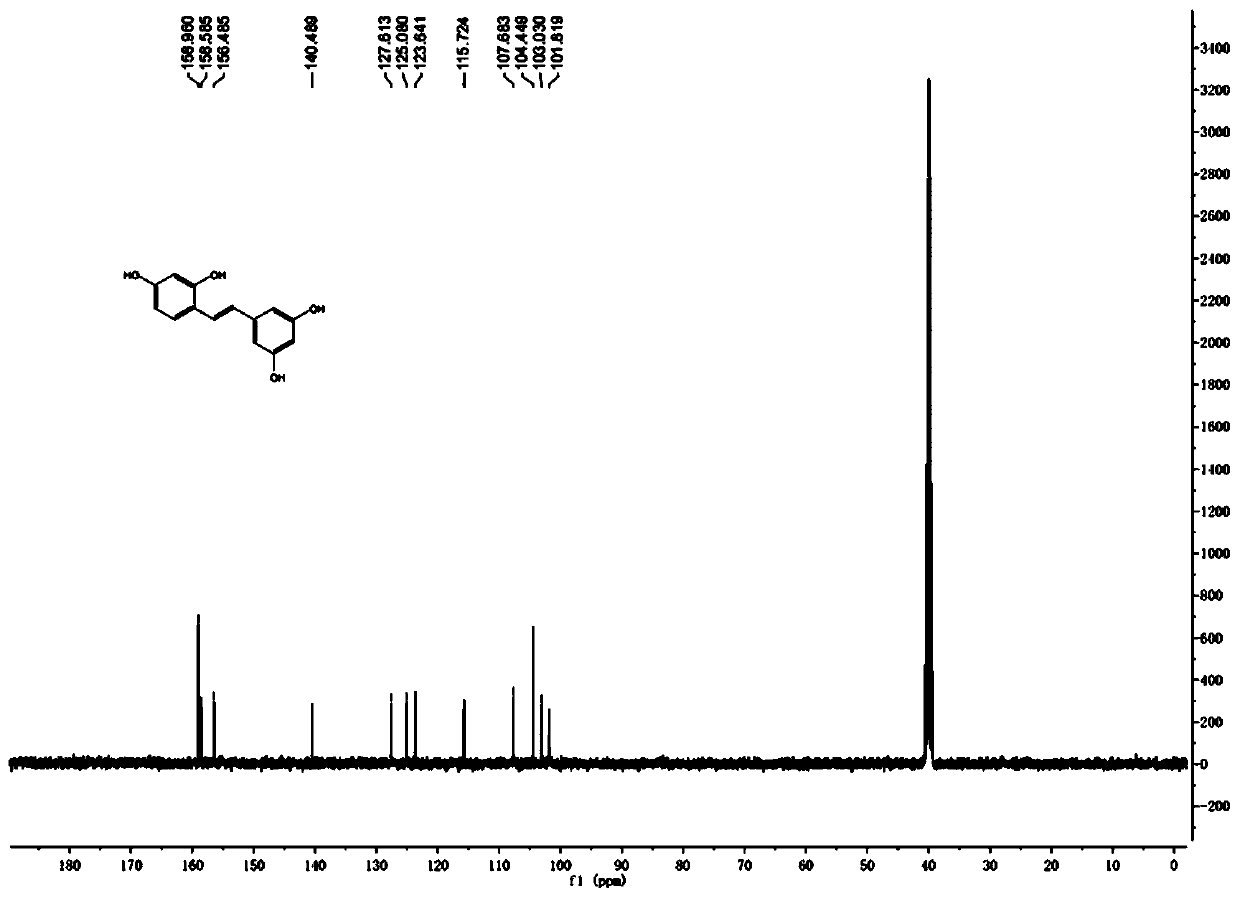
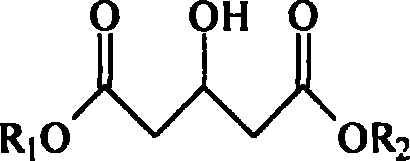
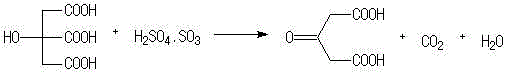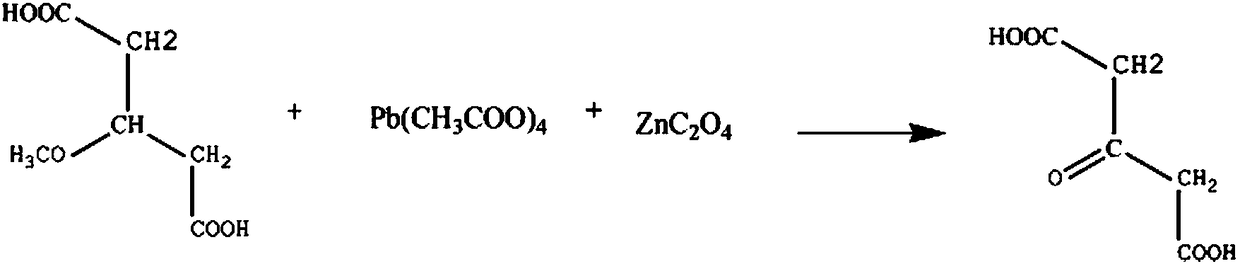Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Acetonedicarboxylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
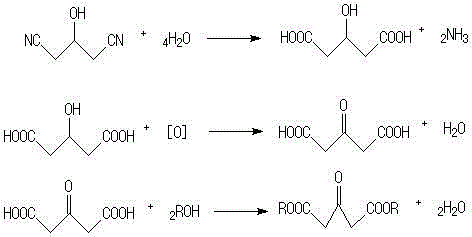
Acetonedicarboxylic acid, 3-oxoglutaric acid or β-ketoglutaric acid is a simple dicarboxylic acid.
Identification method of aliphatic chain isomer alpha-oxoglutarate and 1, 3-acetone dicarboxylic acid
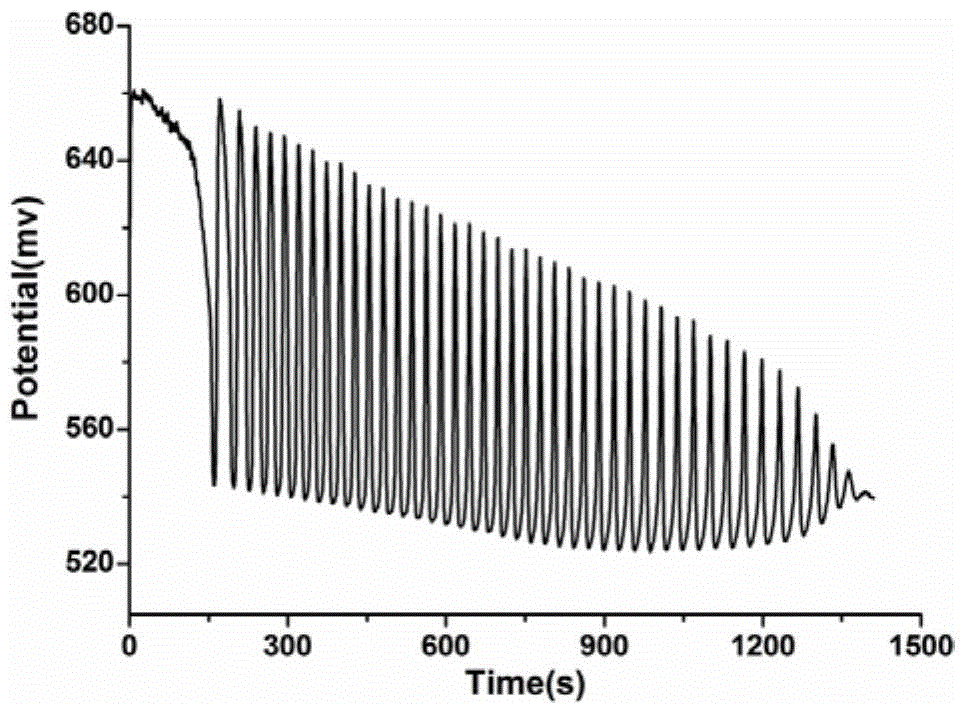
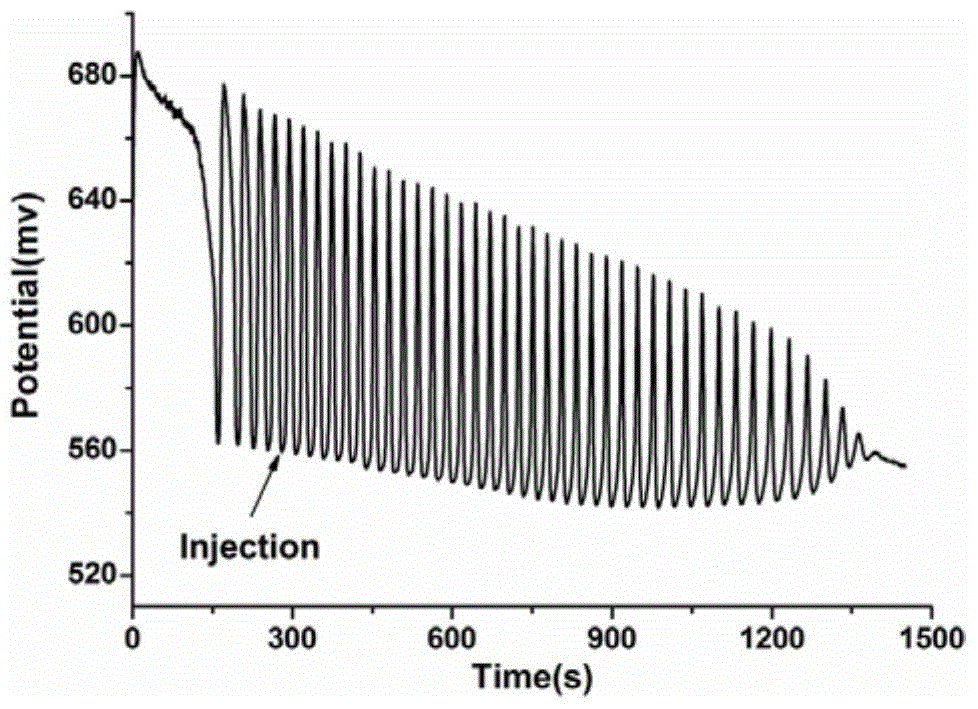
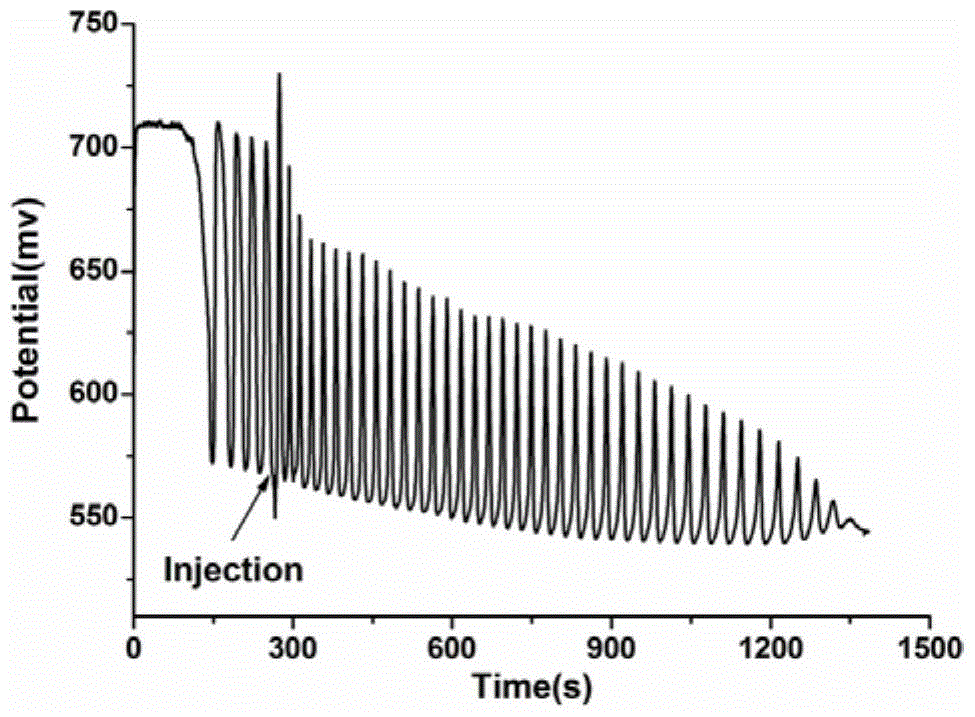
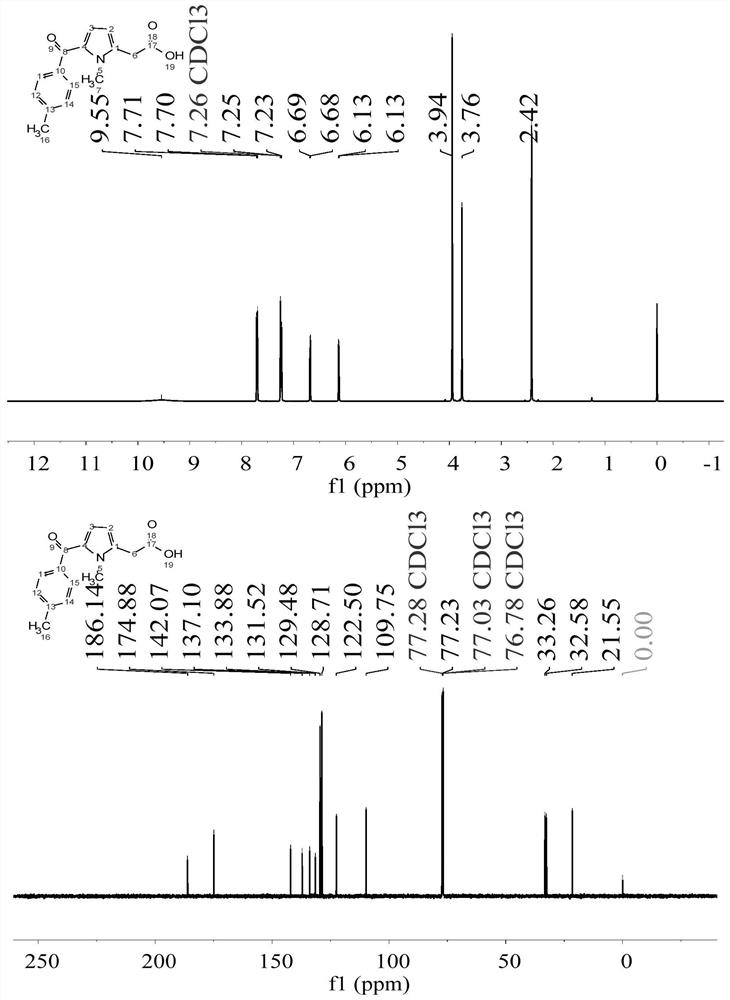
The invention discloses an identification method of aliphatic chain isomer alpha-oxoglutarate and 1,3-acetone dicarboxylic acid. The identification method is characterized by comprising the following steps that with an H<2>SO<4>-KIO<3>-[NiL](ClO<4>)<2>-MA-H<2>O<2> nonlinear chemical oscillation system as an identification solution, differential identification of the aliphatic chain isomer is realized according to an oscillation response of the system generated by the aliphatic chain isomer; L in [NiL](ClO<4>)<2> is 5, 7, 7, 12, 14, 14-hexamethyl-1, 4, 8, 11-tetraaza tetradecyl-4, 11-diene. An oscillation map provided by the identification method is relatively high in intuition; moreover, the aliphatic chain isomer alpha-oxoglutarate and 1,3-acetone dicarboxylic acid can be conveniently and quickly identified, and the method can also be widely used in other isomers; in addition, identification equipment is simple, high in accuracy and easy to operate and observe.
Owner:ANHUI UNIVERSITY
Preparation method of 1,3-cyclohexanedione
ActiveCN111187153AProcess conditions are easy to achieveEasy post-processingOrganic compound preparationCarboxylic acid esters preparationChemical synthesisPtru catalyst
The invention relates to the technical field of chemical synthesis, in particular to a preparation method of 1,3-cyclohexanedione. The preparation method of the 1,3-cyclohexanedione comprises the following steps: with 1,3-acetonedicarboxylic ester and acrylate as raw materials, carrying out condensation and cyclization under the action of a base catalyst to prepare an intermediate; carrying out hydrolysis decarboxylation on the intermediate to obtain a crude product; and recrystallizing the crude product to obtain the 1,3-cyclohexanedione. The preparation method of the 1,3-cyclohexanedione hasthe advantages that process conditions are convenient to realize, post-treatment operation process is simple, yield is as high as 90.9%, reaction selectivity is high, production efficiency is high, operation safety is high, pollution is small, and the preparation method is suitable for industrial scale production.
Owner:山东亘元新材料股份有限公司
Preparation of dimethyl acetone-1,3-dicarboxylate
InactiveCN101475482AHighlight substantiveSignificant progressOrganic compound preparationCarboxylic acid esters preparationEconomic benefitsCarboxylic acid
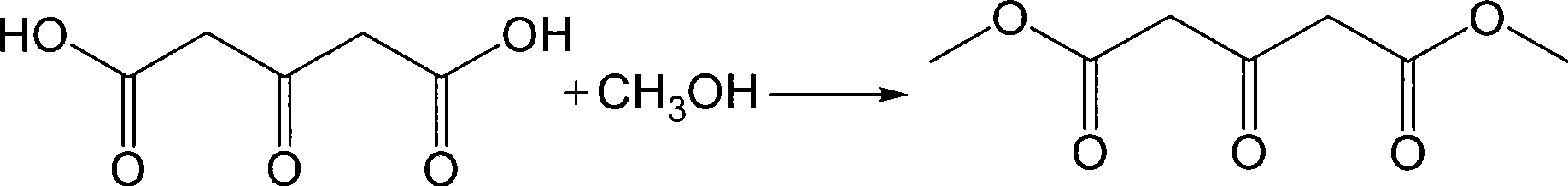
The invention provides a method for preparing Dimethyl 1,3-acetonedicarboxylate. The method comprises: firstly, performing stirring reaction between citric acid and 98 percent concentrated sulfuric acid for 1 to 2 hours at a temperature of between 49 and 52 DEG C, dripping a reaction liquid into water after the reaction is over, stirring the mixture and cooling the mixture to be less than 10 DEG C, separating out crystals, filtrating and washing the crystals, and obtaining acetone dicarboxylic acid; and secondly, dripping anhydrous methanol and thionyl chloride into the prepared acetone dicarboxylic acid, performing reflux reaction for 1 to 3 hours, separating and purifying a reaction liquid after the reaction is over, and obtaining the Dimethyl 1,3-acetonedicarboxylate. The method mainlyhas the advantages of eliminating the prior fuming sulfuric acid and adopting the general concentrated sulfuric acid in the selection of raw materials, shortening the technological flow, reducing theproduction cost and environmental pollution, guaranteeing safe production, realizing high yield and high purity of products, and improving the economic benefit.
Owner:临安市新联线缆材料有限公司
Method for preparing strontium ranelate
Owner:SHANDONG BOYUAN PHARM CO LTD
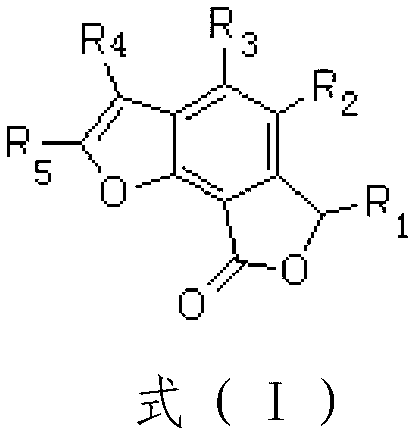
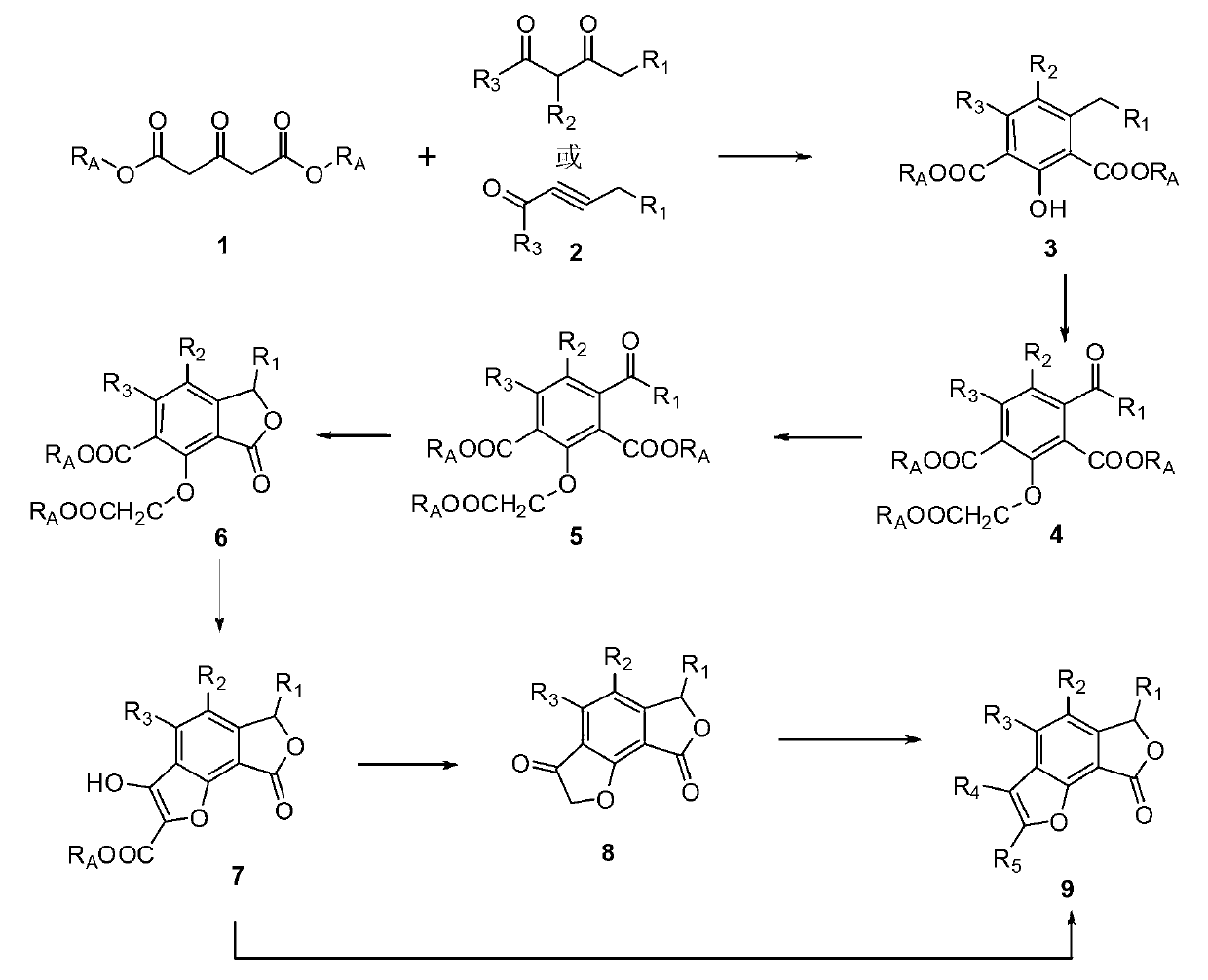
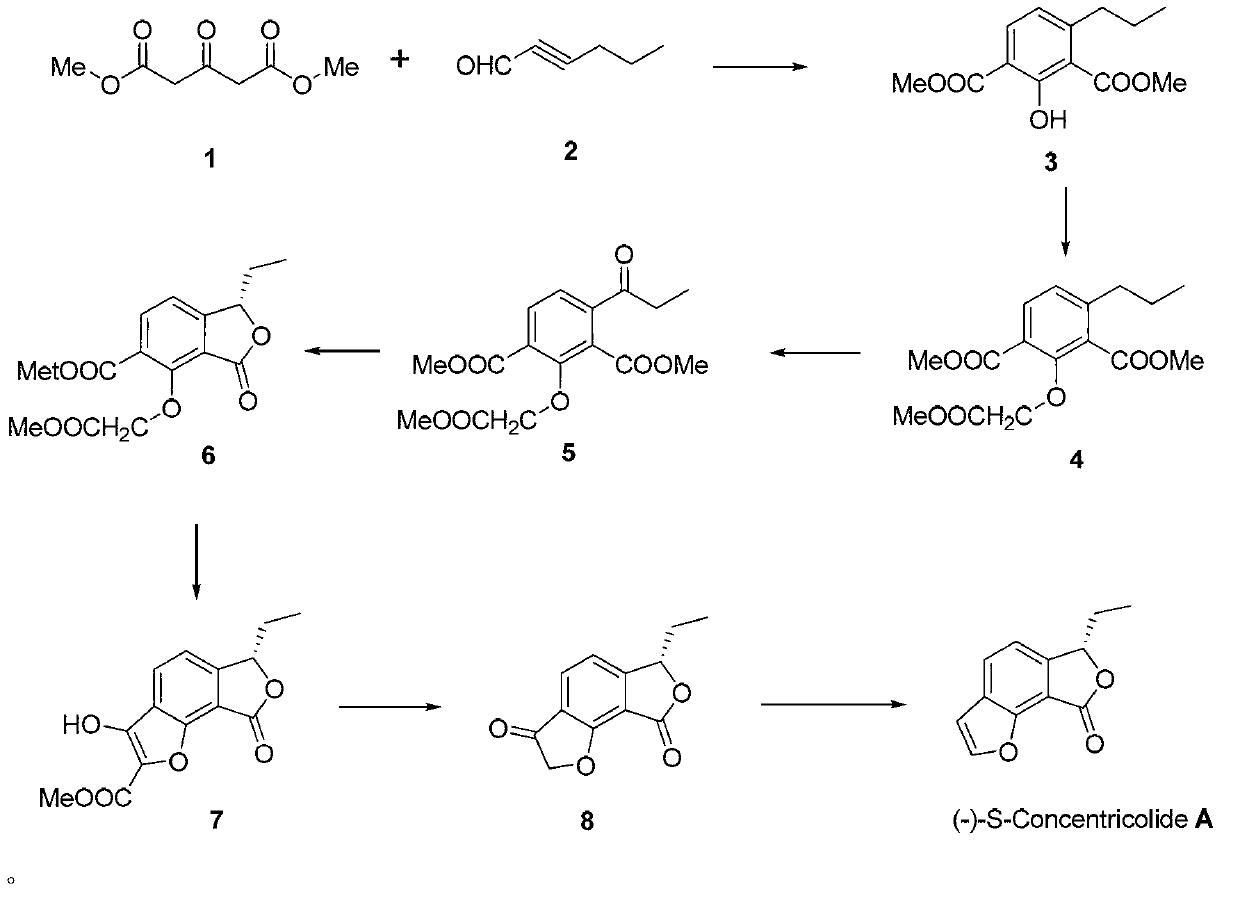
Total synthesis method of natural active product concentricolide and its analogue
The invention relates to a total synthesis method of a natural active product concentricolide and its analogue. The total synthesis method is a universal, simple and effective racemic and asymmetric total synthesis method. The total synthesis method comprises that a 1,3-acetone dicarboxylate compound as a raw material and alkynal undergo a Michael-Aldol reaction to produce a phenyl compound of which the benzene ring contains a phenolic hydroxyl group; through a substitution reaction of the phenolic hydroxyl group and a halogenated carboxylate, and benzyl bit oxidation and reduction reactions, benzofuranone lactone is constructed; under the alkaline condition, through a Diekmann condensation reaction and a Krapho reaction, a benzofuran ring is constructed; and through a series of functional group conversion, racemic total synthesis of the natural active product concentricolide and its analogue is realized. The total synthesis method utilizes a chiral reagent to catalytically reduce a carbonyl group or a double bond thereby constructing a corresponding chiral center so that asymmetric total synthesis of the natural active product concentricolide and its analogue is realized.
Owner:XINXIANG MEDICAL UNIV
Green preparation method of renewable polycarbonate
The invention relates to a green preparation method of renewable polycarbonate. According to the method, citric acid and carbonic ester are taken as raw materials, lithium acetylacetonate is taken as a catalyst, and bulk polymerization is realized under the conditions of high temperature and high vacuum. The method comprises the following steps: (1) using the citric acid to prepare dimethyl acetone dicarboxylate; (2) using the dimethyl acetone dicarboxylate to prepare octahydro-pentalene-2,5-diol and dimethyl (octahydro-pentalene-2,5 diyl) dicarbonate; (3) synthesizing the polycarbonate by using the dimethyl (octahydro-pentalene-2,5 diyl) dicarbonate and different renewable aliphatic diols (including chain type and ring type). The green preparation method has the advantages that the raw materials are low in price and wide in source; furthermore, the preparation method is simple in synthetic process, environment-friendly and degradable in products, and is beneficial to realization of industrial production and structure and function diversification.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Preparation method for polyester with side chain containing dimethyl pyridylamine
ActiveCN110938200AExpand biological applicationsOvercoming the disadvantage of not being able to co-deliver genesPharmaceutical non-active ingredientsPolyesterPolymer science
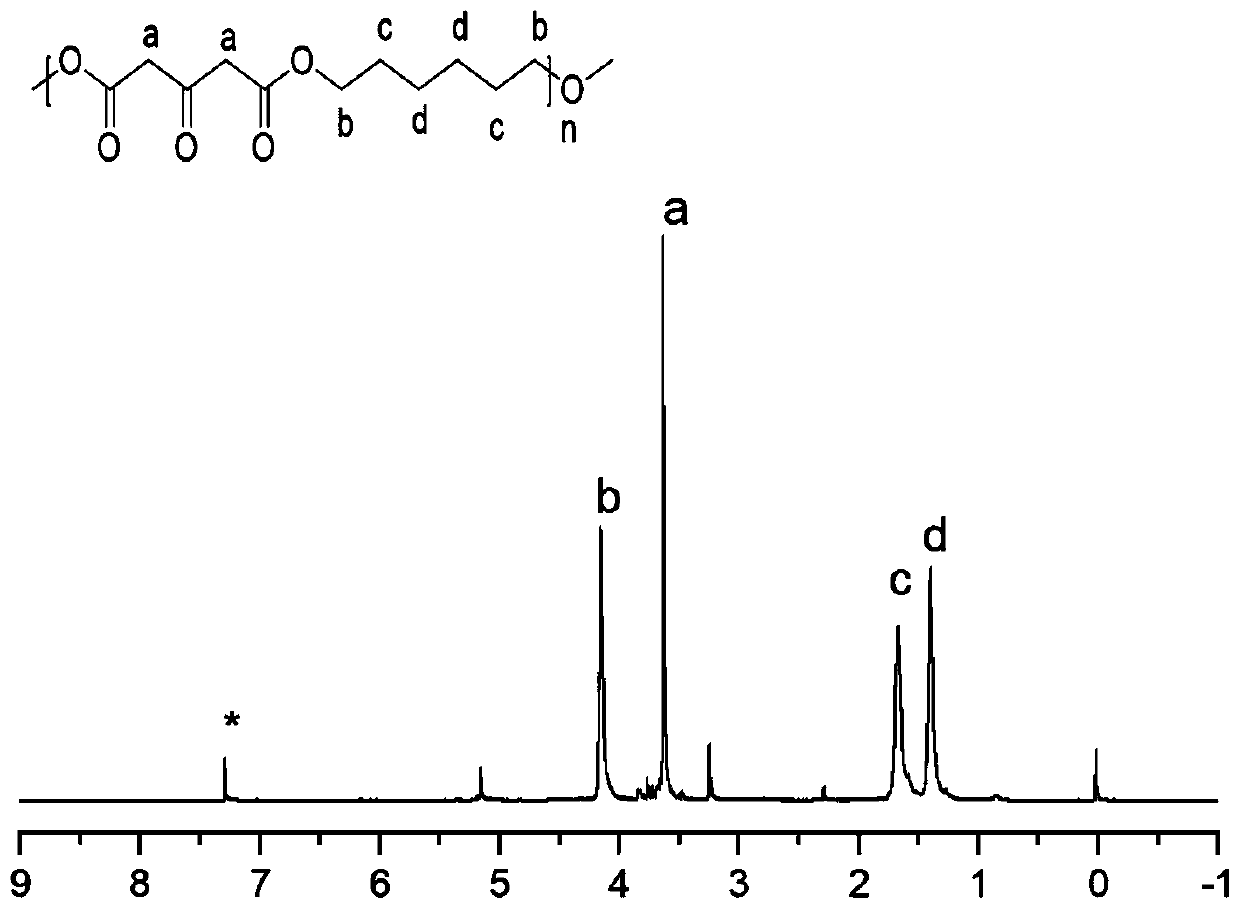
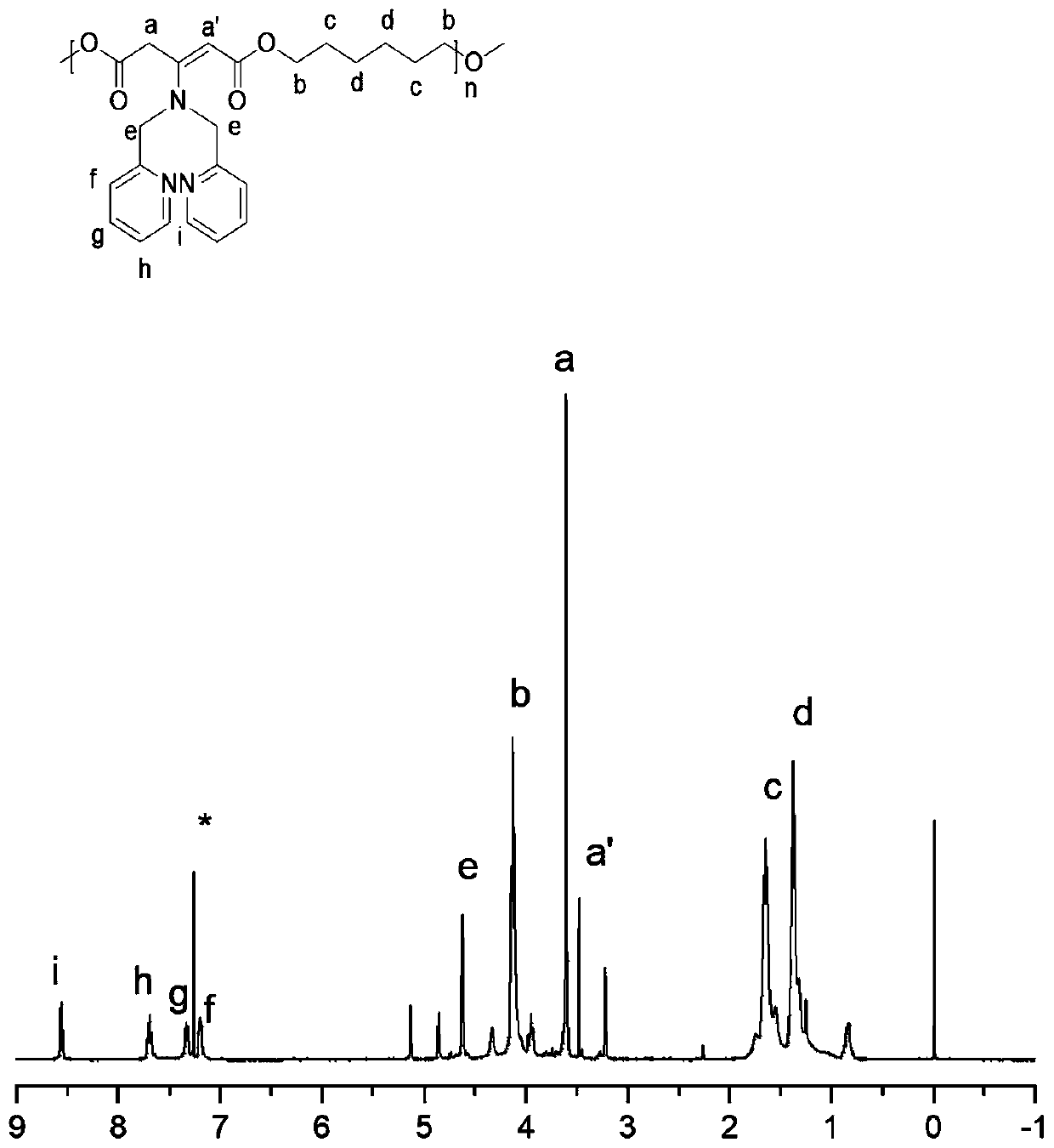
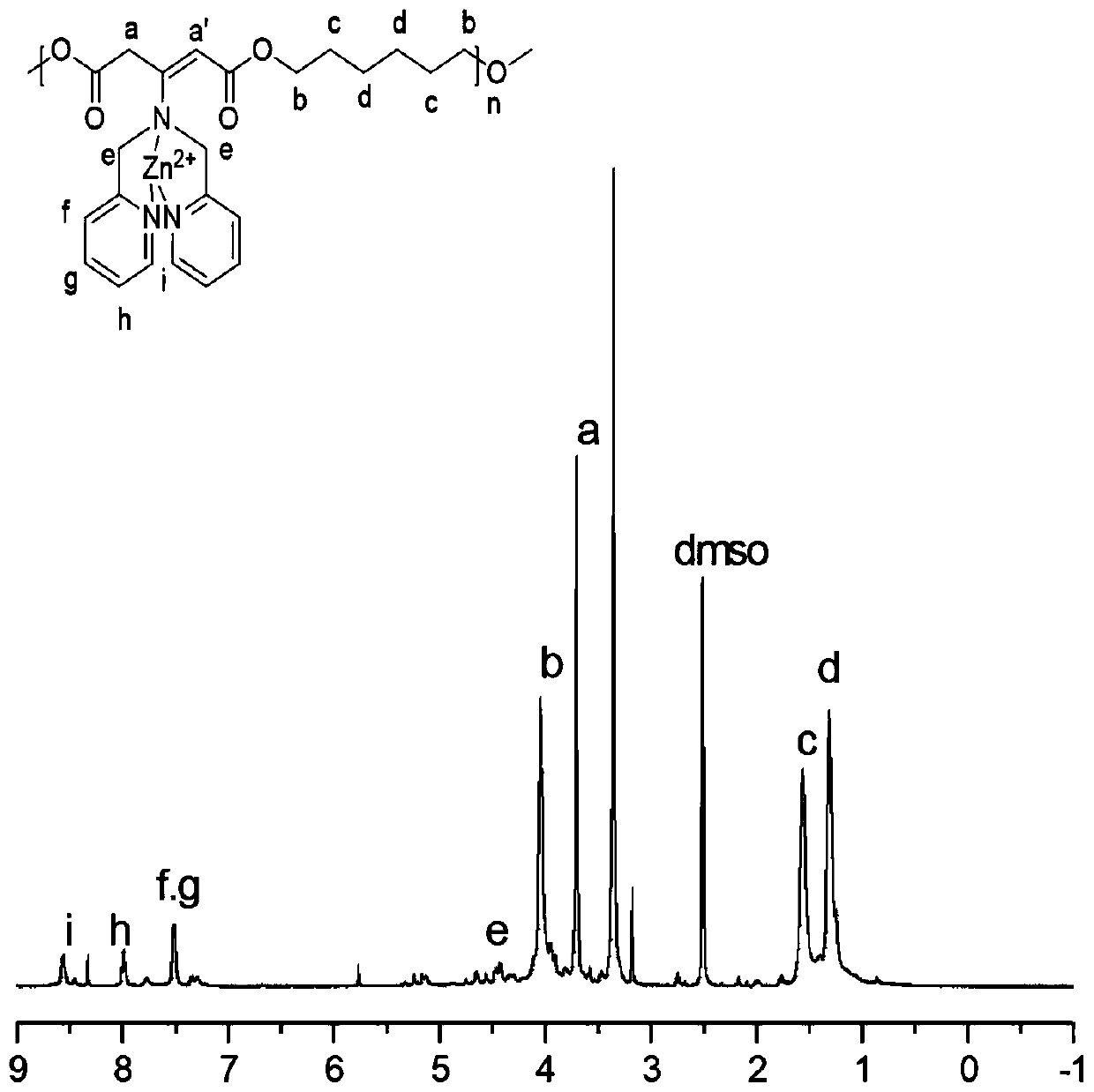
The invention belongs to the technical field of polymer science, and provides a preparation method for polyester with a side chain containing dimethyl pyridylamine. The preparation method comprises the following steps: subjecting a 1,3-acetone dicarboxylic acid monomer and a dihydric alcohol monomer to one-step bulk polymerization under the action of a catalyst, namely tetrabutyl titanate so as toobtain a polyester material; after completion of polymerization, subjecting the polyester material to a grafting reaction with DPA under the catalysis of p-toluenesulfonic acid; and carrying out coordination with zinc nitrate so as to obtain the polyester material with the side chain containing DPA-Zn. The preparation method provided by the invention provides a technical reference for synthesizing a novel polyester material and widens the biological application of the polyester material. According to the invention, dimethyl dicarboxylate containing post-modifiable sites is used as a raw material for polymerization with various types of diols to form polyester; and the formed polyester has good gene recombination capability after coordination between DPA and zinc ions, is expected to be applied to entrapment of hydrophobic drugs and co-delivery of genes, and overcomes the defect that traditional polyester can only deliver hydrophobic drugs but not co-deliver genes.
Owner:DALIAN UNIV OF TECH
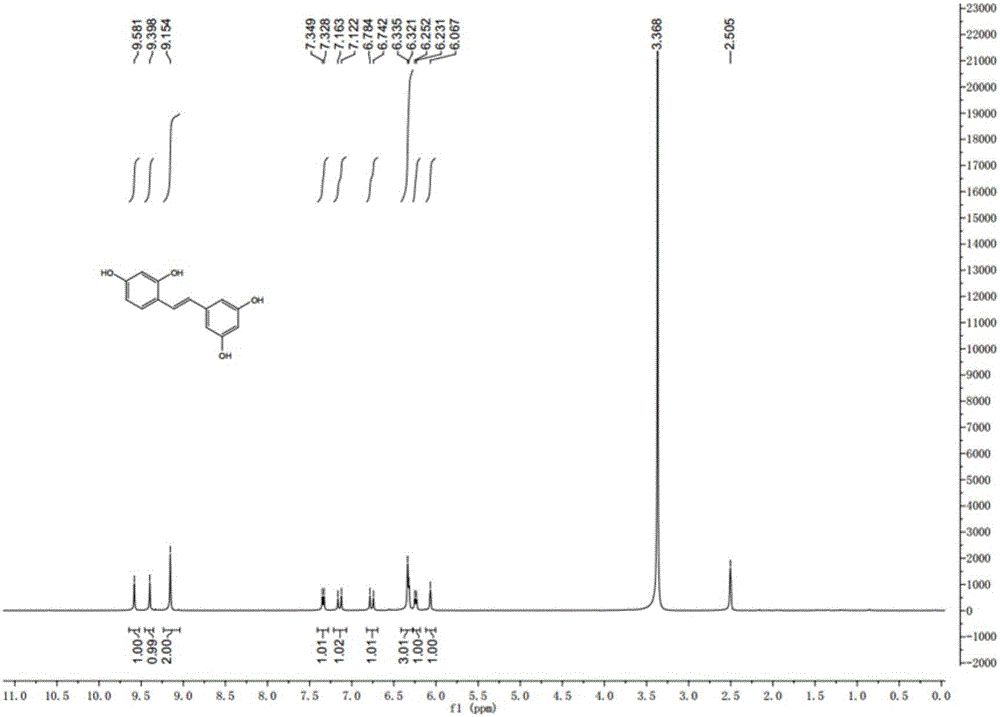
Synthesis method of natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene
ActiveCN106748662ASimple stepsNo protectiveOxygen-containing compound preparationOrganic compound preparationSynthesis methodsBenzaldehyde
The invention discloses a synthesis method of a natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene. According to the method, 1,3-acetone dicarboxylic acid dimethyl ester is used as a starting raw material; condensation and aromatization reactions are performed to obtain 3,5-dihydroxy-2,4-dicarboxylate methyl phenyl acetate; then, hydrolysis and decarboxylation are performed to obtain 3,5-dyhydroxy phenylacetic acid; the 3,5-dyhydroxy phenylacetic acid and 2,4-dihydroxy benzaldehyde take condensation reaction under the existence of alkali to obtain 3-(3,5-dihydroxy phenyl)-7-hydroxy coumarin; next, open loop decarboxylation reaction is performed under the alkaline condition; the natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene is obtained. The method has the advantages that the raw materials are easily obtained; the reaction route is simple and fast; the operation is convenient; the yield is higher.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Synthetic method of high-quality acetonedicarboxylic acid and acetonedicarboxylate
InactiveCN105085242AHigh selectivityEasy to purifyOrganic compound preparationCarboxylic acid esters preparationGlutaric acidCarboxylic acid
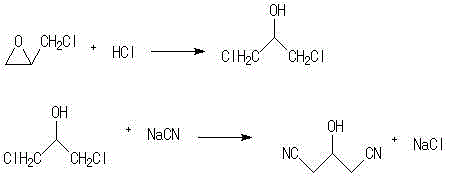
The invention discloses a synthetic method of high-quality acetonedicarboxylic acid and acetonedicarboxylate. The method comprises the following steps: epoxyhalopropane used as an initial raw material and halogen hydride undergo addition to synthesize 1,3-dihalopropanol; 1,3-dihalopropanol reacts with hydrocyanate to generate 3-hydroxy-1,5-pentanedinitrile; the cyan group of 3-hydroxy-1,5-pentanedinitrile is hydrolyzed to form a carboxyl group in order to generate 3-hydroxy-1,5-glutaric acid; 3-hydroxy-1,5-glutaric acid is oxidized by an oxidant to generate acetonedicarboxylic acid; and acetonedicarboxylic acid and alcohol or a hydroxy group-containing organic compound undergo esterification to obtain acetonedicarboxylate. The route of the method is longer than original technological routes, the reaction selectivity of every step is very high, and easy purification is realized, so the content of the finally synthesized acetonedicarboxylic acid and acetonedicarboxylate reaches 99.5% or more, and the single impurity content is smaller than 0.1%. The method has the advantages of light device corrosion, few three wastes, and small environment protection pressure.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
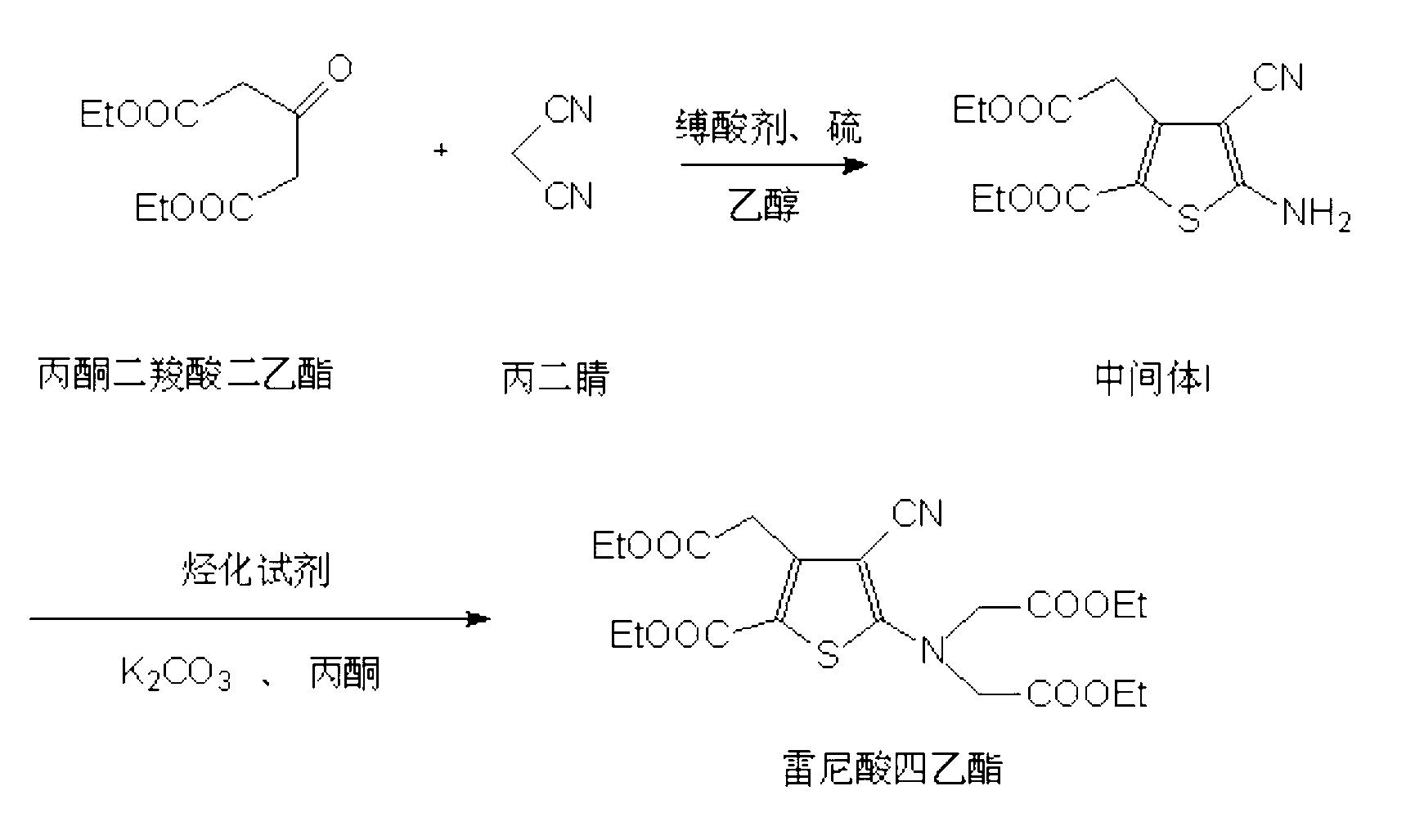
Preparation method of high-purity tetraethyl ranelate and intermediate thereof
InactiveCN103319454AHigh reaction yieldImprove response qualityOrganic chemistryAmmonium sulfideSolvent
The invention relates to a preparation method of 5-amino-4-cyano-3-(2-ethyoxyl-2-carboxymethyl)-thiophene-2-ethyl formate. The preparation method comprises the following steps of: (a) reacting acetonedicarboxylic acid diethyl ester with malononitrile in the presence of an acid-binding agent and ethanol to generate an active intermediate; and (b) after ammonium sulfide is added as a cosolvent, carrying out cyclization reaction on the active intermediate and sulphur under the reflux of the ethanol, cooling, separating out a crystal, filtering and collecting a filter cake to obtain the 5-amino-4-cyano-3-(2-ethyoxyl-2-carboxymethyl)-thiophene-2-ethyl formate. The 5-amino-4-cyano-3-(2-ethyoxyl-2-carboxymethyl)-thiophene-2-ethyl formate prepared by the invention is high in purity, high in yield and low in cost, and three-waste emission is low.
Owner:GUANGDONG ZHONGSHENG PHARMA
A kind of synthetic method of natural product e-2,3',4,5'-tetrahydroxystilbene
ActiveCN106748662BSimple stepsGood atom economyOxygen-containing compound preparationOrganic compound preparationSynthesis methodsPhenylacetic acid
The invention discloses a synthesis method of a natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene. According to the method, 1,3-acetone dicarboxylic acid dimethyl ester is used as a starting raw material; condensation and aromatization reactions are performed to obtain 3,5-dihydroxy-2,4-dicarboxylate methyl phenyl acetate; then, hydrolysis and decarboxylation are performed to obtain 3,5-dyhydroxy phenylacetic acid; the 3,5-dyhydroxy phenylacetic acid and 2,4-dihydroxy benzaldehyde take condensation reaction under the existence of alkali to obtain 3-(3,5-dihydroxy phenyl)-7-hydroxy coumarin; next, open loop decarboxylation reaction is performed under the alkaline condition; the natural product of E-2,3',4,5'-tetrahydroxy diphenyl ethylene is obtained. The method has the advantages that the raw materials are easily obtained; the reaction route is simple and fast; the operation is convenient; the yield is higher.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Process for preparing 3-hydroxyglutarate compound
InactiveCN101143824AEasy to useEconomical to useOrganic compound preparationCarboxylic acid esters preparationPalladium catalystSolvent
The invention discloses a method which utilizes a 3-oxo glutarate ester compound to prepare a 3-hydroxyl glutarate ester compound. Under the conditions of pressure between 0.1 to 3 MPa and temperature between 20 DEG C and 120 DEG C, the 3-oxo glutarate ester compound catalyzed by nickel or palladium catalyzer in ester solvent is heated for half to twenty four hours in a reaction kettle, so that the 3-oxo glutarate ester compound in the class of 3-acetone dicarboxylic acid dimethyl ester (diethyl ester) is hydrogenated and reduced, thus preparing the 3-hydroxyl glutarate ester compound in the class of 3-hydroxyl glutarate dimethyl ester (diethyl ester). The method is carried out under a low perssure, as a result, high pressure is avoided, the requirements on devices is low, and the method can adapt to the fine chemical-scale production. Meanwhile, under the pressure, the use of hydrogen is convenient, safe and economical. The yield rate is high, almost reaching one hundred percent.
Owner:河南豫辰药业股份有限公司
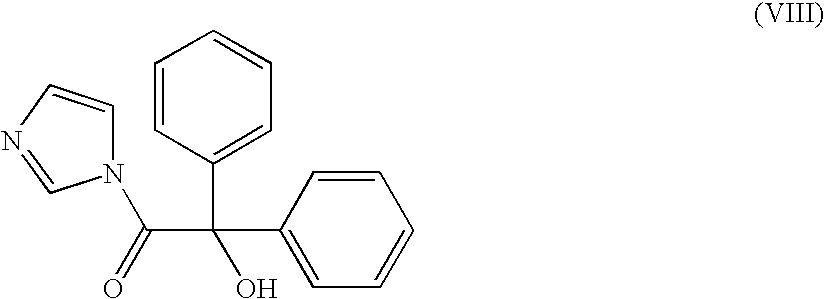
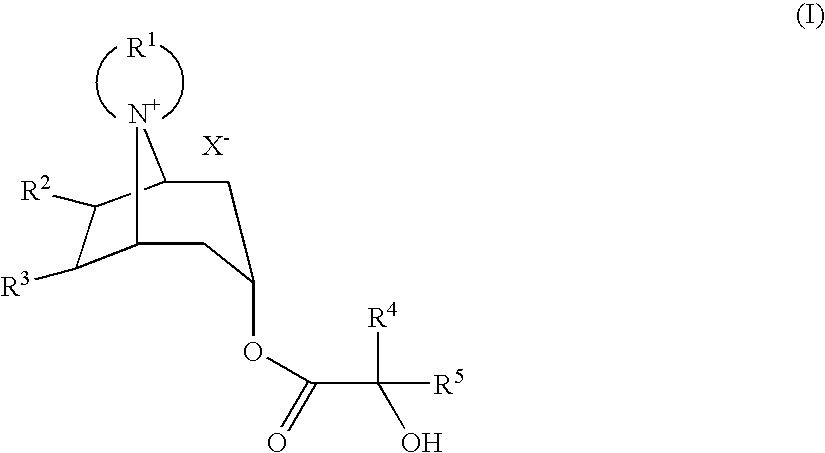
Method For Producing Azoniaspironortropine Esters And Nortropan-3-One Compounds
The present technology relates to an improved one-stage method for producing azoniaspironortropine esters such as trospium chloride by reacting an endonortropine compound with an organic dihalogen compound and an a-hydroxycarboxylic acid in the presence of a base and 1,1′ carbonyldiimidazole or 1,1′ thiocarbonyldiimidazole or thionyldiimidazole. The present technology also relates to an improved method for producing nortropan-3-one compounds or their hydrohalides (such as N-benzyltropanone hydrochloride) by reacting an amine and a protected dialdehyde with a basic aqueous solution of 1,3-acetone dicarboxylic acid.
Owner:K H S PHARMA HLDG
Preparation method of high-purity strontium ranelate
InactiveCN103319455AShortened reaction yieldShortened response massOrganic chemistrySolventPotassium carbonate
The invention relates to a preparation method of high-purity strontium ranelate. The preparation method comprises the following steps of: (a) reacting acetonedicarboxylic acid diethyl ester with malononitrile in the presence of an acid-binding agent and ethanol to generate an active intermediate, and after a cosolvent is added, carrying out cyclization reaction on the active intermediate and sulphur under the reflux of ethanol to prepare 5-amino-4-cyano-3-(2-ethyoxyl-2-carboxymethyl)-thiophene-2-ethyl formate, i.e., an intermediate I; (b) reacting the intermediate I with a hydrocarbonylation reagent in the presence of potassium carbonate and a catalyst to prepare tetraethyl ranelate; and (c) hydrolyzing the tetraethyl ranelate in an alkali metal hydroxide solution to generate salt, adding an insoluble organic solvent to wash impurities, decoloring by using active carbon, filtering, forming strontium salt, and adjusting the pH value of the solution to obtain the strontium ranelate. The strontium ranelate prepared by the invention is high in purity, good in stability and capable of meeting the requirement of bulk drugs for purity, content and strontium content; the preparation process is low in three-waste emission and low in cost.
Owner:GUANGDONG ZHONGSHENG PHARMA
Method for preparing 1,3-acetone dicarboxylic acid diester and intermediate thereof by oxidizing citric acid and hydrogen peroxide
ActiveCN103288628ARaw materials are easy to getLow costOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsFatty alcohol
The invention discloses a method for preparing 1,3-acetone dicarboxylic acid diester and an intermediate thereof by oxidizing citric acid and hydrogen peroxide. The method comprises the steps of: with hydrogen peroxide as an oxidizing agent, oxidizing citric acid in a water solution of citric acid at 0-100 DEG C to obtain 1,3-acetone dicarboxylic acid; and enabling the obtained 1,3-acetone dicarboxylic acid to be subjected to an esterification reaction with low-level fatty alcohol to obtain the 1,3-acetone dicarboxylic acid diester. The method disclosed by the invention is good in selectivity, high in transformation rate, free of environmental pollution and convenient for post treatment, thereby being an environment-friendly synthesis method; the method is a new synthesis method further satisfying atom economy rules; and moreover, the method is suitable for large-scale industrial production and the economic efficiency is greatly improved.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Preparation of bio-based aromatic monomer and preparation method of related homopolyester and copolyester
The invention relates to a preparation method of a citric acid group-based monomer and semi-aromatic homopolyester and copolyester thereof, which takes acetone dicarboxylic acid diethyl ester and various dihydric alcohols (HO-R-OH, -R- comprises at least one of the following structures) as raw materials, adopts a mixed catalyst, and realizes bulk polymerization under the conditions of high temperature and high vacuum. The preparation method comprises the following steps of 1) preparing 2, 5-diethoxycarbonyl-4-hydroxy-3, 4-diphenyl cyclopentadiene ketone from diethyl acetone dicarboxylate, (2)preparing 2, 5-diethoxycarbonyl-3, 4-diphenyl cyclopentadiene ketone from the 2, 5-diethoxycarbonyl-4-hydroxyl-3, 4-diphenyl cyclopentadiene ketone, (3) preparing 2, 3-diphenyl-1, 4-terephthalic aciddiethyl ester from 2, 5-diethoxycarbonyl-3, 4-diphenyl cyclopentadiene ketone, 4) synthesizing homopolyester from 2, 3-diphenyl-1, 4-terephthalic acid diethyl ester and dihydric alcohol with differentcarbon chain lengths, and 5) synthesizing copolyester from 2, 3-diphenyl-1, 4-terephthalic acid diethyl ester, terephthalic acid or ester thereof and various dihydric alcohols. The method has the advantages that the raw materials are low in price and wide in source, the synthesis process is simple and environmentally friendly, the polymerization product has high glass-transition temperature and good thermal stability and can be degraded, and industrial production and structural and functional diversification can be achieved.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Improved low-smoke halogen-free cable rubber jacket and preparation method thereof
InactiveCN107254175ASolve problems that affect the mechanical performance of cablesImprove heat resistanceInsulated cablesCable/conductor manufactureHydrogen SulfateEPDM rubber
The invention discloses a preparation method of an improved low-smoke halogen-free cable rubber jacket. The preparation method includes following steps: A, using EPDM rubber, methyl vinyl silicon rubber, 1, 3-acetone dicarboxylic acid diethyl ester, tetrabutyl ammonium hydrogen phosphate, dimethyl urea, m-caborane, 3-methoxy benzene methanol, 3-nitro-4-aminophenol, calcium sulfate, carbon black and zirconium oxide to preparing a cable inner jacket; B, using polyethylene, EVA rersin, EDTA, dicumyl peroxide, triethanolamine, tertbutyl isosulfate, 3-nitro-4-methyl acetophenone, potassium hydrogen sulfate, dibutyl fumarate and benzoyl acetonitrile to prepare a cable outer jacket; C, socketing the cable inner jacket prepared in the step A and the cable outer jacket prepared in the step B, and filling a flame retardant between the cable inner jacket and the cable outer jacket. By the preparation method, the defects of the prior art can be overcome, and mechanical performance of cables is improved further.
Owner:沧州会友线缆股份有限公司
Preparation method of 2-azanoradamantane-N-Oxyl
ActiveCN106366082AReduce usageThe reaction conditions are mild and safeOrganic chemistryBenzeneSulfohydrazide
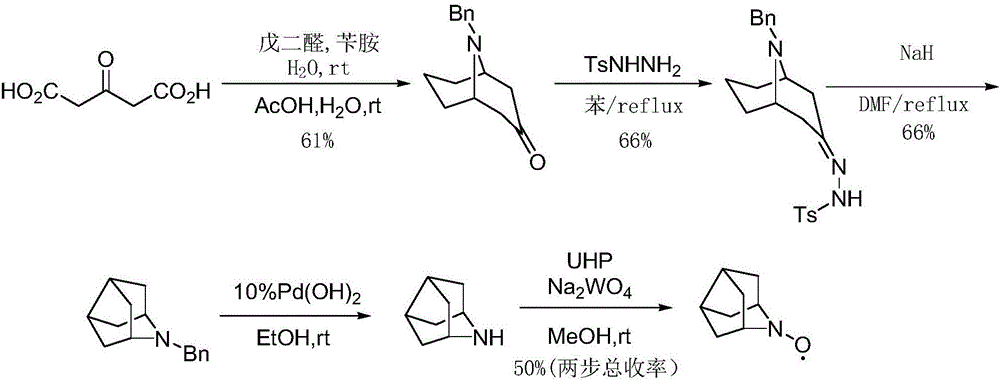
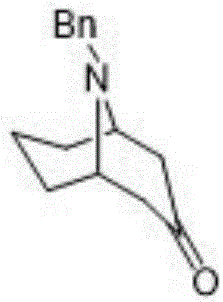
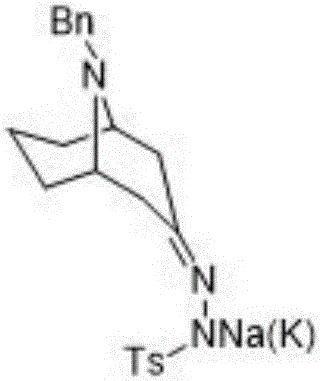
The invention discloses a preparation method of 2-azanoradamantane-N-Oxyl (Nor-AZADO). The method comprises the following steps: placing acetonedicarboxylic acid, glutaraldehyde and benzylamine in an aqueous hydrophosphate solution, and carrying out condensation and decondensation to obtain 9-benzyl-9-azabicyalo-[3,3,1]-nonyl-3-one; carrying out condensation dehydration on 9-benzyl-9-azabicyalo-[3,3,1]-nonyl-3-one and benzene or benzene ring substituted sulfohydrazide, and adding an alkali to obtain 2-(9-benzyl-9-azabicyalo-[3,3,1]-nonane-3-ylidene)-1-benzene or benzene ring substituted sulfohydrazide sodium / potassium salt; carrying out refluxing ring closing on the 2-(9-benzyl-9-azabicyalo-[3,3,1]-nonane-3-ylidene)-1-benzene or benzene ring substituted sulfohydrazide sodium / potassium salt in an organic solvent to obtain N-benzyl-2-azanoradamantane; debenzylating N-benzyl-2-azanoradamantane to obtain 2-azanoradamantane; and oxidizing 2-azanoradamantane by a peroxide oxidant to obtain the Nor-AZADO. The preparation method has the advantages of great increase of the synthesis yield, greenness and environmental protection, high efficiency, low cost, and easiness in industrial large-scale production.
Owner:深圳市宏辉浩医药科技有限公司
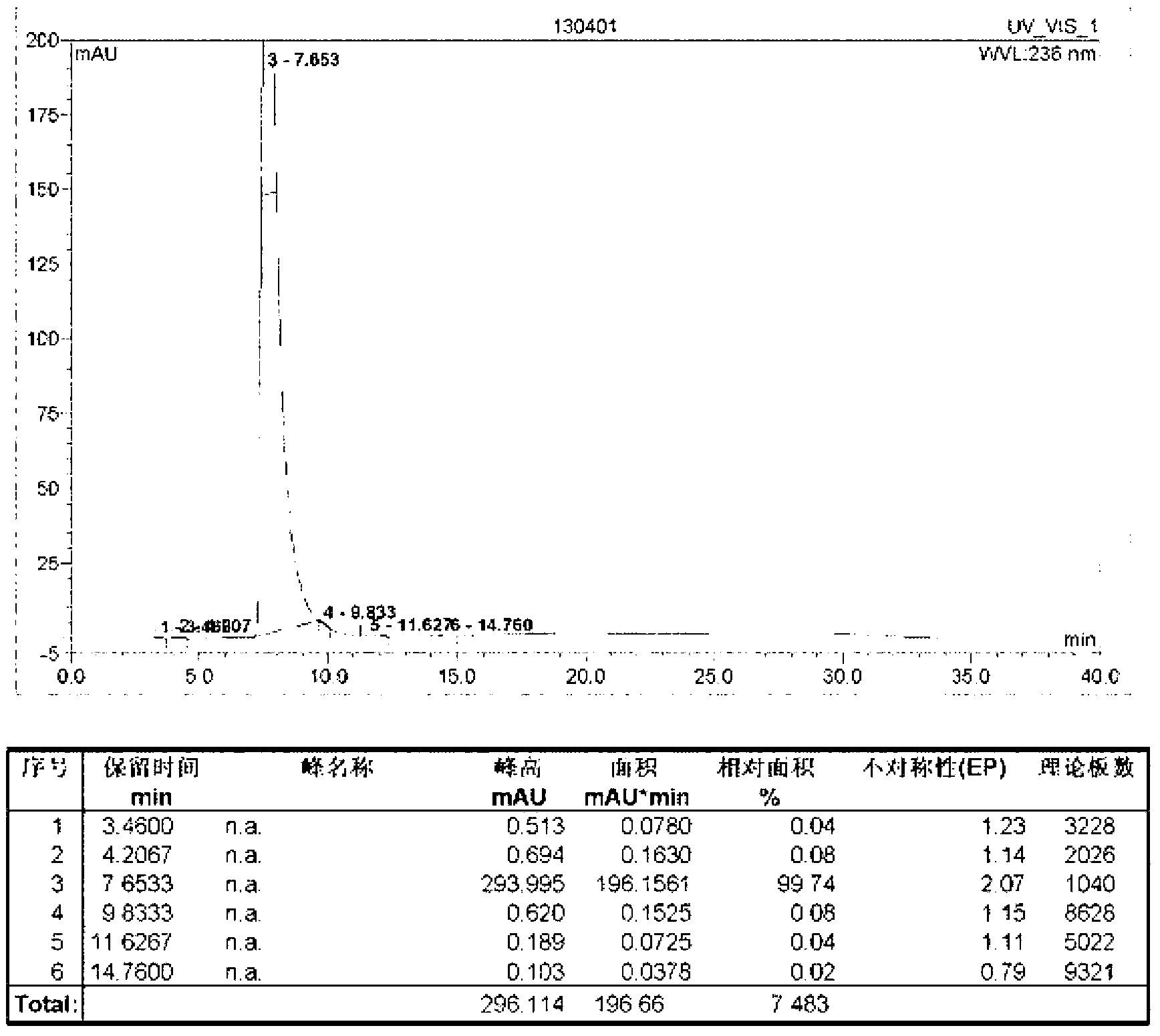
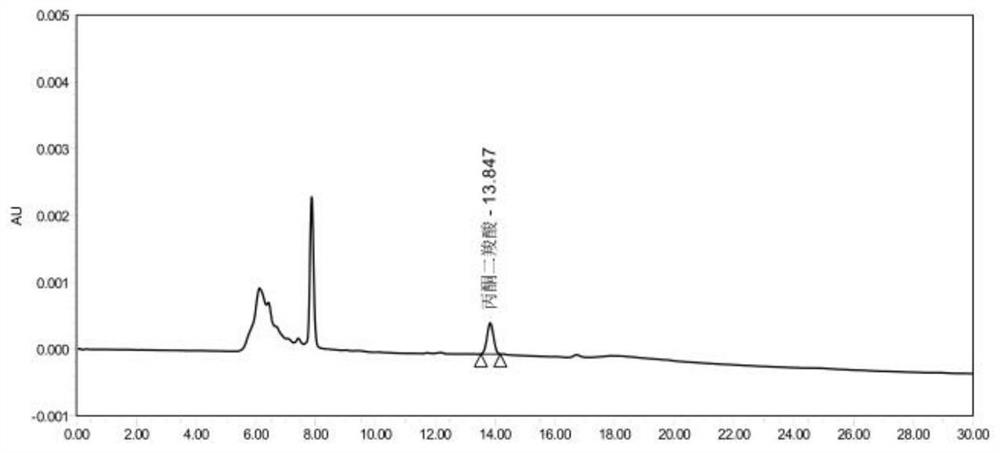
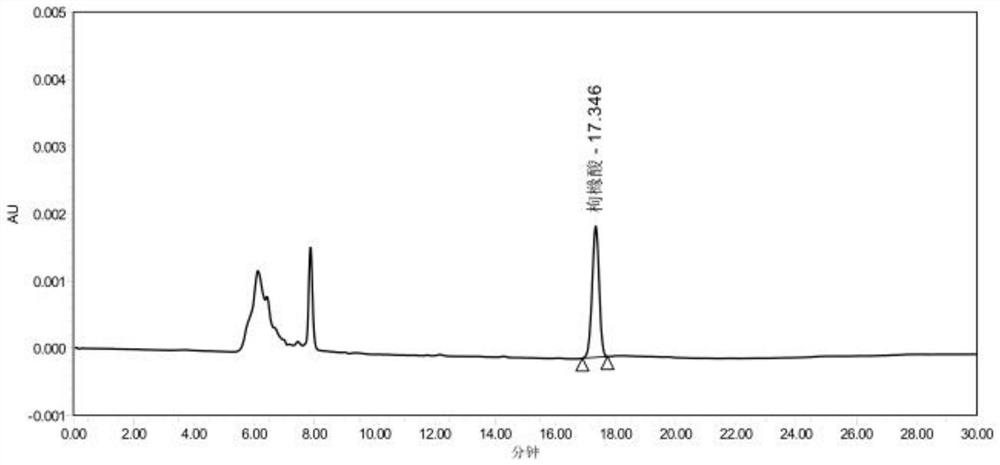
Method for measuring content of acetonedicarboxylic acid in sodium citrate solution
PendingCN113834887AProcess conditions are easy to controlAccurate yieldComponent separationFluid phasePhysical chemistry
The invention discloses a method for determining the content of acetonedicarboxylic acid in a sodium citrate solution. The method comprises the following steps of: 1) preparing a system applicable solution, specifically, taking a proper amount of acetonedicarboxylic acid reference substance and a proper amount of citric acid reference substance, dissolving the acetonedicarboxylic acid reference substance and the citric acid reference substanceby using a mobile phase, and diluting an obtained solution to prepare a solution containing about 100 micrograms of citric acid and 100 micrograms of acetonedicarboxylic acid per 1ml; 2) preparing a test solution, specifically, precisely measuring 2ml of a 2.5% sodium citrate solution, placing the 2.5% sodium citrate solution in a 10ml measuring flask, diluting the 2.5% sodium citrate solution to a scale by using a mobile phase, and performing uniform shaking; and 3) preparing a reference substance solution, specifically, taking a proper amount of an acetonedicarboxylic acid reference substance, dissolving the acetonedicarboxylic acid reference substance with a mobile phase, and diluting an obtained solution to prepare a solution containing about 100 microgramsof acetonedicarboxylic acid per 1ml. According to the method, the content of the acetonedicarboxylic acid in the sodium citrate solution is measured through the high performance liquid chromatography, so that main degradation products of the sodium citrate solution are controlled, the process conditions and storage conditions of the sodium citrate solution are favorably controlled, and meanwhile, the yield in the synthesis process of the acetonedicarboxylic acid can be accurately obtained.
Owner:西安乐析医疗科技有限公司
Dairy product dirt cleaning agent composition and preparation method thereof
InactiveCN106833912ALow corrosion rateImprove cleaning and descaling efficiencyInorganic/elemental detergent compounding agentsCationic surface-active compoundsCleansing AgentsPotassium carbonate
A dairy product dirt cleaning agent composition is characterized by being prepared from the following raw materials in parts by weight: 20-25 parts of acetone-dicarboxylic acid, 13-16 parts of dihydroxytartaric acid, 6-9 parts of sodium dihydroxytartrate, 9-11 parts of sodium di(2-ethyl-hexyl)sulfosuccinate, 1-3 parts of ethosuximide, 11-15 parts of iminodiacetic acid, 15-20 parts of 3-hydracrylic acid, 45-52 parts of ammonium carbonate and 20-25 parts of potassium carbonate. The invention aims to achieve the purpose of cleaning polymer material galalith dirt and water scale during processing and production of dairy products. The dairy product dirt cleaning agent composition is small in addition, non-toxic and corrosion-free, ensures that an effluent generated after cleaning is easy to degrade, and guarantees that dirt cleaning of a dairy product can be finished in one step. The cleaning reaction time of the dairy product is 1-2 h, the descaling rate is larger than 98%, and the dairy product dirt cleaning agent composition has a wide descaling temperature range and can be used within the range from normal temperature to a boiling point.
Owner:GANSU HEIMA PETROCHEM ENG
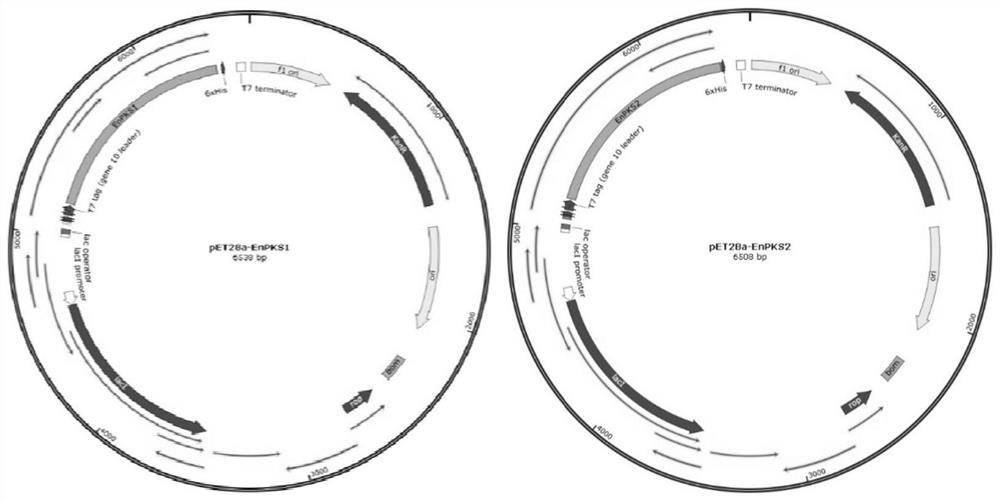
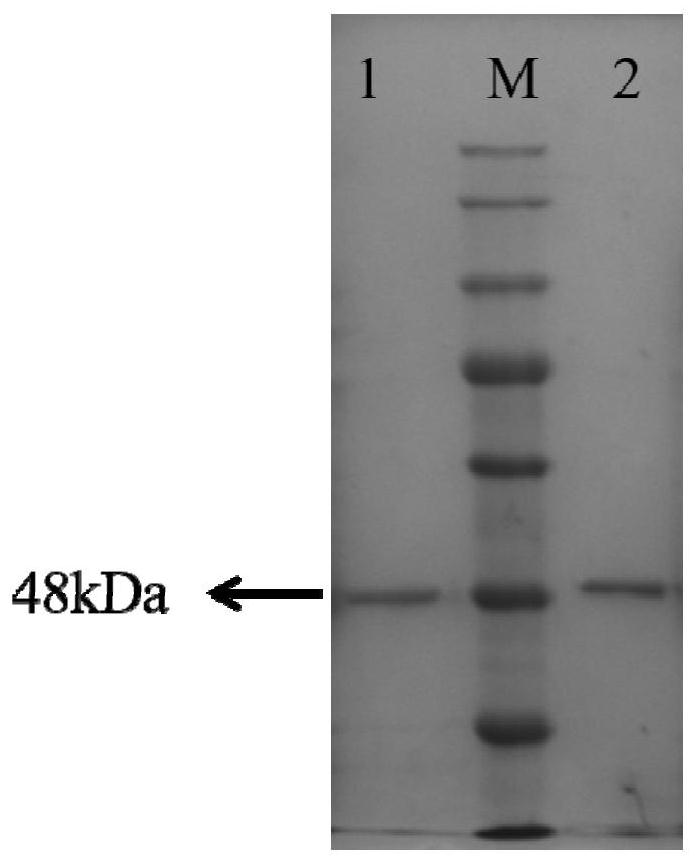
Polyketide synthases EnPKS1 and EnPKS2 from Erythroxylum novogranatense, and gene and application of polyketide synthases EnPKS1 and EnPKS2
ActiveCN113621593ALow similarityPerfect synthetic understandingBacteriaTransferasesL-HyoscyamineEscherichia coli
The invention discloses two polyketide synthases (PKS) EnPKS1 and EnPKS2 from Erythroxylum novogratin, as well as a coding gene and application of the two polyketide synthases. An amino acid sequence of the EnPKS1 is as shown in SEQ ID NO. 4, and a nucleotide sequence of the EnPKS1 is as shown in SEQ ID NO. 3; and an amino acid sequence of the EnPKS2 is as shown in SEQ ID NO. 8, and a nucleotide sequence of the EnPKS2 is as shown in SEQ ID NO. 7. A protein expressed by escherichia coli can be used for catalyzing the condensation of two molecules of malonyl-CoA (malonyl-CoA) to generate acetone dicarboxylic acid. The polyketide synthases can be applied to production of tropane alkaloids (such as hyoscyamine, scopolamine and cocaine) in synthetic biology and production of natural products of acetone dicarboxylic acid intermediates in a biosynthetic route; or the polyketide synthases are used for guiding molecular breeding of related medicinal plants.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Method for preparing 1,3-acetone dicarboxylic acid diester and an intermediate thereof by using citric acid to catalyze oxidization of hydrogen peroxide
ActiveCN103288629ARaw materials are easy to getLow costOrganic compound preparationCarboxylic acid esters preparationStrong acidsFatty alcohol
The invention discloses a method for preparing 1,3-acetone dicarboxylic acid diester and an intermediate thereof by using citric acid to catalyze oxidization of hydrogen peroxide. The method comprises the steps of: with at least one medium-strong acid as a catalyst and hydrogen peroxide as an oxidizing agent, oxidizing citric acid in a water solution of citric acid at 0-100 DEG C to obtain 1,3-acetone dicarboxylic acid; and enabling the obtained 1,3-acetone dicarboxylic acid to be subjected to an esterification reaction with low-level fatty alcohol to obtain the 1,3-acetone dicarboxylic acid diester. The method disclosed by the invention is used for efficiently preparing the 1,3-acetone dicarboxylic acid diester and the intermediate thereof, and is high in yield, small in pollution and short in reaction time; and the method is suitable for large-scale industrial production and the economic efficiency is greatly improved.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Methanol gasoline stabilizer
ActiveCN103666604AImproved resistance to phase separation when exposed to waterSuitable for long term storageLiquid carbonaceous fuelsAllyl phenoxyacetateGasoline
The invention discloses a methanol gasoline stabilizer. The methanol gasoline stabilizer is formed by mixing the following components in percentage by mass: 18.0 to 31.0 percent of 2-octylame, 15.0 to 29.5 percent of N-tert-butyl-piperazine, 15.0 to 24.5 percent of 4-N-butoxy-benzonitrile, 13.0 to 24.0 percent of allyl phenoxyacetate, 10.0 to 20.5 percent of 1,3-acetone dicarboxylate, and 9.0 to 19.5 percent of 3-dimethyl aminoanisole. Before methanol gasoline is prepared, first, 0.4 to 0.8 mass percent of the stabilizer disclosed by the invention is added to the methanol, and the methanol and the stabilizer are mixed uniformly, and then, performing subsequent processes of preparing the methanol gasoline after ultrasonic treatment is performed at a normal temperature. The improvement effect of the storage stability of the methanol gasoline using the methanol gasoline stabilizer disclosed by the invention is remarkable; the anti-water separation performance is improved obviously; through engine stand and actual road driving tests, the gasoline consumption, the output power, the tail gas emission and the like of the methanol gasoline are equivalent to those of the methanol gasoline with the same grade.
Owner:CRPC INNOVATION ENERGY
Method for preparing modified PEF copolyester from citric acid-based aromatic diester
The invention relates to a method for preparing modified PEF copolyester by using 2, 3-diphenyl-diethyl terephthalate, which is used for preparing the PEF copolyester by using dimethyl acetone dicarboxylate and cinnamyl aldehyde as raw materials and zinc acetate and antimonous oxide as catalysts under the conditions of high temperature and high vacuum, and comprises the following steps: 1) preparing the 2, 3-diphenyl-diethyl terephthalate from the diethyl acetone dicarboxylate; and 2) preparing the modified PEF copolyester from the 2, 3-diphenyl-diethyl terephthalate. The preparation method has the advantages that the PEF polyester is wide in raw material source, low in price, renewable and low in production cost; the PEF polyester modification synthesis process is simple, easy to operate,environmentally friendly, high in yield and beneficial to industrial production; the prepared modified PEF copolyester material is good in thermal stability and adjustable in performance, structure and function diversification is easy to achieve, the performance of the modified PEF copolyester material can be comparable with that of polyester based on petroleum raw materials, and the modified PEFcopolyester material has the possibility of large-scale production.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Methanol gasoline stabilizer
ActiveCN103666604BImproved resistance to phase separation when exposed to waterSuitable for long term storageLiquid carbonaceous fuelsAllyl phenoxyacetateGasoline
Owner:CRPC INNOVATION ENERGY
Method for preparing dolasetron mesylate key intermediate based on graphene activation
The invention provides a method for preparing a dolasetron mesylate key intermediate based on graphene activation. The method comprises the following steps: after uniformly mixing 3-cyclopentene-1-methyl formate in a water solution of N-methylmorpholine-N-oxide; adding potassium osmate and phenylboronic acid; after carrying out oxidization reaction, adding sodium periodate; continually carrying out oxidization reaction to obtain a dialdehyde intermediate product; enabling the dialdehyde intermediate product and 1,3-acetonedicarboxylic acid to subjected to Robinson-Schoepf reaction to prepare 3-methoxycarboxyl-7-oxo-9-azabicyclo-nonane-9-methyl acetate; adding a graphene oxide solution into a tetrahydrofuran solution of the 3-methoxycarboxyl-7-oxo-9-azabicyclo-nonane-9-methyl acetate; afteruniformly mixing, carrying out reduction reaction on sodium borohydride and sodium iodide; adding dihydropyran; and carrying out cyclization reaction to obtain a graphene modified (2alpha,6alpha,8alpha,9alphabeta)-hexahydro-8-hydroxyl-2,6-methylene-2H-quinoline-3(4H)-one intermediate.
Owner:武汉军嘉特商贸有限公司
Synthetic method for drug intermediate acetonedicarboxylic acid
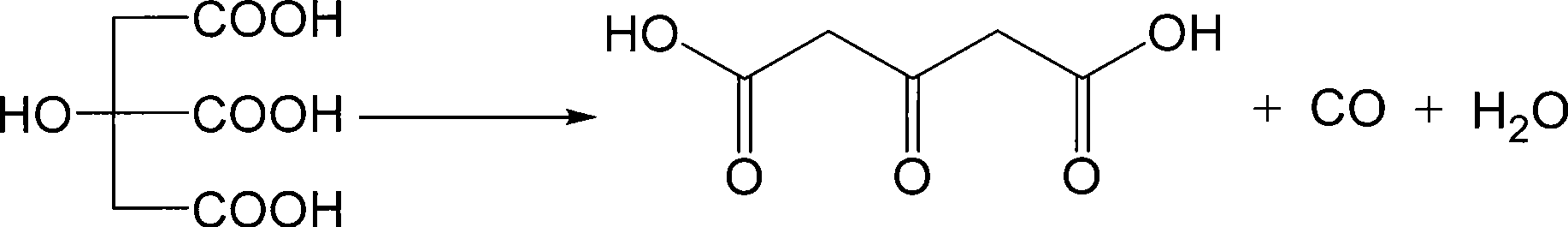
InactiveCN108238929AAvoid health hazardsSolve production safetyOrganic compound preparationCarboxylic compound preparationPotassiumCarboxylic acid
The invention discloses a synthetic method for the drug intermediate acetonedicarboxylic acid. The synthetic method comprises the following steps: adding 2-methoxypropane-1,3-dicarboxylic acid and a sodium nitrate solution into a reaction vessel, increasing the temperature of the obtainded solution, adding lead tetraacetate in batches, controlling a stirring speed and continuing a reaction; and then adding zinc oxalate powder, continuing the reaction, carrying out cooling, carrying out washing with a potassium chloride solution, carrying out washing with a diphenyl ether solution, then carrying out recrystallization in a diethylene glycol solution, and carrying out dehydration with a dehydrating agent so as to obtain the finished acetonedicarboxylic acid.
Owner:CHENGDU AO KA TE TECH CO LTD
A kind of total synthesis method of natural active product charcoccin and its analogs
The invention relates to a total synthesis method of a natural active product concentricolide and its analogue. The total synthesis method is a universal, simple and effective racemic and asymmetric total synthesis method. The total synthesis method comprises that a 1,3-acetone dicarboxylate compound as a raw material and alkynal undergo a Michael-Aldol reaction to produce a phenyl compound of which the benzene ring contains a phenolic hydroxyl group; through a substitution reaction of the phenolic hydroxyl group and a halogenated carboxylate, and benzyl bit oxidation and reduction reactions, benzofuranone lactone is constructed; under the alkaline condition, through a Diekmann condensation reaction and a Krapho reaction, a benzofuran ring is constructed; and through a series of functional group conversion, racemic total synthesis of the natural active product concentricolide and its analogue is realized. The total synthesis method utilizes a chiral reagent to catalytically reduce a carbonyl group or a double bond thereby constructing a corresponding chiral center so that asymmetric total synthesis of the natural active product concentricolide and its analogue is realized.
Owner:XINXIANG MEDICAL UNIV
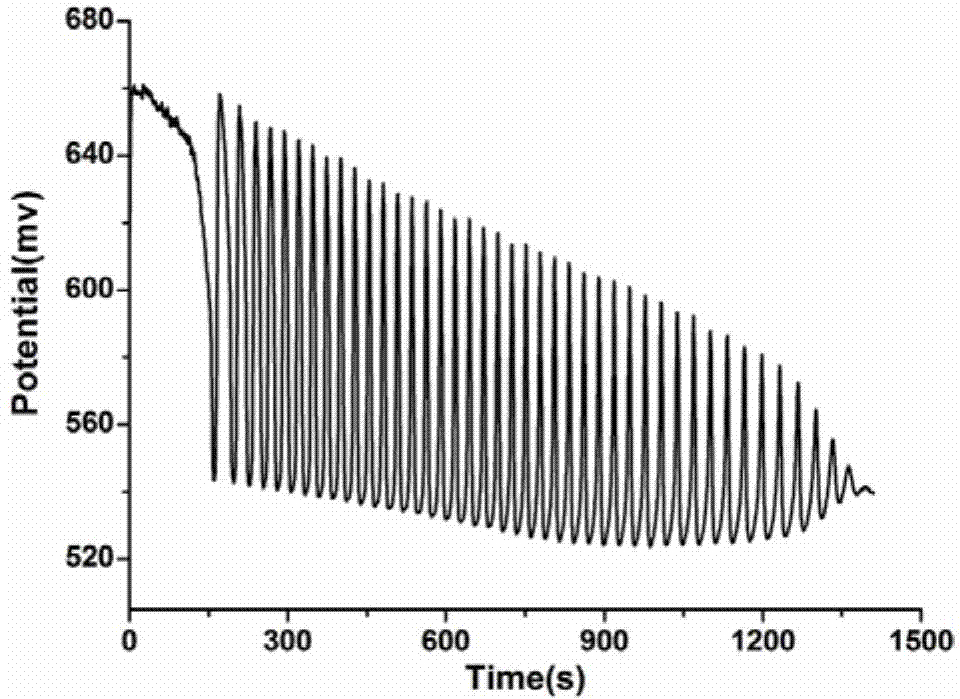
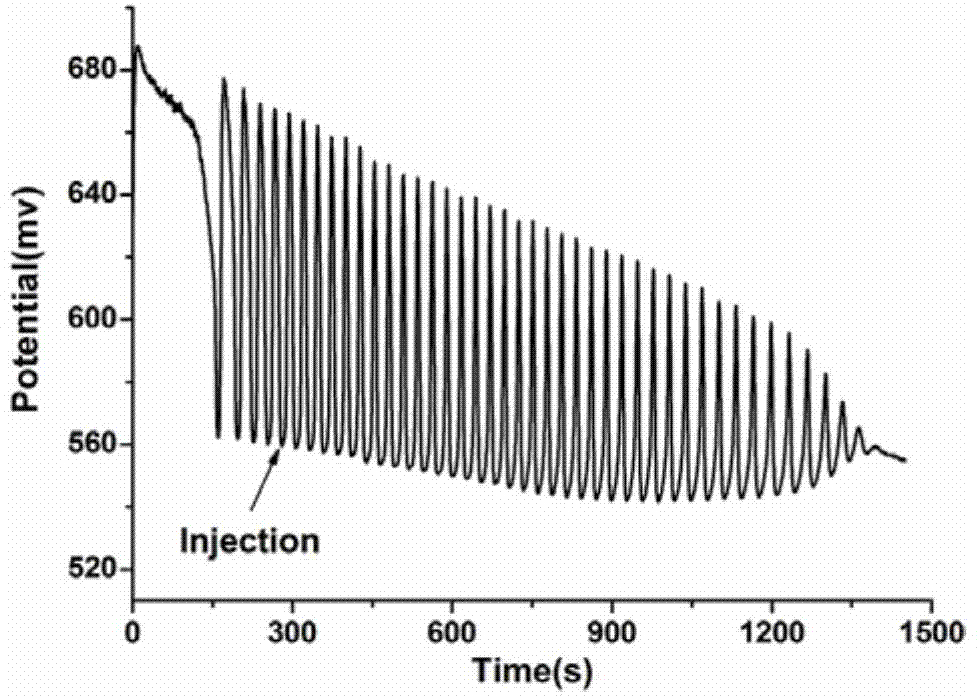
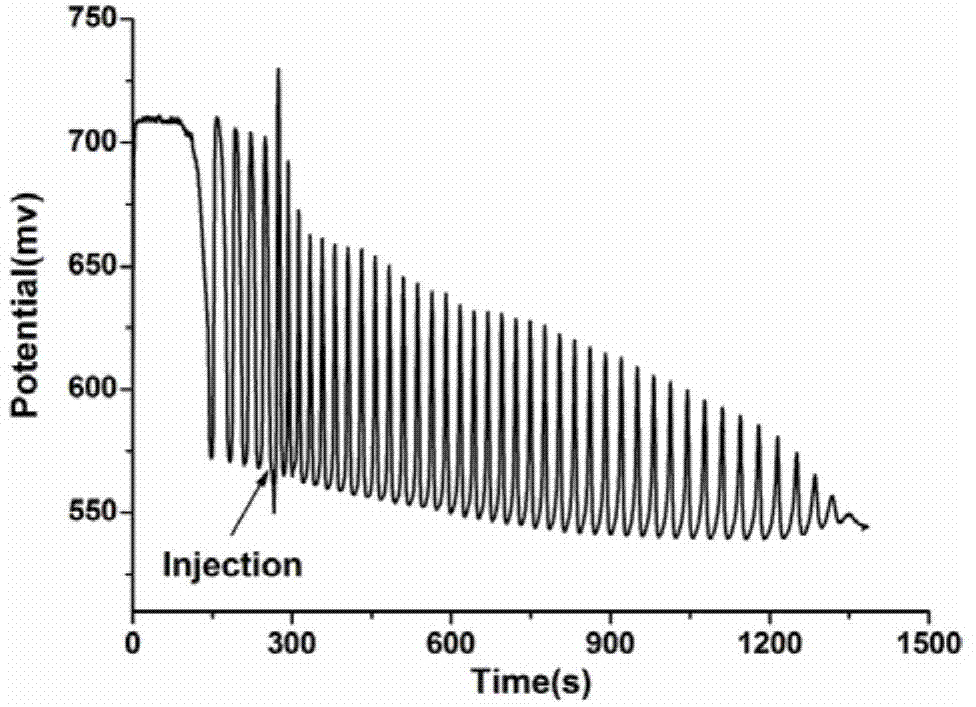
A method for identifying aliphatic chain isomers α-ketoglutaric acid and 1,3-acetone dicarboxylic acid
The invention discloses a method for identifying aliphatic chain isomers α-ketoglutaric acid and 1,3-acetone dicarboxylic acid, which is characterized in that: using "H2SO4-KIO3-[NiL](ClO4) 2‑MA‑H2O2” nonlinear chemical oscillation system is used as the identification solution, and the identification of aliphatic chain isomers can be realized according to the oscillation response of the aliphatic chain isomers to the system; L in [NiL](ClO4)2 is 5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazatetradec-4,11-diene. The oscillation spectrum provided by this identification method is more intuitive, not only can easily and quickly identify the aliphatic chain isomers α-ketoglutaric acid and 1,3-acetone dicarboxylic acid, but also can be widely used In other isomers, and the equipment is simple, high accuracy, easy to operate and observe.
Owner:ANHUI UNIVERSITY
Synthetic method of non-steroidal antiinflammatory drug pain killing
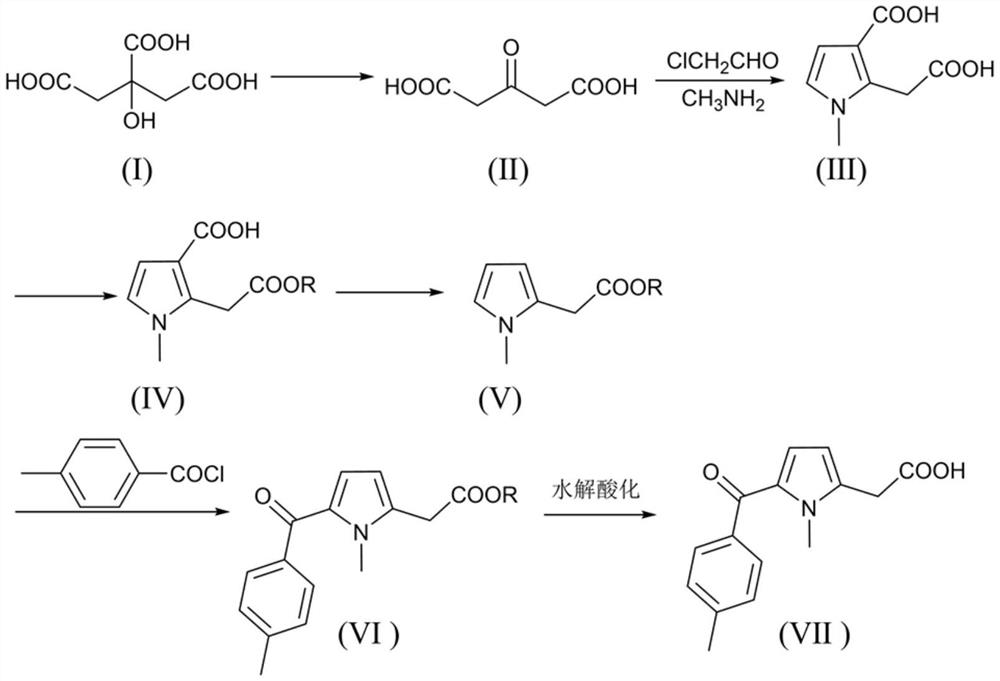
ActiveCN112661688AReduce processing costsProcess raw materials are cheapOrganic chemistryBulk chemical productionTolmetinNon steroid anti inflammatory drug
The invention discloses a synthesis method of non-steroidal anti-inflammatory drug tolmetin. The methodcomprises the following steps: sequentially preparing a sulfuric acid-containing acetonedicarboxylic acid crude product, 2-(2-acetoxy)-1-methyl-1H-pyrrole-3-formic acid and 2-(2-alkyl acetate)-1-methyl-1H-pyrrole-3-formic acid by taking citric acid or citric acid monohydrate as a raw material; and carrying out decarboxylation, acylation, hydrolysis and acidification to obtain tolmetin. The synthetic method provided by the invention overcomes the defect that the existing tolmetin preparation process depends on N-methylpyrrole, and is a low-cost, low-pollution and high-yield synthetic method of non-steroidal anti-inflammatory drug tolmetin.
Owner:HANGZHOU DIKE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com