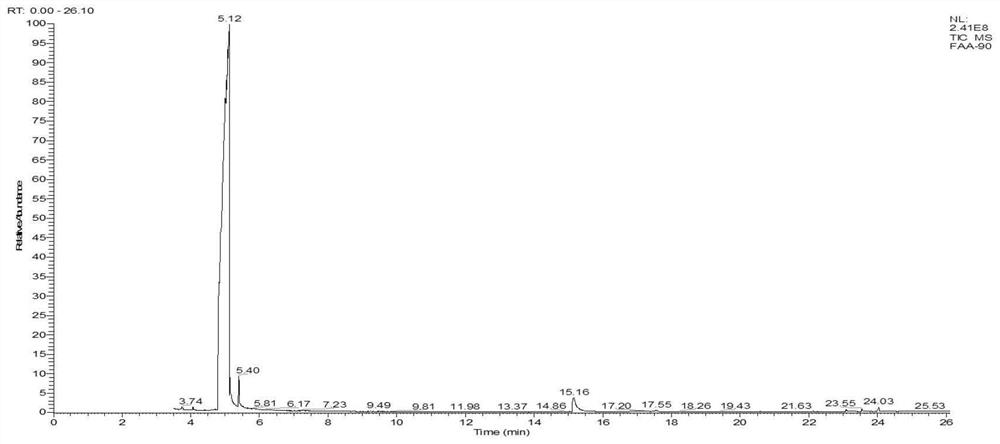
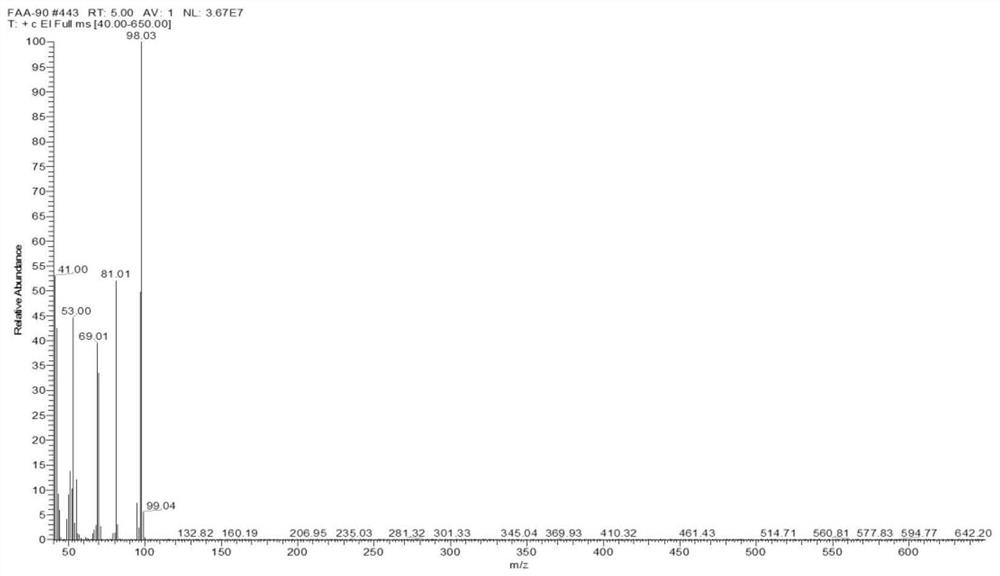
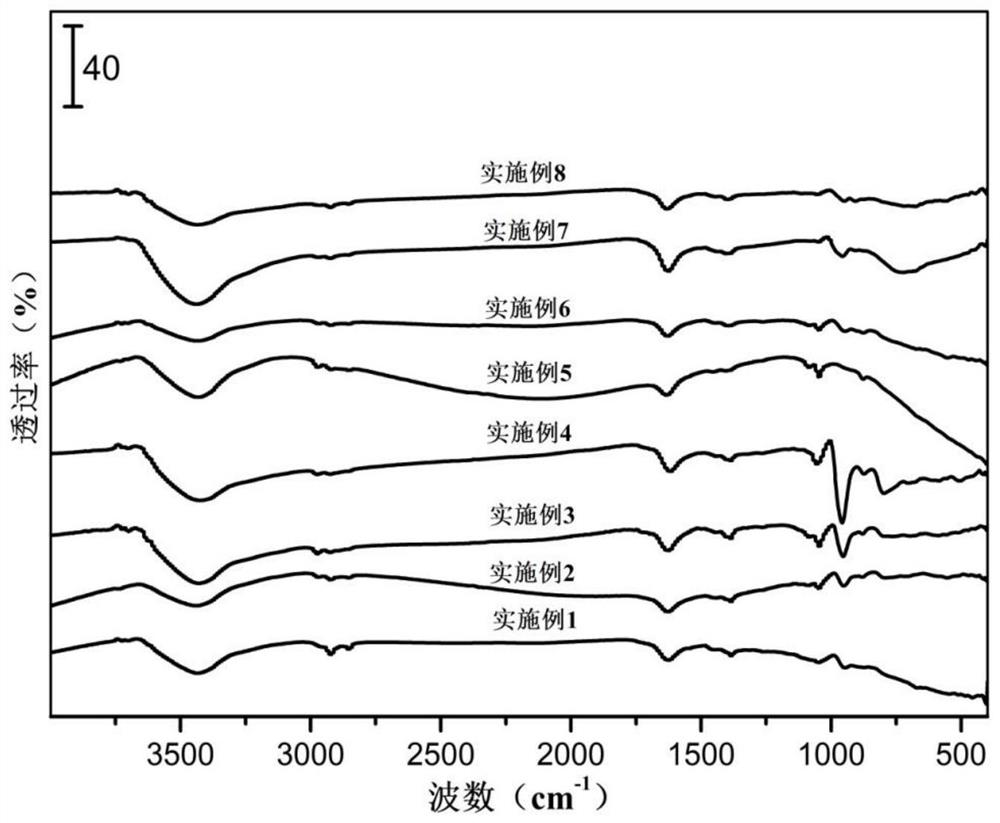
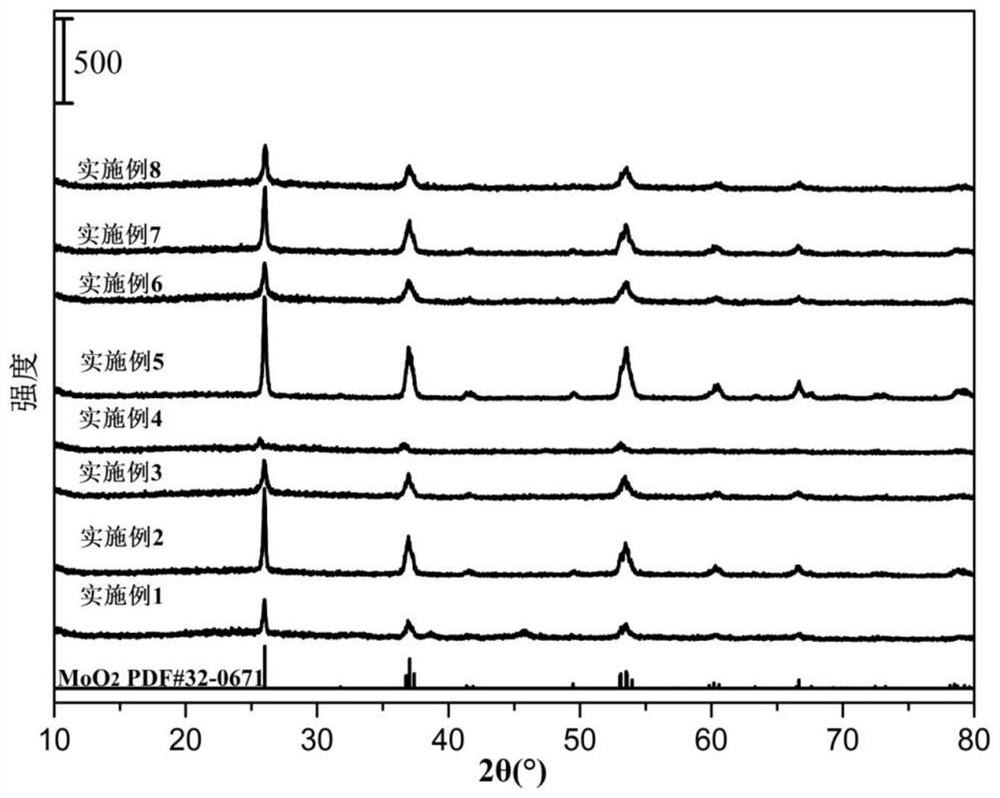
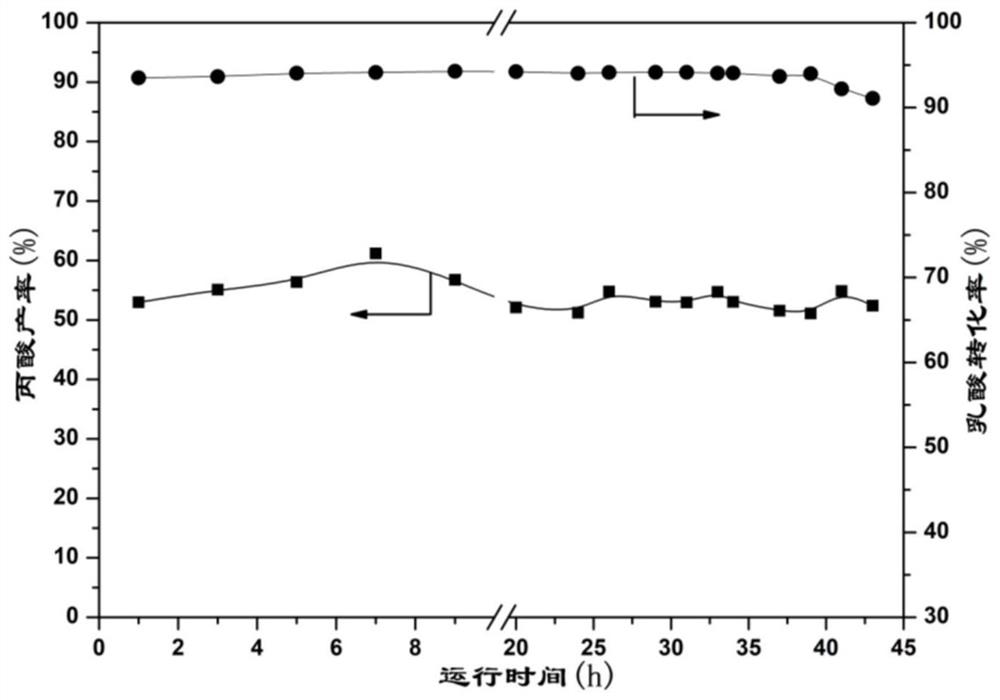
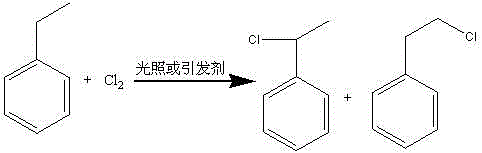
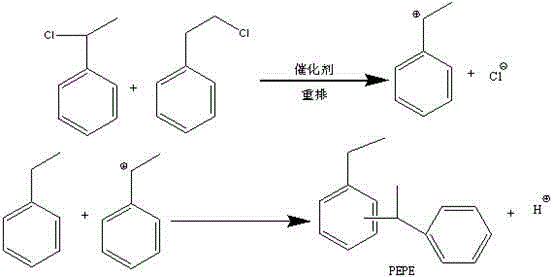
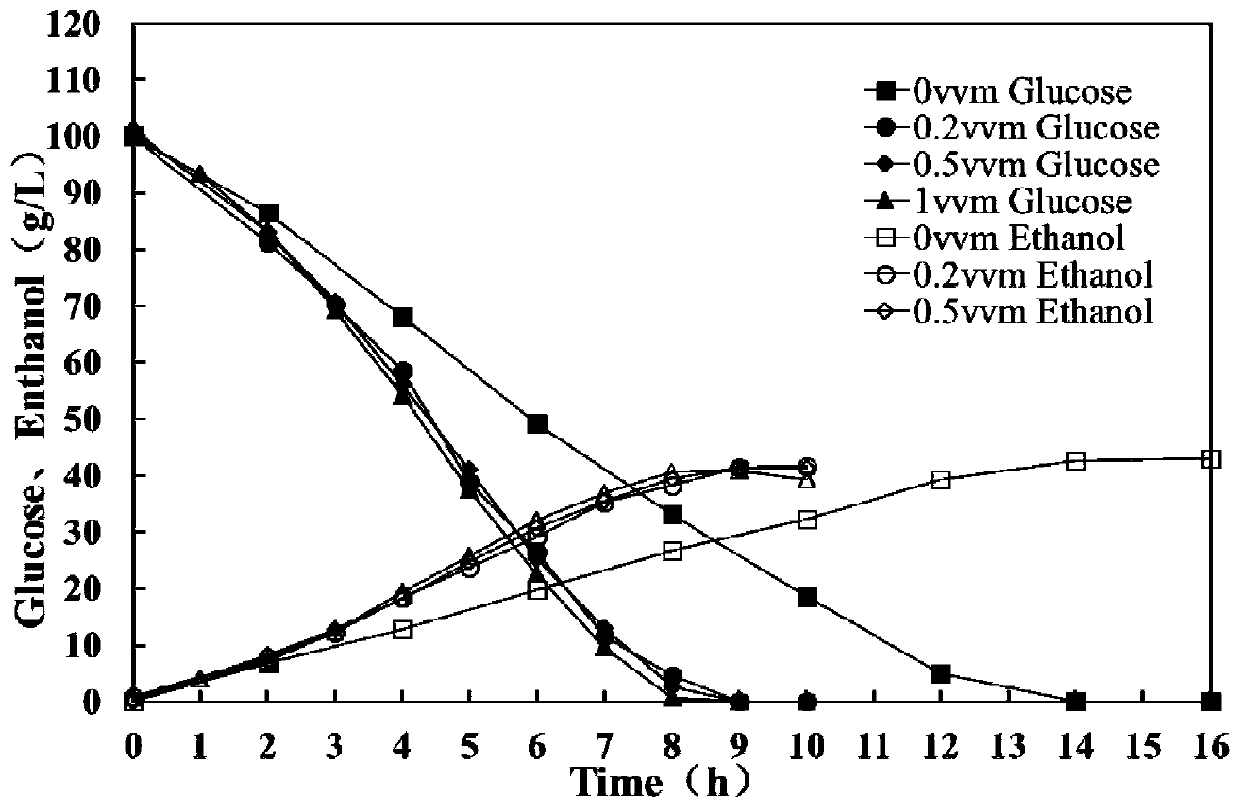
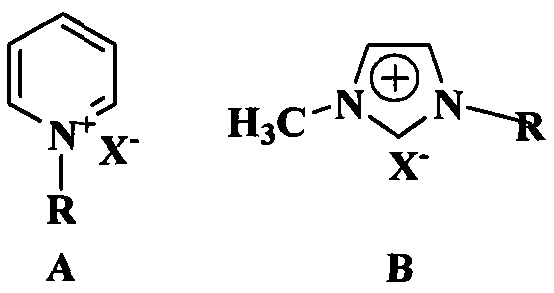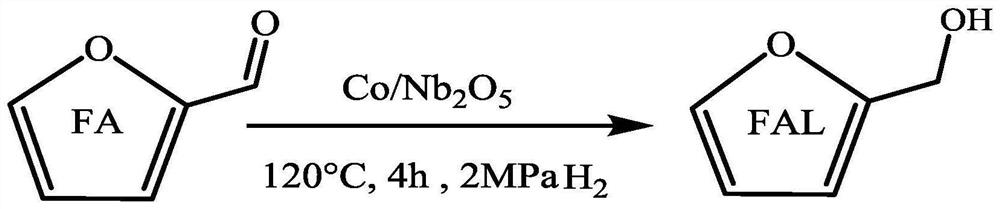Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47results about How to "Yield unchanged" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Microwave-assisted aqueous two-phase extraction and separation method of kudzu root total flavones
The invention relates to a microwave-assisted aqueous two-phase extraction method for separating kudzu root total flavone, which comprises the following steps: 1) preparation of an aqueous two-phase system: taking an inorganic salt, adding water to dissolve the inorganic salt, and adding an organic solvent to obtain a aqueous two-phase extractant; and 2) microwave-assisted aqueous two-phase extraction: adding kudzu root powder into the aqueous two-phase extractant obtained in the step 1), carrying out microwave-assisted extraction, and carrying out vacuum filtration to obtain an obvious phase-separated kudzu root flavone extracting solution. Compared with the prior art, the method provided by the invention has the following advantages: (1) the method is simple to operate and does not need to wait for phase separation; 2) the process is integrated; 3) the extraction process is quick and efficient; 4) the extraction solvent is low in toxicity; 5) the interfacial tension is small, thereby being beneficial to mass transfer between the two phases; 6) abundant impurities can be removed along with solid matters, thereby being beneficial to purifying the sample; and 7) the extraction method does not have obvious scale-up effect, can easily implement technique amplification and continuous operation, and can be directly connected with the subsequent purification procedure without special treatment.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
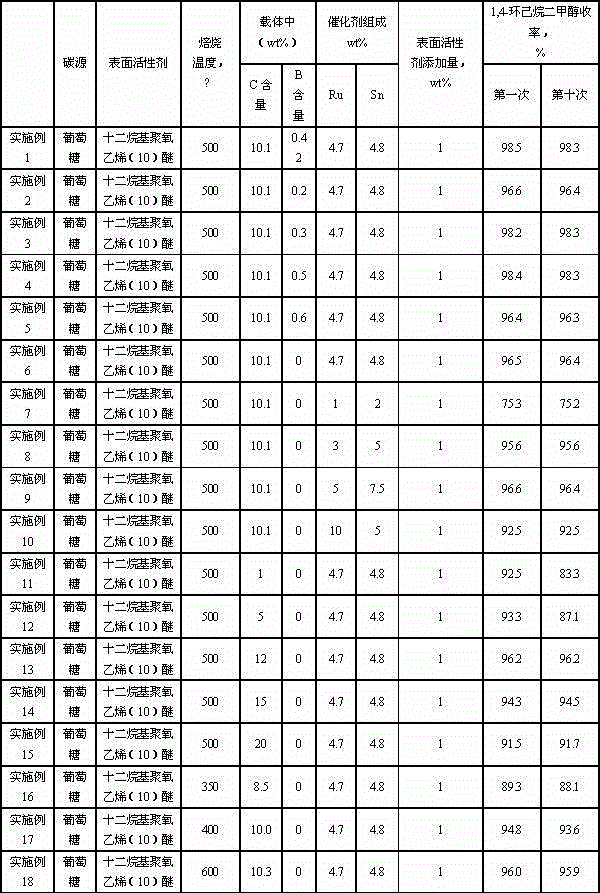
Catalyst for synthesizing 1, 4-cyclohexanedimethanol and preparation method of catalyst
InactiveCN104549251AHigh activityHigh selectivityOrganic compound preparationHydroxy compound preparationCyclohexanedimethanolPolymer science
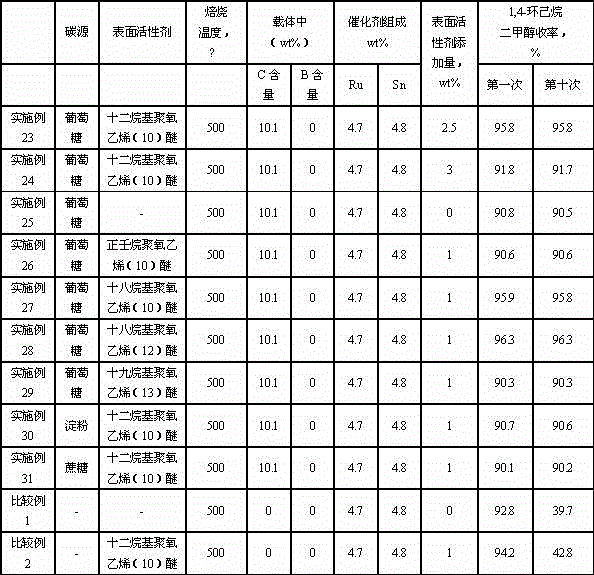
The invention relates to a catalyst for synthesizing 1, 4-cyclohexanedimethanol by using 1,4-cyclohexanedicarboxylic acid through hydrogenation, a preparation method of the catalyst and a method for synthesizing 1, 4-cyclohexanedimethanol in order to mainly solve the problem of short service life of the catalyst in the prior art. The problem is better solved through the technical scheme that the catalyst for synthesizing 1, 4-cyclohexanedimethanol comprises active components Ru and Sn and a carrier C@Al2O3, wherein the catalyst contains 1-10wt% of Ru, the mass ratio of Ru to Sn is 1: (0.5-2), and the carrier contains 1-20wt% of C. The catalyst can be used for industrial production of 1, 4-cyclohexanedimethanol prepared by using 1, 4-cyclohexanedicarboxylic acid through hydrogenation.
Owner:CHINA PETROLEUM & CHEM CORP +1
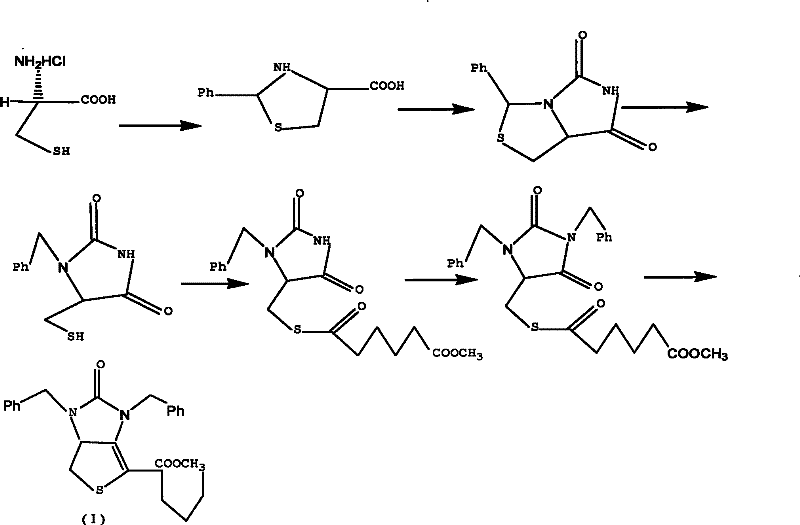
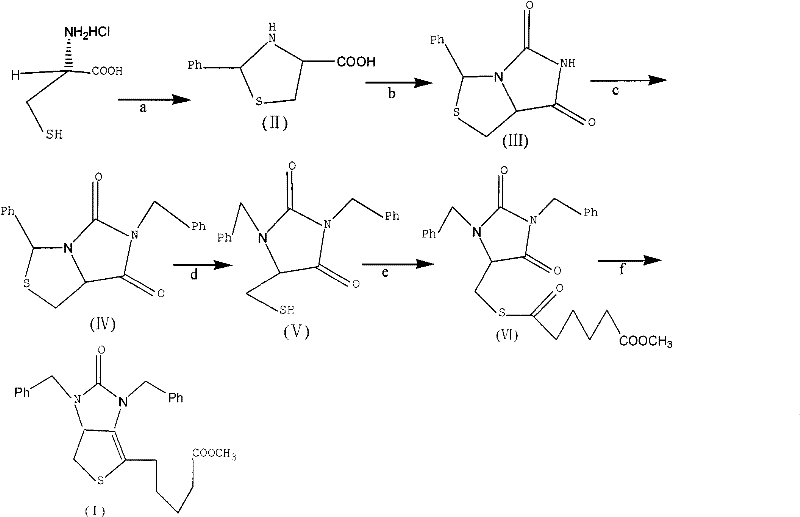
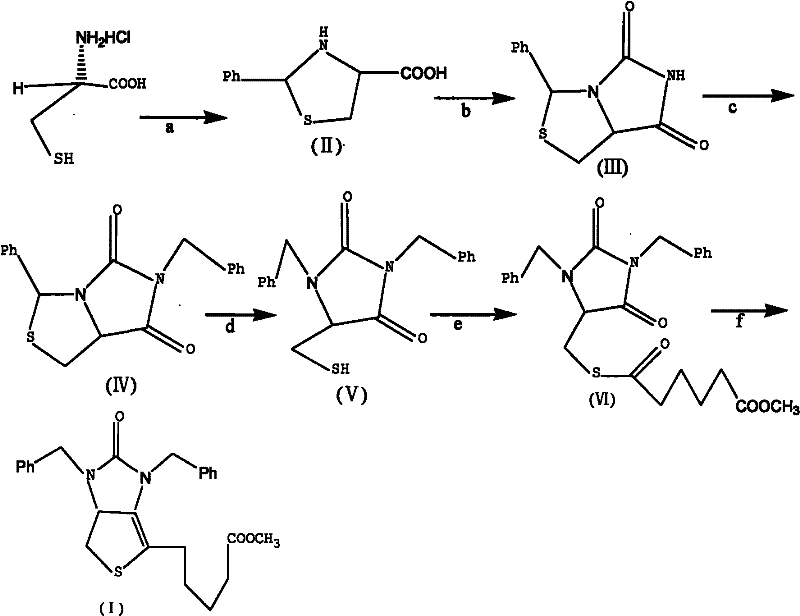
Method for preparing D-(+)-biotin intermediate
ActiveCN101735242AEasy to buyIncreased riskOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsBenzoyl bromideBenzaldehyde
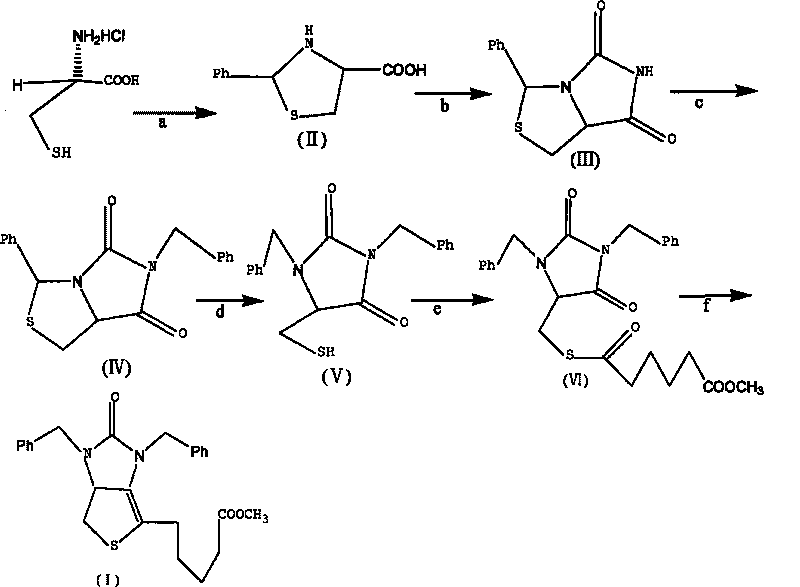
The invention provides a method for preparing a D-(+)-biotin intermediate. The method comprises the steps of: taking L-cysteine monohydrochloride as a starting material; using benzaldehyde and sodium cyanate as a ring closure reagent to synthesize (7aR)-3-phenyl-6-benzyl-1H, 3H-imidazo[1, 5-C]thiazole-(6H, 7aH)-5, 7-dione through the cyclization; then utilizing benzyl bromide to perform benzyl protection on N atoms; then taking zinc as a reducing agent to perform ring-opening synthesis on N, N-dibenzyl-L-sulfhydryl hydantoin; introducing a side chain through an esterification reaction with monomethyl adipate acyl chloride; and taking titanium as the reducing agent to perform reductive ring closure to generate the intermediate. According to the method, the cheap and readily available sodium cyanate is used as the ring closure reagent to replace sodium isocyanate which is toxic and difficult to purchase in an original method, reaction conditions are optimized and reaction order is adjusted, so the disadvantages of harsh reaction conditions and low yield in the original method of ring opening first and then benzyl protection are overcome by the method of performing benzyl protection on the N atoms of an imidazole part first and then performing ring opening; and the total yield reaches 34.0-38.0 percent.
Owner:安徽泰格生物技术股份有限公司
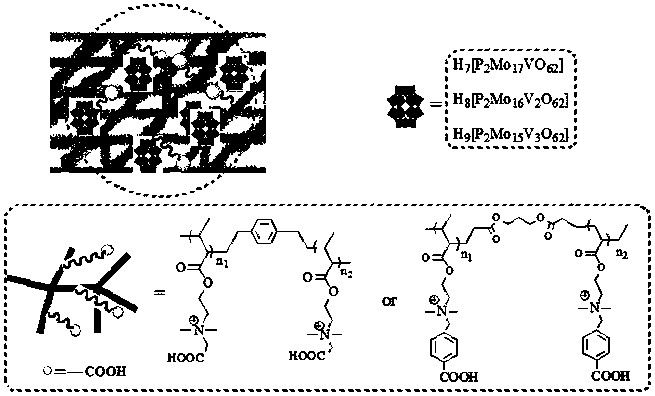
Carboxyl functionalized porous heteropoly acid polyionic liquid and use thereof
ActiveCN109289919AFacilitate the reaction between two phasesSeparation in timeOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkVanadium doping
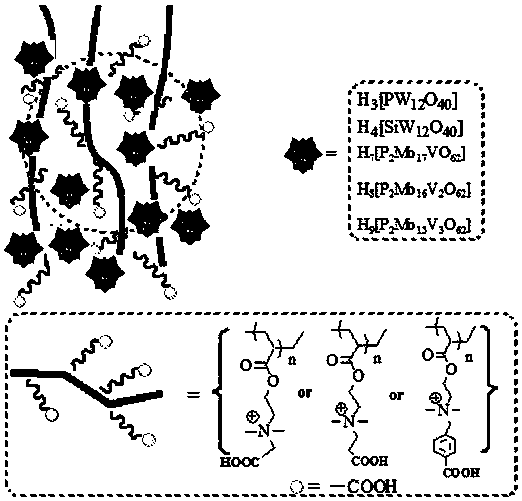
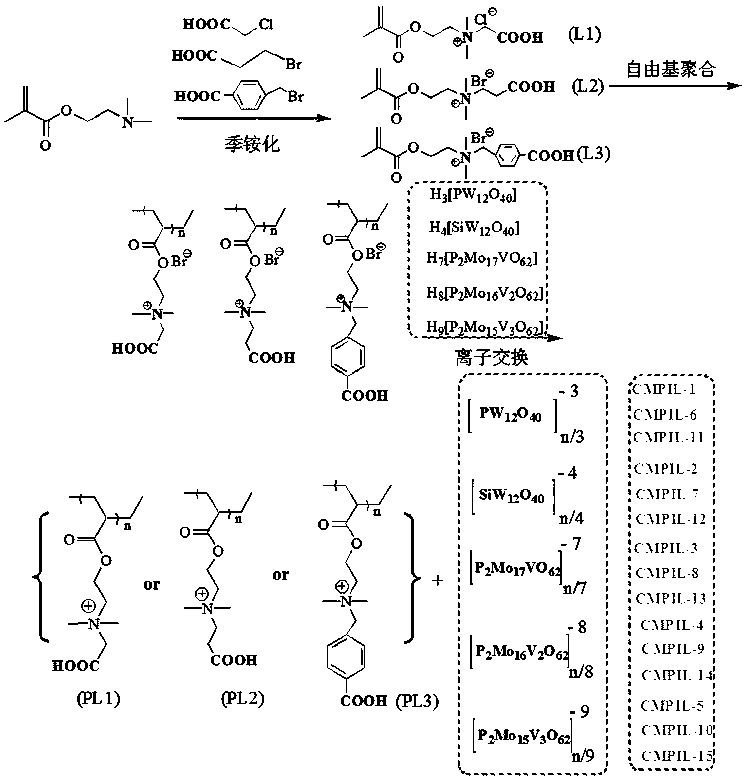
The invention discloses a carboxyl functionalized porous heteropoly acid polyionic liquid and a use thereof. A carboxyl functionalized polyquaternary ammonium salt with a long chain linear structure and a heteropoly acid as building units are self-assembled into a three-dimensional network porous structure under electrostatic interaction so that the polyionic liquid is obtained, or a carboxyl functionalized polyquaternary ammonium with a long chain cross-linked structure and a Dawson-type vanadium-doped heteropoly acid as building units are self-assembled into a three-dimensional network porous structure under electrostatic interaction. The carboxyl functionalized porous heteropoly acid polyionic liquid can be used as a heterogeneous catalyst. Industrial grade hydrogen peroxide is used asan oxidant. A cyclic ketone compound undergoes Baeyer-Villiger oxidation to produce a corresponding cyclic lactone under the solventless condition. The catalytic system has a novel structure, is usedfor a Baeyer-Villiger oxidation reaction, and has the characteristics of good selectivity, mild reaction condition, large operation flexibility and convenient recycling of the catalytic system.
Owner:MINJIANG UNIV
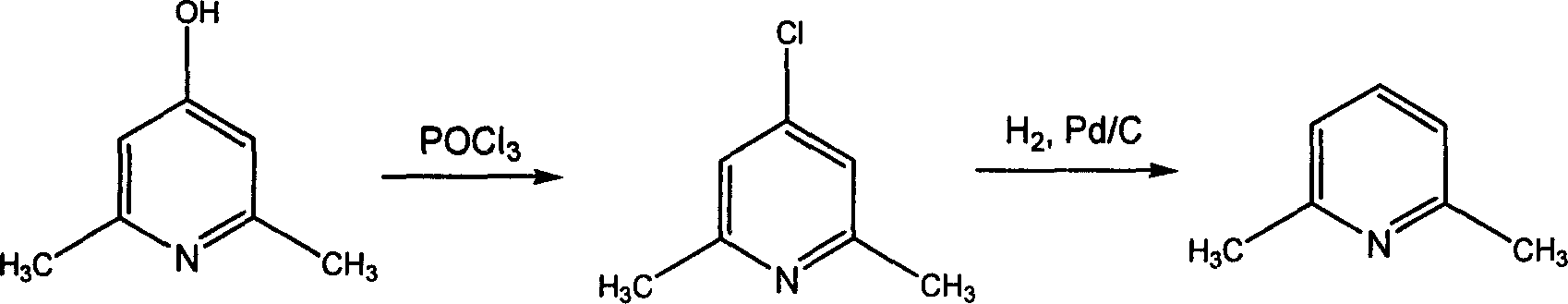
2,6-dimethylpyridine preparation method
InactiveCN1733729AHigh reaction yieldReduce manufacturing costOrganic chemistryAlcoholHydrogen pressure
The invention discloses a process for preparing 2,6-dimethylpyridine comprising the following steps, using Pd / C as catalyst, C1-C4 alcohols as solvent, subjecting 2,6-dimethyl-4-chloropyridine to hydrogenization dechlorination reaction under the conditions of hydrogen pressure 0.1-0.2MPa, 20-55 deg. C, finally carrying out post-treatment to obtain the 2,6-dimethylpyridine.
Owner:ZHEJIANG UNIV OF TECH
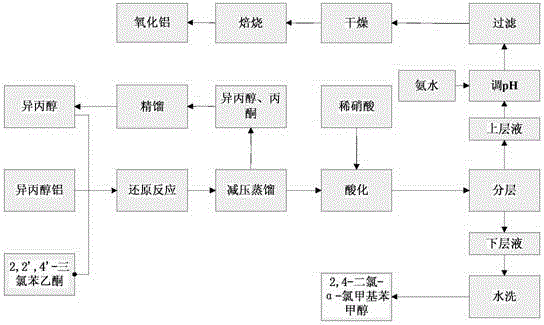
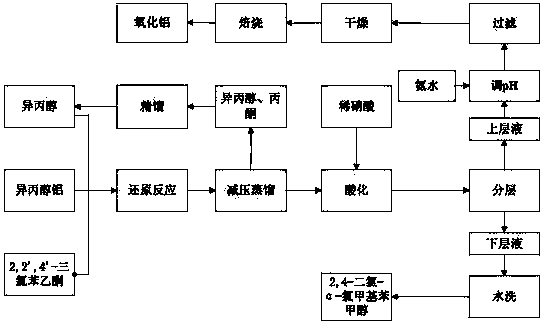
Clean preparation method for 2,4-dichloro-alpha-chloromethyl benzyl alcohol
ActiveCN103664527ANo pollution in the processYield unchangedAluminium compoundsOrganic compound preparationDistillationAluminium hydroxide
The invention discloses a clean preparation method for 2,4-dichloro-alpha-chloromethyl benzyl alcohol and belongs to the field of catalytic synthesis process of 2,4-dichloro-alpha-chloromethyl benzyl alcohol. 2,2',4'-trichloroacetophenone is taken as an initial raw material, aluminium isopropoxide serves as a catalyst, isopropyl alcohol serves as not only a solvent but also a reactant, and reduction reaction, reduced pressure distillation, acidation, water washing and curing are conducted to obtain the 2,4-dichloro-alpha-chloromethyl benzyl alcohol. An isopropyl alcohol and acetone mixed solution obtained through reduced pressure distillation is rectified, 80-84 DEG C fraction is collected for recycling, and the yield is basically kept unchanged. Acidized acid water is neutralized by ammonia water, then filtered to obtain aluminium hydroxide, and finally roasted to acquire aluminium oxide after being dried. The method is mild in reaction conditions, short in reaction time and convenient in after-treatment, and avoids environmental pollution. The conversion rate and the product yield of 2,2',4'-trichloroacetophenone are more than 99%, and the manufacturing cost is low. The method is an efficient and environmental-friendly method for synthesizing 2,4-dichloro-alpha-chloromethyl benzyl alcohol and facilitates large-scale industrial production.
Owner:张家港市乐余科创园投资发展有限公司
Preparation method of high-purity topiramate
InactiveCN106632530AYield unchangedEasy to operateSugar derivativesSugar derivatives preparationSolventChemistry
The invention discloses a preparation method of high-purity topiramate. Under the condition of dissolving the topiramate into an organic solvent, a proper concentration of alkali is added, so that the topiramate slat forms solid to be separated out in the solvent; the solid is dissolved into water, and is acidified into a weak acidic state; the solid is a topiramate coarse product; the topiramate coarse product is recrystallized to obtain the high-purity topiramate. The preparation method of the high-purity topiramate has the advantages that the purity reaches 99.90 percent or higher; under the condition that the product purity reaches 99.9 percent or higher, the yield of the product keeps unchanged; no additional excessive cost is added. The operation is convenient; the process is safe; the industrial production is easy.
Owner:SHAANXI DASHENG PHARMA TECH
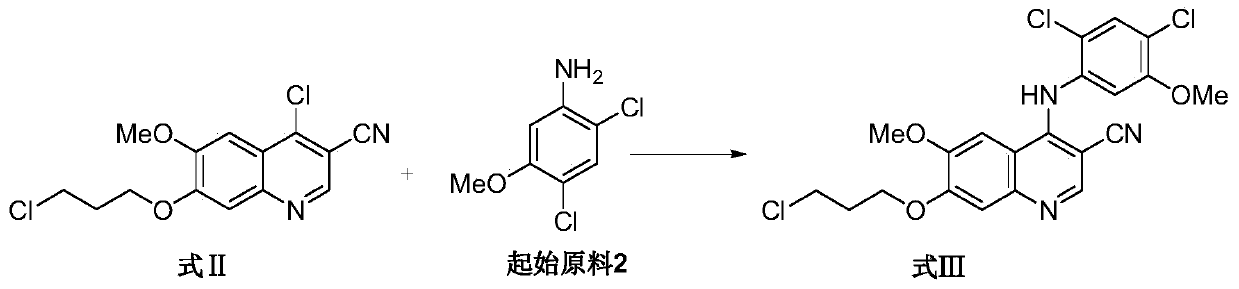
Preparation method of bosutinib
ActiveCN105503719AHigh yieldSuitable for industrial productionOrganic chemistry2-PyrrolidoneBosutinib
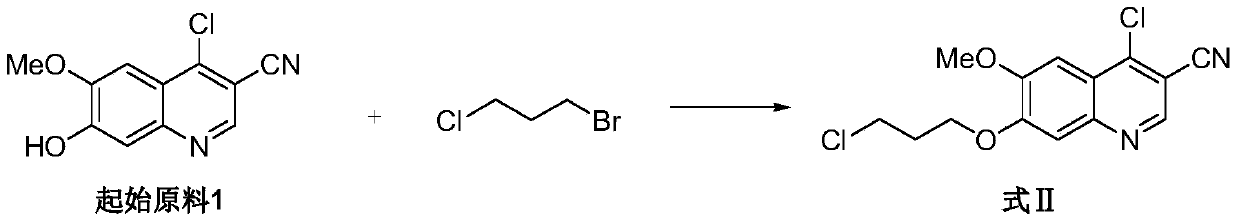
The invention relates to a preparation method of bosutinib. According to the preparation method, 4-chloro-6-methoxyl-7-hydroxyl-3-quinoline formonitrile and 2,4-dichloro-5-methoxyl aniline are taken as the initial raw materials, 1-methyl-2-pyrrolidone is taken as the solvent, and a crude product of bosutinib is obtained through three steps. The preparation method has the advantages of few steps and mild conditions. The reaction conditions of the prior art are improved, the yield is prominently increased, the cost is reduced, and the preparation method is suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
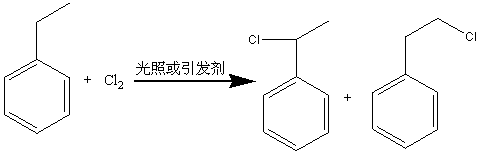
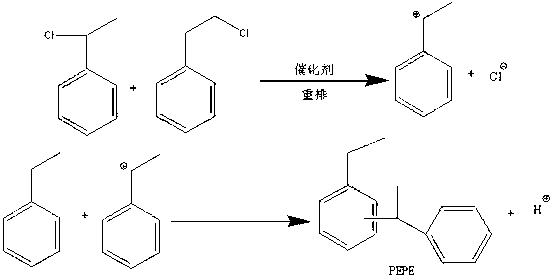
Method for preparing phenyl ethylbenzene ethane capacitor insulating oil through catalysis of acidic ionic liquid
ActiveCN103319297AAffect qualityHigh activityLiquid organic insulatorsHydrocarbon from halogen organic compoundsUltraviolet irradiationIonic liquid
The invention provides a method for preparing phenyl ethylbenzene ethane capacitor insulating oil through catalysis of acidic ionic liquid. The method is characterized in that 2-10mol of ethylbenzene reacts with 1mol of chlorine gas to generate a mixture of alpha-chloroethylbenzene and beta -chloroethylbenzene at 110 DEG C-150 DEG C through the ultraviolet irradiation or under the triggering action of a radical initiator, and the mixture generated through reaction is not separated and is directly mixed with a mixture of 5-20g of acidic ionic liquid catalyst and 2-10mol of ethylbenzene so as to react at 30-130 DEG C to prepare the phenyl ethylbenzene ethane. The phenyl ethylbenzene ethane capacitor insulating oil prepared by the method has excellent characteristics of normal temperature insulation oil, namely phenyl xylyl ethane; the condensation point of the phenyl ethylbenzene ethane capacitor insulating oil is far lower than that of the phenyl xylyl ethane; and the phenyl ethylbenzene ethane capacitor insulating oil has unique low-temperature-resistance performance and is particularly suitable for serving as low-temperature capacitor insulating oil.
Owner:SICHUAN DONGFANG INSULATING MATERIAL
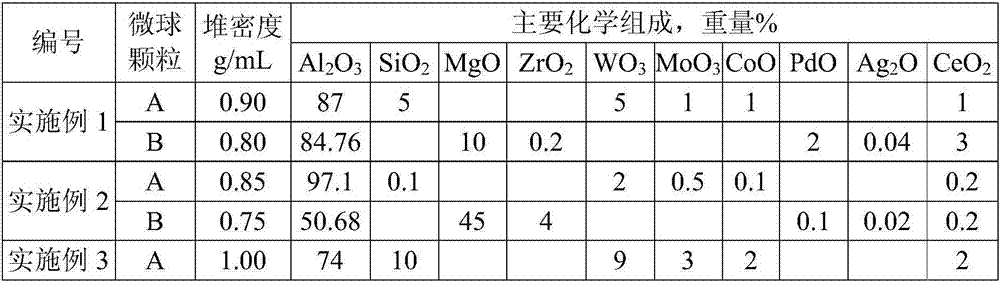
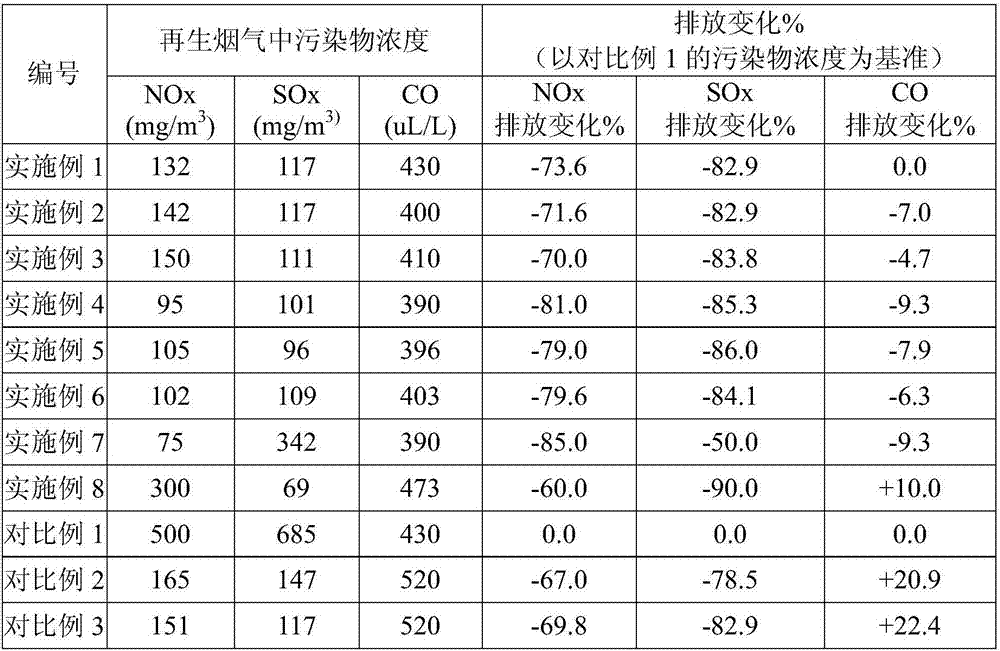
Cocatalyst for reducing FCC (fluid catalytic cracking) regeneration flue gas pollutant discharge and application thereof
ActiveCN106925295AReduce CO contentYield unchangedGas treatmentHeterogenous catalyst chemical elementsRare-earth elementMicrosphere
The invention relates to a cocatalyst for reducing the FCC (fluid catalytic cracking) regeneration flue gas pollutant discharge and application thereof. The cocatalyst comprises microsphere particles A and microsphere particles B, wherein through being metered by 100 percent of the weight of the microsphere particles A, the microsphere particle A is prepared from 80 to 98 percent of aluminum oxide, 0.1 to 10 percent of silicon oxide, 1 to 15 percent of oxide of one or two kinds of metals selected from groups VIB and IB and 0.1 to 3 percent of oxide of rare earth elements; through being metered by 100 percent of the weight of the microsphere particles B, the microsphere particle B is prepared from 50 to 80 percent of aluminum oxide, 10 to 50 percent of oxide of one or several kinds of metals selected from groups IIA, IVB and VIII and 0.1 to 10 percent of oxide of rare earth elements. The cocatalyst is applied to the reduction of nitrogenous pollutant discharge; the discharge of atmospheric pollutants such as NOx, Sox and CO in regeneration flue gas can be obviously reduced.
Owner:HEBEI XINPENG CHEM CO LTD +1
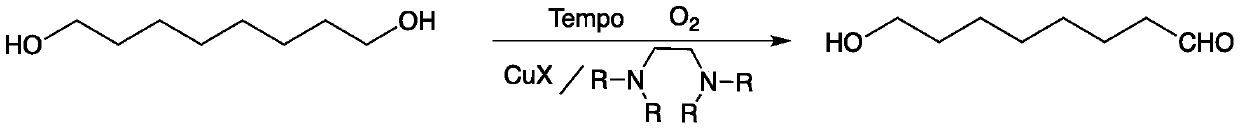
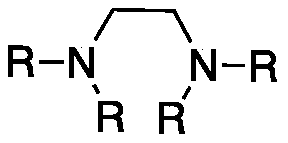
Method for preparing 8-hydroxyl caprylaldehyde
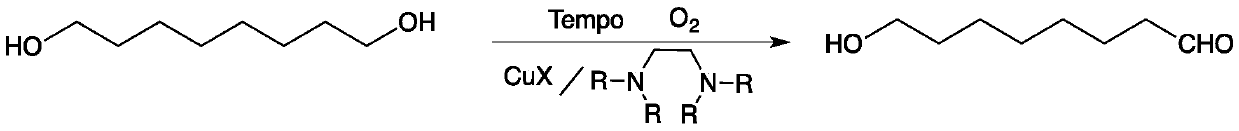
InactiveCN110002974AMild reaction conditionsPollution-free yieldOrganic compound preparationCarbonyl compound preparationSolventChemistry
The invention discloses a method for preparing 8-hydroxyl caprylaldehyde and belongs to the field of chemical synthesis. The method comprises the steps of introducing air to 1,8-octanediol, which serves as a starting raw material, in a solvent at room temperature in the presence of a composite catalyst prepared from cuprous halide, substituted ethylenediamine and Tempo, carrying out a reaction forcertain time, then, carrying out filtering to remove the catalyst, and subjecting filter liquor to reduced-pressure distillation to remove the solvent, so as to obtain the product, i.e., the 8-hydroxyl caprylaldehyde. According to the method for preparing the 8-hydroxyl caprylaldehyde, disclosed by the invention, the technical line has the characteristics of short route, simplicity and convenience in operation and easiness in industrial production, and thus, the method for synthesizing the 8-hydroxyl caprylaldehyde is very economical, simple and convenient.
Owner:JIAXING UNIV
Low-carbon olefin production method
ActiveCN103087766AExtended operating cycleYield unchangedTreatment with plural serial stages onlyHydrocarbon by hydrocarbon crackingChemistryCross over
The invention relates to a low-carbon olefin production method. The method comprises the following steps: 1, preheating a cracking substance A containing a cracking raw material and water vapor to a cross-over temperature; 2, allowing hydrogen having an amount being 0.5-1.5% of the weight of the cracking raw material and oxygen having an amount being 2-12% of the weight of the cracking raw material to contact with the preheated cracking substance A in the presence of a hydrogen selective-combustion catalyst under a hydrogen selective-combustion reaction condition to obtain a cracking substance B; and 3, carrying out steam cracking of the cracking substance B obtained in step 2 to obtain a substance C, and carrying out separating recovery of the substance C to obtain low-carbon olefins. The invention also discloses an another low-carbon olefin production method. The another low-carbon olefin production method is carried out in a cracking system comprising a cracking furnace and a cracking gas header tube, and a hydrogen selective-combustion device is arranged at the bottom of the convection zone of the cracking furnace. The low-carbon olefin production methods can prolong the running period of the cracking furnace.
Owner:CHINA PETROLEUM & CHEM CORP +1
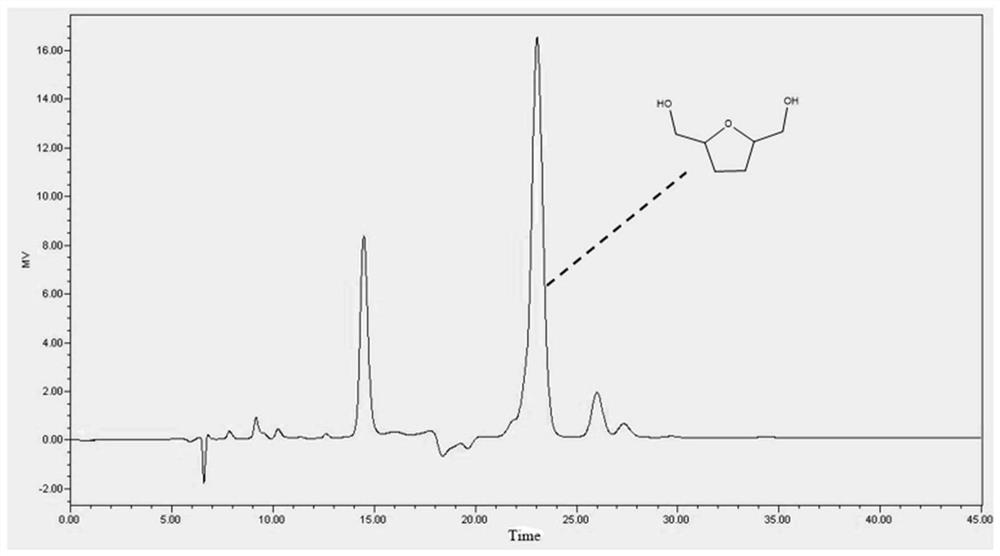
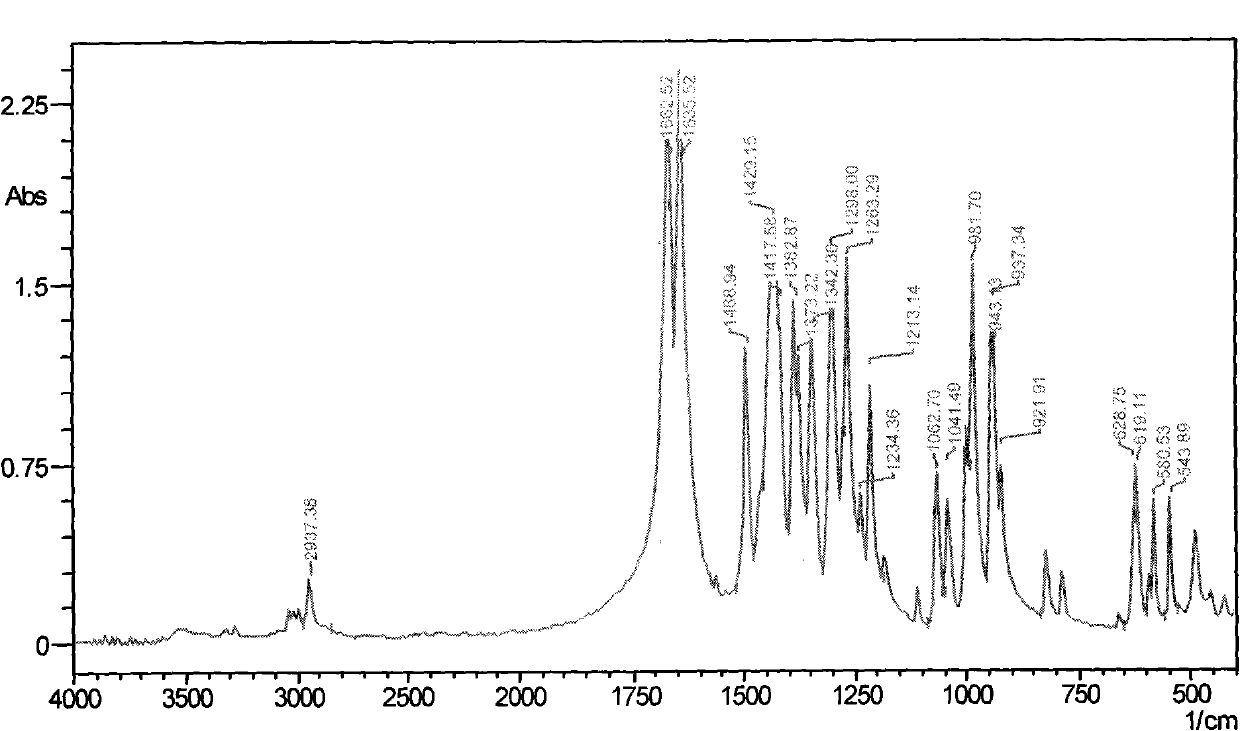
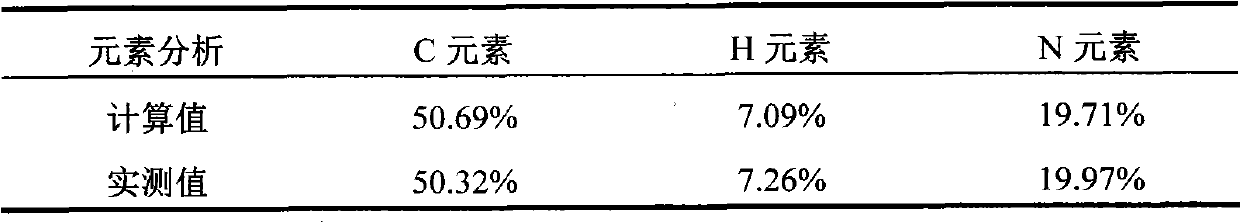
Method for preparing 2, 5-dimethyloltetrahydrofuran through hydrogenation of 5-hydroxymethylfurfural
PendingCN113773284ALow priceImprove protectionOrganic chemistryCatalyst activation/preparationPtru catalystFuraldehyde
The invention discloses a method for preparing 2, 5-dihydroxymethyl tetrahydrofuran (BHMTHF) through catalytic hydrogenation of 5-hydroxymethylfurfural (5-HMF) and application of the 2, 5-dihydroxymethyl tetrahydrofuran (BHMTHF). According to the invention, by using a supported catalyst, a first active component and a second component of the supported catalyst are respectively from corresponding non-noble metal salt solutions and are selected from Ni(NO3)2.6H2O and Co(NO3)2.6H2O; typical acidic oxide gas phase SiO2 is used as a carrier, the loading capacity of the two active components is (4 / 1)-(1 / 1), and the bimetallic catalyst is prepared by adopting a coprecipitation method; and the catalyst is simple and convenient in preparation method, low in price and cyclical, the catalytic reaction system is green, the reaction substrate is renewable, the reaction condition is mild, the input cost is low, and the catalyst has a wide industrial application prospect.
Owner:XIAMEN UNIV
Preparation method of TAT
The invention relates to a preparation method of TAT, comprising the following steps of: slowly dripping acetic anhydride with 1.5-2.5 times of the mass of HA at 5-10 DEG C by using the HA, ammonium acetate and water as raw materials; reacting for 30-50 minutes to obtain DAPA under stirring; reacting for 2-3 hours by using DAPT and the acetic anhydride as the raw materials and increasing temperature to 100-110 DEG C; and reacting for 1-2 hours by reducing the temperature to 30-35 DEG C to obtain a product TAT, in the presence of water as a catalyst, wherein the water accounts for 5-35 percent of the mass of the acetic anhydride raw material. The invention replaces the catalyst in a Lukasavage process method with the inexpensive water, obviously enhances the yield of the TAT, enables the synthesizing process of the TAT to be easy and reduces the production cost.
Owner:ZHONGBEI UNIV
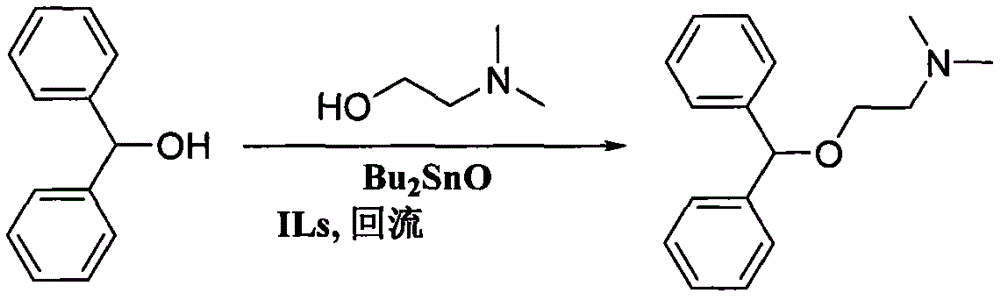
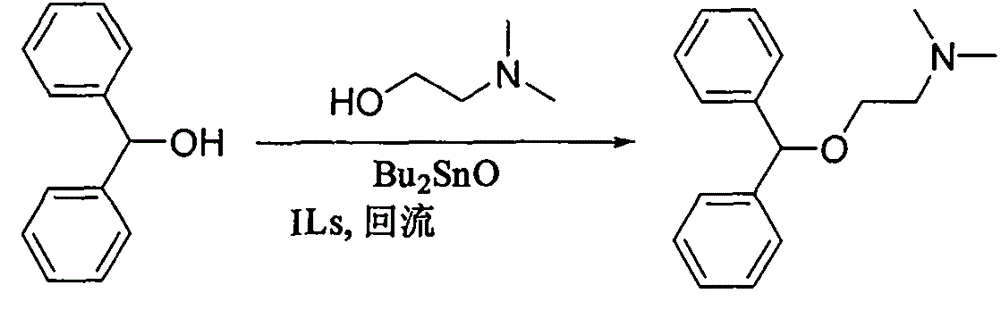
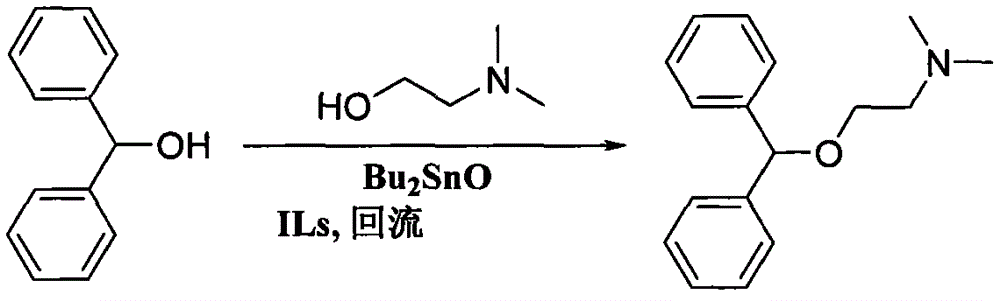
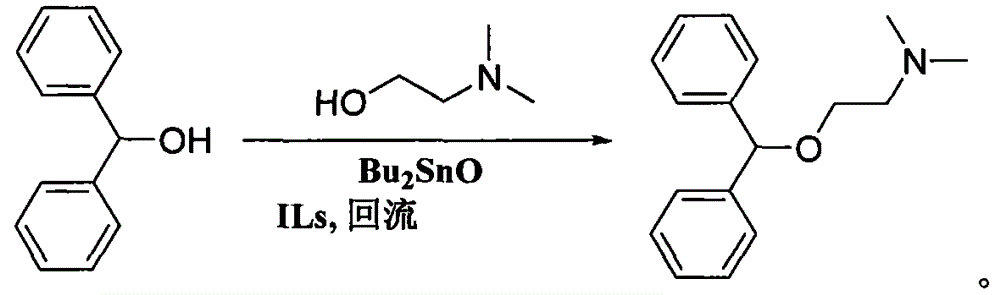
Preparation method of diphenhydramine
InactiveCN104892435ANo pollution in the processYield unchangedOrganic compound preparationAmino-hyroxy compound preparationDibutyltin oxideIon
The invention relates to a preparation method of diphenhydramine, which takes benzhydrol and dimethylaminoethanol as raw materials, under existence of dibutyltin oxide and ion liquid compound, benzhydrol and dimethylaminoethanol are subjected to an intermolecular dehydration reaction to obtain diphenhydramine. The method has the advantages of simple preparation technology, mild reaction condition, low cost, high product yield and high purity.
Owner:JIAXING UNIV
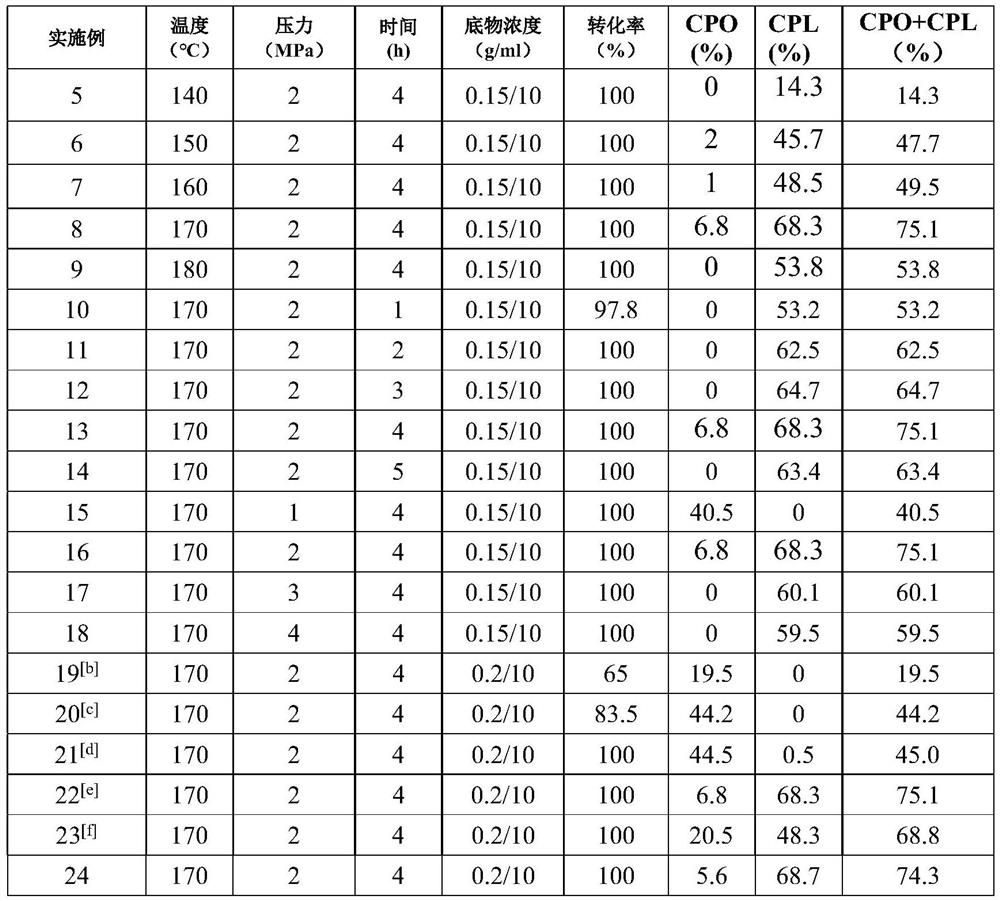
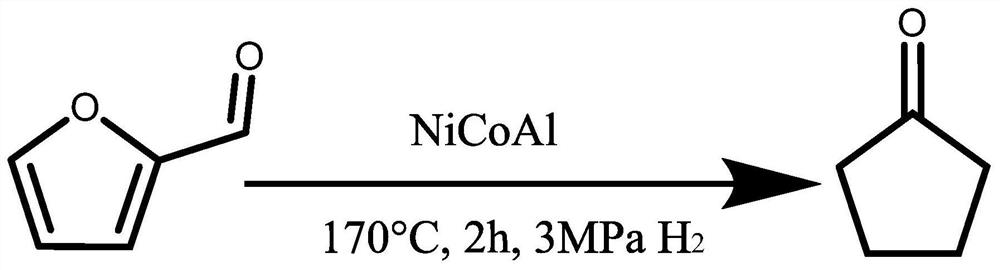
Method for preparing cyclopentanone and cyclopentanol through furfural hydrogenation rearrangement
PendingCN113786837ALow priceImprove protectionPreparation by isomerisationOrganic compound preparationPtru catalystCyclopentanol
The invention discloses a method for preparing cyclopentanone and cyclopentanol through furfural catalytic hydrogenation ring-opening rearrangement and application. Wherein the catalyst is prepared by adopting a urea hydrolysis method, three metals of the catalyst are respectively from corresponding non-noble metal salt solutions and are selected from Ni (NO3) 2.6 H2O, Co (NO3) 2.6 H2O and Al2 (NO3) 2.9 H2O, and the ratio of the three metal atoms Ni / Co / Al is 0.5 / 2.5 / 2. The catalyst is simple and convenient in preparation method, low in price and cyclical, the catalytic reaction system is green, the reaction substrate is renewable, the reaction condition is mild, the input cost is low, and the catalyst has a wide industrial application prospect.
Owner:XIAMEN UNIV
Novel wet corn soaking method
ActiveCN108314743AReduce dosageHigh yieldOn/in inorganic carrierPeptidasesFullereneMagnetic separation
The invention relates to a novel wet corn soaking method. The method comprises pulverizing de-embryonated corn to obtain powder of 20-40 meshes, adding water and a magnetic fullerene immobilized enzyme into the powder, carrying out stirring and soaking at 30-50 DEG C for 5-8 hours, recovering the magnetic fullerene immobilized enzyme through magnetic separation, transferring the remaining soakingmixture into a pulverizer, carrying out fine pulverization, sieving the powder through a sieve of 200 meshes, standing the filtrate over night, carrying out centrifugal separation and carrying out drying to obtain corn starch.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Molybdenum-based catalyst, and preparation method and application thereof
ActiveCN108993479AStrong reductionImprove hydrogenolysis capacityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitrogen atmosphereNitrogen gas
The invention relates to the technical field of catalytic materials, and in particular relates to a molybdenum-based catalyst, and a preparation method and application thereof. The preparation methodof the molybdenum-based catalyst comprises the following steps: adding a molybdenum source into an H2O2 / CH3CH2OH mixed solution, and carrying out stirring to form a reaction system; heating the reaction system to 50-70 DEG C, adding a protein assistant, continuously carrying out stirring, and then evaporating water and ethanol at a temperature of 80-100 DEG C to obtain a catalyst precursor; and filling the catalyst precursor under a nitrogen atmosphere, carrying out heating to 300-450 DEG C, then introducing mixed steam of lactic acid and water into the catalyst precursor, and reducing the catalyst precursor to prepare the molybdenum-based catalyst. The molybdenum-based catalyst prepared by the method is simple to prepare, and the catalyst is clean and non-toxic, is high in catalytic activity, good in stability and low in cost, and has a potential commercial development value.
Owner:CHONGQING UNIV OF TECH
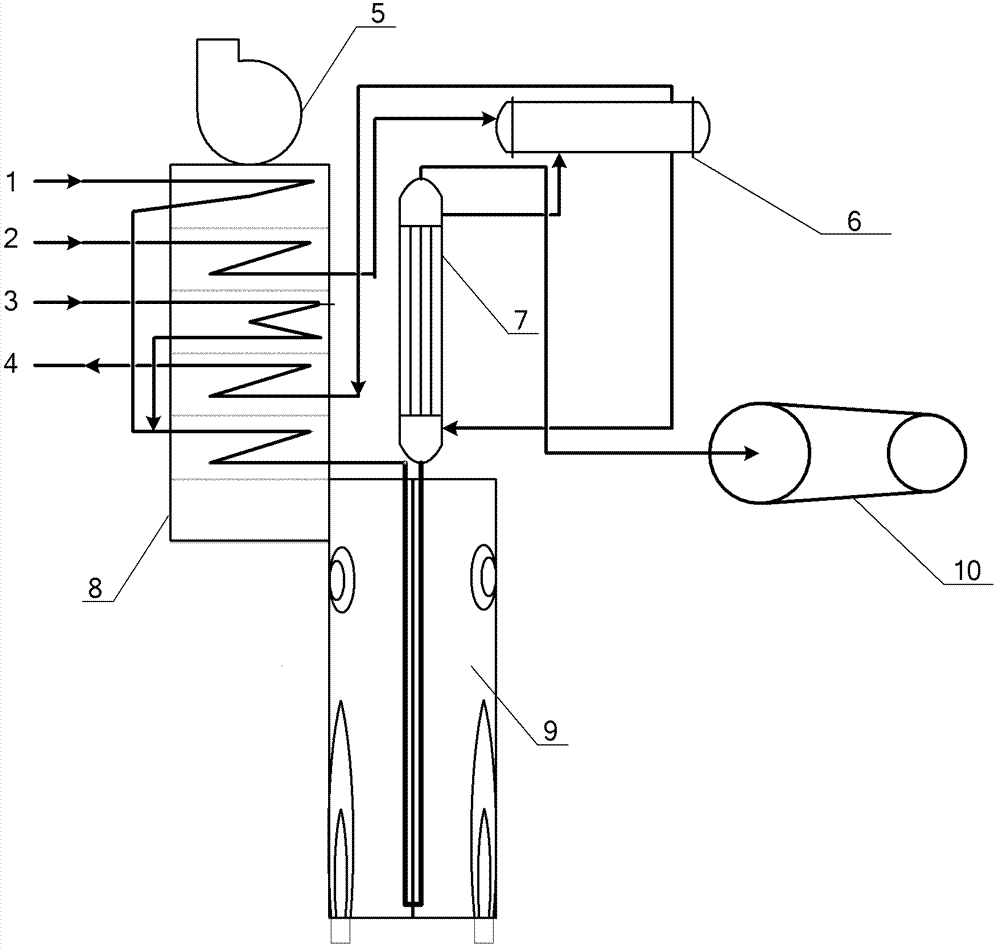
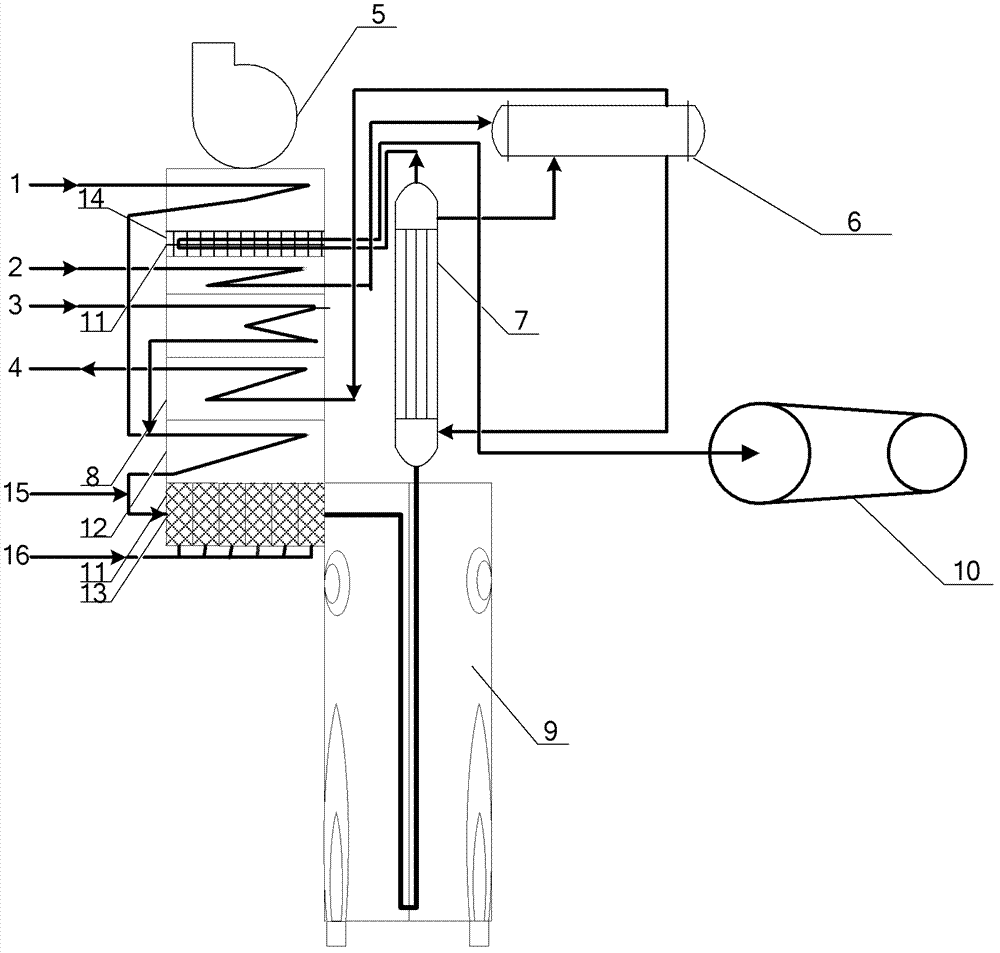
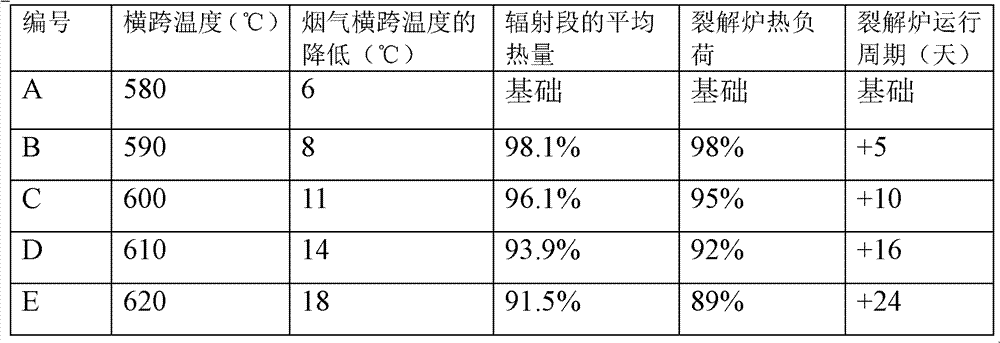
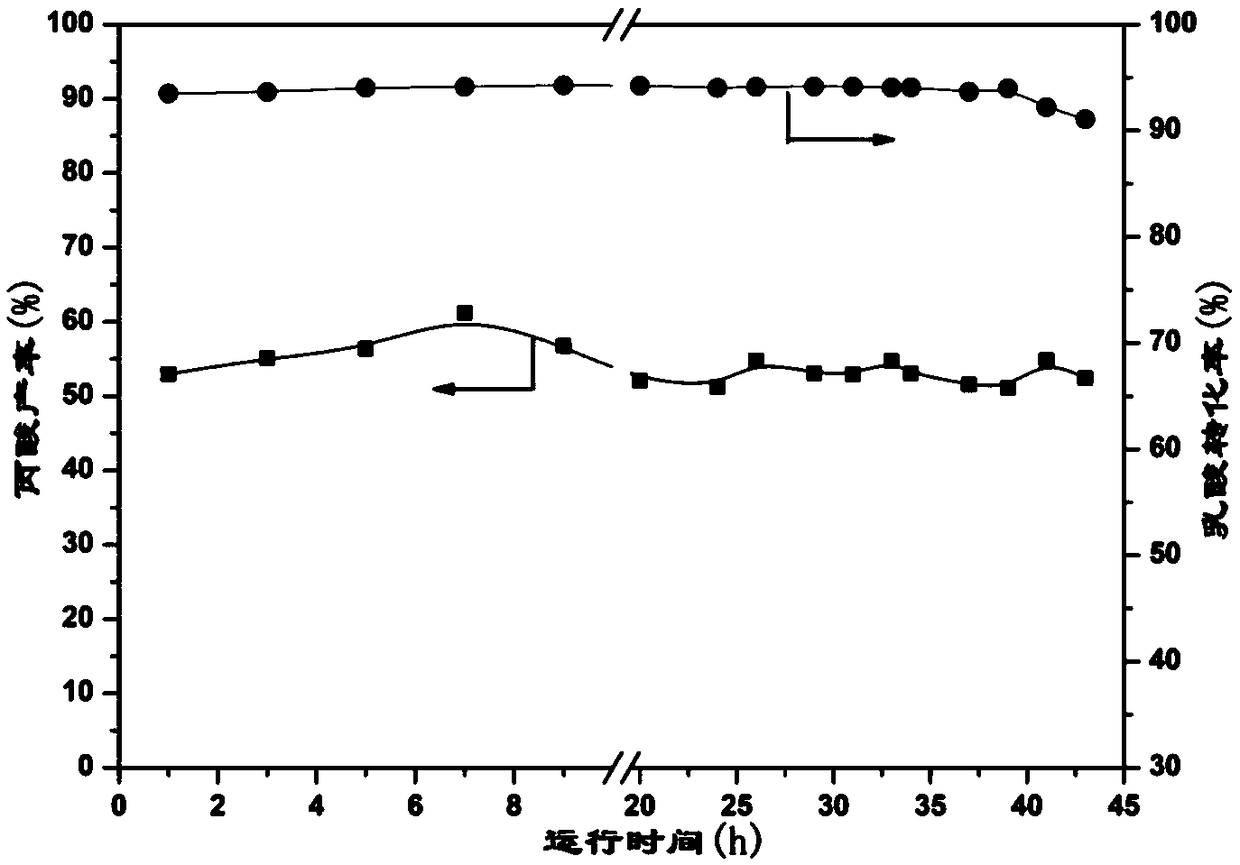
A method for synthesizing benzothieno[3,2-b]chromones through ionic liquid-promoted intramolecular cross dehydrogenation coupling
InactiveCN104725399BImprove economySimple and mild conditionsOrganic chemistryCouplingDehydrogenation
Owner:XINJIANG UNIVERSITY
The preparation method of diphenhydramine
InactiveCN104892435BNo pollution in the processYield unchangedOrganic compound preparationAmino-hyroxy compound preparationBenzeneDibutyltin oxide
The invention relates to a preparation method of diphenhydramine, which takes benzhydrol and dimethylaminoethanol as raw materials, under existence of dibutyltin oxide and ion liquid compound, benzhydrol and dimethylaminoethanol are subjected to an intermolecular dehydration reaction to obtain diphenhydramine. The method has the advantages of simple preparation technology, mild reaction condition, low cost, high product yield and high purity.
Owner:JIAXING UNIV
A method for cleaning and preparing 2,4-dichloro-α-chloromethyl benzyl alcohol
ActiveCN103664527BNo pollution in the processYield unchangedAluminium compoundsOrganic compound preparationAluminium hydroxideDistillation
The invention discloses a clean preparation method for 2,4-dichloro-alpha-chloromethyl benzyl alcohol and belongs to the field of catalytic synthesis process of 2,4-dichloro-alpha-chloromethyl benzyl alcohol. 2,2',4'-trichloroacetophenone is taken as an initial raw material, aluminium isopropoxide serves as a catalyst, isopropyl alcohol serves as not only a solvent but also a reactant, and reduction reaction, reduced pressure distillation, acidation, water washing and curing are conducted to obtain the 2,4-dichloro-alpha-chloromethyl benzyl alcohol. An isopropyl alcohol and acetone mixed solution obtained through reduced pressure distillation is rectified, 80-84 DEG C fraction is collected for recycling, and the yield is basically kept unchanged. Acidized acid water is neutralized by ammonia water, then filtered to obtain aluminium hydroxide, and finally roasted to acquire aluminium oxide after being dried. The method is mild in reaction conditions, short in reaction time and convenient in after-treatment, and avoids environmental pollution. The conversion rate and the product yield of 2,2',4'-trichloroacetophenone are more than 99%, and the manufacturing cost is low. The method is an efficient and environmental-friendly method for synthesizing 2,4-dichloro-alpha-chloromethyl benzyl alcohol and facilitates large-scale industrial production.
Owner:张家港市乐余科创园投资发展有限公司
Pure aqueous phase synthesis method of β-aminocarbonyl compound
InactiveCN103467319BReduce dosageEasy to separateOrganic chemistryOrganic compound preparationKetoneSalicylic acid derivatives
The invention provides a method for synthesizing a beta-amino-carbonyl compound by a pure water phase. Water is used as a reacting solvent, aldehydes, amines and ketones are used as raw materials, and salicylic acid or salicylic acid derivatives is or are adopted as a catalyst to react. The method opens a new low-cost, green and effective way to the preparation of beta-amino-carbonyl compounds, and has the advantages that a target product is good in selectivity and high in yield; the catalyst is small in dose and does not need to be separated from the solvent after the reaction is completed, and can be recycled directly in an aqueous solution manner; experiment results prove that the catalyst still has high catalytic activity after being recycled for 5 times, and the yield of the product is basically the same.
Owner:SHAANXI NORMAL UNIV
Method for preparing D-(+)-biotin intermediate
ActiveCN101735242BEasy to buyIncreased riskOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsBenzoyl bromideBenzaldehyde
The invention provides a method for preparing a D-(+)-biotin intermediate. The method comprises the steps of: taking L-cysteine monohydrochloride as a starting material; using benzaldehyde and sodium cyanate as a ring closure reagent to synthesize (7aR)-3-phenyl-6-benzyl-1H, 3H-imidazo[1, 5-C]thiazole-(6H, 7aH)-5, 7-dione through the cyclization; then utilizing benzyl bromide to perform benzyl protection on N atoms; then taking zinc as a reducing agent to perform ring-opening synthesis on N, N-dibenzyl-L-sulfhydryl hydantoin; introducing a side chain through an esterification reaction with monomethyl adipate acyl chloride; and taking titanium as the reducing agent to perform reductive ring closure to generate the intermediate. According to the method, the cheap and readily available sodium cyanate is used as the ring closure reagent to replace sodium isocyanate which is toxic and difficult to purchase in an original method, reaction conditions are optimized and reaction order is adjusted, so the disadvantages of harsh reaction conditions and low yield in the original method of ring opening first and then benzyl protection are overcome by the method of performing benzyl protection on the N atoms of an imidazole part first and then performing ring opening; and the total yield reaches 34.0-38.0 percent.
Owner:安徽泰格生物技术股份有限公司
Synthetic method and application of magnetic catalyst for preparing furfuryl alcohol through catalytic hydrogenation of furfural
PendingCN113292520ALow toxicityImprove protectionOrganic chemistryCatalyst activation/preparationPtru catalystHydrogen pressure
The invention discloses a method for preparing furfuryl alcohol through catalytic hydrogenation of furfural and a magnetic catalyst thereof. The magnetic catalyst is a supported catalyst, an active component Co of the magnetic catalyst comes from a corresponding non-noble metal salt solution, and a catalyst carrier is an oxide selected from Al or Nb oxides. The catalyst is prepared by adopting a simple excessive impregnation method, and the loading capacity of the active component is 20% of the mass of the corresponding carrier. According to the method, a biomass hydrolysate furfural is adopted as a raw material, commercial hydrogen is adopted as a hydrogen source, deionized water is adopted as a solvent, the reaction temperature is 100-140 DEG C, the hydrogen pressure is 1-2 MPa, the stirring speed is 400 rpm, and the reaction time is 1-4 h. Under optimal conditions, the conversion rate of furfural is close to 100%, and the selectivity of furfuryl alcohol is 96.89%. The catalyst is simple and convenient in preparation method, low in cost and cyclical, the catalytic system is green, the reaction substrate is renewable, the reaction condition is mild, the cost is low, and the catalyst has a wide industrial application prospect.
Owner:XIAMEN UNIV
A new wet corn soaking process
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Molybdenum-based catalyst, preparation method and application thereof
ActiveCN108993479BStrong reductionImprove hydrogenolysis capacityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPhysical chemistry
The invention relates to the technical field of catalytic materials, and in particular relates to a molybdenum-based catalyst, and a preparation method and application thereof. The preparation methodof the molybdenum-based catalyst comprises the following steps: adding a molybdenum source into an H2O2 / CH3CH2OH mixed solution, and carrying out stirring to form a reaction system; heating the reaction system to 50-70 DEG C, adding a protein assistant, continuously carrying out stirring, and then evaporating water and ethanol at a temperature of 80-100 DEG C to obtain a catalyst precursor; and filling the catalyst precursor under a nitrogen atmosphere, carrying out heating to 300-450 DEG C, then introducing mixed steam of lactic acid and water into the catalyst precursor, and reducing the catalyst precursor to prepare the molybdenum-based catalyst. The molybdenum-based catalyst prepared by the method is simple to prepare, and the catalyst is clean and non-toxic, is high in catalytic activity, good in stability and low in cost, and has a potential commercial development value.
Owner:CHONGQING UNIV OF TECH
Acidic ionic liquid catalysis method for preparing phenyl ethyl phenyl ethane capacitor insulating oil
ActiveCN103319297BAffect qualityHigh activityLiquid organic insulatorsHydrocarbon from halogen organic compoundsCatalytic methodIonic liquid
The invention provides a method for preparing phenyl ethylbenzene ethane capacitor insulating oil through catalysis of acidic ionic liquid. The method is characterized in that 2-10mol of ethylbenzene reacts with 1mol of chlorine gas to generate a mixture of alpha-chloroethylbenzene and beta -chloroethylbenzene at 110 DEG C-150 DEG C through the ultraviolet irradiation or under the triggering action of a radical initiator, and the mixture generated through reaction is not separated and is directly mixed with a mixture of 5-20g of acidic ionic liquid catalyst and 2-10mol of ethylbenzene so as to react at 30-130 DEG C to prepare the phenyl ethylbenzene ethane. The phenyl ethylbenzene ethane capacitor insulating oil prepared by the method has excellent characteristics of normal temperature insulation oil, namely phenyl xylyl ethane; the condensation point of the phenyl ethylbenzene ethane capacitor insulating oil is far lower than that of the phenyl xylyl ethane; and the phenyl ethylbenzene ethane capacitor insulating oil has unique low-temperature-resistance performance and is particularly suitable for serving as low-temperature capacitor insulating oil.
Owner:SICHUAN DONGFANG INSULATING MATERIAL
A method of improving the resistance of bacterial strains to inhibitors by using micro-aeration
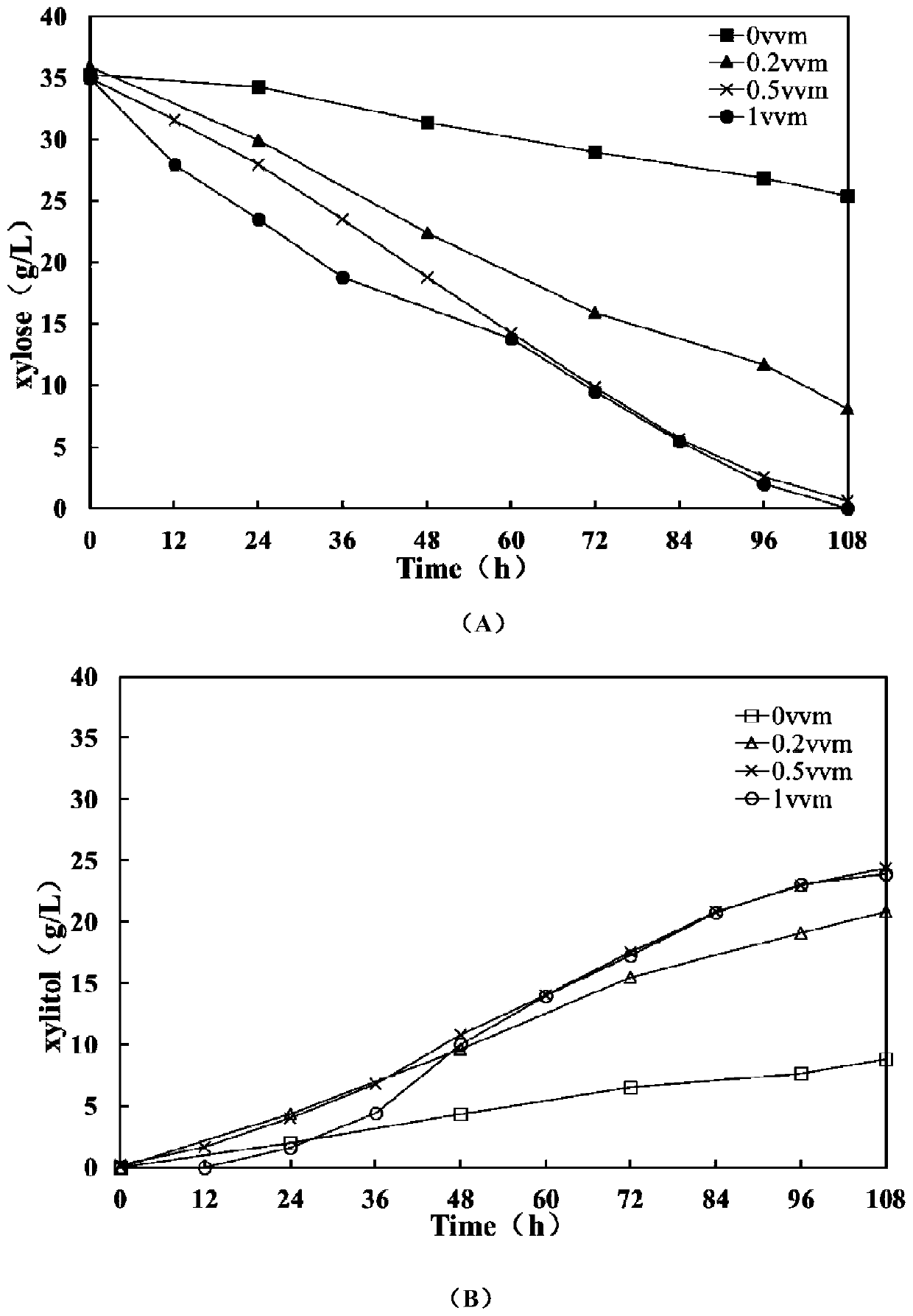
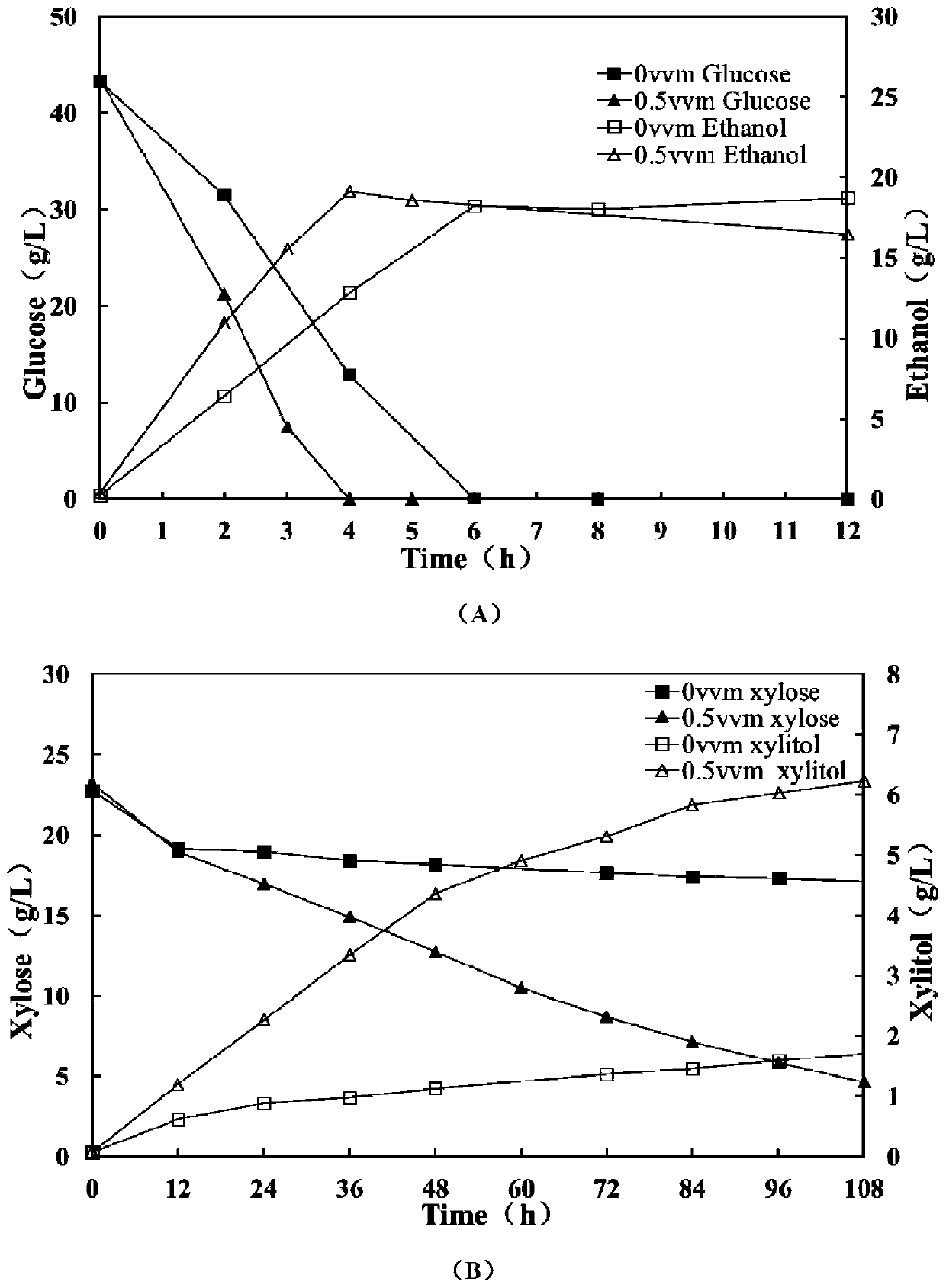
ActiveCN106480106BShorten fermentation timeLow costBiofuelsMicroorganism based processesHigh concentrationXylose fermentation
The invention provides a method for improving resistance of a bacterial strain to inhibitors through micro aeration. The micro aeration is conducted, so the resistance of the bacterial strain to the various inhibitors (formic acid, acetic acid, furfural and hydroxymethylfurfural) in a cellulosic hydrolysate is improved, and the fermentation performance of glucose and xylose are significantly improved. Xylose fermentation conducted by using the method is compared with a control group without ventilation, the xylose has resistance to the various inhibitors in the cellulosic hydrolysate, under the conditions of four kinds of mixed inhibitors with relatively high concentrations, the fermentation time is shortened, residual xylose is decreased greatly and the yield of xylitol is improved. The fermentation of glucose and xylose mixed carbon sources by using the method is compared with a control group without ventilation, under the conditions of four kinds of mixed inhibitors with relatively high concentrations, the method promotes the simultaneous fermentation of glucose and xylose, the yield of ethyl alcohol remains unchanged, and the yield of xylitol is improved.
Owner:DALIAN UNIV OF TECH
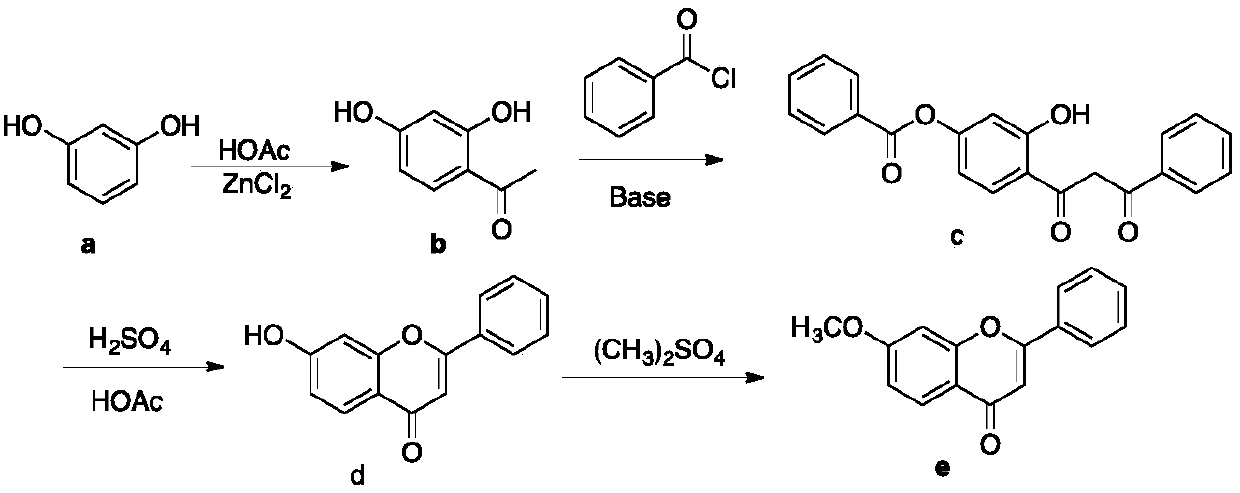
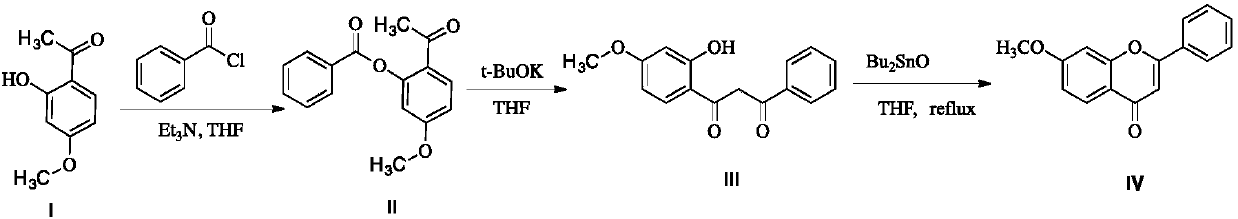
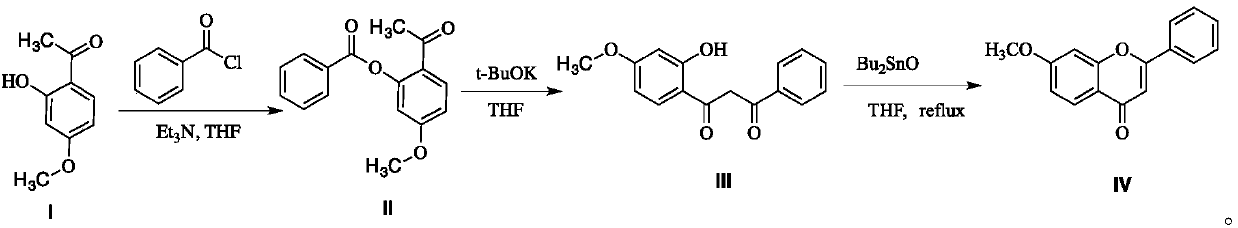
A kind of preparation method of 7-methoxyflavone
InactiveCN106083793BYield unchangedNo pollution in the processOrganic chemistryChemical synthesisFiltration
The invention discloses a preparation method of 7-methoxyflavone and belongs to the field of chemical synthesis. The preparation method comprises that paeonol and benzoyl chloride as raw materials undergo an esterification reaction in the presence of triethylamine base, then the reaction mixed solution is subjected to simple suction filtration so that triethylamine hydrochloride is removed, potassium tert-butoxide is directly added into the filtrate, the mixture undergoes a reaction at the normal temperature, dibutyltin oxide is added into the reaction product, and the formed reaction mixed solution is subjected to heating backflow dehydration so that 7-methoxyflavone is obtained. The technical route has the characteristics of less process, operation simpleness, no pollution, desiccant reuse and industrial production easiness. The preparation method is an economic and simple 7-methoxyflavone preparation method.
Owner:JIAXING UNIV
Catalytic cracking process for reducing content of olefin in gasoline
InactiveCN1141361CReduce olefin contentReduce yieldCatalytic crackingGas compressorReaction temperature
A catalytic cracking process for lowering the olefin content of gasoline features that the condensed oil of rich gas compressor and / or the light gasoline fraction pumped from the top of stablizing tower are filled in the lifting tube reactor at the uppers tream of raw oil nozzle, or in conjunctino with the raw oil from raw oil nozzle to make it in contact with regenerated catalyst and take part in reaction, the resultant flows upward along the lifting tube, the oil gas is quickly separated from the catalyst at outlet of lifting tube to recover the resultant, and the catalyst is stripped and regenerated for reuse. The content of olefin can be reduced by 10-20 v%.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


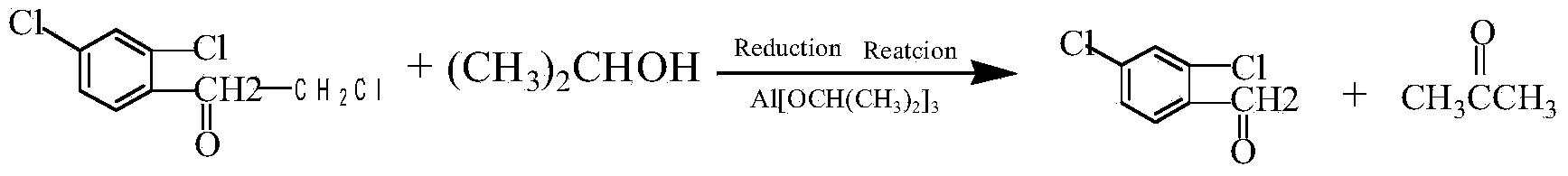
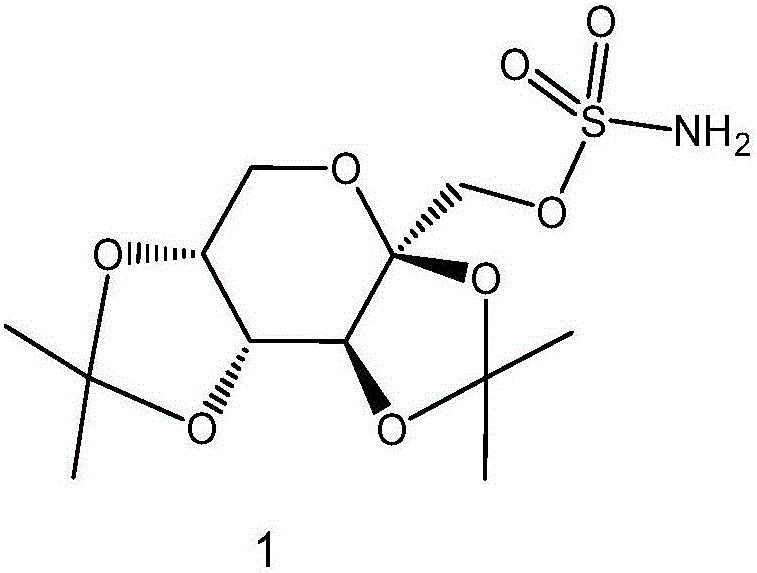

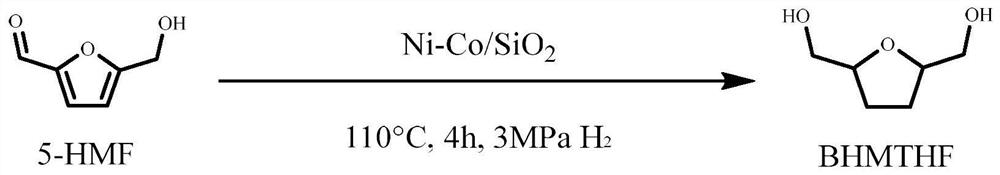

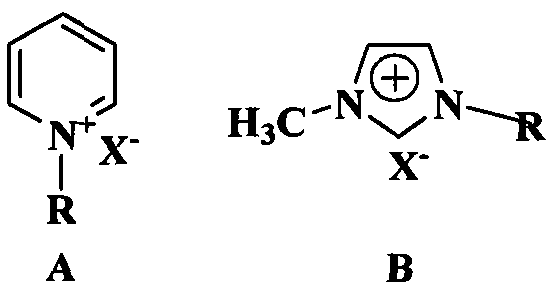
![A method for synthesizing benzothieno[3,2-b]chromones through ionic liquid-promoted intramolecular cross dehydrogenation coupling A method for synthesizing benzothieno[3,2-b]chromones through ionic liquid-promoted intramolecular cross dehydrogenation coupling](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/03bcaf22-5c25-4362-aec3-0b496751083b/150416212310.png)
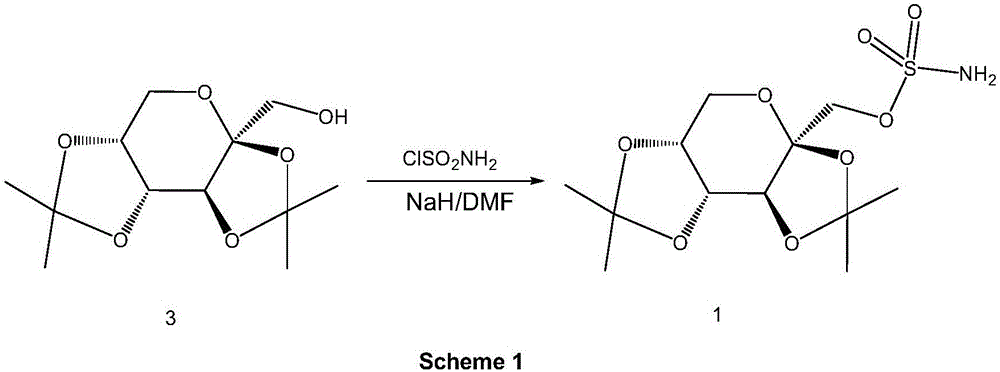
![A method for synthesizing benzothieno[3,2-b]chromones through ionic liquid-promoted intramolecular cross dehydrogenation coupling A method for synthesizing benzothieno[3,2-b]chromones through ionic liquid-promoted intramolecular cross dehydrogenation coupling](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/03bcaf22-5c25-4362-aec3-0b496751083b/150416212334.png)
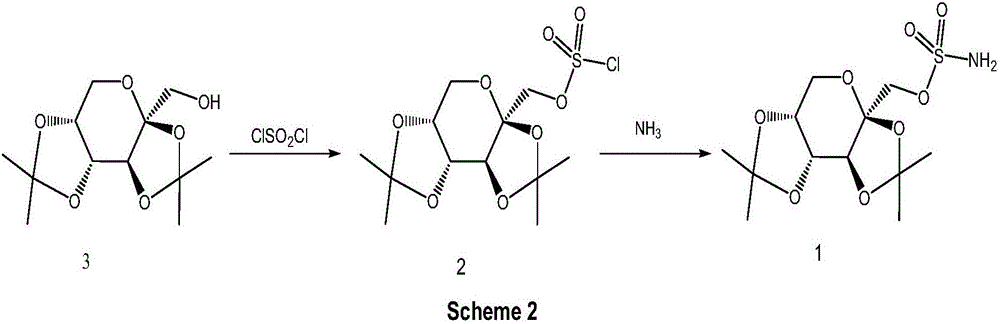
![A method for synthesizing benzothieno[3,2-b]chromones through ionic liquid-promoted intramolecular cross dehydrogenation coupling A method for synthesizing benzothieno[3,2-b]chromones through ionic liquid-promoted intramolecular cross dehydrogenation coupling](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/03bcaf22-5c25-4362-aec3-0b496751083b/150416212344.png)