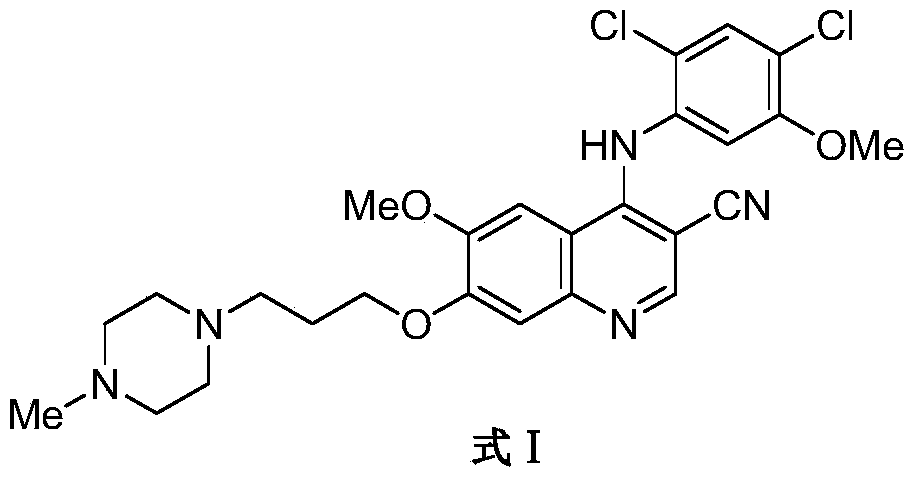
Preparation method of bosutinib
A technology of methoxyl and quinolinecarbonitrile, applied in the field of medicine and chemical industry, can solve the problems of low yield, achieve the effects of improving reaction conditions, stable properties, and facilitating the control of drug quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
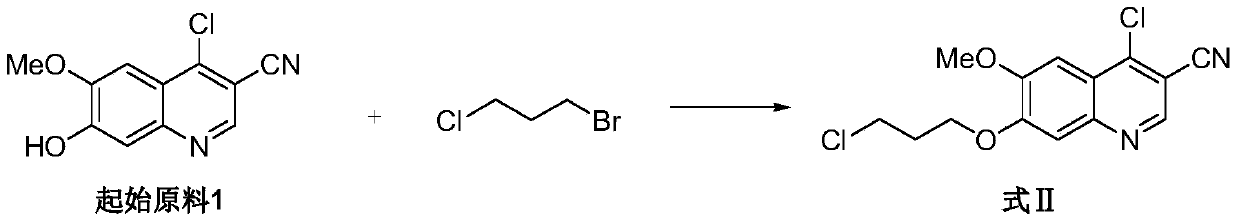
[0034] Embodiment 1: Preparation of 4-chloro-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile
[0035] Under nitrogen protection, add 33L of 1-methyl-2-pyrrolidone into a 50L enamel reaction kettle, and then add 3.30kg of 4-chloro-6-methoxy-7-hydroxy-3-quinolinecarbonitrile, 6.65kg of 1-bromo -3-chloropropane, 5.83kg anhydrous potassium carbonate, 99g dibenzo-18-crown-6-ether (4-chloro-6-methoxy-7-hydroxyl-3-quinolinecarbonitrile and 1-bromo The molar ratio of -3-chloropropane is 1:3); Under the protection of nitrogen, the temperature is controlled at 30°C-40°C and the reaction is stirred for more than 4 hours. TLC tracking monitors to 4-chloro-6-methoxy-7-hydroxyl-3- The quinolinecarbonitrile spots disappeared; the reaction solution was transferred to a 200L glass-lined reactor, and 66L of purified water I was slowly added under stirring at a temperature of 25-40°C; after the addition, the temperature was controlled at 25-35°C and stirred for 30 minutes; Wash with 10 L ...
Embodiment 2
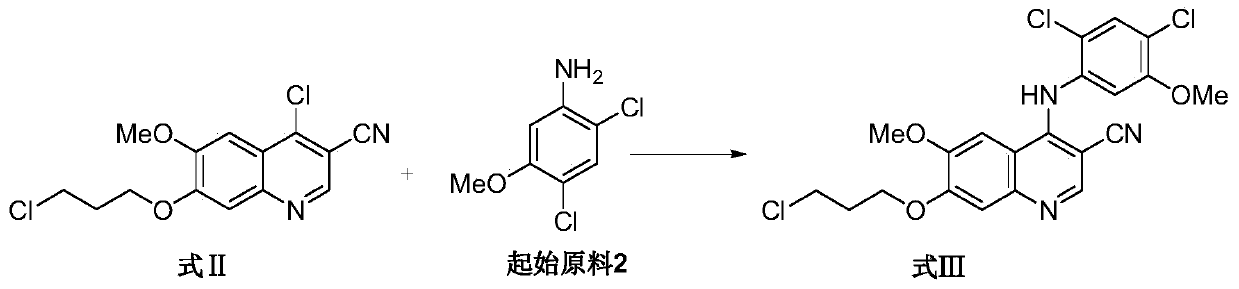
[0036] Example 2: 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile preparation
[0037] Under the protection of nitrogen, 20L dimethylacetamide was added to a 30L glass reactor, and 3.70kg2,4-dichloro-5-methoxyaniline, 1.48kg pyridine hydrochloride, 4.00kg4-chloro- 6-Methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile (4-chloro-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile The molar ratio of nitrile to 2,4-dichloro-5-methoxyaniline is 2:3), stirred and heated at 90-100°C for more than 3 hours, followed by TLC until the spot of intermediate 1 disappeared. Cool the reaction solution to 20-30°C, transfer it to a 200L glass-lined reactor, add 40L ethyl acetate, slowly add 75L purified water under stirring, add 1M hydrochloric acid (prepared by 400mL concentrated hydrochloric acid and 4.5L purified water) to adjust the pH value to 2 , stirred for 30 min, filtered by rejection, and the filter cake was washed with 12L of purified...
Embodiment 3
[0038] Example 3: 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile preparation
[0039] Under the protection of nitrogen, 20L of 1-methyl-2-pyrrolidone was added to a 30L glass reactor, and 3.70kg of 2,4-dichloro-5-methoxyaniline, 1.48kg of pyridine hydrochloride, 4.00kg of 4- Chloro-6-methoxy-7-(3-chloropropoxy)-3-quinolinecarbonitrile (4-chloro-6-methoxy-7-(3-chloropropoxy)-3-quinonitrile The molar ratio of phenonitrile to 2,4-dichloro-5-methoxyaniline is 2:3), stirred, heated and controlled at 90-100°C for more than 3 hours, followed by TLC until the spot of intermediate 1 disappeared. Cool the reaction solution to 20-30°C, transfer it to a 200L glass-lined reactor, add 40L ethyl acetate, slowly add 75L purified water under stirring, add 1M hydrochloric acid (prepared by 400mL concentrated hydrochloric acid and 4.5L purified water) to adjust the pH value to 2 , stirred for 30 min, filtered by rejection, and the filter cake was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com