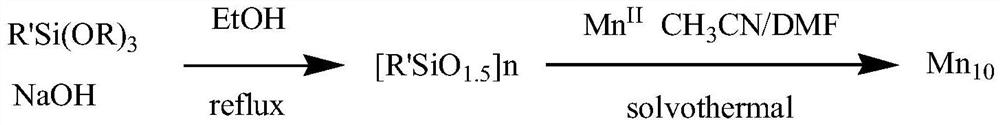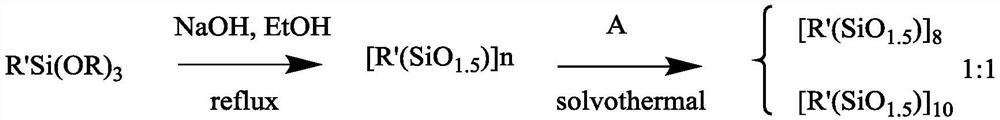Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49results about How to "Synthetic method is convenient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ultrafine protein composite nanoparticle with near-infrared photothermal effect and multi-mode imaging function and preparation method and application thereof
ActiveCN107551279AHigh relaxation rateStrong X-ray attenuation abilityEnergy modified materialsEchographic/ultrasound-imaging preparationsBismuth sulfideComputed tomography
The invention discloses a bismuth sulfide / gadolinium oxide-entrapping ultrafine protein composite nanoparticle with a near-infrared photothermal effect and a multi-mode imaging function and a preparation method and application thereof. By formulation screening and process optimization, the bismuth sulfide / gadolinium oxide albumin ultrafine composite nanoparticle with the near-infrared photothermaleffect and the multi-mode imaging function is prepared in one step. The composite nanoparticle has good physicochemical stability, photostability, biocompatibility and tumor-targeting property, can remarkably enhance near-infrared fluorescence, photoacoustic, magnetic resonance, CT (computed tomography) and thermal signals at a tumor site, and realizes multi-mode-complemented tumor diagnosis, andmoreover, the composite nanoparticle can eliminate tumors under the excitation of near-infrared light; in particular, because the grain size of the nanoparticle is less than 5.5nm, the nanoparticle can be eliminated by the kidneys, and toxic and side effects are minor; a tumor can be diagnosed and treated at the same time, and the composite nanoparticle has a great potential in realizing accuratetumor diagnosis and treatment integration.
Owner:SUZHOU UNIV
Mesoporous polyionic liquid catalyst for normal-pressure CO2 cycloaddition reaction as well as preparation method and application thereof
ActiveCN109939731ALarge specific surface areaThe synthesis method is simpleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsStyrene oxideEpoxy
The invention provides a mesoporous polyionic liquid catalyst for normal-pressure CO2 cycloaddition reaction as well as a preparation method and application thereof and relates to the technical fieldof the preparation of multiphase catalysts in organic chemical industry. A binuclear polyionic liquid polymerization monomer [C1DVIM]Br is prepared from N-vinyl imidazole and dibromomethane through solvothermal reaction, an initiator is added into a polyethylene glycol-water solvent system, and the free radical polymerization reaction is finished by virtue of the polymerization monomer. Accordingto the method, cheap polyethylene glycol is taken as a solvent, a small amount of water is taken as a cosolvent, the mesoporous polyionic liquid prepared through self-polymerization of the initiator has a typical polycationskeleton, rich halogen sites and good heat stability, can be taken as a nonmetal heterogeneous catalyst to be applied to CO2 cycloaddition reaction for oxidizing epoxy compoundssuch as styrene oxide as substrates under a normal pressure and presents excellent catalytic activity and recycling stability.
Owner:HEFEI UNIV
Method for preparing laminated Li-Ni-Mn-O compoiste material and its application
InactiveCN1688045ALow priceIncrease capacityElectrode manufacturing processesLithium compoundsNickelNH3 compound
This invention relates to a preparation method for a laminar composite of LiNiMnO and the application in Li ionic battery. The method includes the following steps: 1, weighing soluble Ni salt and Mn salt to be solved in distilled water and matched to a mixed solution A 2, matching NaOH and ammonia water into solution B in the proportion of 2:1, 3, adding A, B in a reaction pot at the same time to form solution c, regulating its PH value to 11-13 and heating it, 4, filtering and drying the reacted material to get a precursor block D, 5, weighing Li salt in terms of the proportion of D and Li salt to be ground with D to get a mixture E 6, sintering E in two parts to get the final product.
Owner:上海瀛正科技有限公司
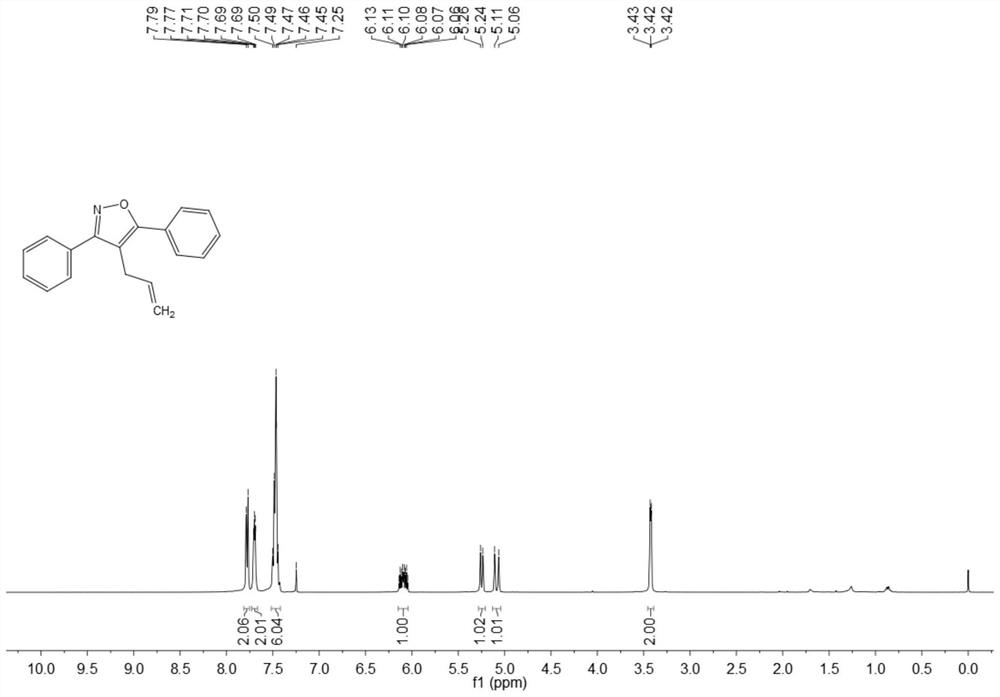
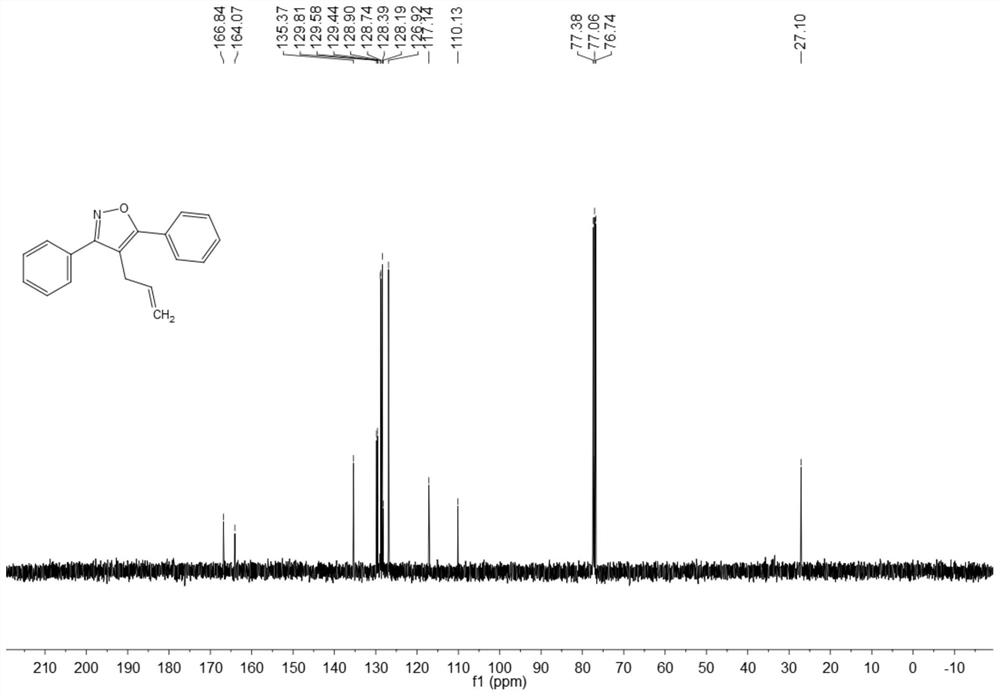
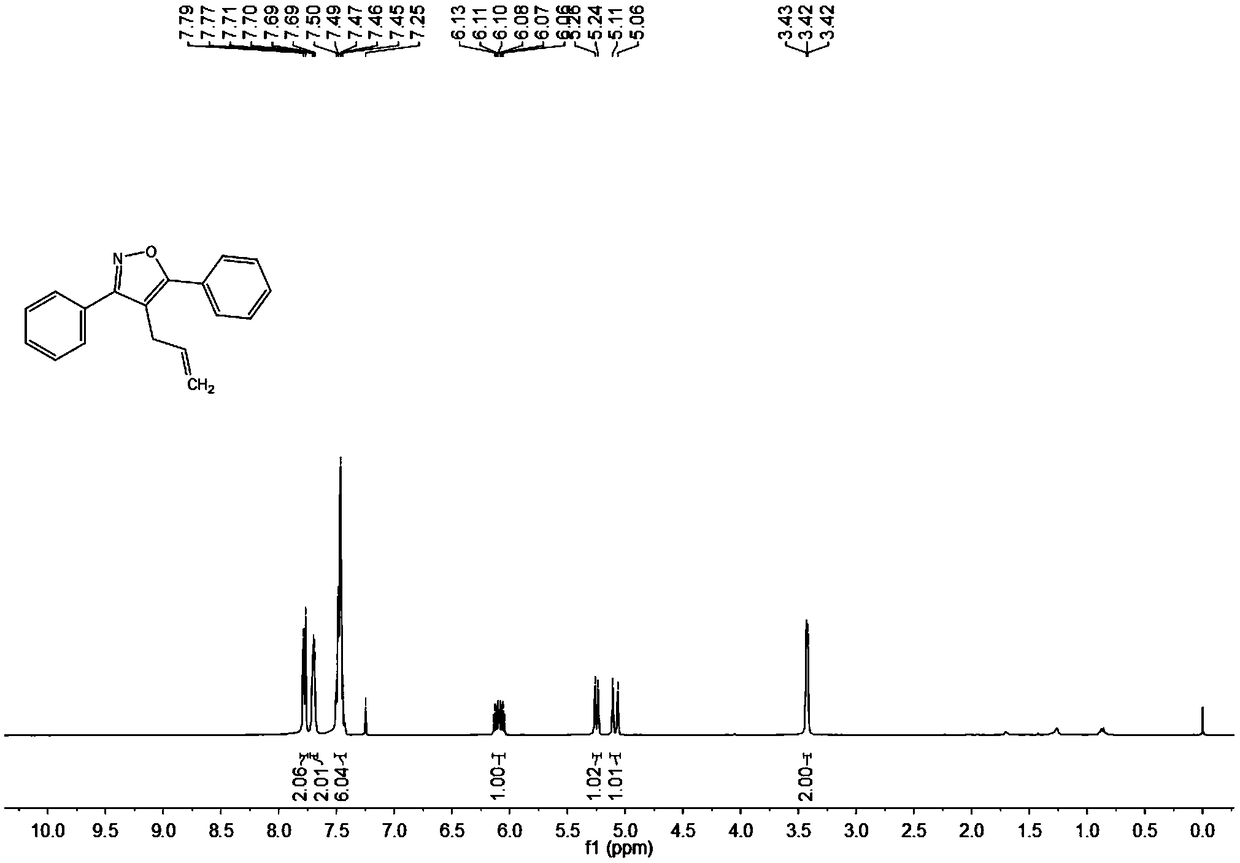
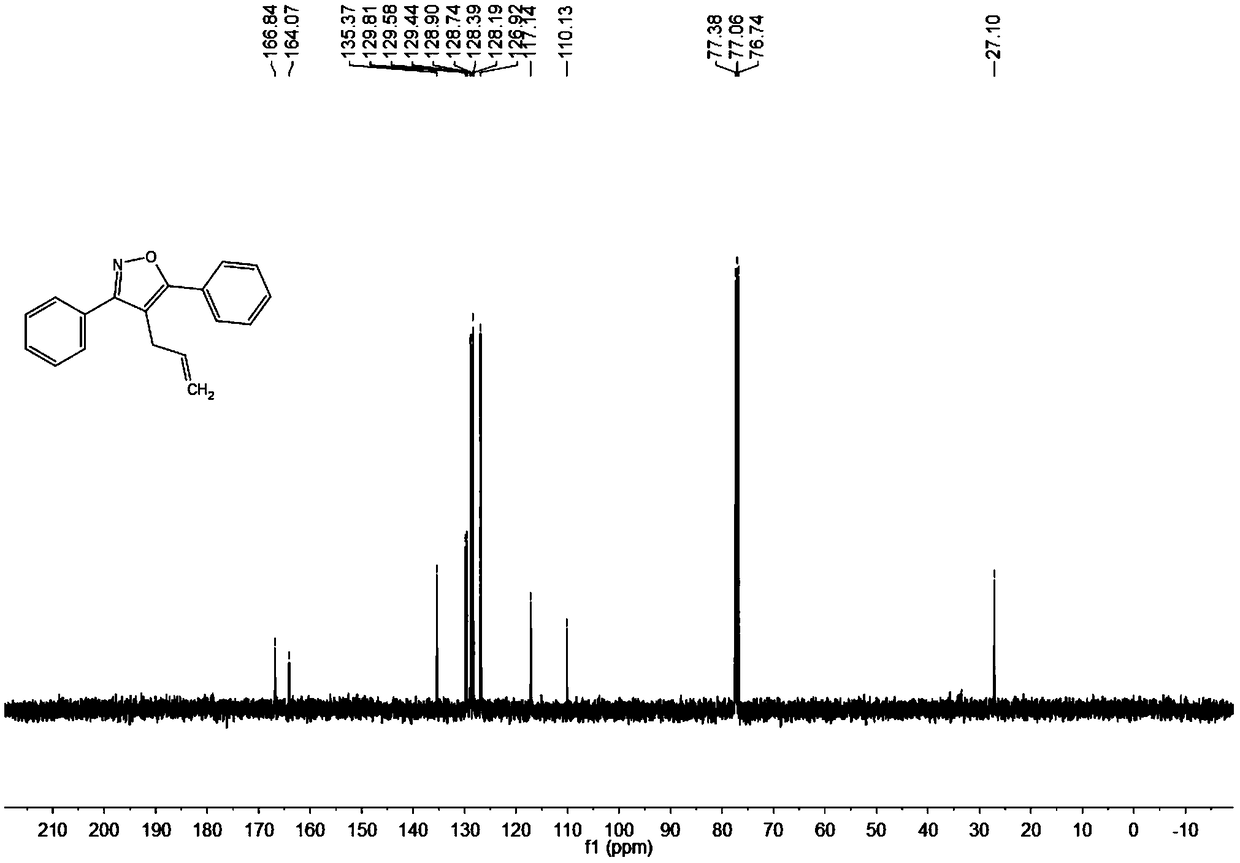
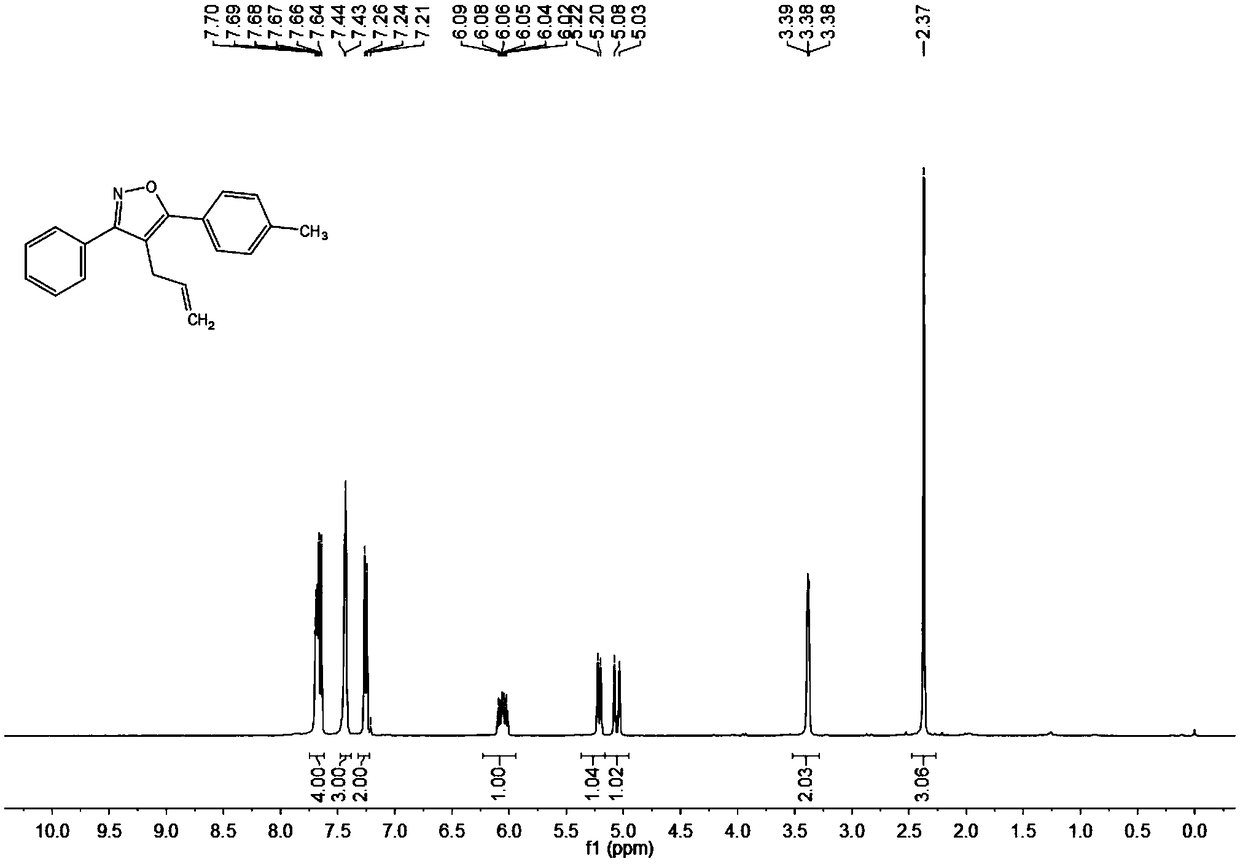
Synthesis method of 4-allyl-3,5-disubstituted isooxazole
ActiveCN108863969ARaw materials are easy to getInnovativeOrganic chemistryOrganic synthesisSynthesis methods
The invention discloses a synthesis method of 4-allyl-3,5-disubstituted isooxazole, and belongs to the technical field of organic synthesis. The synthesis method is characterized in that in a reactor,acetyenic ketone oxime ether substrates, 3-bromopropylene, palladium catalysts, additives and solvents are added; stirring reaction is performed at 70 to 80 DEG C; a reaction product is separated andpurified to obtain the 4-allyl-3,5-disubstituted isooxazole. The method has the advantages that a product obtained by performing Sonogashira coupling on simple and easy-to-obtain acyl chloride and alkyne, and methoxylamine hydrochloride react to obtain a series of norethisteroneoxime ether; the reaction conditions are mild; no environment pollution exists; a potential functional 4-allyl-3,5-disubstituted isooxazole compound is built. The method has innovativeness and atom economy; the conditions are mild; the operation is safe; the scale can be magnified to 5g level scale without influencingthe yield, so that potential practical values are realized.
Owner:SOUTH CHINA UNIV OF TECH
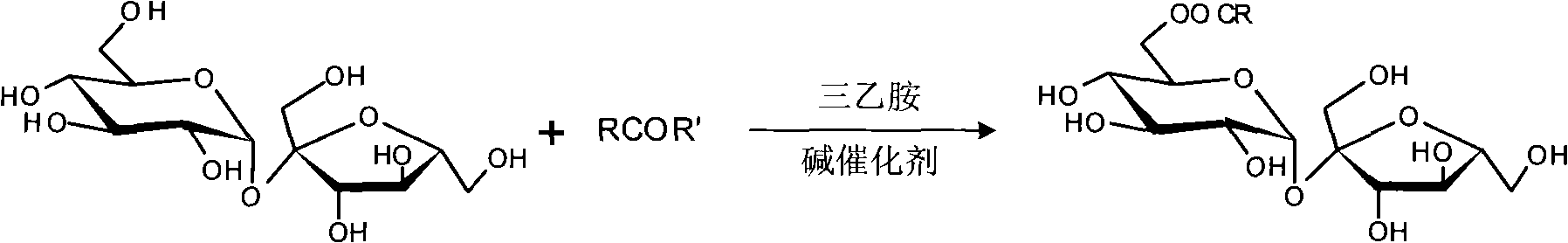
Process for selectively synthesizing sucrose-6-ester
InactiveCN101289475AReduce manufacturing costHigh purityEsterified saccharide compoundsSugar derivativesFood additiveOxime
The invention provides a method for selectively synthesizing sugar-6-ester; wherein, firstly, the secondary amine or oxime (R'H) of five-member ring reacts with acyl chloride (RCOCl) and triethylamine in the environment of aprotic solvent and active amide or active ester is obtained; then the raw material of sugar, the acylating reagent of the prepared active amide or active ester and the catalyst of the triethylamine are stirred and react at indoor temperature, and after the reaction is finished, a base catalyst of intra-molecular shift is added to make the shift of intra-molecular acyl; finally, adhesive column chromatography is adopted for separation to obtain the sugar-6-ester of 6-bit mono-protection. The synthetic method is convenient and simple in operation and the product is high in purity; the heterocyclic parts of 2-thiazole thione, benzotriazole, etc., of the acylating reagent can be recycled, which reduces the production cost of the sugar-6-ester and has a very good practical application meaning for the fields of surfactants, food additives, insecticides, etc.
Owner:CHANGZHOU NIUTANG CHEM PLANT CO LTD +1
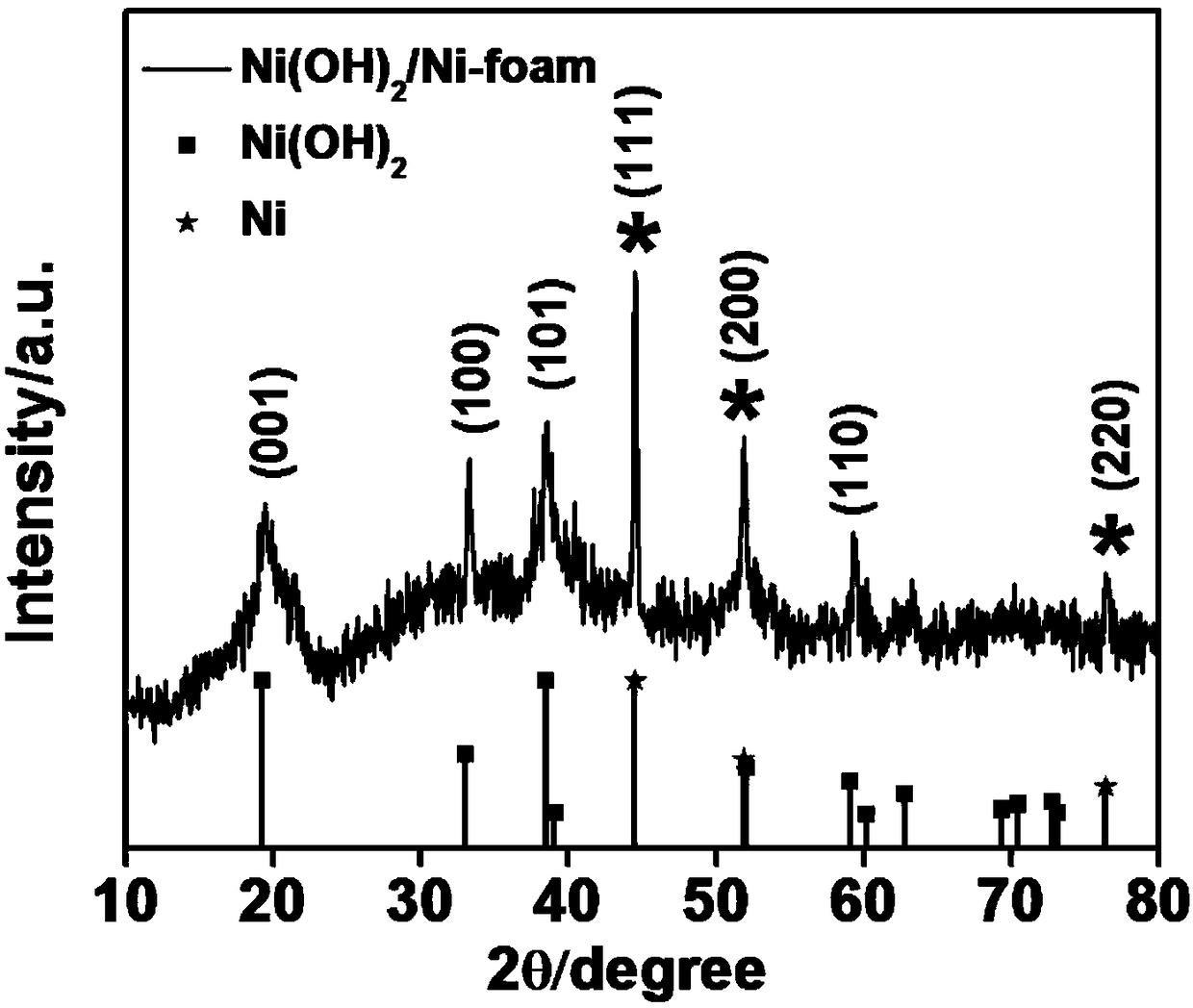
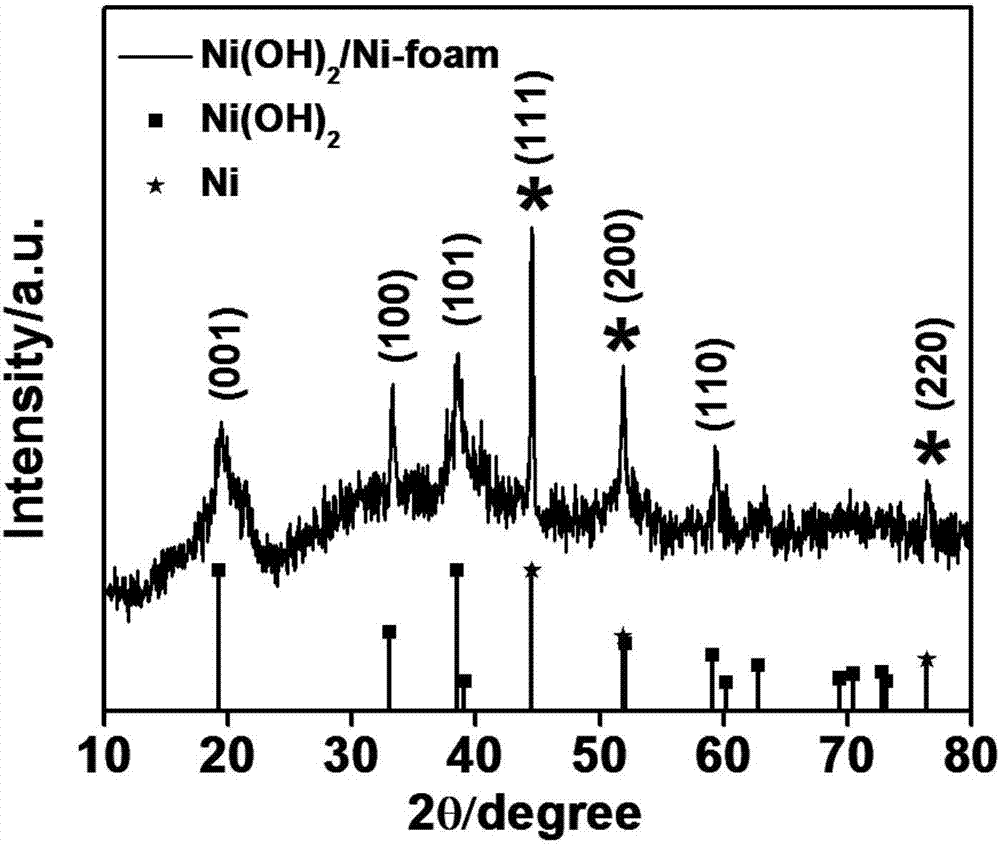
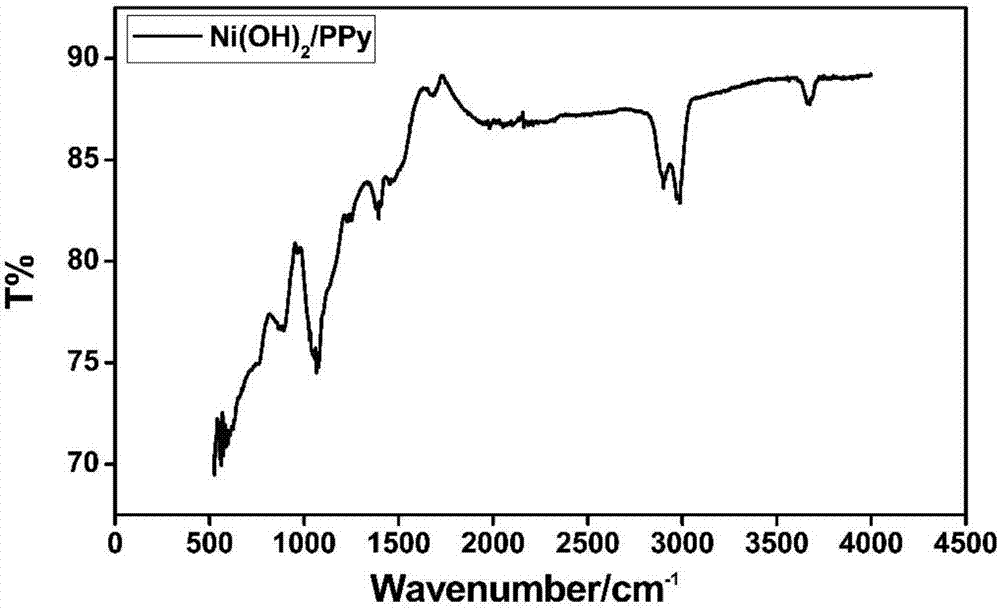
Polypyrrole/nickel hydroxide/foamed nickel integrated electrode and preparation method thereof
ActiveCN106898498AImprove electrochemical performanceGood shape and controllableHybrid capacitor electrodesHybrid/EDL manufactureCapacitanceNitrate
The invention discloses a polypyrrole / nickel hydroxide / foamed nickel integrated electrode and preparation method thereof. The polypyrrole / nickel hydroxide / foamed nickel integrated electrode is formed by loading foamed nickel with polypyrrole and nickel hydroxide, the total load amount of the polypyrrole and the nickel hydroxide on the foamed nickel is 0.9% to 1.4%, and the mass ratio of the polypyrrole to the nickel hydroxide is 1:3 to 2:1. An electrochemical test is performed on the integrated electrode, the electrode has excellent electrochemical performance, as the amount of polypyrrole increases, the specific capacitance of a material increases first and then decreases, and when the molar ratio of nickel nitrate to hexamine is 1:1 and the pyrrole monomer concentration is 0.05mol L<-1>, the specific capacitance of the electrode under the electric current density of 2A g<-1> reaches up to 2174F g<-1>, and the highest specific capacitance retention rate is achieved.
Owner:NANJING UNIV OF SCI & TECH
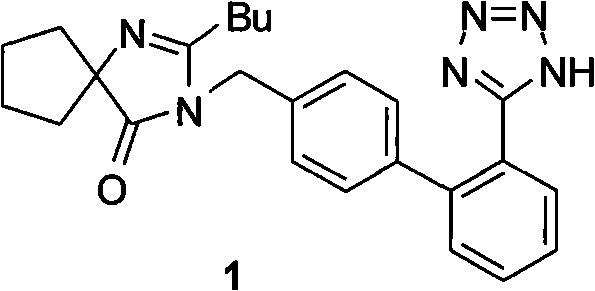
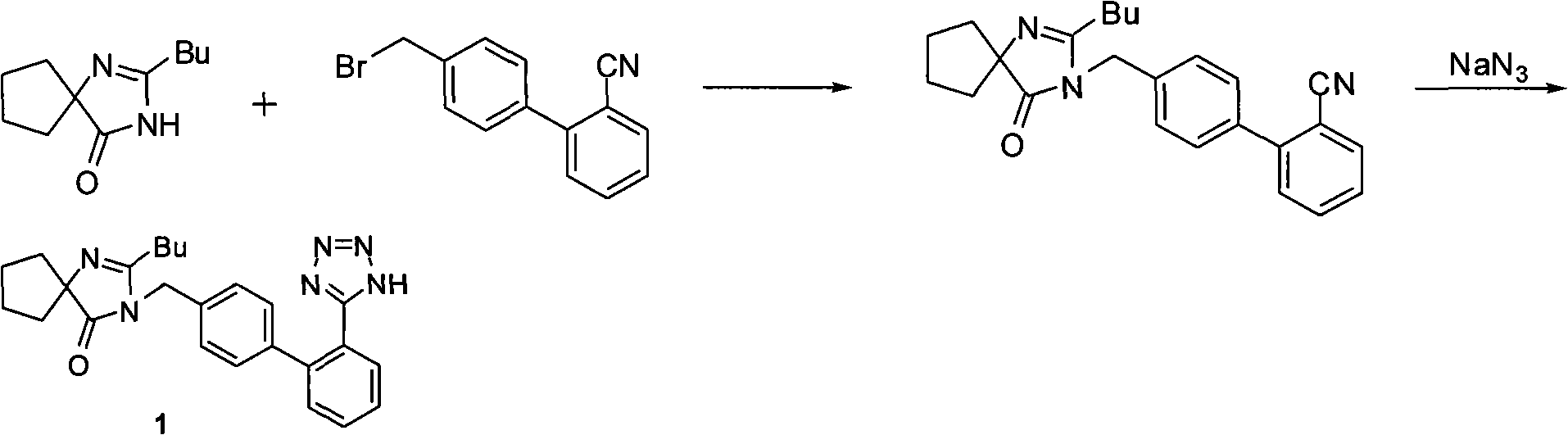
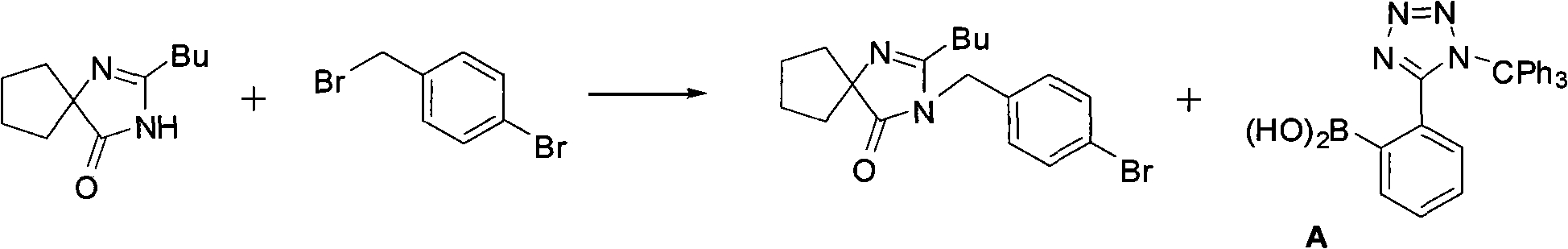
Method of synthesizing losartan and losartan intermediates
InactiveCN102675294APracticalSuitable for industrial productionOrganic chemistrySynthesis methodsDecomposition
The invention relates to a method for synthesizing losartan and losartan intermediates and belongs to the technical field of medicine and medicine intermediates. 5-(4'-bromomethyl biphenyl-2-radical)-2-(1- methyl-1- phenylethyl) tetrazole and 2-butyl-4-chlorine-5-formyl radical imidazole are subjected to condensation, reduction and decomposition protection processes, and the losartan is obtained. The method has the advantages that the synthesis process is suitable for industrial production, in addition, the economic value can be generated, the synthesis process is safe, the raw material cost is saved, subsequent products are easy to treat, the reaction raw materials are single, and the synthesis method is convenient.
Owner:ZHEJIANG TIANYU PHARMA
Boron phosphide single crystals and preparation method and application thereof
ActiveCN110284195AReduce defectsExcellent performancePolycrystalline material growthFrom frozen solutionsHydrogen phosphideSingle crystal
The invention provides boron phosphide single crystal and a preparation method and application thereof. The method comprises the following steps: (1) mixing a boron source, a phosphorus source and a catalyst, placing in a container and vacuumizing, and then sealing; (2) placing the sealed container in step (1) in a reactor, raising the temperature to a first reaction temperature to carry out a reaction, and then lowering the temperature to a second reaction temperature at a rate of temperature fall of 0.06 DEG C / min or below to obtain the boron phosphide single crystal. The preparation method of the boron phosphide single crystal provided by the invention avoids the use of hazardous gases such as hydrogen phosphide; the interaction among the catalyst and the boron source and the phosphorus source is utilized, and high-temperature reaction is first performed and then the temperature is slowly lowered for reaction, so that the separation by crystallization of boron phosphide is gradually realized to obtain the single crystal; the preparation method is a relatively convenient and feasible synthesis method.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Preparation method of visible light response type MIL-100(Fe) photocatalytic composite material
InactiveCN112536070AAvoid the problem of cumbersome thermal preparation processSimple preparation processWater/sewage treatment by irradiationOther chemical processesPhotocatalytic reactionPtru catalyst
The invention relates to preparation of photocatalytic materials, and aims to provide a preparation method of a visible light response type MIL-100(Fe) photocatalytic composite material. The preparation method comprises the following steps: adding trimesic acid and sodium hydroxide in the same parts by mass into deionized water; conducting uniform mixing to obtain a trimesic acid / sodium hydroxidecomposite solution; dropwise adding the composite solution into an equal amount of ferrite solution, then adding a surfactant, and conducting uniform mixing and full reacting to form a turbid liquid;and filtering the turbid liquid, conducting washing, drying the obtained solid to obtain the MIL-100(Fe) photocatalytic composite material. The synthesis method at room temperature is more convenient,the crystallization is more stable, the application stability of the catalyst is greatly improved, and the recycling service life of the catalyst is greatly prolonged. The surfactant surface modification technique effectively reduces the grain size, greatly enhances the specific surface area of the material, and enhances the photocatalytic reaction efficiency. The utilization efficiency of the material on visible light can be remarkably improved, and relatively strong visible light catalytic activity is obtained.
Owner:ZHEJIANG UNIV
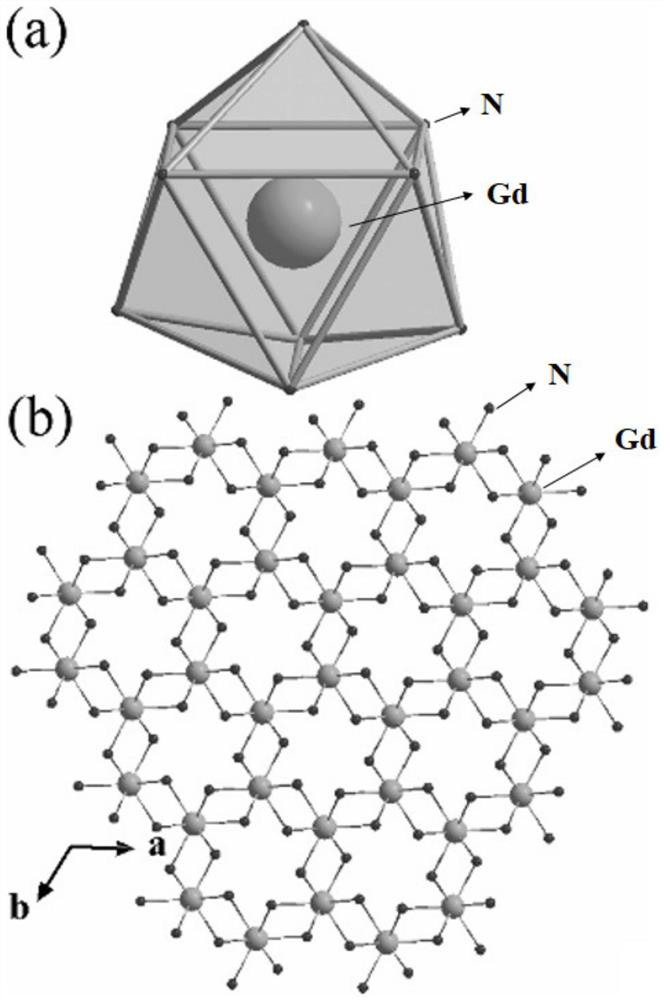
Hydrogen fluoride gadolinium oxide as well as preparation method and application thereof
InactiveCN112456535ASynthetic method is convenientEasy to operateMachines using electric/magnetic effectsRare earth metal compounds preparation/treatmentHydrogen fluorideGadolinium oxide
The invention relates to hydrogen fluoride gadolinium oxide as well as a preparation method and application thereof. The gadolinium oxyfluoride has the following general formula: Gd (OH) yF3-y, wherein y is equal to 0.5-2.5.
Owner:XIAMEN UNIV
Citicoline and synthesizing method of citicoline not using phosphocholine chloride calcium
InactiveCN105693798AReduce usageMild reaction conditionsSugar derivativesSugar derivatives preparationMorpholineChloride
The invention discloses a method for synthesizing citicoline and citicoline without using calcium phosphorylcholine chloride. Acylmorpholine is used as raw material, reacts with ethylene glycol to obtain ethylene glycol ester phosphoromorpholine, and then directly condenses with cytidylic acid to obtain ethylene glycol ester phosphoryl cytidylic acid, and finally tributylamine to ethylene glycol ester The ethylene glycol ester of phosphoromorpholine is ring-opened to give citicoline. There are three steps in the reaction, and the total yield is 78%. The method has cheap and easy-to-obtain raw materials, avoids the use of expensive and toxic DCC and calcium phosphorylcholine chloride, and the yield does not decrease significantly when the reaction scale is expanded to a scale of 500 g. The application of the present invention provides a new synthesis route for the synthesis of citicoline, and has potential application prospects.
Owner:XINXIANG UNIV
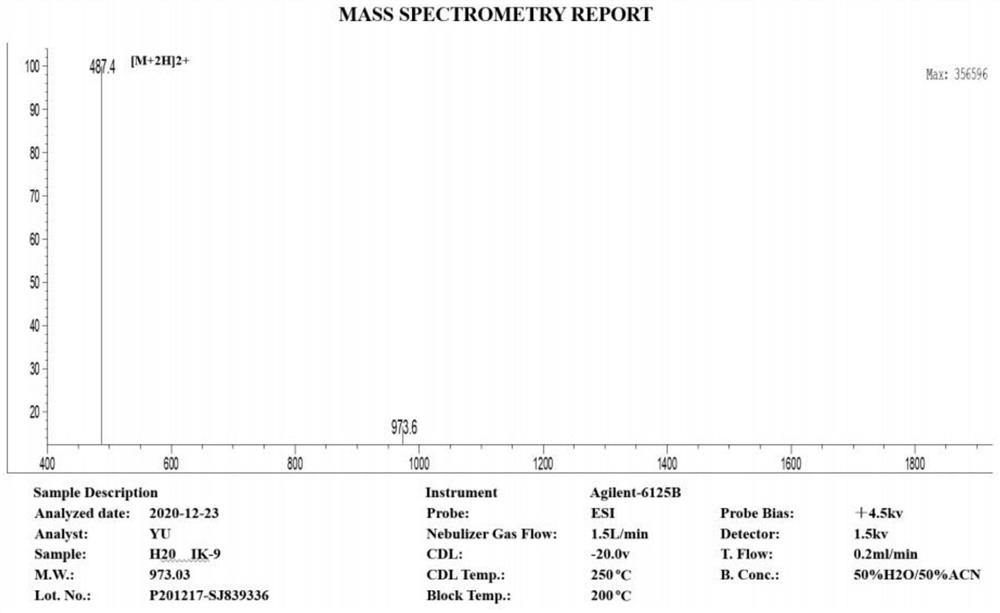
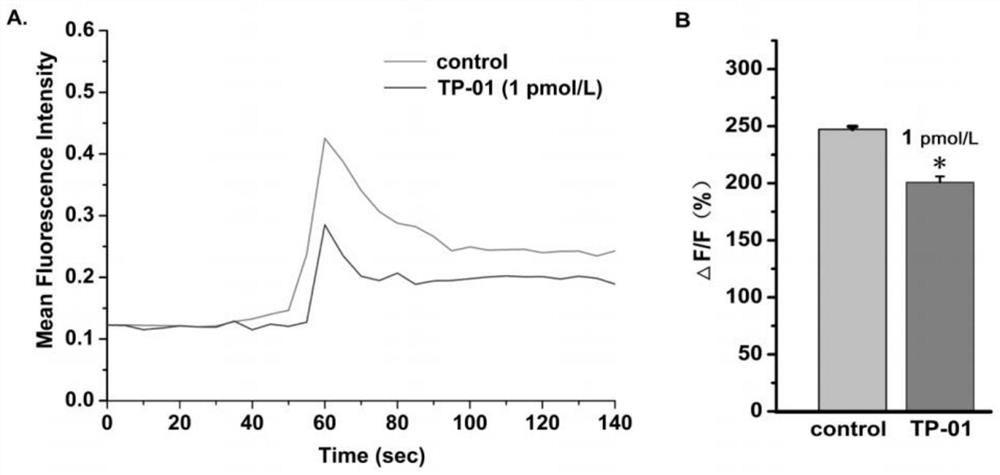
PD-1 targeting peptide with analgesic activity as well as synthesis method and application thereof
ActiveCN113072620ASynthetic method is convenientEasy to operatePeptide/protein ingredientsAntipyreticMolecular biologyAnalgesics drugs
The invention belongs to the technical field of biological medicines, and discloses a small molecule analgesic peptide TP-01 targeting PD-1, the amino acid sequence of the small molecule analgesic peptide TP-01 targeting PD-1 is ISYGGADYK, and the molecular weight of the small molecule analgesic peptide TP-01 targeting PD-1 is 973.04 g / mol. The small molecule polypeptide can be obtained through classical Fmoc-solid phase synthesis, and plays an important role in research and development of analgesic drugs targeting PD-1. The PD-1 targeting peptide has the advantages that the small molecule peptide TP-01 with analgesic activity of the PD-1 is screened by taking the PD-1 as a target and high-throughput screening of an internal peptide library through a molecular docking technology for the first time. The compound provided by the invention can significantly inhibit calcium ion influx induced by a high K < + > solution. Besides, the peptide shows good analgesic activity in a mouse pain model and can effectively relieve inflammatory pain and visceral pain, so that the research blank of analgesic peptide drugs targeting PD-1 is filled, and a new idea and theoretical basis are provided for research and development of the analgesic drugs targeting PD-1.
Owner:NANTONG UNIVERSITY
Functional antibacterial combined medicine and application
PendingCN111658668AImprove bioavailabilityGood biocompatibilityAntibacterial agentsHeavy metal active ingredientsActive agentPhenylboronic acid
The invention provides a functional antibacterial combined medicine and application thereof. The combined medicine comprises the following two independently existing components: aminophenylboronic acid and chloroauric acid trihydrate; and the combined medicine does not contain a surfactant. Aminophenylboronic acid is a medical intermediate; a method for preparing antibacterial gold nanoparticles by using aminophenylboronic acid as a reducing agent in a synthesis process is simple; bacteria is not easily induced to generate drug resistance; relatively high biological safety is achieved; aminophenylboronic acid modified on the surfaces of the gold nanoparticles changes the permeability of bacterial cell walls through the combination of cis-diol and polysaccharides on the surface of bacteriato cause bacterial death, so that the bacterial targeting is relatively high; aminophenylboronic acid and chloroauric acid are stable and easy to store; the whole preparation process is simple and controllable; the prepared antibacterial gold nanoparticles are good in dispersity; and industrial production can be realized.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Composite porous carbon material and preparation method of high-power supercapacitor thereof
InactiveCN108063057ALow costEasy to handleHybrid capacitor electrodesHybrid/EDL manufactureEtchingHigh density
The invention belongs to the technical field of supercapacitors, and particularly relates to a composite porous carbon material and a preparation method of a high-power supercapacitor thereof. The composite porous carbon material comprises the following constituents based on parts by weight: 10-50 parts of graphene oxide, 10-50 parts of sulphonated asphalt and 20-100 parts of potassium hydroxide,wherein the graphene oxide is a product after chemical oxidization and stripping of graphite powder, contains relatively rich oxygen-containing functional groups and is easy to modify, the sulphonatedasphalt has high carbon content and high hydrophilcity, and thus, an asphalt-based porous carbon material with high specific area and high density is easily prepared by alkali activation and etching.A compound is simply dried by a processing preparation method, and the composite porous carbon material can be formed after heating; and moreover, the composite porous carbon material is high in density and large in specific area, and thus, the prepared supercapacitor is high in specific capacity and large in power.
Owner:NINGBO CRRC NEW ENERGY TECH CO LTD
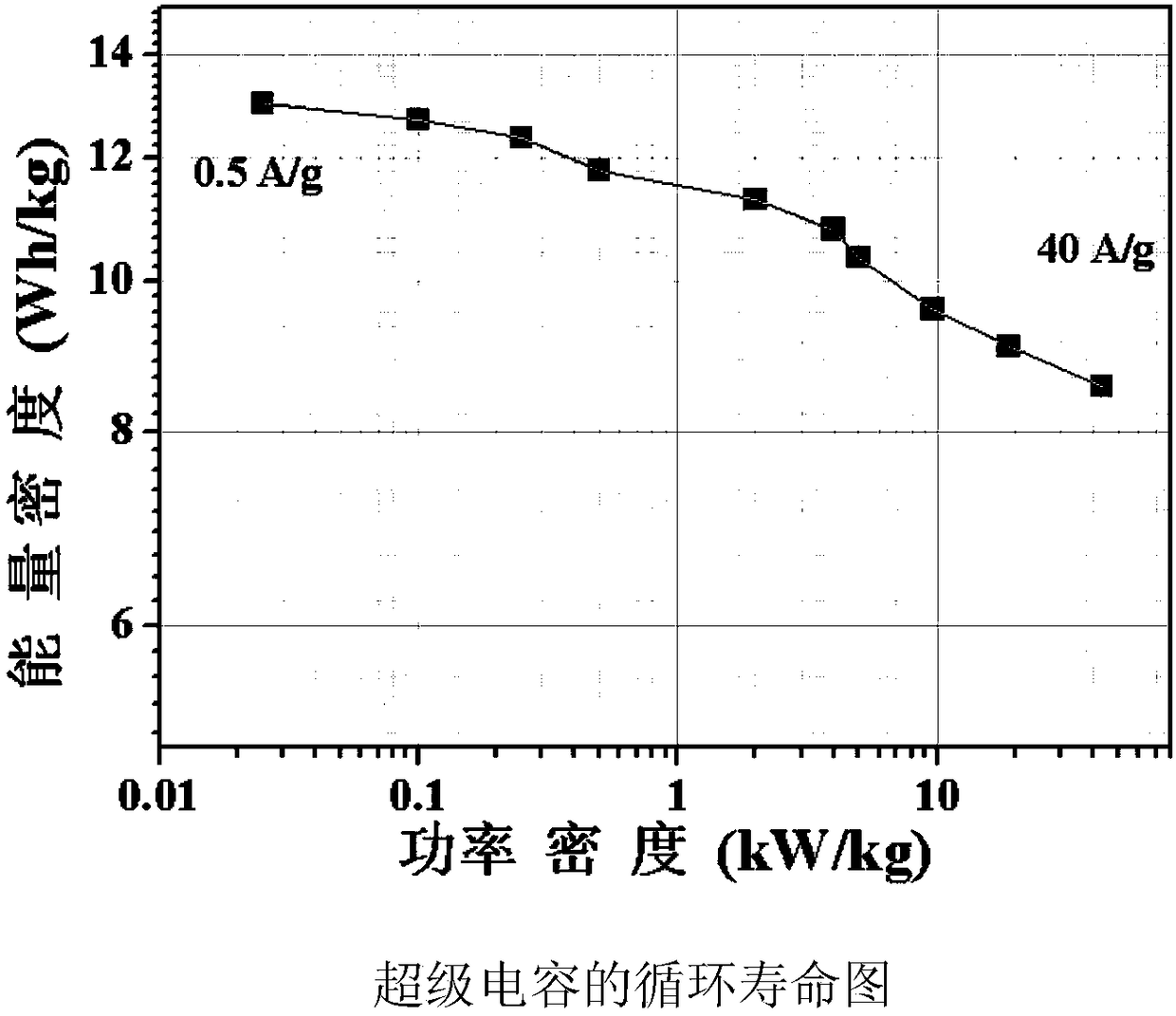
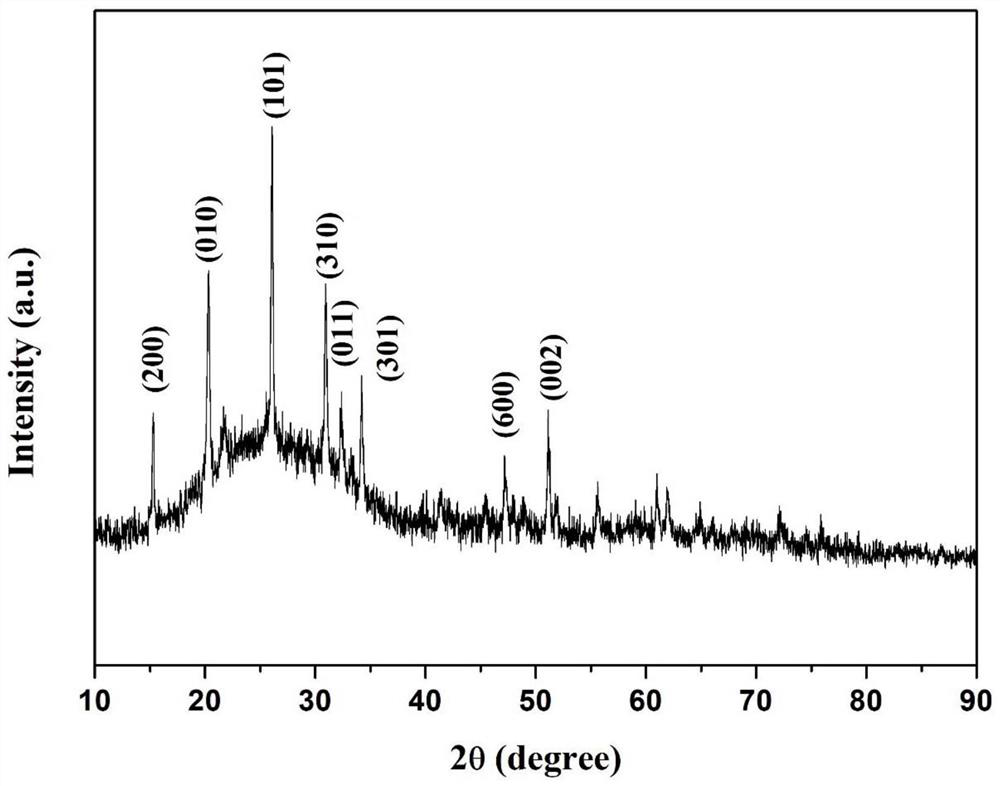
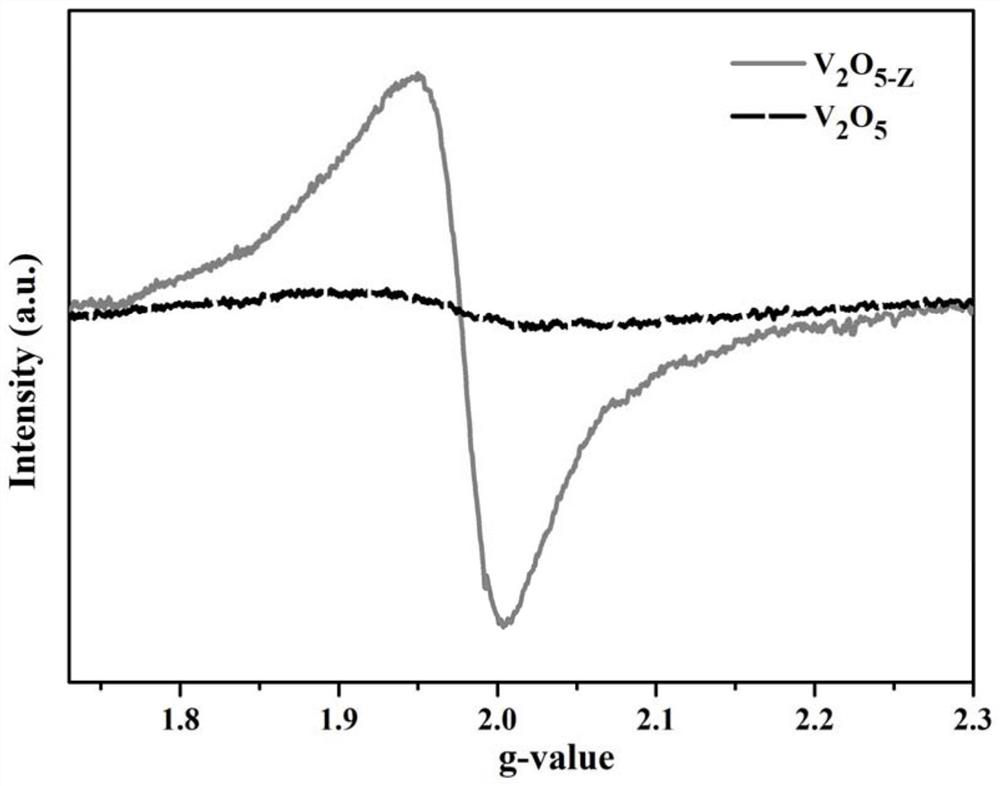
Binder-free oxygen-defect-containing carbon-coated oxide electrode and battery
ActiveCN113380994AImprove electrochemical performanceImprove electronic conductivityCell electrodesSecondary cellsElectrical batteryElectrochemistry
The invention discloses a binder-free oxygen-defect-containing carbon-coated oxide electrode and a battery. The electrode is composed of a current collector and an active material, wherein the active material directly grows on the surface of the current collector and has a three-dimensional honeycomb structure, the active material is a carbon-coated oxide containing oxygen defects, the general formula of the oxide is MXOY-Z, and M comprises at least one of Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Zr, Nb, Mo and Sn. The battery is a magnesium secondary battery, wherein the positive electrode of the battery adopts the binder-free oxygen-defect-containing carbon-coated oxide electrode, and the battery has good electrochemical performance.
Owner:XIAMEN UNIV
Synthesis method of m-phenoxytoluene
InactiveCN101885670AEfficient synthesis methodSynthetic method is convenientEther/acetal/ketal group formation/introductionEther preparationThermal insulationDistillation
The invention discloses a synthesis method of m-phenoxytoluene, relating to a synthesis method of a compound. The synthesis method comprises the following steps of: stirring and mixing m-chlorotoluene, sodium phenate, cuprous chloride, 8-oxyquinoline and polydiglycol in a reaction container at the room temperature, heating until the temperature of mixed liquor is 130-140 DEG C and then reacting for 8-9 hours under thermal-insulation stirring; finally, carrying out high-vacuum reduced pressure distillation and getting 100-110 DEG C of distillates to obtain the m-phenoxytoluene. In the invention, under the catalytic action of the cuprous chloride, the 8-oxyquinoline and the polydiglycol, the sodium phenate and the m-chlorotoluene are directly taken as raw materials to carry out single-step reaction to prepare the m-phenoxytoluene, wherein the m-chlorotoluene is a reactant and meanwhile takes the solvent action so that any solvent does not need to be additionally used, thereby greatly reducing cost.
Owner:YANGZHOU UNIV
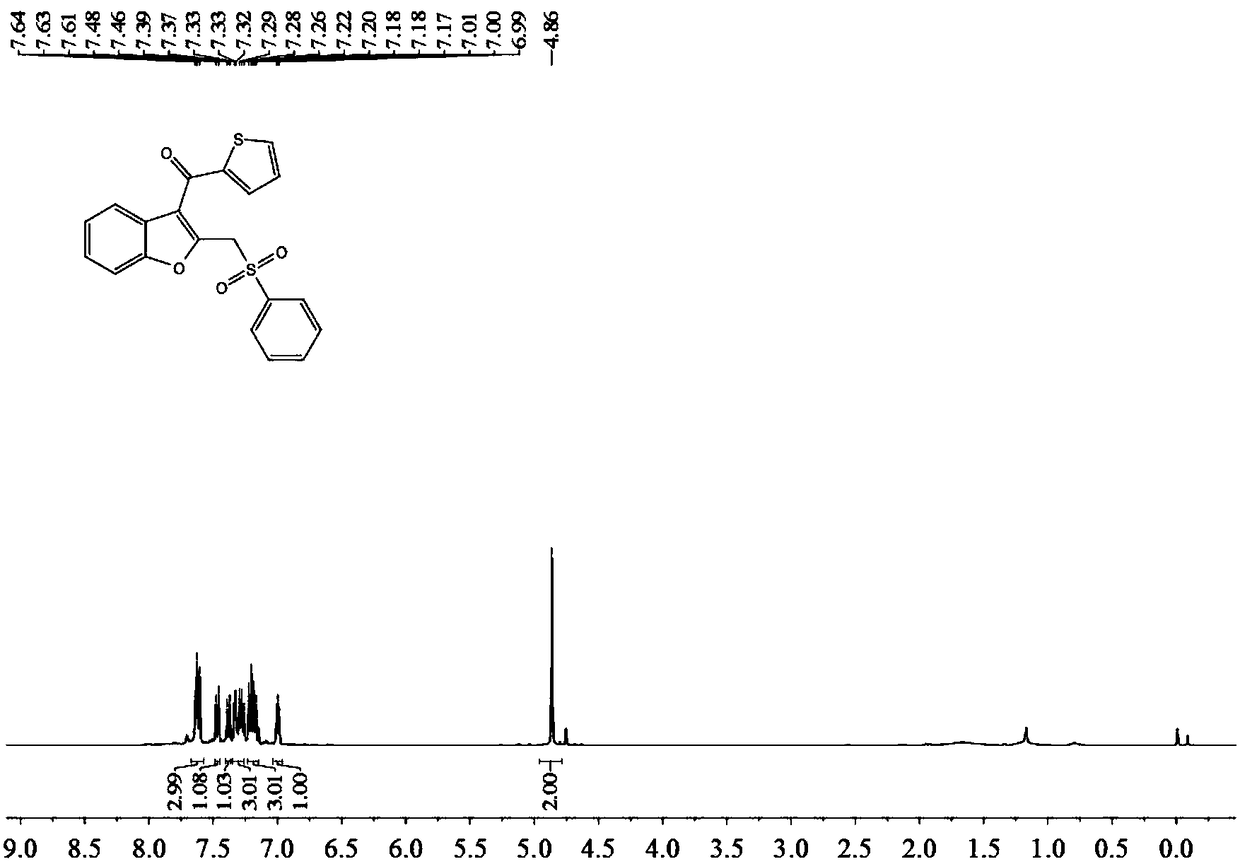
Synthesizing method of benzofuranone compound containing sulfonyl
The invention belongs to the technical field of organic synthesizing and discloses a synthesizing method of a benzofuranone compound containing sulfonyl. The synthesizing method includes: adding substrate with the structure shown in formula 1, sodium sulfinate, silver salt catalyst, oxidizing agent and solvent into a reactor, performing stirring reaction under 80-90 DEG C, and separating and purifying reaction products to obtain the benzofuranone compound containing sulfonyl. The synthesizing method has the advantages that the sodium sulfinate which is simple and easy to obtain is used as the sulfonyl source, the cheap silver salt is used as the catalyst, the oxidizing agent is mild and free of environment pollution, and the functional benzofuranone compound containing sulfonyl is synthesized; the method has innovativeness and atom economy, is mild in condition and safe to operate and has potential practical value.
Owner:SOUTH CHINA UNIV OF TECH
Simple preparation method of Z-type heterojunction photocatalytic material
PendingCN114762829AReduce compound rateSynthetic method is convenientPhysical/chemical process catalystsOrganic compound preparationHeterojunctionPhysical chemistry
The invention discloses a simple preparation method of a Z-type heterojunction composite material. Two semiconductors including Cu2O nanocrystals and g-C3N4 nanosheets are compounded to form a Z-type heterojunction, water is used as a proton source, efficient reduction of carbon dioxide into methanol is realized, and oxygen is generated at the same time. The synthesis method is simple, and water is used as a proton source without other sacrificial agents, so that the cost is effectively reduced, and good application prospects are achieved.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Method for synthesizing irbesartan and intermediate thereof
The invention discloses a method for synthesizing irbesartan and an intermediate thereof, and belongs to the technical field of preparation methods for medicines. The method comprises the following steps of: protecting 5-(4'-methylbiphenyl-2-yl)-2-(1-methyl-1-phenylethyl)tetrazole by using alpha-methyl styrene under the catalysis of organic acid through the novel convenient reaction process, brominating a benzyl group, condensing a solid phase and a liquid phase under phase transfer to obtain irbesartan protected by 1-methyl-1-phenylethyl, performing acid hydrolysis on the protection to obtain the irbesartan and 2-phenyl-2-propanol, and recovering the 2-phenyl-2-propanol to obtain the alpha-methyl styrene. The invention has the advantages that: the synthetic process is suitable for industrial production, economic value can be produced, the synthetic process is safe, the cost for raw materials is saved, the subsequent product can be easily treated, the reaction raw material is single, and the synthetic method is convenient.
Owner:ZHEJIANG TIANYU PHARMA
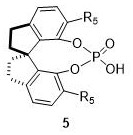
A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method
ActiveCN111217826BEfficient synthesis methodThe synthesis method is simpleOrganic chemistryTetrafluoroborateMethyl palmoxirate
The invention discloses a 2,6-dioxobicyclo[3.3.2]octane derivative and a synthesis method thereof. The synthesis method of the derivative is based on (E)-2-hydroxyl-1-naphthalene vinyl derivative The substrate is under the action of 1-chloromethyl-4-fluoro-1, 4-diazotized bicyclo[2.2.2]octane bis(tetrafluoroborate) salt and cuprous chloride, and the reaction obtains a novel 2,6-Dioxobicyclo[3.3.2]octane skeleton derivative compound. The method of the invention is simple to operate, and the reaction conditions are mild, and a novel 3D skeleton, two rings, three new chemical bonds (two C-O, one C-C) and four chiral centers are constructed in a one-pot reaction And a single stereo configuration was obtained, and a series of 2,6-dioxobicyclo[3.3.2]octane derivatives were constructed.
Owner:NORTHWEST UNIV
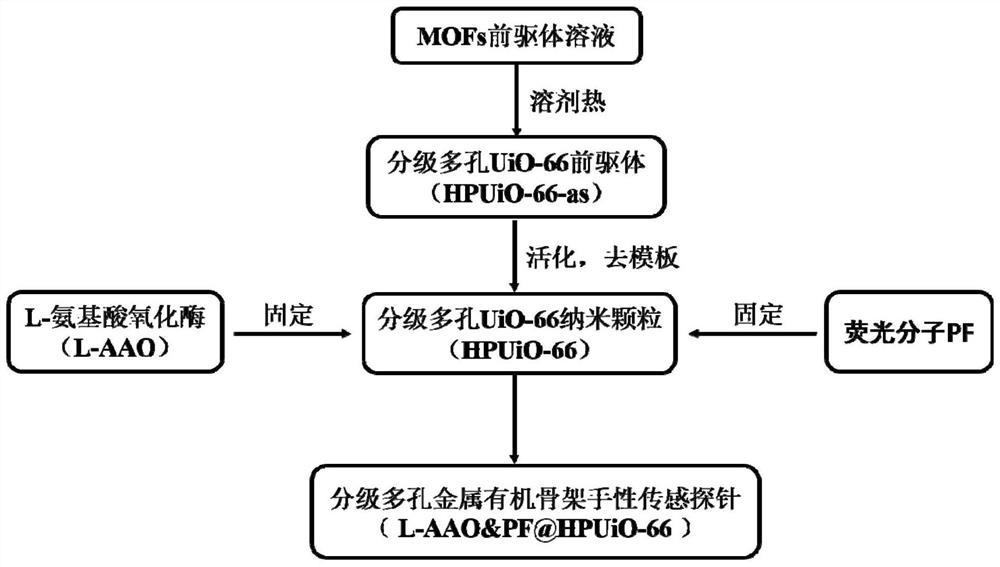
Preparation method of hierarchical porous metal organic framework chiral sensing probe, probe obtained by preparation method and application of probe
ActiveCN113310958AImprove stabilityAvoid aggregation quenchingFluorescence/phosphorescenceMicrosphereMetal-organic framework
The invention relates to a preparation method of a hierarchical porous metal organic framework chiral sensing probe, which comprises the following steps of providing a hierarchical porous metal organic framework microsphere with a regular pore structure, fixing amino acid oxidase on the hierarchical porous metal organic framework microspheres to obtain hierarchical porous metal organic framework microspheres fixed with enzymes, and loading the amino acid oxidase in pore channels of the hierarchical porous metal organic framework microspheres through adsorption, fixing fluorescent molecules on the hierarchical porous metal organic framework microspheres fixed with enzyme to obtain the hierarchical porous metal organic framework chiral sensing probe, and loading the fluorescent molecules in pore channels of the hierarchical porous metal organic framework microspheres through adsorption. The invention also provides the hierarchical porous metal organic framework chiral sensing probe obtained by the preparation method and application thereof. By fixing different amino acid oxidase as a chiral recognition center, the amino acid can be recognized and the content of the amino acid can be analyzed in a high-sensitivity and high-specificity manner.
Owner:EAST CHINA UNIV OF SCI & TECH
Ultra-small protein composite nanoparticles with near-infrared photothermal effect and multimodal imaging functions, its preparation method and application
ActiveCN107551279BHigh near-infrared absorption coefficientImprove spatial resolutionEnergy modified materialsEchographic/ultrasound-imaging preparationsBismuth sulfideTumor targeting
The invention discloses a bismuth sulfide / gadolinium oxide-entrapping ultrafine protein composite nanoparticle with a near-infrared photothermal effect and a multi-mode imaging function and a preparation method and application thereof. By formulation screening and process optimization, the bismuth sulfide / gadolinium oxide albumin ultrafine composite nanoparticle with the near-infrared photothermaleffect and the multi-mode imaging function is prepared in one step. The composite nanoparticle has good physicochemical stability, photostability, biocompatibility and tumor-targeting property, can remarkably enhance near-infrared fluorescence, photoacoustic, magnetic resonance, CT (computed tomography) and thermal signals at a tumor site, and realizes multi-mode-complemented tumor diagnosis, andmoreover, the composite nanoparticle can eliminate tumors under the excitation of near-infrared light; in particular, because the grain size of the nanoparticle is less than 5.5nm, the nanoparticle can be eliminated by the kidneys, and toxic and side effects are minor; a tumor can be diagnosed and treated at the same time, and the composite nanoparticle has a great potential in realizing accuratetumor diagnosis and treatment integration.
Owner:SUZHOU UNIV
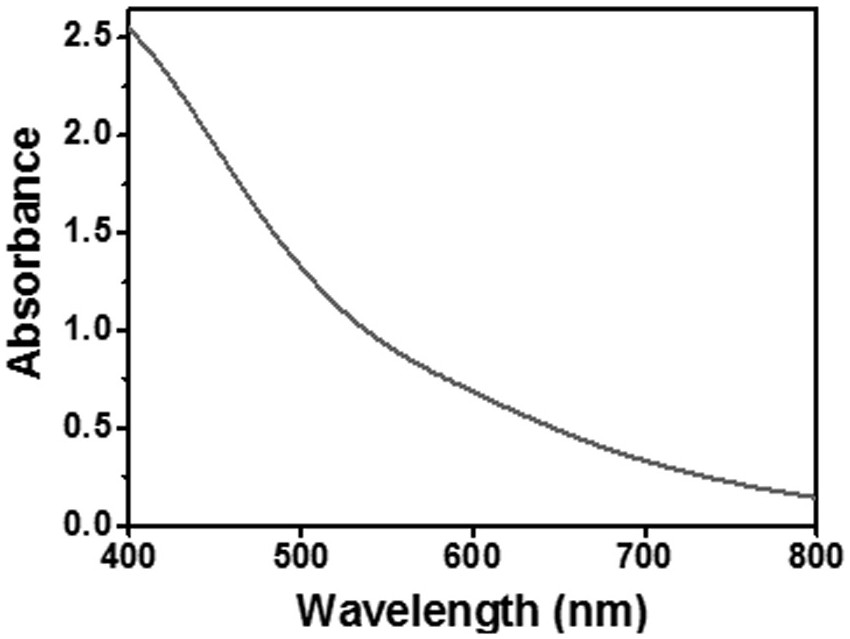
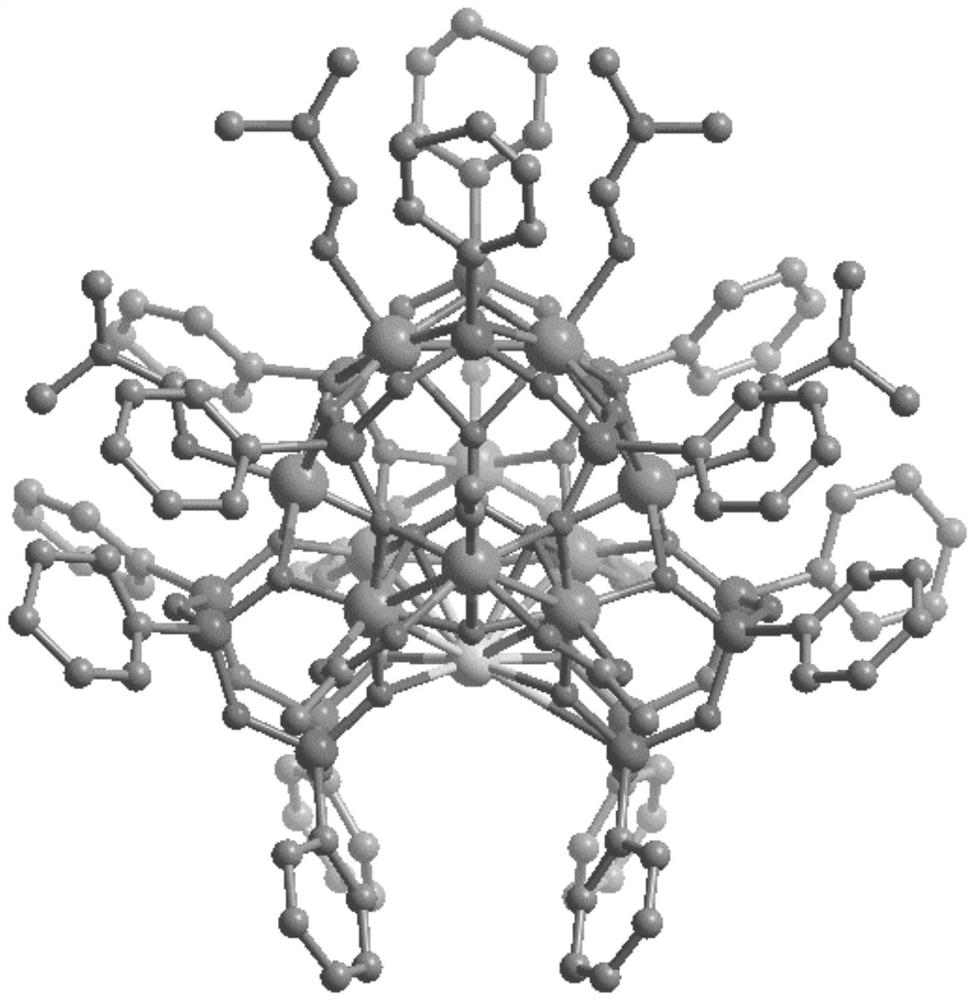
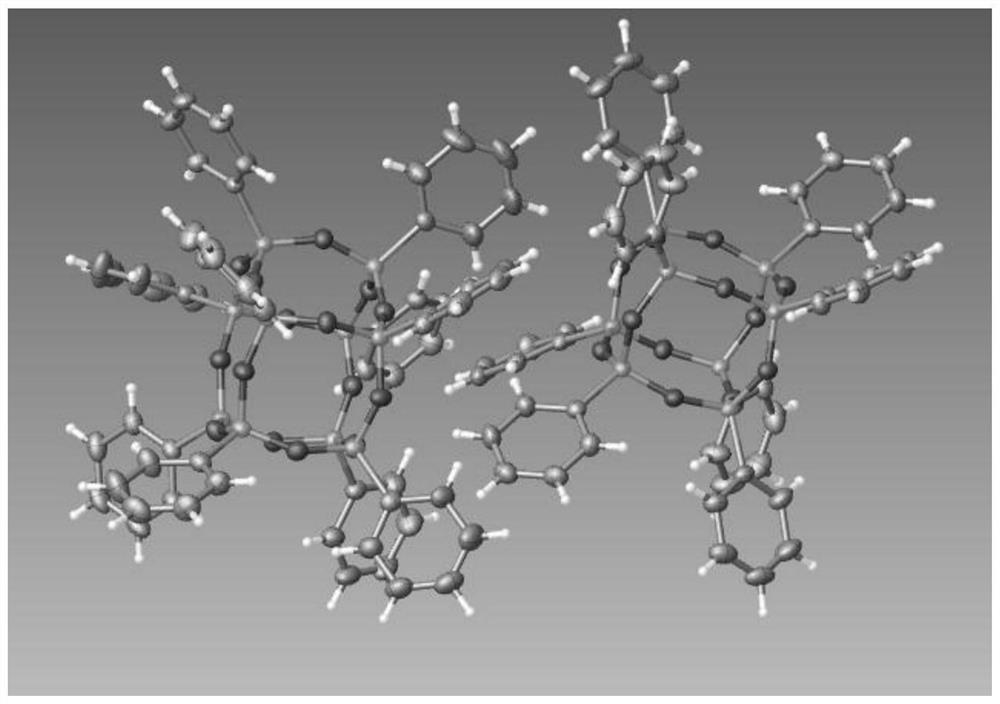
Organosilane ligand protected mixed valence ten-nuclear manganese cluster compound and preparation method thereof
PendingCN113831368ASimple preparation processEase of industrial productionGroup 4/14 element organic compoundsOrganic chemistry methodsAlkoxy groupManganese
The invention relates to an organosilane ligand protected mixed valence ten-nuclear manganese cluster compound and a preparation method thereof. The preparation process comprises the following steps: dissolving alkali in an alcohol solvent, then adding trialkoxysilane containing a substituent group, carrying out reflux reaction, and carrying out prehydrolytic polycondensation to obtain a prepolymer; and then adding a metal salt solution of divalent manganese, transferring the mixture solution into a reaction kettle, heating, and carrying out solvothermal reaction to generate the organosilane protected mixed valence ten-nuclear manganese cluster compound. The invention also provides the mixed-valence ten-nuclear manganese cluster compound prepared by the method. The preparation method is realized through a solvothermal method, reaction conditions are easy to control, and the obtained manganese cluster compound is clear in structure and relatively high in purity, can be directly applied and has a huge application prospect.
Owner:SHANDONG JIAOTONG UNIV
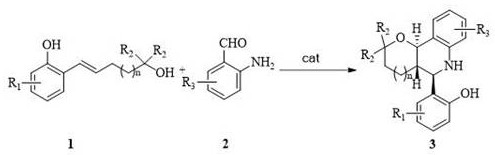
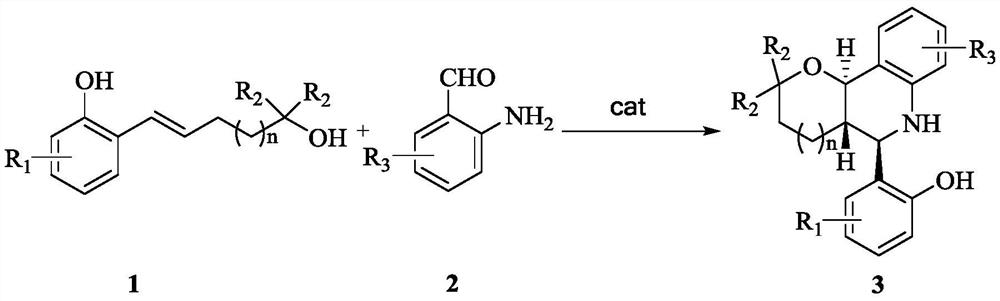
A kind of asymmetric synthesis method of trans-tetrahydrofuran/pyranotetrahydroquinoline derived chiral compound
ActiveCN110684031BEfficient synthesis methodThe synthesis method is simpleOrganic chemistryO-Phosphoric AcidQuinoline
The invention discloses an asymmetric synthesis method of chiral compounds derived from trans-tetrahydrofuran / pyranotetrahydroquinoline. The method uses 5-o-hydroxyphenyl-4-ene-1-pentanol derivatives and o- The aminobenzaldehyde derivative is reacted under the action of a chiral phosphoric acid catalyst to obtain a chiral compound derived from trans-tetrahydrofuran / pyranotetrahydroquinoline. The present invention provides an efficient, simple and convenient method for the first synthesis of trans-tetrahydrofuran / pyranotetrahydroquinoline derived chiral compounds by adopting dearomatization, Prins cyclization and aza-Michael addition series reaction modes resolve resolution. The method of the invention is simple to operate, and the reaction conditions are mild, and two new heterocycles, three new chemical bonds (C-O, C-C, C-N) and three adjacent quaternary carbon chirals are constructed in the one-pot reaction. A series of chiral compounds derived from trans-tetrahydrofuran / pyranotetrahydroquinoline were constructed.
Owner:NORTHWEST UNIV
Nano cuprous oxide/silk fibroin complex and preparation method thereof
Owner:JIAXING UNIV
A kind of synthetic method of 4-allyl-3,5-disubstituted isoxazole
ActiveCN108863969BRaw materials are easy to getInnovativeOrganic chemistryPtru catalystOrganic synthesis
The invention discloses a synthesis method of 4-allyl-3,5-disubstituted isoxazole, belonging to the technical field of organic synthesis. The synthesis method is as follows: in the reactor, add acetylene ketoxime ether substrate, 3-bromopropene, palladium catalyst, additive and solvent, stir and react at 70-80°C, and the reaction product is separated and purified to obtain 4-allyl -3,5-disubstituted isoxazoles. In the method of the present invention, a series of acetylenic ketone oxime ethers are obtained by reacting the product obtained through Sonogashira coupling of simple and easy-to-obtain acyl chlorides and alkynes with methoxyamine hydrochloride, the reaction conditions are mild, and there is no pollution to the environment. Potentially functional 4-allyl-3,5-disubstituted isoxazole compounds, the method is innovative and atom-economical, mild conditions, safe operation, and can be scaled up to 5 g scale without affecting the yield Therefore, it has potential practical value.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing double-cage POSS (Polyhedral Oligomeric Silsesquioxane) by solvothermal method
PendingCN113354817AReaction conditions are easy to controlClear structurePrepolymerSolvothermal reaction
The invention relates to a method for preparing double-cage POSS (Polyhedral Oligomeric Silsesquioxane) by a solvothermal method, which comprises the following steps: dissolving alkali in an alcohol solvent, then adding trialkoxysilane containing substituent groups, carrying out reflux reaction, and carrying out prehydrolytic polycondensation to obtain a prepolymer; and then transferring the prepolymer into a reaction kettle, adding a solvent A, and carrying out solvothermal reaction to generate the double-cage POSS. According to the synthesis method of the double-cage type POSS, the double-cage type POSS is obtained through a solvothermal method, reaction conditions are easy to control, and the obtained double-cage type POSS is clear in structure and high in purity and has a huge application prospect. The prepared double-cage POSS can be directly applied, and can also be used as a raw material for further modification.
Owner:SHANDONG GUIKE NEW MATERIAL CO LTD
A polypyrrole/nickel hydroxide/nickel foam integrated electrode and its preparation method
ActiveCN106898498BImprove electrochemical performanceGood shape and controllableHybrid capacitor electrodesHybrid/EDL manufactureCapacitanceNitrate
Owner:NANJING UNIV OF SCI & TECH
Boron-modified carbon nitride material as well as preparation method and application thereof
ActiveCN114733543AEasy to separateImprove the production effectPhysical/chemical process catalystsOrganic compound preparationModified carbonMuffle furnace
The invention discloses a boron-modified carbon nitride material as well as a preparation method and application thereof, urea and a boric acid solution are mixed and dissolved, a solvent is evaporated, recrystallization is performed, a recrystallized mixture is calcined, a B atom is enabled to enter a g-C3N4 skeleton by substituting a sp2-hybridized C atom in a 3-s-triazine ring, and the boron-modified carbon nitride material is prepared. The synthesis method is convenient, and the boron modified g-C3N4 photocatalyst material can be obtained through simple stirring, evaporation, grinding and air calcination in a muffle furnace. According to the preparation method, boric acid is utilized to enable B atoms to enter a g-C3N4 skeleton by replacing sp2-hybridized C atoms in a 3-s-triazine ring in a thermal polymerization process of urea, so that the g-C3N4 has higher photon-generated carrier separation capacity and reactive oxygen species generation capacity.
Owner:SHANDONG UNIV
A kind of synthetic method of the benzofuranone compound containing sulfone group
The invention belongs to the technical field of organic synthesizing and discloses a synthesizing method of a benzofuranone compound containing sulfonyl. The synthesizing method includes: adding substrate with the structure shown in formula 1, sodium sulfinate, silver salt catalyst, oxidizing agent and solvent into a reactor, performing stirring reaction under 80-90 DEG C, and separating and purifying reaction products to obtain the benzofuranone compound containing sulfonyl. The synthesizing method has the advantages that the sodium sulfinate which is simple and easy to obtain is used as the sulfonyl source, the cheap silver salt is used as the catalyst, the oxidizing agent is mild and free of environment pollution, and the functional benzofuranone compound containing sulfonyl is synthesized; the method has innovativeness and atom economy, is mild in condition and safe to operate and has potential practical value.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



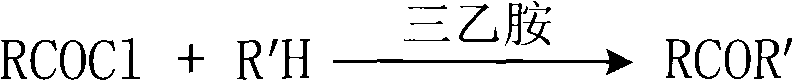
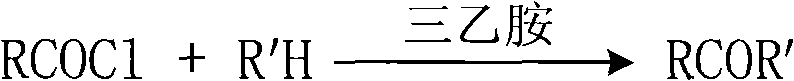











![A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08b7210a-a23a-4304-adc9-1fb6a380f08b/BDA0002265038610000021.png)
![A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08b7210a-a23a-4304-adc9-1fb6a380f08b/BDA0002265038610000022.png)
![A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method A kind of 2,6-dioxabicyclo[3.3.2]octane derivative and its synthesis method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/08b7210a-a23a-4304-adc9-1fb6a380f08b/BDA0002265038610000041.png)