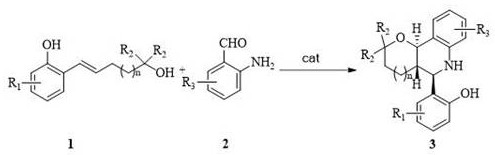
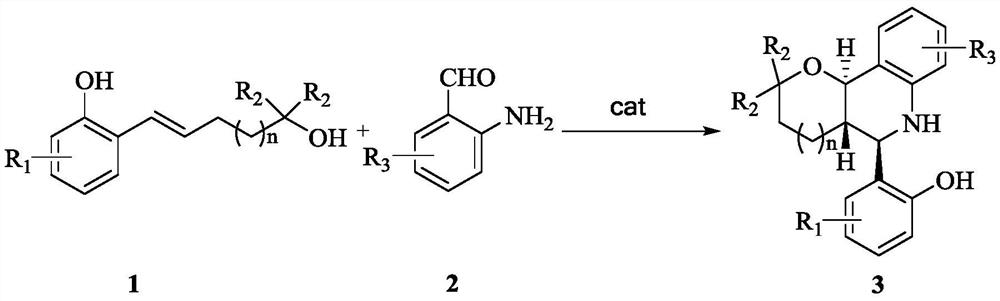
A kind of asymmetric synthesis method of trans-tetrahydrofuran/pyranotetrahydroquinoline derived chiral compound
A technology of tetrahydroquinoline and tetrahydrofuran, which is applied in the direction of organic chemistry, can solve the problems of synthesis difficulty, etc., and achieve the effect of simple operation, convenient synthesis method and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of trans-tetrahydropyranotetrahydroquinoline derivative chiral compounds shown in structural formula 3a
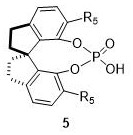
[0029] Compound (0.12mmol) and 2-amino-3,5-dibromo-benzaldehyde (0.1 mmol) and chiral phosphoric acid catalyst (0.01 mmol) as shown in structural formula 5a are added with magnetic Add 1.0mL of dichloromethane to a Kjeldahl flask after vacuuming and filling with nitrogen, then place the reaction system in an oil bath at 40°C for 24 hours, monitor with a TLC plate, after the reaction is complete, remove the solvent under reduced pressure, and separate by column chromatography (petroleum ether: ethyl acetate=15:1), that is, the trans-tetrahydropyranotetrahydroquinoline derivative chiral compound shown in structural formula 3a (white solid, 43.1mg, 98%, 98%ee ); The chemical reaction formula of this method is:
[0030]
[0031] The trans-tetrahydropyranotetrahydroquinoline derived chiral compound shown in structural formula 3a was respectively subjected t...
Embodiment 2
[0038] Synthesis of trans-tetrahydropyranotetrahydroquinoline derivative chiral compounds shown in structural formula 3b
[0039] Compound (0.12mmol) and 2-amino-3,5-dibromo-benzaldehyde (0.1mmol) as shown in structural formula 1b and chiral phosphoric acid catalyst (0.01mmol) as shown in structural formula 5a are added Add 1.0mL of dichloromethane to a Kjeldahl flask after vacuuming and filling with nitrogen, then place the reaction system in an oil bath at 40°C for 24 hours, monitor with a TLC plate, after the reaction is complete, remove the solvent under reduced pressure, and separate by column chromatography (petroleum ether: ethyl acetate = 15:1), that is, to obtain the trans-tetrahydropyranotetrahydroquinoline derivative chiral compound shown in structural formula 3b (white solid, 44.7mg, 98%, 98%ee ); The chemical reaction formula of this method is:
[0040]
[0041] The trans-tetrahydropyranotetrahydroquinoline derived chiral compound shown in structural formula 3...
Embodiment 3
[0048] Synthesis of trans-tetrahydropyranotetrahydroquinoline derivative chiral compounds shown in structural formula 3c
[0049] Compound (0.12mmol) and 2-amino-3,5-dibromo-benzaldehyde (0.1mmol) as shown in structural formula 1c and chiral phosphoric acid catalyst (0.01mmol) as shown in structural formula 5a are added Add 1.0mL of dichloromethane to a Kjeldahl flask after vacuuming and filling with nitrogen, then place the reaction system in an oil bath at 40°C for 24 hours, monitor with a TLC plate, after the reaction is complete, remove the solvent under reduced pressure, and separate by column chromatography (petroleum ether: ethyl acetate=15:1), that is, to obtain the trans-tetrahydropyranotetrahydroquinoline derivative chiral compound shown in structural formula 3c (white solid, 47.4mg, 98%, 98%ee ); The chemical reaction formula of this method is:
[0050]
[0051] The trans-tetrahydropyranotetrahydroquinoline derived chiral compound represented by structural formu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com