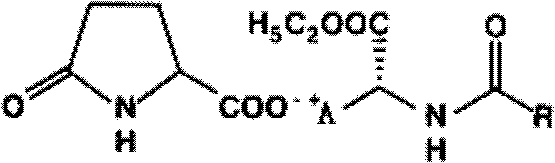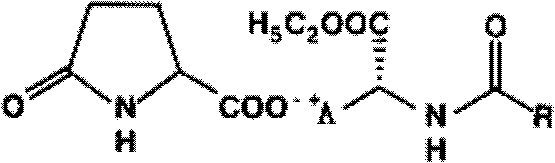Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Ureaplasma urealyticum DNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
It is possible to test for Ureaplasma using a urine sample or a vaginal swab. The urine sample or swab is subjected to a PCR test that looks for the DNA of the bacteria.
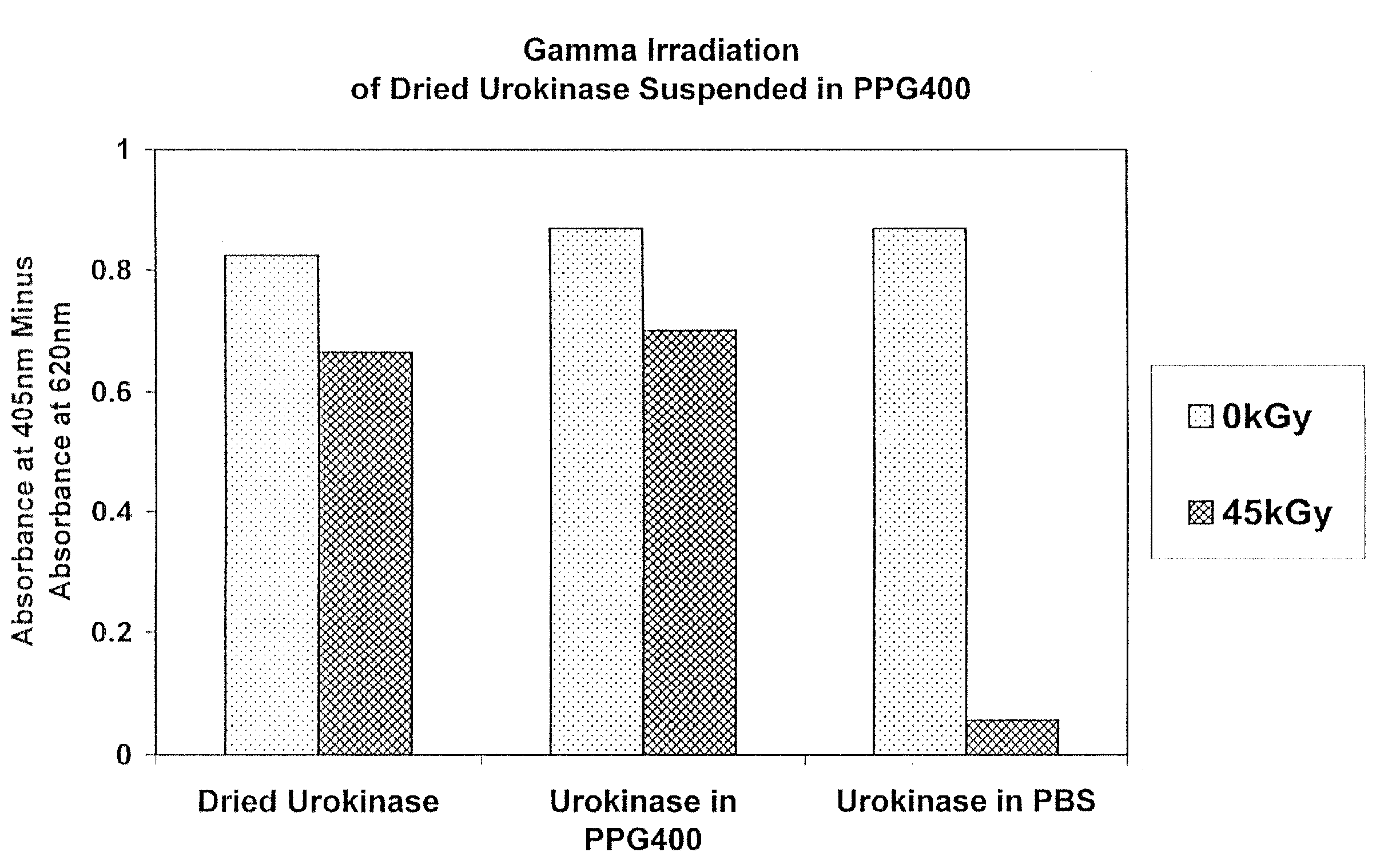
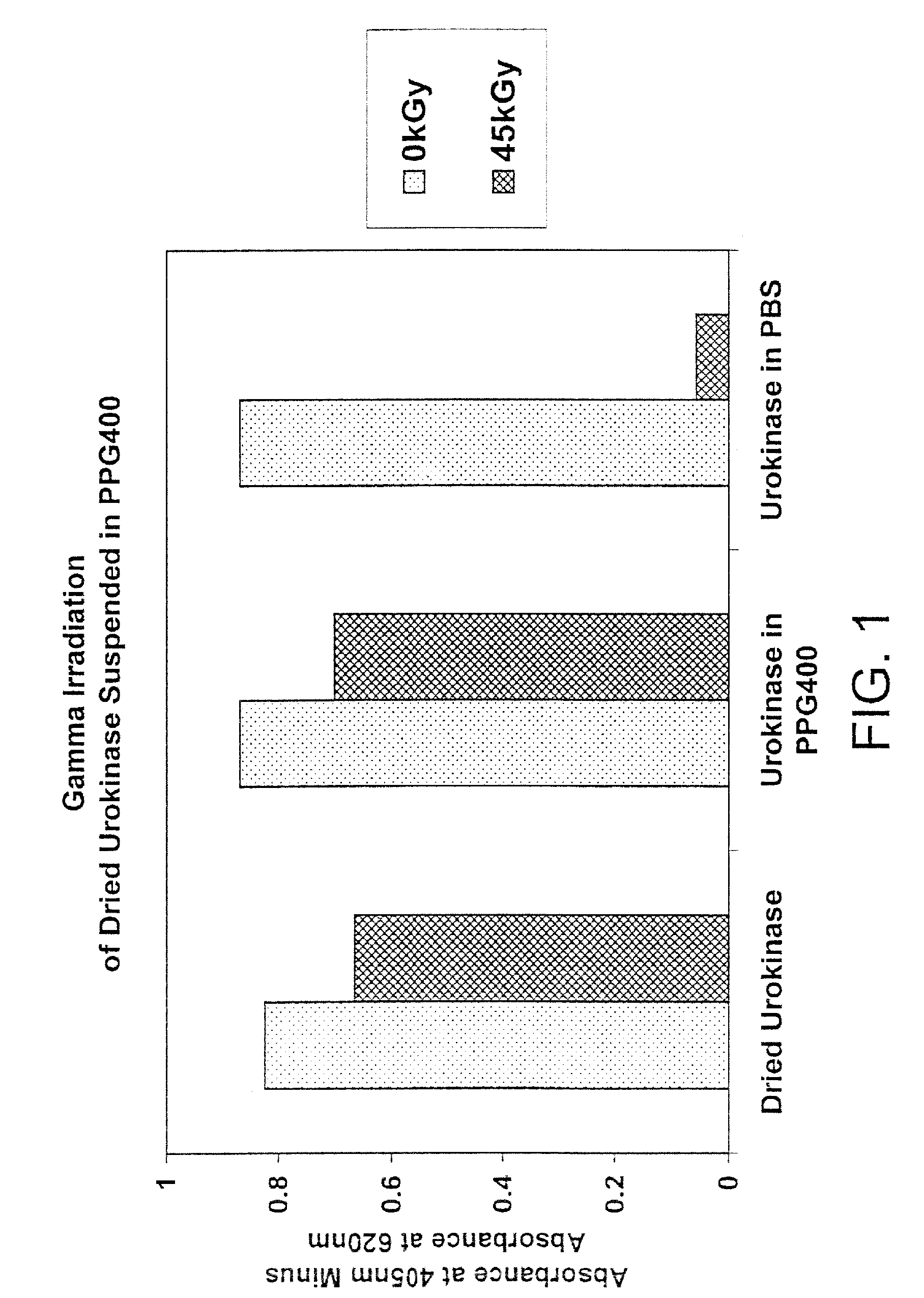
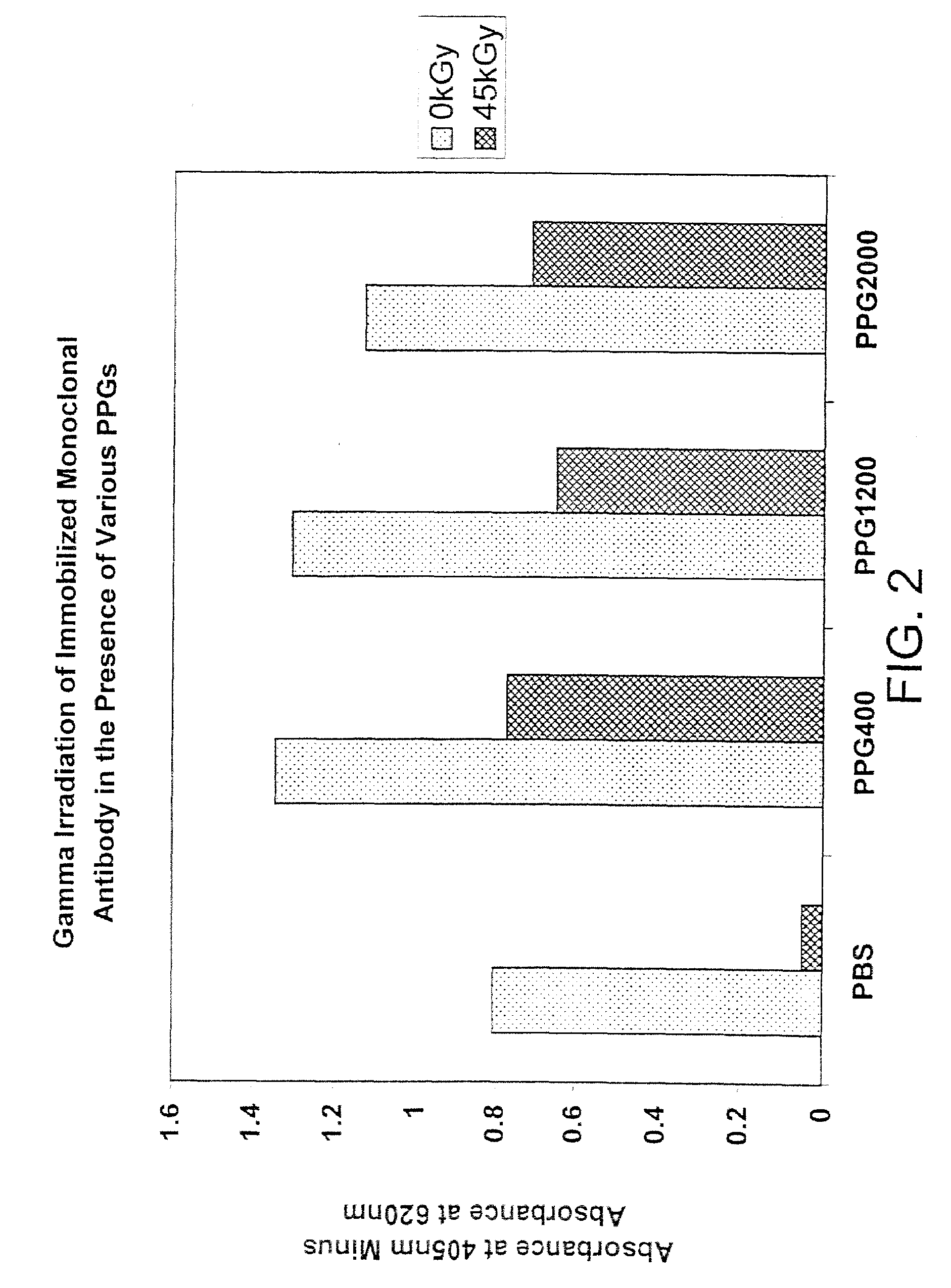
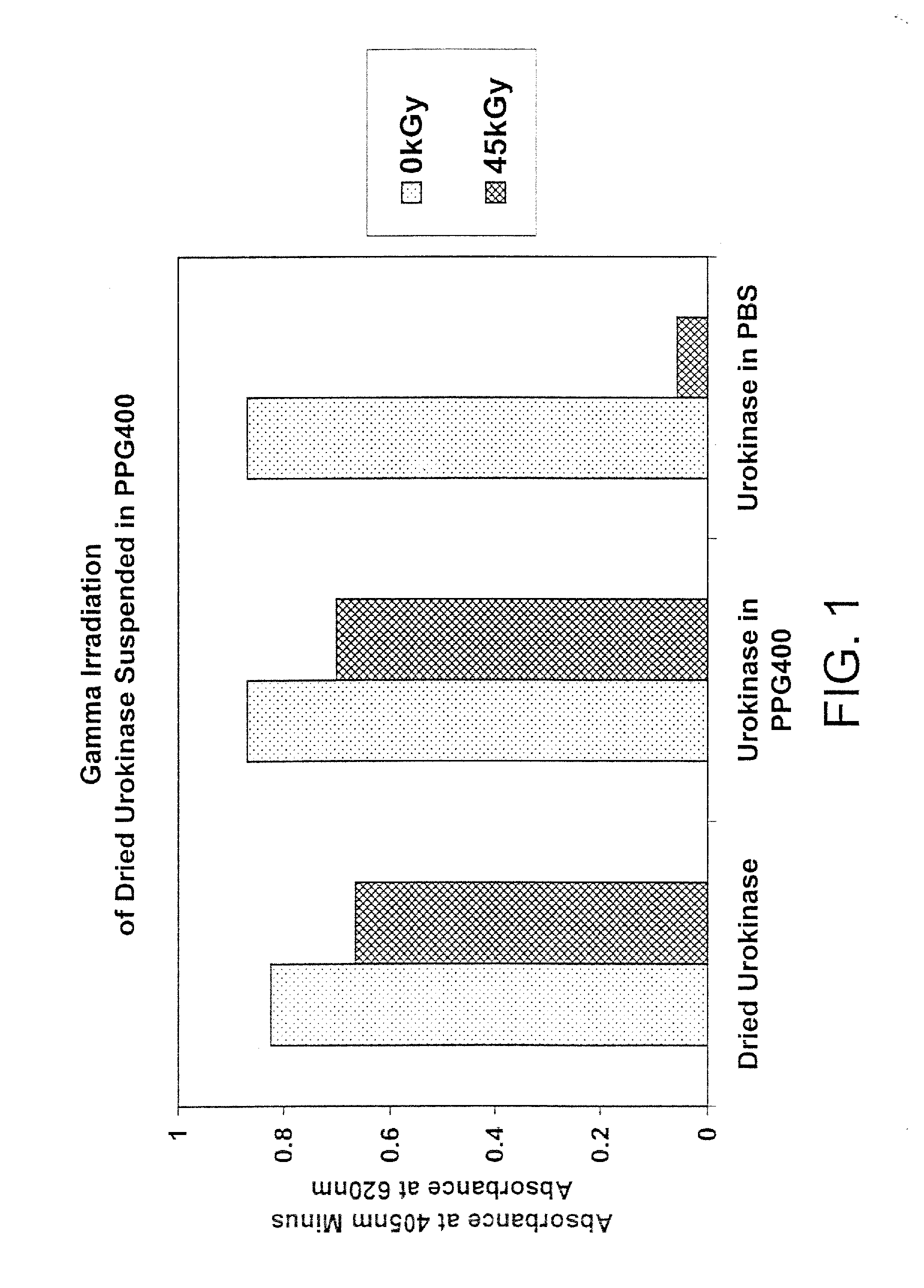
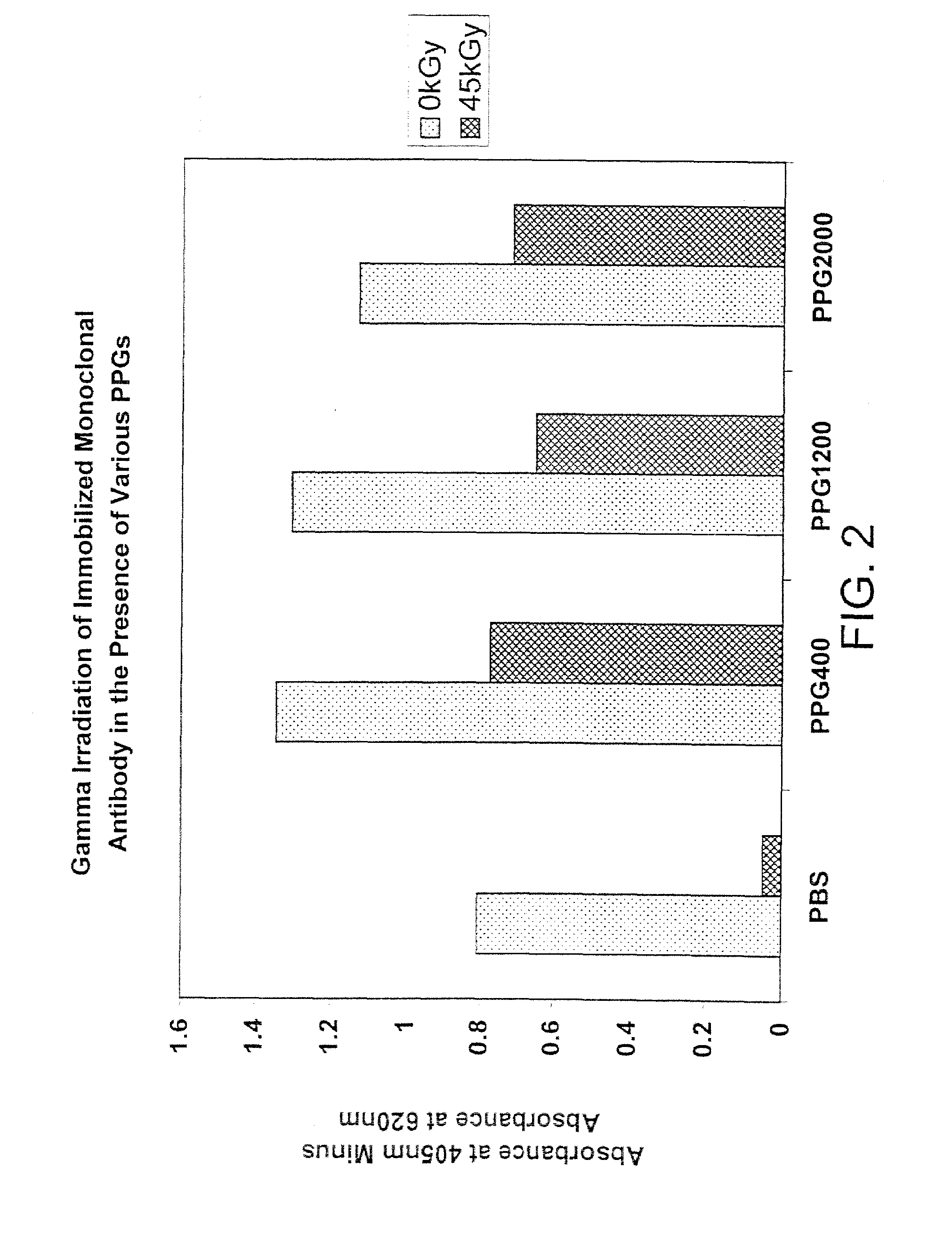
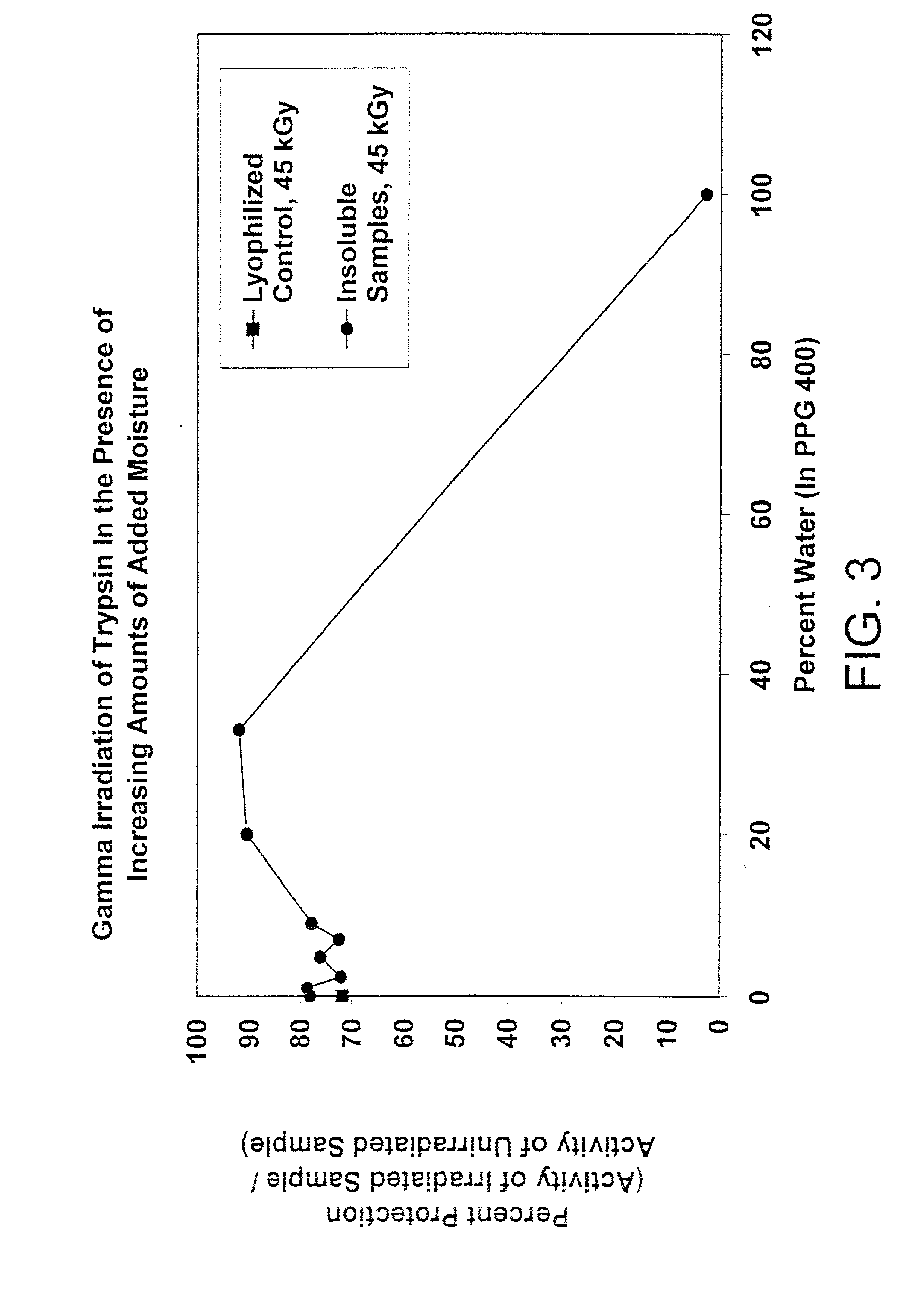
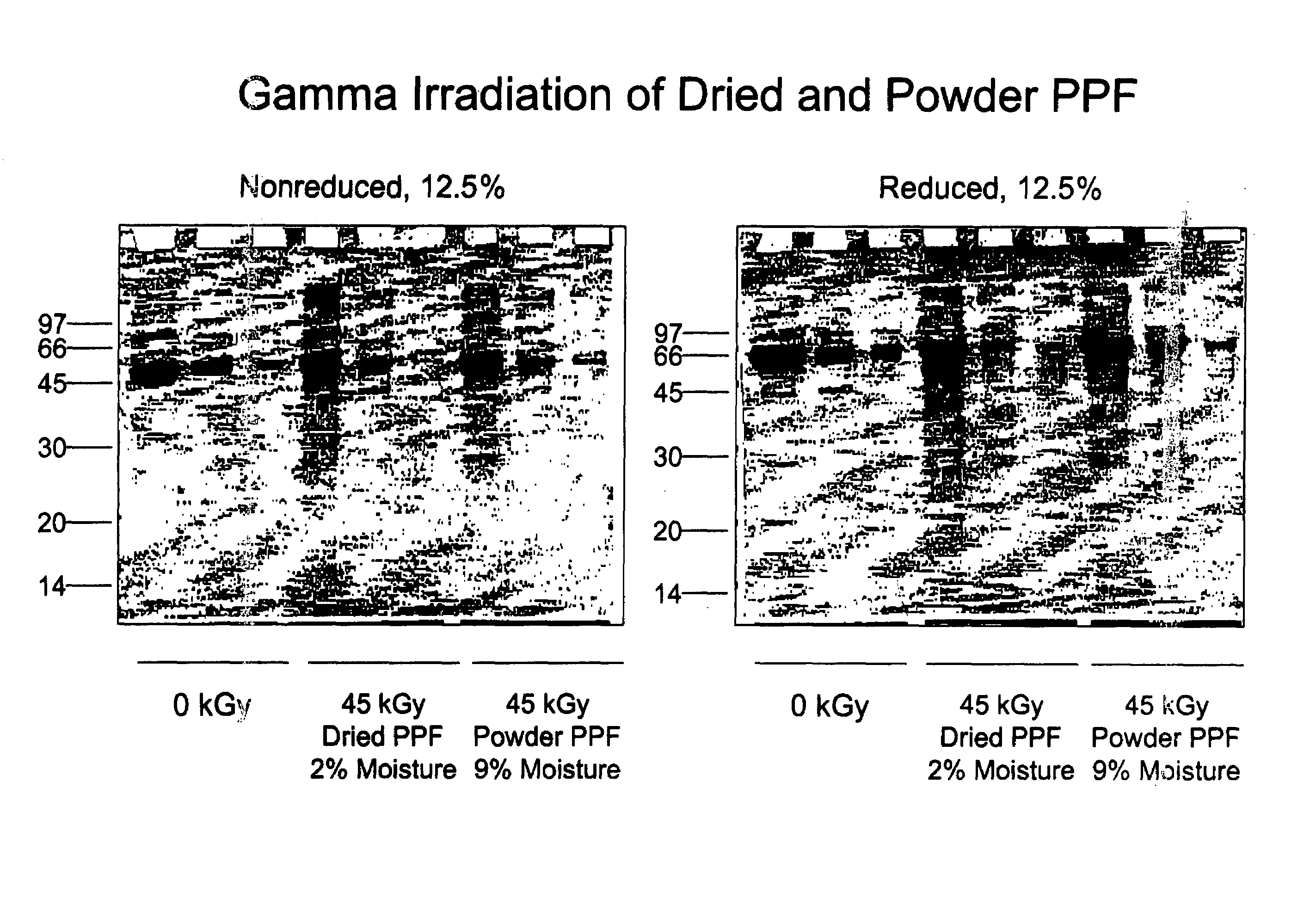
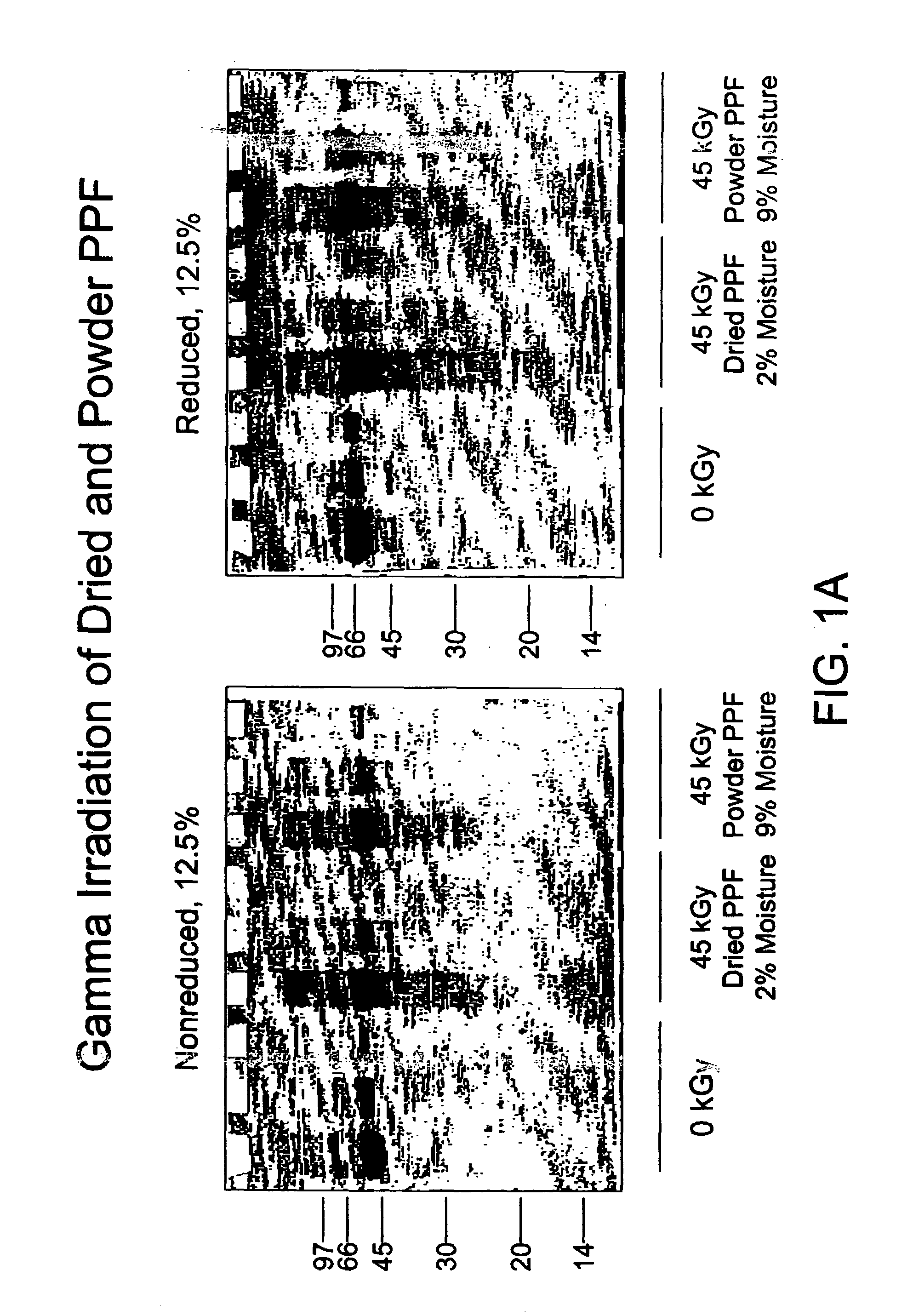
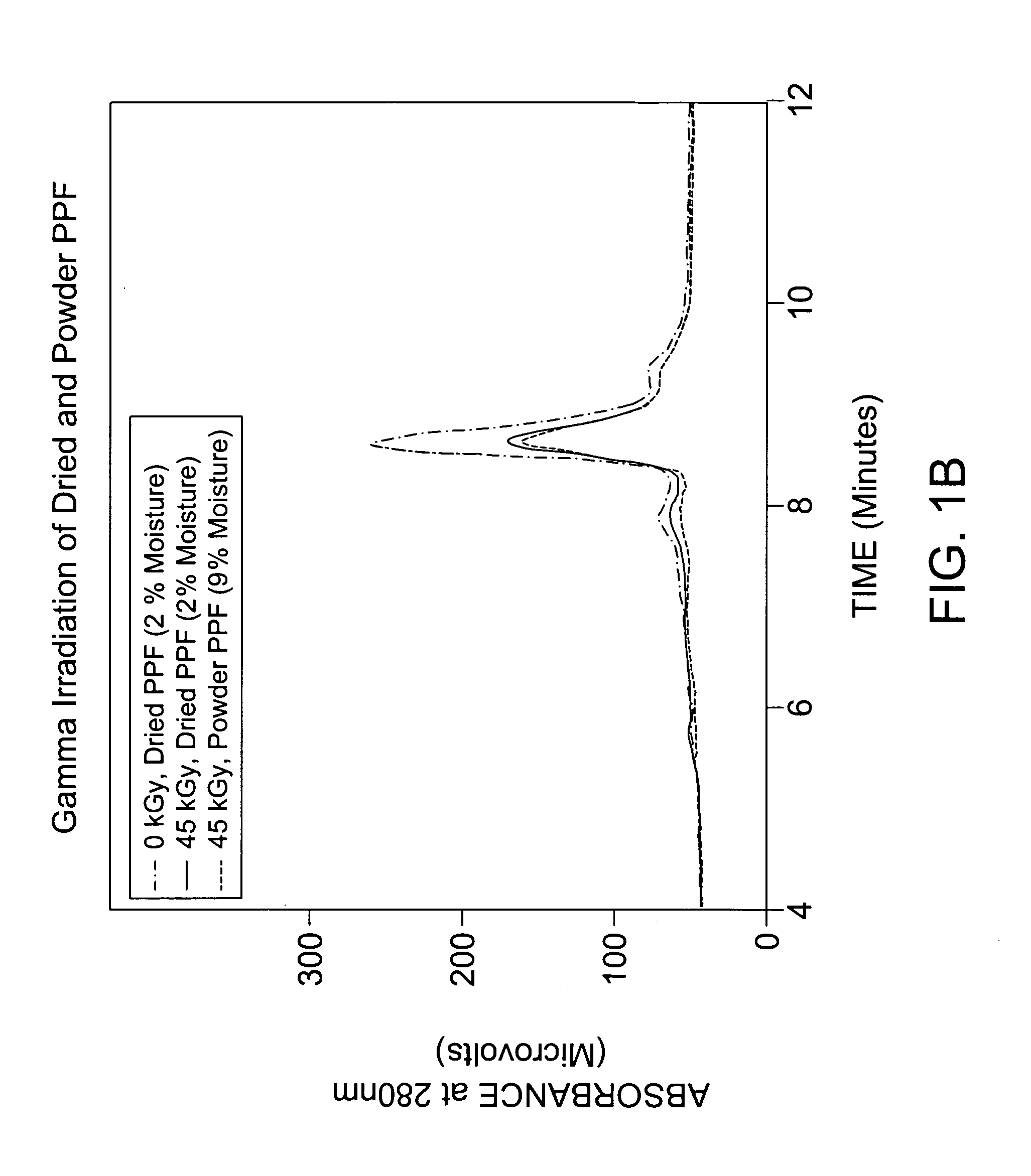
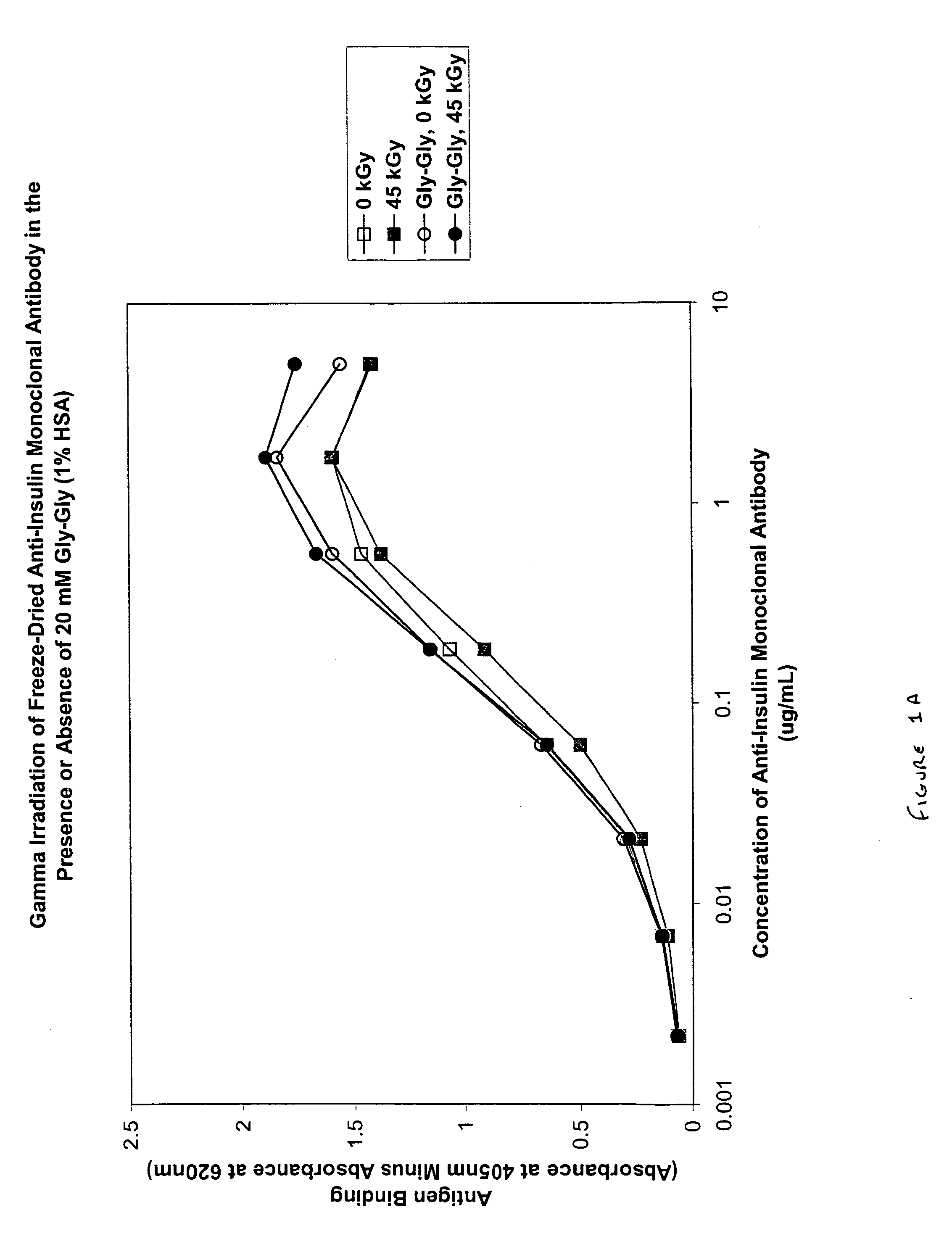
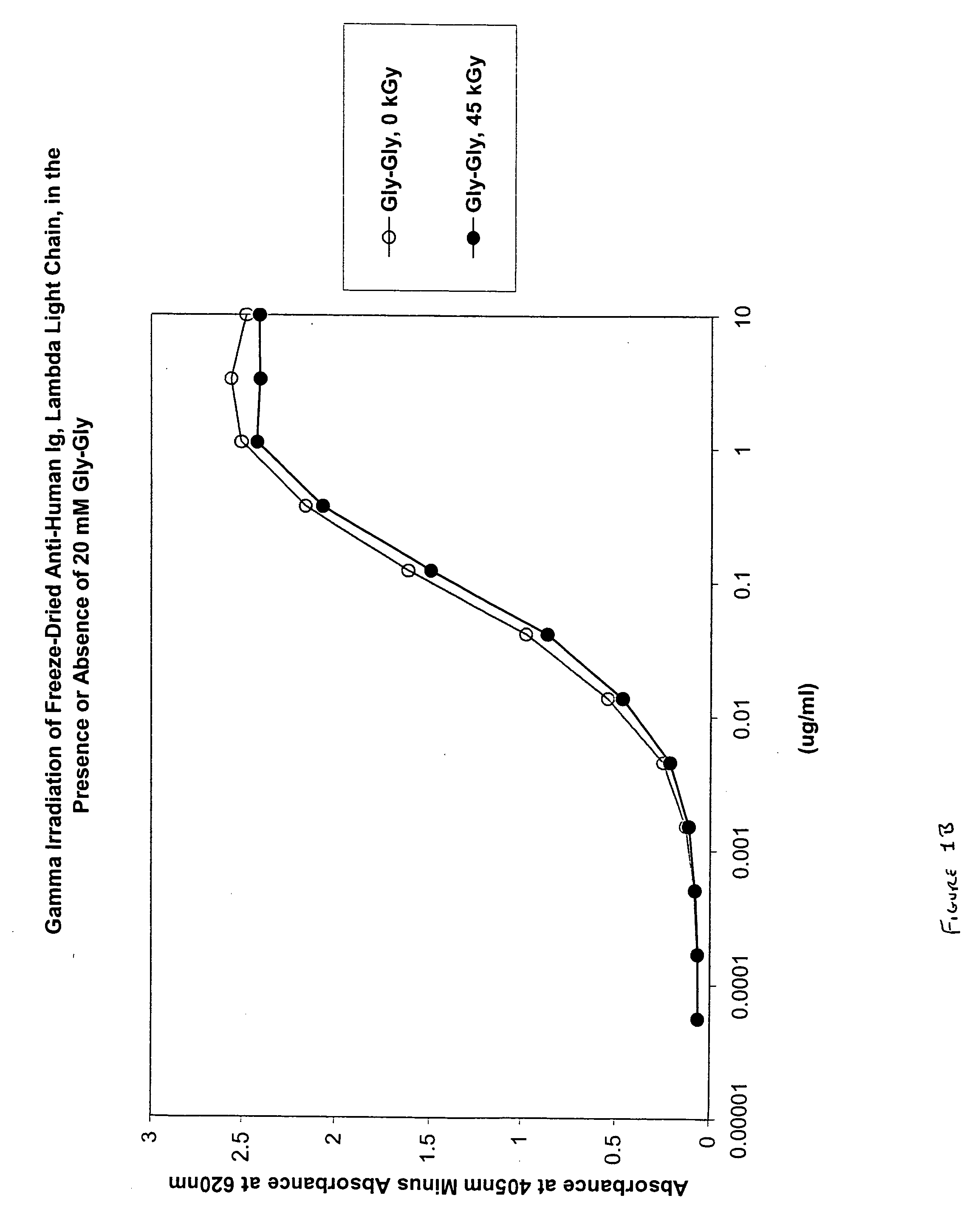
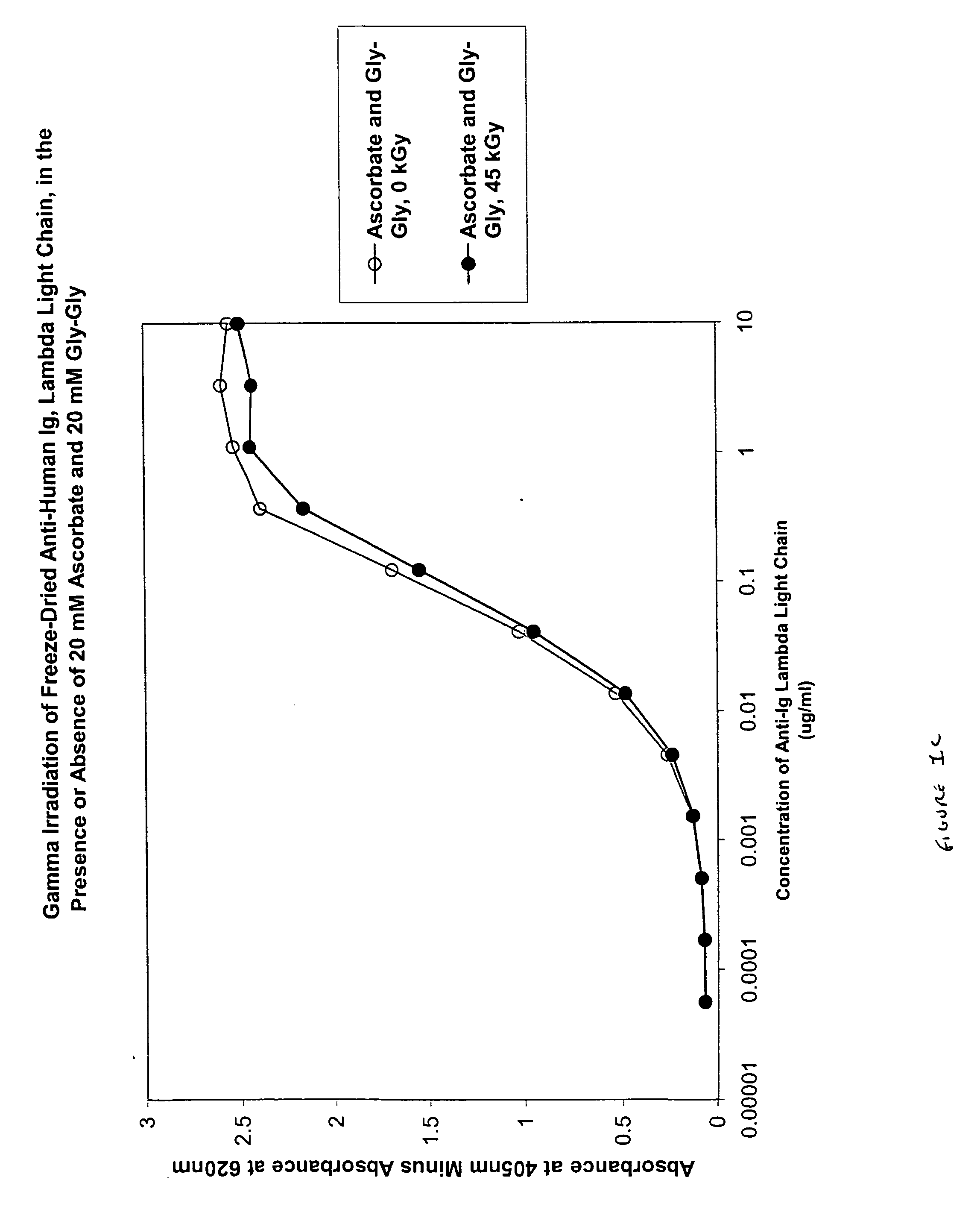
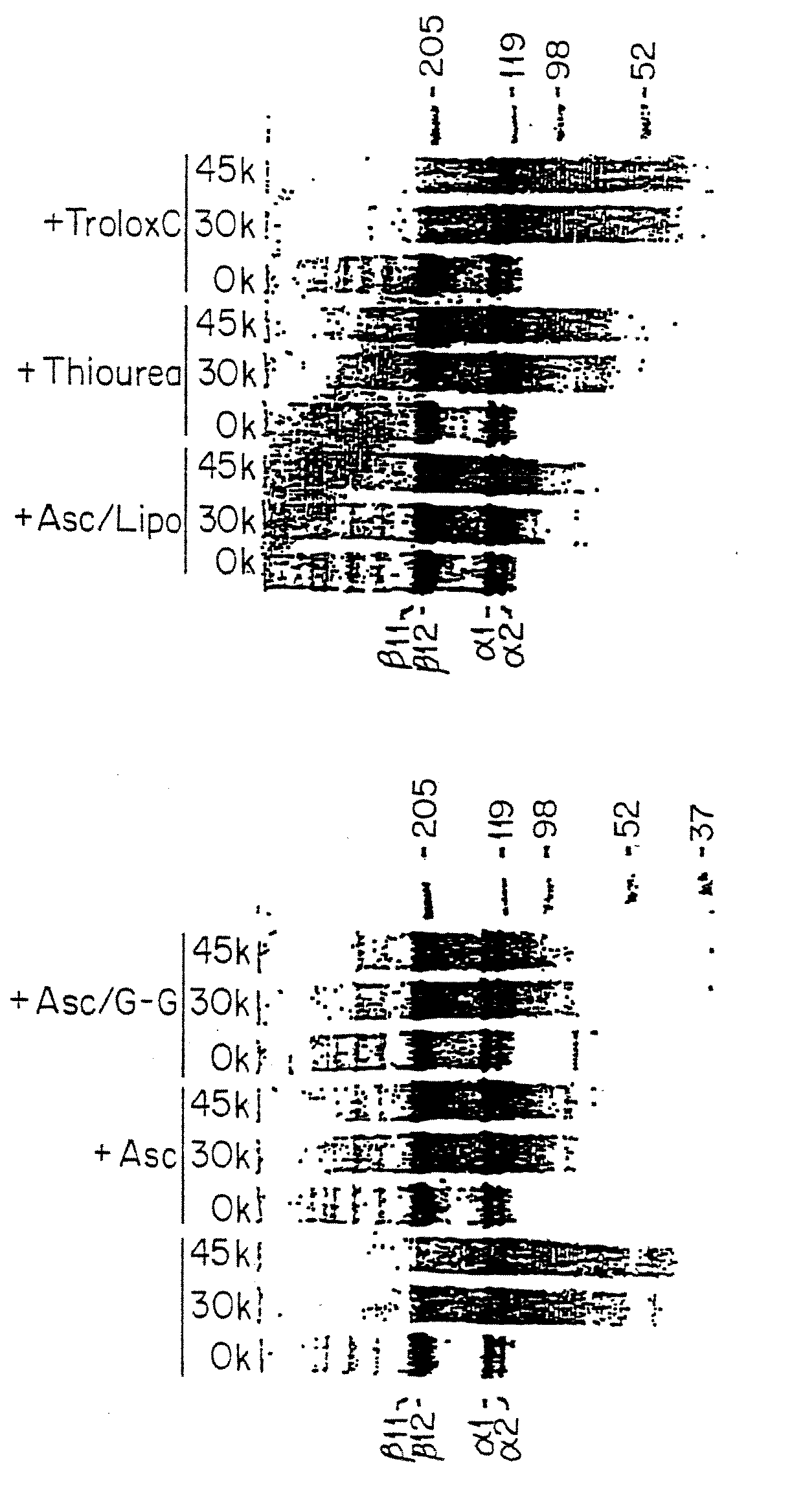
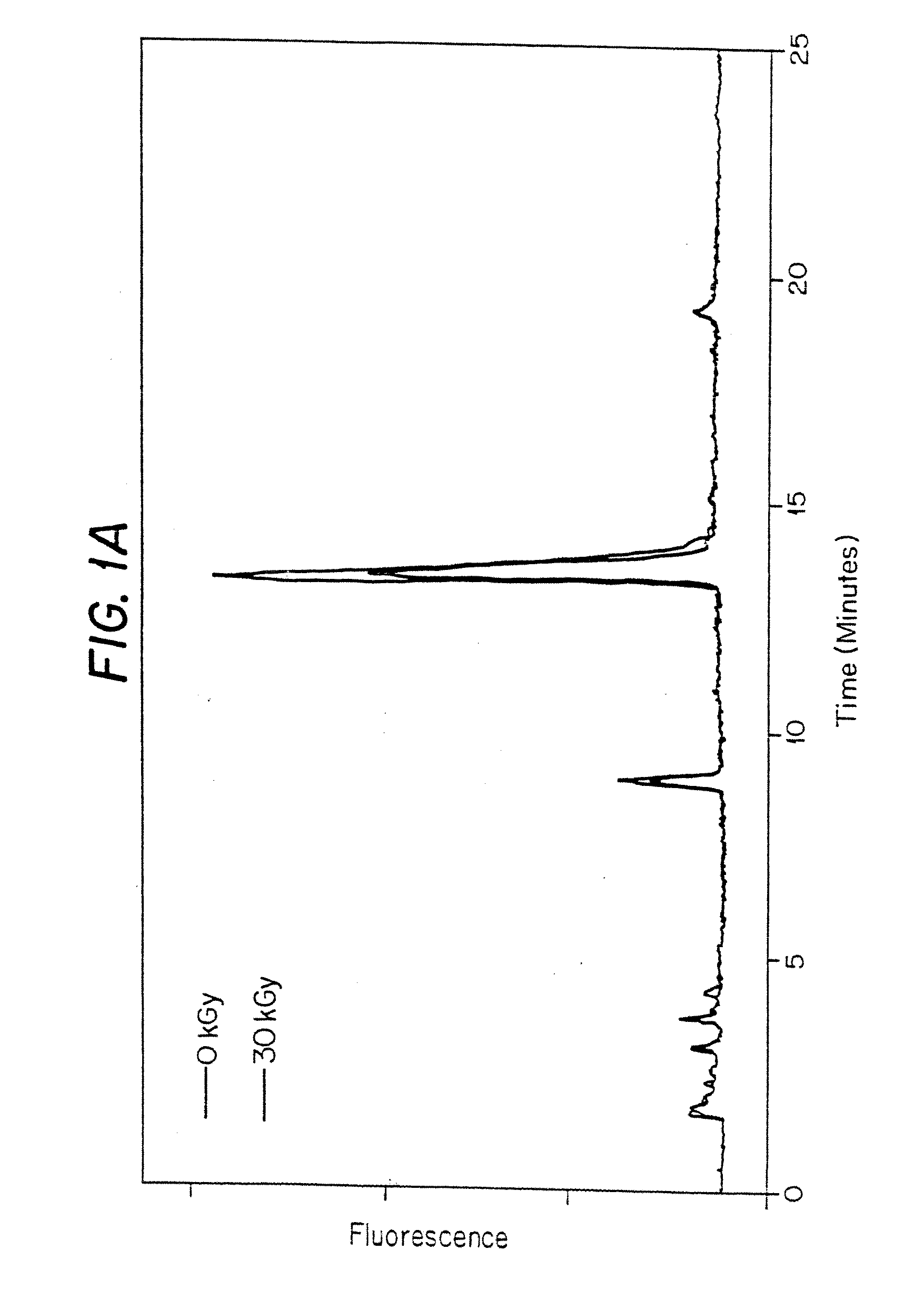
Methods for sterilizing biological materials by irradiation over a temperature gradient
InactiveUS6908591B2Effective sterilizationImprove permeabilityDead animal preservationLavatory sanitoryBiological materialsBiology
Methods are disclosed for sterilizing tissue to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria, (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, spores, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. The methods involve sterilizing one or more tissues with irradiation.
Owner:CLEARANT
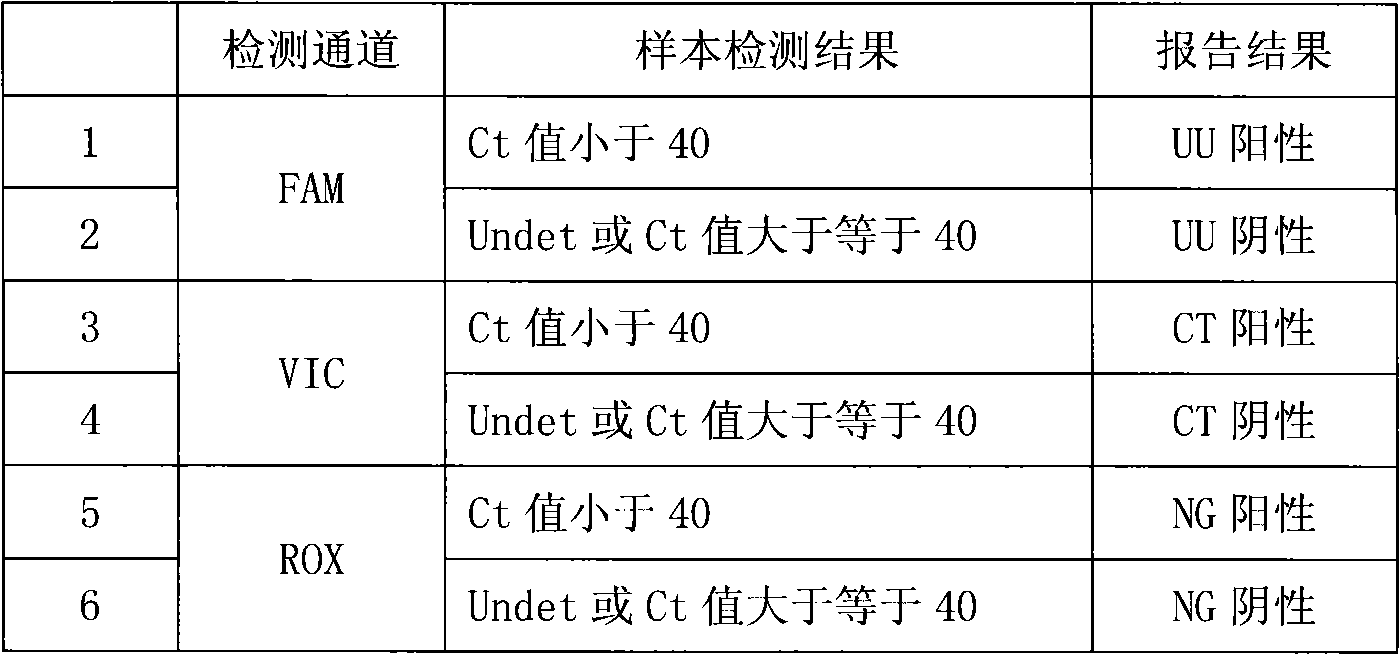
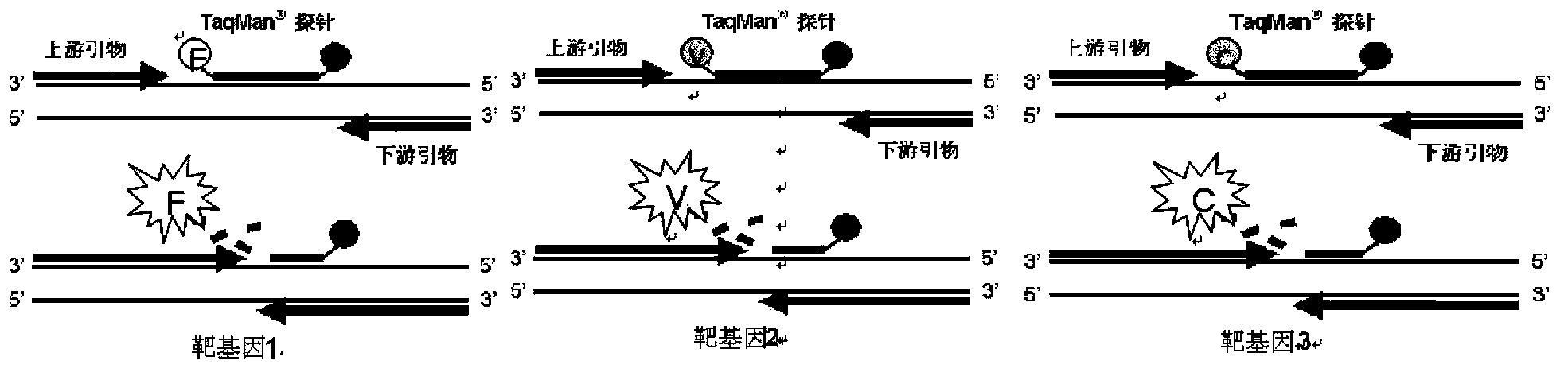
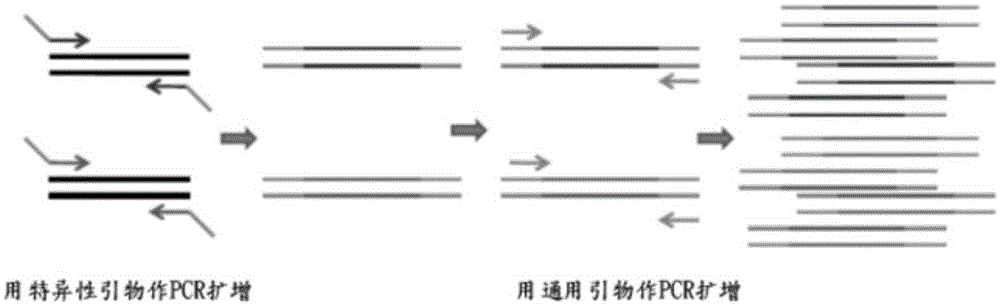
Fluorescence PCR method for diagnosing infection of Chlamydia trachomatis, neisseria gonorrhoeae and ureaplasma urealyticum
ActiveCN101613763AMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
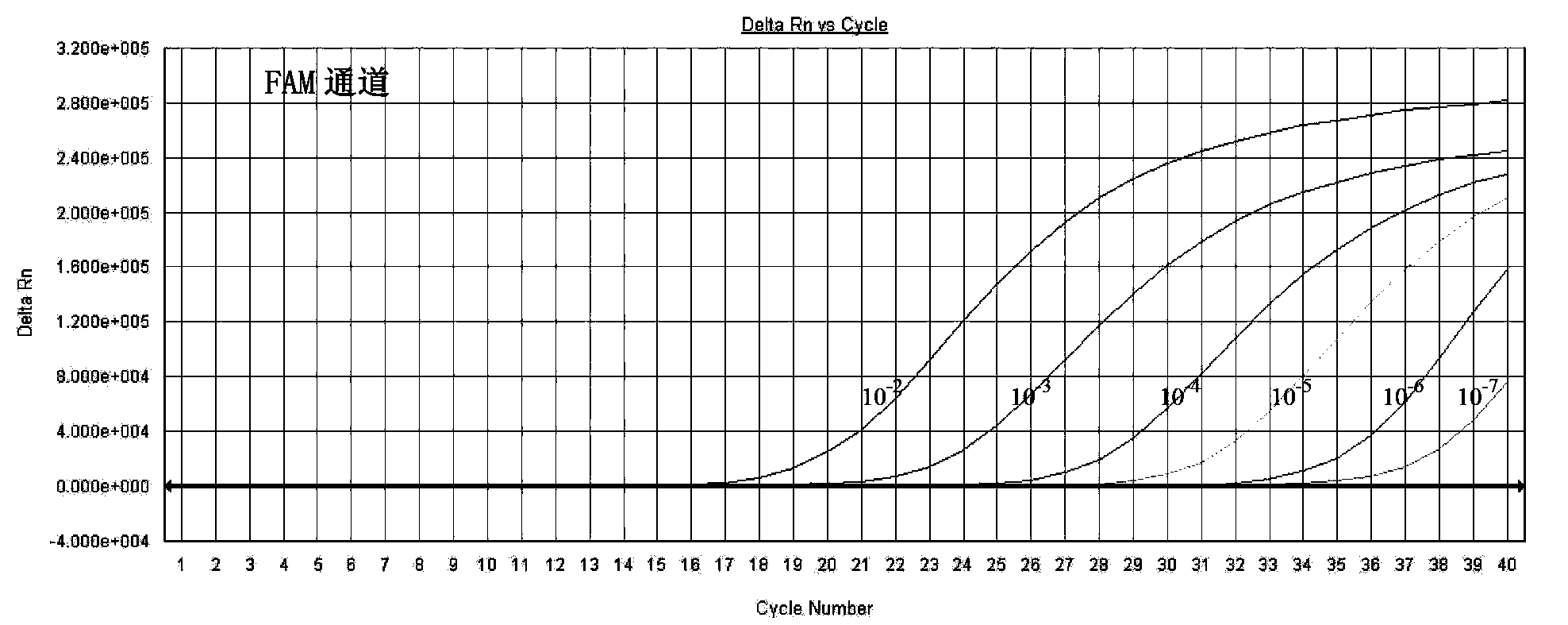
The invention relates to a fluorescence PCR (polymerase chain reaction) detection method for diagnosing infection of Chlamydia trachomatis (CT), neisseria gonorrhoeae (NG) and ureaplasma urealyticum (UU), which belongs to the field of nucleic acid in vitro diagnosis. The method comprises a polymerase chain reaction (PCR) system based on fluorescence PCR technology, contains a forward primer and areverse primer aiming at the CT / the NG / the UU and a fluorescent probe, and can detects DNA of three pathogens such as the CT, the NG, the UU and the like simultaneously in a reaction tub under suitable PCR condition. The method can diagnosing the infection of the CT / the NG / the UU in a clinical sample simply, conveniently and rapidly, has high sensitivity and specificity, and has important clinical value to early control and prevention of relevant genitourinary tract infections, blocking of an infection sources, and infection reduction of related pathogen.
Owner:CITY UNIVERSITY OF HONG KONG
Broad-spectrum and high-efficiency antibacterial washing liquor
InactiveCN102416011ABroad spectrum killingEfficient killingAntibacterial agentsBiocideEscherichia coliArginine
The invention relates to a medicine, in particular to broad-spectrum and high-efficiency antibacterial washing liquor and a preparation method thereof. The invention is characterized in that the antibacterial washing liquor is prepared by the following materials according to the weight ratio: 0.0001-2.0 of N-coconut-oil fatty-acid acyl L-arginine ethyl ester DL-pyrrolidone carboxylate, 0.0001-1.0 of dodecyl dimethyl benzyl ammonium chloride, 10.0-99.995 of water, and the balance of auxiliary materials. The broad-spectrum and high-efficiency antibacterial washing liquor has the following characteristics and advantages that pathogenic microorganisms are killed in a broad-spectrum and high-efficiency manner; ureaplasma urealytium, gonococcocci and trichomonas vaginalis can be killed in one minute; the killing rate for escherichia coli, staphylococcus aureus and candida albicans is respectively more than 99%; non-toxic; no stimulation is caused to the skin; no allergic reaction is caused; no stimulation is caused to the eyes; no stimulation is caused to vaginal mucosa; and the pH value of a preparation is close to that in the normal vagina.
Owner:艾硕特生物科技(昆明)有限公司
Methods of sterilizing biological mixtures using alpha-keto acids
InactiveUS20090214382A1Lower Level RequirementsBiocideDead animal preservationYeastBiological materials
Methods are disclosed for sterilizing biological materials to reduce the level of one or more biological contaminants or pathogens therein, such as viruses, bacterial (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, single or multicellular parasites, and / or prions or similar agents responsible. These methods involve the use of alpha-keto acids in methods of sterilizing biological materials with irradiation.
Owner:CLEARANT
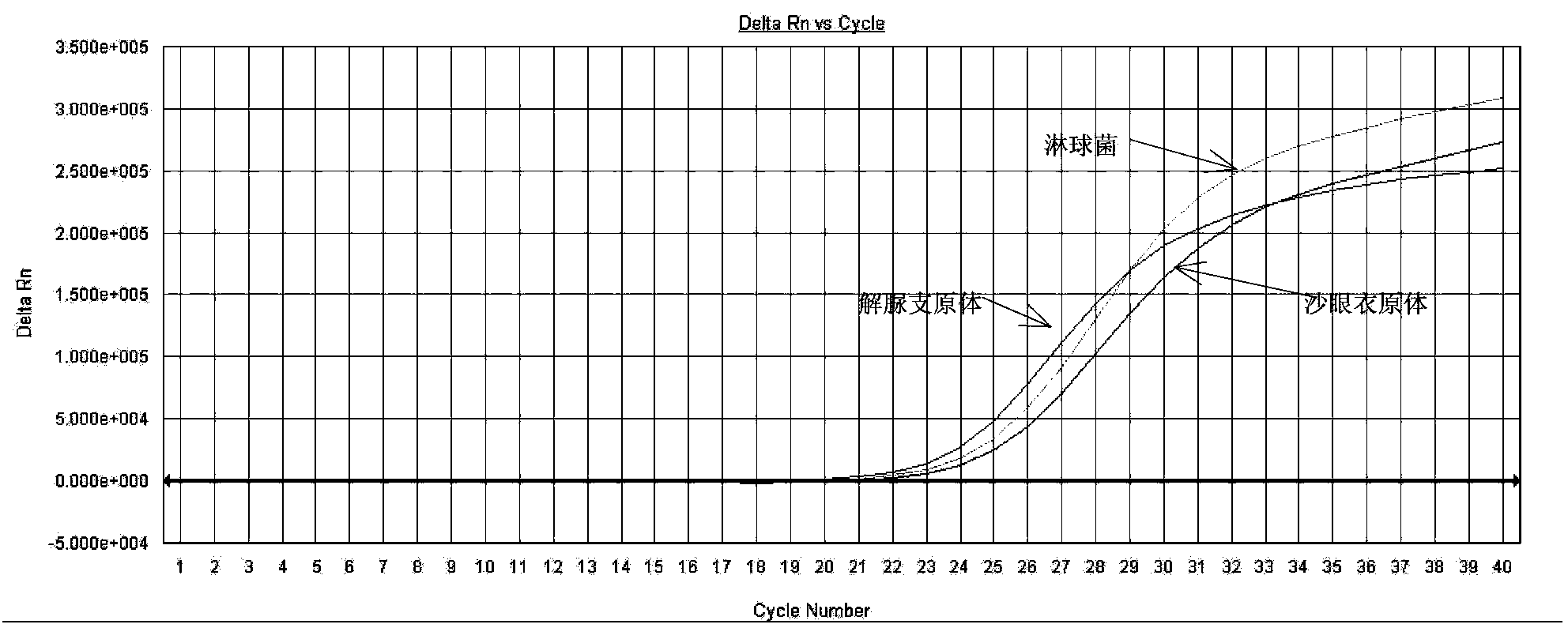
Quintuple fluorescent PCR (polymerase chain reaction) quick and hypersensitive detection kit and application thereof
ActiveCN102888464AGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceMycoplasma hominisPcr method
The invention relates to a real-time PCR (polymerase chain reaction) method for quintuply detecting target nucleic acid in a nucleic acid extracting solution in a single PCR reaction vessel, which is used for detecting Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in a sample.
Owner:苏州华益美生物科技有限公司
Methods for sterilizing biological materials containing non-aqueous solvents
InactiveUS7848487B2Reduce contentLowered residual solventAntibody ingredientsLavatory sanitorySolventBiological materials
Methods are disclosed for sterilizing biological materials to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. The methods involve sterilizing biological materials containing one or more non-aqueous solvents with irradiation.
Owner:CLEARANT
PCR detection reagent kit for sexual disease mixed infection
InactiveCN1865451AStrong specificityMicrobiological testing/measurementImmunodeficiency virusOidiomycin
The invention discloses a synchronizing detection PCR agent box (hepatitis B virus, human immunodeficiency virus, human breast tumor virus, lues helicoid, human cytomegalovirus, simple herpesvirus, white oidiomycin, urea-dissolving mycoplasma, gonotoxin and Chlamydi trachomatis), which consists of blood type and secretion stains type. The user adds predisposed sample in the enlarging pipe to start enlarging reaction, which finishes detecting work simply and rapidly. The invention can detect ten venereal disease causal agents, which is fit for customs quarantine, prevailing monitor and hospital screen examination.
Owner:广州中医药大学热带医学研究所
Methods for Sterilizing Biological Materials Containing Non-Aqueous Solvents
InactiveUS20090202039A1Reduce contentLowered residual solventLavatory sanitoryAntibody ingredientsSolventBiological materials
Methods are disclosed for sterilizing biological materials to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. The methods involve sterilizing biological materials containing one or more non-aqueous solvents with irradiation.
Owner:CLEARANT
Triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum/ chlamydia trachomatis
ActiveCN103409508ASimple and fast operationAvoid pollutionMicrobiological testing/measurementFluorescence/phosphorescenceSynechococcusPositive control
The invention discloses a triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis. The kit comprises an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reaction liquid, an enzyme mixed liquid, a triple reaction liquid for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis, positive control and negative control. The kit disclosed by the invention overcomes the deficiencies that in the prior art, neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis is poor in specificity, lower in sensitivity and the like, effectively prevents pollution, has the advantages of high sensitivity, good specificity, strong repeatability, quick and objective detection result and the like, and has good application prospect for detecting neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Methods for Sterilizing Tissue
InactiveUS20110091353A1Effective sterilizationAvoid radiationLavatory sanitoryChemicalsYeastIrradiation
Methods are disclosed for sterilizing tissue to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria, (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. The methods involve sterilizing one or more tissues with irradiation.
Owner:BURGESS WILSON +3
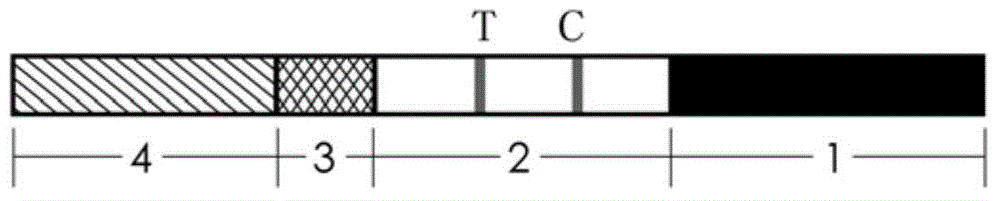
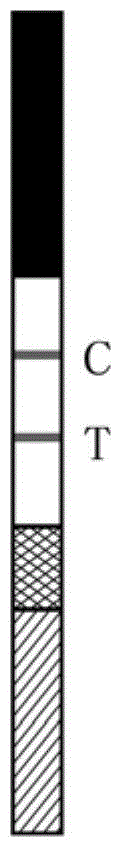
Test strip for quickly detecting ureaplasma urealyticum antibodies in saliva
InactiveCN101655496ASolve the problem that it is not suitable for on-site inspection and the inspection cost is highEasy to operateBiological testingAntigenNitrocellulose
The invention discloses a test strip for quickly detecting ureaplasma urealyticum antibodies in saliva. The test strip comprises a bottom plate on which a sample pad, a collaurum pad, a nitrocellulosefilm and a sample sucking pad are sequentially pasted, wherein the collaurum pad is attached with mouse anti-human IgG antibodies which are labeled by collaurum and can combine with the specificity of the ureaplasma urealyticum antibodies; and a detection line consisting of ureaplasma urealyticum antigens and a quality control line consisting of sheep anti-mouse secondary antibodies which can combine with the specificity of the mouse anti-human IgG antibodies are coated on the nitrocellulose film. The test strip adopts a collaurum labeling technique to detect the ureaplasma urealyticum antibodies in saliva so as to judge whether a testee infects ureaplasma urealyticum or not. The invention has the advantages of simple operation, high reaction speed and sensibility, strong specificity, suitability for on-site detection and self detection, economy, practicability, and the like.
Owner:杭州艾力康医药科技有限公司
PCR method of multiple sex propagate pathogene synchronous detection and kit
InactiveCN101935686AThe detection process is fastSimple stepsMicrobiological testing/measurementMicroorganism based processesDiseaseMycoplasma hominis
The invention discloses a single-tube multiple PCR method for synchronous rapid detection of five sex propagate pathogenes (neisseria gonorrhoeae (NG), mycoplasma hominis (MH), mycoplasma genitalium (MG), chlamydia trachomatis (CT) and ureaplasma urealyticum (UU)) and a kit. The method is characterized by amplifying the five pathogenes and performing gel electrophoresis separation and detection by reasonably designing a primer and optimizing the primer concentration combination and PCR condition in the same reaction tube and under the same heat cycle condition. The invention has the characteristics of sensitivity, rapidness, convenience, and the like of the classic PCR, mainly realizes synchronous detection of the five pathogenes, has lower cost, can be used for developing relative kits and is used for clinical diagnosis and epidemiological survey and control.
Owner:唐文志
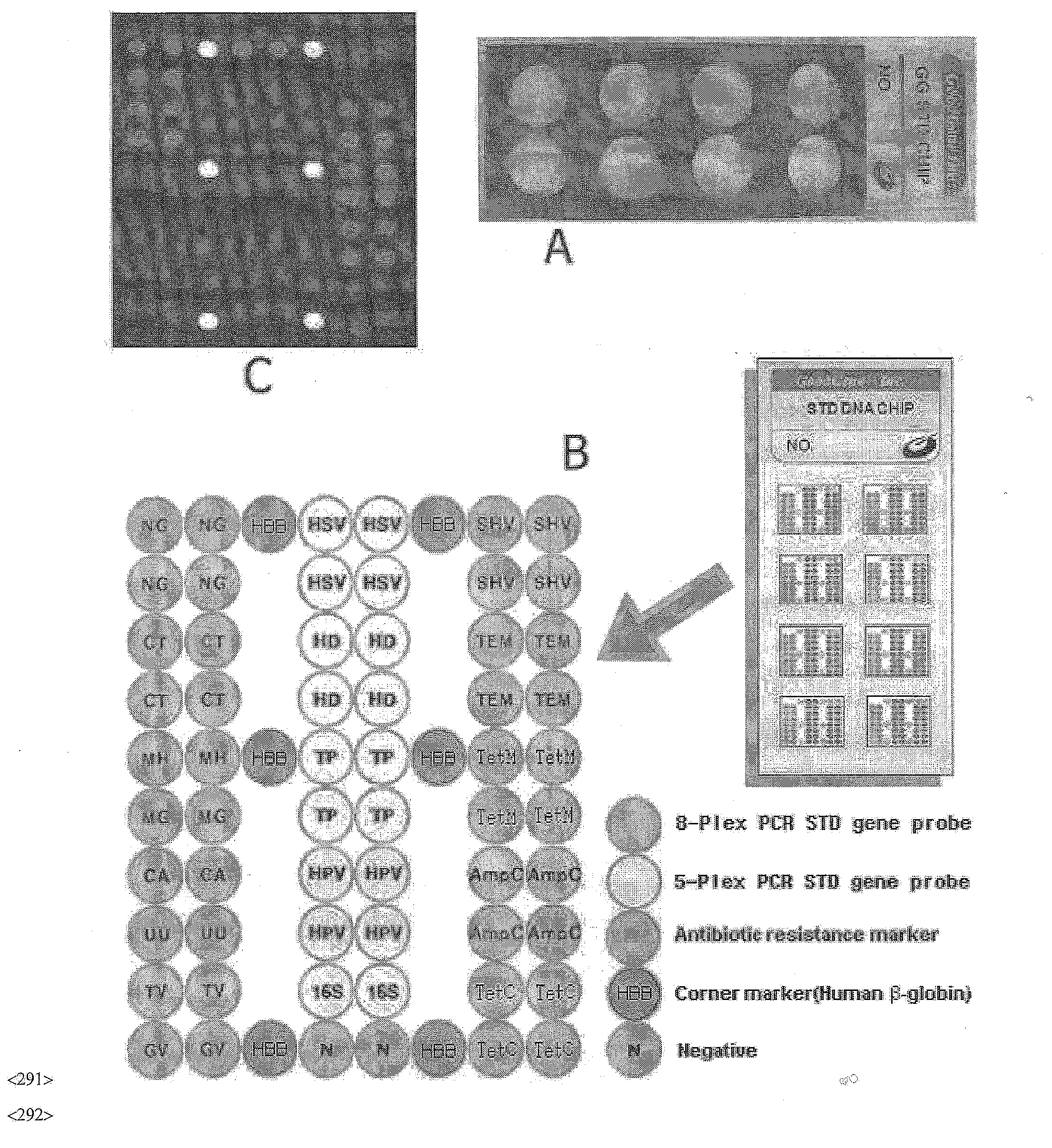
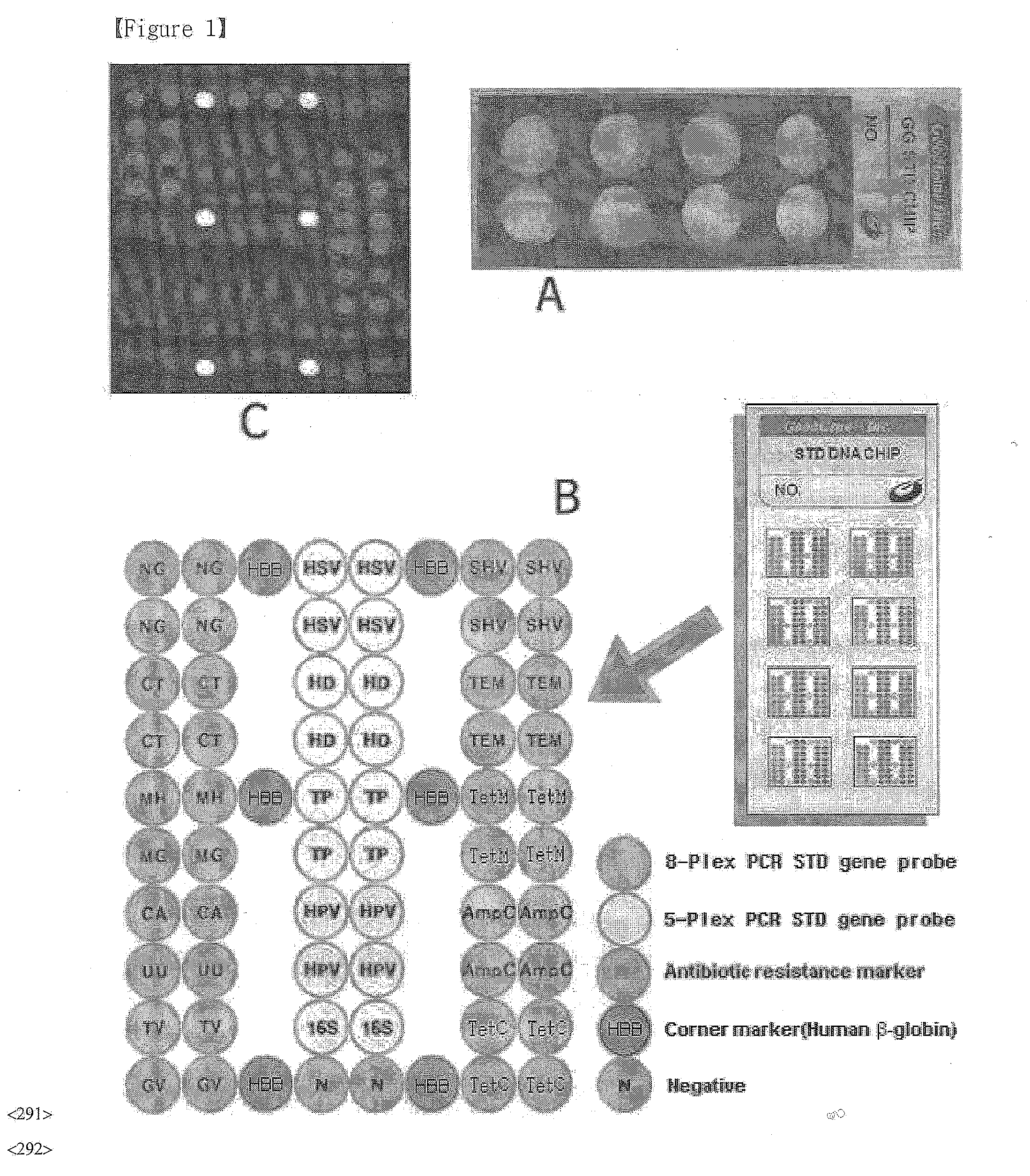
DNA chip, kit for detecting or genotyping bacteria causing sexually transmitted diseases, genotyping antibacterial drug resistance and detecting or genotyping method using the same
InactiveUS20120004113A1Quickly and accurately detectEasy to explainNucleotide librariesMicrobiological testing/measurementDiseaseEscherichia coli
Disclosed are a DNA chip and a kit capable of quickly and accurately detecting or genotyping the highly prevalent and important eleven microbes causing sexually transmitted diseases (STD) Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma genitalium, Mycoplasma hominis, syphilis-causing treponema, pallidum, chancroid-causing Haemophilus ducreyi, genital herpes-causing herpes simplex virus 1 and 2, human papillomavirus (HPV) and Trichomonas vaginalis and three related organisms Candida albicans, Gardnerella vaginalis and coliform bacteria and analyzing antibiotic resistance against tetracycline and lactam antibiotics, and a method for detecting or genotyping using the same. According to the present invention, the presence, genotype and antibiotic resistance of the fourteen organisms can be analyzed quickly and accurately from a DNA sample. With excellent sensitivity, specificity, reproducibility and accuracy of the 14 STD-causing and related microorganisms may be automatically identified quickly and accurately from multiple samples, and selection of antibiotics may be aided.
Owner:GOODGENE
Methods for sterilizing preparations containing albumin
InactiveUS7252799B2Reduce contentLow preparation temperaturePeptide/protein ingredientsAntipyreticYeastBlood plasma
Methods are disclosed for sterilizing preparations containing albumin to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. These methods involve sterilizing preparations containing albumin, such as plasma protein fractions, with irradiation.
Owner:CLEARANT
Combination detection gene chip kit for three kinds of genitourinary infection pathogen
InactiveCN101121946AReduce the number of detection reactionsReduce testing costsMicrobiological testing/measurementBiotechnologyPositive control
The invention relates to a combined test gene chip reagent kit for three urogenital tract infection pathogens, pertaining to the field of biotechnology. The gene chip consists of 18 sites, i.e. 5 specific and conservative test-target sequences of each pathogen of chlamydia trachomatis, mycoplasma urealytium and gonorrhoeae are taken as testing sites, and 2 positive control sites and 1 negative control site are added; PCR method is adopted to expand testing-target sites and DNA segments of the control sites to act as a probe and fixed on the peridium slide to achieve a test gene chip. The matrix of the chip is 9X9, each checking site is repeated by 4 times, and arranged evenly. The reagent kit is capable to meet the requirement of all reagents during the testing process. The testing method involves the combination of PCR expansion, molecular hybridization and fluorescent labeling. The invention has the advantages that the testing cost is low, pollution to testing samples is removed, the specificity and flexibility of testing are excellent. The practical clinic application proves that the flexibility of the invention is as high as 97 to 99.7 percent, and 99 to 99.7 percent for specificity.
Owner:昆明云大生化科技有限责任公司
Methods for sterilizing biological materials using dipeptide stabilizers
InactiveUS20060182652A1Reducing residual solvent contentEffective sterilizationOther blood circulation devicesDead animal preservationYeastDipeptide
Methods are disclosed for sterilizing biological materials to reduce the level of active biological contaminants or pathogens such as viruses, bacteria, nanobacteria, yeasts, molds, mycoplasmas, ureaplasmas, prions and parasites. These methods involve the use of dipeptide stabilizers in methods of sterilizing biological materials with irradiation.
Owner:CLEARANT
Rapid and sensitive genotype identification and nucleic acid detection
InactiveCN105392896AMicrobiological testing/measurementOrgan movement/changes detectionDiseaseUreaplasma parvum
The invention discloses a method, primer, probe and kit for identifying various gene mutations or substance genetic typing. In one embodiment, the disease is pathogenic. In the other embodiment, the invention is applied to detection of multidrug-resistant Mycobacterium tuberculosis, hepatitis B virus, beta-globulin mutation, thrombophilia related mutation; or various sexually transmitted pathogens, consisting of Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum, Treponema pallidum, Herpes simplex virus 1 , Herpes simplex virus 2, Human papillomavirus type 6, and Human papillomavirus type 1.
Owner:DIAGCOR LIFE SCI LTD
Pepper extract with function of resisting infection of ureaplasma urealyticum and mycoplasma hominis
InactiveCN102058709AAnti-Ureaplasma urealyticumHas the effect of Mycoplasma hominis infectionAntibacterial agentsPlant ingredientsTreatment effectMycoplasma hominis
The invention provides a pepper extract with a function of resisting infection of ureaplasma urealyticum and mycoplasma hominis, relating to a pepper extract and aiming at solving the problems that the drug resistance of the ureaplasma urealyticum and the mycoplasma hominis to antibiotic is increased to cause poor treatment effect. The pepper extract is obtained by extraction through the following method: pepper is ground into powder, the powder is put into a supercritical CO2 extraction kettle, the extraction temperature is 30-55 DEG C, the separating temperature is 25-45 DEG C, the extraction pressure is 15-35MPa, the separation pressure is 4-6MPa, the flow of CO2 is 4-12, and the extraction time is 1-2 hours, and then the pepper extract is obtained. The pepper extract is non-toxic, has the functions of resisting the infection of ureaplasma urealyticum and mycoplasma hominis and resisting the infection of drug-resistant ureaplasma urealyticum and drug-resistant mycoplasma hominis, and is applied in the field of resisting the infection of mycoplasma.
Owner:黑龙江省中医研究院
Kit for detecting mycoplasma urealytium and human mycoplasma
ActiveCN106319028AAvoid the inconvenience of re-admission to see the resultsSimple and fast operationMicrobiological testing/measurementMycoplasma hominisAniline
The invention discloses a kit for detecting mycoplasma urealytium and human mycoplasma. The kit comprises four detection reagents: a compound of L-proline and enzyme substrate as chromogen for detecting activity of proline aminopeptidase, a compound of L-leucine and enzyme substrate as chromogen for detecting activity of leucine aminopeptidase, a compound of alpha-D-glucoside and enzyme substrate as chromogen for detecting activity of alpha-glucosidase and a compound of neuraminic acid and enzyme substrate as chromogen for detecting activity of neuraminidase, wherein the chromogen is aniline, naphthaline, naphthol, indoxyl and derivative thereof. According to the invention, a dry chemical enzyme method is adopted for detecting the enzyme compounded in the growth process or the enzyme generated by stimulating host cells, so that the purpose of detecting and identifying mycoplasma urealytium and human mycoplasma can be achieved; and the kit has the advantages of high speed, convenience, accuracy, and the like.
Owner:AUTOBIO DIAGNOSTICS CO LTD
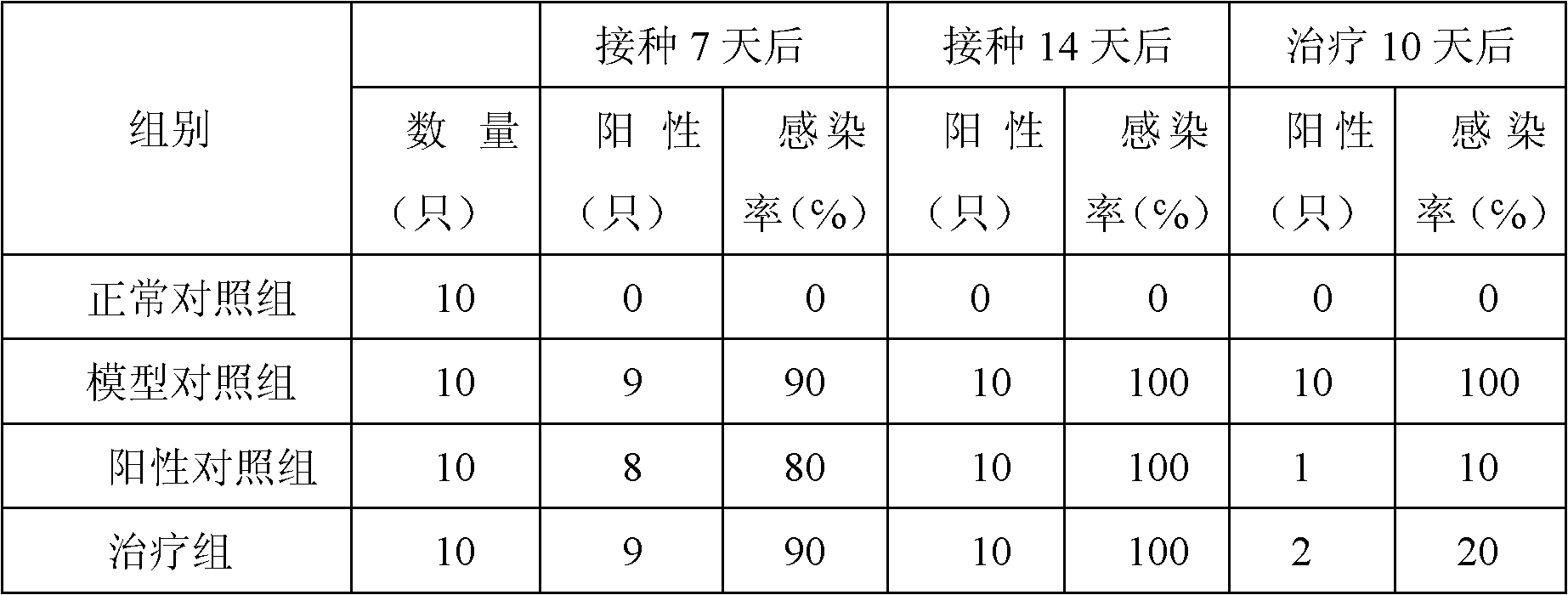
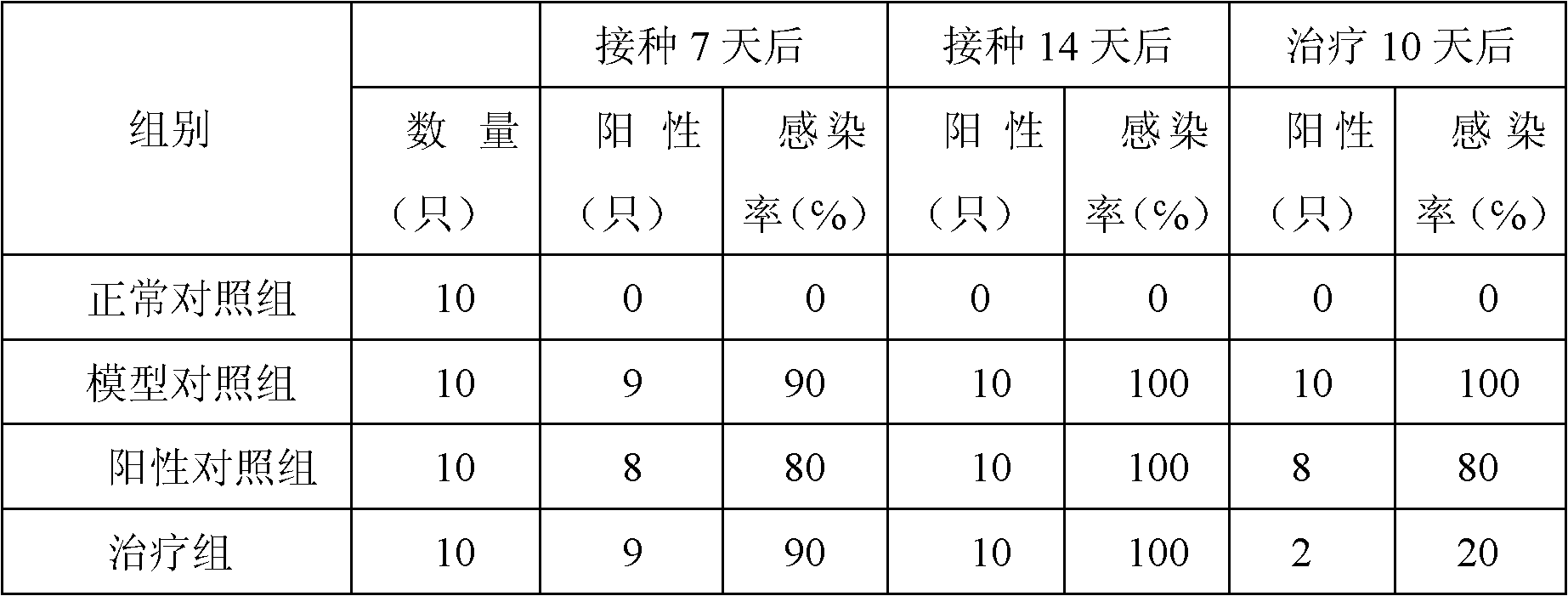
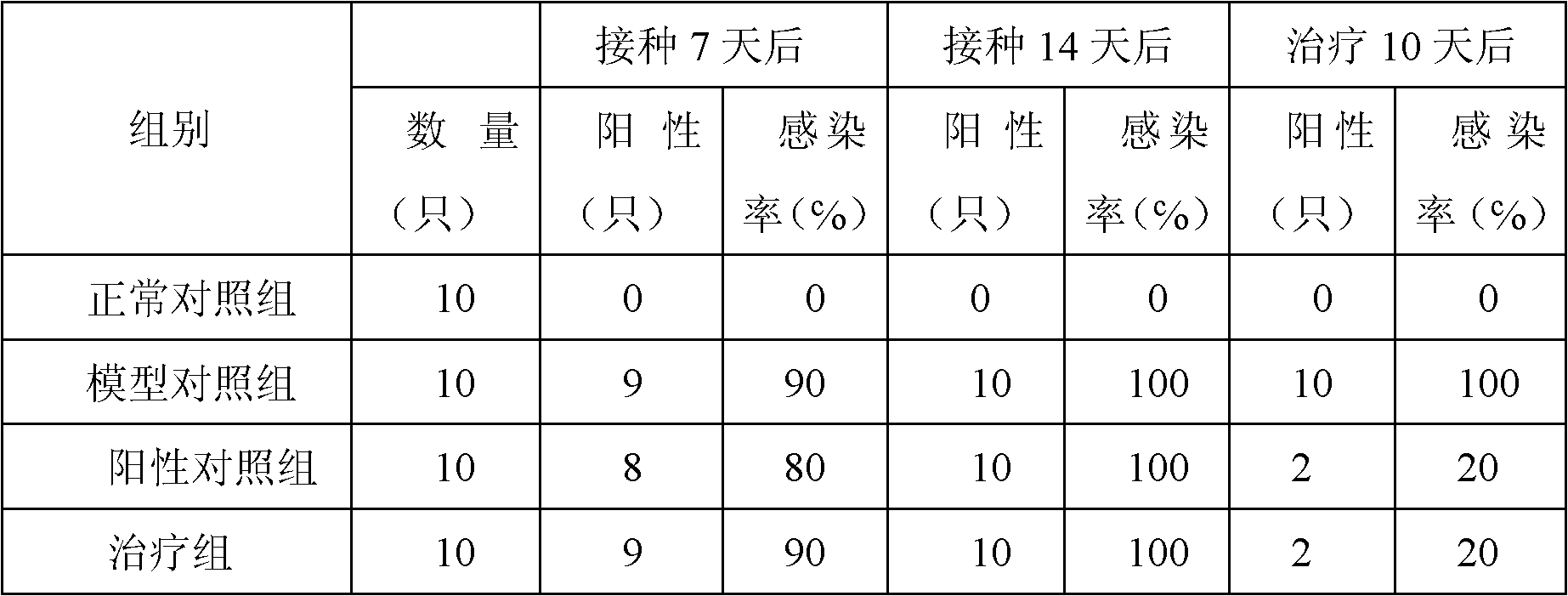
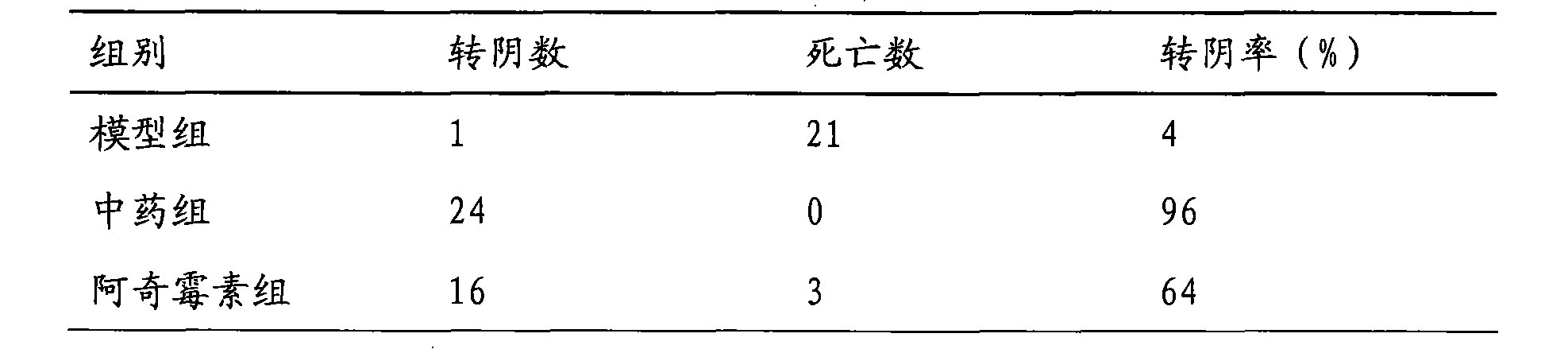
Chinese medicinal composition for treating ureaplasma urealyticum infection and preparation method thereof
InactiveCN101862393AGood antibacterial effectNo side effectsAntibacterial agentsAntiviralsBacteroidesDisease
The invention discloses a Chinese medicinal composition for treating ureaplasma urealyticum infection and a preparation method thereof. The Chinese medicinal composition comprises the following raw material medicaments in part by weight: 30 to 45 parts of szechwon tangshen root, 20 to 45 parts of large-headed atractylodes rhizome, 20 to 45 parts of glossy ganoderma, 30 to 50 parts of houttuynia cordata thumb, 20 to 50 parts of weeping forsythia and 15 to 30 parts of cortex phellodendri. The Chinese medicinal composition has the effect of regulating immune cell factors, and reduces the damage of bacterial pathogens such as tumor necrosis factors, interferon-gamma blocking ureaplasma urealyticum, chlamydia trachomatis and the like to reproductive systems. In-vitro solid and liquid bacteriostatic experiments prove that the Chinese medicinal composition has the obvious bacteriostatic effect, is superior to antibiotic azithromycin applied at home and abroad, and has no toxic or side effect. The Chinese medicinal composition is mainly used for treating male and female internal and external reproductive system diseases caused by the infection of the ureaplasma urealyticum, the chlamydia trachomatis and various bacteria and virus and symptoms of infertility and the like caused by the diseases.
Owner:韩延华 +1
Methods for sterilizing tissue
InactiveUS20080080998A1Effective sterilizationAvoid radiationApparatus sterilizationDead animal preservationFungal microorganismsYeast form
Methods are disclosed for sterilizing tissue to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria, (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. The methods involve sterilizing one or more tissues with irradiation.
Owner:CLEARANT
Yolk bioprotein foam filler and preparation method thereof
InactiveCN105749277AAvoid infectionMaintain a healthy physiological environmentAntibacterial agentsEgg immunoglobulinsEscherichia coliBacteroides
The invention discloses a yolk bioprotein foam filler which is mainly composed of 8-10% of multi-link yolk protein, 0.1-0.3% of preservative, 40-50% of matrix and the balance of a foamer.The multi-link yolk protein is yolk bioprotein capable of being combined with various pathogens, and the pathogens include, but not limited to ureaplasma urealyticum, mycoplasma hominis, staphylococcus, streptococcus, Escherichia coli, Gardnerella vaginalis, gonococcus and Tritirachium album.The yolk bioprotein foam filler is mainly composed of yolk bioprotein aiming at inhibiting pathogenic bacteria, can be combined with corresponding pathogens, can effectively inhibit and remove pathogens, is effective on most bacteria, mycete and mycoplasma, does not have impact on probiotics, can maintain health and physiological environment of a reproductive system, does not enter blood, is higher in safety during local use, is free of irritation and side effect on human body, dependency, drug resistance and hypersensitivity and can be used for various clinical therapeutic products for vaginitis.
Owner:西安昱子生物科技有限公司
Chlamydia trachomatis and ureaplasma urealyticum nucleic acid detection kit
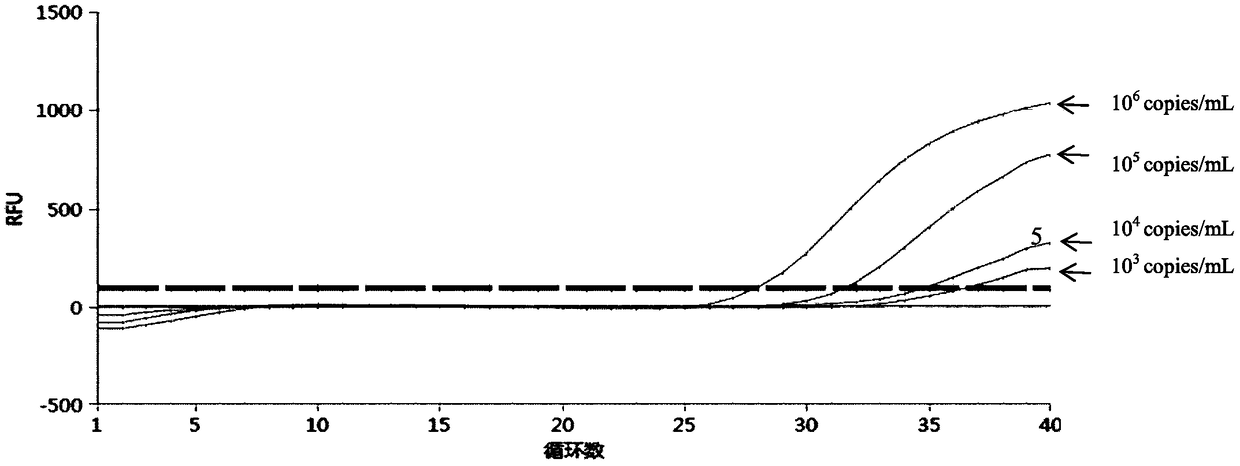
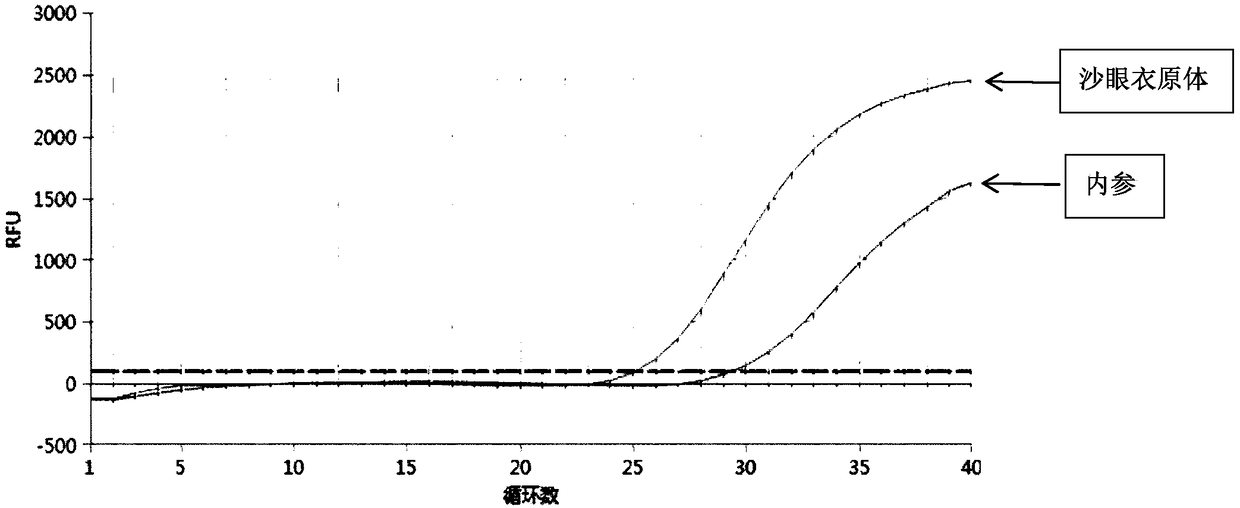
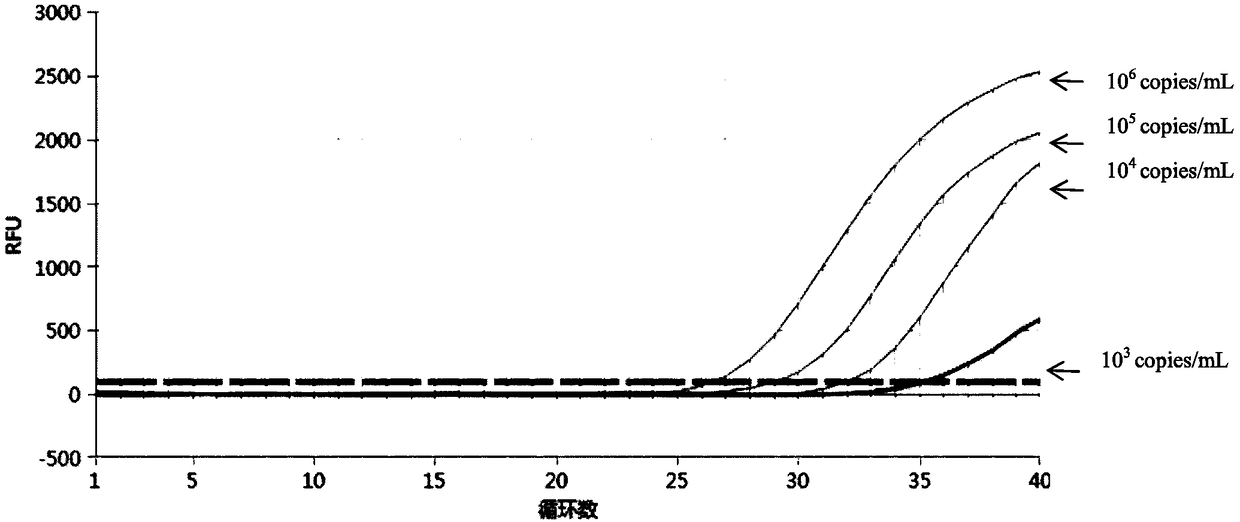
InactiveCN105349660AStrong specificityQuick checkMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionChlamydia trachomatis
The invention provides a chlamydia trachomatis and ureaplasma urealyticum nucleic acid detection kit which comprises a chlamydia trachomatis and ureaplasma urealyticum PCR reaction solution. The PCR reaction solution comprises a reaction buffer solution, deoxyribonucleoside triphosphote, upstream and downstream primers used for target polynucleotide amplification, a probe used for target polynucleotide detection and the like. The probe used for target polynucleotide detection is high in specificity. By the adoption of the kit, chlamydia trachomatis and ureaplasma urealyticum DNA in an unknown sample like genital secretion can be rapidly detected at the same time, the detection sensitivity can reach 200 copies / ml, the detection range is 200 copies / ml-4.00E+10 copies / ml, the reliable experiment basis is provided for diagnosing chlamydia trachomatis and ureaplasma urealyticum infection, and the problems that an existing kit is low in detection efficiency, poor in specificity and low in sensitivity can be solved.
Owner:SANSURE BIOTECH INC
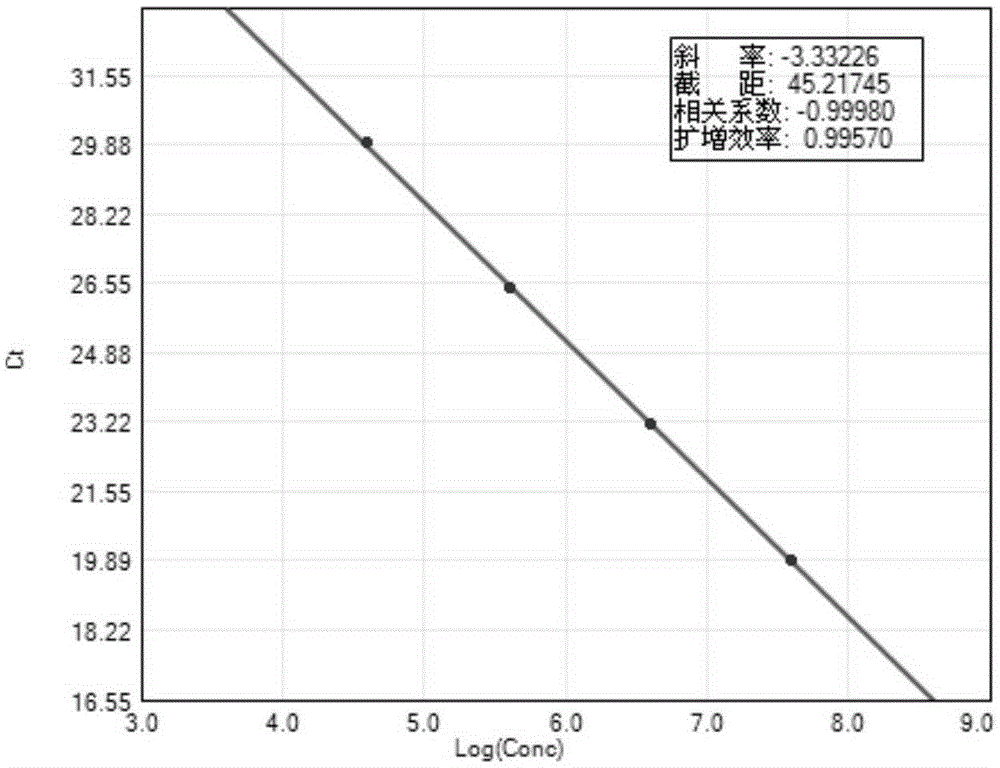
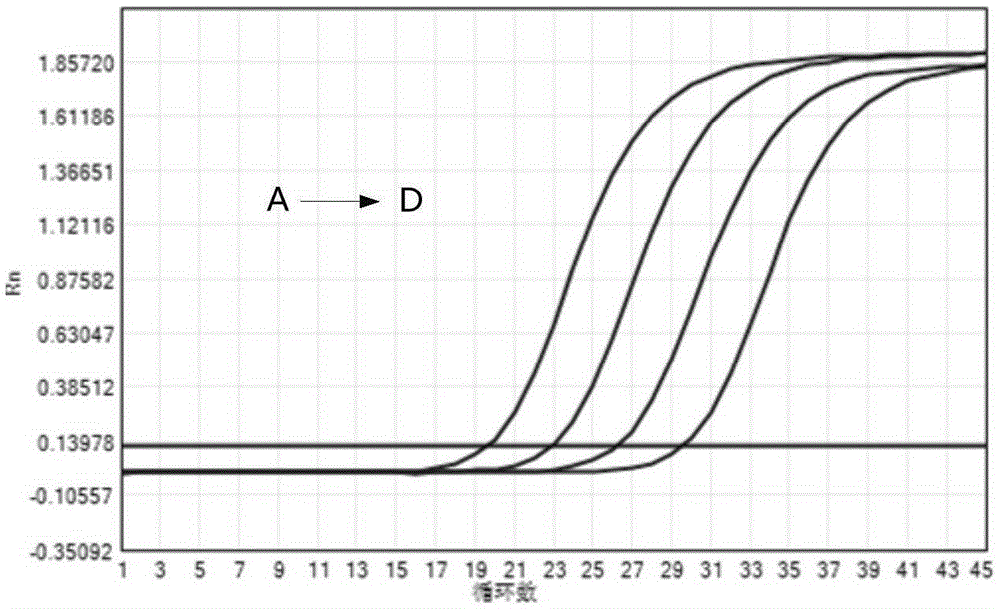
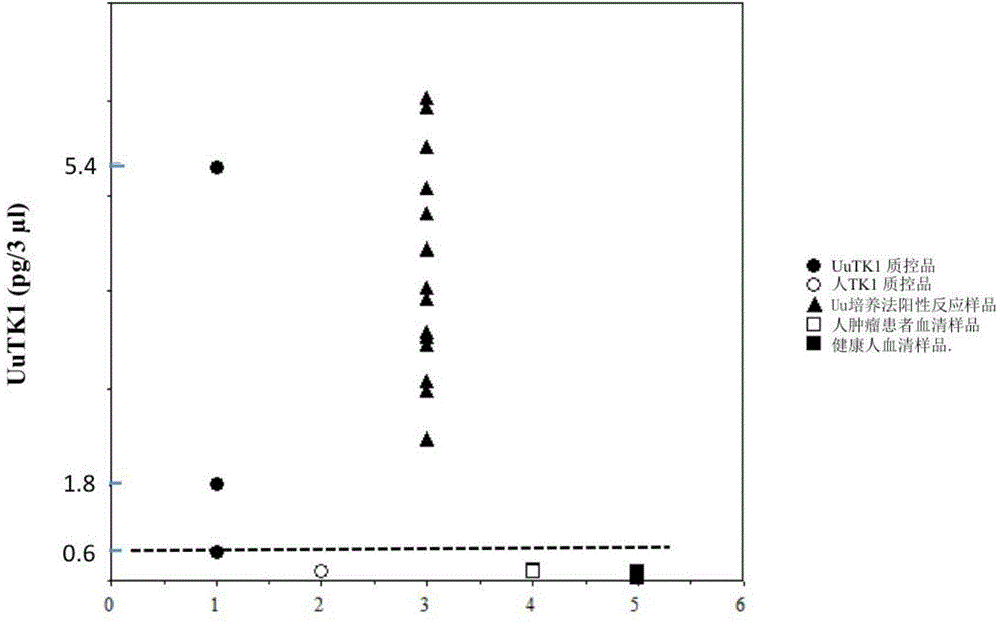
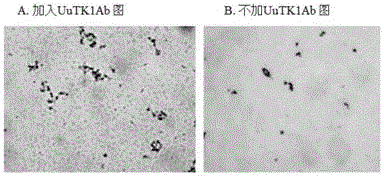
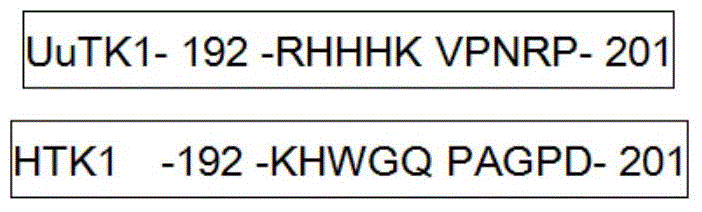
Preparation method of thymidine kinase 1 antibody and application of thymidine kinase 1 antibody in proliferation of ureaplasma urealyticum
ActiveCN104672331AHigh purityIncrease productionTransferasesImmunoglobulins against enzymesAntigenDisease cause
The invention provides design of an antigen for ureaplasma urealyticum thymidine kinase 1 (Ureaplasma urealyticum thymidine kinase1, UuTK1) and a preparation method of an antibody for the antigen. The antibody of UuTK1 and a composition kit of the antibody are capable of specifically identifying a UuTK1 antigen and used for detecting the proliferation degree of the ureaplasma urealyticum. The antibody kit has the characteristics of high sensitivity, high specificity, low cost and the like and is used for being applied to detecting the proliferation speed of the ureaplasma urealyticum in people with human urogenital tract and newborn respiratory tract infection diseases.
Owner:SHENZHEN HUARUI TONGKANG BIOTECHNOLOGICAL
Genital tract pathogen nucleic acid detection kit
InactiveCN109457037AImprove stabilityReduce inconvenienceMicrobiological testing/measurementMicroorganism based processesFreeze-dryingMycoplasma hominis
The invention discloses a genital tract pathogen nucleic acid detection kit which comprises a first detection component, a second detection component and a third detection component which are freeze-dried and simultaneously perform fluorescent quantitative PCR (polymerase chain reaction) detection after detection samples are added. The first detection component is used for detecting Chlamydia trachomatis and Neisseria gonorrhoeae, the second detection component is used for detecting mycoplasma urealytium and mycoplasma hominis, and the third detection component is used for detecting mycoplasmagenitalium. The kit can simultaneously detect five nucleic acids such as the Chlamydia trachomatis, the Neisseria gonorrhoeae, the mycoplasma urealytium, the mycoplasma hominis and the mycoplasma genitalium in a genital tract, detection of five pathogenic microorganisms is integrated into a consumable, reagent stability is improved by the aid of a freeze-drying process, the kit is disposable whenbeing used by each user, only single sampling and one-step operation are needed, laboratory operators are greatly free, detection cost is reduced, inconvenience of a patient is decreased, and the financial burden of the patient is relieved.
Owner:MERLIN BIOMEDICAL (XIAMEN) CO LTD
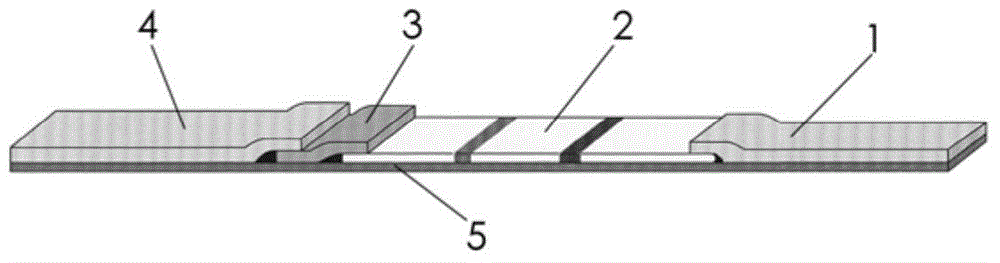
Mycoplasma urealytium immunogold labeling detection kit and detection method
The invention provides a mycoplasma urealytium immunogold labeling detection kit and a detection method. The kit comprises a sample pad, a colloidal gold pad, a nitrocellulose membrane, water absorption filter paper and a reaction supporting substance; the sample pad is arranged at the bottom end of the reaction supporting substance, the top end of the sample pad presses the bottom end of the colloidal gold pad and is stuck to the bottom end of the colloidal gold pad, the top end of the colloidal gold pad presses the bottom end of the nitrocellulose membrane and is stuck to the bottom of the nitrocellulose membrane, and the top end of the nitrocellulose membrane is pressed by the bottom end of the water absorption filter paper and stuck to the bottom end of the water absorption filter paper. The detection processes of mycoplasma urealytium in a human urinary or genital tract secretion sample can be simplified. By utilizing the detection kit to detect the mycoplasma urealytium, detection operation has no dependency on any experiment instrument or environment or operating personnel, operation is easy and convenient, the detection cycle is short, and interpretation is easy; in addition, special instruments or devices are not needed, professional training is not needed, the adaptability is high, and monitoring and inspecting are conveniently conducted at anytime anywhere.
Owner:江苏戴格诺思生物技术有限公司
Anti-3 type mycoplasma urealytium MB protein antibody and immunochromatography kit applying antibody
ActiveCN105968197AReduce manufacturing costStrong specificityBiological material analysisImmunoglobulins against bacteriaLinear epitopeMycoplasma
The invention relates to an anti-3 type mycoplasma urealytium MB protein antibody and an immunochromatography kit applying the antibody to detect the mycoplasma urealytium. The anti-3 type mycoplasma urealytium MB protein antibody is an antibody for identifying linear epitopes consisting of 105 to 118 amino acids of a 3 type mycoplasma urealytium MB protein; a serial number of the 3 type mycoplasma urealytium MB protein in GenBank is AAC41437.1; the sequence of 105 to 118 amino acids of the 3 type mycoplasma urealytium MB protein is KLPREPKPNEQLTI. The rabbit anti-3 type mycoplasma urealytium MB protein antibody provided by the invention has the characteristics of good specificity, high purity, high titer and low preparation cost.
Owner:HUBEI UNIV OF TECH +1
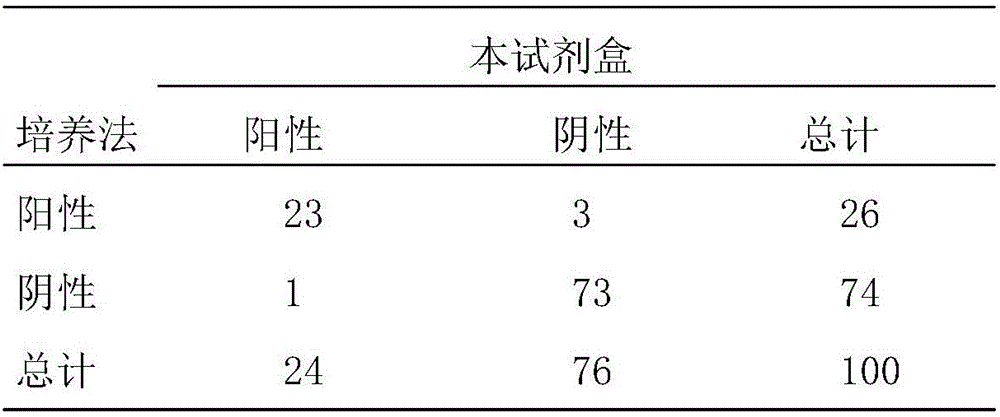
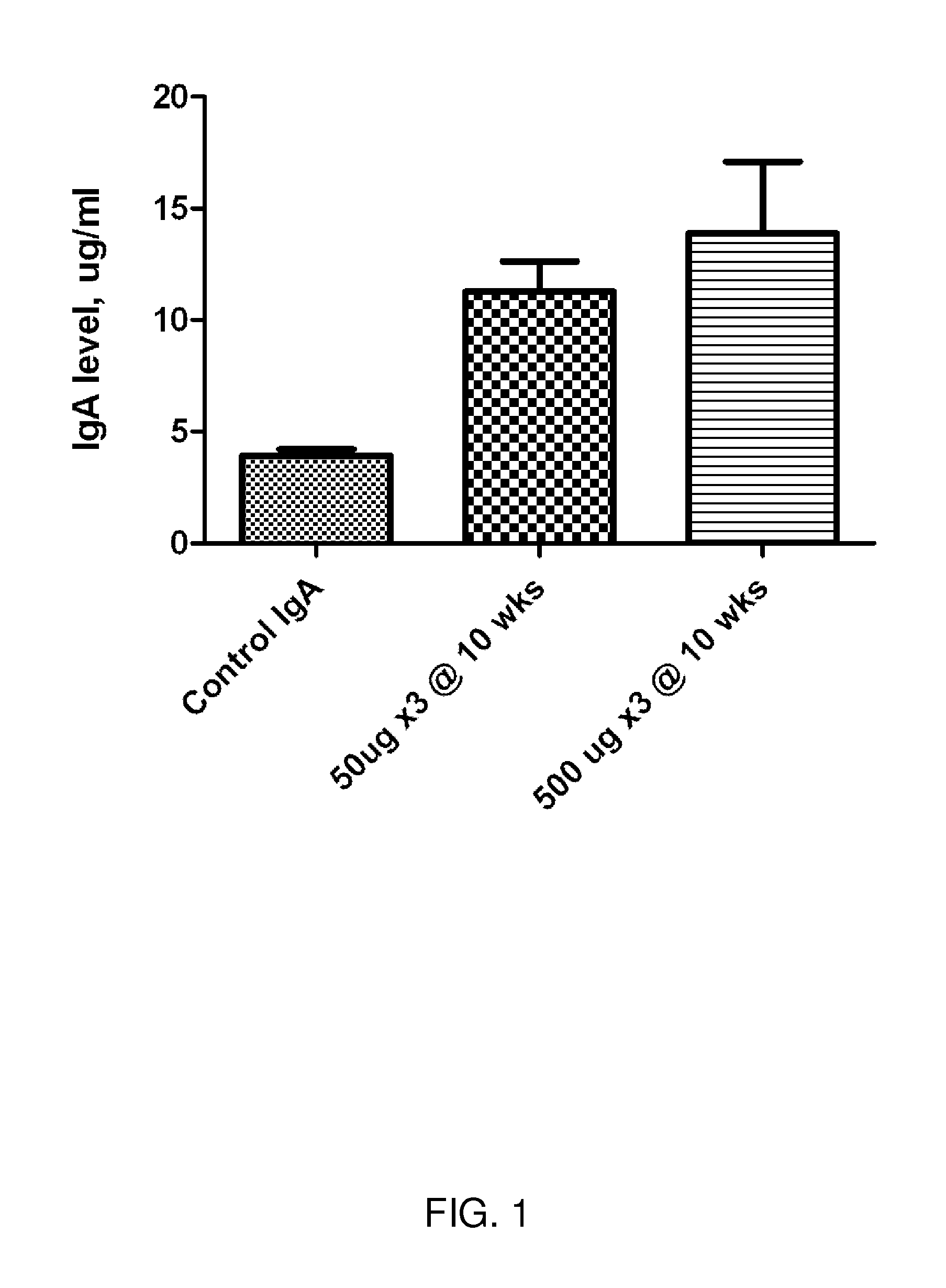
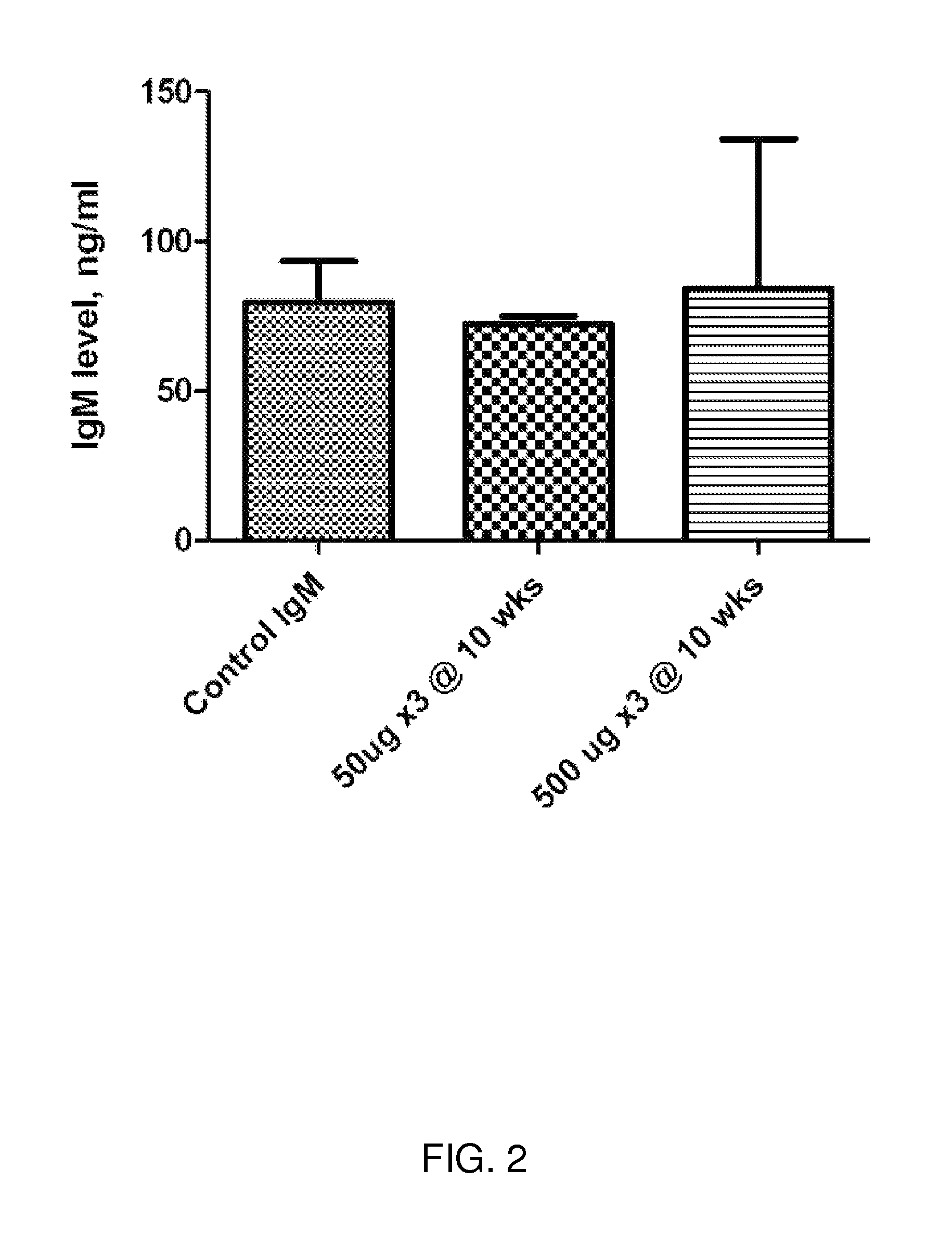
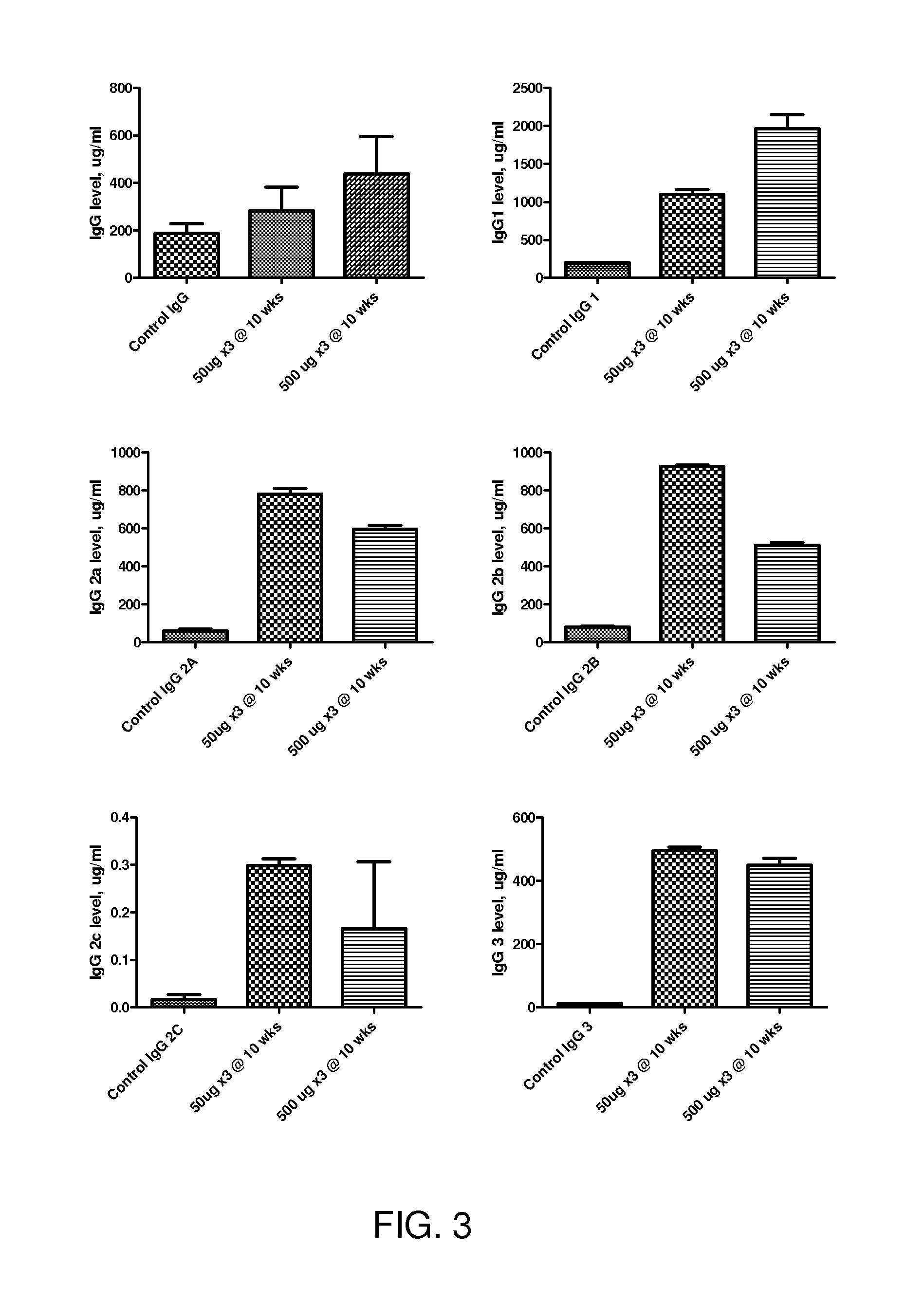
Ureaplasma vaccine and antibody for prevention and treatment of human, animal and cell culture infection
Owner:BAYLOR COLLEGE OF MEDICINE
Method for preventing and treating mycoplasma urealyticum infections
InactiveCN105853673AAntibacterial agentsPlant ingredientsCephalanthus occidentalisMucuna sempervirens
The invention discloses a method for preventing and treating mycoplasma urealyticum infections. The method comprises the following contents: (1) attention should be paid to hygiene, underwear and underpants should be separately washed and preferably be cooked in boiling water for 5 min or above, towels and washbowls should be separately used, and a bathtub and a water closet should be regularly disinfected; (2) a patient should improve his constitution and have good rest; and (3) the patient should take a traditional Chinese medicine decoction prepared from slenderstalk dicranostigma herb, lotus flower, Folium Cephalanthus occidentalis, Rhizoma Disporopsis fuscopicta, Folium Dalbergia mimosoides, Boehmeria gracilis, Mucuna sempervirens, turnip, Radix Pueraria omeiensis, Radix Trapa incise and herb of common achyranthes, wherein results of clinical test show that the total effective rate of the decoction reaches 97.5%.
Owner:滕海斌
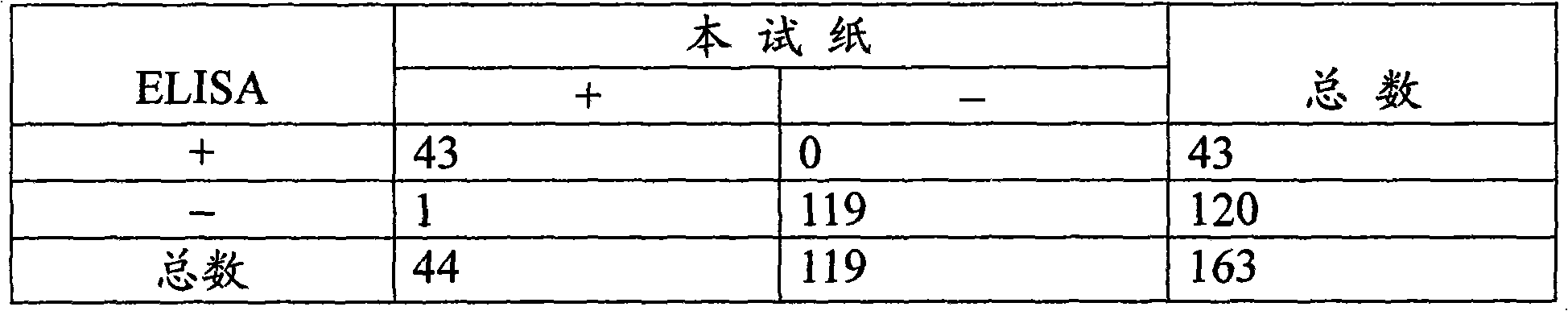
Diagnostic marker for ureaplasma urealyticum infection and preparation method and application of detection kit corresponding to diagnostic marker
PendingCN112946297AImprove throughputHigh reproducibility of resultsMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsMycoplasmaClinical method
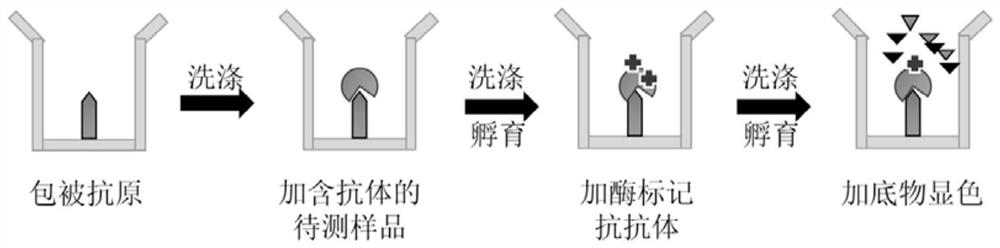
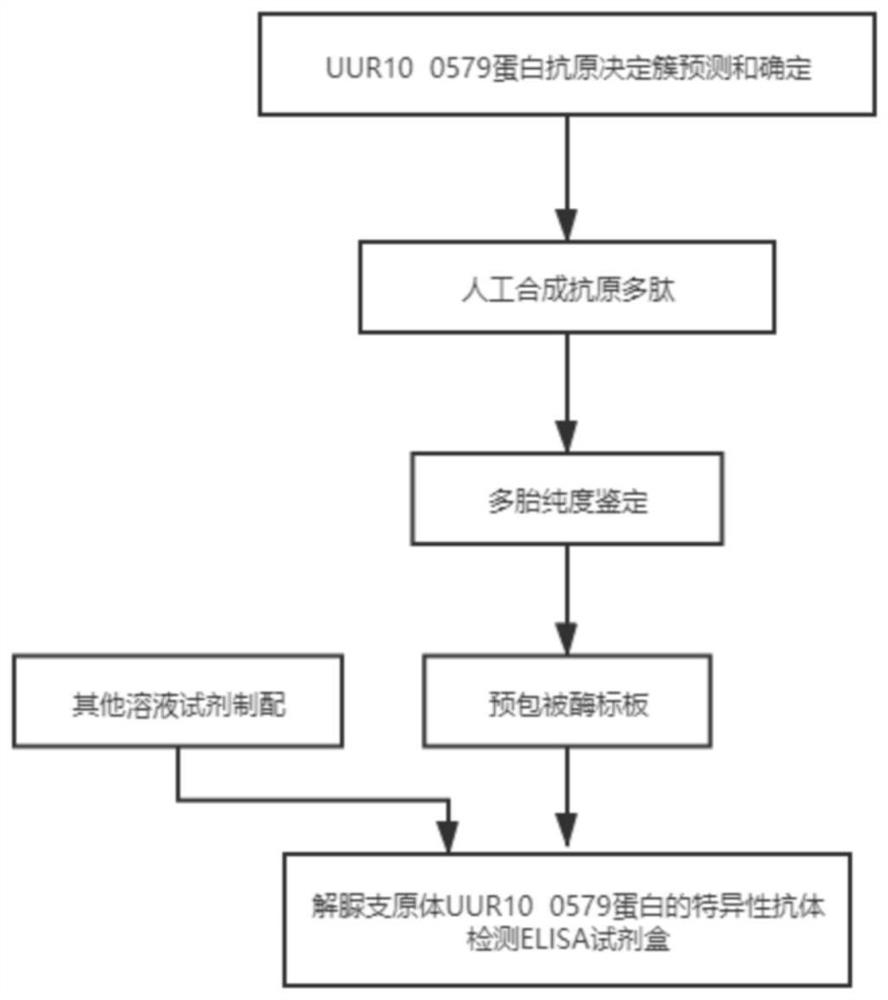
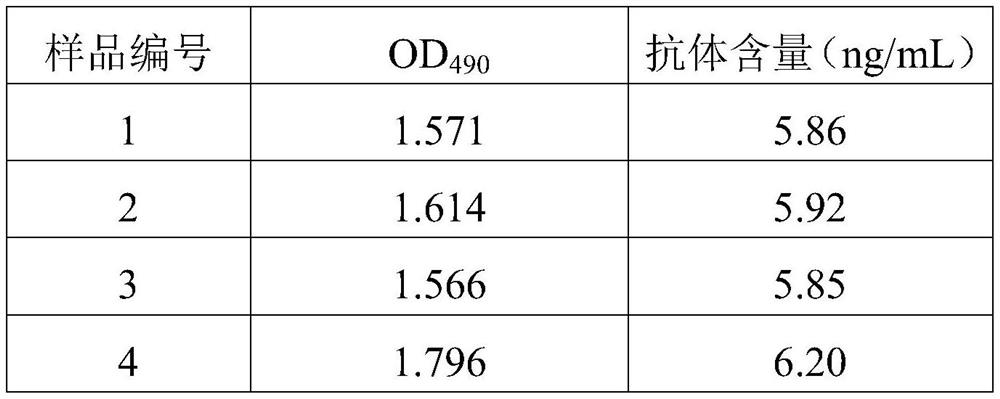
The invention discloses a preparation method and application of a diagnostic marker for ureaplasma urealyticum infection and a detection kit corresponding to the diagnostic marker, the diagnostic marker is a polypeptide containing a UUR10_0579 protein antigenic determinant site, and the amino acid sequence of the diagnostic marker is shown as a sequence 1. According to the invention, pathogen-related specific antibodies are detected through quantitative ELISA, and the kit has the advantages of rapidness, simplicity, high throughput and high result repeatability; and secondly, the UUR10_0579 protein is an important antigen for early infection of the ureaplasma urealyticum and participates in immune response of an organism, so that the UUR10_0579 protein can be used as a specific diagnostic marker for infection of the ureaplasma urealyticum. Compared with a current clinical method for detecting the ureaplasma urealyticum, the method for detecting the ureaplasma urealyticum infection, provided by the invention, has the advantages of early stage, rapidness, sensitivity, specificity, accuracy and the like, and overcomes the defects of the current clinical detection method. The detection method is objective in result judgment and simple and convenient to operate, is suitable for large-scale screening tests, and can be used for final definite diagnosis of ureaplasma urealyticum infection.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com