Chlamydia trachomatis and ureaplasma urealyticum nucleic acid detection kit
A technology for Ureaplasma urealyticum and Chlamydia trachomatis, which is applied in the field of Chlamydia trachomatis/Ureaplasma urealyticum nucleic acid detection kits, which can solve the problems of low detection efficiency, low sensitivity, and poor specificity of kits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment provides a Chlamydia trachomatis / Ureaplasma urealyticum nucleic acid detection kit, the Chlamydia trachomatis / Ureaplasma urealyticum nucleic acid detection kit includes a Chlamydia trachomatis / Ureaplasma urealyticum PCR reaction solution, and the Chlamydia trachomatis The Ureaplasma urealyticum PCR reaction liquid further comprises the following components:
[0038] (1) 0.2 μmol / L-0.4 μmol / L probe for target polynucleotide detection, the probe sequence for target polynucleotide detection is: Chlamydia trachomatis probe: 5'-CTTAAAAGACAATGGATTACCTAT-3 '; Ureaplasma urealyticum probe: 5'-TGGAAGGGGCAAGAGATGGTAAGT-3'.
[0039] (2) 0.2mmol / L deoxyribonucleoside triphosphate.
[0040] (3) Upstream and downstream primers for target polynucleotide amplification of 0.2 μmol / L-0.4 μmol / L, described upstream and downstream primers for target polynucleotide amplification and probes for target polynucleotide detection The needles are primers and probes derived from ...
Embodiment 2
[0052] This embodiment provides the operation steps of the Chlamydia trachomatis / Ureaplasma urealyticum nucleic acid detection kit described in the above-mentioned Example 1 for detecting Chlamydia trachomatis / Ureaplasma urealyticum DNA in unknown samples such as genital secretions:
[0053] 1. Reagent preparation
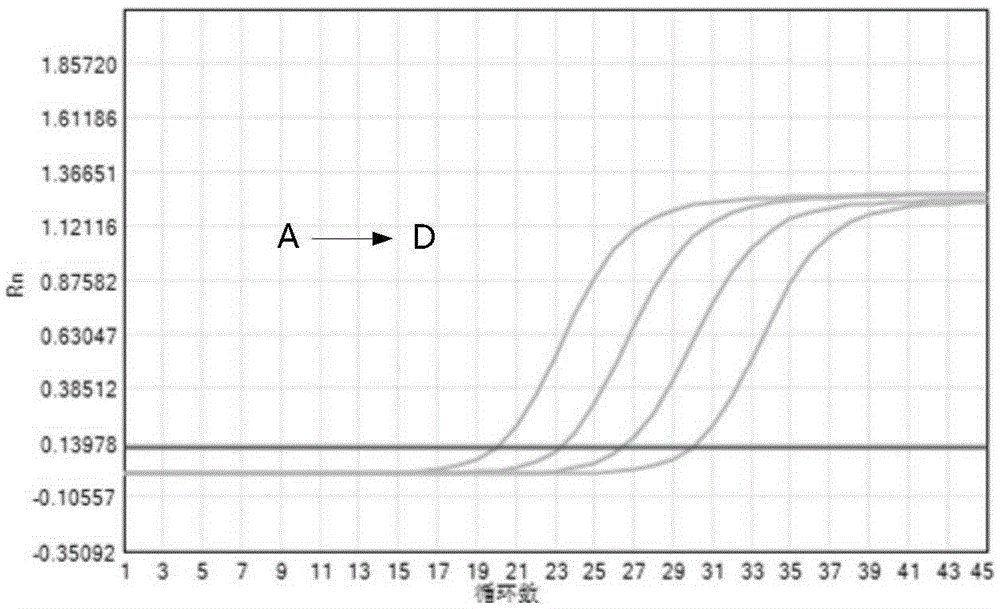
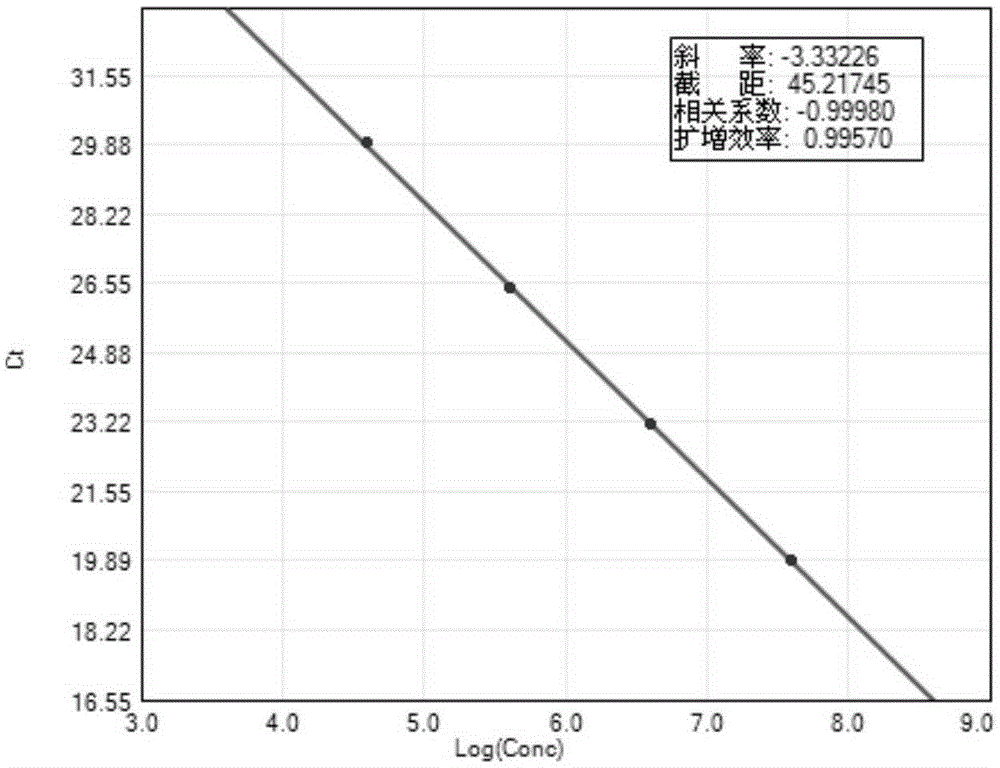
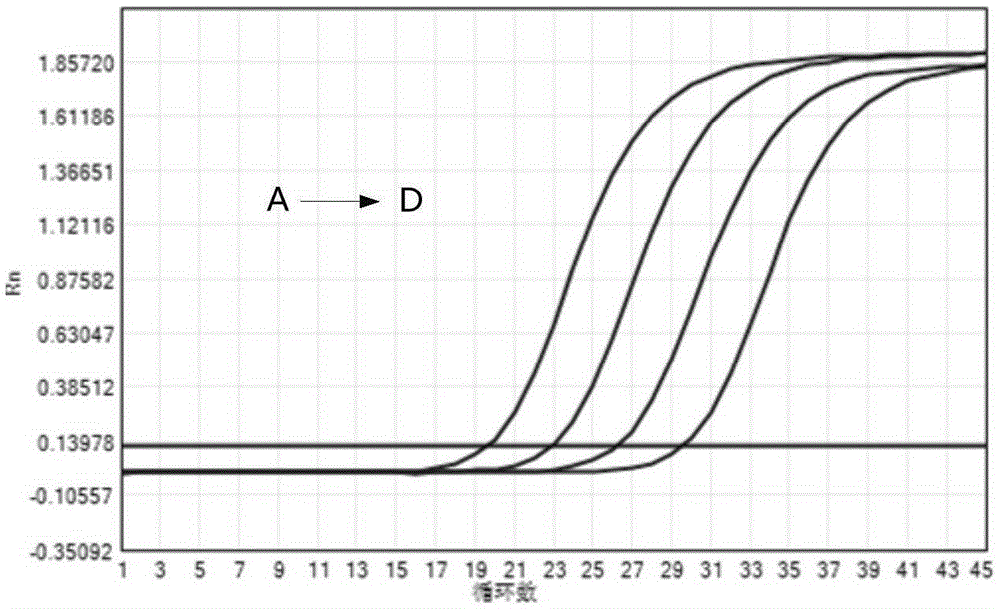
[0054] According to the number of samples to be tested, negative control, positive control, and positive reference products A-D, take the corresponding amount of PCR reaction solution and enzyme mixture in proportion (reaction solution 40 μl / person + enzyme mixture 2 μl / person), and mix thoroughly. Homogenize PCR-mix, centrifuge briefly and set aside.
[0055] 2. Sample processing
[0056] 1. Method A: Nucleic acid extraction by magnetic bead method
[0057](1) Cracking virus: add 200 μl-1ml DNA extraction solution I to each tube, then add 100 μl-1ml sample to be tested, cover the tube cap, shake and mix for 10 seconds, and centrifuge instantaneously;
[0058] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com