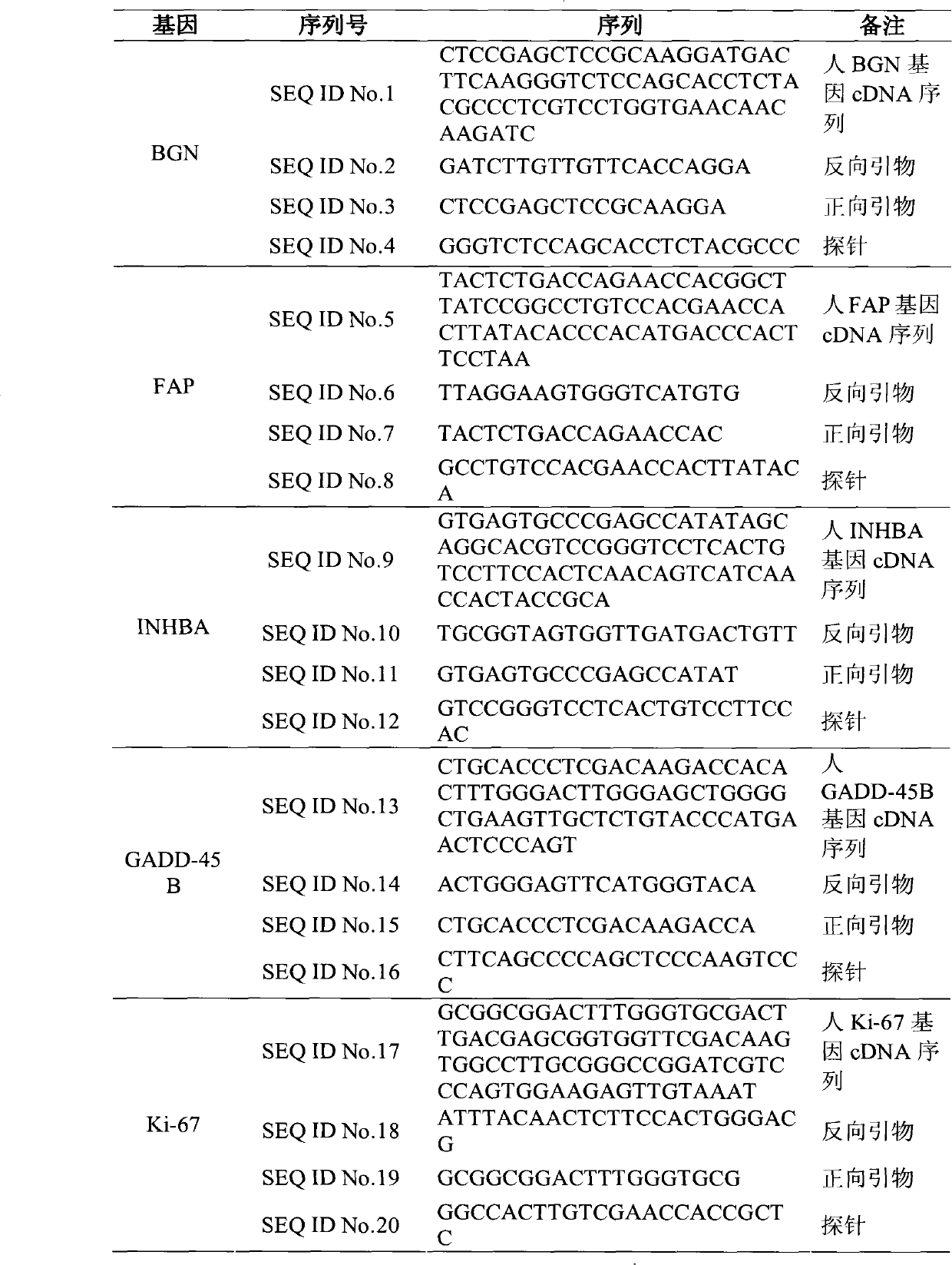
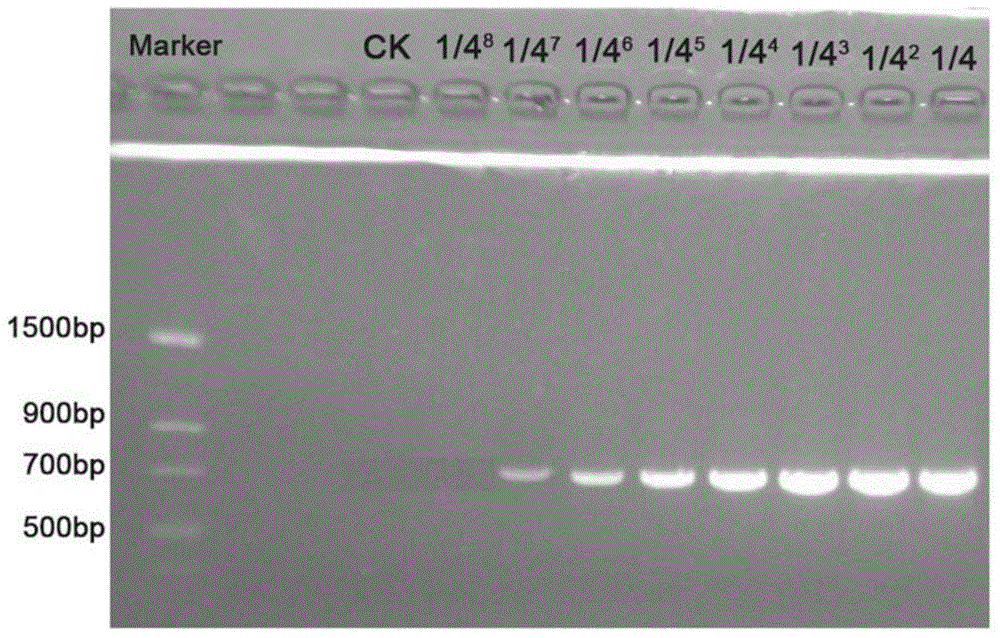
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
132 results about "Deoxyribonucleoside triphosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glycosamine consisting of a base linked to a deoxyribose sugar esterified with triphosphate on its glycose moiety.
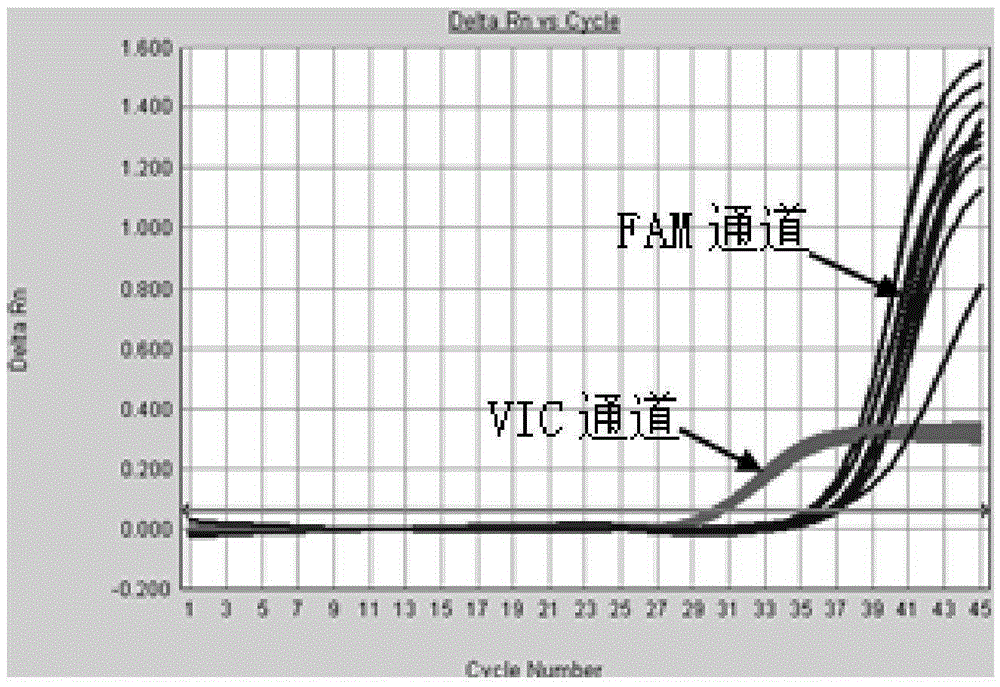
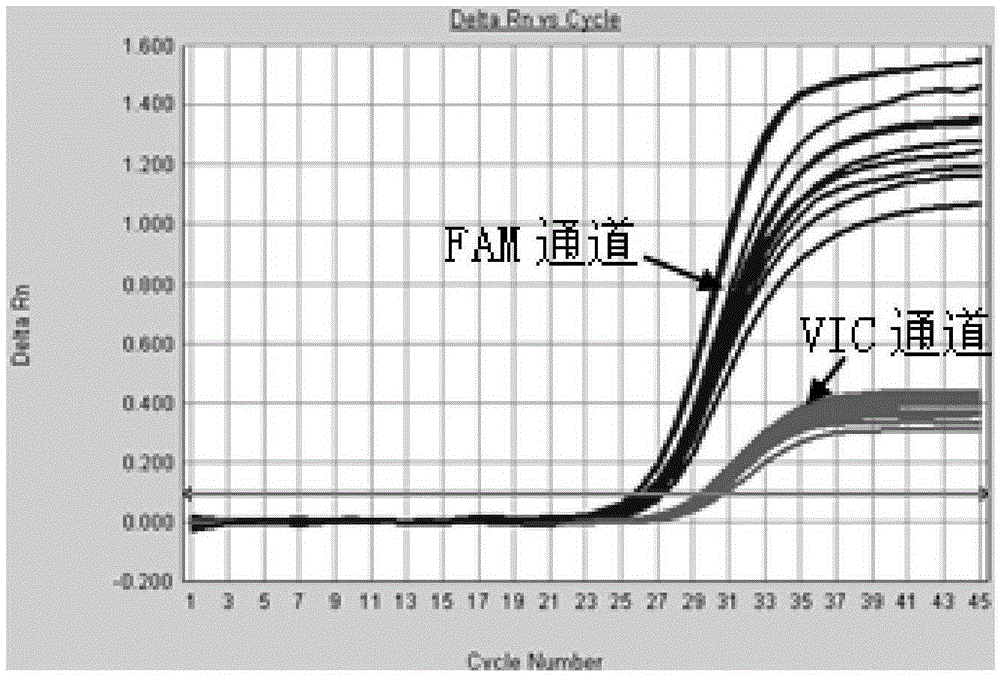
Method and system for digital quantitative analysis of nucleic acid amplification based on micro-droplet
ActiveCN104450891ARealize quantitative analysisRealize quantitative detectionBioreactor/fermenter combinationsBiological substance pretreatmentsPolymerase LAnalysis method
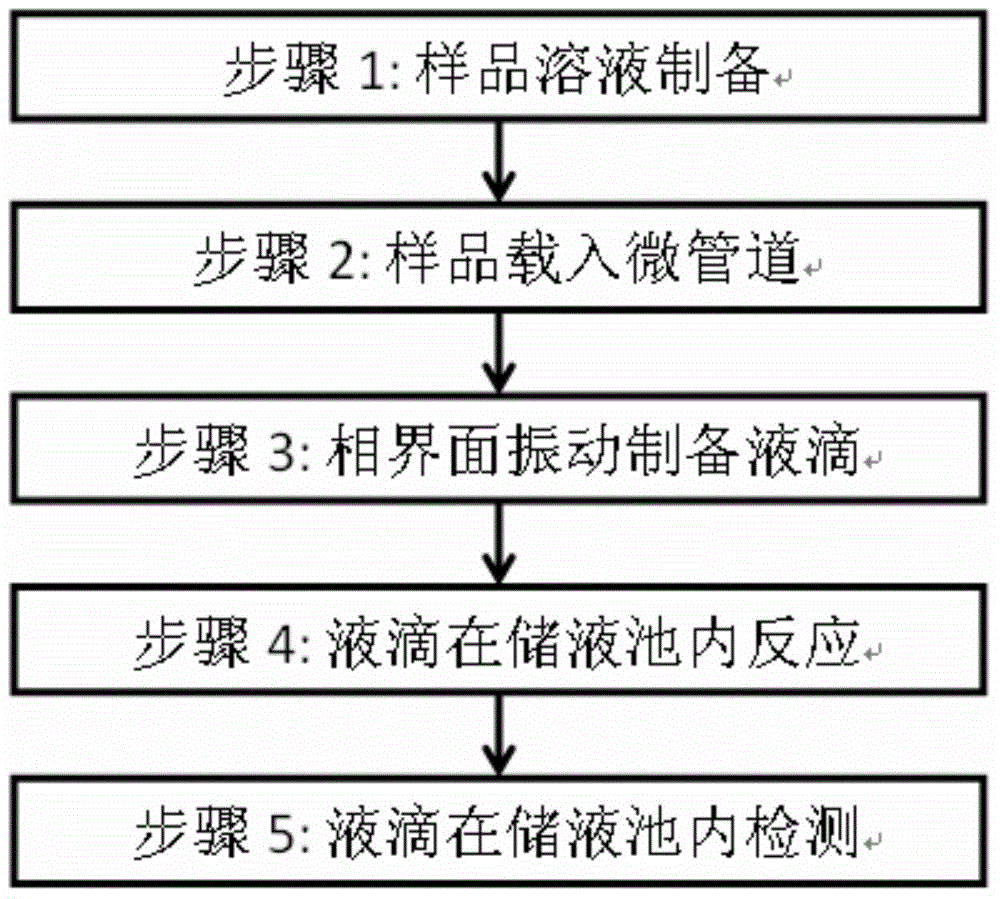
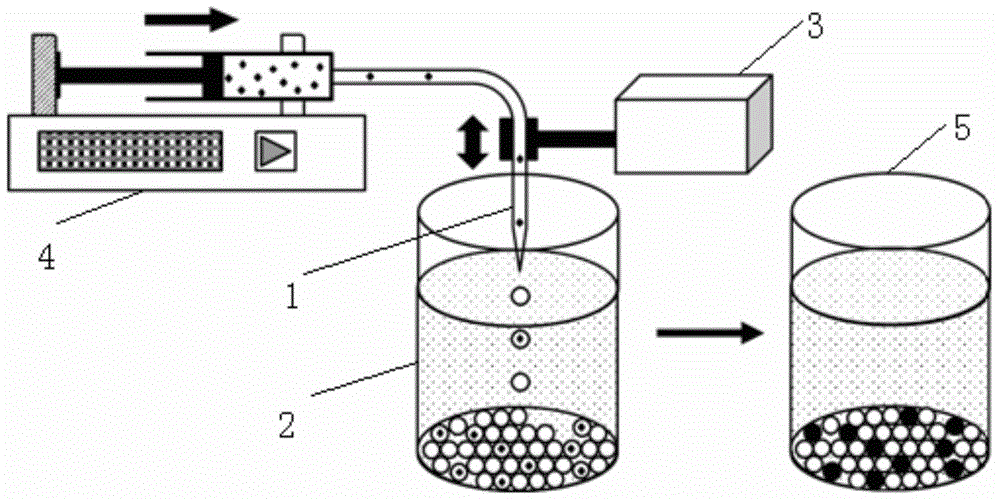
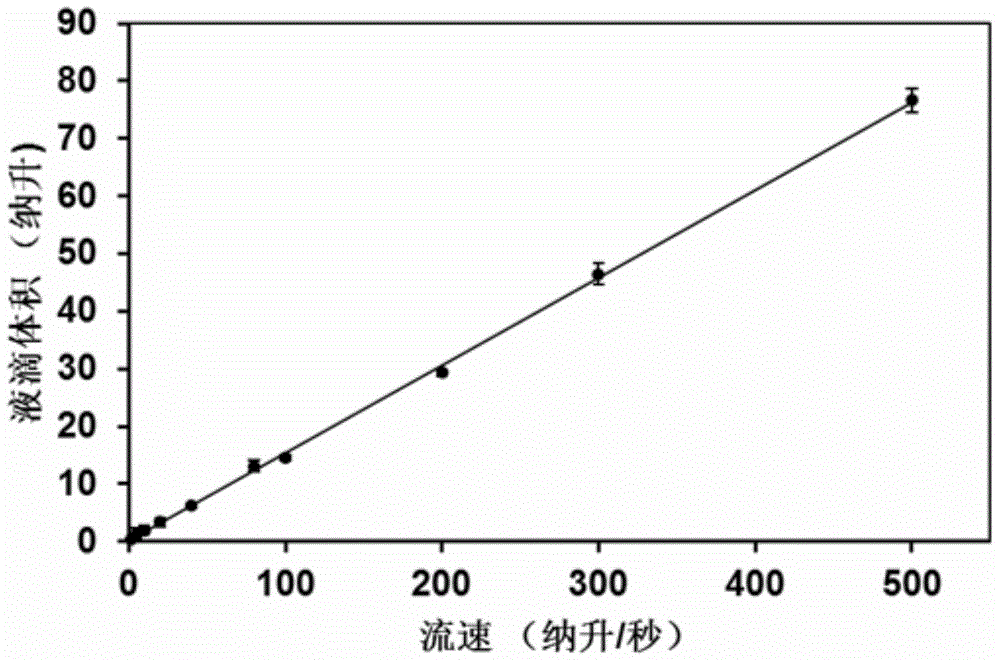
The invention provides a method and a system for digital quantitative analysis of nucleic acid amplification based on micro-droplet. The method comprises the following steps: preparing a to-be-detected nucleic acid amplification reaction liquid which includes a to-be-detected nucleic acid template, a reaction buffer water solution, deoxyribonucleoside triphosphate, a primer, polymerase and a product marking substance; loading the prepared to-be-detected nucleic acid amplification reaction liquid in a micro-pipeline of which the two ends are both provided with an opening, wherein the micro-pipeline is arranged above an open container, and the open container contains an oily liquid containing a surfactant; enabling the opening of one end of the micro-pipeline to do up-and-down reciprocating vibration on the surface of a liquid in the open container or do leftward-and-rightward reciprocating vibration below the liquid surface so as to generate a plurality of droplets which are flatly laid at the bottom of the open container; performing nucleic acid amplification reaction on the plurality of droplets in the open container; acquiring product signals generated after the nucleic acid amplification reaction is ended, and performing quantitative analysis on the nucleic acid template. The digital nucleic acid amplification analysis method can be used for implementing quantitative detection on low-concentration nucleic acid substances in a micro-system and is simple and convenient to operate, high in efficiency and low in cost.
Owner:SICHUAN MACCURA BIOTECH CO LTD
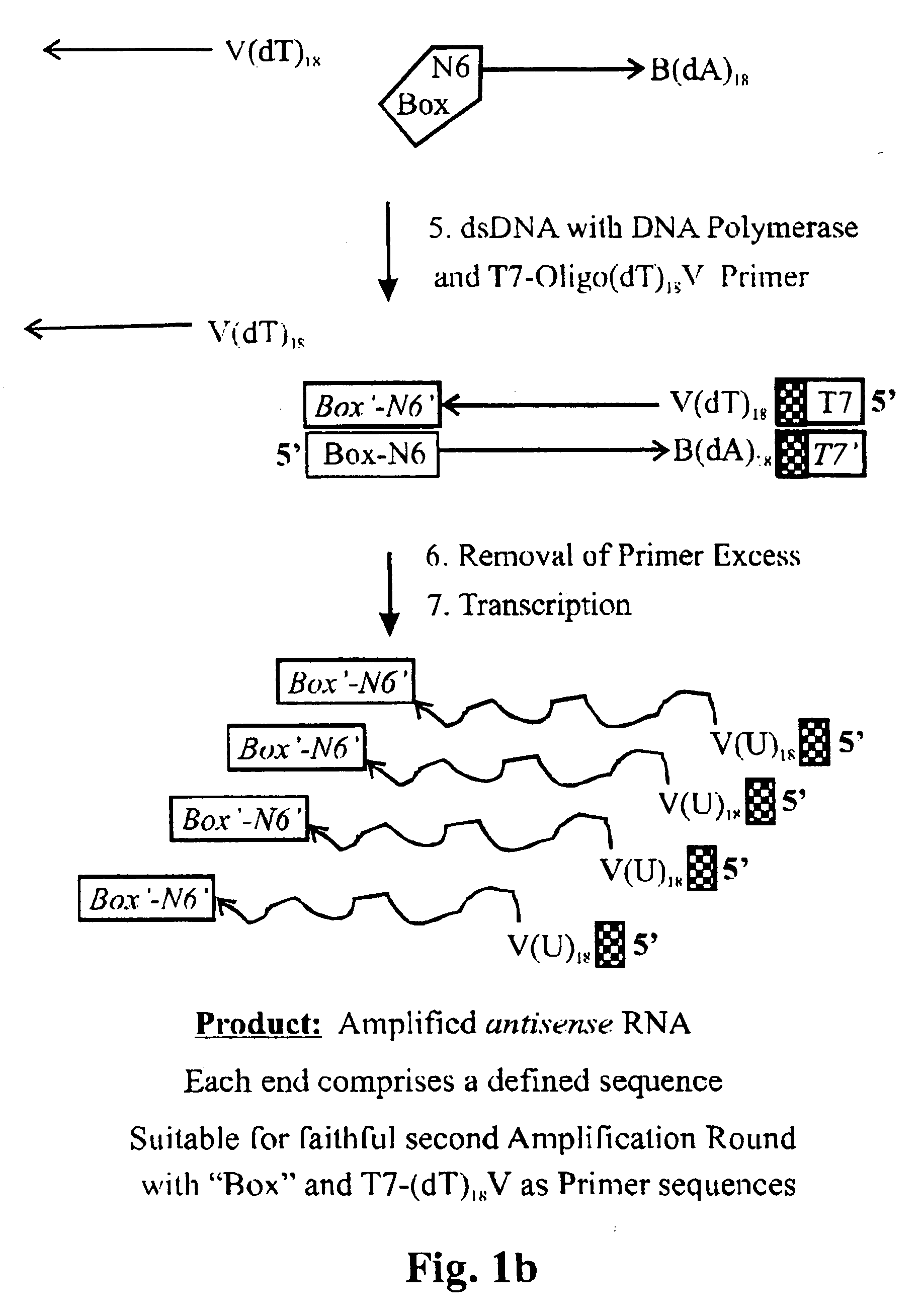
Amplification of ribonucleic acids
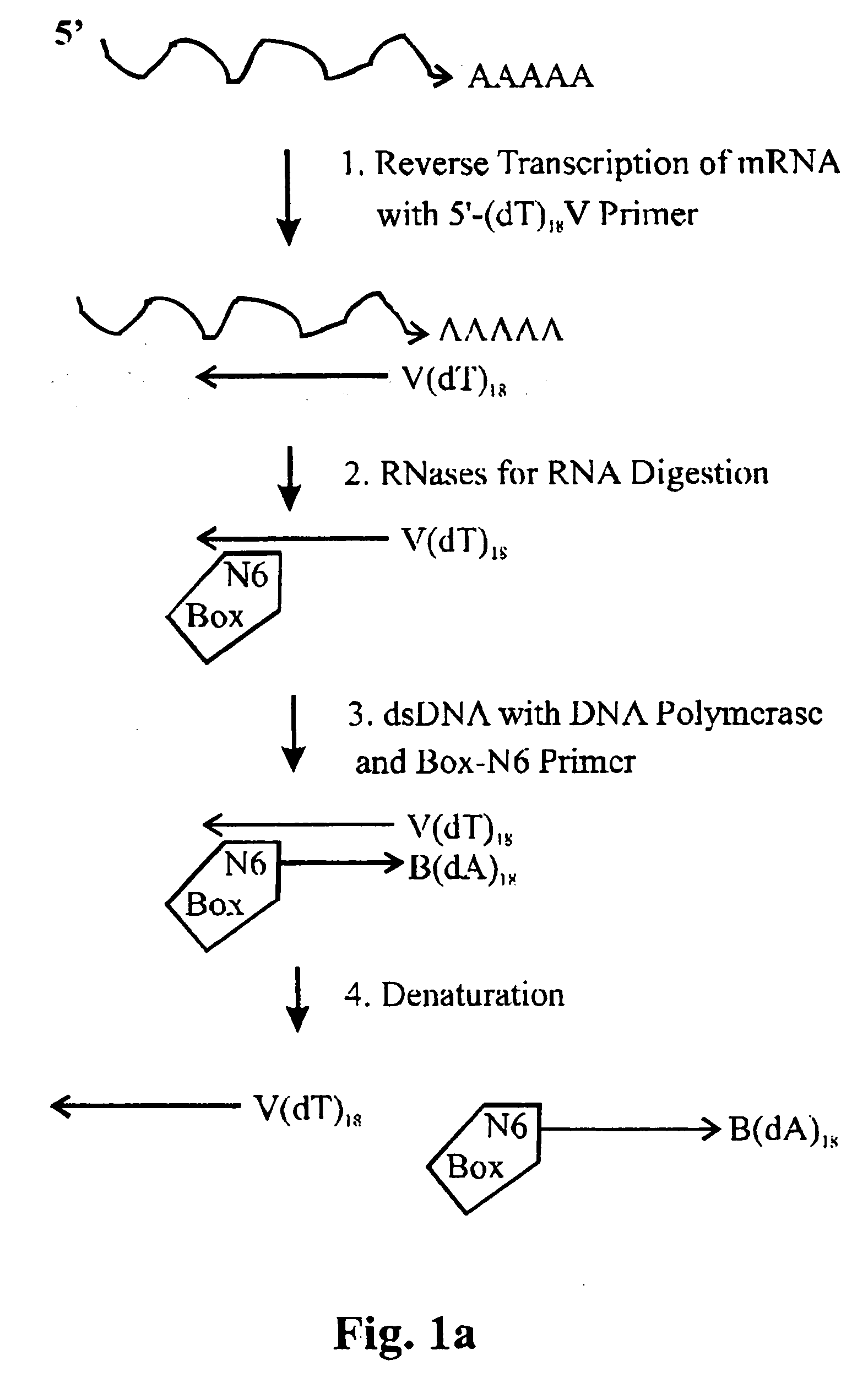
The invention relates to methods for the amplification of ribonucleic acids, comprising the following steps: (a) a single stranded DNA is produced from an RNA by means of reverse transcription, using a single-stranded primer having a defined sequence, an RNA-dependent DNA polymerase and deoxyribonucleoside triphosphates; (b) the template RNA is removed; (c) a DNA duplex is produced by means of a single-stranded primer comprising a box sequence, a DNA polymerase and deoxyribonucleoside triphosphates; (d) the duplex is separated into single-stranded DNAs; (e) DNA duplexes are produced from one of the single-stranded DNAs obtained in step (d) by means of a single-stranded primer comprising a promoter sequence at its 5′end and the same defined sequence as the primer used in step (a) at its 3′end, a DNA polymerase and deoxyribonucleoside triphosphates; (f) a plurality of RNA single strands, both ends of which comprise defined sequences, are produced by means of an RNA polymerase and ribonucleoside triphosphates. The invention also relates to kits for amplifying ribonucleic acids according to one of said methods, said kits comprising the following components: (a) at least at least one single-stranded primer, which contains a promoter sequence; (b) at least one single-stranded primer comprising a box sequence; (c) an RNA-dependent DNA polymerase; (d) deoxyribonucleoside triphosphates; (e) a DNA-dependent DNA polymerase; (f) an RNA polymerase; and (g) ribonucleoside triphosphates.
Owner:AMPTEC
Isothermal amplification of nucleic acid using primers comprising a randomized sequence and specific primers and uses thereof
Methods and kits for amplifying a nucleic acid under isothermal conditions to form an amplified nucleic acid sequence are provided. The methods and kits comprises providing a nucleic acid template, a DNA polymerase, deoxyribonucleoside triphosphates, a primer comprising a randomized sequence, and a specific primer, and amplifying the nucleic acid template.
Owner:GENERAL ELECTRIC CO
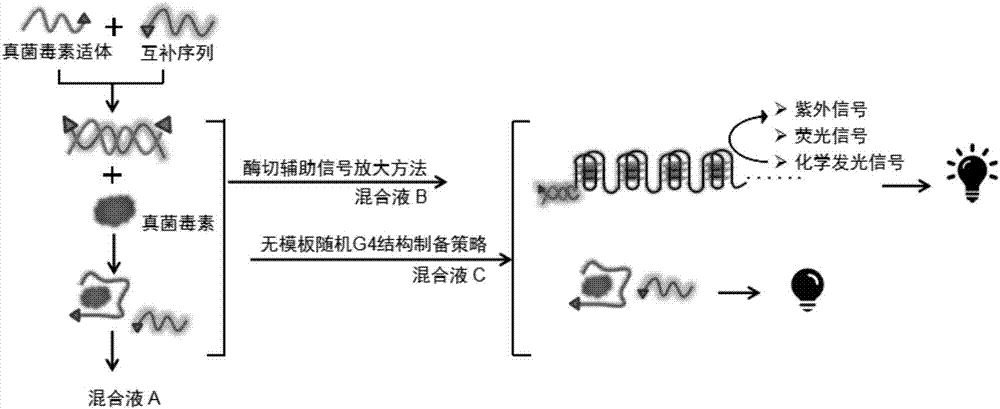
Method for detecting fungaltoxin through multiple signals and kit
ActiveCN107271668ARich identification methodsRich Signal TransformationBiological material analysisBiological testingFluorescenceUltraviolet
The invention discloses a method for detecting fungaltoxin through multiple signals. The method comprises the following steps: 1) combining fungaltoxin with aptamer: adding a to-be-detected sample after the reaction of the fungaltoxin aptamer and complementary sequence thereof, thereby acquiring a mixed solution A; 2) amplifying a digestion auxiliary signal: reacting the mixed solution A with restriction enzyme, thereby acquiring a mixed solution B; 3) preparing a guanine tetramer structure: reacting the mixed solution B with tail-end deoxynucleotide transferase and deoxyribonucleoside triphosphate and reacting with ligand molecules, thereby acquiring a mixed solution C; and 4) detecting and analyzing: performing catalytic oxidation reaction on the mixed solution C and different substrates, generating ultraviolet, fluorescent and chemiluminescent signals and calculating the content of fungaltoxin in the to-be-detected sample according to the relation of the response strength of all the signals and the fungaltoxin concentration. The method disclosed by the invention has the characteristics of quick and simple operation and treatment, short detection time, marker-free effect, low cost, high precision, high sensitivity, and the like.
Owner:ACAD OF NAT FOOD & STRATEGIC RESERVES ADMINISTRATION
High-specificity kit for detecting deafness predisposing genes
InactiveCN102534031AAvoid false positivesMicrobiological testing/measurementTotal DeafnessFluorescence
The invention discloses a fluorescent detection kit for detecting 12 deafness predisposing genes simultaneously. The kit can detect 12 mutational hotspots in the most common deafness associated genes of the Chinese in 3 hours. The kit comprises reagents before amplification and reagents after amplification, wherein the reagents before amplification comprise a polymerase chain reaction (PCR) buffer solution, a reaction mixture of MgCl2 and deoxyribonucleoside triphosphates (DNTPs), Taq DNA polymerase, ultrapure water, and a primer mixture for amplifying loci of detection sites at high specificity; and the reagents after amplification comprise a genotyping standard and an internal standard. Deafness gene loci are simultaneously detected at high sensitivity and high specificity by combining a fluorescent labeling technology, a linolenic acid (LNA) nucleoside monomer doping-primer modification technology and a capillary electrophoresis technology for the first time, manpower and material resources and time are greatly saved, and pollution due to multi-step operation is prevented.
Owner:万戈江
Gene diagnosis and detection core reagent transportable at normal temperature and of high sensitivity and high specificity
InactiveCN102242188AImprove stabilityThe result is stableMicrobiological testing/measurementGlycerolBovine serum albumin
The invention relates to a gene diagnosis and detection core reagent transportable at normal temperature and of high sensitivity and high specificity. The reagent comprises reaction buffer, deoxyribonucleoside triphosphate (DNTP), heat resistant DNA polymerase, glycerin, bovine serum albumin (BSA), dithiothreitol (DTT) and tween-20. The reagent is characterized in that: proper amounts of polysaccharide and disaccharide are mixed into the core reagent; the reaction buffer of the reagent is composed of 5mM-50mM of Tris-HCL, 20mM-500mM of KCL, 1.0mM-5mM of MgC12 and 20mM-200mM of (NH4)2SO4; meanwhile 50uM-300uM of DNTP, 0.01U / ul-0.2U / ul of heat resistant DNA polymerase, 1mM-15mM of polysaccharide, 2M-100M of disaccharide, 3%-30% of glycerin, 10-300ug / ml of BSA, 0.5-10mM of DTT and 0.005-0.05v / v of tween-20 are mixed.
Owner:SHANGHAI XINGHAN SCI&TECH
Mutant A type DNA (deoxyribonucleic acid) polymerase, and encoding gene and application of mutant A type DNA polymerase
ActiveCN107299091AIncorporation efficiency increasedNo loss of amplification efficiencyTransferasesFermentationBiotechnologyPolymerase L
The invention relates to the field of molecular biology and discloses mutant A type DNA (deoxyribonucleic acid) polymerase, and an encoding gene and an application of the mutant A type DNA polymerase. The mutant A type DNA polymerase is generated by amino acid site mutation of conservational motif of A type DNA polymerase in a dNTP (deoxyribonucleoside triphosphate) bond zone. Compared with the A type DNA polymerase not modified or mutated, the mutant A type DNA polymerase has increased dUTP (deoxyuridine triphosphokinase) doping speed; a dUTP doping effect of the mutant A type DNA polymerase is obviously better than control A type DNA polymerase; therefore, the mutant A type DNA polymerase is more applicable to some nucleic acid amplification systems using dUTP to substitute dTTP (deoxy- thymidine triphosphate) and allows the system to avoid nucleic acid amplification product contamination and not to lose amplification efficiency of a target product at the same time; and the mutant A type DNA polymerase meets application requirements of multiple PCR (polymerase chain reaction) fields of food, animal quarantine, human disease screening and the like, a forensic medicine field and a scientific research.
Owner:SUZHOU NUHIGH BIOTECH
Method for determining DNA nucleotide sequence
PCT No. PCT / JP95 / 02254 Sec. 371 Date May 5, 1997 Sec. 102(e) Date May 5, 1997 PCT Filed Nov. 6, 1995 PCT Pub. No. WO96 / 14434 PCT Pub. Date May 17, 1996Disclosed is a method for determining a nucleotide sequence of DNA product amplified by polymerase chain reaction not requiring removal of primers and / or 2'-deoxyribonucleoside-5'-triphosphates and / or derivatives thereof, which comprises reacting ribonucleoside-5'-triphosphates comprising ATP, GTP, CTP, UTP and derivatives thereof and one or more of 3'-deoxyribonucleotide-5'-triphosphates comprising 3'-dATP, 3'-dGTP, 3'-dCTP, 3'-dUTP and derivatives thereof in the presence of an RNA polymerase and a DNA product which has been amplified by polymerase chain reaction and contains a promoter sequence for the RNA polymerase to afford a nucleic acid transcription product, separating the obtained nucleic acid transcription product and determining a nucleic acid sequence from the resluting separated fractions.
Owner:RIKEN
Pseudomonas aeruginosa nucleic acid fluorescent PCR (polymerase chain reaction) detection kit and detection method
InactiveCN106520984AQuick destructionStrong specificityMicrobiological testing/measurementMicroorganism based processesForward primerPositive control
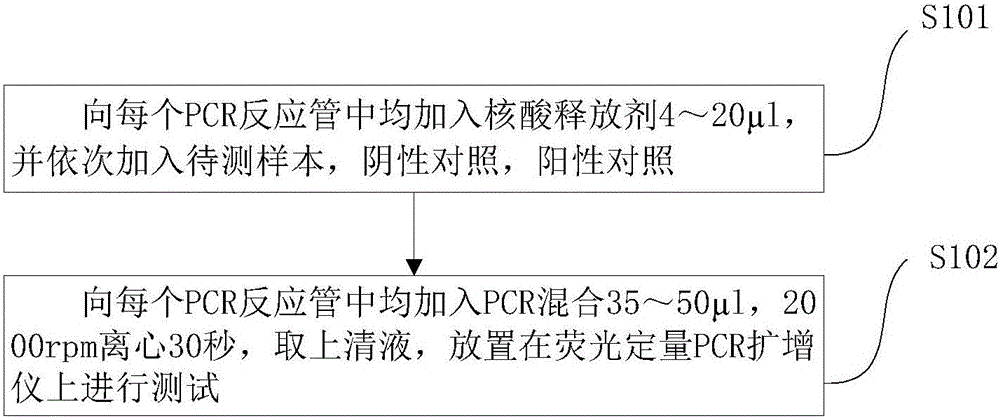
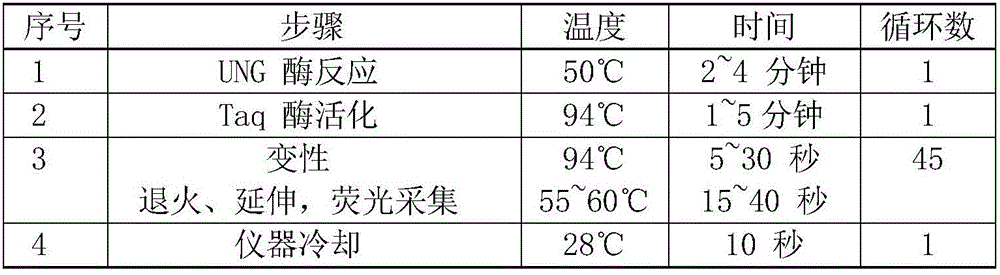
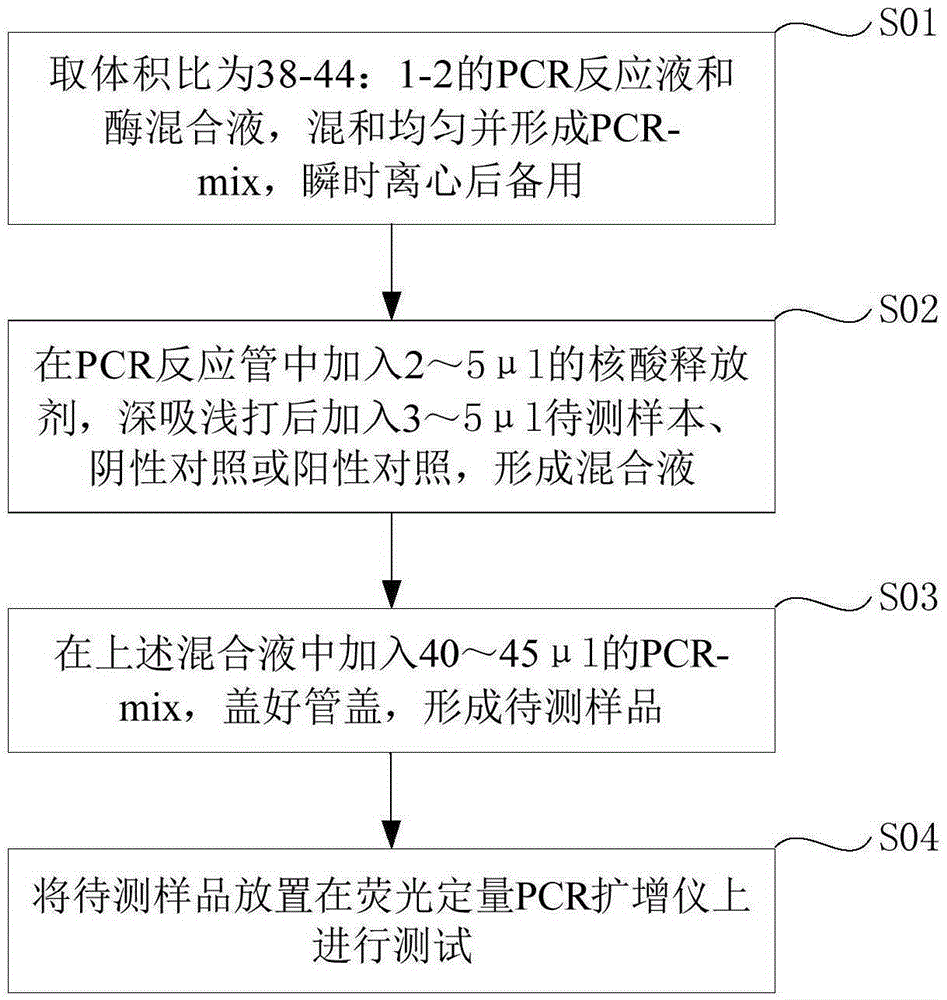
The invention discloses a Pseudomonas aeruginosa nucleic acid fluorescent PCR (polymerase chain reaction) detection kit and detection method. The detection kit comprises a PCR reaction solution, an enzyme mixed solution, a positive control, a negative control and an internal standard, wherein the PCR reaction solution comprises a PCR buffer solution, nucleic acid releaser, deoxyribonucleoside triphosphate, primers for target polynucleotide amplification and probes for target polynucleotide detection; the primers comprise a forward primer and a reverse primer. Or, the detection kit comprises a PCR reaction solution, an enzyme mixed solution and an internal standard, wherein the PCR reaction solution comprises a PCR buffer solution, nucleic acid releaser, deoxyribonucleoside triphosphate, a forward primer for target polynucleotide amplification, a reverse primer for target polynucleotide amplification and probes for target polynucleotide detection. The Pseudomonas aeruginosa nucleic acid fluorescent PCR detection kit shown in the embodiment of the invention with the advantages of no need of nucleic acid extraction, high detection sensitivity, wide detection range and high detection accuracy can quickly and accurately detect Pseudomonas aeruginosa DNA (deoxyribonucleic acid) in plasma, urine and other samples.
Owner:SANSURE BIOTECH INC
HBV nucleic acid quantitative detection system based on micro-droplet digital PCR technology
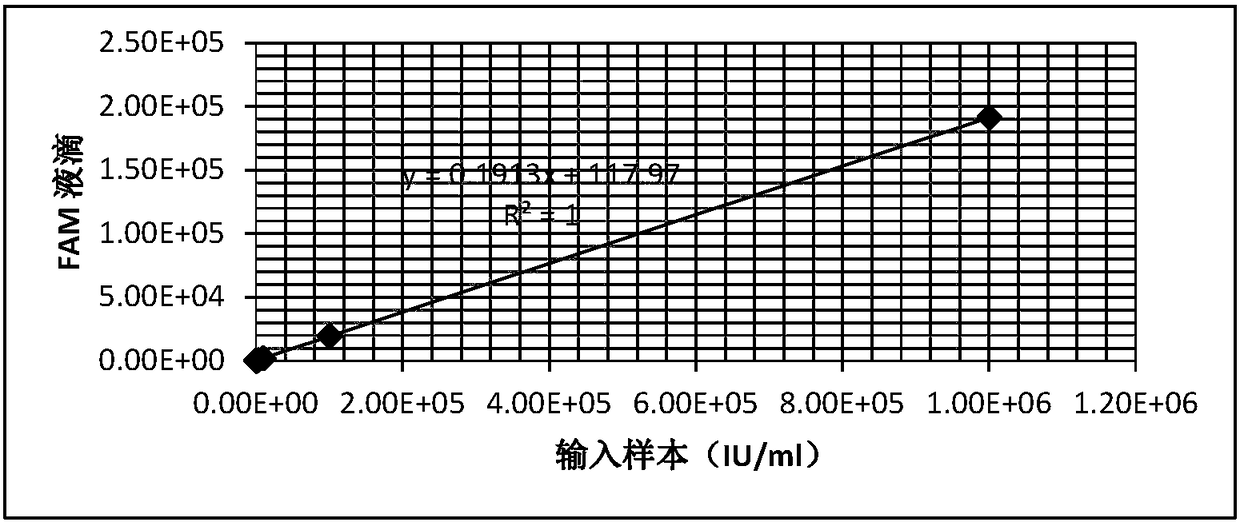
InactiveCN108342507AAccurate Absolute QuantificationMicrobiological testing/measurementHepatitis B virus DNAPcr ctpp
The invention provides a HBV nucleic acid quantitative detection system based on a micro-droplet digital PCR technology, the HBV nucleic acid quantitative detection system comprises an upstream primerand a downstream primer for amplifying hepatitis B virus DNA, a specific probe for detecting the hepatitis B virus DNA; a non-infected linearizing double-stranded plasmid quality control standard product and a probe for detecting the quality control standard product; and a buffer system comprising deoxyribonucleoside triphosphates dATP, dCTP, dGTP, and dUTP, and UNG. The HBV nucleic acid quantitative detection system is based on the micro-droplet digital PCR technology to realize the accurate absolute quantification of HBV DNA, faces the clinical actual demand, and has an important application prospect.
Owner:TARGETINGONE CORP +1
Breast cancer susceptibility gene BRCA1 and BRCA2 detection kit and method
InactiveCN106701936AConducive to risk assessmentImprove featuresMicrobiological testing/measurementBreast cancer susceptibility genesIntein
The invention discloses a breast cancer susceptibility gene BRCA1 and BRCA2 detection kit and method. The kit includes a first-round PCR reagent and a second-round PCR reagent. The first-round PCR reagent contains a buffer solution, magnesium salt, deoxyribonucleoside triphosphate, enzyme mixture liquid and a first-round PCR primer. The second-round PCR reagent contains a buffer solution, magnesium salt, deoxyribonucleoside triphosphate, enzyme mixture liquid and a second-round PCR primer. The first-round PCR primer includes 46 pairs of primers SEQ1-SEQ92 and 67 pairs of primers SEQ93-SEQ226, the 5'end of each upstream primer is added with a sequence SEQ227, and the 5'end of each upstream primer is added with a sequence SEQ228. The primer combination includes 113 pairs of primers and covers all the coding sequences and adjacent at least +5bp intron subdomains of a breast cancer susceptibility genes. An NGS technology is adopted to detect mutations of all the coding sequences and splice sites of the breast cancer susceptibility genes BRCA1 and BRCA2, the kit has very high specificity and accuracy, and the method is simple, convenient and efficient.
Owner:SANSURE BIOTECH INC
Fluorescent quantitation PCR (Polymerase Chain Reaction) detection kit for human papilloma viruses and application method of fluorescent quantitation PCR detection kit
ActiveCN105385786AHigh sensitivityEasy to operateMicrobiological testing/measurementMicroorganism based processes3-deoxyribosePolynucleotide
The invention provides a fluorescent quantitation PCR (Polymerase Chain Reaction) detection kit for human papilloma viruses. The detection kit comprises a PCR reaction solution, wherein the PCR reaction solution comprises a PCR buffering solution, deoxyribonucleoside triphosphate, upstream and downstream primers used for amplifying target polynucleotide, and a probe for detecting the target polynucleotide. The fluorescent quantitation PCR detection kit for the human papilloma viruses, provided by the invention, has very good specificity and has the advantages that the operation is rapid, the method is simple and convenient and the sensitivity is high; meanwhile, an interior label, which is increased in a reaction system, can be used for effectively preventing detection from being false negative. The fluorescent quantitation PCR detection kit for the human papilloma viruses, provided by the invention, adopts a rapid nucleic acid releasing method when DNAs (Deoxyribose Nucleic Acid) are extracted; the method is simple, convenient and rapid, and heating is not needed.
Owner:SANSURE BIOTECH INC
Kit for quantitatively detecting vibrio parahaemolyticus in food and clinic sample
ActiveCN101709331AHigh sensitivityEffective monitoringMicrobiological testing/measurementMicroorganism based processesDiseaseForward primer
The invention discloses a kit for quantitatively detecting vibrio parahaemolyticus in food and clinic sample. The kit comprises respectively packaged DNA extracting solution, PCR amplification reaction liquid, negative standard substance, positive reference material, quantitative standard substance and a plurality of sealed reagent bottles or test tubes. The invention is characterized in that the PCR amplification reaction liquid contains forward primer VP-F, reverse primer VP-R, oligonucleotide probe VP-P, heat-resistant DNA polymerase and deoxyribonucleoside triphosphate for target polynucleotide amplification. The vibrio parahaemolyticus pathogen in food samples and samples of puke, blood and dejection of patient with clinic gastrointestinal inflammation or visceral hemorrhage can be detected, thereby being capable of providing reliable experimental evidences for sensitively and quickly diagnosing vibrio parahaemolyticus pollution and infection and repetitive infection of vibrio parahaemolyticus at an early time; and simultaneously, the amount can be accurately fixed, thereby being capable of effectively monitoring clinic drug administration.
Owner:XIAN TIANLONG SCI & TECH
Kit and method for detecting Acinetobacter baumannii DNA
InactiveCN106434996AQuick destructionStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlAcinetobacter baumannii DNA
The invention discloses a kit and method for detecting Acinetobacter baumannii DNA; the kit comprises: a nucleic acid release agent, a PCR (polymerase chain reaction) liquid, an enzyme mixed liquid, an Acinetobacter baumannii positive control, and an Acinetobacter baumannii negative control, wherein the PCR liquid is made from PCR buffer solution, deoxyribonucleoside, primers for amplifying targeted polynucleotide, a probe for amplifying targeted polynucleotide, primers for amplifying internally labeled fragments and a probe for detecting an internal label. By optimizing conditions in the embodiment of the invention, primers for amplifying targeted polynucleotide and probes for amplifying the targeted polynucleotide, both having optimal amplification, are screened out, the kit is capable of detecting not pathogens of Acinetobacter baumannii but various infections of Acinetobacter baumannii but, is highly specific, and accordingly has greatly improved detection sensitivity, accuracy and stability.
Owner:SANSURE BIOTECH INC
Kit for quickly examining loop SFTSV (severe fever with thrombocytopenia syndrome bunyavirus) mediated isothermal amplification of severe fever with thrombocytopenia syndrome bunyavirus
The invention discloses a kit for quickly examining loop SFTSV (severe fever with thrombocytopenia syndrome bunyavirus) mediated isothermal amplification of severe fever with thrombocytopenia syndrome bunyavirus. The kit consists of components including (1) 2.5 micro-liters of 10X amplification reaction liquid, (2) 2 micro-liters of 25mmol / l dNTP (deoxyribonucleoside triphosphate), (3) 5pmol of F3 outer primer, 5pmol of B3 outer primer, 40pmol of FIP inner primer, 40pmol of BIP inner primer, 20pmol of LF loop primer and 20pmol of LB loop primer, (4) I micro-liter of Bst (bovine somatotropin) DNA (deoxyribonucleic acid) polymerase, (5) 1 micro-liter of reverse transcriptase, (6) 1 micro-liter of fluorescent dye and (7) ddH2O. During usage, RNA (ribonucleic acid) of an examinee is added into the kit, change of colors of a reaction system is observed after amplification, and an examination result is judged accordingly. The examination kit and an examination method have the advantages that examination cost is low, operation is convenient, examination speed is fast, high specificity and high sensitivity identical to that of PCR (polymerase chain reaction) are realized, and scientific evidence for timely diagnosing and treating severe fever with thrombocytopenia syndrome is provided.
Owner:中国人民解放军济南军区联勤部疾病预防控制中心
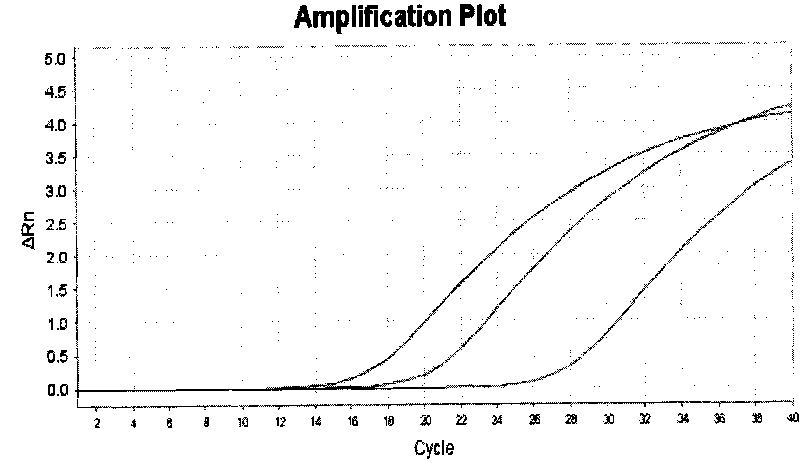
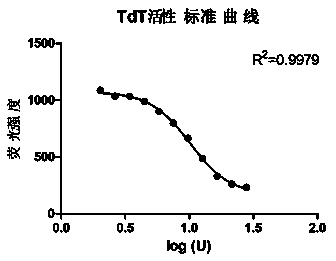
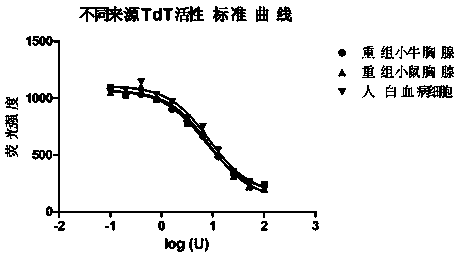
Method for measuring activity of terminal deoxynucleotidyl transferase
InactiveCN104293883ALow costEasy to operateMicrobiological testing/measurementPolymerase LSingle strand
The invention discloses a method for measuring activity of terminal deoxynucleotidyl transferase. The method comprises the steps of: firstly, synthesizing a primer according to a single stranded template, annealing the single stranded template, then performing a 3' end tailing reaction catalyzed by TdT enzyme in the presence of one of four deoxyribonucleoside triphosphates, after the 3' end tailing reaction is completed, performing a polymerization reaction in the presence of enough DNA polymerase without 3'-5 'excision enzyme activity to generate double stranded DNA, finally measuring the relative quantity of the double stranded DNA or single stranded DNA in the reaction system after the polymerization reaction is finished, and deducing the activity degree of the Poly (A) polymerase according to the detection result, wherein the basic group at the first site on the used single stranded template in the 3' direction and the deoxyribonucleoside triphosphate used in the tailing reaction are not complementary. Compared with the existing method, the method disclosed by the invention is free of radioactive pollution, simple and quick to operate, low in reagent preparation cost and high in sensitivity.
Owner:VAZYME BIOTECH NANJING
Fluorescent PCR detecting kit for V600E mutation of BRAF gene
InactiveCN105296666AStrong specificityEasy to operateMicrobiological testing/measurementBraf genesNucleotide
The invention provides a fluorescent PCR detecting kit for V600E mutation of a BRAF gene. The detecting kit comprises a V600E mutation detection reaction solution, wherein the V600E mutation detection reaction solution comprises a 10*PCR buffering solution, deoxyribonucleoside triphosphate, an upstream primer, a downstream primer and a probe, wherein the upstream primmer and the downstream primer are used for amplifying target polynucleotide, and the probe is used for detecting the target polynucleotide. The fluorescent PCR detecting kit for V600E mutation of the BRAF gene is based on a real-time fluorescent quantitative PCR technology and an ARMS-PCR technology, has favorable specificity and is high in operation speed, simple and convenient in method and high in detection precision; and meanwhile an interior label additionally arranged in a reaction system can be used for effectively avoiding that the detected result is false negative.
Owner:HUNAN SHENGWEI GENE TECH
Rapid amplification method of hepatitis B virus nucleic acid
ActiveCN108913811AEasy to operateShort timeMicrobiological testing/measurementMicroorganism based processesPotassiumHepatitis B virus
The invention relates to a rapid amplification method of hepatitis B virus nucleic acid. The method comprises the following steps: mixing a sample containing hepatitis B virus with a nucleic acid release agent, and then adding polymerase chain reaction (PCR) premix to obtain reaction liquid, wherein the nucleic acid release agent is prepared from surfactin, potassium chloride, sodium dodecyl sulfate and ethanol, and the PCR premix is prepared from deoxyribonucleoside triphosphate, an upstream primer shown in a sequence of SEQ ID No. 1, a downstream primer shown in a sequence of SEQ ID No. 2, DNA polymerase and an amplification buffer solution; putting the reaction liquid into a PCR reaction tube to form a thin film having thickness of 0.1 mm or less; putting the PCR reaction tube into a PCR amplification instrument and carrying out PCR amplification according to the following reaction conditions: carrying out pre-degeneration for 10-600s at 90-100 DEG C, carrying out degeneration for 0-1s at 90-100 DEG C, and annealing for 0-1s at 50-65 DEG C. Under the premise of guranteeing the accuracy and effectiveness of amplification, the rapid application method significantly shortens the time required for each cycle, thus achieving the aim of rapidly and simply amplifying the hepatitis B virus nucleic acid.
Owner:SANSURE BIOTECH INC
Novel orthobunyavirus fluorescence quantitative detection kit and detection method of virus
ActiveCN102409109AIncrease positive rateEffective preventionMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseBuffer solution
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION +1
Fluorescent quantitative PCR detecting kit for hepatitis E virus and using method of fluorescent quantitative PCR detecting kit
InactiveCN105296676AEasy to operateSimple methodMicrobiological testing/measurementMagnetic beadPolynucleotide
The invention provides a fluorescent quantitative PCR detecting kit for a hepatitis E virus. The detecting kit comprises a PCR reaction solution, wherein the PCR reaction solution comprises a PCR buffering solution, deoxyribonucleoside triphosphate, a DNA polymerase, an upstream primer, a downstream primer and a probe, wherein the upstream primmer and the downstream primer are used for amplifying target polynucleotide, and the probe is used for detecting the target polynucleotide. The fluorescent quantitative PCR detecting kit for the hepatitis E virus is favorable in specificity, high in operation speed, simple and convenient in method and wide in detection range, and meanwhile an interior label additionally arranged in the reaction system can be used for effectively avoiding that the detected result is false negative. When the fluorescent quantitative PCR detecting kit for the hepatitis E virus is used for extracting RNA, a magnetic bead method which is good in adsorption effect and easy to purify is used, and the method can be used for effectively removing PCR inhibitors in complex samples, so that RNA with high purity and high yield is obtained, and detecting sensitivity, accuracy and stability are greatly improved.
Owner:SANSURE BIOTECH INC
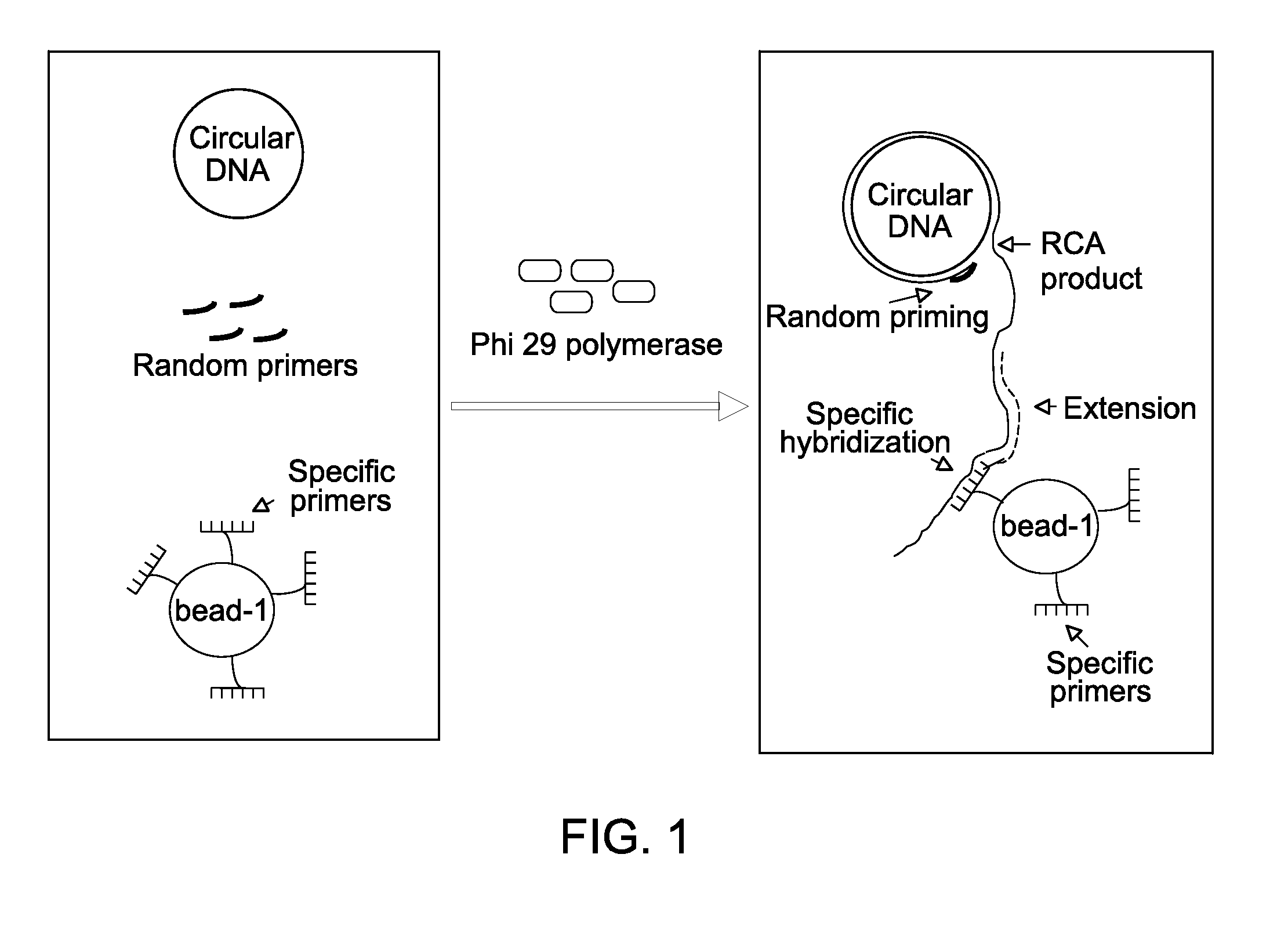
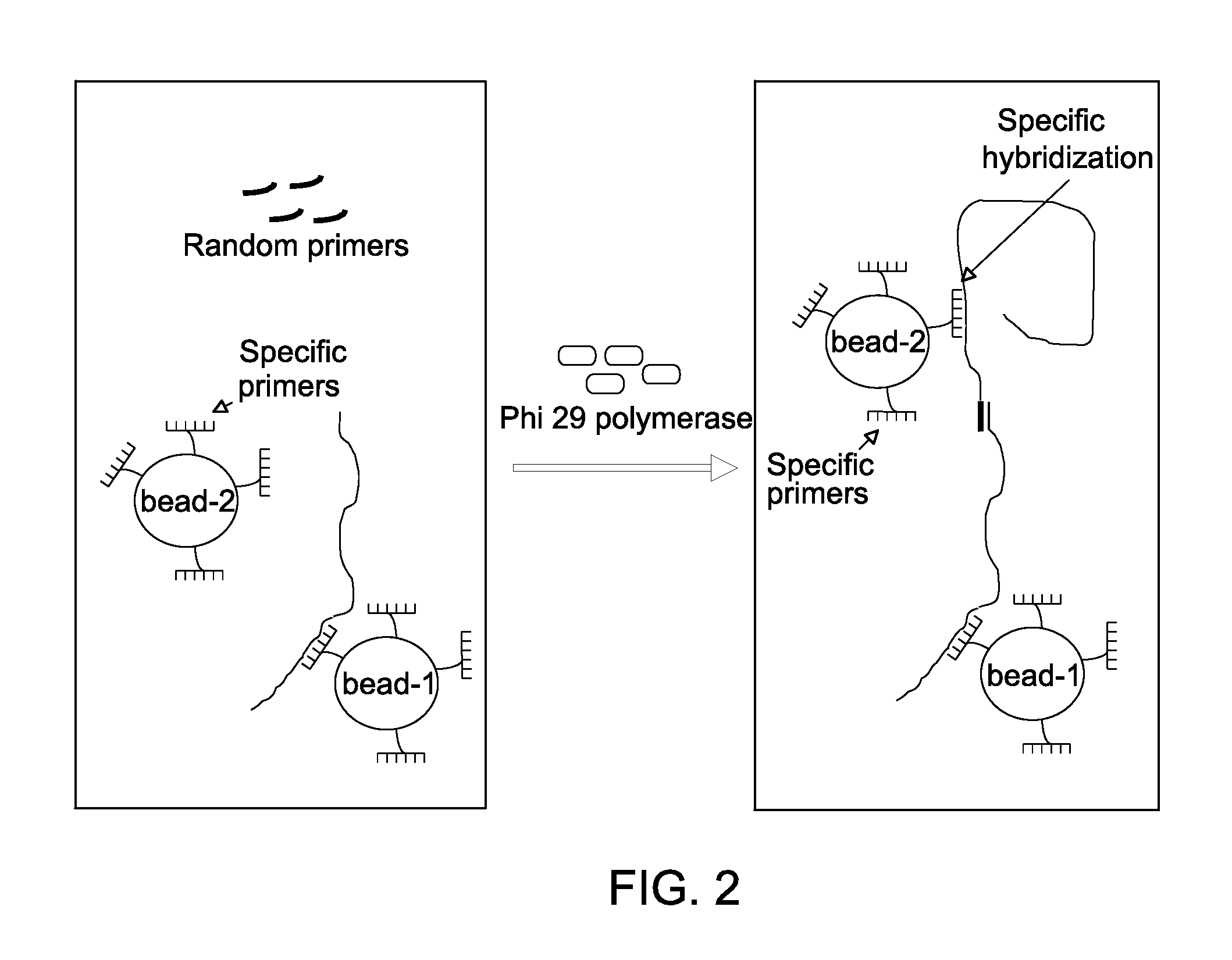
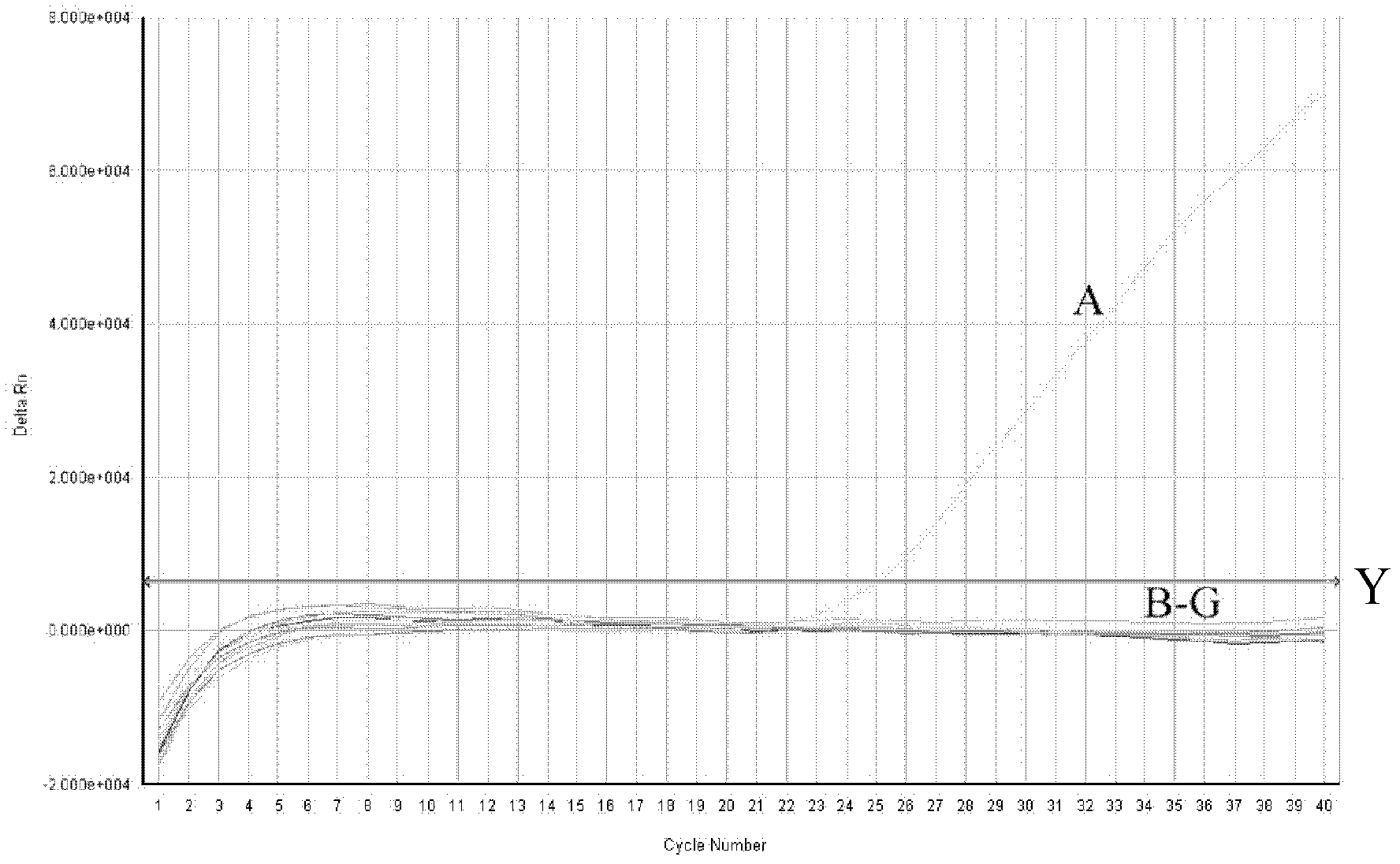
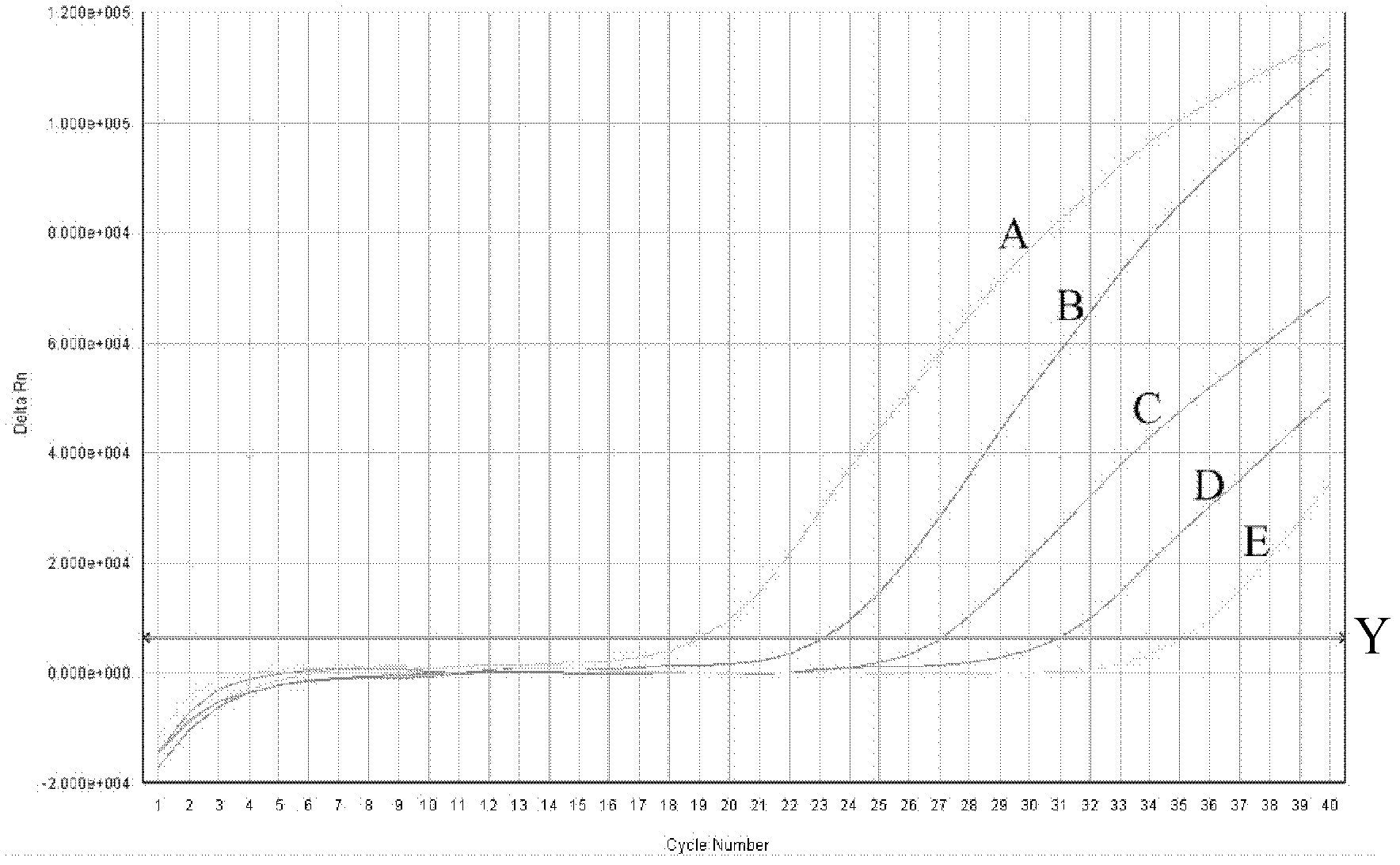
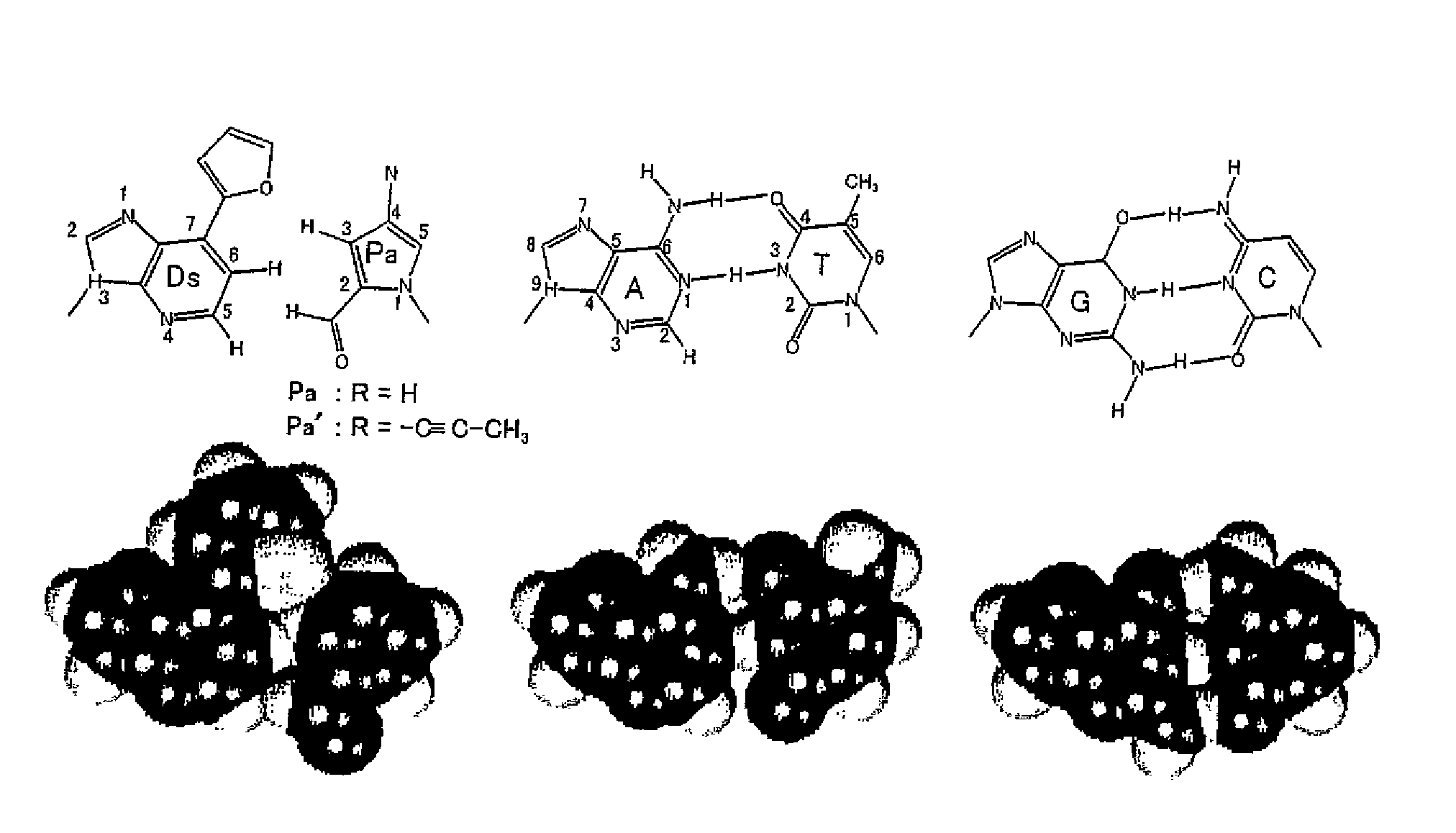
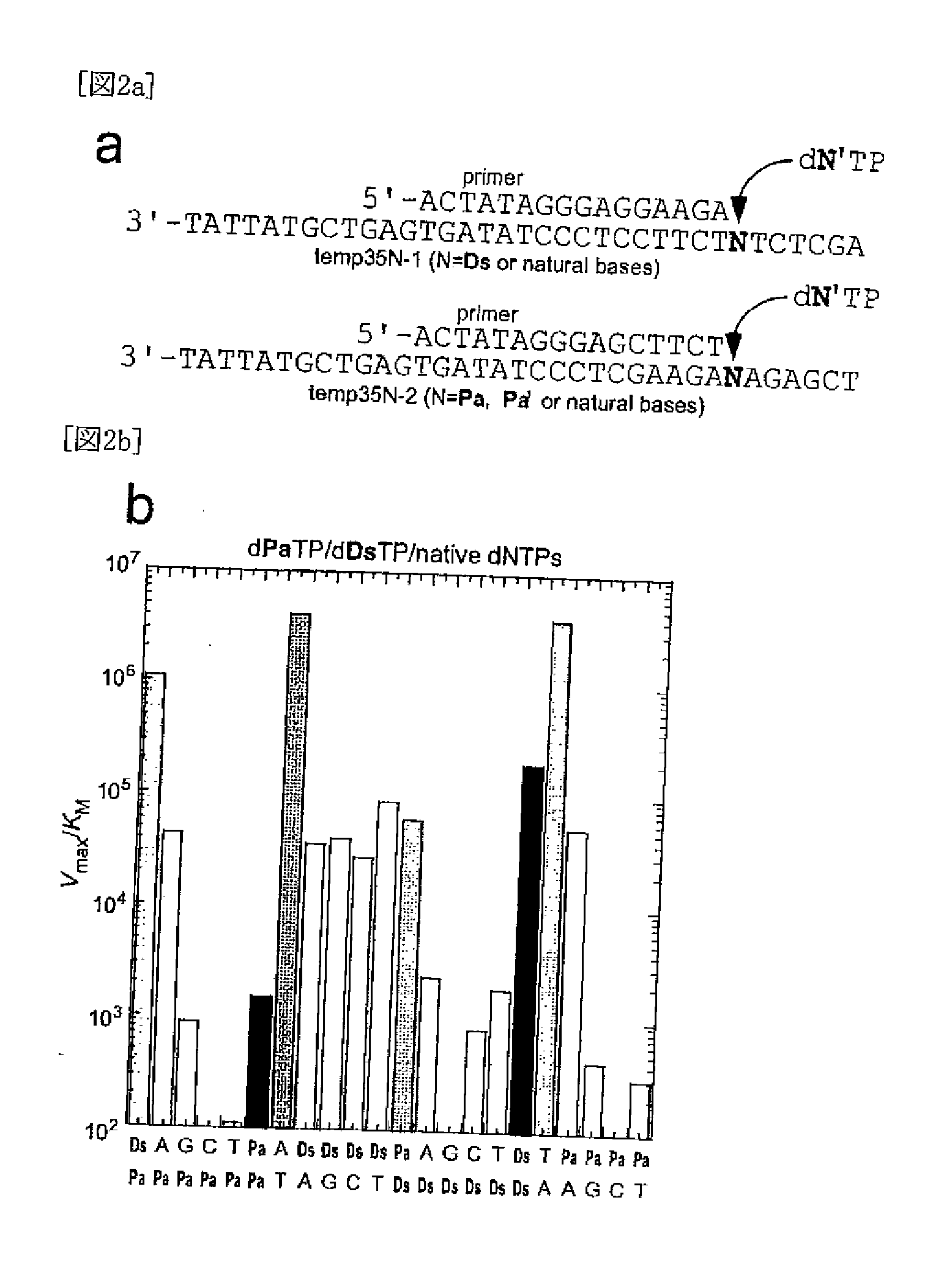
Method for replicating nucleic acids and novel unnatural base pairs
ActiveUS20100036111A1Good efficiency and selectivityEffective transcriptionSugar derivativesMicrobiological testing/measurementPhosphoric acidChemistry
The present invention relates to a method for nucleic acid replication and novel artificial base pairs.The method of the present invention for nucleic acid replication is characterized in that a deoxyribonucleoside 5′-triphosphate, in which the hydroxyl group of phosphoric acid at the γ-position is substituted with a group selected from the group consisting of an amino group, a methylamino group, a dimethylamino group, a mercapto group and a fluoro group, is used as a substrate during replication reaction. The novel artificial base pairs of the present invention are characterized in that 7-(2-thienyl)-imidazo[4,5-b]pyridine (Ds) or an analog thereof forms a base pair with pyrrole-2-carbaldehyde (Pa) or an analog thereof.
Owner:TAGCYX BIOTECHNOLOGIES INC
Solid phase isothermal amplification
ActiveUS20170137874A1Microbiological testing/measurementFermentationPolymerase LReaction temperature
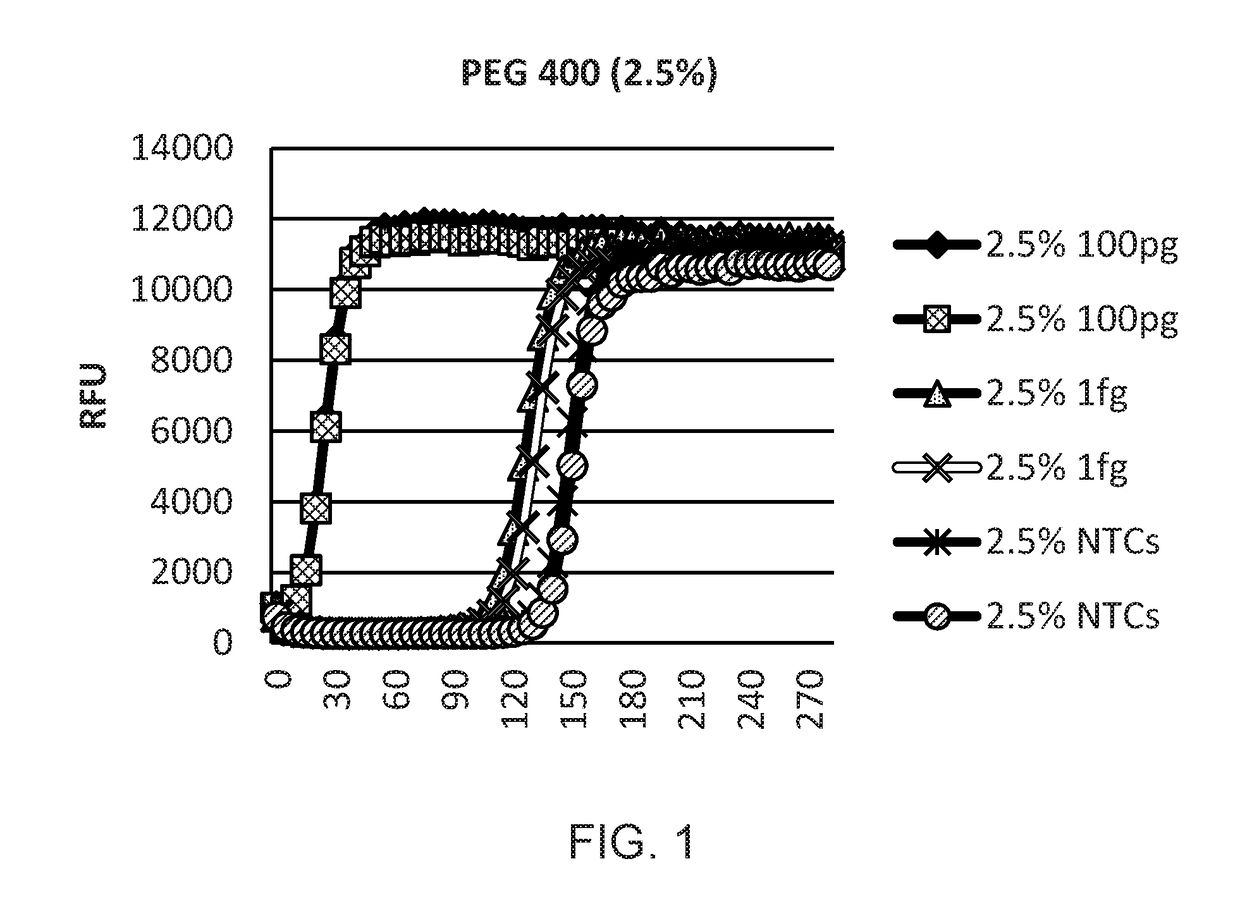
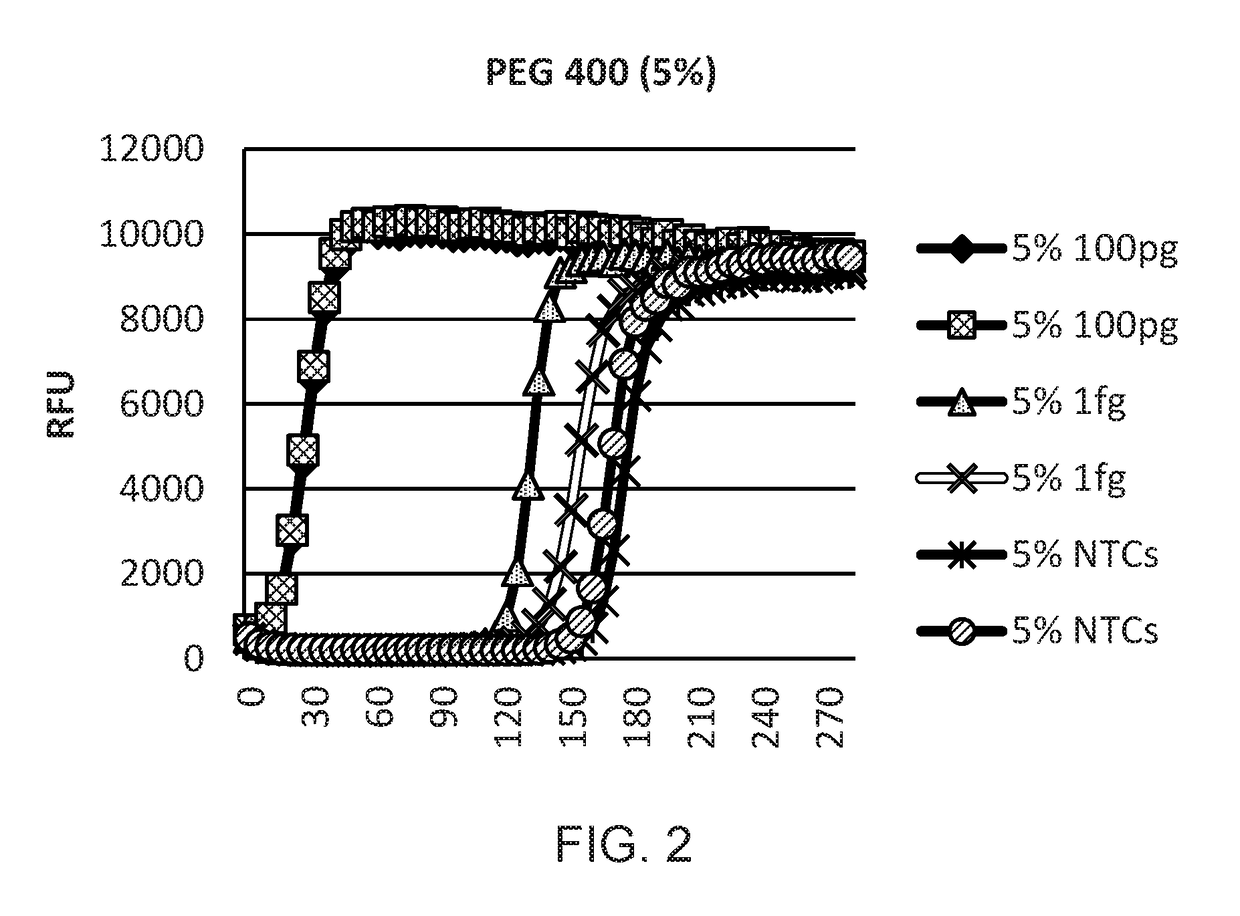
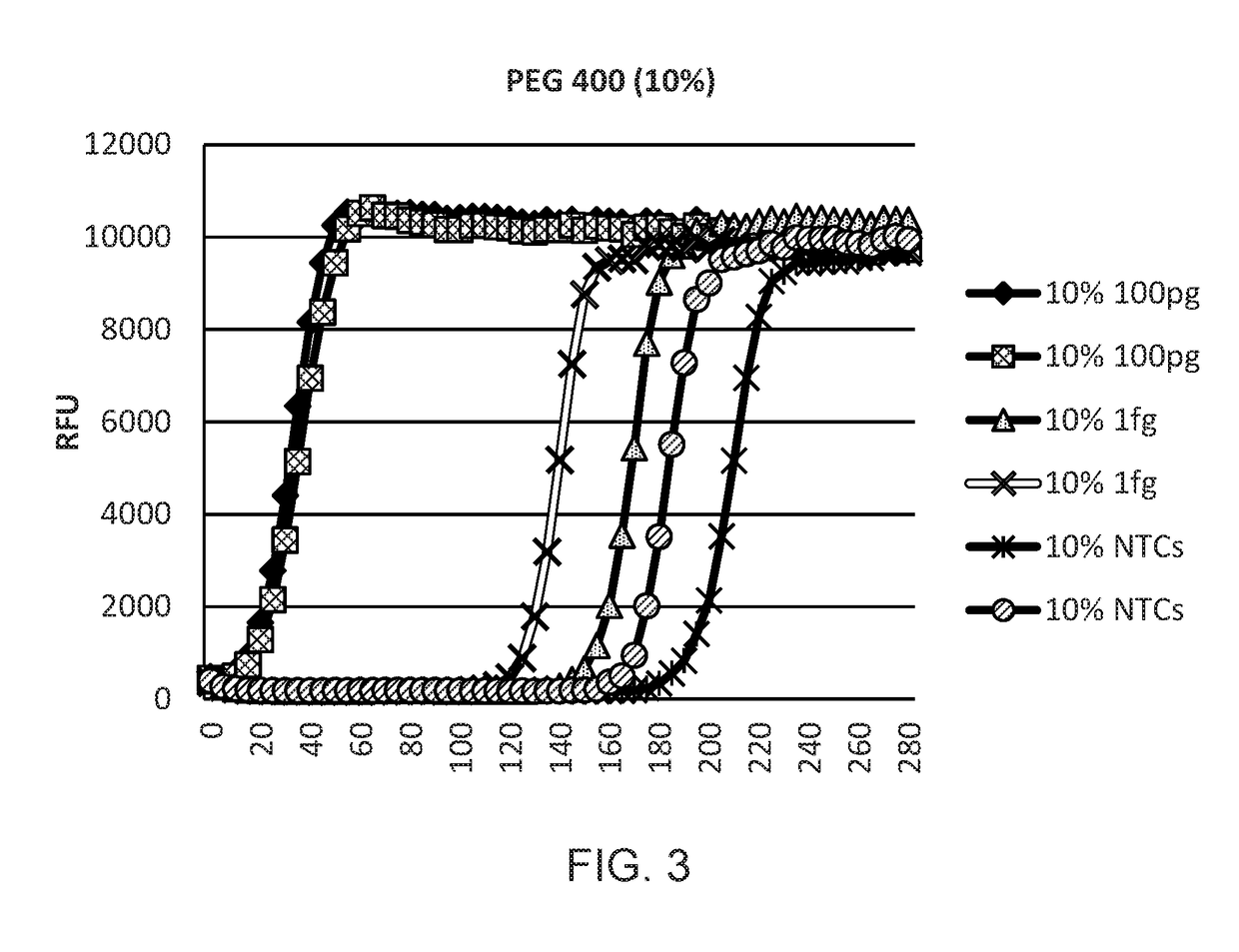
Provided herein are methods and kits for isothermal nucleic acid amplifications that use a target nucleic acid template; a reaction mixture comprising a DNA polymerase having a strand displacement activity, a deoxyribonucleoside triphosphate (dNTP) mixture, a primer with a 3′ end and a 5′ end, a molecular crowding reagent, and a buffer solution for amplifying the target nucleic acid template. The buffer solution maintains a low salt concentration of the reaction mixture, and wherein the salt concentration results in a melting temperature (Tm) of the primer at least 10° C. below the reaction temperature. The amplification is effected under isothermal condition.
Owner:GLOBAL LIFE SCI SOLUTIONS OPERATIONS UK LTD
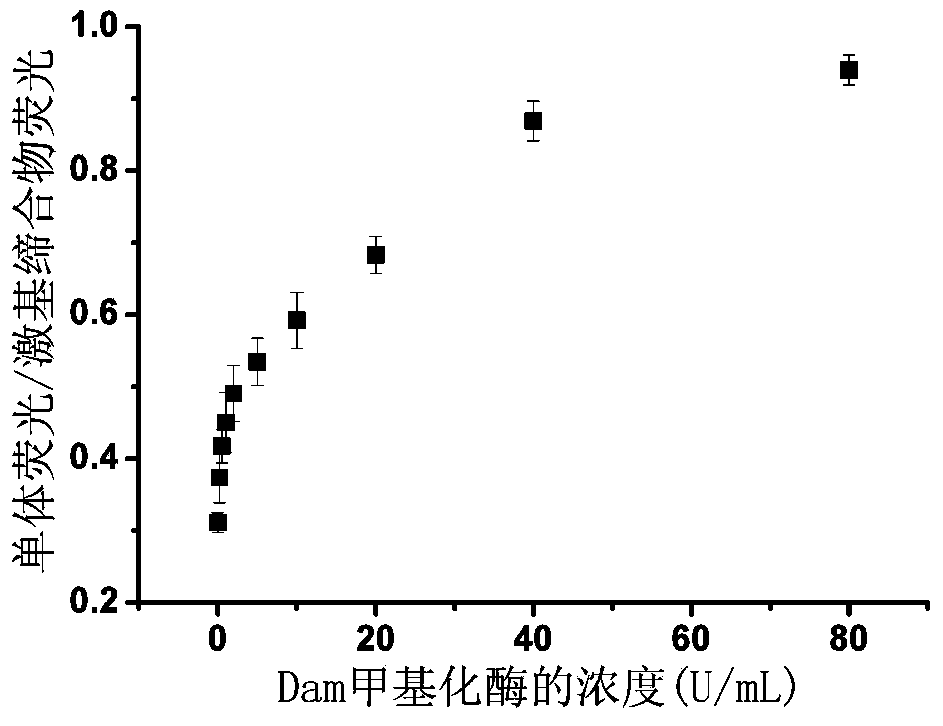
Perylene excimer-based detection method for methylase activity and screening method of methylase inhibitor
ActiveCN103911454ALarge Stokes shiftLong fluorescence lifetimeMicrobiological testing/measurementBiotechnologyS-Adenosyl-l-methionine
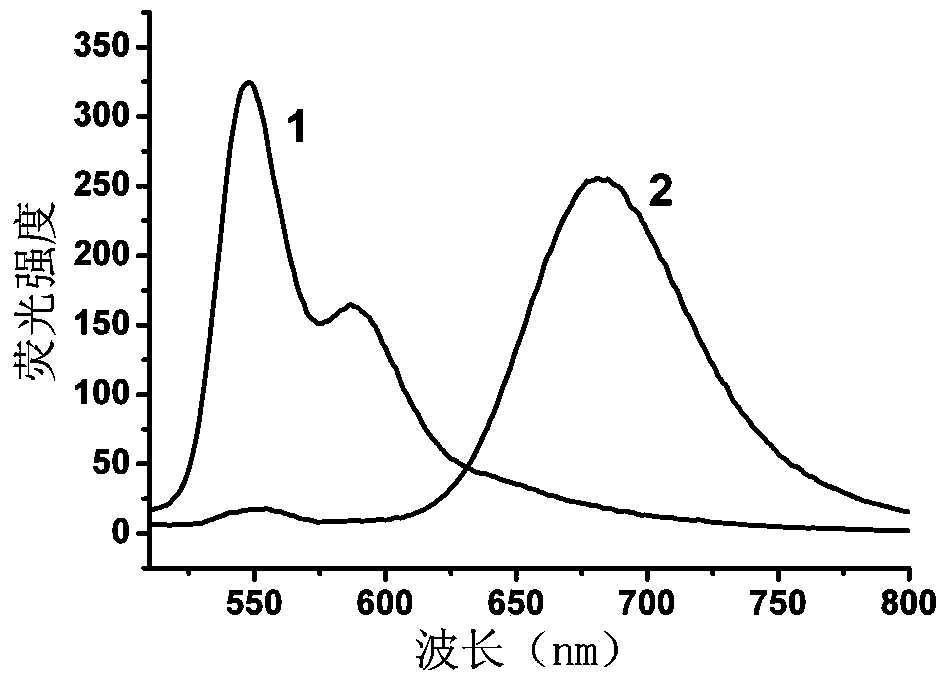
The invention provides a perylene excimer-based detection method for methylase activity and a screening method of a methylase inhibitor, and belongs to the field of biotechnology. The method comprises the following steps: firstly, preparing double-chain DNA, and then enabling the double-chain DNA to react with S-adenosylmethionine, restriction enzyme and different concentration of methylase, so as to obtain a mixed solution; enabling terminal deoxyribonucleotidyl transferase, deoxyribonucleoside triphosphate and TdT reaction buffer to react with the mixed solution, so as to obtain a reaction solution; finally, enabling the mixed solution of a perylene derivative probe and polycation to react with the reaction solution, and carrying out fluorescence detection on the methylase activity. The invention also provides a screening method of the methylase inhibitor. The activity of the methylase and the inhibitor is detected by using the change of ratio of a small molecule probe monomer to the excimer fluorescence intensity, the ratio of two given fluorescence signals is provided from the test, the fluorescence signals are not easily interfered in comparison with a pure fluorescence-intensified or weakened signal, and the sensitivity is higher.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Yak rotavirus detection kit based on constant-temperature isolation type fluorescence PCR (Polymerase Chain Reaction) platform and application
InactiveCN106868227AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationRotavirus RNAFreeze-drying
The invention discloses a yak rotavirus detection kit based on a constant-temperature isolation type fluorescence PCR (Polymerase Chain Reaction) platform and application. The yak rotavirus detection kit is composed of a reaction buffering solution, a fluorescence quantitative PCR reaction solution freeze-drying pipe, a positive reference substance freeze-drying pipe and a negative reference substance freeze-drying pipe. The fluorescence quantitative PCR reaction solution freeze-drying pipe is formed through mixing and freeze-drying a Taq enzyme, reverse transcriptase, a primer for detecting, a probe and dNTPs (deoxyribonucleoside triphosphate). The kit has the advantages of short detection time (reaction time is only 42min), high specificity, high sensitivity and convenience for storage (preservation at 4 DEG C); the kit is matched with a constant-temperature isolation type PCR instrument, is very suitable for on-situ rapid detection of yak rotaviruses and can be widely popularized in grassroots.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
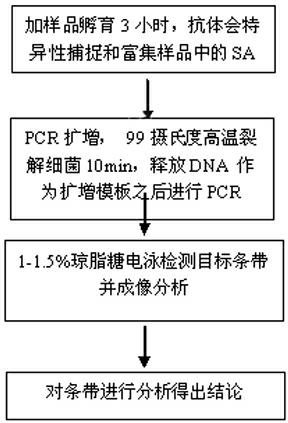
Immunocapture PCR (polymerase chain reaction) detection kit of staphylococcus aureus and using method of kit
InactiveCN102304585AQuick checkEasy to detectMicrobiological testing/measurementMicroorganism based processesForward primerStaphyloccocus aureus
The invention relates to a gene detection kit of staphylococcus aureus, and the detection kit belongs to the field of detection of food-borne pathogenic bacteria and is specially used for detecting the staphylococcus aureus (SA). The immunocapture PCR (polymerase chain reaction) detection kit of the staphylococcus aureus is mainly characterized by comprising 5 mu L of 10*PCR buffer, 2 mu L of dNTPs (deoxyribonucleoside triphosphates) (2.5 mmol / L), 0.25 mu L of Taq DNA (deoxyribonucleic acid) polymerase (5 U / L), 2 mu L of forward primer (10 pmol / L) which refers to primer 5'-GCGATTGATGGTGATACGGTT-3', 2 mu L of reverse primer (10 pmol / L) which refers to primer 5'-AGCCAAGCCTTGACGAACTAAAGC-3' and 99%-100% of double-distilled water, as well as a detection PCR tube coated with a staphylococcus aureus polyclonal antibody, a positive control PCR tube coated with the staphylococcus aureus polyclonal antibody, namely inactivated staphylococcus aureus bacteria liquid, a negative control PCR tube coated with the staphylococcus aureus polyclonal antibody, namely sterilized PBS (phosphate-buffered solution), a positive control 1 tube and a negative control 1 tube. The immunocapture PCR detection kit has the advantages of being capable of quickly, simply and accurately detecting the pathogenic bacteria.
Owner:刘箐 +1
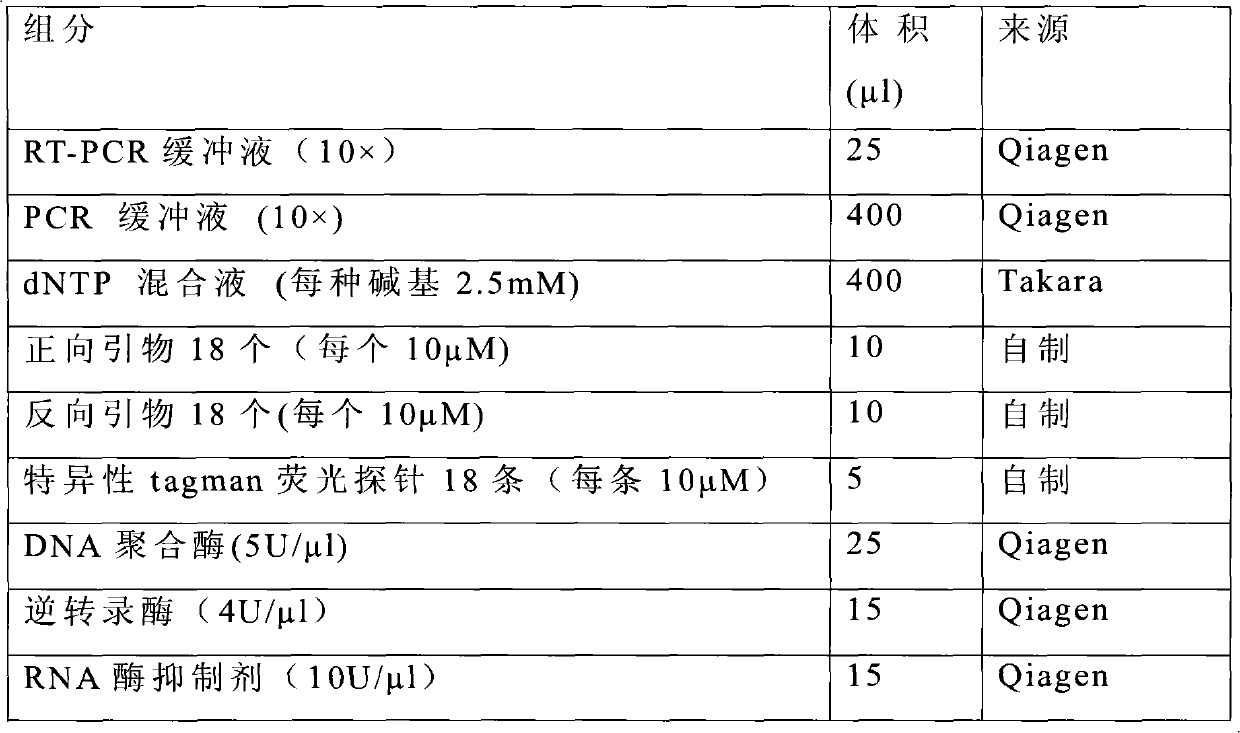
Kit for quantitative evaluation for long-term recurrent risk of colorectal cancer
InactiveCN102586402AWide range of clinical applicationsConsistent amplification efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceGenomicsReverse transcriptase
The invention relates to functional genomics and a gene expression detection and analysis technology, in particular to a kit for quantitative evaluation for long-term recurrent risk of colorectal cancer. The kit is characterized by consisting of 18 pairs of primers, 18 specific taqman fluorescent probes, 10* reverse transcription-polymerase chain reaction (RT-PCR) buffer solution, deoxyribonucleoside triphosphate (dNTP) mixed liquid, reverse transcriptase, deoxyribose nucleic acid (DNA) polymerase, 10* PCR buffer solution and ribonucleic acid (RNA) enzyme inhibitor. By adopting the self-designed and optimized RT primers and integrating a RT-PCR technology and a taqman real-time fluorescent quantitative PCR technology, the kit is simple and rapid in operation, more stable in diction results and lower in detection cost.
Owner:苏州科贝生物技术有限公司
Nested PCR (polymerase chain reaction) kit for detecting duck-manure pollution in water body and detection method thereof
ActiveCN104878002AReduce misjudgmentStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationNeutral Detergent FiberA-DNA
The invention discloses a nested PCR (polymerase chain reaction) kit for detecting duck-manure pollution in a water body and a detection method thereof, and belongs to the field of the detection of pollution in water bodies. The nested PCR kit for detecting the duck-manure pollution in the water body comprises two pairs of nested PCR primers, dNTP (deoxyribonucleoside triphosphate), a PCR buffer, a Taq polymerase, a Mg<2+> solution and ddH2O (double distilled water). Two rounds of PCR amplification are used by a nested PCR method. The detection method comprises the following steps of extracting a DNA (deoxyribonucleic acid) in a to-be-detected water sample, and carrying out a first round of PCR amplification by using the DNA in the to-be-detected water sample as a template, wherein the primers are an SDF (stroma derived factor) and SDR (short-chain dehydrogenase / reductase); after a first reaction is terminated, carrying out a second round of PCR amplification by using a product of the first round of PCR amplification as a template, wherein the primers are an NDF (neutral detergent fiber)and NDR, and after a second reaction is terminated, detecting the existence of a 158bp strip, which shows that the water body is subjected to the duck-manure pollution, through agarose gel electrophoresis. According to the method, the detection sensitivity is greatly increased through nested PCR amplification; a little duck-manure pollution which exists in water can be detected; moreover, the method has a favorable specificity, and can be directly applied to the detection and the preventive treatment work of fecal pollution in the water body.
Owner:NANJING UNIV
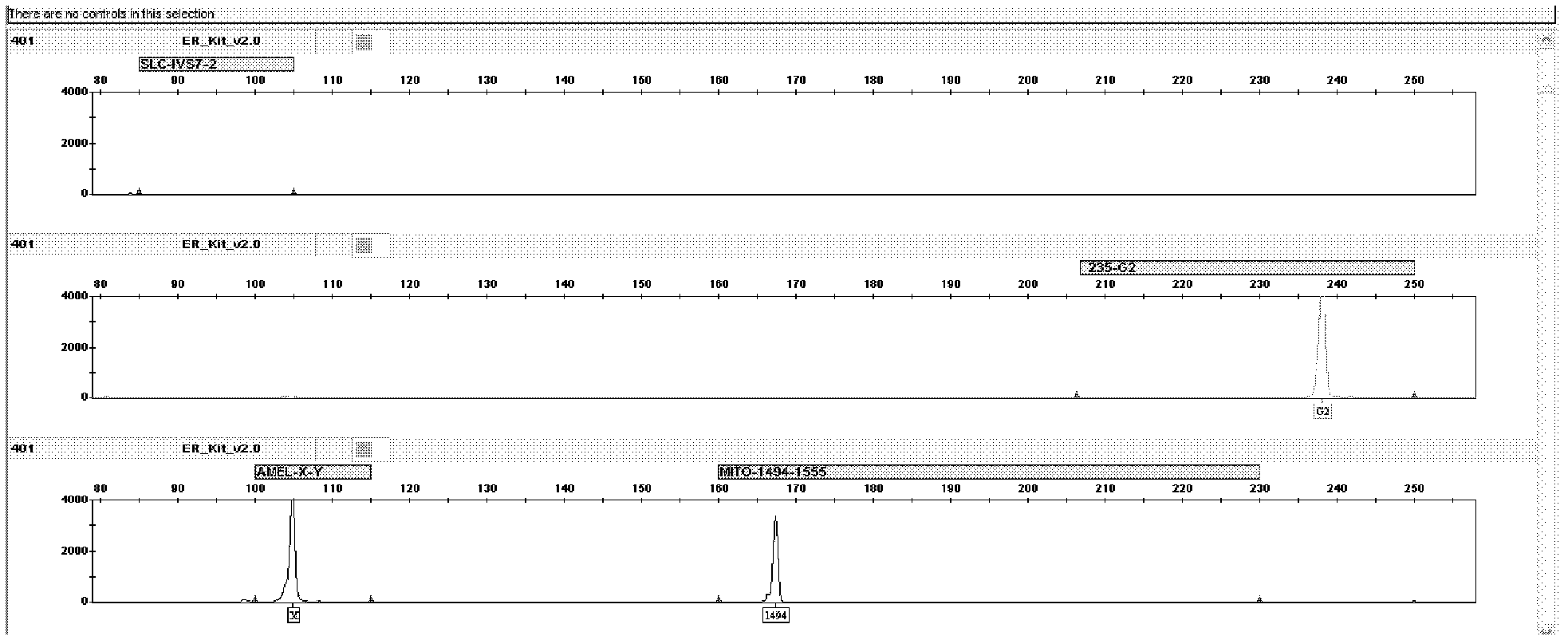
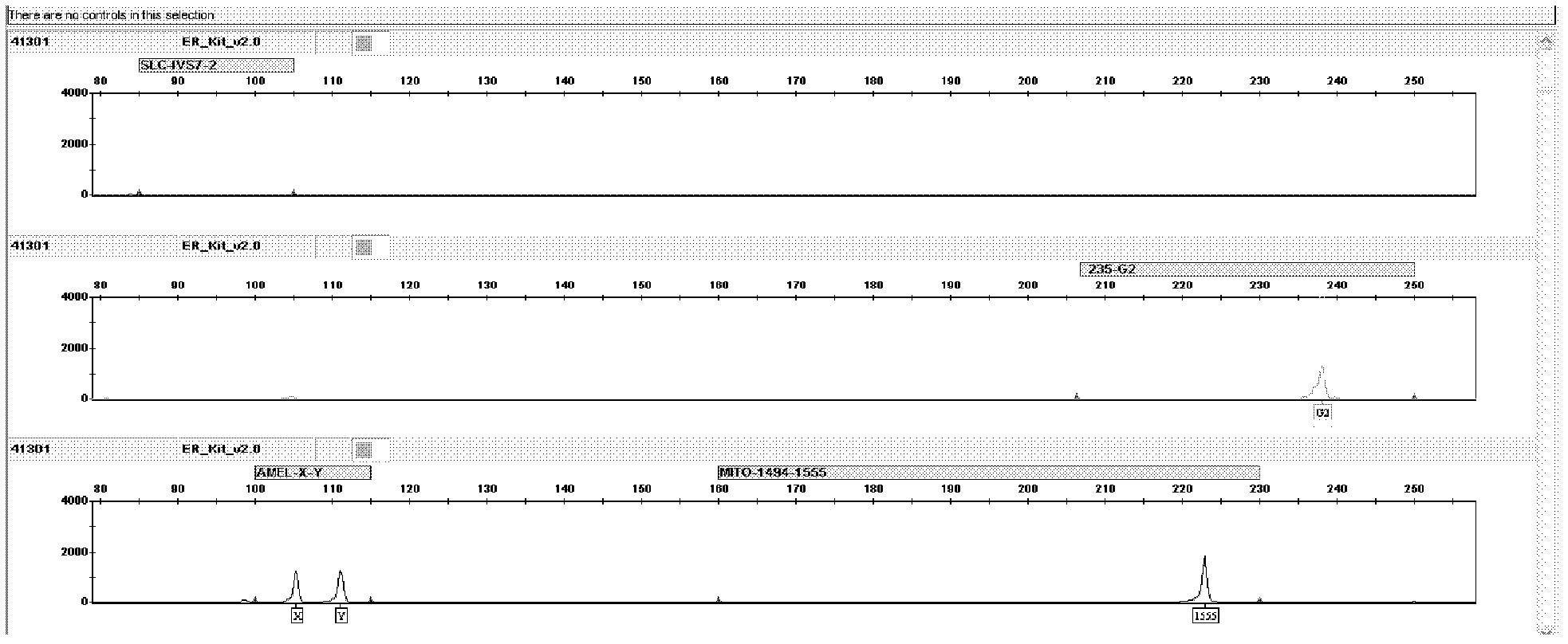
Kit for jointly detecting four deafness predisposing genes and application thereof
InactiveCN102534030AAvoid false positivesMicrobiological testing/measurementFluorescenceLNA nucleoside
The invention discloses a fluorescent detection kit for detecting four deafness predisposing genes simultaneously. The kit comprises reagents before amplification and reagents after amplification, wherein the reagents before amplification comprise a polymerase chain reaction (PCR) buffer solution, a reaction mixture of MgCl2 and deoxyribonucleoside triphosphates (DNTPs), Taq DNA polymerase, ultrapure water, and a primer mixture for amplifying loci of GJB2235delC, 12S rRNA 1555A>G mutation, 1494C>T mutation, IVS7-2A>G and Amelogenin at high specificity; and the reagents after amplification comprise a genotyping standard and an internal standard. Deafness gene loci of the GJB2235delC, 12S rRNA 1555A>G mutation, 1494C>T mutation, IVS7-2A>G and Amelogenin are simultaneously detected at high sensitivity and high specificity by combining a fluorescent labeling technology, a linolenic acid (LNA) nucleoside monomer doping-primer modification technology and a capillary electrophoresis technology for the first time, manpower and material resources and time are greatly saved, and pollution due to multi-step operation is prevented.
Owner:上海芯鑫生物科技有限公司
Reaction mixture for polymerase chain reaction
InactiveUS20120244599A1High sensitivityStrong specificityMicrobiological testing/measurementTransferasesPotassiumHydroxymethyl
A reaction mixture for a polymerase chain reaction (PCR) includes water, DNA polymerase, deoxyribonucleoside triphosphates (dNTPs), tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl), potassium chloride, magnesium chloride, dithiothreitol (DDT), and an additive agent. The reaction mixture is capable of enhancing the sensitivity and specificity of PCR and increasing the yield of correct nucleic acid sequence copies when being used in PCR.
Owner:GENEREACH BIOTECH CORP
Isothermal PCR nucleic acid augmentative method based on dI modified primer and endonuclease V
InactiveCN101182514AIncrease compositing speedHigh synthesis efficiencyRecombinant DNA-technologyForward primerHelicase
The invention relates to an isothermal PCR nucleic acid amplification method based on dI modified primer and endonucleaseV. The amplified target nucleic acid primer is completely matched with the template; 1 to 5 dI nucleic acids are on the 10 to 30 site at the 5' end; 10 to 30 nucleic acids are at the downstream of the dI nucleic acids. The reaction components of isothermal PCR comprise dI modified forward primer and reverse primer, target nucleic acid template, four kinds of deoxyribonucleoside triphosphates dATP, dTTP, dCTP and dGTP, DNA polymerase, endonucleaseV, single DNA chain binding protein and helicase. After the initial thermal denaturation and annealing hybridization, the mixture of isothermal PCR carries out the PCR reaction. During the amplification process, the circulation and regeneration of the primer-template compound is realized by endonucleaseV, which induces the DNA synthesis reaction; the single DNA chain binding protein and helicase can improve the efficiency of isothermal PCR. The amplification of the target nucleic acid of the invention does not rely on the thermal cycler; the invention has easy and convenient operation, which can be used for a large scale and high yield amplification of the target nucleic acid.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com