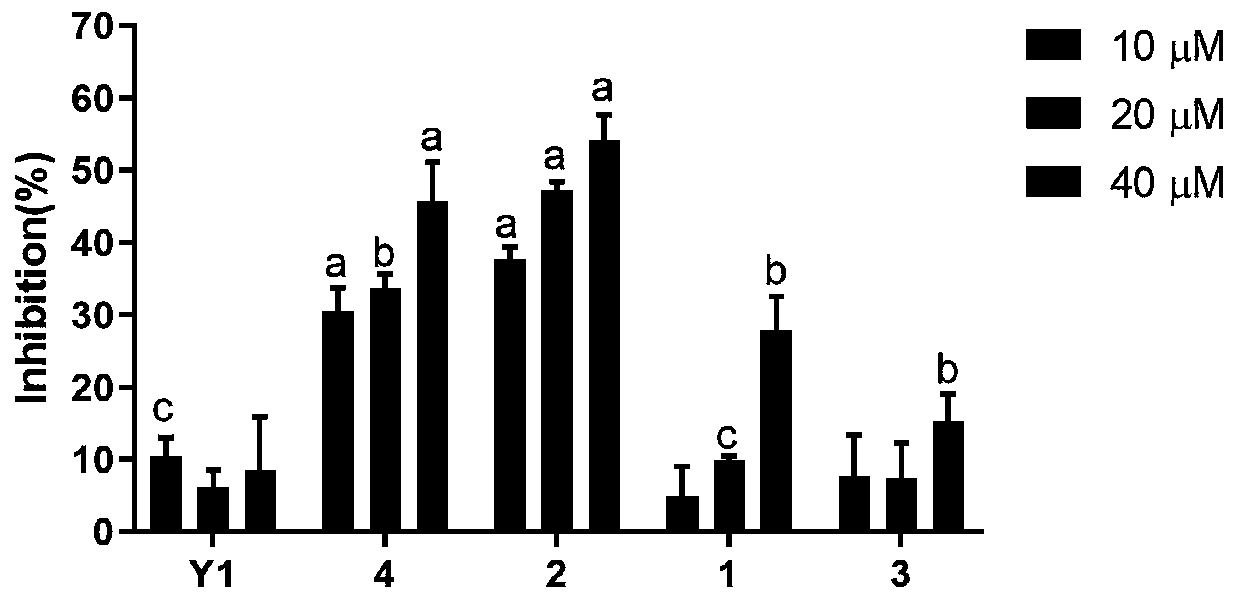
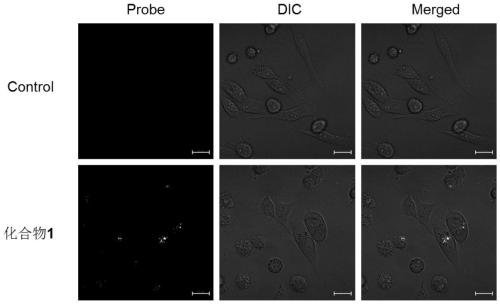
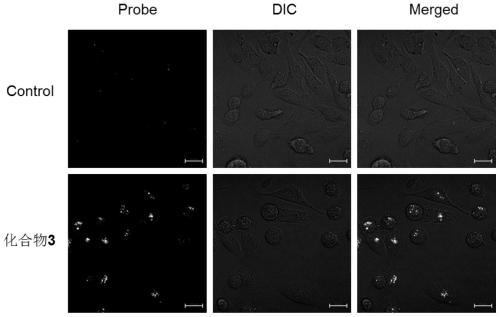
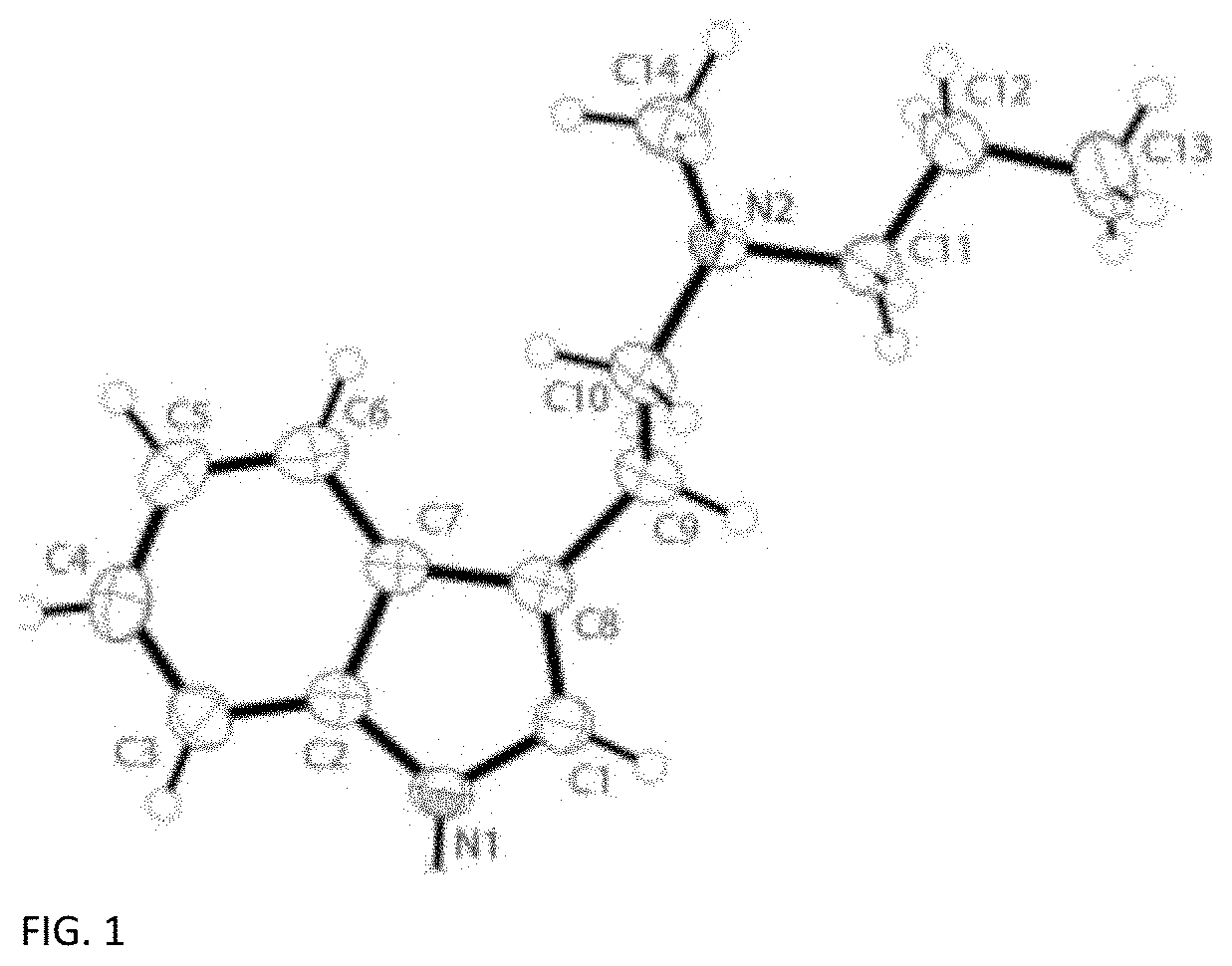
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
160 results about "Tryptamines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
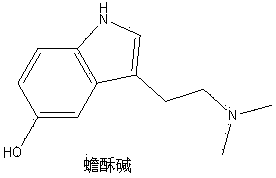
Decarboxylated monoamine derivatives of TRYPTOPHAN.
Lactobacillus plantarum with function of reducing contents of biogenic amines in foods and application of lactobacillus plantarum
ActiveCN105132308AAcid resistantHas the ability to clearBacteriaMicroorganism based processesBiotechnologyFermentation
The invention belongs to the technical field of microorganisms and discloses lactobacillus plantarum with a function of reducing contents of biogenic amines in foods and application of the lactobacillus plantarum. The lactobacillus plantarum is resistant to acids and capable of strongly removing eight types of biogenic amines (including tryptamine, phenethylamine, putrescine, cadaverine, histamine, tyramine, spermidine and spermine) in vitro. After 1*1010CFU / ml of the lactobacillus plantarum and the eight types of biogenic amines are co-cultured for 24h, wherein the concentration of each type of the biogenic amines is 100mg / L, and the total amine concentration is 800mg / L, the total amine content is reduced by 70% approximately, the content of each type of the biogenic amines is reduced by 30%-100%, and the removal rate of histamine highest in toxicity is up to 96.69%. Further, the lactobacillus plantarum is capable of lowering pH to 4 in eight hours owing to quickness in acid generation, thereby having favorable fermentation potential. The lactobacillus plantarum is used for degradation of the biogenic amines in foods, especially in fermented foods and extensive in application prospect.
Owner:JIANGNAN UNIV
Use of tryptanthrin compound as indoleamine 2,3-dioxygenase (IDO) inhibitor
InactiveCN103054870AExcellent inhibitory effectOrganic active ingredientsNervous disorderDiseaseInhibition constant
The invention belongs to the medicinal field, and concretely relates to a use of a 8-nitrotryptanthrin compound as an IDO inhibitor. The 8-nitrotryptanthrin compound is a reversible IDO inhibitor, has an inhibition constant Ki of 0.054muM, has in-vitro and cell-based median effective inhibition concentrations IC50 of 0.103muM and 1.80*10<-5>muM respectively, and has an inhibition effectiveness obviously better than a present inhibitor 1-methyltryptophan (Ki of 34muM and IC50 of 340muM). 8-nitrotryptanthrin disclosed in the invention can effectively lower the abnormally-increasing IDO activity in a tumor animal model as the IDO inhibitor, and also has tumor treatment effects comprising tumor growth delaying, tumor volume reduction and in-vitro tumor cell killing. 8-nitrotryptanthrin disclosed in the invention has a wide application prospect, and can be used for treating serious diseases having the IDO mediated tryptophan metabolism approach pathology characteristics, such as cancers, the Alzheimer disease, tristimania, cataract and the like as the IDO inhibitor.
Owner:FUDAN UNIV
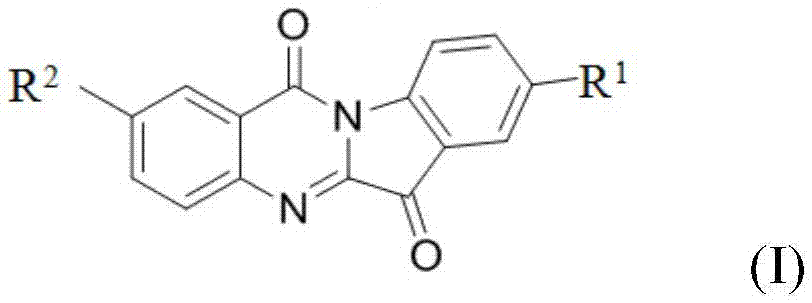
Novel tryptanthrin derivative, synthetic method and medicinal application thereof
ActiveCN105330666AImprove solubilityStrong inhibitory activityAntibacterial agentsOrganic active ingredientsCancer cellMorpholine
The invention relates to a novel tryptanthrin (indole[2,1-b] quinazoline-6,12-diketone) derivative, a synthetic method and a medicinal application thereof. A general structural formula is as follows (see the description). The novel tryptanthrin derivative is synthesized through biomimetic synthesis, the general chemical structural formula of the novel derivative is shown in Figure 1, wherein in the formula, R,R1,R2,R3 are halogen, nitro, amido, hydroxy, alkyl, alkoxy and aryl, R1is morpholine, piperidine, N-methylpiperidine, N-ethylpiperidine, isonipecotic acid, piperazine, 1-(2-pyridyl) piperazine, dimethylamine, diethylamine, 1-(2-ethoxy) piperazine and 1-(2-furoyl) piperazine, and an o-aminobenzoic acid derivative reacts with an indole quinone derivative in solvents of methylbenzene, methyl alcohol, ethyl alcohol, dichloromethane, isopropanol and the like, so as to prepare the novel tryptanthrin derivative. The novel tryptanthrin derivative which has a good inhibiting effect and a good antibacterial effect on cancer cells is preliminarily screened through an MTT experiment and an antibacterial experiment, and the medicine has pharmacological effects in the aspects of tumor resistance, antibiosis and inflammation diminishing.
Owner:NORTHWEST UNIV
Therapeutic Compounds
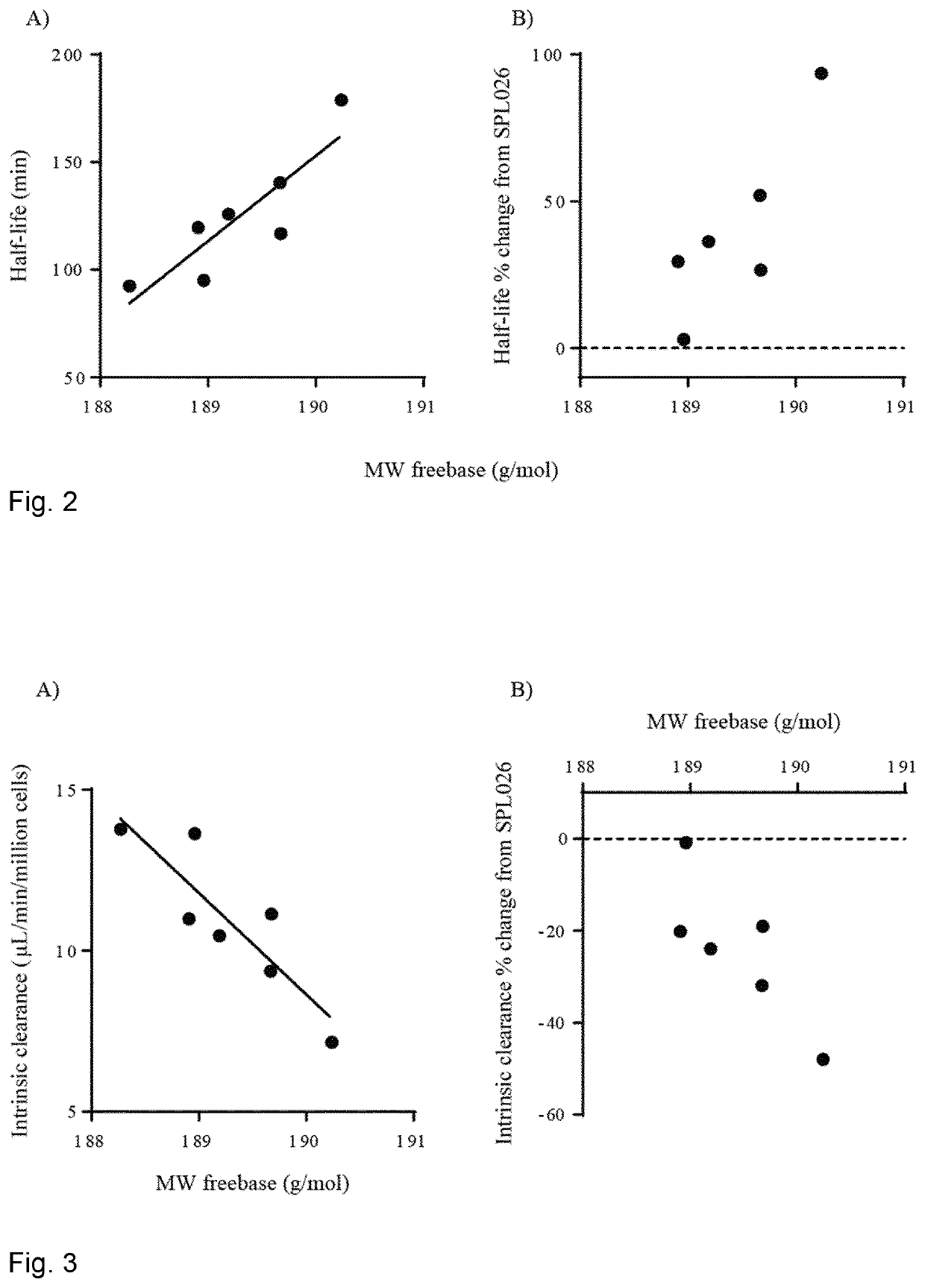
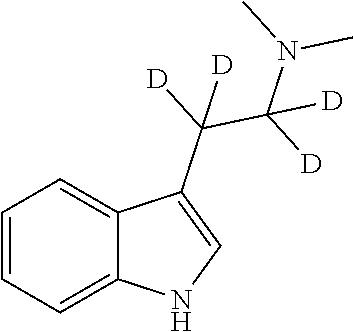
PendingUS20200390746A1High purityImprove efficiencyOrganic active ingredientsNervous disorderTryptaminesMedicinal chemistry
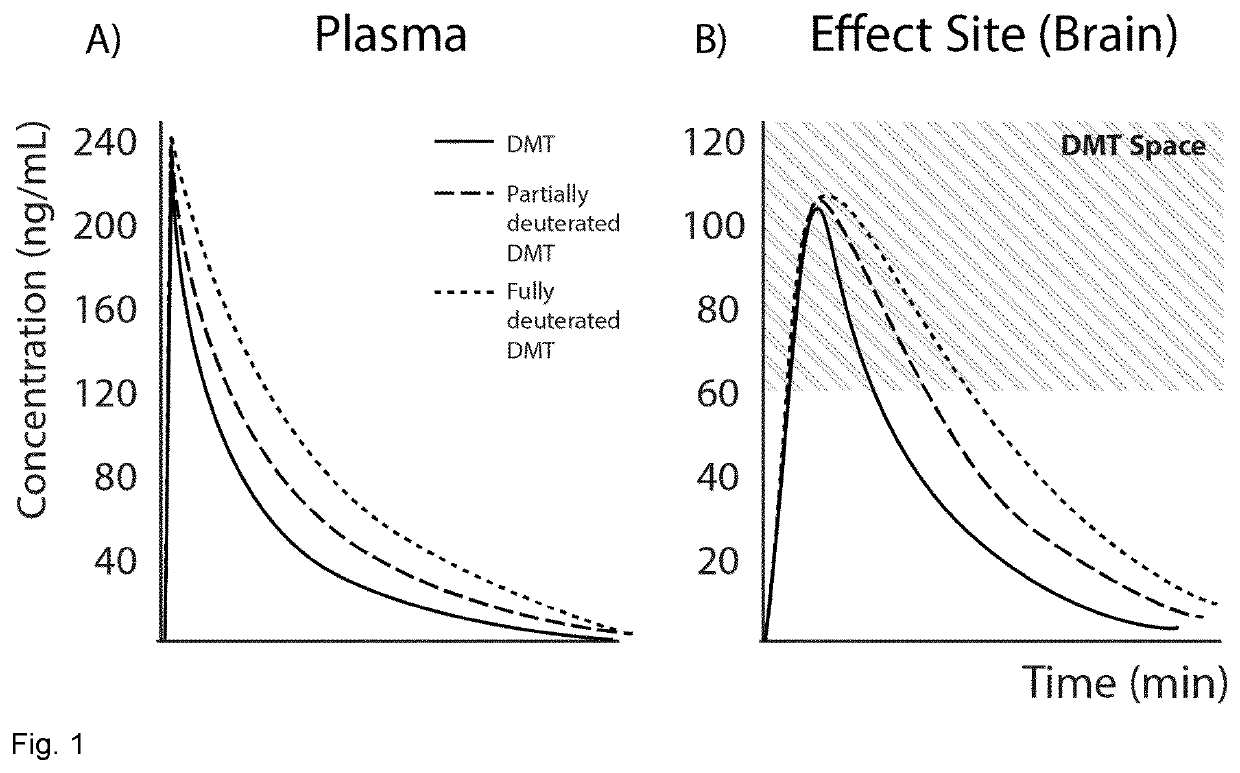
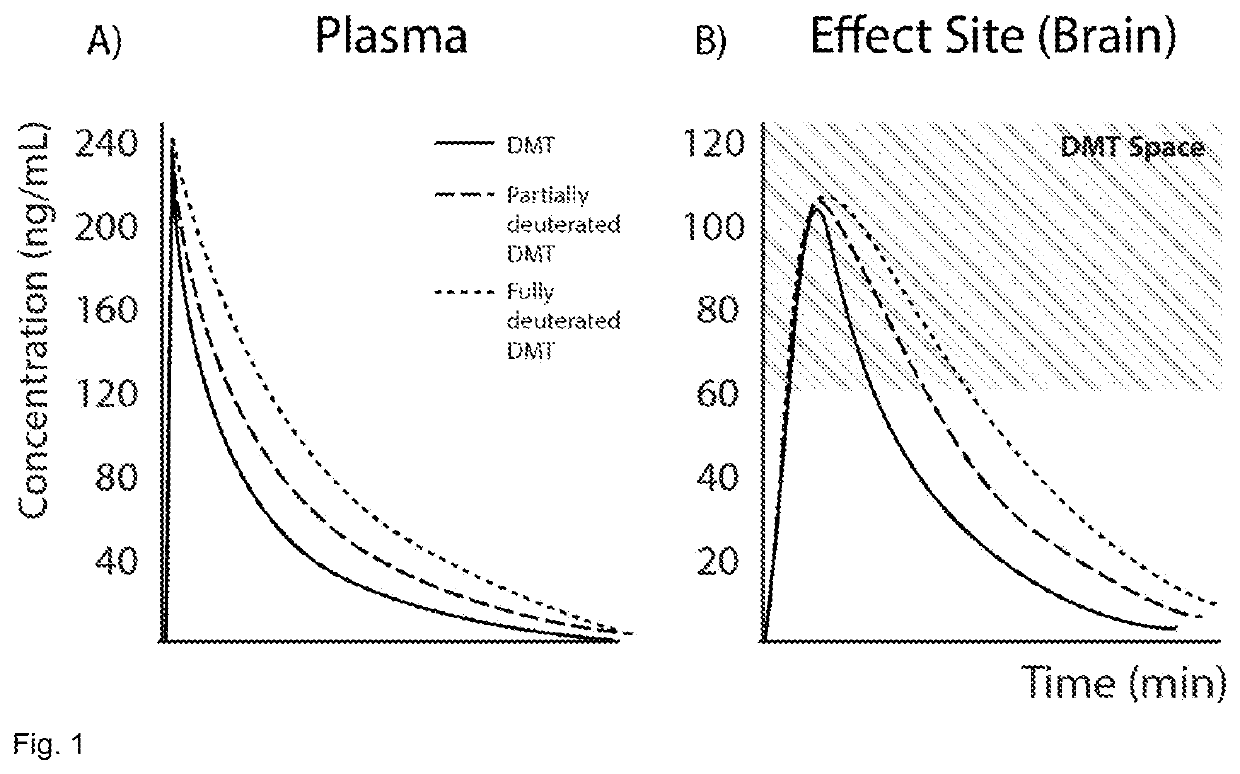
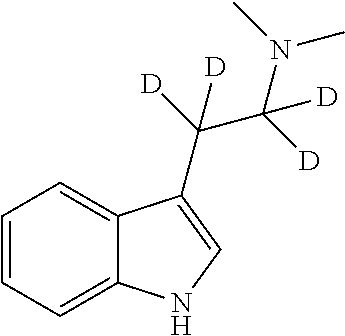
The present invention relates to compositions comprising N,N-dimethyltryptamine, deuterated N,N-dimethyltryptamine and / or partially deuterated N,N-dimethyltryptamine. In particular, the present invention relates to compositions comprising a combination of N,N-dimethyltryptamine and 2% or more by weight of one or more deuterated N,N-dimethyltryptamine compound selected from α,α-dideutero-N,N-dimethyltryptamine and α,α,β,β-tetradeutero-N,N-dimethyltryptamine. Additional and alternative compositions of the present invention comprise a combination of N,N-dimethyltryptamine and 2% or more by weight of one or more partially deuterated N,N-dimethyltryptamine compound selected from α,β,β-trideutero-N,N-dimethyltryptamine, α,β-dideutero-N,N-dimethyltryptamine, and α-deutero-N,N-dimethyltryptamine. Methods of synthesising compositions of the present invention, and methods of use of presently described compositions in treating psychiatric or psychocognitive disorders, such as major depressive disorder, are also provided.
Owner:SMALL PHARMA LTD
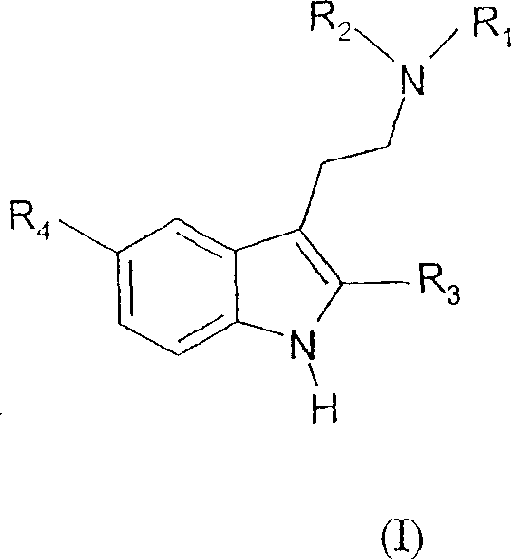
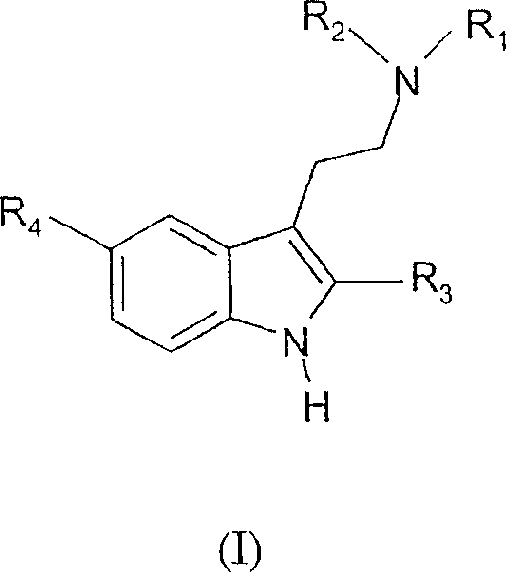
Tryptamine prodrugs
The present invention provides a tryptamine prodrug compound. A compound represented by the formula (I)where each symbol is as described in the specification, or a salt or zwitterion thereof, is converted to an active which has 5HT2A receptor agonist activity, and is useful as an agent for the treatment of depression.
Owner:REUNION NEUROSCIENCE CANADA INC
Use of beggarweed alkaloid monomer component
InactiveCN103816150AStrong inhibitory activityImproved stress behaviorOrganic active ingredientsNervous disorderAmine oxidase inhibitorsPharmaceutical drug
Owner:LANZHOU UNIVERSITY OF TECHNOLOGY
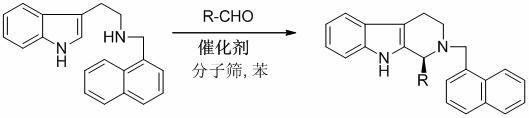
Method for synthesizing optically active tetrahydro-beta-carboline derivative through catalysis of chiral spirocyclic phosphoric acid
InactiveCN102432608AHigh enantioselectivityMild reaction conditionsAsymmetric synthesesO-Phosphoric AcidPtru catalyst
The invention discloses a method for synthesizing an optically active tetrahydro-beta-carboline derivative through the catalysis of chiral spirocyclic phosphoric acid. The method comprises the following steps of: reacting at the temperature of between 20 and 40 DEG C for 3 to 50 hours by taking Nb-alpha-naphthylmethyl tryptamine and aldehyde as raw materials, the chiral spirocyclic phosphoric acid as a catalyst and benzene as a reaction solvent in the presence of 4 angstrom molecular sieve powder under the protection of nitrogen, and performing column chromatographic purification and separation to obtain the optically active tetrahydro-beta-carboline derivative. Reaction conditions are mild, and the process is simple and convenient to operate; and the obtained optically active tetrahydro-beta-carboline derivative has high potential bioactivity, and can be used as an intermediate for pharmaceutical synthesis.
Owner:ZHEJIANG UNIV
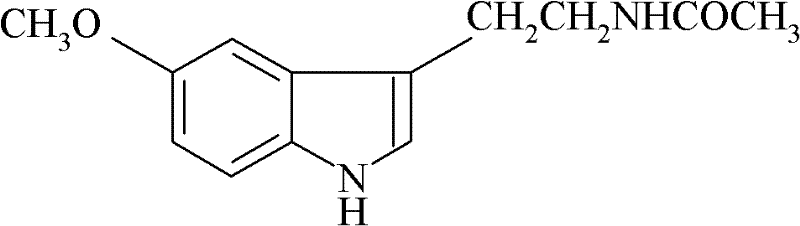
Preparation method of N-acetyl-5-methoxytryptamine
InactiveCN102329263AReduce dosageLess side effectsOrganic chemistryAcetic anhydrideN-Acetyl-5-methoxytryptamine
The invention discloses a preparation method of N-acetyl-5-methoxytryptamine. The preparation method comprises the following steps: adding a raw material, namely 5- methoxytryptamine into a dichloromethane solvent, dripping mixed solution of acetic anhydride and dichloromethane at the dripping time of 0.5-1.0 hour at the temperature of 5-10 DEG C, adding a catalyst, namely 4-DMAP (4-dimethylaminophenol), reacting for 1-2 hours at the normal temperature and collecting a target product from reaction products. Through testing, the product purity is not less than 98%, the yield is not less than 94% and the melting point is 114-117 DEG C. The catalyst, namely the 4-DMAP is adopted for performing N-acylation reaction, the using quantity is low, the speed is fast, the side reactions are few, the reaction conditions are mild, the yield is high, the reaction is performed under normal pressure, the operation is safe, simple and convenient, and the preparation method is suitable for industrial production.
Owner:SHANGHAI CHEM REAGENT RES INST
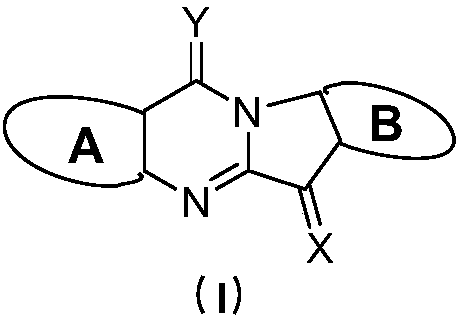
Tryptanthrin derivatives and application thereof
InactiveCN109956942AStrong inhibitory activityGood treatment effectOrganic active ingredientsSenses disorderDiseaseHead and neck tumors
The invention discloses compounds represented by a general formula (I) shown in the description, wherein in the formula (I), A and B are respectively a six-membered cyclic compound containing D1, D2,D3 and D4 and a six-membered cyclic compound containing D5, D6, D7 and D8. The invention also disclose an indoleamine-2,3-dioxygenase and / or tryptophan-2,3-dioxygenase inhibitor containing the above compounds and the application of the compounds in preparation of medicaments for treating cancers. The compounds provided by the invention can effectively inhibit cell proliferation, and have good therapeutic effects on various diseases such as the cancers, significant therapeutic effects on breast cancer, cervical cancer, colon cancer, lung cancer, stomach cancer, rectal cancer, pancreatic cancer,brain cancer, skin cancer, oral cancer, prostate cancer, bone cancer, kidney cancer, ovarian cancer, bladder cancer, liver cancer, fallopian tube tumors, ovarian tumors, peritoneal tumors, stage IV melanoma, glioma, neuroblastoma, hepatocellular carcinoma, mastoid renal tumors, head and neck tumors, leukemia, lymphoma, myeloma and non-small cell lung cancers, and very broad application prospects.
Owner:SHANGHAI SHENGYUE PHARM TECH CO LTD
Recombinant broad-spectrum metarhizium as well as preparation method and application thereof
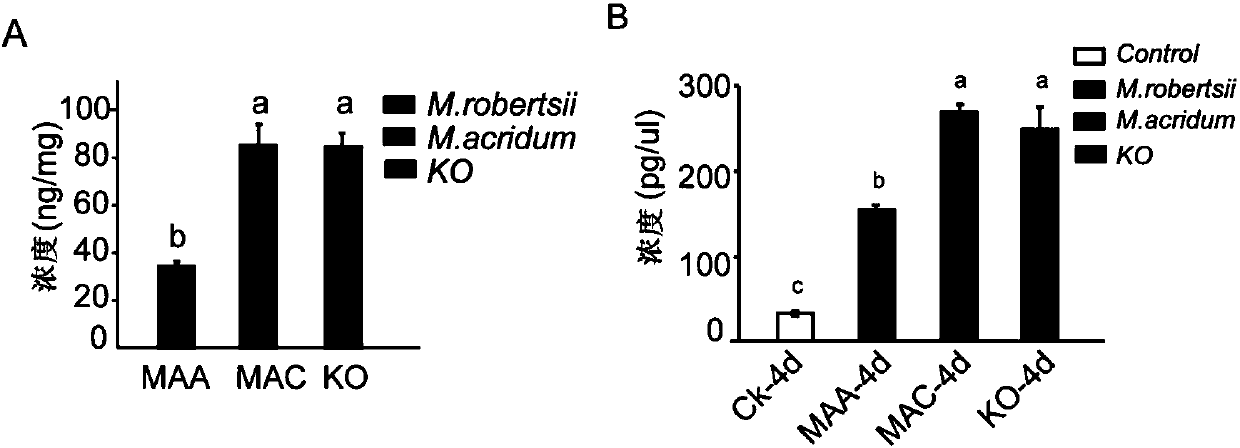
ActiveCN107916232AIncrease the concentration of tryptamineImprove insecticidal efficiencyBiocideFungiBiotechnologyTryptamine
The invention provides recombinant broad-spectrum metarhizium; the recombinant broad-spectrum metarhizium down-regulates the expression of monoamine oxidase or does not express the monoamine oxidase,or the content of tryptamine in a recombinant broad-spectrum metarhizium body is higher than that of tryptamine in wild type broad-spectrum metarhizium; the recombinant broad-spectrum metarhizium is the strain per se, the progenies of the strain, the conidia produced by the strain, the mycelia produced by the strain, or any combination of the progenies, the conidia and the mycelia. A monoamine oxidase gene in the recombinant broad-spectrum metarhizium is knocked out, so that the concentration of tryptamine in a broad-spectrum metarhizium robertsii is significantly increased, the insecticidal efficiency is further obviously increased, and the half lethal time LT50 of the wild-type broad-spectrum metarhizium is shortened to 6.136+ / -0.488 days from 7.33+ / -0.445 days; furthermore, the recombinant broad-spectrum metarhizium is harmless to the environment, good in biological safety and non-toxic to human beings.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Diazene directed modular synthesis of compounds with quaternary carbon centers
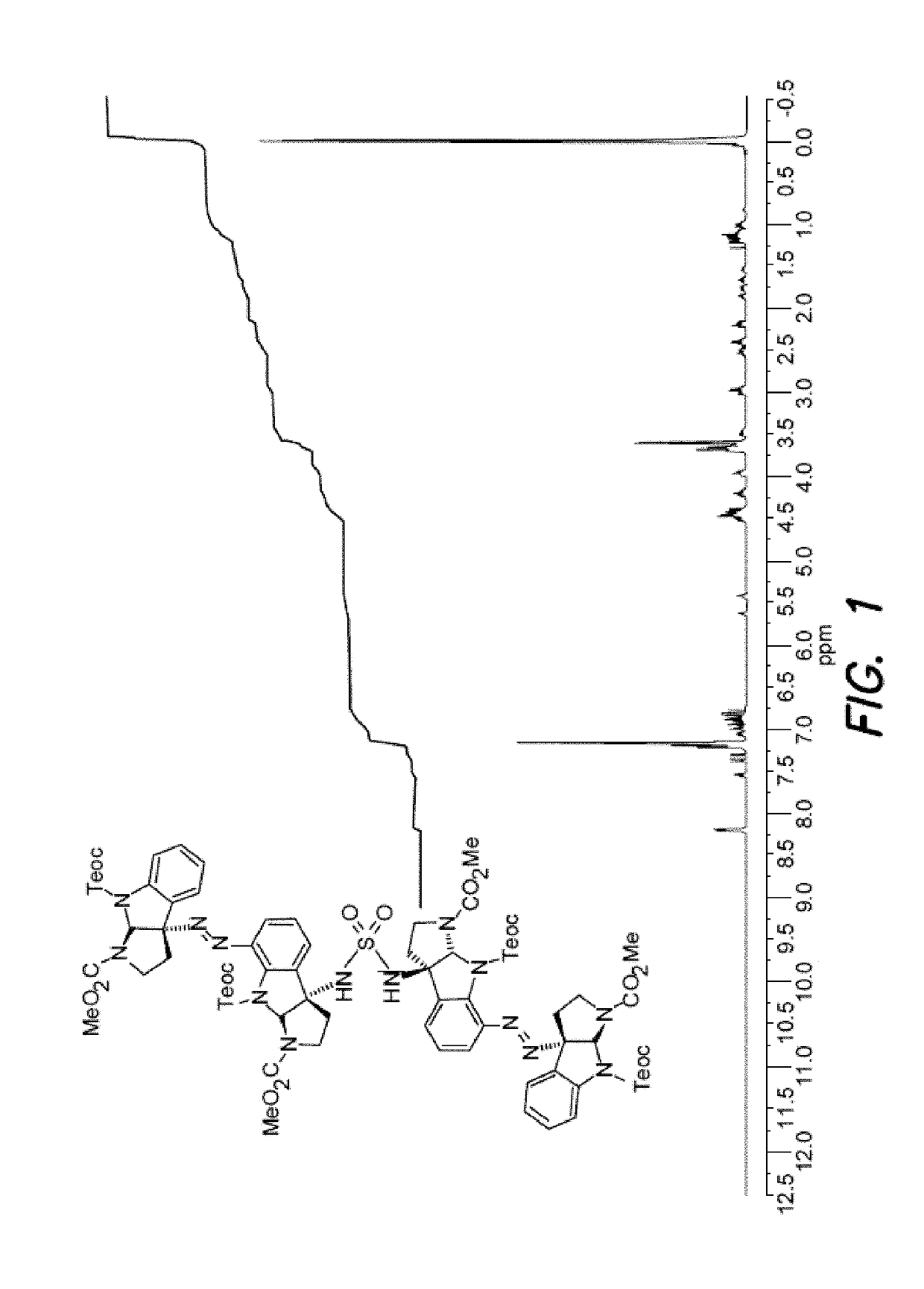
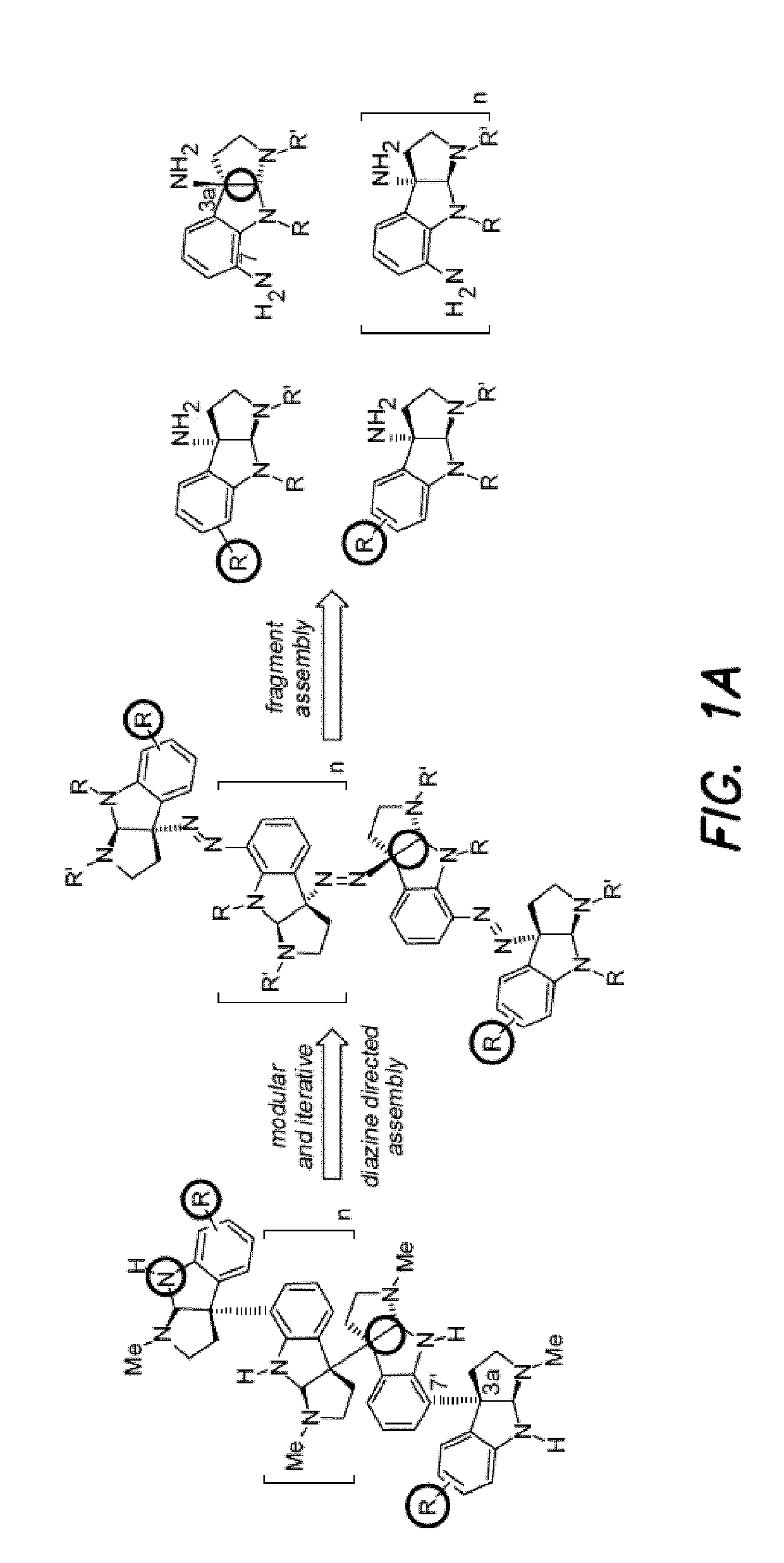
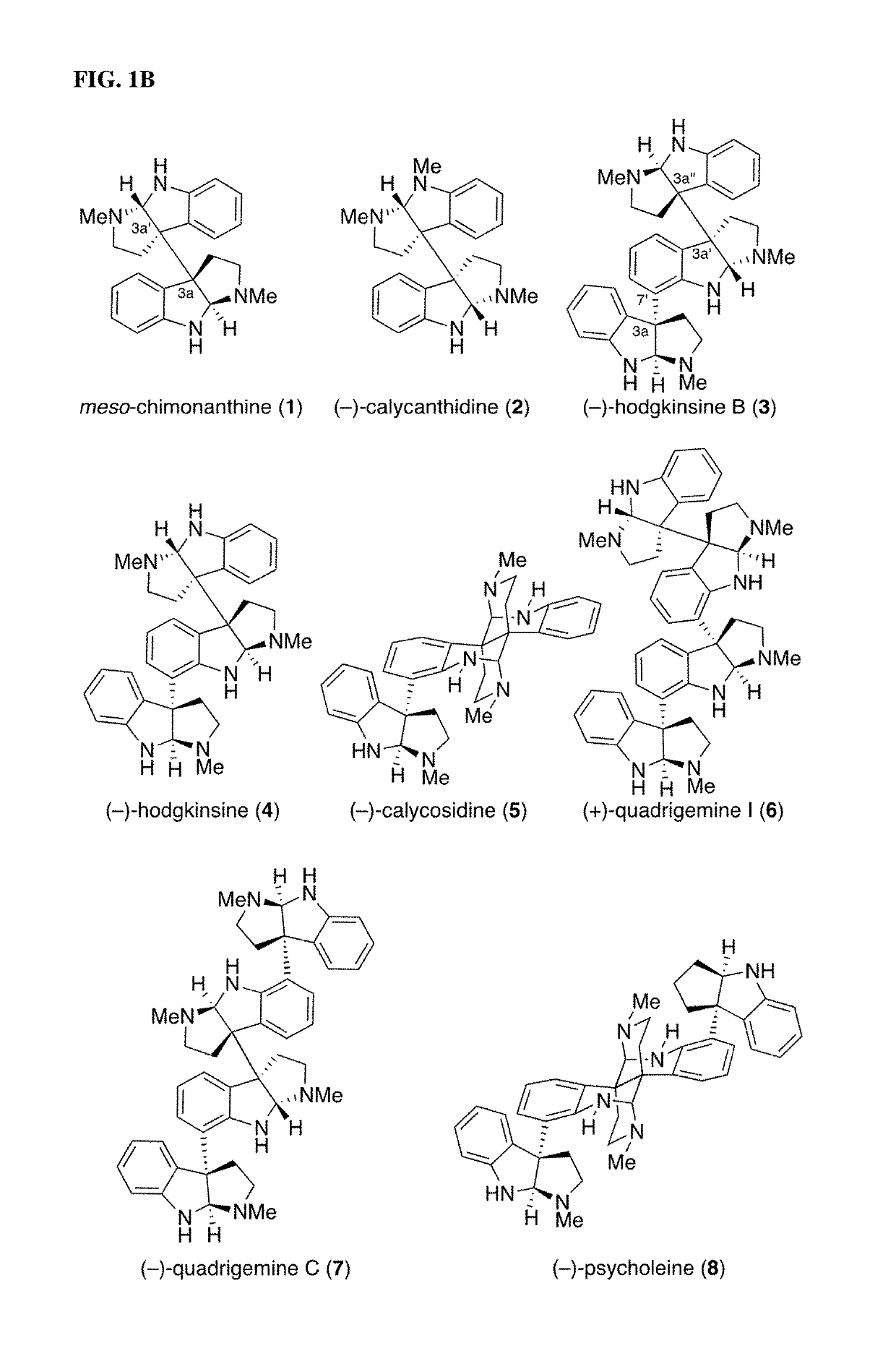
Diazene-directed modular synthesis is described for the preparation Csp2-Csp3 and Csp3-Csp3 linkages where one or more stereogenic quaternary carbon centers are formed. The disclosed methods are directed to the preparation of compounds of Formula (I), or a pharmaceutically acceptable salt, tautomer or stereoisomer thereof, from compounds of Formula (II):wherein R1-R5 and q are as defined independently for each occurrence herein. A wide variety of compounds can be accessed in this manner, including oligocyclotryptamines, where the stereochemistry of each subunit is beneficially secured before fragment coupling.
Owner:MASSACHUSETTS INST OF TECH
Method of Inducing Dendritic and Synaptic Genesis in Neurodegenerative Chronic Diseases
The present invention discloses a method to recover and restore dendritic and synaptic neuron connections that have been degraded or destroyed by neurodegenerative diseases. In the present invention tryptamines are used to induce neuro plasticity and restore both dendritic density and synaptic connections of neurons in the brain. In the preferred embodiment LSD given in micro doses can induce dendritic and synaptic genesis in neuronal networks and improve the quality of life of people with neurodegenerative diseases such as Alzheimer's, Huntington's, Multiple Sclerosis, Parkinson's and Frontotemporal dementia.
Owner:PETCAVICH ROBERT JOSEPH
Tryptamine prodrugs
The present invention provides a tryptamine prodrug compound. A compound represented by the formula (I)where each symbol is as described in the specification, or a salt or zwitterion thereof, is converted to an active which has 5HT2A receptor agonist activity, and is useful as an agent for the treatment of depression.
Owner:REUNION NEUROSCIENCE CANADA INC
Crystalline forms of psilacetin
ActiveUS20210292278A1Organic active ingredientsOrganic chemistry methodsCrystallographyPharmaceutical drug
Owner:CAAMTECH LLC
Novel tryptamine derivative and preparation method and application thereof
PendingCN111116449AStrong inhibitory activityHas a neuroprotective effectOrganic active ingredientsNervous disorderInflammatory factorsSide effect
The invention discloses a novel tryptamine derivative. The novel tryptamine derivative is characterized in that the chemical structural formula of the novel tryptamine derivative is as shown in the specification. According to the invention, an intracerebral tryptamine hormone fragment with an anti-neuroinflammation effect and a non-steroidal anti-inflammatory drug salicylic acid and derivatives thereof are subjected to molecular combination; on one hand, COX effect can be inhibited; and on the other hand, the generation of iNOS and inflammatory factors and the activation of immune cells can beinhibited, and the occurrence and development of inflammations are jointly inhibited through multiple mechanisms. Meanwhile, carboxylic acid groups of salicylic acid non-steroidal anti-inflammatory drugs can be removed, so gastrointestinal side effects caused by acidity are reduced. On such a basis, a series of compounds are designed and synthesized; and enzyme inhibition tests, various immune cell tests and animal in-vivo experimental studies prove that the synthesized compounds have good anti-inflammatory and analgesic activity.
Owner:LANZHOU UNIVERSITY
Chiral 3-indolyl-3,3'-disubstituted oxoindole compound and preparation method thereof
ActiveCN111646931ARich varietyImprove biological activityOrganic chemistry methodsChemical recyclingChemical synthesisPharmaceutical Substances
The invention discloses a chiral 3-indolyl-3,3'-disubstituted oxoindole compound and a preparation method thereof and belongs to the field of organic chemical synthesis; the preparation method comprises the following steps: dissolving 2-nitroindole (I) and 3-substituted oxindole (II) in an organic solvent, adding a chiral catalyst, carrying out a stirring reaction at 0-25 DEG C for 5-7 days, adding a solvent and an organic acid into the reaction system after the reaction is completed, continuously carrying out a reaction on the reaction mixed solution at 0-25 DEG C for 10-15 h, and carrying out separation and purification. According to the preparation method disclosed by the invention, through asymmetric dearomatization / heavy aromatization reaction of 2-nitroindole, the preparation of chiral 3-indolyl-3'-alkyl oxindole and 3-indolyl-3'-aryl oxindole derivatives is realized; the compound is a key synthetic intermediate of cyclic tryptamine alkaloid, and can provide more candidate molecules for research and development of new drugs and screening of drugs. The preparation method disclosed by the invention has the advantages of novelty, simplicity, simplicity in operation, mild reaction conditions, high yield, high stereoselectivity and the like.
Owner:CHENGDU UNIV
Application of evodiamine derivative in preparation of medicine for treating superficial fungal infection
ActiveCN113768936ALightens dark red erythemaReduce hardeningOrganic active ingredientsAntimycoticsEvodiae FructusCutis
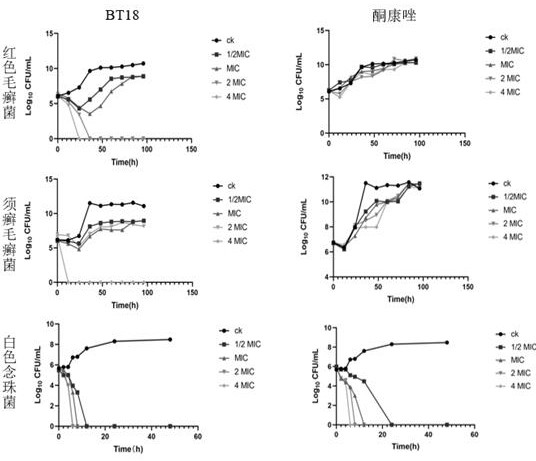
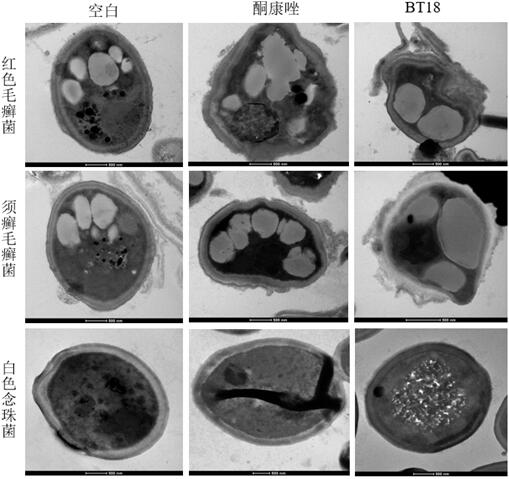
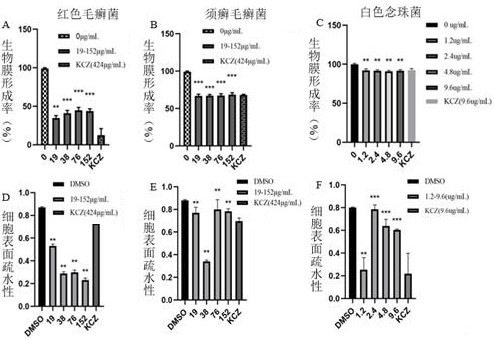
The invention provides application of an evodiamine derivative in preparation of a medicine for treating superficial fungal infection. The evodiamine derivative disclosed by the invention is prepared by taking a methyl anthranilate compound and tryptamine as initial raw materials through amine-ester exchange and a one-step ring closing reaction catalyzed by Lewis acid. The evodiamine derivative disclosed by the invention can show good antibacterial activity on three fungi, namely trichophyton rubrum, trichophyton mentagrophytes and candida albicans. Moreover, cell walls, cell membranes and organelles of fungal spores are destroyed to a certain extent, the hydrophobicity of fungal biomembranes can be influenced to a certain extent, phenomena of dark red erythema, skin hardening and generation of a large amount of scurf caused by errhysis spots of skin subjected to dermabrasion treatment can be obviously relieved, and phenomena of thickened epidermal layer of skin tissue, small-area epidermal layer necrosis, cuticle small-area parakeratosis and the like are avoided. The evodiamine derivative has low toxicity and low drug resistance and can be applied to preparation of drugs for treating the superficial fungal infection.
Owner:NANHUA UNIV
5-halo-tryptamine derivatives used as ligands of 5-HT 6 and/or 5-HT7 serotonin receptors
Compounds of Formula (I): (I); wherein: R1 and R2 either the same or different, are H or linear or branched C1-C6 alkyl; R3=linear or branched C1-C6 alkyl; R4=halogen, and pharmaceutically acceptable salts thereof are useful as active ingredients in the preparation of medicaments used as ligands of the 5-HT6 and / or 5-HT7 serotoninergic receptors.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
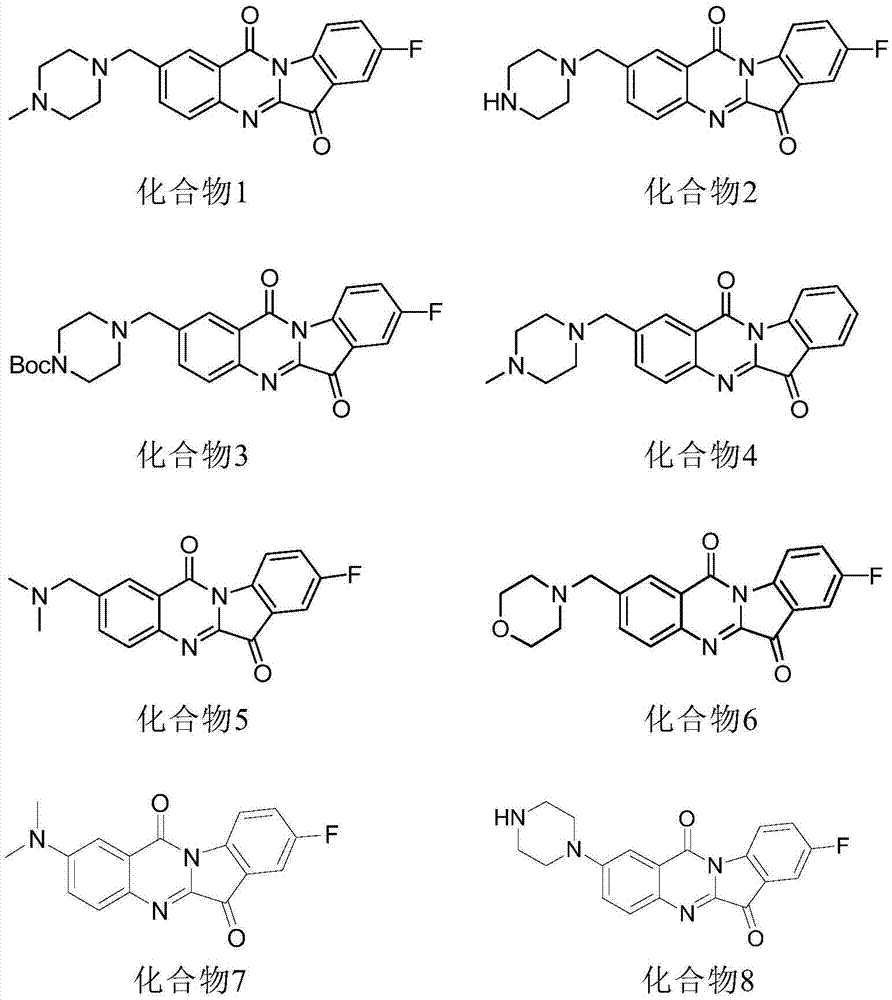
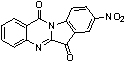
6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound
The invention discloses a 6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound,a preparation method and applications thereof, wherein the structural general formula (I) of the compoundis defined in the specification, R1 is hydrogen atom, morpholinomethyl, piperazinomethyl or substituted piperazinomethyl, R2 is hydrogen atom, butyl, morpholine substituted alkyl, benzyl, substitutedbenzyl, tryptamine, substituted tryptamine or N,N-dimethylamino, R3 is-C(O)R4 or-SO2R5, R4 and R5 are alkyl (C3-C6 alkyl), cycloalkyl (3-6-membered ring), alkoxy or alkyl substituted amino, and n is 1, 2, 3 or 4. The compound of the invention has inhibition activity on breast cancer, prostate cancer and human neuroblastoma.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
Evodiamine derivative as well as preparation and application thereof
PendingCN113683615AInhibition of invasion and migrationGrowth inhibitionOrganic chemistryAntineoplastic agentsEvodiamineApoptosis
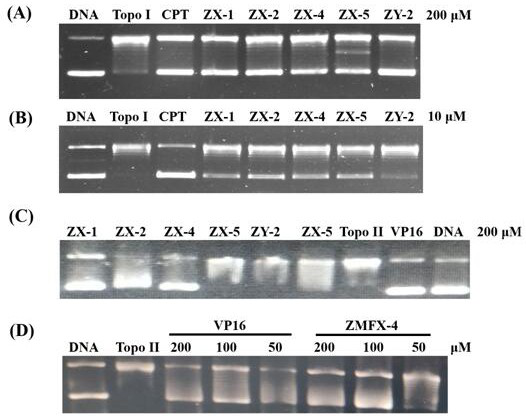
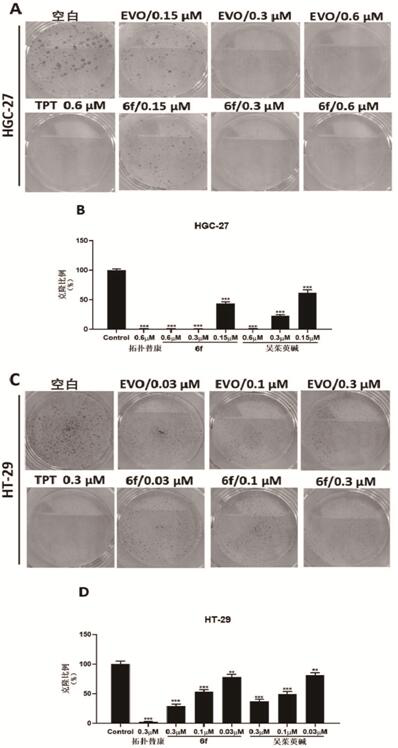
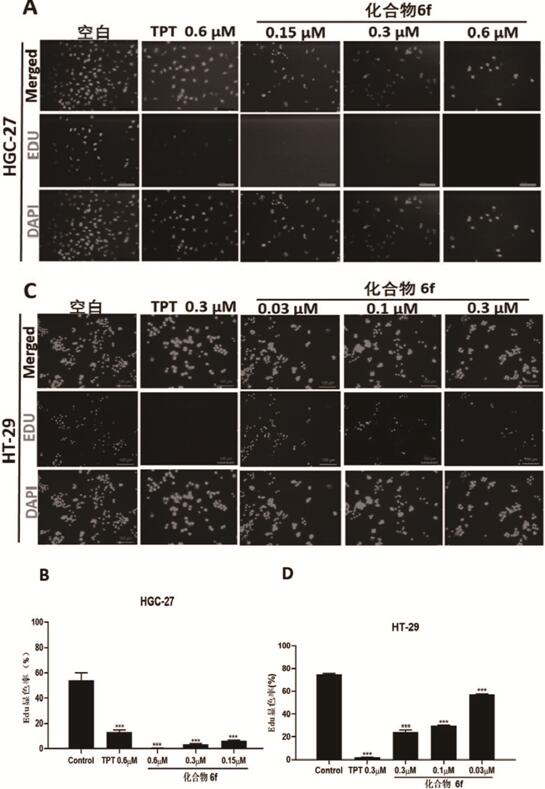
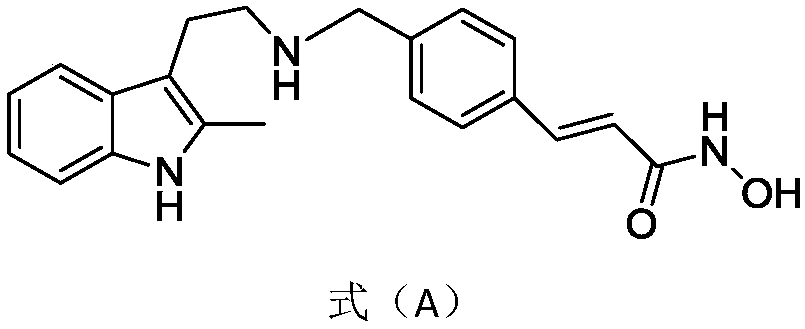
The invention provides an evodiamine derivative. The evodiamine derivative is prepared by taking an anthranilic acid compound and tryptamine as initial raw materials through a condensation reaction and a one-step ring closing reaction catalyzed by lewis acid. The evodiamine derivative prepared by the preparation method disclosed by the invention has dual inhibition effects on topoisomerase I / II, so a broad-spectrum anti-tumor effect is achieved. The derivative can induce apoptosis of cancer cells and retard proceeding to a G2 / M period in a concentration-dependent manner, and has an effect of inhibiting invasion and migration of cancer cells in vitro. The derivative has a good tumor growth inhibition effect in a nude mouse transplantation tumor model, has no obvious toxicity at a cell level and an animal level, has good safety and can be applied to preparation of anti-tumor drugs.
Owner:NANHUA UNIV
Panobinostat intermediate as well as synthesis and application thereof
InactiveCN108794375AImprove quality controlMild reaction conditionsOrganic chemistryPanobinostatBenzaldehyde
The invention belongs to the field of medicine synthesis and in particular relates to a panobinostat intermediate as well as synthesis and application thereof. The intermediate is shown as a formula II and is obtained by taking 2-methyltryptamine and 4-chloromethylbenzaldehyde to react; raw materials are cheap and easy to obtain, reaction conditions are moderate and the operation is simple; the intermediate is used as a raw material to prepare panobinostat, the cost is low, reaction steps are few, the purity is high, the reaction conditions are moderate and the operation is simple; the qualitycontrol and cost reduction of panobinostat crude drugs are facilitated. (The formula II is shown in the description.).
Owner:CHONGQING MEDICAL UNIVERSITY
Tetrahydro-beta-carboline dimer as well as preparation method and application thereof
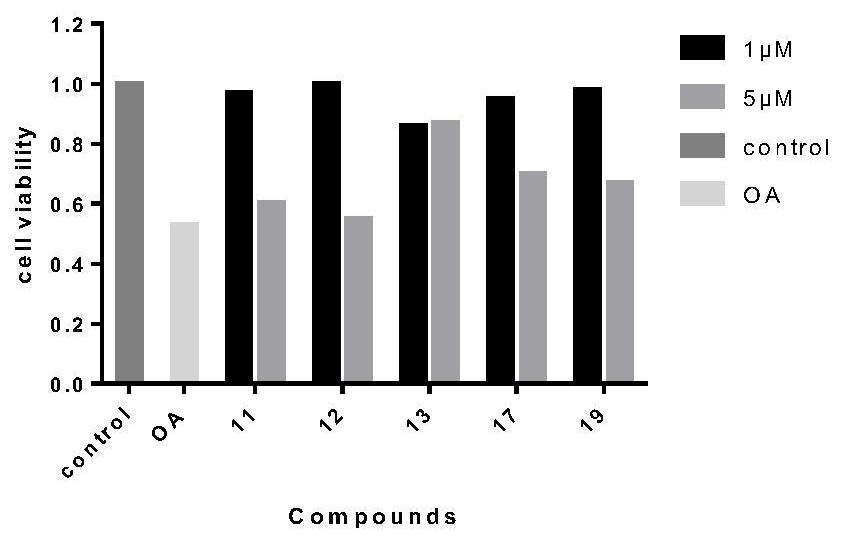
ActiveCN111675725AEasy to operateGood repeatabilityOrganic active ingredientsNervous disorderDimerDisease
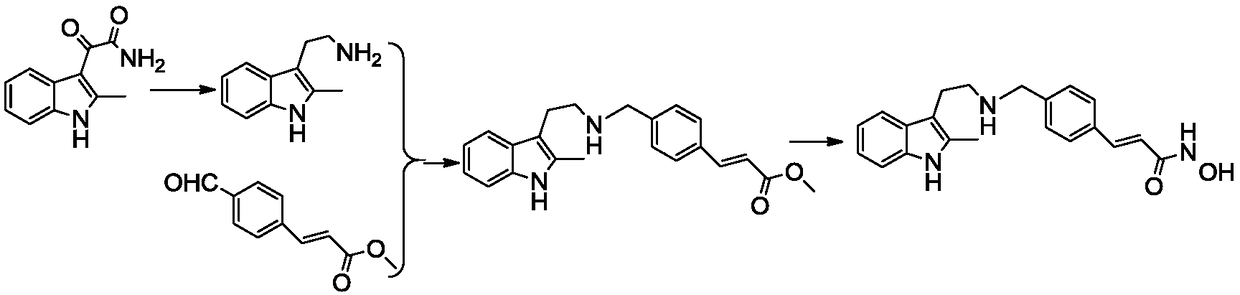
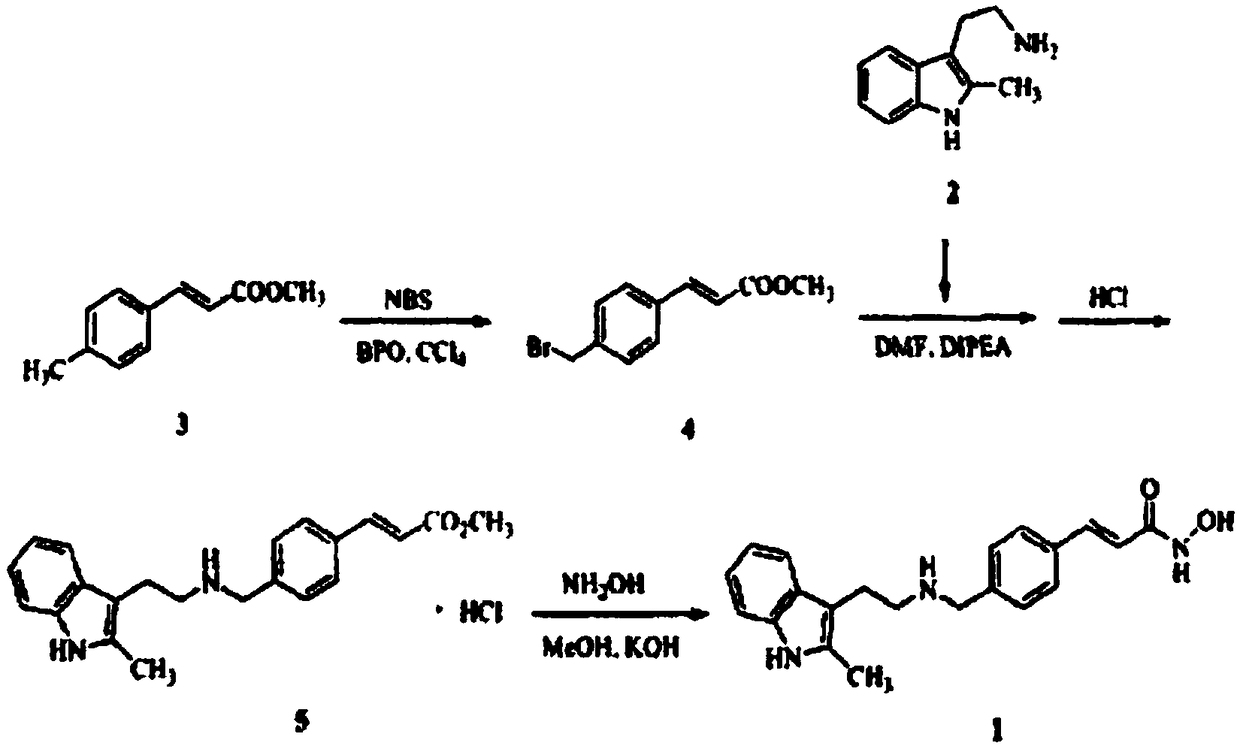
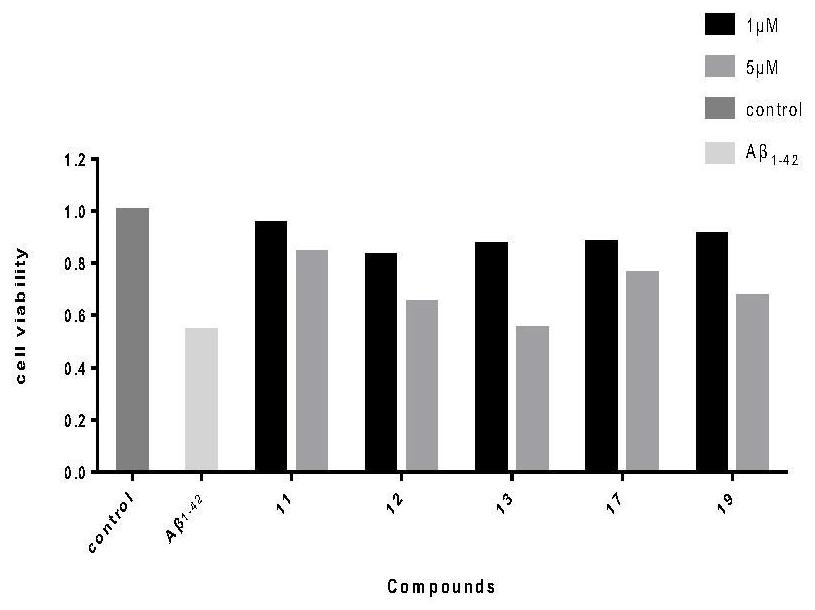
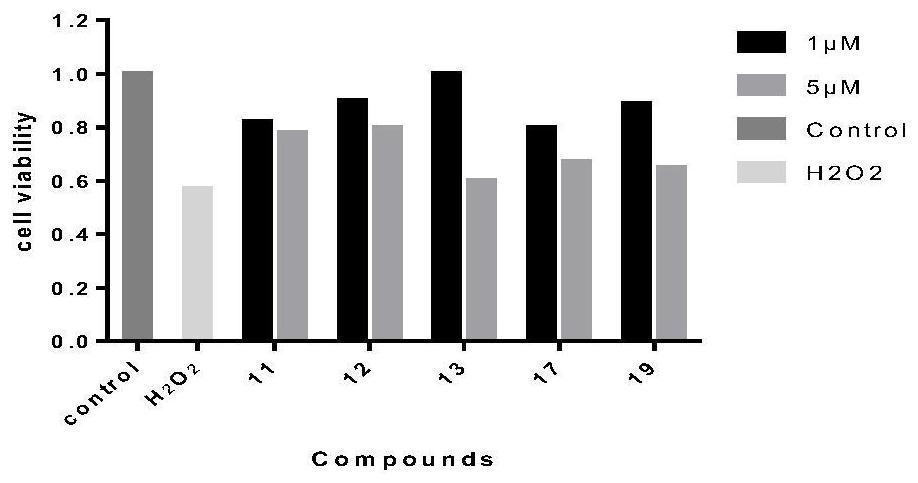
The invention discloses an application of a tetrahydro-beta-carboline dimer derivative in the preparation of a medicine for preventing or treating Alzheimer's disease. The tetrahydro-beta-carboline dimer derivative is as shown in a structural formula I, which is described in the specification, L-tryptophan and tryptamine are used as raw materials; a tetrahydro-beta-carboline monomer is generated through a Picet-Spengler reaction and aldehyde cyclization, then a tetrahydro-beta-carboline dimer is generated through acyl chloride connection and a click chemical reaction, the synthesis steps are simple, the raw materials are easy to obtain, the yield is high, and the tetrahydro-beta-carboline dimer has remarkable inhibitory activity on butyrylcholine esterase and A[beta]1-42 aggregation. According to the neural protective effect under three AD models on cells, after the cells are independently treated by A[beta]1-42, H2O2 and OA, the survival rate of the cells is low, and apoptosis or necrosis of the cells occurs. The compounds 11, 12, 13, 17 and 19 are used for co-treatment, the compounds 11, 12, 13, 17 and 19 successfully block A[beta]1-42, H2O2 and OA induced cell death, and it is proved that the compounds 11, 12, 13, 17 and 19 have a remarkable neural protective effect.
Owner:CHINA PHARM UNIV +1
Pervone vincamine injection and preparation process thereof
InactiveCN101721361AUnique dosage formEasy to useOrganic active ingredientsPharmaceutical delivery mechanismSolubilityInfusion solution
The invention provides a pervone vincamine injection and a preparation process thereof, relating to the technical field of pervone vincamine injections and preparation processes thereof. The injection is a solution type injection or frozen powder injection, and the solution type injection is divided into a water injection and a drug-carried type infusion solution, wherein the contained active constituent is the pervone vincamine, and the pervone vincamine can exit in the form of soluble salt. The invention has the advantages that compared with the prior art, the injection has the characteristics of unique formulation, convenient use, fast curative effect, safety, reliability and the like, and is applied to the clinical treatment, in particular to the intravenous injection for some patients who can not swallow drugs due to all kinds of reasons or can not carry out the oral administration due to the gastrointestinal tract dysfunction; and the preparation process is simple, convenient and economical, is easy for industrial production, has high economic and social benefits, and solves the key problem that the solubility of the pervone vincamine is lower in water because the pervone vincamine belongs to alkaloids in tryptophane system. It is especially significant to develop a pervone vincamine aqueous solution applied to the clinical treatment.
Owner:李荣立
Nitrogen-phosphorus efficient flame retardant containing p-hydroxybenzaldehyde and tryptamine structures, preparation method and application
InactiveCN111303483AImprove flame retardant performanceHinder meltingGroup 5/15 element organic compoundsP-hydroxybenzaldehydeTryptamines
The invention discloses a nitrogen-phosphorus efficient flame retardant containing p-hydroxybenzaldehyde and tryptamine structures, a preparation method and an application. The preparation method comprises the following steps: S1, adding p-hydroxybenzaldehyde and tryptamine into a solvent for reaction under the protection of nitrogen to obtain a p-hydroxybenzyltryptamine Schiff base solution; S2,adding 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide into the p-hydroxybenzyltryptamine Schiff base solution obtained in the step S1 for reaction to obtain a p-hydroxybenzyltryptamine phosphaphenanthrene solution; and S3, cooling the p-hydroxybenzyltryptamine phosphaphenanthrene solution obtained in the S2 to room temperature, adding deionized water to separate out a white solid, filtering,washing the white solid with deionized water for 2-3 times, and drying the white solid to obtain the nitrogen-phosphorus efficient flame retardant containing p-hydroxybenzaldehyde and tryptamine structures. The high polymer material prepared by the invention has good transparency.
Owner:WEST ANHUI UNIV
Therapeutic compositions comprising deuterated or partially deuterated n,n-dimethyltryptamine compounds
PendingUS20220168275A1High purityImprove efficiencyOrganic active ingredientsNervous disorderTryptaminesMedicinal chemistry
The present invention relates to compositions comprising N,N-dimethyltryptamine, deuterated N,N-dimethyltryptamine and / or partially deuterated N,N-dimethyltryptamine. In particular, the present invention relates to compositions comprising a combination of N,N-dimethyltryptamine and 2% or more by weight of one or more deuterated N,N-dimethyltryptamine compound selected from α,α-dideutero-N,N-dimethyltryptamine and α,α,β,β-tetradeutero-N,N-dimethyltryptamine. Additional and alternative compositions of the present invention comprise a combination of N,N-dimethyltryptamine and 2% or more by weight of one or more partially deuterated N,N-dimethyltryptamine compound selected from α,β,β-trideutero-N,N-dimethyltryptamine, α,β-dideutero-N,N-dimethyltryptamine, and α-deutero-N,N-dimethyltryptamine. Methods of synthesising compositions of the present invention, and methods of use of presently described compositions in treating psychiatric or psychocognitive disorders, such as major depressive disorder, are also provided.
Owner:SMALL PHARMA LTD
Application of N-aryl, benzyl tryptanthrin and derivatives thereof in preparation of hIDO2 inhibitors
The invention provides an indoleamine 2, 3-dioxygenase 2 inhibitor and application thereof, and particularly provides a formula-I compound or pharmaceutically-acceptable sodium thereof. The compound has excellent effects of inhibiting indoleamine 2, 3-dioxygenase 2 activity and resisting tumors. The invention further provides a drug composition containing the compound and application of the drug composition in resisting indoleamine 2, 3-dioxygenase.
Owner:SUZHOU ROEING BIOPHARMACEUTICALS CO LTD
Tryptophan decarboxylase (TDC) in Morus notabili and application thereof
PendingCN111849950AHigh activityIncrease enzyme activityFermentationVector-based foreign material introductionEnzyme GeneProkaryotic expression
The invention discloses a TDC in Morus notabili and application thereof. The amino acid sequence of the TDC in Morus notabili is as shown in SEQ ID NO. 4; after a Morus notabili TDC gene is cloned, aprokaryotic expression system is constructed, then enzymatic determination is conducted, and high TDC activity is determined; and thus, the Morus notabili TDC can be used as a target gene of engineering bacteria. The Morus notabili TDC can also be used as a catalyst for catalyzing conversion of L-tryptophan into tryptamine in vitro, so the Morus notabil TDC has good application prospects.
Owner:SOUTHWEST UNIV
Eudistomins Y derivative with anti-tumor activity as well as preparation method and application of Eudistomins Y derivative
ActiveCN111423438APrevent proliferationExpand the scope of useOrganic chemistryFluorescence/phosphorescenceChemical compoundOncology
The invention discloses a Eudistomins Y derivative with anti-tumor activity as well as a preparation method and application of the Eudistomins Y derivative. A tryptamine derivative (i) is used as a raw material and is subjected to a feeding reaction with acetophenone derivatives (ii), I2 and H2O2 to obtain a compound represented by a general formula (iii), and the compound represented by the general formula (iii) is subjected to a feeding reaction with R-X and K2CO3 according to the molar ratio of 1:(2-6):(2-6) to obtain a compound represented by a general formula (I). The invention provides the Eudistomins Y derivative represented by a general formula (I) and a medically acceptable salt thereof. The Eudistomins Y derivative has the antitumor activity, and is obviously superior to an Eudistomin Y1 prototype compound in the aspect of inhibiting tumor cell proliferation; compared with the non-fluorescence activity of a natural Eudistomins Y compound, the Eudistomins Y derivative represented by the general formula (I) provided by the invention has a fluorescence characteristic and can effectively trace the distribution of the Eudistomins Y derivative in cells and tissues.
Owner:YANTAI UNIV
Crystalline n-methyl tryptamine derivatives
Crystalline N-methyl tryptamine derivatives, compositions containing those crystalline forms and their methods of use are disclosed. The crystalline N-methyl tryptamine derivatives according to the invention include crystalline N-methyl-N-propyltryptamine (MPT), crystalline N-methyl-N-isopropyltryptammonium fumarate (MiPT fumarate) and crystalline 4-hydroxy-N-methyl-N-isopropyltryptammonium fumarate monohydrate (HO-MiPT fumarate monohydrate).
Owner:CAAMTECH LLC
Derivatives of tryptamine and analogous compounds, and pharmaceutical formulations containing them
This invention relates to novel substituted tryptamines and related derivatives, as well as pharmaceutical compositions formulated therefrom. These compounds, compositions and their salts can be used in the manufacture of medicaments for interacting with the melatoninergic system. These compounds and conditions can be used for treating several types of medical conditions, such as central nervous system and psychiatric disorders (sleep disorders, epilepsy and other convulsive disorders, anxiety, neurodegenerative diseases), chronobiological-based disorders (jet lag, delayed sleep syndrome, shift-work, seasonal affective disorder), neoplastic conditions, and conditions associated with senescence.
Owner:NEURIM PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

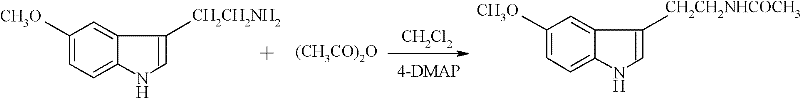






![6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound 6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1cec6fc8-0ba6-41cc-b986-c53efce53163/191014134543.png)
![6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound 6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1cec6fc8-0ba6-41cc-b986-c53efce53163/191014134545.png)
![6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound 6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazoline-4(3H)-one compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1cec6fc8-0ba6-41cc-b986-c53efce53163/BDA0002243933080000031.png)