Chiral 3-indolyl-3,3'-disubstituted oxoindole compound and preparation method thereof
A technology of oxidizing indole and indolyl group, applied in the direction of organic chemistry method, organic chemistry, etc., can solve the problems of limiting the universality of the substrate, limiting the application of the reaction, low reaction yield and the like, achieving low catalyst dosage, Simple operation and good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of Compound (III-a)
[0040]
[0041] Asymmetric synthesis of compound III-a:
[0042]Method 1: In a dry reaction tube, Chiral Catalyst A (0.02 mmol) was dissolved in 1 mL of toluene. 2-Nitroindole I-a (0.1 mmol) and 3-substituted oxindole II-a (0.12 mmol) were then added. The reaction mixture was stirred at room temperature for 2 days. Then 1 mL of dichloromethane and p-toluenesulfonic acid (0.12 mmol) were added to the reaction system, and the reaction was continued to be stirred at room temperature for 12 h. After the reaction is completed, the crude product is separated and purified by column chromatography (petroleum ether:ethyl acetate=10:1~6:1) to obtain compound III-a. The yield was 90%, 6% ee.
[0043] Method 2: In a dry reaction tube, the chiral catalyst B (0.02 mmol) was dissolved in 1 mL of toluene. 2-Nitroindole I-a (0.1 mmol) and 3-substituted oxindole II-a (0.12 mmol) were then added. The reaction mixture was stirred at room ...
Embodiment 2
[0047] Example 2: Synthesis of Compound (III-b)
[0048]
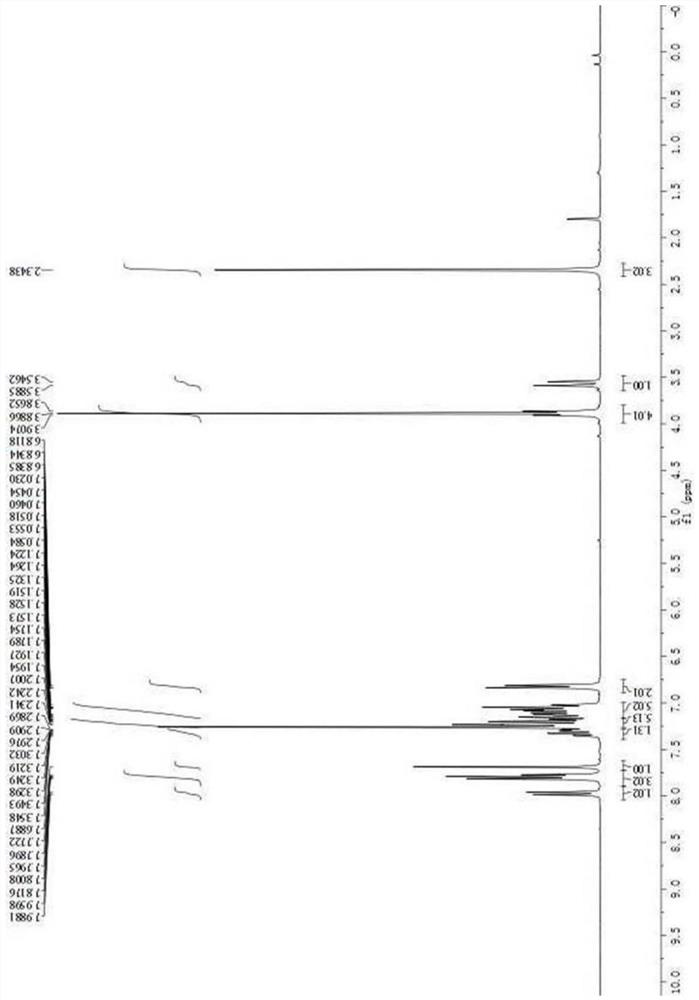
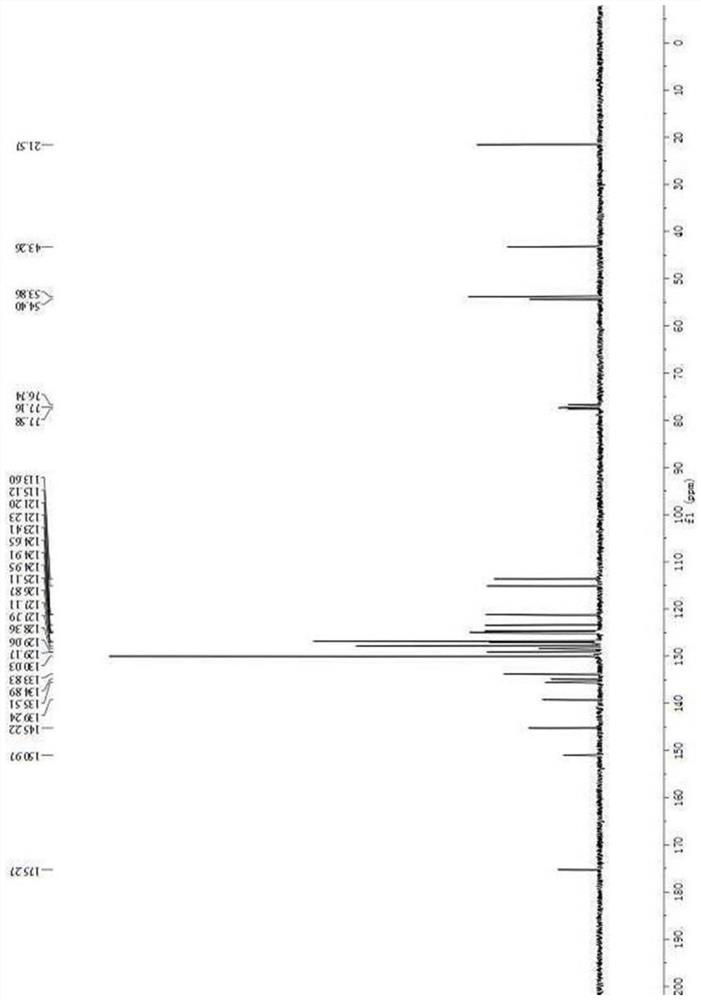
[0049] Asymmetric synthesis of compound III-b: In a dry reaction tube, 50 mg of activated Molecular sieves and chiral catalyst D (0.02 mmol) were dissolved in 1 mL of toluene. After cooling to 0°C, 2-nitroindole I-a (0.1 mmol) and 3-substituted oxide indole II-b (0.12 mmol) were added. The reaction mixture was stirred at 0°C for 5 days. The reaction system was warmed to room temperature, 1 mL of dichloromethane and methanesulfonic acid (0.12 mmol) were added to the reaction system, and the reaction was continued to stir at room temperature for 15 h. After the reaction is completed, the crude product is separated and purified by column chromatography (petroleum ether:ethyl acetate=10:1~6:1) to obtain compound III-b. White solid, 54.7 mg, 97% yield; 97% ee; [α] D 20 =+82.5(c 1.0, CH 2 Cl 2 ); m.p.261.8-262.7℃.Theee was determined by HPLC analysis using a ChiralpakAD-H column(70 / 30hexane / i-PrOH;flow rate:1.0mL / ...
Embodiment 3
[0050] Example 3: Synthesis of Compound (III-c)
[0051]
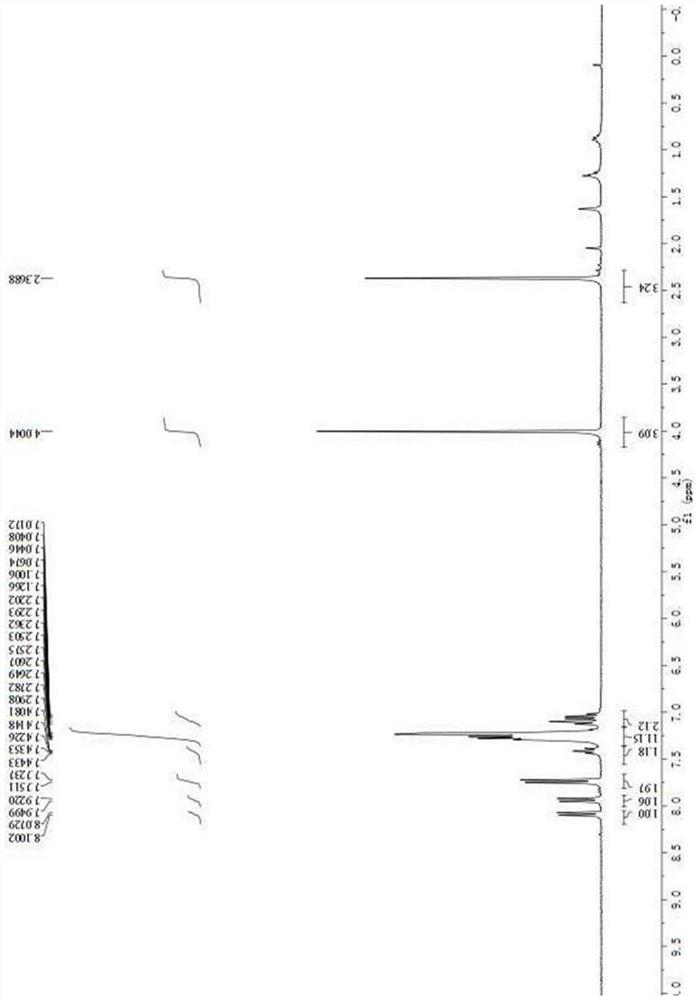
[0052] Asymmetric synthesis of compound III-c: In a dry reaction tube, 50 mg of activated Molecular sieves and chiral catalyst D (0.02 mmol) were dissolved in 1 mL of chlorobenzene. After cooling to 0°C, 2-nitroindole I-a (0.1 mmol) and 3-substituted oxide indole II-c (0.12 mmol) were added. The reaction mixture was stirred at 0°C for 5 days. The reaction system was raised to room temperature, 1 mL of chloroform and p-toluenesulfonic acid (0.12 mmol) were added to the reaction system, and the reaction was continued to stir at room temperature for 12 h. After the reaction is complete, the crude product is separated and purified by column chromatography (petroleum ether:ethyl acetate=10:1~6:1) to obtain compound III-c. White solid, 52.8 mg, 91% yield; 92% ee; [α] D 20 =+87.3(c 1.0, CH 2 Cl 2 ); m.p.204.3-205.1℃.The ee was determined by HPLC analysis using a ChiralpakAD-H column(70 / 30hexane / i-PrOH;flowrate:1.0m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com