Use of beggarweed alkaloid monomer component
A technology of alkaloids and leech, applied in the field of alkaloid monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
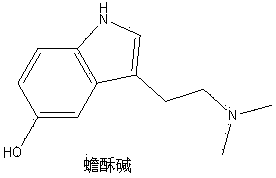
[0024] Example 1 The alkaloid monomer components of leeches alkaloids bufynine, 5-Hydroxy-N, N-dimethyl tryptamine N 12 Preparation of -oxide, 5-methoxy-N,N-dimethyltryptamine
[0025]10kg of leeches, ground into powder, reflux extraction with 6 times of industrial ethanol in 80°C water bath twice, each time for 2 hours. After the extraction is completed, the ethanol extracts are combined, filtered, and the filtrate is evaporated to an extract sample under reduced pressure. The alcohol extraction extract is suspended in 10L of distilled water, and after stirring, the suspension is adjusted to about pH=2-3 with 2mol / L hydrochloric acid solution. After the acid solution was left to stand for 8 hours, filter it, adjust the filtrate to pH=10-11 with 4mol / L sodium hydroxide, immediately extract it three times with an equal volume of chloroform, combine the organic phases, and evaporate to dryness under reduced pressure to obtain the alkaloid extract . A total of 7.5g. Perform m...
Embodiment 2
[0026] Example 2 Determination of the monoamine oxidase inhibitory activity of the alkaloid monomer components of the leeches by enzyme labeling method
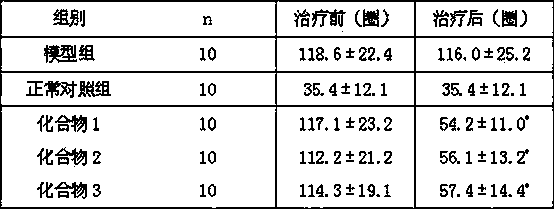
[0027] Determination of the alkaloid monomer components of the mountain leeches by enzyme labeling method: compounds 1 - 3 (Concentrations are all 100 μ g / ml) inhibition rate, iproniazid phosphate is the positive control drug. The specific experimental process is as follows:
[0028] (1) Preparation of monoamine oxidase:
[0029] One female wistar rat was killed by cervical dislocation, the liver was taken out, cut into pieces, washed several times with phosphate buffer (pH=7.6), after washing, 20ml of 0.3M sucrose buffer was added for homogenization After fully homogenizing, take 10ml each and pour into two 50ml centrifuge tubes. After washing the homogenizer with 20ml of 0.3M sucrose buffer, 10ml of each was poured into the aforementioned centrifuge tubes. After balancing with a tray balance, centrifuge at 1000×g for 1...
Embodiment 3
[0036] Example 3 Forced swimming test (depressed mouse model)
[0037] Put male SD rats individually into a glass cylinder with a water depth of 15cm (height: 40cm and diameter: 18cm), the water temperature is (24±2)°C, and the water depth can be slightly adjusted so that the rear feet of the rats can just touch the bottom of the cylinder but are not enough to support Body is appropriate. When first put in, the rats were forced to swim and struggled to climb up or dive into the bottom of the cylinder. After 2-3 minutes, the activity gradually weakened, and the intermittent immobility state appeared, and the time became longer and longer. About 80% of the total time, the rats were dried after 15 minutes of the pre-test. Experimental grouping: excluding the unqualified ones in the preliminary test, 50 qualified rats were randomly divided into 5 groups, 10 rats in each group. The blank control group and the fluoxetine positive control group were intragastrically given normal sal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com