Eudistomins Y derivative with anti-tumor activity as well as preparation method and application of Eudistomins Y derivative
A technology of antineoplastic drugs and derivatives, applied in antineoplastic drugs, chemical instruments and methods, organic chemistry, etc., can solve the problems of individual differences in drug action and drug resistance, and achieve the effect of expanding the scope of drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
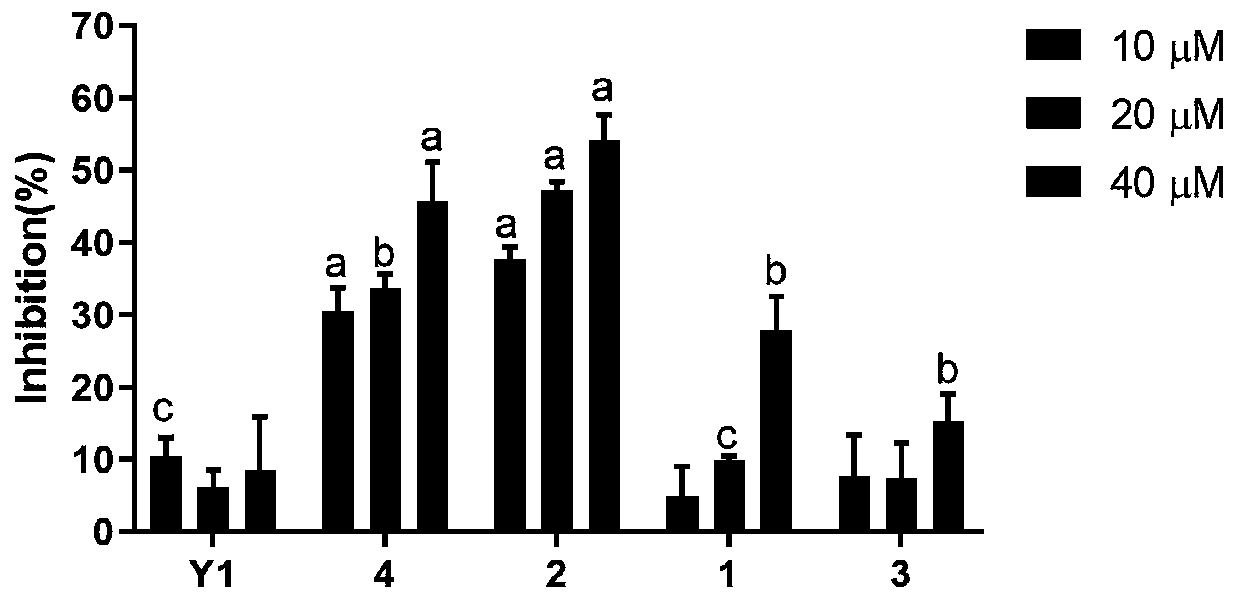
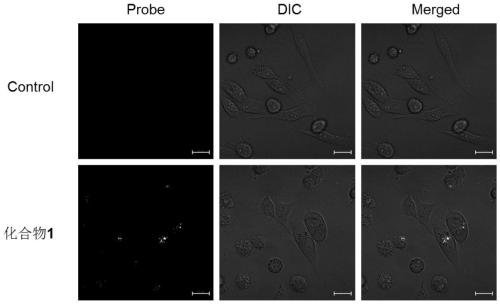
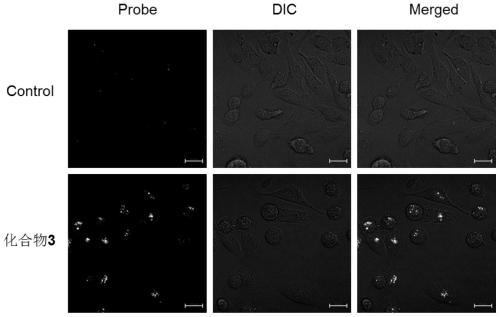
Image
Examples
Embodiment 1
[0039]
[0040] Tryptamine (128mg, 0.8mmol), p-hydroxyacetophenone (96mg, 0.8mmol) and I 2 (162 mg, 0.64 mmol) was dissolved in DMSO (3 mL), and H was added at room temperature 2 o 2 (30%, 1.5eq), after stirring and dissolving, the temperature rose to 110°C, and TLC tracked and detected the reaction. After the reaction was completed, the reaction was cooled to room temperature, ethyl acetate, saturated brine, 10% Na 2 S 2 o 3 , saturated ammonium chloride, extracted, dried with anhydrous sodium sulfate, filtered, concentrated, separated and purified by column chromatography to obtain EudistominY1 (138mg, 0.48mmol, 60%) as a yellow solid.
[0041] Eudistomin Y1, 1 H NMR (400MHz, DMSO-d 6 )δ11.90(s,1H),8.48(d,J=5.0Hz,1H),8.36(d,J=4.9Hz,1H),8.27(d,J=7.9Hz,1H),8.22(ddd, 2H),7.75(d,J=8.2Hz,1H),7.55(ddd,J=8.3,7.1,1.2Hz,1H),7.25(td,1H),6.90(ddd,2H).
[0042]
[0043] Under argon protection, EudistominY1 (289mg, 1mmol) and K 2 CO 3 (690mg, 5mmol) was dissolved in anh...
Embodiment 2
[0047] Preparation of compound 3
[0048]
[0049] Under argon protection, compound 2 (30mg, 0.09mmol) and K 2 CO 3 (62mg, 0.45mmol) was dissolved in anhydrous DMF (1mL), and methyl iodide (28μL, 0.45mmol) was slowly added in an ice bath, stirred at room temperature, and the reaction was monitored by TLC. It was extracted with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatographed to obtain compound 3 (27mg, 0.08mmol, 90%) as a white solid.
[0050] Compound 3, 1 HNMR (400MHz, CDCl 3 )δ8.51(d,J=5.1Hz,1H),8.19(d,J=7.9Hz,1H),8.13–8.10(m,1H),8.09–8.04(m,2H),7.69–7.61(m ,1H),7.49(d,J=8.3Hz,1H),7.37–7.30(m,1H),7.09–7.04(m,2H),4.78(d,J=2.4Hz,2H),3.77(s, 3H),2.59–2.52(m,1H).
Embodiment 3
[0053]
[0054] Tryptamine (161mg, 1mmol), p-methoxyacetophenone (150mg, 1mmol) and I 2 (203 mg, 0.8 mmol) was dissolved in DMSO (4 mL), and H was added at room temperature 2 o 2 (30%, 1.5eq), stir to dissolve, raise the temperature to 120°C, and track and detect the reaction by TLC. After the reaction was completed, the reaction was cooled to room temperature, ethyl acetate, saturated brine, 10% Na 2 S 2 o 3 , saturated ammonium chloride, extracted, dried over anhydrous sodium sulfate, filtered, concentrated, separated and purified by column chromatography to obtain yellow solid compound 4 (151 mg, 0.5 mmol, 50%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com