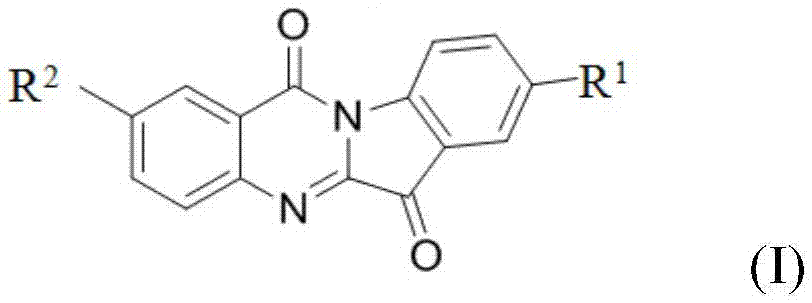
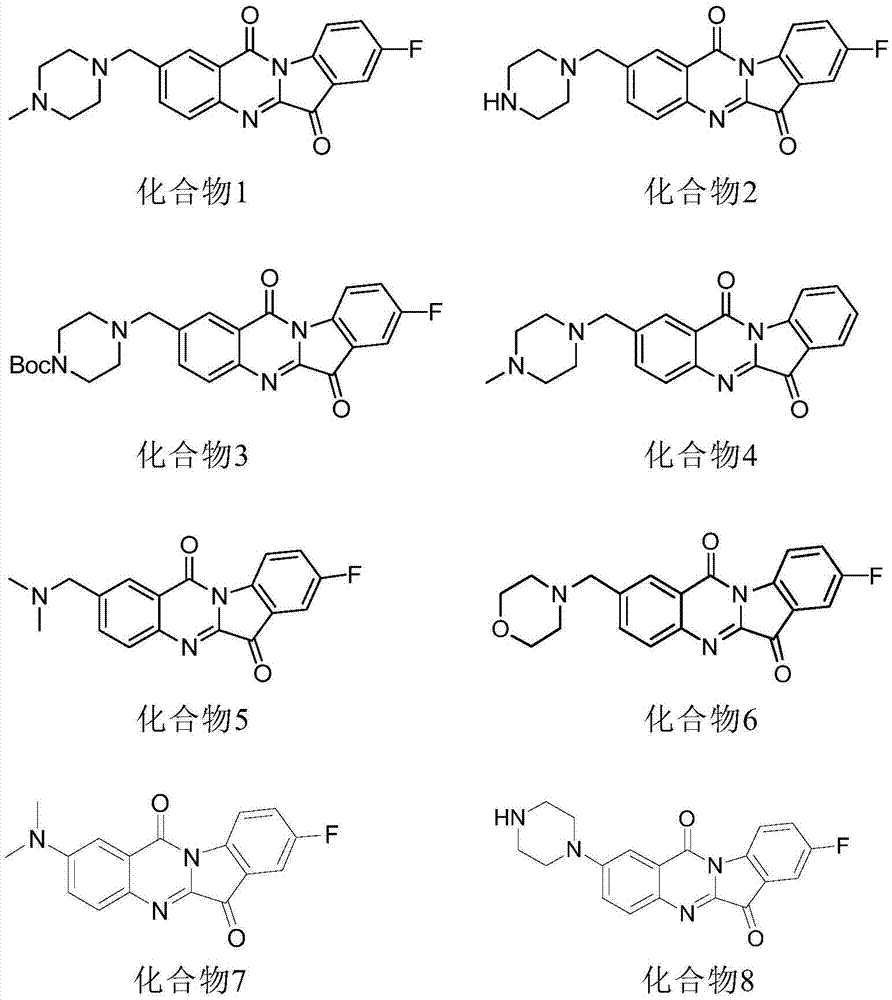
Application of N-aryl, benzyl tryptanthrin and derivatives thereof in preparation of hIDO2 inhibitors
A technology of use and preparation, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
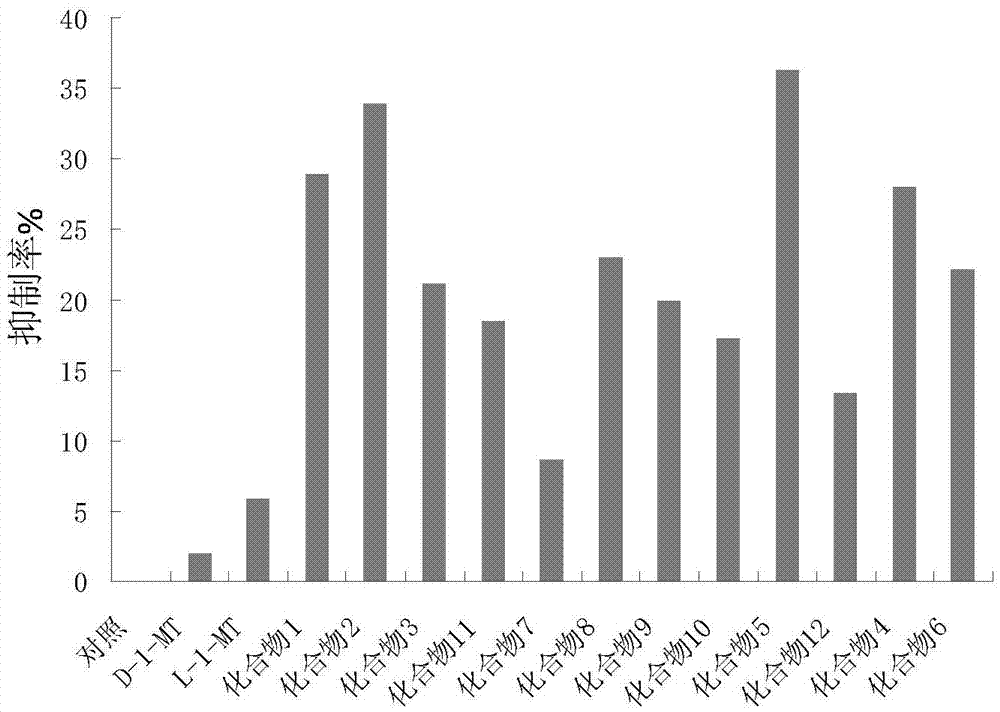
[0092] Preliminary Screening of IDO2 Inhibitors at Enzyme Level
[0093] In a 500 μL standard detection system, 50 mmol / L potassium phosphate buffer (pH 7.5), 200 μg / mL catalase, 40 mmol / L ascorbic acid, 20 μmol / L methylene blue, a suitable concentration of the substrate L-tryptophan and the final Mix the IDO2 inhibitor to be tested (including the compound of the present invention, L-1-MT and D-1-MT) with a concentration of 10 μM, put the mixed solution in a water bath at 37°C for 5 minutes, add IDO2 to the above mixed solution, and react at 37°C for 30 minutes After the end of the enzymatic reaction, 200 μL of 30% (w / v) trichloroacetic acid was added to stop the reaction, and then heated in a water bath at 65°C for 15 minutes to complete the reaction product from N-formylkynurenine to kynurenine transform. Centrifuge at 138,000×g for 10 minutes, draw 100 μL of the supernatant and mix well with an equal volume of 2‰ (w / v) acetic acid solution of p-dimethylaminobenzaldehyde, k...
Embodiment 2
[0096] Determination of inhibition type and Ki value of compounds of the present invention
[0097] In the 500 μL detection system described in Example 1, the substrate L-tryptophan of different concentrations (20, 30, 40 mM or 20, 25, 35 mM) were added, and at each substrate concentration, different concentrations Gradient inhibitors to be tested (including the compound of the present invention, L-1-MT and D-1-MT), no inhibitor was added to the control group, 10 μL IDO2 (about 1 μM) was added to the mixed solution in a water bath at 37°C for 5 minutes, and 37°C React for 30 minutes, add 200 μL of 30% (w / v) trichloroacetic acid to terminate the reaction after the enzymatic reaction, and then heat in a water bath at 65°C for 15 minutes to complete the reaction product from N-formylkynurenine to kynurenine acid conversion. Centrifuge at 138,000×g for 10 minutes, draw 100 μL of the supernatant and mix well with an equal volume of 2‰ (w / v) acetic acid solution of p-dimethylaminob...
Embodiment 3
[0103] The half effective inhibitory concentration IC of the compound of the present invention 50 Determination of value
[0104] The ICs of compounds of the present invention inhibiting IDO1 and IDO2 were measured in vitro and at the cellular level respectively 50 value.
[0105] In vitro level (enzyme level): In the 500 μ L detection system described in embodiment 1, add the substrate L-tryptophan of 30 mM and the inhibitor of different concentration gradient (comprising compound of the present invention, L-1-MT and D- 1-MT), the control group did not add inhibitors, and other treatments were the same as in Example 1. After the reaction, a microplate reader was used to detect the absorbance at 492nm. The inhibition rate was plotted against the concentration of the inhibitor, and the IC was calculated using the modified Cole's method 50 value.
[0106] Cell level: U87MG cell line (ATCC No.: HTB-14), cultured in DMEM high glucose containing 10% fetal bovine serum at 37°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com